Abstract
Objective:
To assess whether pregnancies achieved with trophectoderm biopsy for preimplantation genetic testing (PGT) have different risks of adverse obstetric and neonatal outcomes compared to pregnancies achieved with In Vitro Fertilization (IVF) without biopsy.
Design:
Observational cohort
Setting:
University-affiliated fertility center
Patients:
Pregnancies achieved via IVF with PGT (n=177) and IVF without PGT (n=180) that resulted in a live birth.
Interventions:
None
Main Outcome Measures:
Maternal outcomes including preeclampsia and placenta previa; neonatal outcomes including birth weight and birth defects.
Results:
There was a statistically significant increase in the risk of preeclampsia among IVF+PGT pregnancies compared to IVF-without-PGT pregnancies, with an incidence of 10.5% versus 4.1% (adjusted odds ratio [aOR] 3.02; 95% confidence interval [95%CI] 1.10, 8.29; P=0.02). The incidence of placenta previa was 5.8% in IVF+PGT pregnancies versus 1.4% in IVF-without-PGT pregnancies (aOR 4.56; 95%CI 0.93, 22.44). Similar incidences of gestational diabetes, preterm premature rupture of membranes, and postpartum hemorrhage were observed. IVF+PGT and IVF-without-PGT neonates did not have a significantly different gestational age at delivery or rate of preterm birth, low birth weight, NICU admission, neonatal morbidities, or birth defects. All trends, including the significantly increased risk of preeclampsia in IVF+PGT pregnancies, persisted upon stratification of analysis to only singleton live births.
Conclusion:
To date, this is the largest and most extensively controlled study examining maternal and neonatal outcomes after trophectoderm biopsy. There was a statistically significant three-fold increase in the odds of preeclampsia associated with trophectoderm biopsy. Given the rise in PGT utilization, further investigation is warranted.
Keywords: Preimplantation genetic testing, trophectoderm biopsy, in vitro fertilization, maternal outcomes, neonatal outcomes, preeclampsia
Capsule:
Trophectoderm biopsy was associated with a statistically significant increase in preeclampsia. Thus given the rise in PGT utilization, further investigation is warranted.
INTRODUCTION
The utilization of Preimplantation Genetic Testing (PGT), including Preimplantation Genetic Screening (PGS) for aneuploidy and Preimplantation Genetic Diagnosis (PGD) for single gene disorders, is increasing rapidly (1). In fact, the rate of PGT in the United States has increased from 4% of all cycles in 2005 to over 22% in 2015. In 2015 alone, 26,201 retrieval cycles conducted in the United States included PGT, resulting in 8529 live births (1). Current indications for PGT include aneuploidy assessment for recurrent pregnancy loss (2), advanced maternal age (3), sex selection, HLA-matched siblings (4), and testing for genetic disorders such as unbalanced translocations and single gene mutations (5–10).
Despite this increasing utilization, there has been very little examination of the maternal and neonatal outcomes for pregnancies achieved via IVF with PGT. Because trophectoderm biopsy removes cells that are destined to form the placenta, there is potential for increased risk of adverse pregnancy outcomes that are associated with abnormal placentation. Shallow or otherwise abnormal initial placentation has been strongly suggested to be involved in later development of preeclampsia and restricted fetal growth, conditions which are associated with significant maternal and infant morbidity (11,12). A few international studies have investigated such maternal and infant consequences post-PGT (13–17), but no study in the US has directly compared the outcomes of IVF+PGT to IVF alone. Therefore, we conducted a cohort study, the largest to date, comparing the maternal and infant outcomes of IVF with and without trophectoderm biopsy.
METHODS
Patients
Women receiving fertility care at Stanford were enrolled after confirmation of a viable pregnancy at around 8 weeks of gestation. All women with a viable pregnancy at approximately 8 weeks of gestation were eligible to participate with recruitment beginning in October 2011 and ending with deliveries projected to occur by the end of December 2017. To maximize the number of PGT cases which could be included in the analysis, we performed a retrospective chart review. Of the 177 cases of PGT included in this analysis, 26 PGT cases were identified by retrospective chart review. Demographics and past pregnancy history were obtained from participant questionnaires or medical records. Past medical history, fertility treatment, and prenatal, delivery, and postpartum data were collected from medical records. The Institutional Review Board of Stanford University approved the study protocol. Statistical analyses of the raw data were performed by a professional biostatistician within Stanford Medicine’s Quantitative Science Unit; the biostatistician was not a part of the study design or data collection.
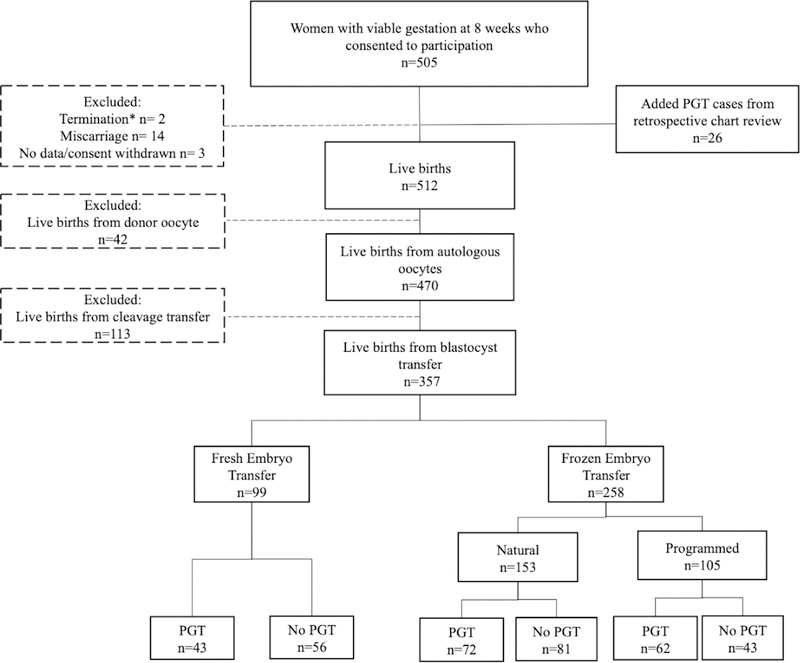
Live births resulting from autologous oocytes with embryos transferred at the blastocyst stage were included in this analysis. An additional analysis was performed limited to only singleton live births resulting from blastocyst transfers to minimize confounding by the comorbidities associated with multiple gestation. There were two participants who had more than one viable pregnancy during the study timeframe; only the first pregnancy was included in this analysis. We excluded miscarriages, terminations for fetal anomalies or maternal health, with no miscarriages or terminations due to development of preeclampsia. Participants were also excluded if they were lost to follow-up, requested to be withdrawn from the study, or if pregnancy outcome was not available. All PGT cycles utilized trophectoderm biopsy. A total of 357 live births, 177 IVF+PGT and 180 IVF-without-PGT, were included in our analyses (Figure 1).
Figure 1 –
Flow diagram of study participants
*One termination for fetal anomaly (IVF-without-PGT cohort) and one for maternal health (IVF+PGT cohort).
PGT and IVF treatment
The embryo biopsy was performed by pipette removal of 5–8 trophectoderm cells from day 5 or day 6 fully expanded blastocysts through a small opening in the zona pellucida, which was created by laser. Clinical pregnancy was confirmed by the presence of the fetal heartbeat within the gestational sac at a 6 or 8-week ultrasound.
Study outcomes
The primary aim of our study was to examine obstetric outcomes, specifically the incidence of preeclampsia. Data on placental complications (specifically placental abruption, placenta previa, and placenta accreta) were also collected. Hypertensive disorders were adjudicated by a trained obstetrician-gynecologist (author F.V.V.H.) who had no knowledge of the participants’ PGT status or medical history. Data collection instruments for pregnancy outcome were separate from those describing demographics and treatment. This study uses the current American College of Obstetricians and Gynecologists’ (ACOG) definitions for hypertensive disorders in pregnancy (18). Preeclampsia was defined by the presence of hypertension (persistent systolic blood pressure (BP) 140 mmHg or higher and/or diastolic BP 90 mmHg or higher) after 20 weeks of gestation in a previously normotensive woman and proteinuria (≥ 300 mg protein in a 24-hour urine collection, protein/creatinine ratio > 0.3, or dipstick reading of at least 1+). New onset hypertension in the absence of proteinuria, but with at least one of the following symptoms, also qualified for the diagnosis of preeclampsia: thrombocytopenia (< 100,000/µl), renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms. These symptoms also qualified for the diagnosis of preeclampsia with severe features. Additional criteria defining preeclampsia with severe features were a systolic BP of ≥ 160 mmHg and/or a diastolic BP of ≥ 110 mmHg, HELLP syndrome (hemolysis [LDH increase], elevated liver enzymes [liver transaminases levels twice as normal], and low platelet count [< 100,000/µl]). If a preeclamptic woman developed new onset grand mal seizures, then eclampsia was diagnosed. Hypertension diagnosed before 20 weeks or prior to conception was defined as chronic hypertension while gestational hypertension was defined as newly onset hypertension in the absence of proteinuria after 20 weeks. Additional obstetric outcomes examined were the development of gestational diabetes, preterm premature rupture of membranes, postpartum hemorrhage, caesarean section delivery, and induction of labor.
The secondary aim of our study was to examine the neonatal outcomes. We studied the incidence of preterm birth (<37 weeks), birth weight, birth defects, Apgar score at 5 minutes, neonatal intensive care unit (NICU) admission, neonatal jaundice, and neonatal morbidity (defined by the presence of hypoglycemia, hypothermia, intraventricular hemorrhage, necrotizing enterocolitis, seizure, infection, sepsis, or respiratory distress syndrome).
Statistical Analysis
Study data were captured and managed in Stanford’s REDCap electronic data tool (19). Categorical variables underwent Chi-square analysis (or Fisher’s exact test) to compare the significance of differences in proportions while continuous variables underwent t-test (or Mann-Whitney test) to compare the significance of differences in mean. Multivariate logistic and linear regression models were performed to determine the adjusted odds ratios (aOR) of obstetric and neonatal outcomes. The obstetric outcome models were adjusted for the following covariates: parity, maternal age, maternal BMI, prior history of hypertension, PCOS infertility diagnosis, natural versus programmed frozen embryo transfer (FET) (20), and neonate sex. The neonatal outcome models were adjusted for the following covariates: parity, maternal age, and maternal BMI. We also performed subanalyses on index pregnancies achieved solely from frozen embryo transfers (Supplemental Table 2) due to evidence that frozen embryo transfer is associated with a higher risk of hypertensive disorders in pregnancy (21–26). Statistical analyses were conducted using the R statistical software 3.1.0 (26). To account for the multiple statistical tests, we applied a Bonferroni correction to our significance threshold for all secondary outcomes. P<0.05 was considered statistically significant.
RESULTS
Participant characteristics
By the time we closed our dataset for analysis on December 31, 2017, there were 512 live births which were available to be included in the analysis. There were 505 women with viable pregnancies at approximately 8 weeks gestation who consented to participate in our study; three withdrew from the study, 14 had spontaneous pregnancy losses before 20 weeks, one terminated the pregnancy due to anomaly (from the IVF-without-PGT cohort), and one terminated for maternal health (from the IVF+PGT cohort). In these 16 non-viable pregnancies, preeclampsia and placental disorders were not present. 26 PGT cases were added to our cohort from retrospective chart review. Donor oocyte (n=42) births were excluded from our analysis due to both well-demonstrated increased risk of preeclampsia with oocyte donation and limited sample size. Because all PGT cases were performed with trophectoderm biopsy followed by blastocyst transfer, only pregnancies from blastocyst transfers were included in the IVF-without-PGT cohort (113 pregnancies from cleavage transfers were excluded). Thus, a final 357 live births conceived by autologous, blastocyst transfer (177 with PGT and 180 without PGT) were used in our analyses (Figure 1).
The indications for PGT were: aneuploidy (n=74), monogenetic disorder, (n=27), recurrent pregnancy loss (n=39), advanced maternal age (n=35), sex selection (n=26), previous IVF failure (n=10), and elective (n=8). Some women had multiple indications for PGT.
Baseline clinical and demographic characteristics of the women in our study are presented in Table 1. Between the IVF+PGT and IVF-without-PGT cohorts, there were no significant differences in the women’s age, BMI, parity, race/ethnicity, incidence of hypertensive disease in previous pregnancy, or rate of chronic hypertension. Compared with the controls, IVF+PGT women had a lower mean number of embryos transferred (1.2 vs. 1.5, P=0.0001). There were 26 pairs of twins (no triplets), of which 11 pairs were in the IVF+PGT cohort (12.4% of cohort) and 15 pairs were in the IVF-without-PGT cohort (16.7%). The most common indication for fertility treatment for the IVF-without-PGT cohort was male factor (43.5%) while the most common indication for the IVF+PGT cohort was split between male factor (27.2%) and monogenetic disorder (both 23.9%). There were no smokers in either cohort.
Table 1 -.
Baseline demographic and clinical characteristics of all participants with live births from autologous oocytes transferred at the blastocyst stage. Data are presented as mean ± SD or N (%).
| IVF n=180 | IVF-PGT n=177 | |
|---|---|---|
| Maternal age at delivery (y) | 36.5±4.1 | 36.9±3.9 |
| Age >40 years | 38(21.1) | 44(24.9) |
| Maternal BMI (kg/m2) | 24.7±4.9 | 23.4±4.0 |
| BMI >30 | 23(12.8) | 13(7.3) |
| Nulliparous | 110(61.1) | 116(65.5) |
| Parity | 0.4±0.6 | 0.5±0.7 |
| History of hypertensive disease in previous pregnancy | 3(1.7) | 2(1.1) |
| History of chronic hypertension | 5(2.8) | 9(5.1) |
| Maternal race/ethnicity | ||
| White: | 75(41.7) | 80(45.2) |
| Asian: | 82((45.6) | 71(40.1) |
| Hispanic | 10(5.6) | 7(4.0) |
| African American: | 1(0.6) | 2(1.1) |
| Pacific Islander: | 0(0) | 0(0) |
| Other: | 10(5.6) | 13(7.3) |
| Unknown: | 1(0.6) | 4(2.3) |
| Maternal smoking in pregnancy | 0(0) | 0(0) |
| Infertility Diagnosis | ||
| Age: | 9(5.1) | 7(3.9) |
| Diminished ovarian: reserve: | 23(13.0) | 29(16.1) |
| Male: | 77(43.5) | 49(27.2) |
| Polycystic Ovarian Syndrome (PCOS): | 24(13.6) | 19(10.6) |
| Ovulatory disorder: | 7(4.0) | 7(3.9) |
| Tubal/Uterine: | 19(2.5) | 9(5.0) |
| Endometriosis: | 7(4.0) | 5(2.8) |
| Recurrent Pregnancy Loss: | 16(9.0) | 12(6.7) |
| Monogenetic Disorder: | 6(3.4) | 43(23.9) |
| Sex Selection: | 0(0) | 25(13.9) |
| Single or Lesbian Female: | 2(1.1) | 14(7.8) |
| Unexplained: | 32(18.1) | 31(17.2) |
| Other: | 6(3.4) | 1(0.6) |
| Number of embryos transferred | 1.5±0.7 | 1.2±0.4 |
| Number of twins | 30(16.7) | 22(12.4) |
| Mode of conception | ||
| Fresh IVF | 56(31.1) | 43(24.3) |
| Frozen natural | 81(45.0) | 72(40.7) |
| Frozen programmed | 43(23.9) | 62(35.0) |
The participant demographic characteristics for those included in the subanalysis of index pregnancy stratified by mode of conception (frozen versus fresh embryo transfer) are shown in Supplemental Table 1, and such stratification revealed similar patterns to Table 1. Within this FET subgroup, there were similar numbers of natural and programmed FET cycle live births (153 and 105, respectively).
Obstetric outcomes
The incidence of preeclampsia among IVF+PGT women was 10.5% compared to 4.1% among IVF-without-PGT women, demonstrating a statistically significant increase in the risk of preeclampsia with PGT (aOR 3.02; 95% CI 1.10, 8.29; P=0.02). When analysis was restricted to only singleton live births (Table 3), the incidence of preeclampsia among pregnancies achieved via IVF+PGT was 9.3% compared to 3.7% among pregnancies achieved via IVF-without-PGT (aOR 2.95; 95% CI 0.98, 8.92; P=0.04). The incidences of placenta previa were 5.8% for IVF+PGT vs. 1.4% for IVF-without-PGT (aOR 4.56; 95% CI 0.93, 22.44; P=0.28). There were no observed statistically different incidences of other placental disorders or hypertensive disorders of pregnancy. PGT-IVF women had similar incidences of gestational diabetes, preterm premature rupture of membrane, postpartum hemorrhage, C-section, and induction of labor to IVF-without-PGT women. Similar trends in obstetric outcomes were observed upon further stratification by singleton-only live births (Table 3) and by method of conception: FET (Supplemental Table 2) and fresh embryo transfer (data not shown).
Table 3 –
Obstetric and neonatal outcomes after IVF with and without PGT. Only singleton live births from autologous oocytes transferred at the blastocyst stage were included. Data are presented as mean ± SD or N (%).
| IVF n=150 | IVF-PGT n=155 | Adjusted Odds Ratio* (95% CI) | P-value | |
|---|---|---|---|---|
| OBSTETRIC OUTCOMES | ||||
| Gestational hypertension | 3 (2.2) | 3 (1.9) | 1.0 | |
| Preeclampsia | 5 (3.7) | 15 (9.3) | 2.95 (0.98, 8.92) | 0.04 |
| Preeclampsia with severe features | 3 (2.2) | 7 (4.3) | 1.59 (0.34, 7.36) | 1.0 |
| Placenta disorder | ||||
| Abruption: | 0 (0.0) | 1 (0.6) | ||
| Previa: | 2(1.5) | 8(5.0) | 3.73 (0.74, 18.92) | 0.65 |
| Accreta: | 1(0.7) | 2(1.2) | ||
| Gestational diabetes | 34 (25.4) | 38 (23.6) | 0.96 (0.55, 1.69) | 1.0 |
| Preterm premature rupture of membranes | 15 (11.2) | 12 (7.5) | 0.67 (0.28, 1.57) | 1.0 |
| Postpartum hemorrhage | 6 (4.5) | 11 (6.8) | 1.29 (0.47, 3.56) | 1.0 |
| Caesarean section | 61 (45.5) | 85 (52.8) | 1.23 (0.75, 2.07) | 1.0 |
| Induction of labor | 40 (29.9) | 40 (24.8) | 0.71 (0.40, 1.23) | 1.0 |
| NEONATAL OUTCOMES | ||||
| Gestational age (days) | 271.7±16.6 | 272.3±13.9 | 0.38 (−3.17, 3.93) Mean difference (95% CI) | 1.0 |
| Preterm birth (<37 weeks) | 17 (12.7) | 19 (12.3) | 0.98 (0.48, 1.99) | 1.0 |
| Sex of neonates | ||||
| Male: | 59 (45.4) | 101 (63.5) | 2.05 (1.26, 3.32) | 0.04 |
| Birth weight (g) | 3273.6±610.7 | 3325.4±542.0 | 43.02 (−93.78, 179.82) Mean difference (95% CI) | 1.0 |
| Low birth weight (<2,500 g) | 13 (10.2) | 13 (8.4) | 0.85 (0.37, 1.93) | 1.0 |
| Very low birth weight (<1,500g) | 1 (0.8) | 1 (0.6) | 0.91 (0.05, 15.70) | 1.0 |
| Birth defects | 3 (2.2) | 9 (5.6) | 3.10 (0.80, 12.01) | 0.97 |
| NICU admission | 16 (11.9) | 11 (7.0) | 0.58 (0.25, 1.32) | 1.0 |
| Apgar score at 5 min | 8.9±0.4 | 8.9±0.6 | −0.10 (−0.22, 0.03) Mean difference (95% CI) | 1.0 |
| Apgar score at 5 min <7 | 1 (0.8) | 1 (0.7) | 1.22 (0.07, 20.90) | 1.0 |
| Neonatal morbidity** | 22 (16.4) | 27 (16.8) | 1.00 (0.53, 1.89) | 1.0 |
| Jaundice/hyperbilirubinemia | 18 (13.4) | 21 (13.0) | 0.94 (0.47, 1.88) | 1.0 |
Obstetric Odds Ratio (95% CI) adjusted for the following confounders: parity, age, BMI, prior history of hypertension, blood pressure disorder in previous pregnancy, PCOS infertility diagnosis, mode of conception, and neonate sex.
Neonatal Odds Ratio (95% CI) and Mean difference (95% CI) adjusted for the following confounders: parity, age, BMI. Some outcomes were too low for regression modeling, and thus do not have an adjusted odds ratio.
Neonatal morbidity defined by the presence of hypoglycemia, hypothermia, intraventricular hemorrhage, necrotizing enterocolitis, seizure, infection, sepsis, or respiratory distress syndrome
Neonatal outcomes
The mean gestational age of IVF+PGT neonates was similar to that of IVF-without-PGT neonates (269.1 days vs. 270.3; P=1.0), and there were no significant differences in the rate of preterm birth, birth weight, NICU admission, mean Apgar score at 5 minutes, and neonatal morbidity. There was a significantly higher proportion of male neonates in the IVF+PGT group (63.0% vs. 45.5%; P=0.04). When the 26 participants with “sex selection” as the PGT indication were removed, the sex imbalance persisted with 61% male and 39% female. There were no statistically significant differences in the incidences of birth defects in our main analysis (Table 2 and 3), but subanalysis of only the FET cohort (Supplemental Table 2) showed a significantly increased odds of birth defects among IVF+PGT neonates (aOR 11.90, 95% CI 1.40, 100.87; P=0.04). The IVF+PGT cohort’s birth defects were: Arachnoid cyst, two cases of ventricular septal defect, auditory canal malformation, right-sided inguinal hernia, Marfan Syndrome, lack of helical fold of left ear, left kidney cyst, hypospadias, and bilateral pyelektasis. The birth defects for the IVF-without-PGT cohort were: Imperforate anus, abdominal cyst, and Tetralogy of Fallot. Otherwise, similar trends in neonatal outcomes were observed upon further stratification by singleton-only live births (Table 3) and by method of conception: FET (Supplemental Table 2) and fresh embryo transfer (data not shown).
Table 2 -.
Obstetric and neonatal outcomes after IVF with and without PGT. All live births (including multiple gestations) from autologous oocytes transferred at the blastocyst stage were included. Data are presented as N (%).
| IVF n=180 | IVF-PGT n=177 | Adjusted Odds Ratio* (95% CI) | P-value | |
|---|---|---|---|---|
| OBSTETRIC OUTCOMES | ||||
| Gestational hypertension | 3 (2.0) | 3 (1.7) | 1.0 | |
| Preeclampsia | 6 (4.1) | 18 (10.5) | 3.02 (1.10, 8.29) | 0.02 |
| Preeclampsia with severe features | 6 (4.1) | 8 (4.7) | 0.87 (0.27, 2.84) | 1.0 |
| Placenta disorder | ||||
| Abruption: | 0 (0.0) | 1 (0.6) | 1.0 | |
| Previa: | 2(1.4) | 10(5.8) | 4.56 (0.93, 22.44) | 0.28 |
| Accreta: | 1(0.7) | 2(1.2) | 1.0 | |
| Gestational diabetes | 35 (24.0) | 42 (24.4) | 1.07 (0.62, 1.85) | 1.0 |
| Preterm premature rupture of membranes | 17 (11.6) | 12 (7.0) | 0.63 (0.28, 1.42) | 1.0 |
| Postpartum hemorrhage | 7 (4.8) | 13 (7.6) | 1.29 (0.47, 3.56) | 1.0 |
| Caesarean section | 69 (46.9) | 95 (55.2) | 1.28 (0.79, 2.07) | 1.0 |
| Induction of labor | 43 (29.3) | 40 (23.3) | 0.66 (0.39, 1.14) | 1.0 |
| NEONATAL OUTCOMES | ||||
| Gestational age (days) | 269.1±17.0 | 270.3±14.6 | 1.08 (−2.35, 4.52) Mean difference (95% CI) | 1.0 |
| Preterm birth (<37 weeks) | 26 (16.4) | 29 (16.4) | 1.01 (0.56, 1.82) | 1.0 |
| Sex of neonates | ||||
| Male: | 70 (45.5) | 114 (63.0) | 1.96 (1.25, 3.07) | 0.04 |
| Birth weight (g) | 3151.0±654.9 | 3245.4±577.3 | 91.18 (−44.68, 227.05) Mean difference (95% CI) | 1.0 |
| Low birth weight (<2,500 g) | 24 (15.8) | 22 (12.6) | 0.77 (0.41, 1.45) | 1.0 |
| Very low birth weight (<1,500g) | 2 (1.3) | 1 (0.6) | 0.44 (0.04, 5.02) | 1.0 |
| Birth defects | 3 (1.9) | 10 (5.5) | 3.69 (0.97, 14.03) | 0.45 |
| NICU admission | 23 (14.4) | 13 (7.3) | 0.44 (0.21, 0.91) | 0.29 |
| Apgar score at 5 min | 8.9±0.4 | 8.8±0.6 | −0.08 (−0.20, 0.03) Mean difference (95% CI) | 1.0 |
| Apgar score at 5 min <7 | 1 (0.7) | 1 (0.6) | 1.08 (0.07, 17.95) | 1.0 |
| Neonatal morbidity** | 30 (18.8) | 29 (15.8) | 0.78 (0.44, 1.38) | 1.0 |
| Jaundice/hyperbilirubinemia | 20 (12.5) | 21 (11.5) | 0.86 (0.44, 1.68) | 1.0 |
Obstetric Odds Ratio (95% CI) adjusted for the following confounders: parity, age, BMI, prior history of hypertension, blood pressure disorder in previous pregnancy, PCOS infertility diagnosis, mode of conception, and neonate sex.
Neonatal Odds Ratio (95% CI) and Mean difference (95% CI) adjusted for the following confounders: parity, age, BMI. Some outcomes were too low for regression modeling, and thus do not have an adjusted odds ratio.
Neonatal morbidity defined by the presence of hypoglycemia, hypothermia, intraventricular hemorrhage, necrotizing enterocolitis, seizure, infection, sepsis, or respiratory distress syndrome
DISCUSSION
Our study is one of the largest to report detailed maternal and neonatal outcomes comparing IVF+PGT to IVF-without-PGT. For obstetric outcomes, there was a three-fold increase in the odds of preeclampsia in the IVF+PGT cohort (aOR 3.02, 95% CI 1.10, 8.29; P=0.02) that persisted even when analysis was stratified to only singleton live births to minimize confounding by the comorbidities associated with multiple gestation. No statistically significant differences in adverse neonatal outcomes were found in our main analysis. The observation regarding an increase in the incidence of birth defects with PGT in the subanalysis of FETs should be interpreted very cautiously, given the small number of cases and wide confidence interval.
Despite the increased use of PGT, there are only a few studies that have investigated neonatal outcomes for pregnancies achieved with IVF+PGT, and even fewer have reported maternal outcomes. In fact, worldwide there have only been a total of 347 PGT mothers and 1534 PGT neonates studied (13–17). In almost all of these studies, the PGT biopsy procedure was polar body or cleavage-stage blastomere removal, which limits applicability to current US clinical settings as blastocyst-stage biopsy of trophectoderm cells (method used in our study) has become the predominant method within the United States.
A 2012 Belgian study found similar birth weight, perinatal death, and major malformation rates between children born after PGD and ICSI (13), and a more recent 2014 Israeli study found similar rates of low birth weight and intrauterine growth restriction, despite lower gestational age, in children conceived after PGD (14). However, the latter study compared their PGD cohort to children conceived spontaneously. In a 2016 Danish study that similarly made comparisons with spontaneously conceived pregnancies, Bay et al. reported that PGD (n=126) had increased risk of placenta previa, caesarean section, preterm birth, shorter gestation, and neonatal admission. The authors reported, however, that risks were no longer different in a subanalysis once PGD outcomes were compared to IVF/ICSI outcomes (15). It is important to note that for the above studies, two factors known to increase the risk of adverse pregnancy and neonatal outcomes were not adjusted for in the analyses. Firstly, frozen embryo transfer has been associated with increased risk of hypertensive disorders in pregnancy (21–23), preeclampsia (24–26), and placental disorders (28, 29). Secondly, donor oocyte has been associated with increased risk of preeclampsia (30–32), lower gestational age (33), and lower birth weight (33).
Two clinical trials have investigated the outcomes of pregnancy and children born after PGT. A 2013 trial in the United States found that PGT neonates (n=61) had lower risks of preterm delivery, low birthweight, and NICU admission (16), but this likely resulted from the study design in which researchers transferred 1 embryo if PGT was performed and 2 embryos if not. In fact, the control (non-biopsied) group had 29 twins and 1 triplet compared to only 1 twin in the PGT group. In a 2016 trial from China, Jing et al. found that blastocyst embryo biopsy and subsequent frozen transfer (n=166) was associated with a higher rate of gestational hypertension when compared with cleavage embryo biopsy and fresh transfer (n=129) (17). However, this higher rate is confounded by the indirect comparison of frozen to fresh transfer. As previously mentioned, frozen embryo transfer has been associated with higher risks of hypertensive and placental disorders (21–26, 28, 29). Additionally, the investigators obtained pregnancy and neonatal outcomes via phone survey of patients rather than medical records, leaving open the possibility of recall bias.
Thus, our cohort study is unique in several aspects. We have included only participants with live births from autologous oocytes and adjusted for multiple confounders that have been previously demonstrated to increase the risk for adverse pregnancy outcomes, such as preeclampsia and placental disorders. In fact, this study has a more extensive control than any previous study for covariates that could potentially confound pregnancy and neonatal outcomes; most significantly, we controlled for frozen embryo transfers, excluded donor oocytes, and performed subanalysis on only singleton live births. Other strengths of this study include the rigorous medical record review of study outcomes to minimize recall bias as well as the blinded adjudication and careful diagnosis of hypertensive disorders. Lastly, the results of this study lend more applicability than previous studies to current clinical settings by only investigating trophectoderm biopsy for PGT.
The main limitation of our study is the sample size, as the participant pool was a single university center. Thus, larger studies are needed to add more insight into the safety of PGT. The lack of a unified medical record system in the United States currently presents a significant challenge to accessing detailed pregnancy and neonatal medical records for a larger cohort of women who have undergone PGT (34). Nonetheless, our study is one of only a few, and the largest yet, to provide detailed data regarding maternal and neonatal outcomes following trophectoderm biopsy compared with outcomes following IVF-without-PGT.
In conclusion, there was a statistically significant three-fold increase in the odds of preeclampsia associated with trophectoderm biopsy and no statistically significant differences in adverse neonatal outcomes. The utilization of PGT has been increasing (1) because PGT reduces the risk of aneuploid pregnancy loss and aneuploid pregnancy (10), may shorten the time to live birth for couples (35), and enables couples who carry single gene disorders to have an unaffected child. Despite these advantages, it is important that potential risks are considered. With the increasing utilization of PGT, we hope that this study provides not only emerging data, but also an impetus for further investigation into the potential risks of trophectoderm biopsy.
Supplementary Material
Supplemental Table 1 - Baseline demographic and clinical characteristics of all participants with live births from autologous oocytes oocytes transferred at the blastocyst stage, separated by frozen and fresh IVF cycles. Data are presented as mean ± SD or N (%).
Supplemental Table 2 - Obstetrical and neonatal outcomes following IVF with and without PGT among only frozen embryo transfers. Participants with live births from autologous oocytes transferred at the blastocyst stage were included. Data are presented as mean ± SD or N (%).
*Obstetric Odds Ratio (95% CI) adjusted for the following confounders: parity, age, BMI, prior history of hypertension, blood pressure disorder in previous pregnancy, PCOS infertility diagnosis, mode of conception, and neonate sex.
Neonatal Odds Ratio (95% CI) and Mean difference (95% CI) adjusted for the following confounders: parity, age, BMI. Some outcomes were too low for regression modeling, and thus do not have an adjusted odds ratio.
** Neonatal morbidity defined by the presence of hypoglycemia, hypothermia, intraventricular hemorrhage, necrotizing enterocolitis, seizure, infection, sepsis, or respiratory distress syndrome
Acknowledgments
The authors would like to thank all participants and hospitals that supported the collection of these data. This study was funded by Award Number P01 HD 065647–01A1 from the National Institute of Child and Human Development (V.L.B.), a Heisenberg Fellowship Award from the German Research Foundation (VE490/8–1; F.V.V.H.) and a MedScholars fellowship from the Stanford Medical Scholars Research Program (W.Y.Z.). The use of REDCap was supported by Stanford CTSA award number UL1 TR001085 from NIH/NCRR.
Funding: This study was funded by Award Number P01 HD 065647–01A1 from the National Institute of Child and Human Development, a Heisenberg Fellowship Award from the German Research Foundation (VE490/8–1), and a MedScholars fellowship from the Stanford Medical Scholars Research Program. The use of REDCap was supported by Stanford CTSA award number UL1 TR001085 from NIH/NCRR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Society for Reproductive Assisted Technology. National Summary Report for 2015 Retrieved November 18, 2018, from https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?#patient-cumulative.
- 2.Munné S, Chen S, Fischer J, Colls P, Zheng X, Stevens J, et al. Preimplantation genetic diagnosis reduces pregnancy loss in women aged 35 years and older with a history of recurrent miscarriages. Fertil Steril 2005;84:331–335. [DOI] [PubMed] [Google Scholar]
- 3.Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril 2017. May;107(5):1122–1129. [DOI] [PubMed] [Google Scholar]
- 4.Verlinsky Y, Rechitsky S, Sharapova T, Morris R, Taranissi M, Kuliev A. Preimplantation HLA Testing. JAMA 2004;291(17):2079–2085. [DOI] [PubMed] [Google Scholar]
- 5.Otani T, Roche M, Mizuike M, Colls P, Escudero T, Munné S. Preimplantation genetic diagnosis significantly improves the pregnancy outcome of translocation carriers with a history of recurrent miscarriage and unsuccessful pregnancies. Reprod Biomed Online 2006;6:869–874. [DOI] [PubMed] [Google Scholar]
- 6.Fischer J, Colls P, Escudero T, Munné S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of a recurrent losses. Fertil Steril 2010;94:283–289. [DOI] [PubMed] [Google Scholar]
- 7.Franssen M, Korevaar JC, van der Veen F, Leschot NJ, Bossuyt PM, Goddijn M. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: case-control study. BMJ 2006; 332:759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verlinsky Y, Cohen J, Munne S, Gianaroli L, Simpson JL, Ferraretti AP, et al. Over a decade of experience with preimplantation genetic diagnosis: A multicenter report. Fertil Steril 2004;82:292–294. [DOI] [PubMed] [Google Scholar]
- 9.Monni G, Peddes C, Iuculano A, Ibba RM. From Prenatal to Preimplantation Genetic Diagnosis of β-Thalassemia. Prevention Model in 8748 Cases: 40 Years of Single Center Experience. J Clin Med 2018. ;February 20;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munné S, Magli C, Cohen J, Morton P, Sadowy S, Gianaroli L, et al. Positive outcome after preimplantation diagnosis of aneuploidy in human embryos. Hum Reprod 1999;14:2191–2199. [DOI] [PubMed] [Google Scholar]
- 11.Furuya M, Ishida J, Aoki I, Fukamizo A. Pathophysiology of placentation abnormalities in pregnancy-induced hypertension. Vasc Health Risk Manag 2008. December; 4(6): 1301–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of Preeclampsia. Annual Review of Pathology: Mechanisms of Disease 2010. February; 5: 173–192. [DOI] [PubMed] [Google Scholar]
- 13.Desmyttere S, De Rycke M, Staessen C, Liebaers I, De Schrijver F, Verpoest W, et al. Neonatal follow-up of 995 consecutively born children after embryo biopsy for PGD. Hum Reprod 2012. January;27(1):288–93. [DOI] [PubMed] [Google Scholar]
- 14.Eldar-Geva T, Srebnik N, Altarescu G, Varshaver I, Brooks B, Levy-Lahad E, et al. Neonatal outcome after preimplantation genetic diagnosis. Fertil Steril 2014. October;102(4):1016–21. [DOI] [PubMed] [Google Scholar]
- 15.Bay B, Ingerslev HJ, Lemmen JG, Degn B, Rasmussen IA, Kesmodel US. Preimplantation genetic diagnosis: a national multicenter obstetric and neonatal follow-up study. Fertil Steril 2016. November;106(6):1363–1369. [DOI] [PubMed] [Google Scholar]
- 16.Forman E, Hong K, Franasiak J, Scott R Jr. Obstetrical and neonatal outcomes from the BEST Trial: single embryo transfer with aneuploidy screening improves outcomes after in vitro fertilization without compromising delivery rates. Am J Obstet Gynecol 2014;210(2). [DOI] [PubMed] [Google Scholar]
- 17.Jing S, Luo K, He H, Lu C, Zhang S, Tan Y, et al. Obstetric and neonatal outcomes in blastocyst-stage biopsy with frozen embryo transfer and cleavage-stage biopsy with fresh embryo transfer after preimplantation genetic diagnosis/screening. Fertil Steril 2016. July;106(1):105–112. [DOI] [PubMed] [Google Scholar]
- 18.Pregnancy ACoOaGATFoHi. Hypertension in Pregnancy2013. The American College of Obstetricians and Gynecologists 2013. [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Versen-Höynck F, Schaub AM, Chiu KH, Liu J, Lingis M, Williams RS, et al. Increased Preeclampsia Risk and Reduced Aortic Compliance With In Vitro Fertilization Cycles in the Absence of a Corpus Luteum. Hypertension 2019. March;73(3):640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad PR, et al. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum Reprod 2015. July;30(7):1724–31. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril 2014;101:128–33. [DOI] [PubMed] [Google Scholar]
- 23.Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, et al. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update 2018. January 1;24(1):35–58. [DOI] [PubMed] [Google Scholar]
- 24.Sites CK, Wilson D, Barsky M, Bernson D, Bernstein IM, Boulet S, et al. Embryo cryopreservation and preeclampsia risk. Fertil Steril 2017;108:784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N Engl J Med 2016;375:523–33. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B, Wei D, Legro RS, Shi Y, Li J, Zhang L, et al. Obstetric complications after frozen versus fresh embryo transfer in women with polycystic ovary results from a randomized trial. Fertil Steril 2018. February;109(2):324–329. [DOI] [PubMed] [Google Scholar]
- 27.R Development Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, URL http://www.R-project.org, 2006. [Google Scholar]
- 28.Liebaers I, Desmyttere S, Verpoest W, De Rycke M, Staessen C, Sermon K, et al. Report on a consecutive series of 581 children born after blastomere biopsy for preimplantation genetic diagnosis. Hum Reprod 2010. January;25(1):275–82. [DOI] [PubMed] [Google Scholar]
- 29.Kaser DJ, Melamed A, Bormann CL, Myers DE, Missmer SA, Walsh BW, et al. Cryopreserved embryo transfer is an independent risk factor for placenta accreta. Fertil Steril 2015. May;103(5):1176–84. [DOI] [PubMed] [Google Scholar]
- 30.Schwarze JE, Borda P, Vasquez P, Ortega C, Villa S, Crosby JA, et al. Is the risk of preeclampsia higher in donor oocyte pregnancies? A systematic review and meta-analysis. JBRA Assist Reprod 2017. December 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dorp W, Rietveld AM, Laven JS, van den Heuvel-Eibrink MM, Hukkelhoven CW, Schipper I. Pregnancy outcome of non-anonymous oocyte donation: a case-control study. Eur J Obstet Gynecol Reprod Biol 2014. November;182:107–12. [DOI] [PubMed] [Google Scholar]
- 32.Levron Y, Dviri M, Segol I, Yerushalmi GM, Hourvitz A, Orvieto R, et al. The ‘immunologic theory’ of preeclampsia revisited: a lesson from donor oocyte gestations. Am J Obstet Gynecol 2014. October;211(4):383.e1–5. [DOI] [PubMed] [Google Scholar]
- 33.Adams DH, Clark RA, Davies MJ, de Lacey S. A meta-analysis of neonatal health outcomes from oocyte donation. J Dev Orig Health Dis 2015. November 27:1–16. [DOI] [PubMed] [Google Scholar]
- 34.Floyd EG, von Versen-Höynck F, Liu J, Chi YY, Fleischmann RR, Baker VL. Collection of pregnancy outcome records following infertility-challenges and possible solutions. J Assist Reprod Genet 2016. August;33(8):993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franasiak JM, Hong KH, Werner MD, Juneau Cr, Morin SJ, Neal SA, et al. Preimplantation genetic screening (PGS) in low responders shortens time to pregnancy: a randomized controlled trial. Fertil Steril 2017. September;108(3):e60–e61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 - Baseline demographic and clinical characteristics of all participants with live births from autologous oocytes oocytes transferred at the blastocyst stage, separated by frozen and fresh IVF cycles. Data are presented as mean ± SD or N (%).
Supplemental Table 2 - Obstetrical and neonatal outcomes following IVF with and without PGT among only frozen embryo transfers. Participants with live births from autologous oocytes transferred at the blastocyst stage were included. Data are presented as mean ± SD or N (%).
*Obstetric Odds Ratio (95% CI) adjusted for the following confounders: parity, age, BMI, prior history of hypertension, blood pressure disorder in previous pregnancy, PCOS infertility diagnosis, mode of conception, and neonate sex.
Neonatal Odds Ratio (95% CI) and Mean difference (95% CI) adjusted for the following confounders: parity, age, BMI. Some outcomes were too low for regression modeling, and thus do not have an adjusted odds ratio.
** Neonatal morbidity defined by the presence of hypoglycemia, hypothermia, intraventricular hemorrhage, necrotizing enterocolitis, seizure, infection, sepsis, or respiratory distress syndrome