Abstract
Background:
Prescription tranquilizer/sedative (e.g., alprazolam, zolpidem) misuse (i.e., use in ways not intended by the prescriber or without a prescription) is understudied, with little research identifying misuse correlates. Identification of key correlates could identify subgroups more likely to engage in misuse, allowing for targeted treatment. This work examines tranquilizer/sedative use and misuse prevalence rates and misuse correlates across U.S. age cohorts, using nationally representative data.
Methods:
Data were from the 2015–16 National Survey on Drug Use and Health (n=114,043). Analyses used design-based logistic regression for past-year tranquilizer/sedative misuse correlates across participants or those engaged in past-year use; past-month misuse correlates were also examined in those with past-year misuse.
Results:
Young adults (18–25 years) had the highest prevalence of past-year and past-month tranquilizer/sedative misuse, with 42.8% of those with past-year use also engaged in misuse. Mental health correlates were associated with past-year misuse, while substance use, particularly opioid misuse, was associated with both past-year and past-month misuse. Substance use correlate strength was most likely to vary by age group, with older adults (65 years and older) having fewer significant correlates overall.
Conclusions:
This work highlighted young adults and those with other substance use as most likely to engage in tranquilizer/sedative misuse. In particular, those endorsing suicidality and reporting opioid misuse are a subgroup of concern, given their especially elevated rates of misuse and the increased risk for overdose imparted by tranquilizer/sedative medication. Workplace-based interventions for young adults and school-based universal prevention may be warranted to limit tranquilizer/sedative misuse in these groups.
Keywords: Tranquilizer, benzodiazepine, sedative, misuse, age cohorts
1. INTRODUCTION
Prescription drug misuse (PDM) has received increasing attention recently, with commentators labelling PDM as an epidemic (Kanouse & Compton, 2015; Von Korff & Franklin, 2016). Much of the focus has been on opioid PDM, given its outsized role in PDM prevalence and consequences, including overdose. As a result, prescription tranquilizer (i.e., primarily capturing benzodiazepine medication, such as alprazolam, used often for anxiety treatment) and sedative PDM (i.e., medications primarily indicated for insomnia, such as zolpidem) remains understudied (e.g., Maree, Marcum, Saghafi, Weiner, & Karp, 2016). In the US, tranquilizer/sedative use disorder treatment increased by 67% from 2003 to 2012 (SAMHSA, 2014), and adult fatal benzodiazepine overdose increased by over 400% from 1996 to 2013 (Bachhuber, Hennessy, Cunningham, & Starrels, 2016), with a nearly 300% increase due to benzodiazepine and opioid co-ingestion (Jones & McAninch, 2015). Data also indicate a 90% increase in US emergency department visits involving benzodiazepines from 2005 to 2011 (Day, 2014), with an increase of over 300% from benzodiazepine and opioid co-ingestion (Jones & McAninch, 2015).
Adolescent tranquilizer and sedative PDM is associated with poor psychosocial correlates, including major depression, poorer academic achievement, and problematic substance use (Hall, Howard, & McCabe, 2010; McCabe & West, 2014; Rigg & Ford, 2014; Schepis & Krishnan-Sarin, 2008). In adults, research on such misuse has occurred primarily in non-US samples (Fride Tvete, Bjorner, & Skomedal, 2015; McLarnon, Monaghan, Stewart, & Barrett, 2011; Nattala, Murthy, Thennarasu, & Cottler, 2014; Tahiri et al., 2017), with similarly concerning correlates.
Many aspects of tranquilizer/sedative PDM remain unexplored. No tranquilizer PDM research could be found comparing PDM and correlates across the lifespan in a US sample, with only one US study on zolpidem PDM (Schepis, 2014). Research evaluating tranquilizer/sedative PDM across the lifespan could have significant clinical utility, as characterization of older adults engaged in tranquilizer/sedative PDM could help prevent significant associated consequences (e.g., falls and fractures, neurocognitive impairments, and increased overdose risk; Airagnes, Pelissolo, Lavallee, Flament, & Limosin, 2016; Maree et al., 2016) by identifying those most likely to misuse. Identification of tranquilizer/sedative PDM correlates across age cohorts could establish whether correlates differ and require different foci by age, or are similar across ages, allowing for consistent prevention targets.
1.1. Aims
We aimed to fill these gaps in the literature through analyses of the 2015–16 National Survey on Drug Use and Health (NSDUH), with tranquilizer/sedative use and PDM combined, due to low sedative use/misuse prevalence and per previous research (Schepis & Hakes, 2013; Tetrault et al., 2008). First, we estimated the prevalence of lifetime and past-year tranquilizer/sedative use and PDM across six age groups: adolescents (12–17), young adults (18–25), and adults aged 26–34, 35–49, 50–64, and 65 and older. Second, we evaluated past-year tranquilizer/sedative misuse correlates across the population and in those engaged in any past-year tranquilizer/sedative use. Third, we examined past-month tranquilizer/sedative misuse correlates among those endorsing past-year misuse, with those 50 and older aggregated due to sample size concerns.
2. METHODS
The NSDUH is an annual survey of substance use and associated behaviors in a representative sample of the US non-institutionalized population. It uses an independent, multistage area probability sample with population-based weights to provide nationally- representative estimates. All sensitive questions (e.g., those on PDM) were asked via audio computer-assisted self-interviewing (ACASI) to maximize honest reporting, with skip-outs and consistency checks to promote full responding and data consistency. More information on the NSDUH, including on psychometrics, is available elsewhere (CBHSQ, 2016; 2017; SAMHSA, 2010).
2.1. Participants
For 2015–16, 114,043 respondents were included in the NSDUH public use files. Females composed 51.3% of the sample, with Caucasians (63.5%), Hispanic/Latinos (16.4%) and African-Americans (12.0%) comprising the three largest racial/ethnic groups (all weighted). For characteristics by age group, see online-only Supplemental Table A.
2.2. Measures
2.2.1. Primary Outcomes
To aid recall, the NSDUH used trade and generic drug names and medication pictures, including Xanax®, Valium® or alprazolam for tranquilizers, and Ambien®, Lunesta® or zolpidem for sedatives. Initially, lifetime tranquilizer/sedative use and past-year tranquilizer/sedative use were assessed. Then, in those with lifetime but not past-year use, only lifetime tranquilizer/sedative misuse was assessed; in those with past-year tranquilizer/sedative use, past-year tranquilizer/sedative misuse was assessed instead. For both timeframes, this instruction is used: “The next question asks about using [drug class] in any way a doctor did not direct you to use them…including: Using it without a prescription of your own; Using it in greater amounts, more often, or longer than you were told to take it; Using it in any other way a doctor did not direct you to use it.” Past-month tranquilizer/sedative misuse was assessed among respondents who reported past-year tranquilizer/sedative misuse.
2.2.2. Age Categories
Current age groups were restricted by the NSDUH public use file variables: the six-level CATAG6 variable used for the first three tables (ages 12–17, 18–25, 26–34, 35–49, 50–64, and 65 and older) or the five-level CATAG3 variable for Table 4 (12–17, 18–25, 26–34, 35–49, and 50 and older).
Table 4.
Correlates of past month prescription sedative/tranquilizer misuse by age group among those engaged in past year misuse
| 12−17 years (adolescents) (a) |
18−25 years (young adults) (b) |
26−34 years (c) |
35−49 years (d) |
50 years and older (e) |
Post Hoc Differences | |
|---|---|---|---|---|---|---|
| Sample Size | 523 | 1,543 | 687 | 541 | 262 | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Sociodemographic Correlates | ||||||
| Male Sex | 1.76 (1.16−2.65)** | 1.25 (0.97−1.61) | 0.83 (0.64−1.08) | 0.99 (0.72−1.35) | 1.24 (0.76−2.03) | t = −1.53, p = 0.13 |
| White Ethnicity | 0.95 (0.68−1.35) | 1.20 (0.96−1.50) | 1.04 (0.73−1.46) | 1.33 (0.89−2.00) | 1.23 (0.70−2.15) | t = −0.33, p = 0.74 |
| Poverty | 1.04 (0.65−1.64) | 1.22 (0.91−1.63) | 1.08 (0.74−1.56) | 1.26 (0.83−1.91) | 0.96 (0.53−1.71) | t = −1.44, p = 0.15 |
| Large Metro Area | 0.65 (0.42−0.99)* | 0.69 (0.52−0.92)* | 0.57 (0.42−0.79)*** | 0.90 (0.66−1.21) | 0.82 (0.57−1.17) | t = −0.42, p = 0.68 |
| In School/College Graduate | 1.60 (0.84−3.02) | 0.60 (0.49−0.73)*** | 0.88 (0.65−1.20) | 0.66 (0.44−0.99)* | 1.03 (0.69−1.56) | t = −0.95, p = 0.35 |
| Religiosity | 1.02 (0.97−1.07) | 0.98 (0.95−1.02) | 0.98 (0.94−1.02) | 0.94 (0.91−0.99)* | 0.91 (0.86−0.96)** | t = −4.61, p < 0.001 |
| Physical Health Correlates | ||||||
| Self−reported Fair or Poor Health | 1.22 (0.54−2.76) | 1.57 (0.98−2.54) | 2.30 (1.20−4.44)* | 1.84 (1.00−3.37)* | 0.86 (0.43−1.73) | t = 0.96, p = 0.34 |
| Overweight/Obese BMI | 1.02 (0.61−1.73) | 1.14 (0.88−1.48) | 1.21 (0.76−1.91) | 0.86 (0.54−1.39) | 1.03 (0.51−2.11) | t = 0.17, p = 0.86 |
| Past−year Hospitalization | 0.77 (0.36−1.63) | 1.06 (0.66−1.68) | 1.61 (0.87−2.99) | 1.22 (0.60−2.49) | 0.79 (0.37−1.68) | t = 0.01, p = 0.99 |
| Mental Health Correlates | ||||||
| Past−year Mental Health Treatment | 0.98 (0.61−1.59) | 1.21 (0.92−1.61) | 1.36 (0.84−2.19) | 1.75 (1.01−3.06)* | 0.80 (0.40−1.60) | t = 1.32, p = 0.19 |
| Past−year Major Depression | 0.65 (0.43−0.98)* | 0.88 (0.61−1.26) | 1.25 (0.78−2.01) | 1.26 (0.77−2.07) | 0.78 (0.36−1.73) | t = 0.46, p = 0.65 |
| Past−year Serious Psychological Distress | 0.90 (0.65−1.24) | 1.57 (1.05−2.33)* | 1.32 (0.84−2.07) | 1.23 (0.56−2.74) | t = 2.31, p = 0.025 | |
| Past−year WHO Disability Assessment Scale | 1.00 (0.97−1.02) | 1.02 (0.99−1.05) | 1.02 (0.98−1.05) | 1.01 (0.96−1.07) | t = 1.48, p = 0.14 | |
| Past−year Suicidal Ideation | 0.99 (0.67−1.46) | 1.44 (0.88−2.34) | 0.94 (0.58−1.52) | 1.25 (0.55−2.56) | t = 1.38, p = 0.18 | |
| Substance Use Correlates | ||||||
| Past−month Binge Alcohol Use | 1.80 (1.11−2.93)* | 1.37 (1.02−1.83)* | 0.90 (0.61−1.32) | 1.34 (0.87−2.07) | 1.15 (0.64−2.07) | t = 0.90, p = 0.37 |
| Past−year Marijuana Use | 1.66 (0.88−3.12) | 1.29 (0.91−1.82) | 1.76 (1.22−2.54)** | 0.96 (0.56−1.66) | 0.98 (0.41−2.38) | t = 0.97, p = 0.34 |
| Past−year Opioid Use | 1.80 (0.93−3.46) | 1.68 (1.10−2.55)* | 1.16 (0.71−1.90) | 1.44 (0.91−2.29) | 1.18 (0.53−2.61) | t = 1.36, p = 0.18 |
| Past−year Stimulant Use | 1.35 (0.91−2.02) | 1.30 (0.99−1.71) | 1.42 (0.93−2.18) | 1.08 (0.64−1.82) | 0.96 (0.39−2.40) | t = 0.81, p = 0.42 |
| Past−year Opioid Misuse | 1.89 (1.06−3.37)* | 2.05 (1.45−2.90)*** | 1.85 (1.20−2.86)** | 1.97 (1.21−3.19)** | 2.99 (1.59−5.61)*** | t = 5.01, p < 0.001 |
| Past−year Stimulant Misuse | 1.79 (1.12−2.86)* | 1.30 (0.96−1.76) | 2.09 (1.38−3.17)*** | 1.56 (0.86−2.84) | 1.61 (0.43−6.02) | t = 2.51, p = 0.015 |
| Past−year DSM−IV SUD | 1.56 (0.94−2.62) | 1.64 (1.13−2.36)** | 1.53 (1.05−2.25)* | 1.48 (0.99−2.19) | 1.80 (0.88−3.70) | t = 3.34, p = 0.002 |
Source: NSDUH, 2015−16 cohorts.
Notes: BMI = Body Mass Index; WHO = World Health Organization; SUD = Substance Use Disorder
Past−year, but not past−month, misuse was coded as 0, while past−month misuse was coded as 1. Non−tranquilizer/sedative users and appropriate users were not included.
Listed odds ratios indicate the odds of the characteristic in the first column (e.g., male sex) being present in those engaged in past−month tranquilizer/sedative misuse, versus those engaged in past−year, but not past−month, misuse.
2.2.3. Correlates
Correlate selection used previous PDM research, with greater attention to research assessing PDM by age cohort across the lifespan (e.g., Mowbray & Quinn, 2015; Schepis, 2014) and past work on tranquilizer/sedative misuse (e.g., Boyd, West, & McCabe, 2018; Rigg & Ford, 2014). Correlates were grouped into sociodemographics, physical health, mental health, and substance use.
Sociodemographics:
sex, ethnicity (white versus non-white), poverty status, metro area size, educational status or attainment (currently in school/college graduate versus not in school/non-college graduate), and religiosity. Religiosity was a four-item variable used by Grucza et al. (2016) with good psychometrics (α > 0.9 for 2015–16).
Physical health:
self-reported health (poor versus fair to excellent), overweight/obese body mass index (BMI; ≥ 25), and past-year hospitalization.
Mental health:
past-year major depression, past-year mental health treatment, past-year serious psychological distress (SPD; adult only), past-year level of impairment from mental health symptoms (adult only), and past-year suicidal ideation (adult only). Major depression was assessed based on the DSM-IV (American Psychiatric Association [APA], 2000), with good psychometrics (Zanarini & Frankenburg, 2001). SPD comes from the K6 assessment (Kessler et al., 2003) for the worst month in the past year. Scores ≥13 (of 24) are positive for SPD. Past-year mental health-related impairment comes from the World Health Organization’s Disability Assessment Scale (WHODAS), a continuous 13-item assessment in the NSDUH (CBHSQ, 2016; Novak, Colpe, Barker, & Gfroerer, 2010). Suicidal ideation is queried by asking adults if in the past year “did you seriously think about trying to kill yourself?”
Substance use:
past-month binge drinking, past-year marijuana use, past-year prescription opioid use, past-year prescription stimulant use, past-year prescription opioid misuse, past-year prescription stimulant misuse, and past-year any DSM-IV substance use disorder (SUD) diagnosis. Past-month binge drinking was an occasion (“at the same time or within a couple of hours”) of consuming 5/4 (men/women) or more alcoholic drinks. Prescription opioid or stimulant use and PDM were assessed via similar questions to those for tranquilizers/sedatives (above). Past-year SUD is assessed using DSM-IV criteria (APA, 2000), with strong psychometrics (SAMHSA, 2010).
2.3. Analyses
Analyses utilized STATA 15.1 (College Station, TX). Data were weighted, clustered on primary sampling units, and stratified; adjusted person-level weights (weight/2) created unbiased population-based estimates. The Taylor series approximation, with adjusted degrees of freedom, was used to create robust variance estimates. Initial analyses (Table 1) employed weighted cross-tabulations to estimate prevalence and 95% confidence intervals for tranquilizer/sedative use and PDM; Bonferroni-corrected post hoc design-based logistic regressions tested for age cohort-based differences.
Table 1.
Prevalence of lifetime and past year sedative/tranquilizer use and lifetime, past year and past 30-day misuse by age group
| 12–17 years (adolescents) (a) |
18–25 years (young adults) (b) |
26–34 years (c) |
35–49 years (d) |
50–64 years (e) |
65 and older (f) |
Post hoc Comparisons | |
|---|---|---|---|---|---|---|---|
| Sample Size | 27,857 | 28,213 | 17,835 | 22,530 | 10,398 | 7,210 | |
| Lifetime use | 2,229 | 5,388 | 4,871 | 7,395 | 3,901 | 2,549 | a < b < c < f < d, e |
| weighted % of population (95% CI) | 7.9 (7.5−8.3) | 19.0 (18.3−19.7) | 27.3 (26.4−28.2) | 31.6 (30.8−32.3) | 37.2 (36.1−38.4) | 35.3 (34.0−36.6) | |
| Lifetime misuse | 638 | 2,143 | 1,385 | 1,279 | 549 | 167 | f < a < e < d < b < c |
| weighted % of population (95% CI) | 2.3 (2.1−2.5) | 7.8 (7.4−8.3) | 8.1 (7.6−8.6) | 5.4 (5.1−5.8) | 5.3 (4.8−5.9) | 2.2 (1.8−2.7) | |
| weighted % of those with lifetime use (95% CI) | 29.6 (27.3−31.9) | 41.3 (39.5−43.2) | 29.6 (27.9−31.3) | 17.2 (16.0−18.4) | 14.3 (13.0−15.8) | 6.3 (5.2−7.6) | f < e < d< a < b, c |
| Past year use | 1,682 | 3,816 | 2,943 | 4,445 | 2,413 | 1,574 | a < b < c-f; c, f < e |
| weighted % of population (95% CI) | 6.0 (5.6−6.4) | 13.5 (13.0−14.0) | 16.6 (15.9−17.3) | 18.8 (18.2−19.4) | 22.8 (21.8−23.8) | 21.9 (20.7−23.1) | |
| Past year misuse | 523 | 1,543 | 687 | 541 | 197 | 65 | f < a, e < d < b, c |
| weighted % of population (95% CI) | 1.9 (1.7−2.1) | 5.8 (5.4−6.2) | 4.0 (3.7−4.4) | 2.4 (2.1−2.6) | 1.9 (1.6−2.3) | 0.9 (0.7−1.2) | |
| weighted % of those with past-year use (95% CI) | 31.4 (28.4−34.6) | 42.8 (40.6−45.2) | 24.2 (22.5−26.0) | 12.6 (11.5−13.8) | 8.4 (7.0−10.0) | 4.2 (3.1−5.5) | b < a, c < d < e < f |
| Past 30-day misuse | 171 | 471 | 232 | 205 | 62 | 18 | f < a-e; a < b-d; d, e < b, c |
| weighted % of population (95% CI) | 0.7 (0.5−0.8) | 1.8 (1.5−2.0) | 1.4 (1.2−1.6) | 0.9 (0.8−1.0) | 0.5 (0.4−0.7) | 0.3 (0.1−0.6) |
Source: NSDUH, 2015–16 cohorts.
Notes: Post hoc comparisons controlled for race/ethnicity and sex and were only noted when the p-value was at or below a Bonferroni-corrected value of 0.00333 (0.05/15 = .00333). Unweighted samples and weighted percentages are provided, with 95% confidence intervals following the weighted percentages.
Further analyses used design-based logistic regression to examine the relationships between correlates and tranquilizer/sedative use and tranquilizer/sedative PDM. Correlates were entered in a univariable fashion for each age group, followed by analysis across the age groups including an interaction term (i.e., correlate * age groups) to examine whether correlate strength varied by age group. These analyses examined correlates of past-year tranquilizer/sedative PDM across the population (Table 2) and in only those endorsing past-year tranquilizer/sedative use (use= 0, PDM= 1; Table 3). Finally, analyses examined past-month tranquilizer/sedative PDM correlates in those endorsing past-year tranquilizer/sedative misuse (Table 4). Analyses for physical health, mental health and substance use correlates controlled for the sociodemographics.
Table 2.
Correlates of past year prescription sedative/tranquilizer misuse by age group across the US population
| 12–17 years (adolescents) | 18–25 years (young adults) | 26–34 years | 35–49 years | 50–64 years | 65 and older | Interaction term | |
|---|---|---|---|---|---|---|---|
| Sample Size | 27,857 | 28,213 | 17,835 | 22,530 | 10,398 | 7,210 | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Sociodemographic Correlates | |||||||
| Male Sex | 1.14 (0.96−1.34) | 0.76 (0.68−0.87)*** | 0.67 (0.57−0.80)*** | 0.83 (0.72−0.97)* | 0.79 (0.62−1.03) | 0.61 (0.37−1.03) | t = −1.91, p = 0.062 |
| White Ethnicity | 0.93 (0.77−1.12) | 1.45 (1.26−1.66)** | 1.46 (1.22–1.76)*** | 1.90 (1.58−2.29)*** | 0.92 (0.71−1.19) | 0.76 (0.42−1.39) | t = −3.85, p < 0.001 |
| Poverty | 1.15 (0.92−1.44) | 0.96 (0.86−1.06) | 1.11 (0.92–1.33) | 1.36 (1.10−1.69)** | 1.76 (1.26−2.46)*** | 1.45 (0.51−4.11) | t = 5.73, p < 0.001 |
| Large Metro Area | 0.88 (0.74−1.05) | 0.83 (0.75−0.93)** | 0.90 (0.78–1.03) | 0.94 (0.81−1.08) | 1.20 (0.95−1.51) | 1.78 (1.03−3.07)* | t = 2.15, p = 0.036 |
| In School/College Graduate | 0.54 (0.42−0.70)*** | 0.66 (0.59−0.73)*** | 0.59 (0.51–0.68)*** | 0.77 (0.63−0.93)** | 0.76 (0.55−1.06) | 1.19 (0.68−2.07) | t = 8.43, p < 0.001 |
| Religiosity | 0.93 (0.91−0.95)*** | 0.92 (0.91−0.94)*** | 0.91 (0.90–0.93)*** | 0.92 (0.90−0.94)*** | 0.92 (0.90−0.96)*** | 0.98 (0.93−1.04) | t = 0.65, p = 0.65 |
| Physical Health Correlates | |||||||
| Self-reported Poor Health | 1.47 (0.95−2.26) | 1.30 (1.04−1.63)* | 1.59 (1.19–2.12)** | 1.65 (1.21−2.25)** | 1.36 (0.85−2.18) | 1.79 (0.95−3.36) | t = −6.68, p < 0.001 |
| Overweight/Obese BMI | 1.22 (0.89−1.67) | 0.74 (0.66−0.84)*** | 0.81 (0.67–0.99) | 0.92 (0.75−1.13) | 1.07 (0.69−1.67) | 1.24 (0.66−2.31) | t = −13.07, p < 0.001 |
| Past-year Hospitalization | 2.75 (1.97−3.85)*** | 1.46 (1.18−1.80)*** | 1.15 (0.86–1.54) | 1.65 (1.21−2.24)** | 1.64 (1.06−2.54)* | 1.78 (0.93−3.43) | t = −7.43, p < 0.001 |
| Mental Health Correlates | |||||||
| Past-year Mental Health Treatment | 2.66 (2.16−3.27)*** | 2.71 (2.25−3.27)*** | 2.86 (2.31−3.56)*** | 3.69 (2.92−4.65)*** | 3.98 (2.63−6.02)*** | 5.23 (3.02−9.05)*** | t = 2.42, p = 0.019 |
| Past-year Major Depression | 2.78 (2.15−3.60)*** | 2.38 (2.01−2.83)*** | 3.13 (2.50−3.92)*** | 4.23 (3.32−5.38)*** | 3.52 (2.13−5.81)*** | 3.12 (1.20−8.10)* | t = −2.08, p = 0.043 |
| Past-year Serious Psychological Distress | 2.62 (2.25−3.04)*** | 3.20 (2.66−3.85)*** | 4.10 (3.25−5.18)*** | 3.45 (1.96−6.06)*** | 2.12 (0.84−5.89) | t = −0.22, p = 0.830 | |
| Past-year WHO Disability Assessment Scale | 1.08 (1.07−1.09)*** | 1.10 (1.08−1.11)*** | 1.12 (1.10−1.13)*** | 1.14 (1.11−1.17)*** | 1.10 (1.04−1.16)*** | t = 20.01, p < 0.001 | |
| Past-year Suicidal Ideation | 2.98 (2.59−3.42)*** | 2.95 (2.26−3.85)*** | 4.94 (3.64−6.69)*** | 4.78 (2.50−9.12)*** | 2.83 (0.82−9.79) | t = −4.68, p < 0.001 | |
| Substance Use Correlates | |||||||
| Past-month Binge Alcohol Use | 9.21 (7.07−12.00)*** | 3.03 (2.60−3.52)*** | 2.27 (1.86−2.76)*** | 2.28 (1.76−2.95)*** | 2.50 (1.80−3.47)*** | 2.14 (0.95−4.86) | t = 2.06, p = 0.044 |
| Past-year Marijuana Use | 19.61 (14.96−25.72)*** | 9.40 (8.04−10.98)*** | 5.90 (4.53−6.91)*** | 5.39 (4.32−6.72)*** | 3.22 (2.02−5.15)*** | 5.06 (2.08−12.33)*** | t = 9.21, p < 0.001 |
| Past-year Opioid Use | 8.39 (6.23−11.28)*** | 5.43 (4.67−6.31)*** | 5.00 (4.11−6.08)*** | 4.74 (3.68−6.10)*** | 3.19 (2.22−4.58)*** | 3.91 (2.09−7.31)*** | t = 5.17, p < 0.001 |
| Past-year Stimulant Use | 14.43 (10.95−19.03)*** | 7.08 (6.20−8.08)*** | 5.90 (4.56−7.12)*** | 5.47 (4.31−6.94)*** | 4.68 (3.06−7.15)*** | 3.77 (1.79−7.97)*** | t = −4.68, p < 0.001 |
| Past-year Opioid Misuse | 31.05 (22.51−42.81)*** | 16.35 (13.99−19.11)*** | 18.89 (15.90−22.44)*** | 22.11 (17.66−27.67)*** | 16.15 (11.09−23.54)*** | 15.96 (6.21−41.03)*** | t = 14.30, p < 0.001 |
| Past-year Stimulant Misuse | 29.52 (22.59−38.58)*** | 9.28 (7.96−10.81)*** | 9.69 (7.50−12.52)*** | 16.72 (11.16−25.03)*** | 16.15 (7.16−36.42)*** | all cases engaged | t = 5.26, p < 0.001 |
| Past-year DSM-IV SUD | 25.47 (18.89−34.33)*** | 8.55 (7.24−10.10)*** | 7.01 (5.72−8.59)*** | 9.45 (7.76−11.52)*** | 8.71 (5.95−12.76)*** | 7.88 (3.17−19.58)*** | t = 11.22, p < 0.001 |
Source: NSDUH, 2015–16 cohorts.
Notes: BMI = Body Mass Index; WHO = World Health Organization; SUD = Substance Use Disorder
Non-use or appropriate use was coded as 0, while misuse was coded as 1.
Listed odds ratios indicate the odds of the characteristic in the first column (e.g., male sex) being present in those engaged in past-year tranquilizer/sedative misuse, versus those not engaged in past-year misuse.
Table 3.
Correlates of past year prescription sedative/tranquilizer misuse by age group among past year sedative/tranquilizer users only
| 12–17 years (adolescents) (a) |
18–25 years (young adults) (b) |
26–34 years (c) |
35–49 years (d) |
50–64 years (e) |
65 and older (f) |
Post Hoc Differences | |
|---|---|---|---|---|---|---|---|
| Sample Size | 1,682 | 3,816 | 2,943 | 4,455 | 2,413 | 1,574 | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Sociodemographic Correlates | |||||||
| Male Sex | 1.01 (0.84−1.20) | 0.53 (0.46–0.61)*** | 0.48 (0.40–0.58)*** | 0.68 (0.60–0.80)*** | 0.70 (0.54–0.92)* | 0.60 (0.36–0.99)* | t = 0.45, p = 0.66 |
| White Ethnicity | 0.90 (0.74−1.08) | 1.30 (1.14−1.48)*** | 1.25 (1.05−1.49)* | 1.51 (1.26−1.84)*** | 0.79 (0.62−1.02) | 0.66 (0.36−1.20) | t = −23.76, p < 0.001 |
| Poverty | 1.13 (0.89−1.45) | 0.94 (0.83−1.08) | 0.95 (0.78−1.16) | 1.20 (0.96−1.50) | 1.48 (1.03−2.11)* | 1.56 (0.54−4.47) | t = −17.43, p < 0.001 |
| Large Metro Area | 0.95 (0.78−1.17) | 0.90 (0.80−1.02) | 1.01 (0.87−1.17) | 1.05 (0.92−1.26) | 1.34 (1.08−1.68)** | 1.91 (1.09−3.34)* | t = −16.83, p < 0.001 |
| In School/College Graduate | 0.69 (0.51−0.94)* | 0.77 (0.69−0.86)*** | 0.77 (0.65−0.91)** | 0.93 (0.77−1.13) | 0.93 (0.66−1.31) | 1.23 (0.69−2.20) | t = −17.91, p < 0.001 |
| Religiosity | 0.94 (0.92−0.96)*** | 0.92 (0.90−0.93)*** | 0.91 (0.90−0.93)*** | 0.93 (0.91−0.95)*** | 0.94 (0.91−0.97)*** | 0.99 (0.93−1.05) | t = −19.51, p < 0.001 |
| Physical Health Correlates | |||||||
| Self−reported Fair or Poor Health | 0.78 (0.48−1.28) | 0.76 (0.56−1.03) | 0.86 (0.62−1.20) | 0.83 (0.59−1.16) | 0.66 (0.42−1.04) | 1.11 (0.55−2.23) | t = −12.98, p < 0.001 |
| Overweight/Obese BMI | 0.99 (0.68−1.46) | 0.63 (0.54−0.73)*** | 0.81 (0.66−1.01) | 0.83 (0.67−1.03) | 1.12 (0.70−1.79) | 1.34 (0.71−2.50) | t = −19.80, p < 0.001 |
| Past−year Hospitalization | 0.87 (0.57−1.32) | 0.78 (0.61−1.01) | 0.80 (0.58−1.12) | 0.84 (0.61−1.14) | 0.92 (0.59−1.43) | 1.11 (0.55−2.23) | t = −13.62, p < 0.001 |
| Mental Health Correlates | |||||||
| Past−year Mental Health Treatment | 0.76 (0.58−0.99)* | 0.55 (0.44−0.67)*** | 0.59 (0.45−0.76)*** | 0.85 (0.67−1.08) | 1.13 (0.77−1.67) | 1.58 (0.92−2.74) | t = −2.08, p = 0.043 |
| Past−year Major Depression | 1.02 (0.74−1.41) | 1.05 (0.86−1.29) | 1.30 (1.01−1.67)* | 1.64 (1.28−2.09)*** | 1.36 (0.82−2.27) | 1.20 (0.45−3.21) | t = −13.81, p < 0.001 |
| Past−year Serious Psychological Distress | 1.10 (0.92−1.31) | 1.42 (1.18−1.70)*** | 1.55 (1.20−2.01)*** | 1.42 (0.80−2.53) | 0.80 (0.31−2.07) | t = −10.77, p < 0.001 | |
| Past−year WHO Disability Assessment Scale | 1.01 (1.00−1.02) | 1.02 (1.01−1.04)** | 1.04 (1.02−1.05)*** | 1.06 (1.02−1.09)*** | 1.01 (0.95−1.07) | t = 0.80, p = 0.427 | |
| Past−year Suicidal Ideation | 1.43 (1.16−1.77)*** | 1.45 (1.05−1.99)* | 1.95 (1.38−2.75)*** | 2.27 (1.16−4.46)* | 1.20 (0.33−4.36) | t = −9.72, p < 0.001 | |
| Substance Use Correlates | |||||||
| Past−month Binge Alcohol Use | 3.54 (2.46−5.09)*** | 2.25 (1.87−2.72)*** | 1.79 (1.47−2.18)*** | 1.92 (1.51−2.44)*** | 2.49 (1.72−3.60)*** | 1.91 (0.84−4.34) | t = −5.73, p < 0.001 |
| Past−year Marijuana Use | 7.52 (5.49−10.29)*** | 4.88 (3.97−6.01)*** | 3.44 (2.70−4.38)*** | 3.50 (2.77−4.41)*** | 1.95 (1.23−3.09)** | 3.22 (1.13−9.18)* | t = −2.72, p = 0.009 |
| Past−year Opioid Use | 1.51 (1.09−2.09)* | 1.60 (1.33−1.92)*** | 1.59 (1.25−2.03)*** | 1.55 (1.20−1.99)*** | 1.06 (0.72−1.58) | 1.56 (0.82−2.97) | t = −14.19, p < 0.001 |
| Past−year Stimulant Use | 2.64 (1.92−3.63)*** | 2.35 (1.94−2.84)*** | 2.49 (1.93−3.22)*** | 2.32 (1.78−3.03)*** | 1.94 (1.20−3.13)** | 1.46 (0.63−3.37) | t = −9.64, p < 0.001 |
| Past−year Opioid Misuse | 6.03 (4.34−8.40)*** | 5.44 (4.44−6.66)*** | 8.15 (6.53−10.17)*** | 11.60 (9.08−14.82)*** | 7.90 (5.11−12.20)*** | 6.76 (2.57−17.82)*** | t = 2.17, p = 0.035 |
| Past−year Stimulant Misuse | 7.08 (4.94−10.14)*** | 5.21 (4.13−6.57)*** | 5.56 (3.84−8.04)*** | 8.28 (5.38−12.75)*** | 7.39 (3.18−17.15)*** | all cases engaged | t = −5.17, p < 0.001 |
| Past−year DSM−IV SUD | 8.11 (6.07−10.84)*** | 4.70 (3.75−5.89)*** | 4.16 (3.22−5.36)*** | 5.57 (4.47−6.94)** | 6.17 (4.19−9.09)*** | 3.78 (1.35−10.59)* | t = −0.70, p = 0.489 |
Source: NSDUH, 2015−16 cohorts.
Notes: BMI = Body Mass Index; WHO = World Health Organization; SUD = Substance Use Disorder
Appropriate use was coded as 0, while misuse was coded as 1. Non−tranquilizer/sedative users were not included.
Listed odds ratios indicate the odds of the characteristic in the first column (e.g., male sex) being present in those engaged in past−year tranquilizer/sedative misuse, versus those engaged in past−year medical use only.
3. RESULTS
3.1. Tranquilizer/Sedative Use and Misuse Prevalence by Age Group
Per Table 1, adolescents aged 12–17 had the lowest lifetime and past-year tranquilizer/sedative use prevalence rates (7.9% and 6.0%, respectively), with the highest rates in adults aged 50–64 (37.2% and 22.8%, respectively) or 65 and older (35.3% and 21.9%, respectively). Conversely, tranquilizer/sedative PDM prevalence across age cohorts displayed an inverted U-shaped pattern: adults aged 65 and older had the lowest rates (lifetime: 2.2%, past-year: 0.9%), followed by adolescents (lifetime: 2.3%, past-year: 1.9%). Lifetime misuse rates were highest in adults aged 26–34 (8.1%), but past-year and past-month tranquilizer/sedative PDM rates were highest in young adults aged 18–25 (5.8% and 1.8%, respectively).
One of every 2.4 young adults (or 41.3%) endorsing lifetime and 1 of every 2.3 young adults (or 42.8%) endorsing past-year tranquilizer/sedative use also engaged in misuse (the NSDUH does not separate use from combined PDM and use). Conversely, adults 65 and older who used tranquilizer/sedative medication were least likely to engage in lifetime (6.3%) or past-year (4.2%) PDM.
3.2. Past-Year Tranquilizer/Sedative Misuse Correlates by Age Group Across the Population
White ethnicity was associated with elevated odds of past-year tranquilizer/sedative misuse in three of six age groups (18–25, 26–34, 35–49; please see Table 2). In contrast, male sex (18–25, 26–34, 35–49), being in school or a college graduate (12–17, 18–25, 26–34, 35–49) and higher levels of religiosity (except those 65 and older) were associated with lowered odds of past-year tranquilizer/sedative misuse in at least three groups. White ethnicity, poverty status, metro area residence and being in school or a college graduate all interacted with age group, suggesting different relationships by age.
Self-reported poor physical health and past-year hospitalizations were associated with increased odds of past-year tranquilizer/sedative misuse in three (18–25, 26–34, 35–49) or four age groups (12–17, 18–25, 35–49, 50–64), respectively. Increased odds were also found in the five mental health outcomes examined across ages, except for past-year SPD or suicidality in adults 65 and older; odds were highest for past-year suicidal ideation in three of five groups. Similarly, all examined substance use outcomes were associated with increased past-year tranquilizer/sedative misuse odds, except for binge drinking in adults 65 and older. The greatest odds elevations were for opioid PDM, with at least 15 times greater odds for each age group. All physical health, mental health (except for past-year SPD) and substance use correlates evidenced a significant interaction with age group.
Additional analyses of past-year tranquilizer/sedative PDM, opioid PDM, and suicidal ideation found increasing suicidality with increasing PDM engagement. As captured in Figure 1, only 3.4% of those without PDM endorsed past-year suicidal ideation, while 11.8% endorsing opioid-only PDM and 12.6% endorsing tranquilizer/sedative-only PDM endorsed suicidal ideation. In contrast, 20.8% of those engaged in both opioid and tranquilizer/sedative PDM endorsed past-year suicidal ideation.
Figure 1.
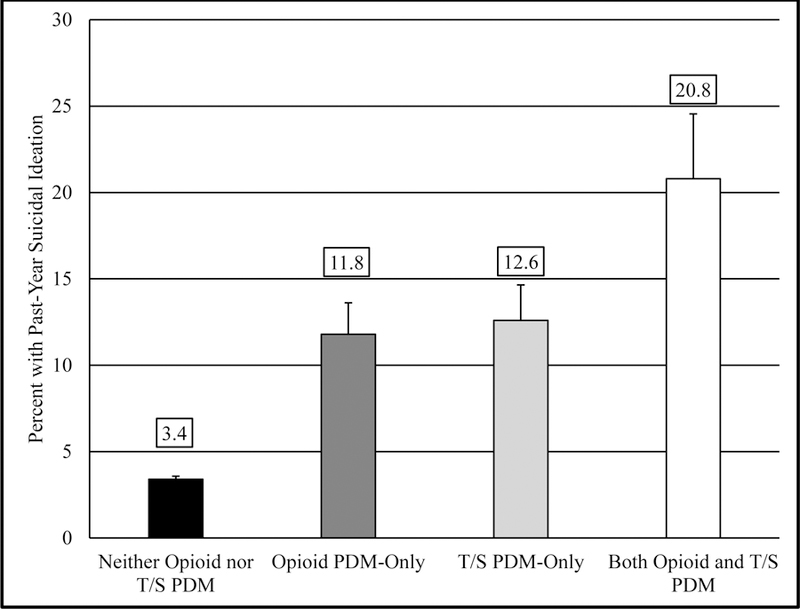
Prevalence of Past−Year Suicidal Ideation by Past−Year Prescription Drug Misuse Status
Notes: Error bars represent 95% confidence intervals; T/S = Tranquilizer/Sedative
3.3. Past-Year Tranquilizer/Sedative Misuse Correlates by Age Group among Those Engaged in Past-Year Use
After restricting the sample to only those endorsing any past-year tranquilizer/sedative use (n= 16,883; Table 3), a similar pattern emerged to that for correlates across the population. In particular, male sex and religiosity were protective against past-year tranquilizer/sedative misuse among those with any past-year use, with five age groups evidencing significantly lowered odds (all groups except adolescents and adults 65 and older, respectively). Educational enrollment/attainment was associated with lower odds of past-year misuse among all engaged in use in three groups (12–17, 18–25, 26–34), while white race/ethnicity conferred increased odds in three age groups (18–25, 26–34, 35–49). All examined sociodemographic correlates, except for sex, significantly interacted with age group.
Physical health correlates were generally not associated with past-year tranquilizer/sedative misuse in those with past-year use, while the examined mental health correlates were often associated with increased odds. This was especially true for the 26–34 and 35–49 age groups and for suicidal ideation. Conversely, receiving past-year mental health treatment was associated with lower misuse odds for three age groups: 12–17, 18–25 and 26–34. Substance use correlates were generally associated with increased odds of past-year tranquilizer/sedative misuse among those with past-year use. Relationships were less likely to be significant in adults 65 and older, and past-year opioid misuse often had the highest odds ratios. All physical health, mental health and substance use correlates (except for impairment from mental health symptoms and SUD diagnosis) evidenced a significant interaction with age group.
3.4. Past-Month Tranquilizer/Sedative Misuse Correlates by Age Group among Those Engaged in Past-Year Misuse
Notably fewer correlates were associated with past-month tranquilizer/sedative misuse among those with past-year misuse (n=3,556; Table 4). Living in a large metro area was associated with lower odds in three of five groups (12–17, 18–25, 26–34), and school enrollment (18–25, 35–49) and religiosity (35–49, 50 and older) were protective in two age groups. Religiosity also interacted with age group. While the examined physical and mental health correlates generally were not associated with past-month tranquilizer/sedative misuse, the substance use correlates had some notable associations. Past-year opioid misuse was associated with elevated odds of past-month tranquilizer/sedative misuse across age groups, and past-year opioid misuse, past-year stimulant misuse and past-year SUD diagnosis interacted with age group.
4. DISCUSSION
This research found significant differences in tranquilizer/sedative use, misuse, and correlates of misuse by age. While older age was associated with higher lifetime and past-year tranquilizer/sedative use prevalence, lifetime misuse peaked in young adults aged 18–25 years (7.8%) and those aged 26–34 years (8.1%). Indeed, 41.3% of young adults reporting lifetime tranquilizer/sedative use also engaged in lifetime misuse. Past-year tranquilizer/sedative misuse was most likely in young adults, at 5.8%; 42.8% of all young adults engaged in past-year use also engaged in past-year misuse. Similar results were found in 18 year-old participants in the nationally-representative Monitoring the Future survey, with 44.9% of those with lifetime medical tranquilizer/sedative use also engaged in nonmedical misuse (McCabe, Veliz, Boyd, & Schulenberg, 2017). Young adults also had the highest rates of past-month misuse. Thus, young adults are most in need of tranquilizer/sedative misuse prevention and intervention efforts.
Across ages, females and those of white race/ethnicity had greater odds of tranquilizer/sedative PDM, while those in school or with college degrees and those with higher religiosity generally had lower odds. The finding that females had greater odds of tranquilizer/sedative PDM is at odds with most work indicating greater use across a variety of substances in men (e.g., Kuhn, 2015), but it is more consistent with the lack of clear sex differences in PDM (Han, Compton, Jones, & Cai, 2015; Saha et al., 2016). Physical health correlates did not consistently highlight tranquilizer/sedative PDM, perhaps because the predominant tranquilizer/sedative indications are for mental (e.g., anxiety and insomnia) and not physical health conditions.
In contrast, mental health correlates highlighted those engaged in past-year misuse within the general population, and substance use correlates were most effective at differentiating those endorsing past-year misuse from either the general population or the sample engaged in any past-year tranquilizer/sedative use. Both the mental health and substance use correlates evidenced a diminishing relationship with tranquilizer/sedative PDM in older age groups, especially those 65 and older and especially for the mental health correlates. This finding may be an issue of statistical power and/or it may reflect qualitative age-based differences in PDM processes. Opioid PDM was a particularly robust correlate of tranquilizer/sedative PDM across timeframes and age groups, consistent with Huang and colleagues (2006). The regular co-occurrence of opioid and tranquilizer/sedative PDM is a concerning finding, given their synergistic depressant effects and co-use in many intentional overdoses (McClure, Niles, Kaufman, & Gudin, 2017).
On a related note, perhaps most alarming was the near dose-response relationship between past-year tranquilizer/sedative and/or opioid PDM and suicidal ideation, with 8 to 9% increases in ideation for each medication class misused. This finding is consistent with past work linking opioid PDM to suicidal ideation in adolescents and adults (Ford & Perna, 2015; Guo et al., 2016; Zullig & Divin, 2012). The current work adds to the literature by linking tranquilizer/sedative PDM to suicidal ideation across the population, further highlighting the risk of such PDM, not least because of the overdose potential inherent in tranquilizer/sedative medication (Warner, Trinidad, Bastian, Minino, & Hedegaard, 2016).
Among older cohorts, the examined correlates had fewer significant relationships with past-year or past-month tranquilizer/sedative misuse. While substance use correlates were still most likely to be associated with misuse in those 65 and older, with opioid PDM remaining the most salient correlate, few correlates in general were significant in older adults. The relative lack of significant correlates could stem from at least two non-mutually exclusive factors. One, while fewer older adults engaged in tranquilizer/sedative misuse, the direction of the results were often the same as those found in younger groups. Thus, the limited significant findings likely result from lower statistical power in older adults.
Conversely, and as we (Schepis, 2014) and others (Maree et al., 2016) have speculated, older adult tranquilizer/sedative misuse processes may qualitatively differ from those in younger individuals. While the relationship directions were similar to those of younger groups, the magnitude was often lower. Older adults had the highest tranquilizer/sedative use prevalence, consistent with past research (Olfson, King, & Schoenbaum, 2015) and increasing rates of insomnia (Roth, 2007) and depression (where these medications may support antidepressants; Olfson et al., 2015). Despite this, older adults had the lowest PDM rates, supporting the idea of qualitative differences from younger groups. Maree et al. (2016) noted increased physical health conditions and insomnia in older adults as potential drivers of tranquilizer/sedative misuse. Insomnia is not assessed in the NSDUH, and other physical health conditions are poorly assessed. It may be that limited NSDUH physical health assessment obscured identification of correlates; furthermore, as insomnia increases with age (Roth, 2007), the lack of an insomnia assessment may have excluded a key older adult PDM correlate.
4.1. Limitations and Future Directions
First, the data are cross-sectional and self-report. Self-report bias was possible, and no causal inferences should be made. In particular, the data cannot indicate whether suicidal ideation preceded PDM or the converse. Nonetheless, research indicates that self-report substance use data are reliable and valid, though underreporting and participant misclassification is likely (Johnston & O’Malley, 1985; O’Malley, Bachman, & Johnston, 1983); in addition, use of medication pictures, numerous trade and generic medication names and ACASI methods reduce self-report bias (Center for Behavioral Health Statistics and Quality, 2014, 2015). Second, self-selection bias was likely, as some selected individuals refused participation in either the screening or interview phases. Third, while the NSDUH makes extra efforts to sample older adults in assisted living or other controlled access dwellings, older adults in non-household settings were probably undersampled (Cunningham et al., 2015). Finally, insomnia is not assessed, physical health conditions are poorly assessed, and only limited (and generally past-year) mental health variables are assessed in the NSDUH, limiting the potential for understanding their relationships with tranquilizer/sedative misuse.
In addition to research addressing these limitations, future work that further explores the suicidal ideation and PDM interface would have great clinical value. Also, future work should address PDM of multiple tranquilizer/sedative medications and its correlates, as well as the influence of mental health treatment on these findings.
4.2. Clinical Implications and Summary
Young adults aged 18–25 and adults aged 26–34 are most likely to engage in tranquilizer/sedative misuse. Furthermore, younger individuals not currently in school or a college graduate evidenced increased misuse odds. Workplace-based interventions may be most efficacious in reaching these transitional-age groups (Bray, Galvin, & Cluff, 2011). A brief, internet-based intervention to limit workplace PDM (Lucas, Neeper, Linde, & Bennett, 2017) appears promising, but needs further evaluation. Combined school- and family-based prevention approaches that teach life skills and increase parental engagement are also recommended, as they cost-effectively delay PDM initiation through adolescence (Crowley, Jones, Coffman, & Greenberg, 2014). Finally, screening and brief motivational interviewing-based interventions that promote appropriate medication use should be considered, as they can lower opioid PDM risk and can be delivered by non-prescribers (Chang, Compton, Almeter, & Fox, 2015). More significant interventions may be needed (e.g., cognitive-behavioral therapy; Darker, Sweeney, Barry, Farrell, & Donnelly-Swift, 2015) for those with signs of PDM (e.g., early refill requests).
While older adults had the lowest tranquilizer/sedative PDM rates, despite the highest use rates, their increased likelihood of consequences makes them an important population for intervention. The American Geriatrics Society listed tranquilizer/sedative medications as generally inappropriate for older adult use (American Geriatrics Society Beers Criteria Update Expert Panel, 2015), and our finding of past-year use in nearly 22% of those 65 and older suggests that a first step is shifting to safer treatments for insomnia and anxiety, such as cognitive-behavioral therapies (Kaczkurkin & Foa, 2015; Taylor & Pruiksma, 2014). Attention to opioid use and misuse may highlight older adults at-risk for tranquilizer/sedative PDM and is particularly warranted, given the risks associated with co-use of these medications (Sun et al., 2017). Finally, those with suicidal ideation and engaged in opioid misuse are a group of particular concern, given that tranquilizer/sedative medication potentiates the overdose risk of opioid medication (Park, Saitz, Ganoczy, Ilgen, & Bohnert, 2015).
Supplementary Material
REFERENCES
- Airagnes G, Pelissolo A, Lavallee M, Flament M, & Limosin F (2016). Benzodiazepine Misuse in the Elderly: Risk Factors, Consequences, and Management. Curr Psychiatry Rep, 18(10), 89. [DOI] [PubMed] [Google Scholar]
- American Geriatrics Society Beers Criteria Update Expert Panel. (2015). American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc, 63(11), 2227–2246. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Bachhuber MA, Hennessy S, Cunningham CO, & Starrels JL (2016). Increasing Benzodiazepine Prescriptions and Overdose Mortality in the United States, 1996–2013. American Journal of Public Health, 106(4), 686–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd CJ, West B, & McCabe SE (2018). Does misuse lead to a disorder? The misuse of prescription tranquilizer and sedative medications and subsequent substance use disorders in a U.S. longitudinal sample. Addictive Behaviors, 79, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JW, Galvin DM, & Cluff LA (2011). Young Adults in the Workplace: A Multisite Initiative of Substance Use Prevention Programs Research Triangle Park, NC: RTI Press. [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2014). National Survey on Drug Use and Health (NSDUH): Summary of Methodological Studies, 1971–2014 Rockville, MD: Substance Abuse and Mental Health Services Administration. [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2015). 2014 National Survey on Drug Use and Health: Methodological summary and definitions Rockville, MD:: Substance Abuse and Mental Health Services Administration. [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2016). 2015 National Survey on Drug Use and Health public use file codebook Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2017). 2016 National Survey on Drug Use and Health: Methodological summary and definitions Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Chang YP, Compton P, Almeter P, & Fox CH (2015). The Effect of Motivational Interviewing on Prescription Opioid Adherence Among Older Adults With Chronic Pain. Perspect Psychiatr Care, 51(3), 211–219. [DOI] [PubMed] [Google Scholar]
- Crowley DM, Jones DE, Coffman DL, & Greenberg MT (2014). Can we build an efficient response to the prescription drug abuse epidemic? Assessing the cost effectiveness of universal prevention in the PROSPER trial. Prev Med, 62C, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D, Flicker L, Murphy J, Aldworth J, Myers S, & Kennet J (2015). Incidence and Impact of Controlled Access Situations on Nonresponse. American Association for Public Opinion Research 60th Annual Conference. Miami Beach, FL. [Google Scholar]
- Darker CD, Sweeney BP, Barry JM, Farrell MF, & Donnelly-Swift E (2015). Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev(5), Cd009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C (2014). Benzodiazepines in Combination with Opioid Pain Relievers or Alcohol: Greater Risk of More Serious ED Visit Outcomes. The CBHSQ Report Rockville (MD): Substance Abuse and Mental Health Services Administration; Available from: https://www.ncbi.nlm.nih.gov/books/NBK384672/. [PubMed] [Google Scholar]
- Ford JA, & Perna D (2015). Prescription drug misuse and suicidal ideation: Findings from the National Survey on Drug Use and Health. Drug Alcohol Depend, 157, 192–196. [DOI] [PubMed] [Google Scholar]
- Fride Tvete I, Bjorner T, & Skomedal T (2015). Risk factors for excessive benzodiazepine use in a working age population: a nationwide 5-year survey in Norway. Scand J Prim Health Care, 33(4), 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Agrawal A, Krauss MJ, Bongu J, Plunk AD, Cavazos-Rehg PA, & Bierut LJ (2016). Declining Prevalence of Marijuana Use Disorders Among Adolescents in the United States, 2002 to 2013. J Am Acad Child Adolesc Psychiatry, 55(6), 487–494 e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Xu Y, Deng J, Huang J, Huang G, Gao X, … Lu C (2016). Association Between Nonmedical Use of Prescription Drugs and Suicidal Behavior Among Adolescents. JAMA Pediatr, 170(10), 971–978. [DOI] [PubMed] [Google Scholar]
- Hall MT, Howard MO, & McCabe SE (2010). Subtypes of adolescent sedative/anxiolytic misusers: A latent profile analysis. Addict Behav, 35(10), 882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Jones CM, & Cai R (2015). Nonmedical Prescription Opioid Use and Use Disorders Among Adults Aged 18 Through 64 Years in the United States, 2003–2013. JAMA, 314(14), 1468–1478. [DOI] [PubMed] [Google Scholar]
- Huang B, Dawson DA, Stinson FS, Hasin DS, Ruan WJ, Saha TD, … Grant BF (2006). Prevalence, correlates, and comorbidity of nonmedical prescription drug use and drug use disorders in the United States: Results of the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry, 67(7), 1062–1073. [DOI] [PubMed] [Google Scholar]
- Johnston LD, & O’Malley PM (1985). Issues of validity and population coverage in student surveys of drug use. NIDA Research Monograph, 57, 31–54. [PubMed] [Google Scholar]
- Jones CM, & McAninch JK (2015). Emergency Department Visits and Overdose Deaths From Combined Use of Opioids and Benzodiazepines. Am J Prev Med, 49(4), 493–501. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, & Foa EB (2015). Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci, 17(3), 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanouse AB, & Compton P (2015). The epidemic of prescription opioid abuse, the subsequent rising prevalence of heroin use, and the federal response. J Pain Palliat Care Pharmacother, 29(2), 102–114. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, … Zaslavsky AM (2003). Screening for serious mental illness in the general population. Arch Gen Psychiatry, 60(2), 184–189. [DOI] [PubMed] [Google Scholar]
- Kuhn C (2015). Emergence of sex differences in the development of substance use and abuse during adolescence. Pharmacol Ther, 153, 55–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas G, Neeper M, Linde B, & Bennett J (2017). Preventing Prescription Drug Misuse in Work Settings: Efficacy of a Brief Intervention in Health Consciousness. Journal of Medical Internet Research, 19(7), e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maree RD, Marcum ZA, Saghafi E, Weiner DK, & Karp JF (2016). A Systematic Review of Opioid and Benzodiazepine Misuse in Older Adults. Am J Geriatr Psychiatry, 24(11), 949–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Veliz P, Boyd CJ, & Schulenberg JE (2017). Medical and nonmedical use of prescription sedatives and anxiolytics: Adolescents’ use and substance use disorder symptoms in adulthood. Addict Behav, 65, 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, & West BT (2014). Medical and nonmedical use of prescription benzodiazepine anxiolytics among U.S. high school seniors. Addict Behav, 39(5), 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure FL, Niles JK, Kaufman HW, & Gudin J (2017). Concurrent Use of Opioids and Benzodiazepines: Evaluation of Prescription Drug Monitoring by a United States Laboratory. J Addict Med, 11(6), 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon ME, Monaghan TL, Stewart SH, & Barrett SP (2011). Drug misuse and diversion in adults prescribed anxiolytics and sedatives. Pharmacotherapy, 31(3), 262–272. [DOI] [PubMed] [Google Scholar]
- Mowbray O, & Quinn A (2015). Prescription pain reliever misuse prevalence, correlates, and origin of possession throughout the life course. Addict Behav, 50, 22–27. [DOI] [PubMed] [Google Scholar]
- Nattala P, Murthy P, Thennarasu K, & Cottler LB (2014). Nonmedical use of sedatives in urban Bengaluru. Indian J Psychiatry, 56(3), 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak SP, Colpe LJ, Barker PR, & Gfroerer JC (2010). Development of a brief mental health impairment scale using a nationally representative sample in the USA. Int J Methods Psychiatr Res, 19 Suppl 1, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley PM, Bachman JG, & Johnston LD (1983). Reliability and consistency in self-reports of drug use. International Journal of Addiction, 18, 805–824. [DOI] [PubMed] [Google Scholar]
- Olfson M, King M, & Schoenbaum M (2015). Benzodiazepine use in the United States. JAMA Psychiatry, 72(2), 136–142. [DOI] [PubMed] [Google Scholar]
- Park TW, Saitz R, Ganoczy D, Ilgen MA, & Bohnert ASB (2015). Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ : British Medical Journal, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigg KK, & Ford JA (2014). The misuse of benzodiazepines among adolescents: psychosocial risk factors in a national sample. Drug Alcohol Depend, 137, 137–142. [DOI] [PubMed] [Google Scholar]
- Roth T (2007). Insomnia: Definition, Prevalence, Etiology, and Consequences. Journal of Clinical Sleep Medicine : JCSM : official publication of the American Academy of Sleep Medicine, 3(5 Suppl), S7–S10. [PMC free article] [PubMed] [Google Scholar]
- Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, … Grant BF (2016). Nonmedical Prescription Opioid Use and DSM-5 Nonmedical Prescription Opioid Use Disorder in the United States. J Clin Psychiatry, 77(6), 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS (2014). Age cohort differences in the nonmedical use of prescription zolpidem: findings from a nationally representative sample. Addict Behav, 39(9), 1311–1317. [DOI] [PubMed] [Google Scholar]
- Schepis TS, & Hakes JK (2013). Dose-related effects for the precipitation of psychopathology by opioid or tranquilizer/sedative nonmedical prescription use: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Addict Med, 7(1), 39–44. [DOI] [PubMed] [Google Scholar]
- Schepis TS, & Krishnan-Sarin S (2008). Characterizing adolescent prescription misusers: a population-based study. J Am Acad Child Adolesc Psychiatry, 47(7), 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2010). Reliability of Key Measures in the National Survey on Drug Use and Health (Office of Applied Studies, Methodology Series M-8, HHS Publication No. SMA 09–4425). Rockville, MD. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2014). Treatment Episode Data Set (TEDS): 2002–2012. National admissions to substance abuse treatment services BHSIS Series S-71, HHS Publication No. (SMA) 14–4850. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, & Mackey S (2017). Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ, 356, j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiri Z, Kellici S, Mone I, Shabani D, Qazimi M, & Burazeri G (2017). Prevalence and correlates of inappropriate use of benzodiazepines in Kosovo. Int J Clin Pharm, 39(4), 669–673. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, & Pruiksma KE (2014). Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int Rev Psychiatry, 26(2), 205–213. [DOI] [PubMed] [Google Scholar]
- Tetrault JM, Desai RA, Becker WC, Fiellin DA, Concato J, & Sullivan LE (2008). Gender and non-medical use of prescription opioids: results from a national US survey. Addiction, 103(2), 258–268. [DOI] [PubMed] [Google Scholar]
- Von Korff MR, & Franklin G (2016). Responding to America’s Iatrogenic Epidemic of Prescription Opioid Addiction and Overdose. Med Care, 54(5), 426–429. [DOI] [PubMed] [Google Scholar]
- Warner M, Trinidad JP, Bastian BA, Minino AM, & Hedegaard H (2016). Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2010–2014. Natl Vital Stat Rep, 65(10), 1–15. [PubMed] [Google Scholar]
- Zanarini MC, & Frankenburg FR (2001). Attainment and maintenance of reliability of axis I and II disorders over the course of a longitudinal study. Compr Psychiatry, 42(5), 369–374. [DOI] [PubMed] [Google Scholar]
- Zullig KJ, & Divin AL (2012). The association between non-medical prescription drug use, depressive symptoms, and suicidality among college students. Addictive Behaviors, 37(8), 890–899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


