Abstract
Purpose:
To assess whether there are differences in Alzheimer’s disease (AD)-associated atrophy regions in Chinese and white AD patients versus cognitively normal older adults, and to test whether associations between clinical severity and gray matter volume are similar or different across these ethnic groups in a cross-sectional analysis.
Methods:
Chinese and white AD patients, individuals with mild cognitive impairment (MCI), and cognitively normal (CN) controls (n = 46 white and 48 Chinese) were clinically evaluated at an academic center within one year of MRI acquisition. Clinical severity was assessed using the Clinical Dementia Rating scale sum of boxes and cortical atrophy was measured via voxel-based morphometry as well as Freesurfer. Chinese and white cohorts were demographically matched for age, sex, and education.
Results:
Clinical severity by diagnosis was similar across ethnicities. Chinese and white patient groups showed similar amounts of atrophy in the regions most affected in AD after accounting for demographic variables and head size. There was no significant difference between ethnic groups when compared by atrophy and clinical severity.
Conclusion:
Our study suggests that Chinese and white AD patients, when matched demographically, are clinically and neuroanatomically similar on normalized measures of cortical atrophy and clinical severity.
Keywords: Alzheimer’s disease, neuroimaging, diversity, Chinese Americans, clinical trial design
INTRODUCTION
Understanding the similarities and differences in Alzheimer’s disease (AD) across ethnic groups (e.g., Asian versus European) could improve clinical evaluation and management of AD around the globe. Prior studies have found differences in AD risk across ethnicities; for example, older African Americans and Hispanics are more likely than older whites to have AD and other dementias1 and may have longer survival compared with white AD patients2. Interestingly, the AD genetic risk factor APOE-ε4 has weaker effects in African Americans and Hispanics but stronger effects in Japanese compared to white individuals3. However, despite evidence for differences in AD risk across ethnicities and the ~3.4 million ethnic Chinese in the U.S.4, few studies of dementia have focused on Chinese-Americans and the similarities and differences to whites across the AD spectrum.
While neuropsychiatric symptoms (e.g., anxiety, delusions and depression)5,6 and some subscales of neuropsychological screens7 are different between Chinese and white AD populations, fewer studies have explored differences in imaging biomarkers between these ethnic groups. Two prior studies found morphological brain features and differences between cognitively normal (CN) English-speaking whites and Chinese-speaking Asians8,9. We previously found that APOE-ε4 is associated with lower volume in AD-affected regions in cognitively normal Chinese but not whites10. The goals of our study are two-fold: (1) to examine the association between clinical severity (assessed with the Clinical Dementia Rating Sum of Boxes [CDR-SB]) and gray matter (GM) volume loss in Chinese and white individuals and (2) to assess whether there are atrophy differences in AD-associated regions between the two groups across the AD spectrum. To ensure our volumetric findings were robust across neuroanatomically diverse individuals, we measured volume loss using two analytically different, independent image analysis pipelines. Understanding the similarities and differences between Chinese and white AD patients is important because any ethnic-specific differences could impact the interpretation of research or clinical trials utilizing diverse participants, particularly if clinical or neuroimaging measures are used to assess treatment outcome.
MATERIALS AND METHODS
Study Participants and Assessment
All participants were recruited from the San Francisco Bay Area as part of on-going studies of healthy aging and AD administered by the University of California, San Francisco (UCSF) Memory and Aging Center (MAC). All participants underwent a multi-step screening process requiring at least one in-person visit to the MAC or a MAC-sponsored location (Chinese participants were eligible to be seen at alternate locations described below). To be eligible for this study, a participant required a 1.5 Tesla (T) or 3T MRI scan at our center as well as clinical and cognitive assessments within one year of the MRI scan. During the screening process, all participants underwent a neurologic exam, detailed cognitive assessment11,12, and medical history. The tests included in the cognitive assessment varied depending upon whether each study participant chose to undergo testing in English or Chinese (Mandarin or Cantonese). For those testing in English, the cognitive assessment included all tests administered as part of the National Alzheimer’s Disease Coordinating Center (NACC) Uniform Data Set (UDS)13. For participants testing in Chinese, the cognitive assessment included the Chinese version of the Cognitive Abilities Screening Instrument (CASI-C)14, which assesses memory, language, visuospatial, and executive function. Using the CASI-C, it is possible to derive a Chinese language Mini-Mental State Exam (MMSE) score. Beyond the CASI-C, participants who chose to test in Chinese underwent the Clinical Dementia Rating Scale (CDR)15; Geriatric Depression Scale (GDS)16; the Chinese Version of the Verbal Learning Test (CVVLT)17 or the Common Objects Memory Test (COMT)18; Stroop Color Word Test19; a design fluency task from the Ruff Figural Fluency Test (RFFT)20; Filled Dots trial of the Delis-Kaplan Executive Function System Design Fluency (D-KEFS DF)21; Benson figure22; digit span forward and backward23; Color Trails Form A, trials 1 (CTT-1) and 2 (CTT-2)24; and category verbal fluency test (animals, vegetables)25. Each participant had a study partner who was interviewed to help evaluate the participant’s functional abilities. Informants also completed the CDR, a well-validated test sensitive to the changes most often seen in AD26 and a clinically useful staging instrument27. The CDR has been shown to be valid in studies of Asian individuals28. A multidisciplinary team composed of a neurologist, neuropsychologist, and nurse then reviewed each participant’s data and determined the participant’s clinical diagnosis. Mild cognitive impairment (MCI) was diagnosed according to criteria as described in Petersen et al29. AD was diagnosed according to criteria as described in McKhann et al30.
Chinese participants were recruited through the Chinese Outreach portion of the MAC Alzheimer’s Disease Research Center (ADRC). Chinese participants were evaluated at the MAC or at two alternate locations in San Francisco’s Chinatown neighborhood. Chinese participants were consented and received cognitive tests in their preferred language (Mandarin, Cantonese, or English). All printed materials were also available in Chinese.
Chinese and white study participants were selected to maximize similarities across demographic variables (sex, age, education) by diagnosis to reduce confounding from factors outside of race that may contribute to brain volume and clinical severity. We first included all Chinese participants with imaging data available that passed post-processing quality control and then selected white participants to best match the available Chinese cohort. All participants or proxies provided IRB-approved, written informed consent prior to participation, and all tests were approved by the University of California, San Francisco Committee on Human Research.
Structural Image Acquisition
Study participants underwent MRI scanning within one year of CDR administration and had at least one T1-weighted MR image available for analysis. Individuals were scanned at the UCSF Neuroscience Imaging Center (NIC) or at the UCSF Veterans Affairs Medical Center (SFVA). Scans from the UCSF NIC were acquired using a 3.0 Tesla Siemens (Siemens, Iselin, NJ) TIM Trio scanner equipped with a 12-channel head coil using a magnetization prepared rapid gradient echo (MPRAGE) sequence (160 sagittal slices; slice thickness, 1.0 mm; field of view (FOV), 256 × 230 mm2; matrix 256 × 230; voxel size, 1.0 × 1.0 × mm3; repetition time (TR), 2,300 ms; echo time (TE), 2.98 ms; flip angle, 9°). Scans from the SFVA were acquired on a 1.5 Tesla Siemens Magnetom VISION system (Siemens, Iselin, NJ) equipped with a quadrature head coil using an MPRAGE sequence (164 coronal slices; slice thickness, 1.5mm; FOV, 256 × 256 mm2; matrix, 256 × 256; voxel size, 1.0 × 1.5 × 1.0 mm3, TR, 10 ms; TE, 4 ms; flip angle, 15°).
SPM Image Processing
All T1-weighted MR images were visually inspected for movement and other artifacts prior to analysis. The images were then bias corrected and segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) using Statistical Parametric Mapping (SPM) 1231 under the developers’ recommended (default) preprocessing parameters. Each segmented image was manually validated for accurate segmentation. The segmented scans were then warped to a cohort-specific template using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) tool box32. DARTEL-processed GM images were then linearly co-registered to the MNI152 template and smoothed using an 8 mm FWHM kernel. All Chinese and white participants’ images were preprocessed together to minimize batch effects due to differences in morphology.
Freesurfer (FS) Image Processing
All images were processed using Freesurfer version 5.3 using the default settings. Prior to skull stripping, all images were bias corrected33. The Freesurfer pipeline estimates white matter and cortical surfaces, which enables the estimation of cortical thickness across multiple brain regions34. The standard Freesurfer segmentation is also able to estimate volumes of subcortical regions35. Following initial surface estimations, we inspected each patient’s segmentation visually and corrected errors by hand. Of the 94 individuals included in the study, 4 Freesurfer segmentations did not pass quality control after two separate attempts to segment and edit the image, leaving 90 individuals with Freesurfer data available for analysis. Following segmentation, we extracted regional cortical thicknesses from participant images using the Desikan-Killiany cortical atlas36. In brief, the atlas contains 34 gyrally-defined cortical regions of interest (ROIs). Given the strong relationship between atrophy of the hippocampus and amygdala in AD37, we also extracted volumes for these subcortical ROIs. We used ROIs from both hemispheres, yielding a total of 72 ROIs.
Statistical Analyses
Comparing Clinical Characteristics
We first assessed whether clinical impairment varied by diagnosis in Chinese and white study participants across the AD spectrum. We used linear regression to compare CDR-SB scores by diagnosis and ethnic grouping, adjusting for age, sex, and education.
Voxel Based Morphometry (VBM)
All voxel-based testing was conducted using SPM. An aggregate region of AD-associated atrophy was generated using general linear models. The model was fit to each voxel and tested the relationship between GM tissue density and an individual’s CDR-SB score, under the one-tailed assumption that clinical impairment in MCI and AD patients would be associated with lower volume compared to controls. In this analysis, covariates included race (Chinese or white), sex, education, total intracranial volume (TIV), and scan type (1.5T or 3T). Chinese and white individuals were analyzed together to ensure identification of a ‘common’ set of regions across all study participants that could then be assessed at the individual level. For consideration as a statistically meaningful finding, a cluster of associated voxels was required to be significant at a family-wise error (FWE) corrected p-value ≤ 0.05 and have a cluster size greater than 50 voxels. GM volumes were then extracted from significant clusters and summed to make a composite AD ROI. The composite AD ROI was then used in secondary analyses as the outcome variable in a linear model, with race (Chinese or white), CDR-SB score, age, sex, education, TIV, and scan type (1.5T or 3T) as predictors.
Cortical Thickness Analysis
Using linear regression models, we assessed whether regional cortical thickness was associated with CDR-SB score across all Desikan-Killiany ROIs as well as hippocampus and amygdala. Covariates included race (Chinese or white), sex, education, and scan type (1.5T or 3T). TIV was included as a covariate when analyzing hippocampal and amygdalar volumes. For these analyses, we defined statistical significance using the Bonferroni technique at a p-value of 0.00069 (0.05 / 72 ROIs).
The top regions of atrophy identified in regional analyses were utilized in secondary analyses with the same covariates.
All statistical analyses were performed using R unless otherwise stated.
RESULTS
Cohort Composition
Ninety-four individuals were eligible for this study and were seen between 2005 and 2016 (Table 1). Nearly half of the participants (n=46) were white and the remainder were Chinese (n=48). Comparisons by race in the overall cohort, CN group, and AD group revealed no significant differences in age, education, sex, CDR-SB score, CDR total score, MMSE score, or scan type (all p>0.05). In the MCI group, there were no significant differences by race in age, education, sex, CDR score, and MMSE score (Table 1), but there were significant differences between Chinese and whites in CDR-SB score and scan type.
Table 1.
Cohort description by ethnic and diagnostic groupings
| Overall Cohort | Cognitively Normal | Mild Cognitive Impairment | Alzheimer’s Disease | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Chinese | p-value | White | Chinese | p-value | White | Chinese | p-value | White | Chinese | p-value | |
| N | 46 | 48 | 23 | 24 | 15 | 16 | 8 | 8 | ||||
| Age (Mean ± SD) | 69 ± 9.2 | 69 ± 8.5 | 0.77 | 69 ± 8.7 | 69 ± 8.1 | 0.99 | 67 ± 9.5 | 66 ± 9.0 | 0.83 | 75.8 ± 8.2 | 74.5 ± 6.5 | 0.74 |
| Female Sex (N; %) | 25 (54.3%) | 25 (52.1%) | 0.99 | 13 (56.5%) | 13 (54.2%) | 1 | 9 (60%) | 9 (56.2%) | 1 | 3 (37.5%) | 3 (37.5%) | 1 |
| CDR-SB Score (Mean ± SD) | 1.5 ± 2.1 | 1.5 ± 2.8 | 0.94 | 0 ± 0 | 0.12 ± 0.3 | 0.08 | 1.7 ± 1.2 | 0.6 ± 0.5 | 0.002 | 5.31 ± 1.4 | 7.1 ± 3.0 | 0.15 |
| CDR Score (Mean ± SD) | 0.3 ± 0.4 | 0.4 ± 0.5 | 0.53 | 0 ± 0 | 0.06 ± 0.2 | 0.08 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.18 | 0.9 ± 0.2 | 1.3 ± 0.6 | 0.11 |
| MMSE (Mean ± SD) | 27.1 ± 3.7 | 26.9 ± 3.6 | 0.78 | 29.3 ± 0.9 | 28.9 ± 1.1 | 0.20 | 27.5 ± 1.6 | 27.5 ± 1.4 | 0.95 | 20.0 ± 2.5 | 19.9 ± 2.6 | 0.92 |
| Education (Years; Mean ± SD) | 15.2 ± 2.5 | 14.9 ± 3.3 | 0.52 | 15.3 ± 1.9 | 15.5 ± 2.9 | 0.75 | 15.5 ± 3.2 | 14.9 ± 2.8 | 0.59 | 14.6 ± 2.6 | 12.8 ± 4.5 | 0.33 |
| 3T MRI Scan (N; %) | 36 (78.3%) | 45 (93.8%) | 0.06 | 22 (95.7%) | 23 (95.8%) | 1 | 9 (60%) | 16 (100%) | 0.02 | 5 (62.5%) | 6 (75%) | 1 |
Cohort demographic and clinical characteristics. MMSE: Mini–mental state examination; CDR-SB: Clinical Dementia Rating – Sum of Boxes score; CDR: Clinical Dementia Rating; T: Tesla; SD: Standard deviation.
Chinese and white cohorts are clinically similar in MCI and AD
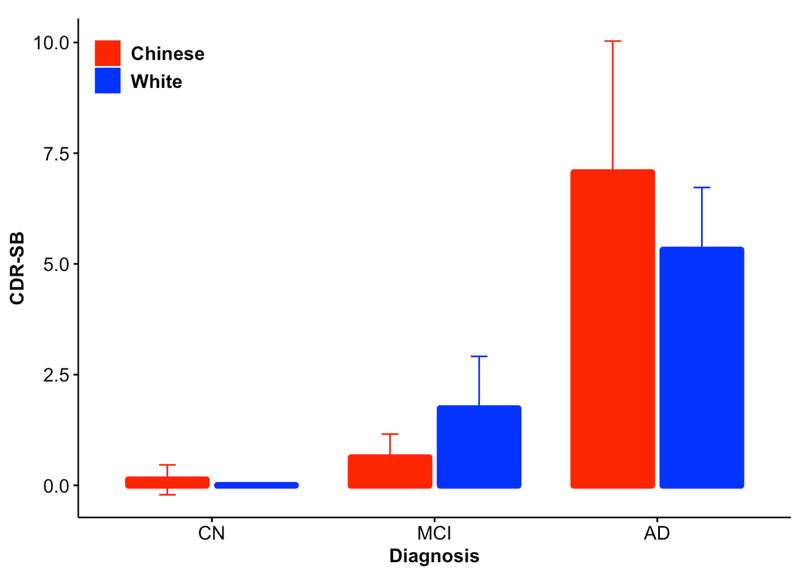
We first tested whether there were clinical differences between MCI and AD versus CN controls in the Chinese and white cohorts. There was a significant difference in clinical severity (measured by CDR-SB score) by diagnostic grouping in both the white (MCI p=2.83×10−7; AD p<2×10−16) and Chinese (MCI p=0.19; AD p=3.17×10−16) cohorts as well as when the two races were combined together (MCI p=1.41×10−4; AD p<2×10−16). CDR-SB scores were not associated with age, race, sex, or education (see table Supplemental Digital Content 2, Figure 1).
Figure 1: CDR-SB score by race and diagnosis.
Clinical Dementia Rating Sum of Boxes (CDR-SB) scores were significantly different across diagnostic groups (see Table, Supplemental Digital Content 1), but showed no significant difference across races (p=0.96). The plotted points are best linear unbiased predictions (BLUPs). See Supplemental Digital Content 1 and the text for details. CN – Control; MCI – Mild Cognitive Impairment; AD – Alzheimer’s disease.
VBM across the AD spectrum identifies common areas of regional atrophy in Chinese and white individuals
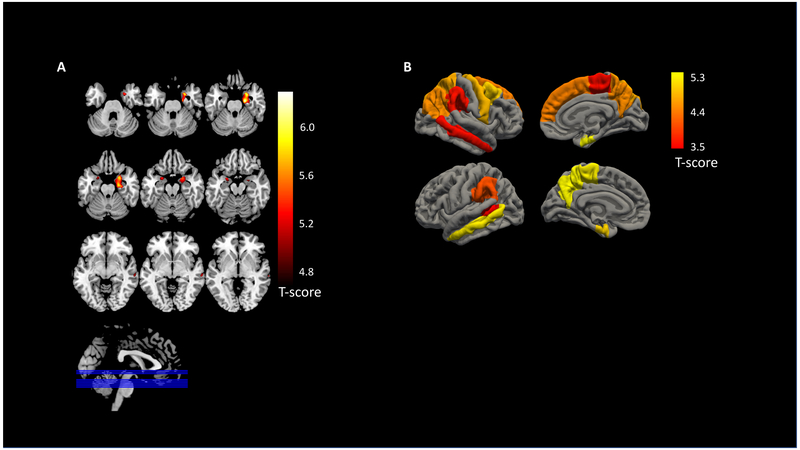
In the VBM analysis of all participants contrasting CN to MCI and AD individuals, three clusters were significant after multiple testing correction. As expected, both the right and left hippocampus, amygdala, and parahippocampal area showed significant atrophy along with the right middle temporal lobe (pFWE<0.05) (Figure 2A).
Figure 2: AD-associated atrophy measured by VBM and FS.
Atrophy varied significantly across the AD spectrum (measured by Clinical Dementia Rating Sum of Boxes (CDR-SB) scores). A) The voxel-based morphometry (VBM) regions of interest (ROI) included the right and left hippocampus, amygdala, and parahippocampal areas along with the right middle temporal lobe. B) The Freesurfer (FS)-based AD ROIs included the bilateral hippocampi and amygdalae volumes along with bilateral entorhinal, middle temporal, paracentral gyrus, precuneus, and supramarginal thicknesses as well as left banks of the superior temporal sulcus, right caudal middle frontal, right inferior parietal, right precentral, right superior frontal, and right superior parietal thicknesses.
As expected, there were significant volumetric differences in the combined AD ROI by CDR-SB score in the Chinese (p=8.21×10−5) and white (p=4.00×10−5) cohorts as well as the combined cohort (p=4.73×10−10) (Figure 2A). Volume within the AD ROI was also significantly associated with age, education, and TIV. Race, sex, and scan type were not significant predictors of volume (see Table, Supplemental Digital Content 2).
Cortical thickness analysis across the AD spectrum identifies common areas of regional atrophy in Chinese and white individuals
In the regional cortical thickness analysis across all participants comparing CN to MCI and AD, 19 regions were significantly associated with CDR-SB score (p<0.00069). As expected, and consistent with the VBM analysis, both the right and left hippocampus as well as amygdalar volumes were associated with CDR-SB scores. Additionally, the bilateral entorhinal, middle temporal, paracentral gyrus, precuneus, and supramarginal thicknesses as well as left banks of the superior temporal sulcus, right caudal middle frontal, right inferior parietal, right precentral, right superior frontal, and right superior parietal thicknesses were associated with CDR-SB scores (Figure 2B). Full analysis results for all 72 ROI are available in Supplemental Digital Content 3.
We next tested whether Freesurfer-derived hippocampal volumes and entorhinal cortex thicknesses varied by diagnosis, as these regions had the most significant bilateral volumetric and thickness findings, respectively. Hippocampal volumes were significantly associated with CDR-SB score in the Chinese (right p=7.26×10−5; left p=2.21×10−5) and white cohorts (right p=0.005; left p=0.006), as well as the combined cohort (see Table, Supplemental Digital Content 2). Similarly, entorhinal cortex thicknesses were significantly associated with CDR-SB score in the Chinese (right p=6.22×10−5; left p=0.004) and white cohorts (right p=0.01; left p=0.001), as well as the combined cohort (see Table, Supplemental Digital Content 2). However, race was not a significant predictor of hippocampal volume or entorhinal cortex thickness after multiple testing correction (p>0.00069).
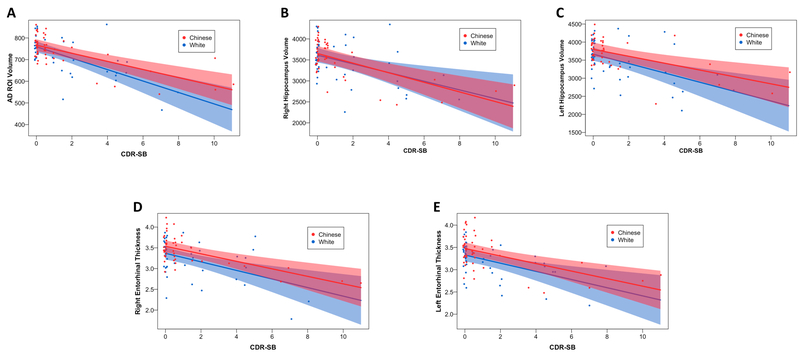
Although not significant after multiple testing, Chinese participants showed greater entorhinal thicknesses than white participants (raw p-value <0.05). To specifically test whether GM volume loss varied as a function of clinical progression and race, we added an interaction term to the VBM and FS models and found that volume loss did not significantly vary between races across the AD spectrum for the VBM-generated AD ROI or top Freesurfer regions (Table 2, Figure 3).
Table 2.
Interaction analyses of AD regions of interest across the AD spectrum
| VBM ROI | FS Right Hippocampus | FS Left Hippocampus | FS Right Entorhinal | FS Left Entorhinal | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β ± SE | p-value | β ± SE | p-value | β ± SE | p-value | β ± SE | p-value | β ± SE | p-value | |
| Age | −3.34 ± −0.79 | 6.33E-05 | −37.17 ± 5.38 | 1.03E-09 | −34.92 ± 5.67 | 2.64E-08 | −0.02 ± 0.01 | 0.001 | −0.01 ± 0.004 | 0.24 |
| Sex | 18.05 ± 17.49 | 0.30 | 0.76 ± 119.42 | 0.99 | 285.84 ± 125.69 | 0.03 | 0.02 ± 0.08 | 0.77 | 0.10 ± 0.08 | 0.21 |
| Education | −6.83 ± 2.38 | 0.005 | 19.58 ± 18.66 | 0.30 | −8.62 ± 19.63 | 0.66 | −0.03 ± 0.02 | 0.08 | 0.001 ± 0.02 | 0.95 |
| Scan Type | 26.05 ± 22.38 | 0.25 | −191.06 ± 151.43 | 0.21 | −272.02 ± 159.375 | 0.09 | 0.05 ± 0.13 | 0.70 | 0.11 ± 0.12 | 0.38 |
| TIV | 405.45 ± 62.94 | 6.75E-09 | 6.38E-04 ± 3.667E-04 | 0.86 | 0.001 ± 3.860E-04 | 0.002 | - | - | - | - |
| CDR-SB | −26.390 ± 4.94 | 7.49E-07 | −103.56 ± 33.13 | 0.002 | −129.48 ± 34.87 | 3.74E-04 | −0.10 ± 0.03 | 5.34E-04 | −0.10 ± 0.03 | 0.001 |
| Race | 7.11 ± 16.06 | 0.66 | 39.50 ± 107.07 | 0.71 | 132.67 ± 112.69 | 0.24 | 0.17 ± 0.09 | 0.07 | 0.15 ± 0.09 | 0.09 |
| CDR-SB X Race | 7.70 ± 5.73 | 0.18 | 10.91 ± 39.56 | 0.78 | 34.13 ± 41.64 | 0.41 | 0.01 ± 0.03 | 0.71 | 0.01 ± 0.03 | 0.83 |
There was no significant difference in AD-associated grey matter volume loss between Chinese and whites after accounting for age, sex, education, TIV, scan type, and race. For the interaction analyses, we used the same five regions of interest generated in our primary volumetric analyses, a voxel-based morphometry (VBM) aggregate region of interest (ROI) and Freesurfer volumetric measures (bilateral hippocampi and entorhinal cortices). ROI: regions of interest; TIV: total intracranial volume; CDR-SB: Clinical Dementia Rating – Sum of Boxes score; VBM: Voxel-based morphometry; FS: Freesurfer; β: Beta coefficient from regression model; SE: Standard error.
Figure 3: No difference in AD-associated volume loss between Chinese and white individuals across the AD spectrum.
Interaction analyses of Clinical Dementia Rating Sum of Boxes (CDR-SB) score by race versus volume or thickness for the top AD regions identified by voxel-based morphometry (VBM) and Freesurfer (FS) are shown. Volume was used for the VBM region of interest (ROI) along with FS hippocampal measurements (A-C) while thickness was used for the FS entorhinal cortex measurements (D-E). There was no significant association between AD-associated volume loss and race across the AD spectrum. The plotted points are partial residuals with 95% confidence bands provided in shading. See Table 2 for details.
DISCUSSION
In this study, we compared GM loss with clinical measures of disease severity between demographically similar groups of Chinese and white CN, MCI and AD participants living in the San Francisco area. We found no significant differences in disease severity as measured by CDR-SB score. Similarly, using two independent image processing pipelines, we found no significant volumetric or cortical thickness differences in GM atrophy between Chinese and white participants across the AD spectrum. The correlation between volume loss and degree of clinical severity was the same in both Chinese and white individuals. Taken together, this suggests that Chinese and white AD patients, when matched demographically, look neuroanatomically and clinically similar on normalized measures of GM atrophy and clinical severity.
The incidence of AD in European populations was recently estimated at 11.08 cases per 1,000 person-years, and was 6.25 cases per 1,000 person-years in the most populated country in the world, China38,39. While some cohort studies suggest that age-specific incidence of dementia has decreased in the past 20 years in European populations40 and in the United States41, the prevalence and incidence of dementia has increased in China due to a growing elderly population, rising dementia awareness, and growing research in less developed regions38. This increasing prevalence and incidence of dementia could have important ramifications given the accelerated growth of the oldest segments of the Chinese population42. Differences in cultural stigma, prediction of dementia risk, early and accurate diagnosis, and therapeutic treatment and response across populations all likely contribute to these differences in AD epidemiology in diverse groups. Despite the high numbers of Chinese-Americans in the U.S., this population has not been included in many studies of AD and other dementias. Taken together, these factors can lead to challenges in predicting the severity of cognitive impairment as well as providing appropriate diagnoses and treatments for people with AD, particularly in underserved groups and ethnic populations in developing countries.
In our study, CDR-SB scores were significantly associated with diagnosis and age, consistent with prior studies in different Asian populations43,44 and providing further evidence that the CDR-SB is a reliable as well as valid classification tool to evaluate severity of dementia and is sensitive to cognitive decline in diverse populations45. Our neuroimaging analyses identified several brain regions that showed GM volume loss in both Chinese and white AD patients. As expected, these regions included many regions classically associated with AD, such as the hippocampus, amygdala, parahippocamal regions, and temporal cortex46,47.
Previous studies suggest that MRI volumetric data can aid clinical evaluation as a complementary diagnostic tool48. Indeed, two studies conducted separately in Chinese and white cohorts have examined atrophy patterns in AD and MCI patients and consistently demonstrated that hippocampal volume derived from MRI scans predicts future cognitive decline and conversion to AD among individuals with MCI46,49. GM volume loss in medial temporal lobe, hippocampus, and parahippocampus was also shown to accurately distinguish AD from normal controls and other neurodegenerative diseases in Chinese individuals50,51, as well as other populations48,49. Our results suggest that volume loss in the AD-associated ROIs we identified using both VBM and Freesurfer — which included hippocampal and parahippocampal structures — could be a good predictor for clinical progression from MCI to AD in diverse populations.
Our data suggests that Chinese and white individuals do not show significant brain atrophy differences in the context of AD neurodegeneration. In our study, both cohorts showed similar patterns of clinical and neuroanatomical vulnerability, suggesting similar pathophysiological processes occur in both ethnic groups. We replicated this finding using two independent data processing pipelines (after normalizing regional volume by TIV, scan type, and demographic variables). While not definitively conclusive, our study has important implications for studies evaluating AD progression with measures such as CDR-SB score or hippocampal atrophy. Our findings suggest that clinical and neuroimaging data from Chinese and white individuals can be combined and analyzed as a single group without confounding due to differential disease effects by racial groupings. Despite differences in population-based genetic background, this suggests that the patterns of neurodegeneration are similar in both groups, supporting a common ‘trajectory’ of conversion from healthy aging to MCI and AD that may be amenable to similar types of interventions.
Our cohorts were clinically well characterized and we used this information to minimize bias across cohorts. This included comparing baseline assessments of CN controls, MCI, and AD patients and matching Chinese and white individuals within each diagnostic group by age, sex, education, and scan type to the greatest extent possible. To ensure our results were not driven by a single image processing modality, we performed our analyses using both VBM and Freesurfer. These two analytically different pipelines converged upon the same findings, suggesting our results are not driven by technical artifacts or biased by an individual pipeline’s reference atlas. In our study, scanner type was not associated with brain morphology, although variation introduced by the difference in distribution of the two scanner types across diagnostic and population subgroups cannot be wholly discounted. This is important for clinical practice and research when using 1.5T MRI53.
The main caveat of our study is the limited sample size, raising the possibility that more varied environmental and/or genetic influences could mask effects in other populations in China or other European countries. Although the number of white research participants at our center is larger, we specifically designed our study to directly match all available Chinese participants with demographically similar white participants. One of the contributors to this limited sample size is the low participation rate of ethnic minorities in clinical research and intervention trials54,55. This study is also limited by the comparatively small number of cognitive assessments for participants who chose to test in Chinese, some of which remain to be fully validated for neuroanatomic correlates in Mandarin and Cantonese-speaking individuals. It is possible that more detailed cognitive testing could reveal subtle differences between study groups. Furthermore, we did not have sufficient data to account for potential modifying contributions of lifestyle and/or environment across the Chinese-American cohort. Future work will benefit from broader samples, additional language-validated cognitive testing, and inclusion of systematic measures of lifestyle and environmental factors that may modify trajectories in cognitive health and neurodegeneration.
Our study provides cross-sectional evidence that Chinese and white individuals across the AD spectrum show the same disease-associated patterns of neuroimaging and clinical severity. This research highlights the importance of implementing strategies to improve diversity in clinical research and therapeutic trials. Gaining a better understanding of ethnically diverse communities in AD clinical research will generate rich information about contributions of genetic variation, environment, diet, and culture among ethnic groups that may differentially contribute to AD risk and may influence future treatment decisions as therapeutic interventions become available. Evaluation of diverse aging populations will be critical for effectively addressing public health policy issues related to AD and in recruitment for clinical trials, and could ultimately inform the design and interpretation of intervention trials in multi-ethnic cohorts.
Supplementary Material
ACKNOWLEDGMENTS
We thank all participants for their contributions to our research. Primary support for data analysis was provided by the NIA K01 AG049152 (JSY), Larry L. Hillblom Foundation 2016-A-005-SUP (JSY), Tau Consortium (JSY), Bluefield Project to Cure FTD (JSY), John Douglas French Alzheimer’s Foundation (JSY), and Radiological Society of North America (RSNA) RMS1741 (LWB). Additional support was provided by NIH grants P50-AG023501 and P01-AG1972403 (BLM), the Larry L. Hillblom Foundation (JHK), and the National Center for Advancing Translational Sciences of the NIH under Award Number TL1 TR001871 (JSC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
DISCLOSURE STATEMENT
The authors have no conflicts of interest to disclose.
Contributor Information
Jia Fan, Email: liufanxiaolin@163.com.
Marian Tse, Email: marian.tse@ucsf.edu.
Jessie S. Carr, Email: jessie.carr@ucsf.edu.
Bruce L. Miller, Email: bruce.miller@ucsf.edu.
Joel H. Kramer, Email: joel.kramer@ucsf.edu.
Howard J. Rosen, Email: howard.rosen@ucsf.edu.
Luke W. Bonham, Email: luke.bonham@ucsf.edu.
REFERENCES
- 1.Dilworth-Anderson P, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S. Diagnosis and assessment of Alzheimer’s disease in diverse populations. Alzheimer’s Dement. 2008;4(4):305–309. doi: 10.1016/j.jalz.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Mehta KM, Yaffe K, Pérez-Stable EJ, et al. Race/ethnic differences in AD survival in US Alzheimer’s Disease Centers. Neurology. 2008;70(14):1163–1170. doi: 10.1212/01.wnl.0000285287.99923.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. doi: 10.1001/jama.1997.03550160069041. [DOI] [PubMed] [Google Scholar]
- 4.United States Census Bureau. Profile of General Population and Housing Characteristics: 2010.
- 5.Chow TW, Liu CK, Fuh JL, et al. Neuropsychiatric symptoms of Alzheimer’s disease differ in Chinese and American patients. Int J Geriatr Psychiatry. 2002;17(1):22–28. doi: 10.1002/gps.509. [DOI] [PubMed] [Google Scholar]
- 6.Chao SZ, Matthews BR, Yokoyama JS, et al. Depressive symptoms in Chinese-American subjects with cognitive impairment. Am J Geriatr Psychiatry. 2014;22(7):642–652. doi: 10.1016/j.jagp.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan AS, Choi MK, Salmon DP. The effects of age, education, and gender on the Mattis Dementia Rating Scale performance of elderly Chinese and American individuals. Journals Gerontol Ser B-Psychological Sci Soc Sci. 2001;56(6):356–363. [DOI] [PubMed] [Google Scholar]
- 8.Crinion JT, Green DW, Chung R, et al. Neuroanatomical markers of speaking Chinese. Hum Brain Mapp. 2009;30:4108–4115. doi: 10.1002/hbm.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochunov P, Fox P, Lancaster J, et al. Localized morphological brain differences between English-speaking Caucasians and Chinese-speaking Asians: new evidence of anatomical plasticity. Neuroreport. 2003;14:961–964. doi: 10.1097/01.wnr.0000075417.59944.00. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama JS, Lee AKL, Takada LT, et al. Apolipoprotein ε4 is associated with lower brain volume in cognitively normal Chinese but not white older adults. PLoS One. 2015;10(3):e0118338. doi: 10.1371/journal.pone.0118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen HJ, Alcantar O, Rothlind J, et al. Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. Neuroimage. 2010;49:3358–3364. doi: 10.1016/j.neuroimage.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer JH, Jurik J, Sha SJ, et al. Distinctive Neuropsychological Patterns in Frontotemporal Dementia, Semantic Dementia, and Alzheimer Disease. Cogn Behav Neurol. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin K-N, Wang P-N, Liu C-Y, Chen W-T, Lee Y-C, Liu H-C. Cutoff scores of the cognitive abilities screening instrument, Chinese version in screening of dementia. Dement Geriatr Cogn Disord. 2002;14(4):176–182. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/WNL.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 16.Sheikh RL, Yesavage JA. Geriatric Depression Scale (GDS). Recent Evidence and Development of a Shorter Version. Clin Gerontol. 1986;5:165–173. doi: 10.1300/J018v05n01_09. [DOI] [Google Scholar]
- 17.Chang CC, Kramer JH, Lin KN, et al. Validating the Chinese version of the Verbal Learning Test for screening Alzheimer’s disease. J Int Neuropsychol Soc. 2010;16:244–251. doi: 10.1017/S1355617709991184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kempler D, Teng EL, Taussig M, Dick MB. The common objects memory test (COMT): a simple test with cross-cultural applicability. J Int Neuropsychol Soc. 2010;16:537–545. doi: 10.1017/S1355617710000160. [DOI] [PubMed] [Google Scholar]
- 19.Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, IL: Skoelting; 1978. [Google Scholar]
- 20.Ruff R Ruff Figural Fluency Test. Odessa, FL: Psychological Assessment Resources, Inc.; 1996. [Google Scholar]
- 21.Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 22.Possin KL, Laluz VR, Alcantar OZ, Miller BL, Kramer JH. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer’s disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49(1):43–48. doi: 10.1016/j.neuropsychologia.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woods DL, Kishiyamaa MM, Lund EW, et al. Improving digit span assessment of short-term verbal memory. J Clin Exp Neuropsychol. 2011;33(1):101–111. doi: 10.1080/13803395.2010.493149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Elia L, Satz P, Uchiyama C, White T. Color Trails Test. Odessa, FL: Psychological Assessment Resources, Inc.; 1996. [Google Scholar]
- 25.Mok EHL, Lam LCW, Chiu HFK. Category Verbal Fluency Test Performance in Chinese Elderly with Alzheimer’s Disease. Dement Geriatr Cogn Disord. 2004;18(2):120–124. doi: 10.1159/000079190. [DOI] [PubMed] [Google Scholar]
- 26.Gauthier S, Leuzy A, Rosa-Neto P. How can we improve transfer of outcomes from randomized clinical trials to clinical practice with disease-modifying drugs in Alzheimer’s disease? Neurodegener Dis. 2014;13(2–3):197–199. doi: 10.1159/000353748. [DOI] [PubMed] [Google Scholar]
- 27.Balsis S, Benge JF, Lowe DA, Geraci L, Doody RS. How Do Scores on the ADAS-Cog, MMSE, and CDR-SOB Correspond? Clin Neuropsychol. 2015;29(7):1002–1009. doi: 10.1080/13854046.2015.1119312. [DOI] [PubMed] [Google Scholar]
- 28.Yeo CYY, Chan MPC, Lim WS, Chong MS. Clinical utility of the clinical dementia rating sum of boxes in mild cognitive impairment and dementia in an Asian population. Alzheimer’s Dement J Alzheimer’s Assoc. 2010;6(4):S342. doi: 10.1016/j.jalz.2010.05.1146. [DOI] [Google Scholar]
- 29.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. [DOI] [PubMed] [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimerʼs disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimerʼs Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 31.Ashburner J, Ridgway GR. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci. 2013;(February). doi: 10.3389/fnins.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashburner J A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Ségonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 34.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 35.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 36.Desikan RS, Seggonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC. Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Res - Neuroimaging. 2011;194(1):7–13. doi: 10.1016/j.pscychresns.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan KY, Wu JJ, Liu L, et al. Epidemiology of alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet. 2013;381(9882):2016–2023. doi: 10.1016/S0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Xu Y, Nie H, et al. Prevalence of dementia and major dementia subtypes in the Chinese populations: A meta-analysis of dementia prevalence surveys, 1980–2010. J Clin Neurosci. 2012;19(10):1333–1337. doi: 10.1016/j.jocn.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Scheltens P, Blennow K, Breteler MMB, et al. Alzheimer’s disease. Lancet. 2017;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 41.Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimer’s Dement. 2011;7(1):80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng Z, Liu C, Guan X, Mor V. China’s rapidly aging population creates policy challenges in shaping a viable long-term care system. Health Aff. 2012;31(12):2764–2773. doi: 10.1377/hlthaff.2012.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meguro K, Shimada M, Yamaguchi S, et al. Neuropsychosocial features of very mild Alzheimer’s disease (CDR 0.5) and progression to dementia in a community: The tajiri project. J Geriatr Psychiatry Neurol. 2004;17(4):183–189. doi: 10.1177/0891988704269812. [DOI] [PubMed] [Google Scholar]
- 44.Lim WS, Chin JJ, Lam CK, Lim PPJ, Sahadevan S. Clinical dementia rating: Experience of a multi-racial Asian population. Alzheimer Dis Assoc Disord. 2005;19(3):134–142. doi: 10.1097/01.wad.0000174991.60709.36. [DOI] [PubMed] [Google Scholar]
- 45.Morris JC. Clinical Dementia Rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatrics. 1997;9(S1):173–176. doi: 10.1017/S1041610297004870. [DOI] [PubMed] [Google Scholar]
- 46.Schuff N, Woerner N, Boreta L, et al. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain. 2009;132(Pt 4):1067–1077. http://brain.oxfordjournals.org/content/132/4/1067.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller MI, Younes L, Ratnanather JT, et al. Amygdala Atrophy in MCI/Alzheimer’s Disease in the BIOCARD cohort based on Diffeomorphic Morphometry. Med Image Comput Comput Assist Interv. 2012. [PMC free article] [PubMed] [Google Scholar]
- 48.Duara R, Loewenstein DA, Potter E, et al. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology. 2008;71(24):1986–1992. doi: 10.1212/01.wnl.0000336925.79704.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jack CR, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52(7):1397–1403. doi: 10.1212/WNL.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Y, Zhang Z, Zhou B, et al. Grey-matter volume as a potential feature for the classification of Alzheimer’s disease and mild cognitive impairment: An exploratory study. Neurosci Bull. 2014;30(3):477–489. doi: 10.1007/s12264-013-1432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He J, Iosif AM, Lee DY, et al. Brain structure and cerebrovascular risk in cognitively impaired patients: Shanghai community brain health initiative-pilot phase. Arch Neurol. 2010;67(10):1231–1237. doi: 10.1001/archneurol.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He D, Yu H, Chen Y. Equity in the distribution of CT and MRI in China: A panel analysis. Int J Equity Health. 2013;12:39. doi: 10.1186/1475-9276-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang Y, Hojatkashani C, Dinov ID, et al. The construction of a Chinese MRI brain atlas: A morphometric comparison study between Chinese and Caucasian cohorts. Neuroimage. 2010;51(1):33–41. doi: 10.1016/j.neuroimage.2010.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodríguez-Gómez O, Palacio-Lacambra ME, Palasí A, Ruiz-Laza A, Boada-Rovira M. Prevention of Alzheimer’s disease: a global challenge for next generation neuroscientists. J Alzheimers Dis. 2014;42(S4):S515–S523. doi: 10.3233/JAD-141479. [DOI] [PubMed] [Google Scholar]
- 55.Faison WE, Schultz SK, Aerssens J, et al. Potential ethnic modifiers in the assessment and treatment of Alzheimer’s disease: challenges for the future. Int Psychogeriatr. 2007;19(3):539–558. doi: 10.1017/S104161020700511X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.