SUMMARY
Salmonella is a leading cause of bacterial foodborne illness. We report the collaborative investigative efforts of US and Canadian public health officials during the 2013–2014 international outbreak of multiple Salmonella serotype infections linked to sprouted chia seed powder. The investigation included open-ended interviews of ill persons, traceback, product testing, facility inspections, and trace forward. Ninety-four persons infected with outbreak strains from 16 states and four provinces were identified; 21% were hospitalized and none died. Fifty-four (96%) of 56 persons who consumed chia seed powder, reported 13 different brands that traced back to a single Canadian firm, distributed by four US and eight Canadian companies. Laboratory testing yielded outbreak strains from leftover and intact product. Contaminated product was recalled. Although chia seed powder is a novel outbreak vehicle, sprouted seeds are recognized as an important cause of foodborne illness; firms should follow available guidance to reduce the risk of bacterial contamination during sprouting.
Key words: Disease outbreak, foodborne infections, Salmonella enterica
INTRODUCTION
Salmonella is a leading cause of bacterial foodborne illness in both the USA and Canada, resulting in a combined estimated 1·1 million illnesses, 20 000 hospitalizations, and 400 deaths each year [1, 2]. Salmonella is found in the intestinal tract of animals, and human infections are often caused by consuming contaminated foods of animal origin as well as animal contact [3]. Increasingly, contaminated raw produce has been associated with outbreaks of salmonellosis [4].
Consuming raw sprouted seeds is recognized as an important cause of foodborne illness [5–7]. In the last two decades, sprouted seeds have been associated with at least 55 foodborne outbreaks resulting in more than 1500 illnesses worldwide [8]. The conditions under which seeds are sprouted favor proliferation of any bacteria that may be present on the seeds, and sprouted seeds are often consumed raw without undergoing processing that could kill foodborne pathogens [9, 10]. This paper describes the collaborative efforts of the US CDC (Centers for Disease Control and Prevention), Public Health Agency of Canada (PHAC), US Food and Drug Administration (FDA), Canadian Food Inspection Agency (CFIA), and state and provincial partners to investigate an international outbreak of multiple Salmonella serotype infections linked to a novel vehicle of foodborne illness, sprouted chia seed powder, and summarizes the ongoing risk of foodborne illness for persons consuming raw, sprouted foods.
METHODS
Outbreak detection, case-finding, and hypothesis generation
On 6 May 2014 PulseNet USA, the national molecular subtyping network for foodborne disease surveillance, identified nine Salmonella Newport isolates from five states with an indistinguishable pulsed-field gel electrophoresis (PFGE) pattern (US XbaI restriction enzyme pattern JJPX01·1645). This PFGE pattern was novel in the USA. Although different PFGE naming conventions are used in the USA and Canada, four isolates from two Canadian provinces were identified during a similar timeframe with a PFGE pattern indistinguishable from the US isolates (Canada XbaI pattern NewpXAI.0413). This PFGE pattern was also novel to PulseNet Canada.
Case definitions were expanded in the USA and Canada during the course of the investigation as additional Salmonella serotypes and PFGE patterns were identified through product testing. Criteria for including PFGE patterns in the case definition differed slightly between countries. In Canada, all PFGE patterns isolated from intact product testing which had also been identified in human isolates were included in the case definition and investigated. In the USA, PFGE patterns were only included in the case definition if additional epidemiological data collected through patient interviews supported the relationship of the PFGE pattern to the outbreak. For common strains identified through product testing in the USA, a second restriction enzyme (BlnI) analysis was conducted and interviews were sought only for ill persons with clinical isolates that matched the two enzyme pattern combination.
In the USA, a confirmed case was defined as a person with a culture-confirmed infection with Salmonella Newport, Salmonella Hartford, or Salmonella Oranienburg after 1 January 2014 indistinguishable by PFGE to the outbreak strains. The outbreak strains included four PFGE patterns: US XbaI patterns JJPX01·1645 (Newport), JJPX01·4815 (Newport), JHAX01·0291 (Hartford), and JJXX01·0027 (Oranienburg). In Canada, a confirmed case was defined as a person with a culture-confirmed infection with Salmonella Newport, S. Hartford, S. Oranienburg, or S. Saintpaul on or after 1 December 2013 indistinguishable by PFGE to the outbreak strains. The outbreak strains included 13 PFGE patterns: CAN XbaI/BlnI patterns NewpXAI.0413 (Newport), NewpXAI.0416 (Newport), NewpXAI.0418 (Newport), NewpXAI.0423 (Newport), NewpXAI.0424 (Newport), HartXAI.0038 (Hartford), HartXAI.0040 (Hartford), OraniXAI.0005/OraniBNI.0007 (Oranienburg), OraniXAI.0005/OraniBNI.0073 (Oranienburg), OraniXAI.0005/OraniBNI.0075 (Oranienburg), OraniXAI.0006 (Oranienburg), SainXAI.0005/SainBNI.0073 (Saintpaul), and SainXAI.0005/SainBNI.0010 (Saintpaul).
Initial hypothesis generation in the USA consisted of open-ended interviews of ill persons, followed by a series of supplemental questionnaires to refine hypotheses. Iterative interviews were conducted with consenting recently ill persons or proxies to obtain food history for the 7 days before illness onset. Three different supplemental questionnaires in the USA and one in Canada were developed and deployed during the investigation to gather increasingly detailed information on foods, products, and brands consumed. Supplemental questionnaires focusing on foods commonly reported during the first several open-ended interviews (i.e., smoothies, supplements, seeds, and fruit) were implemented starting on 26 May 2014 in the USA. A focused questionnaire based on the CDC questionnaire was implemented on 28 May 2014 in Canada. The questionnaires were revised to further focus on chia seeds and chia seed powder during the investigation.
Although there were no existing data on expected exposure frequencies in the general population for chia seeds and chia seed powder products in the USA or Canada, PHAC was in the process of conducting a national population-based 7-day food consumption study, and temporarily added questions to the survey tool in order to estimate baseline consumption of chia and chia-containing foods. The proportion of case-patients reporting exposure to specific foods was compared with the proportion reported in the PHAC survey of healthy persons. A binomial probability distribution was used to determine which food exposures reported by case-patients were significantly higher than those reported by healthy persons in Canada.
Product traceback, facility inspections, and trace forward
Public health agencies collected detailed product information, including purchase dates, lot codes, and product photographs during open-ended interviews, which were provided to food regulatory officials in the USA and Canada.
CalFERT, a partnership between the California Department of Public Health and the FDA's San Francisco and Los Angeles District Offices, and CFIA inspected suppliers and processors of chia seed powder and chia-containing products identified through the epidemiological and traceback investigations. Additional traceback investigation determined the source-country of the raw agricultural product (chia seeds) used to produce the chia seed powder; FDA and CFIA conducted trace forward of these products to discern the extent of distribution of potentially contaminated products as well as the multitude of brand names used.
Laboratory investigation
State, provincial, and federal public health and food safety laboratories performed serotyping on all Salmonella isolates identified. Clinical isolates were obtained from ill persons and food isolates from their opened food samples, intact food samples collected at retail, as well as from samples taken during inspection activities.
Clinical and food isolates were subtyped using PFGE at state, provincial, and federal laboratories, and results were electronically submitted to PulseNet USA and PulseNet Canada. Four outbreak isolates in the USA were tested for susceptibility to 15 antimicrobial agents by the National Antimicrobial Resistance Monitoring System (NARMS) laboratory at CDC. PulseNet International was queried for the outbreak strains to determine if there were other countries identifying similar PFGE patterns.
RESULTS
Case-finding and demographics
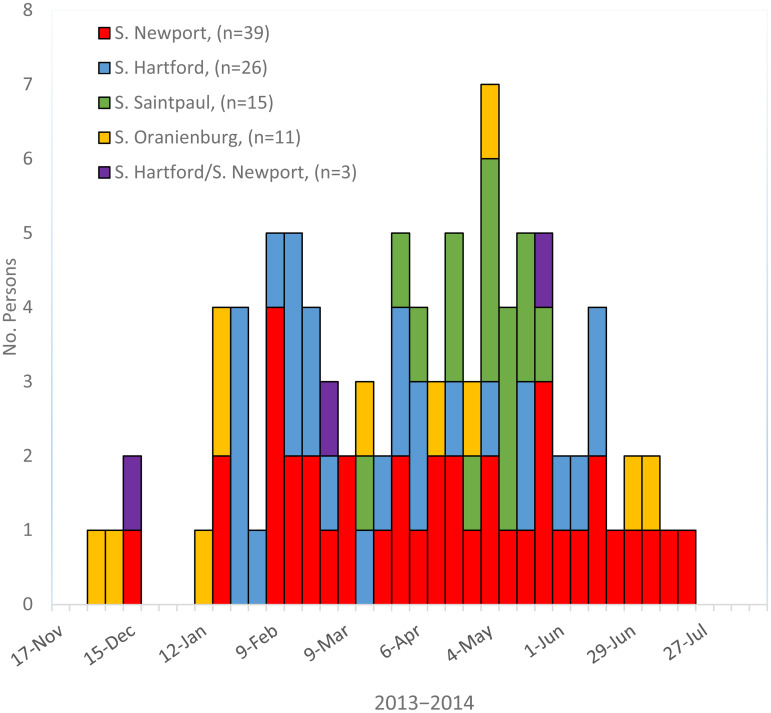
A total of 94 persons infected with the outbreak strains of Salmonella Newport (39 persons), S. Hartford (26 persons), S. Oranienburg (11 persons), S. Saintpaul (15 persons), or co-infected with two outbreak strains (3 persons) were identified in the USA and Canada (Fig. 1). Ill persons were reported from 16 states and four provinces (Fig 2). Dates of illness onset ranged from 4 December 2013 to 22 July 2014. Ill persons ranged in age from 6 months to 89 years, with a median of 42 years; 66% (n = 62) were female. Seventeen (21%) of 80 ill persons for whom information was available were hospitalized; no deaths were reported (Table 1).
Fig. 1.
Persons infected with the outbreak strains of Salmonella Newport, S. Hartford, S. Saintpaul, S. Oranienbrurg, and coinfected with S. Newport and S. Hartford in the USA and Canada by week of illness onset, 2013–2014 (n = 94).
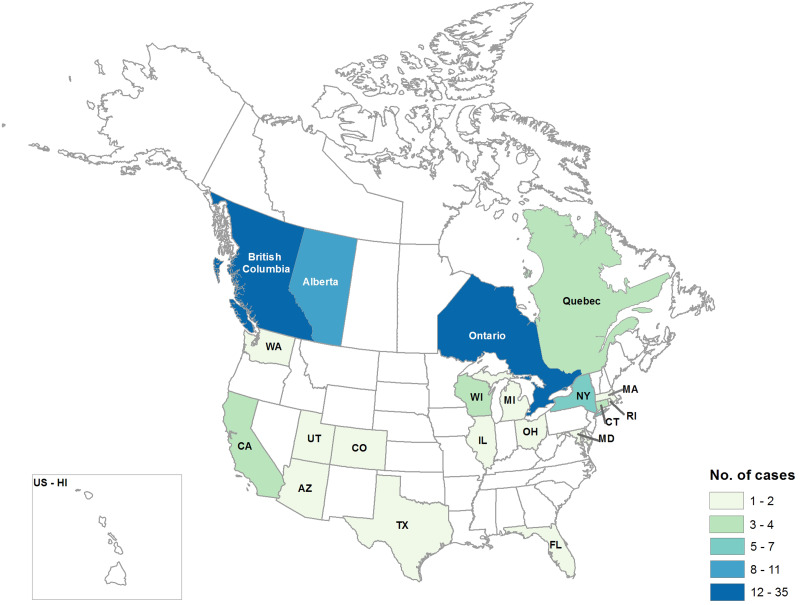
Fig. 2.
Persons infected with the outbreak strains of Salmonella Newport, S. Hartford, S. Saintpaul, S. Oranienbrurg, and coinfected with S. Newport and S. Hartford in the USA and Canada by state and province, 2013–2014, (n = 94) (case count map) USA: Arizona (1), California (4), Colorado (1), Connecticut (3), Florida (1), Illinois (2), Maryland (1), Massachusetts (1), Michigan (1), New York (7), Ohio (1), Rhode Island (1), Texas (2), Utah (1), Washington (1), and Wisconsin (3); Canada: Ontario (35), British Columbia (14) Alberta (10), and Quebec (4).
Table 1.
Demographic characteristics and clinical outcomes of persons infected with the outbreak strains of Salmonella Newport, S. Hartford, S. Saintpaul, S. Oranienbrurg, and coinfected with S. Newport and S. Hartford in the USA and Canada, 2013–2014, (n=94)
| No. (%) | |
|---|---|
| Demographic characteristics, n = 94 | |
| Age (years), median (range) | 42 (<1–89) |
| Female | 62 (66%) |
| Outcomes, n = 80 | |
| Hospitalizations | 17 (22%) |
| Deaths | 0 |
Vehicle identification
Before CDC began open-ended interviews with ill persons in the USA, a local health department in Wisconsin identified ‘chia flour’ as an exposure of interest from a patient interview using a routine enteric illness questionnaire. This, combined with several case-patients reporting vegetarian and dairy-free diets during other initial state or local health department interviews, prompted the decision to perform open-ended interviews. Seven open-ended interviews were completed, with all ill persons reporting consumption of chia seed powder before illness onset. Six of these reported consumption of a single brand of chia seed powder (Brand X).
After combining information from the open-ended interviews with data from the supplemental questionnaires deployed in both countries, 66 (86%) of 77 ill persons with available information reported exposure to chia seed products (47 in Canada; 19 in the USA). Fifty-six (73%) ill persons reported exposure to chia seed powder (41 in Canada; 15 in the USA). Of these, 54 or 96% of ill persons reported brands of chia seed powder produced by a single firm (Firm Y), spanning 13 different brands. Of the 11 persons with exposure information who did not report chia seed products, nine were in Canada and two were in the USA. Most (72%) ill persons in the USA who reported eating chia seed powder reported consuming a single brand, Brand X. The percentage of ill persons in this outbreak reporting chia seed (13%) and chia seed powder (73%) was significantly higher than the percentage of Canadian food consumption study respondents (N = 157) that reported exposure to chia seeds (6·4%) and chia seed powder (0·6%) (P values 0·025 and <0·001, respectively) [11].
Laboratory investigation
Fourteen samples of leftover sprouted powder product provided by ill persons in seven states and two provinces yielded outbreak strains of Salmonella (Newport, Hartford, Oranienburg, and Saintpaul). All outbreak strains were identified in intact, retention, and warehouse sprouted chia seed samples collected by investigators in both countries. In the USA, additional strains of Salmonella Oranienburg (XbaI JJXX01·0028) and S. Saintpaul (XbaI JN6X01·0048) were identified in product testing. Because these strains were very common, CDC requested state public health laboratories to perform second restriction enzyme (BlnI) analyses. There were no clinical isolates matching the two enzyme pattern combination Salmonella Saintpaul XbaI/BlnI pattern JN6X01·0048/JN6A26·0001. There was one clinical isolate matching the two enzyme pattern combination Salmonella Oranienburg XbaI/BlnI pattern JJXX01·0028/JJXA26·0015, but no epidemiological data were available from the patient. Therefore, these strains were not included in the case definition in the USA. Similarly, intact product testing in Canada also identified additional Salmonella serotypes that were not linked to any clinical illnesses. These included Salmonella Sandiego, Salmonella Gaminara, Salmonella Freetown, Salmonella Carrau, Salmonella II48:z10:[1,5], and Salmonella i:6,7:b:- (Table 2). No samples of unsprouted chia seeds (opened product from ill persons or retail samples) yielded Salmonella.
Table 2.
Salmonella Newport, S. Hartford, S. Saintpaul, and S. Oranienbrurg positive samples identified from clinical specimens, leftover product from ill persons’ homes, and product from regulatory investigations, by serotype and PFGE pattern, 2013–2014
| Serotype (outbreak strain/PFGE pattern) | Clinical specimens (n = 94) | Product from ill persons’ homes (n = 24) | Product from regulatory investigations (n = 40) |
|---|---|---|---|
| Newport | |||
| JJPX01·1645/NewpXAI.0413 | 35 | 14 | 18 |
| JJPX01·4815 | 1 | 0 | 1 |
| NewpXAI.0416 | 0 | 1 | 0 |
| NewpXAI.0418 | 1 | 0 | 0 |
| NewpXAI.0423 | 1 | 0 | 0 |
| NewpXAI.0424 | 1 | 0 | 0 |
| Hartford | |||
| JHAX01·0291/HartXAI.0038 | 24 | 4 | 1 |
| HartXAI.0040 | 2 | 0 | 0 |
| Oranienburg | |||
| JJXX01·0027/OraniXAI.0006 | 6 | 0 | 0 |
| OraniXAI.0005/OraniBNI.0007 | 3 | 0 | 1 |
| OraniXAI.0005/OraniBNI.0073 | 1 | 0 | 0 |
| OraniXAI.0005/OraniBNI.0075 | 1 | 0 | 0 |
| Saintpaul | |||
| SainXAI.0005/SainBNI.0073 | 14 | 1 | 2 |
| SainXAI.0005/SainBNI.0010 | 1 | 1 | 0 |
| Coinfections | |||
| NewpXAI.0413 and HartXAI.0038 | 2 | 0 | 0 |
| NewpXAI.0416 and HartXAI.0038 | 1 | 0 | 0 |
| Additional serotypes/PFGE patterns* | – | 3 | 17 |
Included Salmonella Newport, S. Hartford, S. Saintpaul, S. Oranienburg, S. Sandiego, S. Freetown, S. Carrau, S. Gaminara, S. II 48:z10:[1,5], and S. I:6,7:b:-
None of the outbreak-associated strains of Salmonella were identified in PulseNet International; however, a genetically similar isolate with a PFGE pattern differing by one band was isolated in 2006 from a South American country.
NARMS conducted antimicrobial susceptibility testing on four US isolates collected from ill persons infected with the outbreak strains of Salmonella Hartford (two isolates) and S. Newport (two isolates). All four isolates tested were susceptible to all antibiotics tested.
Traceback investigation
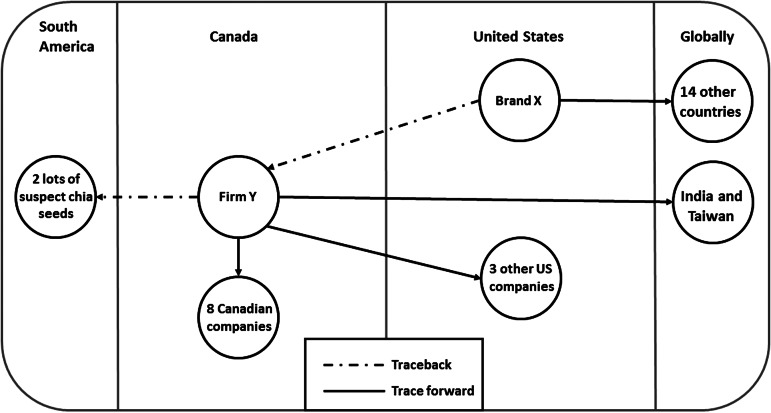
Brand X identified in the US investigation was distributed from a single facility in California, and was traced back to Firm Y, a processing facility located in Canada, where the chia seeds were sprouted and then made into chia seed powder (Fig. 3). Firm Y was determined to be the common supplier of chia seed powder for all 13 brands reported by ill persons in the USA and Canada. Based on lot code information, two lots of chia seeds were used to produce the implicated chia seed powder. These two lots were imported from the same South American country from which the 2006 isolate was identified; the chia seeds were grown and harvested by up to two farms in June–July 2013.
Fig. 3.
Traceback and trace forward investigations of Brand X and Firm Y.
Facility inspections
CalFERT inspected the Brand X facility on 27 May 2014. This facility, located in California, received bulk chia seed powder from Firm Y, and Firm Y was the sole source of Brand X chia seed powder during the timeframe of interest. Although the bulk chia seed powder was imported via a company located in New York, it was shipped directly from the original manufacturer (Firm Y) to the Brand X facility. Brand X facility blended, repackaged, and distributed the finished chia powder products nationwide. There was no evidence observed that indicated contamination occurred at the Brand X facility. Environmental, raw ingredient, and retention (raw ingredient and finished product) samples were collected. Salmonella Newport matching the outbreak strain was detected in both the raw ingredient retention samples and the raw ingredients. Salmonella was not detected in the environmental samples. Samples of Brand X were also collected at various retail locations in California and the matching outbreak strain of Salmonella Newport was detected in some of these retail samples.
CFIA initiated its food safety investigation at Firm Y in Canada on 28 May 2014. Firm Y manufactured the chia seed powder by sprouting, drying, and grinding raw chia seeds. The chia seeds were not treated with chlorine or by any other means before sprouting, which was performed in warm water. Retention and environmental samples were obtained during the investigation. Salmonella serotypes with matching PFGE patterns to the outbreak strains were detected in retention samples; all environmental samples collected at Firm Y were negative for Salmonella.
Trace forward
Trace forward of the implicated chia seed powder from Firm Y indicated the chia seed powder was distributed to a number of companies in both the USA and Canada. Twelve companies purchased chia seed powder from Firm Y, and distributed the product under 13 different brand names (Fig. 3). Trace forward of sprouted chia seed powder produced at Firm Y indicated that potentially contaminated product may have been directly distributed to India and Taiwan in addition to the USA, and that Brand X product may have been distributed to an additional 14 countries (Bahrain, Bermuda, Brazil, China, Costa Rica, Estonia, Iceland, Israel, Jamaica, Lebanon, Netherlands, New Zealand, Singapore and Slovenia). Several brands were also available for purchase on the internet and could therefore have been distributed more widely.
Regulatory actions
Brand X issued a recall on 28 May 2014. Five additional recalls occurred during 4 June–1 July 2014 from the four US companies that repacked and distributed Firm Y chia seed powder under five separate brand names. In Canada, nine public recall warnings occurred during 30 May–25 June 2014 from the eight Canadian companies repacking and distributing Firm Y chia seed powder under eight different brand names.
On 11 June 2014, FDA placed Firm Y on Import Alert denying admission of Firm Y chia seeds and sprouted chia seed powder into the USA unless the importer provided evidence that the products were not contaminated with Salmonella.
Both Canada and the USA submitted notifications of a potential Public Health Emergency of International Concern (PHEIC) to the Pan American Health Organization in accordance with the International Health Regulations on 17 June and 26 June 2014, respectively. The World Health Organization did not declare the outbreak a PHEIC.
On 3 June 2014, the International Food Safety Authorities Network (INFOSAN) notified Canada and the USA about inquiries they had received from various network members regarding the recalls. Following this, both countries provided INFOSAN with the requested information, including details on the outbreak and recalls, and international distribution of recalled products.
Communications
During the investigation, several communication strategies were employed to inform consumers of investigation findings, product actions, and steps to protect themselves. These included investigation web updates [12], social media messages, and targeted outreach to reporters [13]. Despite such efforts, widespread interest in the outbreak investigation remained low, and case-patients who became ill after the recalls reported not being aware of them. Although the investigation concluded in August 2014, many of the recalled products had long shelf lives extending for at least another year. The recalls and public alerts ensured products would be removed from the marketplace for sale; however, people unaware of the recalls could continue to consume potentially contaminated products long after the recalls if they remained in the home.
DISCUSSION
Epidemiological, traceback, and laboratory investigations indicated that this 2013–2014 international outbreak of multiple Salmonella serotypes was caused by contaminated sprouted chia seed powder, becoming the first known foodborne illness outbreak linked to this product. Although sprouted chia seed powder is a novel outbreak vehicle, sprouts are recognized as an important cause of foodborne illness. This outbreak emphasizes the ongoing concern of foodborne illness for persons consuming raw, sprouted foods, and the need for continued consumer education on the potential risks of such behavior. Furthermore, this outbreak investigation highlights the importance of collaboration between public health and regulatory, state, provincial, and federal partners to identify disease sources and prevent illness internationally.
Chia, or Salvia hispanica, is a species of flowering plant native to Central and South America [14]. Although chia has been a staple food in these regions for generations, chia containing products have become increasingly popular in North America more recently, often touted as ‘superfoods’ with many claimed health benefits. Chia can be consumed in a variety of ways, including consuming the seeds directly or following sprouting. Because chia seeds and powder products are added to food products that are not further processed, any bacteria present will likely persist.
The exact source of contamination of the chia seeds from this outbreak is unknown. Contamination could have occurred during growing and/or harvesting at farms in South America, during transport, or during processing at Firm Y. We hypothesize that the chia seeds were likely contaminated before arriving at Firm Y for several reasons. First, the original outbreak strain of Salmonella Newport was a newly recognized strain in North America and a similar strain with one band difference from the main outbreak strain was isolated in South America in 2006. Also, 10 (13%) ill persons in this investigation reported consuming only chia seeds, and not sprouted chia seed powder. While this finding could have been due to unreported chia seed powder consumption, two of these persons in Canada reported consuming a brand of chia seeds that were traced back to the same seed lot as used to produce recalled Firm Y products, providing evidence of possible seed contamination. Therefore, contaminated unsprouted chia seed products likely arrived at Firm Y.
While no chia seeds sampled (opened product from ill persons’ homes or retail samples) yielded Salmonella during our investigation, we suspect chia seeds were contaminated with a low number of Salmonella bacteria from numerous serotypes and subtypes, given the large number of Salmonella strains isolated from ill persons and products tested during the investigation. Also, isolating pathogens from implicated seeds during previous outbreaks associated with sprouts has proven quite difficult [15, 16]. As such, low-level chia seed contamination could be the cause of sporadic infections of Salmonella and other enteric pathogens that are not recognized as outbreaks.
Regardless of the source of contamination, Salmonella was likely amplified during the sprouting process [17]. CFIA determined during the facility inspection of Firm Y that several steps during chia powder production were conducive to pathogen growth and that the process lacked any treatment that would be lethal to Salmonella. Namely, the chia seeds were not chlorinated or treated by any other means before sprouting. Sprouting was performed in warm water, creating an ideal environment for bacterial growth. The sprouted seeds were then slowly dried and ground, without a step (i.e., sufficient heat) to eliminate bacteria before distribution to consumers. As indicated during case-patient interviews, chia seed powder is generally consumed without further processing; therefore consumers would be exposed to any bacteria present following sprouting.
Consumer and industry awareness of the risk related to consuming and producing sprouts remains low [8]. Furthermore, these particular chia seed products were dried and ground following sprouting, a process after which they no longer maintain the appearance of a raw agricultural product. In this case, it is important for all involved stakeholders, including government agencies and industry, to communicate evidence-based information about the risks of illness that sprouted products can pose to protect consumers, especially those at highest risk for severe illness. In addition, this investigation raises questions about how to effectively communicate ongoing risk to consumers about contaminated products with long shelf lives after investigations conclude.
Due to the possibility that low levels of bacterial contamination may be present on raw agricultural products, combined with a lack of consumer awareness of the risks of eating raw, sprouted products, especially those that may not appear to be sprouted, additional attention is required by industry to minimize risk. Federal governments in both countries provide guidance for the sprouting industry, which includes, in part, the use of good agricultural practices, seed disinfection treatment, and microbial testing [18]. Such guidelines were originally developed for traditional sprouting practices, and did not take into consideration the novel process used by Firm Y to produce a sprouted, dried and ground product. Chia, unlike most other seeds used for sprouting, absorbs water at an accelerated rate [14], making conventional surface sterilization methods impractical. Regardless if sprouts are consumed fresh, or dried and ground, standard disinfection treatments have not been shown to completely eliminate pathogens [17]. Therefore, the risk of foodborne illness outbreaks associated with sprouted seeds continues. In Canada, CFIA is reviewing industry practices through establishment inspections as well as implementing various sampling programmes for sprouted and dried products in order to inform a risk mitigation strategy. In the USA, two Food Safety Modernization Act rules finalized in 2015 provide standards for firms manufacturing such products: the Produce Safety Rule covers the sprouting aspect of the process, and the grinding of the dried sprouts would be subject to the Preventive Controls Rule [19, 20].
To the best of our knowledge, this is the first identified foodborne outbreak associated with chia seed products. Chia seeds and chia seed powder consumption represent a growing trend in healthy eating in North America, and these and similar ‘superfoods’ are not routinely included in initial case follow-up questionnaires or hypothesis-generating questionnaires, but could be considered during future investigations. We identified this novel foodborne illness outbreak vehicle after pursuing open-ended interviews of ill persons. While time consuming, we chose open-ended interviews following an early healthy eater ‘signal’ from the initial interviews, which included a report of ‘chia flour’ consumption. Open-ended interviews were critical to identifying the vehicle during this outbreak investigation. This approach could be considered in future foodborne outbreak investigations when more common approaches, such as using hypothesis-generating questionnaires, may be less likely to successfully narrow the search for disease source.
ACKNOWLEDGEMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or other institutions. The authors would like to acknowledge state, provincial, and local health officials for their assistance during the course of this investigation. We also thank the staff from the Canadian Food Inspection Agency Ontario Operations Branch, Food Microbiology Laboratory Services, and Office of Food Safety and Recall for their support in the food safety investigation.
DECLARATION OF INTEREST
None.
References
- 1.Scallan E, et al. Foodborne illness acquired in the United States – major pathogens. Emerging Infectious Diseases 2011; 17: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas MK, et al. Estimates of foodborne illness-related hospitalizations and deaths in Canada for 30 specified pathogens and unspecified agents. Foodborne Pathogens and Disease 2015; 12: 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mead PS, et al. Food-related illness and death in the United States. Emerging Infectious Diseases 1999; 5: 607–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch MF, Tauxe RV, Hedberg CW. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiology and Infection 2009; 137: 307–315. [DOI] [PubMed] [Google Scholar]
- 5.Harb J, et al. Outbreak of Salmonella enteritidis phage type 11B in the provinces of Alberta and Saskatchewan, June 2000. Canada Communicable Disease Report 2003; 29: 125–128. [PubMed] [Google Scholar]
- 6.Ahmed R, et al. Outbreak of Salmonella enteritidis phage type 13a associated with mung bean sprouts in Ontario, 2005. (http://www.sproutnet.com/pdfs/toronto-mung-2005.pdf). Accessed 6 May 2015.
- 7.Taormina PJ, Beuchat LR, Slutsker L. Infections associated with eating seed sprouts: an international concern. Emerging Infectious Diseases 1999; 5: 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdozain M, et al. Failures in sprouts-related risk communication. Food Control 2013; 30: 649–656. [Google Scholar]
- 9.Abadias M, et al. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. International Journal of Food Microbiology 2008; 123: 121–129. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JL, et al. Industry practices and compliance with U.S. Food and Drug Administration guidelines among California sprout firms. Journal of Food Protection 2003; 66: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 11.Public Health Agency of Canada. Foodbook Report. 2015. (http://healthycanadians.gc.ca/publications/eating-nutrition/foodbook-2015/alt/pub-eng.pdf). Accessed 15 January 2016.
- 12.Centers for Disease Control and Prevention. Multistate outbreak of Salmonella infections linked to organic sprouted chia powder. 2014. (http://www.cdc.gov/salmonella/newport-05-14/index.html). Accessed 6 May 2016.
- 13.Centers for Disease Control and Prevention. CDC investigates ongoing Salmonella outbreak linked to chia products. 2014. (http://www.cdc.gov/media/releases/2014/a0613-chia-products.html). Accessed 6 May 2016.
- 14.Chia Seeds – History and Origin. 2015. (http://www.ahdintl.com/_oldsite-archive/ChiaSeeds-HistoryAndOrigin.html). Accessed 6 November 2015.
- 15.Van Beneden CA, et al. Multinational outbreak of Salmonella enterica serotype Newport infections due to contaminated alfalfa sprouts. Jama 1999; 281: 158–162. [DOI] [PubMed] [Google Scholar]
- 16.Mahon BE, et al. An international outbreak of Salmonella infections caused by alfalfa sprouts grown from contaminated seeds. The Journal of Infectious Diseases 1997; 175: 876–882. [DOI] [PubMed] [Google Scholar]
- 17.Microbiological Safety Evaluations and Recommendations on Sprouted Seeds. National Advisory Committee on Microbiological Criteria for Foods. International Journal of Food Microbiology 1999; 52: 123–153. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. Guidance for industry: reducing microbial food safety hazards for sprouted seeds. 1999. (http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ProducePlantProducts/ucm120244.htm). Accessed 28 October 2015.
- 19.US Food and Drug Administration. Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food. 2015. (http://www.regulations.gov/document?D=FDA-2011-N-0920-1979). Accessed 16 November 2015.
- 20.US Food and Drug Administration. Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption. 2015. (http://www.fda.gov/downloads/Food/GuidanceRegulation/FSMA/UCM470746.pdf) Accessed 28 October 2015.