ABSTRACT
The driver mutations in exon 9 of the calreticulin protein have only been identified in patients with myeloid cancers. We recently demonstrated that healthy individuals display strong and frequent T-cell responses towards this mutation. This memory T-cell response is likely evidence of the elimination of mutated cells in healthy individuals.
KEYWORDS: Neoantigens, memory T-cells, calreticulin, hematological cancer, cancer immunoediting
Text
The Philadelphia chromosome negative chronic myeloproliferative neoplasms (MPN) are disorders of the hematopoietic stem cells in the bone marrow.1 Approximately 15% of patients with MPN harbor a driver mutation in exon 9 of the calreticulin (CALR) gene, which results in the generation of a novel mutant CALR protein with a 36 amino acid C-terminus.1,2 We have previously demonstrated that T cells from MPN patients with mutant CALR recognize epitopes in the mutant C-terminus,3 and that T cells isolated and expanded from MPN patients are able to recognize and kill autologous CALR mutant cells.4 The CALR mutations have only been identified in patients with myeloid cancers, never in healthy individuals.2,5 Thus, healthy individuals are not expected to harbor immune responses to epitopes from the mutant CALR C-terminus, as the immune system in these individuals has never been challenged with mutant CALR epitopes.
However, we just recently demonstrated that healthy individuals display strong and frequent T-cell responses to several epitopes derived from the mutant CALR C-terminus.6 Both in vitro stimulated peripheral blood mononuclear cell (PBMC) cultures and PBMC analyzed directly ex vivo displayed strong responses to several mutant CALR epitopes. These CALR-mutant-specific T-cell responses identified in ex vivo experiments are most interesting as, even in cancer patients, it is extremely rare to detect tumor-associated-antigen-specific T cells without prior stimulation of cells in vitro.7 Thus, these frequent ex vivo responses show that healthy donors harbor a high frequency of circulating T cells specific to mutant CALR epitopes. Additionally, the responses in healthy donors were even stronger and more frequent than the responses in patients with CALR-mutant MPN. This finding fits well with the theory of cancer immunoediting, which stipulates that patients with established cancer have a decreased cancer-specific immune response.8
The surprising amount of strong T-cell responses to the mutant CALR epitopes spurred us to investigate whether the responding T cells were naïve, or if they could possibly be antigen-experienced memory T cells. This was investigated by enriching memory T cells using either magnetically activated cell sorting (MACS) or fluorescence-activated cell sorting (FACS) and then by analyzing CALR-mutant-specific responses in the memory T cell enriched cultures. Surprisingly, memory T cells were activated upon stimulation with mutant CALR epitopes while naïve T cells isolated by MACS or FACS were not activated upon stimulation with mutant CALR epitopes. Additionally, we showed that CD4+ T-cell clones isolated from CD4+ memory T cells from a healthy donor were able to kill autologous dendritic cells presenting mutant CALR epitopes. We had a great interest investigating the phenotype of the T cells specific to the mutant CALR epitopes, as healthy donors do not harbor CALR-mutations, and thus should not have been challenged with mutant CALR epitopes. Additionally, Pittet and colleagues demonstrated that the immune responses in healthy donors specific to the tumor-associated-antigen MART-1 were indeed from naïve T-cell responses.9 Nevertheless, we showed that the CALR-mutant-specific T-cell responses in healthy donors were indeed memory T-cell responses. We investigated if a potential sequence homology between the mutant CALR C-terminus and other known epitopes could explain the high frequency of T-cell responses to the mutant C-terminus. However, the mutant CALR C-terminus did not share sequence homology with any other epitopes.
One likely explanation for the frequently occurring CALR mutant-specific immune responses in healthy individuals is that healthy persons occasionally acquire a CALR exon 9 mutation. However, due to the high immunogenic potential of these mutations, the mutant cells are cleared by the immune system and consequently memory T cells specific to the CALR mutations are generated (Figure 1). For a long time, it has been speculated that the immune system is able to spontaneously eliminate neoplastic cells before the establishment of overt malignancy, but such a feat has never been shown in man.8,10 Additionally, memory T-cell responses to tumor-specific-antigens, such as the CALR-mutations, have never been identified in healthy individuals. We believe our detection of these memory T-cell responses in healthy donors likely provided evidence of the first “E” – elimination – in the theory of cancer immunoediting, and that the immune system in man is able to spontaneously clear neoplastic cells.
Figure 1.
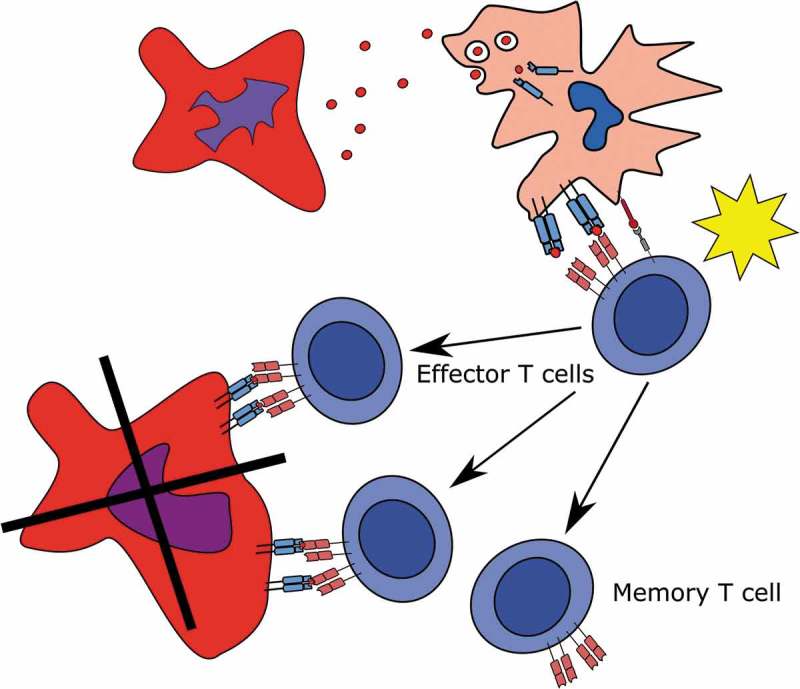
Generation of T cell memory to the CALR-mutations.
(Top to down). A CALR-mutant cell dies and sheds its mutant CALR epitopes to an antigen presenting cell which phagocytoses, process and presents mutant CALR epitopes to a naïve T cell. The naïve T cell is primed, starts proliferating and differentiating into either effector T cells, which kill the CALR-mutant cells, or to memory T cells.
Funding Statement
This study was supported in part by grant from Danish Cancer Society to H.C.H under grant [R149-A10159] in addition to support from Herlev Hospital.
Disclosure of Potential Conflicts of Interest
No authors have conflicts of interest to disclose. However, it should be noted that Morten Orebo Holmström, Hans Carl Hasselbalch, and Mads Hald Andersen have filed a patent regarding the CALR exon 9 mutation as a target for cancer immune therapy. The patent has been transferred to University Hospital Zealand, Zealand Region and Copenhagen University Hospital at Herlev, Capital Region according to Danish Law concerning inventions made at public research institutions.
References
- 1.Spivak JL. Myeloproliferative neoplasms. N Engl J Med. 2017;376(22):2168–2181. doi: 10.1056/NEJMra1406186. [DOI] [PubMed] [Google Scholar]
- 2.Ortmann CA, Kent DG, Nangalia J, Silber Y, Wedge DC, Grinfeld J, Baxter EJ, Massie CE, Papaemmanuil E, Menon S, et al. Effect of mutation order on myeloproliferative neoplasms. N Engl J Med. 2015;372(7):601–612. doi: 10.1056/NEJMoa1412098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmström MO, Riley CH, Svane IM, Hasselbalch HC, Andersen MH. The CALR exon 9 mutations are shared neoantigens in patients with CALR mutant chronic myeloproliferative neoplasms. Leukemia. 2016;30(12):2413–2416. doi: 10.1038/leu.2016.233. [DOI] [PubMed] [Google Scholar]
- 4.Holmstrom MO, Martinenaite E, Ahmad SM, Met O, Friese C, Kjaer L, Riley CH, Thor Straten P, Svane IM, Hasselbalch HC, et al. The calreticulin (CALR) exon 9 mutations are promising targets for cancer immune therapy. Leukemia. 2018;32(2):429–437. doi: 10.1038/leu.2017.214. [DOI] [PubMed] [Google Scholar]
- 5.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NCC, Berg T, Gisslinger B, Pietra D, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 6.Holmström MO, Ahmad SM, Klausen U, Bendtsen SK, Martinenaite E, Riley CH, Svane IM, Kjær L, Skov V, Ellervik C, et al. High frequencies of circulating memory T cells specific for calreticulin exon 9 mutations in healthy individuals. Blood Cancer J. 2019;9(2):8. doi: 10.1038/s41408-018-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Wolfgang I, Hofmann W, Uharek L, Thiel E, Scheibenbogen C, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113(26):6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 8.Dunn GP, Old LJ, Schreiber RD. The Three Es of Cancer Immunoediting. Annu Rev Immunol. 2004;22(1):329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 9.Pittet MJ, Valmori D, Dunbar PR, Speiser DE, Liénard D, Lejeune F, Fleischhauer K, Cerundolo V, Cerottini JC, Romero P. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190(5):705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting : from immuno- surveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]


