Abstract
Oncology has recently undergone a revolutionary change with widespread adoption of immunotherapy for many cancers. Immunotherapy using monoclonal antibodies against “checkpoint” molecules, including PD-1, PD-L1, and CTLA-4, is highly effective in a significant subset of patients. However, immune-related adverse events (irAEs) have emerged as frequent complications of checkpoint blockade, likely due to the physiologic role of checkpoint pathways in regulating adaptive immunity and preventing autoimmunity. As immunotherapy becomes more common, a better understanding of the etiology of irAEs and ways to limit these events is needed. At the same time, studying these new therapy-related disorders provides an opportunity to better understand naturally occurring human autoimmune and inflammatory disorders, with the potential to improve therapies for cancer and autoimmune diseases.
Keywords: cancer immunotherapy, immune-related adverse events, checkpoint blockade, autoimmunity, tolerance
Blocking the inhibitors of adaptive immunity can increase both protective and pathogenic immune responses
The immune system evolved to protect against microbial infections. The vast repertoire of specificities of T and B lymphocytes can recognize most antigens, including those present on normal tissues. Mechanisms of immunological tolerance (see Glossary) evolved to limit reactions to healthy self-tissues, commensal organisms, and harmless environmental antigens. Collectively, central tolerance and peripheral tolerance mechanisms provide multiple layers of protection against pathogenic autoimmune responses, acting as fail-safes such that if one mechanism fails, back ups are in place to prevent tissue damage. If enough mechanisms fail, pathologic manifestations of immune-mediated responses can follow, such as autoimmunity or allergy, potentially leading to organ failure and/or death.
Cancer poses a unique problem for the immune system. Because cancer cells arise from self, these cells may evade immune destruction by engaging (and even enhancing) the mechanisms of immune tolerance that protect normal cells. The mammalian immune system can recognize and respond to cancer if adequately activated; however, immune responses are generally not sufficient to cure cancer without therapeutic intervention. Fortunately, new immunotherapeutic approaches can augment anti-tumor responses. Antibodies that block immune “checkpoint” inhibitory receptors are the most widely adopted class of immunotherapeutic drugs, and have shown striking anti-tumor responses in a subset of cancer patients [1, 2] (Box 1). Currently, Cytotoxic T-lymphocyte Associated Protein-4 (CTLA-4), Programmed Death-1 (PD-1), and Programmed Death Ligand-1 (PD-L1) are the only inhibitory molecules targeted by Food and Drug Administration (FDA) approved drugs, though newer therapies targeting others -- including Lymphocyte Activation Gene-3 (LAG-3), T-Cell Immunoglobulin and Mucin-Domain Containing-3 (TIM-3), and T Cell Immunoreceptor With Ig and ITIM Domains (TIGIT) -- are under development or in clinical trials (clinicaltrials.gov) for cancer immunotherapy. Additionally, hundreds of different combinations are now being investigated, and PD-1 blockade is a cornerstone of these combination treatments [2].
Box 1: Checkpoint blockade in patients: cancer burden and survival.
Significant efforts are being made to define the correlates of response to checkpoint blockade [1, 2, 57]. PD-1 pathway inhibitors show a range of response rates depending on the cancer type; from high (53–87%, e.g. MSIhi cancers, Merkel cell carcinoma, and Hodgkin’s lymphoma), intermediate (15–40% e.g. skin melanoma, NSCLC, RCC, and head and neck cancers), and low (<15%, pancreatic, prostate, and ovarian carcinoma, triple negative breast cancer, MSS colorectal cancer [1]. Patients with CD8+ T cells at the invasive tumor margin [20], high PD-L1 expression in the tumor [20, 58–61], and high numbers of somatic mutations (e.g. MSIhi, defects in DNA repair mechanisms) [62, 63] tend to respond better to PD-1 blockade than patients lacking an immune infiltrate in the tumor, have low PD-L1 expression, and low numbers of somatic mutations. However, these criteria are not presently sufficient to guide the clinical use of PD-1 inhibitors.
The development of exhaustion in the tumor microenvironment can limit T cell functions [11]. Studies in both mouse tumor models (MC38 colon adenocarcinoma, B16 melanoma, and D4M.3A melanoma) and human melanoma have suggested that PD-1 checkpoint blockade may work in part by relieving T cell exhaustion in the tumor microenvironment [11, 12, 64–66]. Moreover, the presence of a “stem-like” population of exhausted CD8+ T cells expressing the transcription factor TCF1 tends to correlate with better clinical outcomes following checkpoint blockade in mouse models, as well as in melanoma and NSCLC patients [65, 67–70]. Ultimately, considering both the state of the tumor and the immune response will be important. Adaptive resistance can result when increased inflammation drives upregulation of immunoregulatory molecules including PD-L1 [71, 72], and work in advanced melanomas demonstrated that tumor evolution can occur [73]. Since increasing immune pressure on the tumor may lead to resistance to checkpoint blockade, assessing tumor evolution in patients will likely be essential moving forward.
The future of cancer immunotherapy appears to be combination therapy with anti-PD-1, aiming to achieve better clinical outcomes for more cancer patients. The types of potential combinations are incredibly diverse, including pairing with other immunotherapies or traditional cancer therapies (e.g chemotherapy or radiation) [2, 74]. Strategies can combine two therapies targeting similar targets (e.g. multiple coinhibitory receptors targeting exhausted CD8+ T cells), or different pathways (e.g. pairing immunotherapy and chemotherapy). Indeed, efforts to improve innate immunity by blocking CD47 have shown synergy with anti-PD-L1 in preclinical models (RENCA tumors in BALB/c mice) [75, 76]. CD47 inhibitors have demonstrated promising activity in non-Hodgkin’s lymphoma patients in combination with rituximab [77], and the CD47 plus PD-1 pathway blockade combination is under investigation. However, the benefit of anti-tumor efficacy must be weighted against the risk of added toxicity, and the frequency, severity, and nature of irAEs will likely vary substantially with new and diverse combinations [2].
Checkpoint blockade may act in part by relieving T cell exhaustion and boosting effector functions in the tumor microenvironment (Box 1). However, these inhibitory receptors also play critical roles in inducing anergy, maintaining peripheral tolerance, and preventing over-activation of adaptive immune responses [3, 4]. Thus, one of the major risks of checkpoint blockade is inducing off-tumor inflammatory responses, many of which are autoimmune. These clinical manifestations, referred to as immune-related adverse events (irAEs), have been observed in a number of checkpoint blockade patients. While checkpoint blockade has generally been well-tolerated, life threatening toxicities do occur [5], presenting a major clinical challenge to safely administering these inhibitors. IrAEs are extremely varied in terms of the tissues affected, severity and time of onset relative to treatment. Some irAEs develop quickly after initiation of checkpoint blockade (e.g. rash, colitis), while others appear later (e.g. liver toxicity, hypophysitis) [6–8]. Additionally, some irAEs may cause permanent tissue damage, such as when endocrine organs are destroyed (e.g. insulin, adrenal corticosteroid deficiency), while others are largely reversible due to the intrinsic regenerative capacity of the involved organ (e.g. colitis, pneumonitis, or dermatitis) [6–9]. This diversity likely reflects differences in both the underlying mechanisms driving these irAEs and the physiology of the underlying organ. Understanding what drives these irAEs is not only relevant for cancer immunotherapy, but also provides an opportunity to define mechanisms of sporadic autoimmunity and therapeutic strategies to better treat autoimmune disorders.
The complex relationship between cancer immunotherapy and irAEs, the causes of irAEs, and what we can learn from irAEs about immune regulation and autoimmunity are becoming increasingly important issues as FDA approvals for cancer immunotherapies continue to increase. This article discusses the current state of the field regarding irAEs and cancer immunotherapy, and highlights key questions to address, as more and more patients receive these inhibitors.
Basic functions of immune checkpoint molecules
CTLA-4, PD-1 and PD-L1 were chosen as targets for immunotherapy based on extensive basic research showing that these molecules are physiological inhibitory regulators of T cell responses. PD-1 and CTLA-4 are upregulated on all conventional CD4+ and CD8+ T cells during acute activation (e.g. by pathogens or tumors), rapidly acting as brakes to temper the activation process. The underlying mechanisms of action of PD-1 and CTLA-4 are non-redundant, as early studies examining genetic knockout mice for these two receptors highlighted differences between these two pathways in terms of peripheral tolerance. Specifically, CTLA-4 knockout (Ctla4−/−) mice develop lethal multi-organ autoimmunity early in life, whereas PD-1 knockout (Pdcd1−/−) mice develop accelerated autoimmune diseases later in life varying according to the specific genetic background [3]. The broad expression of PD-1 on T cells in non-lymphoid tissues and its main ligand PD-L1 in inflamed tissue sites positions this pathway to be a critical regulator of effector T cell responses locally in peripheral tissues, while CTLA-4 and its ligands (B7–1 and B7–2) tend to be more abundant in secondary lymphoid organs in humans and in mice [3, 4]. Additionally, persistent antigenic stimulation during chronic viral infections (including lymphocytic choriomeningitis virus (LCMV) clone 13 in mice, and human immunodeficiency virus, hepatitis B virus, and hepatitis C virus in humans); and likely during tumor progression (including MC38, B16, and MB49 in mice and melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and others in humans) leads to elevated and sustained expression of PD-1 [3, 10–12]. CTLA-4 plays a lesser role than PD-1 in regulating CD8+ T cell exhaustion, a finding described in chronic LCMV infection (Clone 13) in mice, where anti-PD-L1 treatment reduced viral load and reinvigorated exhausted CD8+ T cells relative to controls, and anti-CTLA-4 treatment had little effect on virus-specific CD8+ T cell numbers or viral load [10]. This concept has also been shown in mouse tumor models, including MC38, B16, and A20HA [12–14], where PD-1 blockade played a role in reversing CD8+ T cell exhaustion, and CTLA-4 blockade had more of a role in T cell activation and priming [12, 13]. Thus, CTLA-4 may be more involved during conventional T cell priming and/or Treg cell functions in general [4, 14–16]. Emerging evidence suggests these non-redundant functions of PD-1 and CTLA-4 might also extend to human cancers, including melanoma [17–20], though this is still an active area of research.
Checkpoint molecules play critical roles in regulating Treg functions, and this has important consequences for immune homeostasis and tolerance. Treg cells exhibit elevated expression of both CTLA-4 and PD-1 compared to naïve conventional CD4+ T cells. On the one hand, mice selectively lacking CTLA-4 on Treg cells (Foxp3-IRES-Cre Ctla4f/f mice) develop multi-organ autoimmunity similar to that of complete CTLA-4 knockout mice [21], indicating that CTLA-4 can promote Treg suppressive functions. However, timing of deletion is likely critical for the effect of CTLA-4 on Treg cells, since inducible deletion of CTLA-4 in adulthood does not result in widespread autoimmunity [22]. The PD-1 pathway appears to control multiple types of Treg cells. In addition to its roles in regulating cellular immune responses, PD-1 can restrain the generation and function of T follicular regulatory (TFR) cells, linking this pathway to the control of humoral immune responses, including antibodies [23]. Specifically, in a co-transfer setting, a mixture of wild type (WT) or Pdcd1−/− TFR (from Pdcd1−/− mice), and WT TFH, was transferred into T cell-deficient mice (Tcra−/−); Pdcd1−/− TFR suppressed WT TFH more than WT TFR, leading to reduced antibody production [24]. In addition, PD-L1 can induce Foxp3 expression in both mouse and human CD4+ T cells [25, 26], suggesting that this pathway can play a role in inducible Treg (iTreg) development. Recent data in mice suggest that PD-1 signaling can impart a regulatory phenotype to iTreg and transcription factor T-bet+ iTreg cells, at least in part through PD-1-mediated maintenance of Foxp3 protein expression via downregulation of asparaginyl endopeptidase -- an endo-lysosomal protease cleaving Foxp3, leading to Foxp3 instability [27].
In addition to conventional and regulatory T cell lineages, PD-1 and CTLA-4 can both be expressed by a number of other cell types in both mice and humans. For instance, CTLA-4 can also be expressed on B cells, natural killer (NK) cells, dendritic cells, monocytes, granulocytes, some stem cells, and cells of the pituitary gland [4]. PD-1 can be expressed by B cells, natural killer (NK) cells, NKT cells, innate lymphoid cell (ILC) progenitors, and some cancer cells, including melanoma [3]. A better understanding of how each receptor affects these cell types will likely be critical for understanding both anti-tumor immunity as well as irAEs induced following checkpoint blockade.
Immune checkpoint blockade-induced irAEs in cancer patients
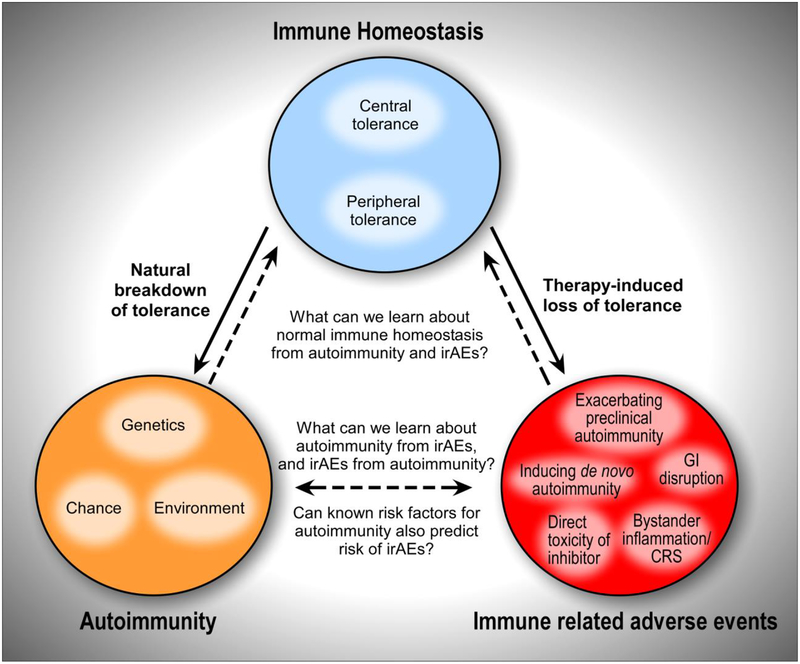
The first United States FDA approved drug targeting an immune checkpoint inhibitor for cancer therapy was an anti-CTLA-4 monoclonal antibody for non-resectable or metastatic melanoma (ipilimumab, Bristol-Myers Squibb). This was followed by approval of anti-PD-1 (nivolumab, Bristol-Myers Squibb; pembrolizumab, Merck; cemiplimab, Regeneron), and anti-PD-L1 monoclonal antibodies (atezolizumab, Genentech; avelumab EMD Serono, and durvalumab, AstraZeneca). PD-1 pathway inhibitors are currently approved for 14 types of cancer including melanoma, NSCLC, RCC, and microsatellite instability high (MSIhi) or mismatch repair deficient (dMMR) cancers, among others [1]. The combination of anti-CTLA-4 (ipilimumab) plus anti-PD-1 (nivolumamb) has been FDA approved for use in melanoma, RCC, as well as MSIhi or dMMR metastatic colorectal cancer. These immune checkpoint inhibitors have shown remarkable efficacy in a subset of cancer patients (Box 1) [1]. However, since PD-1 and CTLA-4 contribute to immune homeostasis, perturbing these pathways can facilitate the loss of immunological tolerance in patients (Key Figure, Figure 1) [3, 7]. These therapy-induced irAEs have presented a major obstacle for safely administering these inhibitors, particularly when used in certain combinations [6–8]. We are only beginning to appreciate the depth and breadth of irAEs, which are more frequent and varied than was apparent in initial clinical trials.
Key Figure, Figure 1: Pathogenic immune responses and the onset of autoimmunity or immune-relative adverse events.
Perturbed immune homeostasis can provoke pathogenic immune responses and the onset of autoimmunity or immune-relative adverse events (IrAE). (Top) Immune homeostasis involves major contributions from both central and peripheral tolerance mechanisms, acting as fail safes to limit aberrant immune activation against self tissues. (Bottom Left) Autoimmunity manifests when there is a natural breakdown of immune tolerance. While the underlying mechanisms contributing to autoimmunity remain elusive, they are thought to involve a complex interplay between host genetics, environmental factors, and some element of probability. Some autoimmune diseases can be caused by single gene mutations/loss of function, but generally, autoimmunity is caused by perturbations in a number of pathways. (Bottom Right) IrAE can occur following therapy-induced loss of tolerance. The mechanisms may share some overlap with natural autoimmunity in different organ systems, and may also operate via distinct mechanisms. Unlike spontaneous autoimmunity, therapy-induced loss of tolerance occurs following a sudden, widespread inhibition of either a single pathway (e.g. anti-PD-1 or anti-CTLA-4 monotherapy) or several pathways (e.g. combination therapy with anti-PD-1 plus anti-CTLA-4), and in a way not commonly found in the natural breakdown of tolerance.
Consistent with the non-redundant functions of PD-1/PD-L1 and CTLA-4, the types of irAEs related to single drug therapy targeting CTLA-4 or the PD-1 pathway differ. For example, hypophysitis is a common endocrine irAE observed following CTLA-4 blockade in cancer patients, but rarely seen following PD-1 pathway blockade [8, 29–31]. Conversely, thyroiditis in cancer patients is more common with PD-1 blockade than CTLA-4 blockade [8]. In general, irAEs in cancer patients are more common with anti-CTLA-4 (60–85%) than anti-PD-1 (16–37%) or anti-PD-L1 (12–24%) at standard doses of these drugs [6–8]. Although experience with combined use of anti-CTLA-4 and anti-PD-1 or anti-PD-L1 is limited mainly to melanoma patients, the frequency and severity of irAEs appear greater in combination-treated patients than in patients treated with single agents; indeed, up to 60% of patients on anti-PD-1 plus anti-CTLA-4 therapy can develop severe irAEs (including inflammation in the nervous system and heart) [32]. Additional studies in a different cohort of melanoma patients receiving the ipilimumab plus nivolumab combination reported that the frequency of events that require emergency room visits and hospitalization may be even higher than previous reports (approximately 91% of patients in [33] compared to 60% of patients developing severe irAEs in [32]); this highlights the potential toxicity of this combination. Long-term follow-up on patients receiving this combination therapy showed only modest benefits in overall survival compared to sequential therapy, starting with anti-PD1 alone (clinicaltrials.gov IDs NCT01844505 and NCT0184450) [32, 34]. Determining how to balance toxicity while maximizing anti-tumor responses is a critical goal for the field and may hinge on developing novel approaches to prevent or treat irAEs. The following sections discuss key aspects of irAEs in checkpoint blockade, with an emphasis on critical gaps in knowledge that need to be addressed to limit irAEs.
Mechanisms driving irAEs.
The diverse clinical manifestations of irAEs likely reflect different mechanisms inducing disease pathology. IrAEs may be caused by activation of immune responses unrelated to those targeting the tumor. Alternatively, on target/off tumor responses may occur (e.g. checkpoint blockade reactivating exhausted T cells that cross-react with both the tumor and self tissues, causing autoimmune tissue damage). Overall, the types of irAEs that occur following checkpoint blockade do not appear to be specific to the type of cancer [7, 8], which suggests that the cause of irAEs is a drug-induced loss of immune tolerance unrelated to the tumor. While the precise mechanisms leading to irAEs following checkpoint blockade remain largely unclear, potential mechanisms include: (i) exacerbating a pre-existing but subclinical autoimmune condition in the patient (e.g., through reactivation of autoreactive T cells, enhanced T cell help to B cells leading to autoreactive antibody production, or Treg depletion); (ii) initiating a new autoimmune or inflammatory condition (e.g. through aberrant activation of autoreactive lymphocytes, wider scale changes in global cytokine production resulting in cytokine-mediated toxicities, or exaggerated responses to pathogenic microbes); (iii) gastrointestinal (GI) disruption by altering commensal microbiota in the GI tract and/or immune interactions with environmental microorganisms, leading to pathology where there was previously tolerance; (iv) bystander tissue injury due to anti-tumor responses; and/or (v) aberrant reactions to the checkpoint inhibitor itself (e.g. CTLA-4 is expressed in the pituitary gland, where direct antibody binding has been proposed as a potential mechanism of ipilimumab-induced hypophysitis [6–8]) (Key Figure, Figure 1). The degree to which checkpoint blockade-induced loss of tolerance (e.g. irAEs) relates to the spontaneous loss of tolerance (e.g. autoimmune diseases) in humans remains unknown (Key Figure, Figure 1), and is an important area of active investigation.
The fraction of irAEs that stem from an underlying autoimmune disorder is not yet clear (Box 2). Patients with pre-existing autoimmune diseases have largely been excluded from cancer immunotherapy trials to date, but they are not excluded from treatment by approved checkpoint inhibitors based on the product labels. Some retrospective studies of post-approval cohorts have begun to address the relationship between baseline autoimmune disease and checkpoint inhibitors. One retrospective review of advanced melanoma patients with pre-existing autoimmune disorders (including rheumatoid arthritis, psoriasis, inflammatory bowel disease, multiple sclerosis, systemic lupus erythematosus, and autoimmune thyroiditis) showed that 8 out of 30 patients had a worsening of their pre-existing autoimmune condition requiring treatment following immunotherapy with ipilimumab [35]. These events were generally well managed with corticosteroids [35]. In another retrospective analysis of 119 advanced melanoma patients who received anti-PD-1 antibodies (most patients received pembrolizumab, some received nivolumab) from 13 academic centers, 52 were identified with pre-existing autoimmune diseases (including rheumatoid arthritis, polymyalgia rheumatica, psoriasis, Sjögren’s syndrome, and thrombocytopeinc purpura) and/or a major irAE on prior ipilimumab treatment (including grade 3 or 4 colitis, hypophysitis, or hepatitis); of these patients, 38% had a flare requiring immunosuppression [36]. These data demonstrate that not all patients with a pre-existing autoimmune condition show flares following checkpoint blockade. In the aforementioned study, autoimmune relapses were observed in melanoma patients with rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, psoriasis, and immune thrombocytopenic purpura, but not with gastrointestinal or neurological disorders [36]. Why some autoimmune or inflammatory conditions flare and some do not is unclear. A better understanding of what drives these events is needed. Due to the clinical complexity surrounding autoimmune disease development, experts in autoimmune disorders should be involved in the care of cancer patients before, during, and after checkpoint blockade.
Box 2: Lessons from autoimmune progression during cancer immunotherapy.
Autoimmunity in patients is generally the result of a complex interplay between genetics (e.g., HLA haplotypes, IL23R, TNFAIP3, IL2RA), environmental factors (e.g., diet, infections), and probability, leading to the loss of tolerance (Key Figure, Figure 1) [78]. Some mechanisms are so important to immune homeostasis that loss of a single gene causes autoimmunity, including FOXP3 and AIRE, both causing severe multi-organ human autoimmune diseases (Immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy (APECED), respectively [78]. More commonly, autoimmunity can result from partial disruption of several pathways, with autoimmunity from a single gene deficiency being uncommon. Polymorphisms in PD-1 and CTLA-4 in humans have been associated with increased autoimmunity risk [4]. Additionally, human CTLA-4 mutations can be associated with features of autoimmunity, including the diseases CTLA-4 Haploinsufficiency with Autoimmune Infiltration (CHAI) and LRBA deficiency with autoantibodies, Treg cell defects, autoimmune infiltration, and enteropathy (LATAIE) [79]. CHAI patients have a heterozygous loss of function mutation in the CTLA4 gene, leading to lymphocytic infiltration of multiple organs [79]. LATAIE is caused by LRBA deficiency, with loss of CTLA-4 on the cell surface due to disrupted CTLA-4 trafficking within the cell [79]. Despite these genetic associations, spontaneous autoimmunity (SA) due to exclusive loss of PD-1 or CTLA-4 signaling outside of checkpoint blockade is likely rare, due to redundancy in peripheral tolerance pathways.
Historically, it has been difficult to tease apart the contribution of individual pathways in autoimmune disease progression. The use of checkpoint blockade in cancer patients represents a unique opportunity to determine how blocking one mechanism of tolerance in isolation impacts human health. The fraction of pathogenic autoimmune responses actively held in check by PD-1 and/or CTLA-4 at any given time in patients is largely unclear. By extension, the consequences of checkpoint blockade on the breakdown of tolerance are difficult to predict. IrAEs might represent a rapid onset version of SA, or a completely new etiology presenting with similar symptoms. Checkpoint-induced diabetes resembles T1D by a number of parameters, including insulin-dependence, serum A1C concentrations, the presence of autoantibodies, and certain Human Leukocyte Antigen (HLA) associations (including HLA-DR4) [81]. Generally, in checkpoint-induced diabetes, the time between initiating checkpoint inhibition and diabetes onset is faster than in T1D [81]. Checkpoint colitis bears similarities to ulcerative colitis, including edema, erythema, friability, and superficial ulcerations, with differences in pathology and distribution of tissues affected (e.g. continuous inflammation from the anus to the cecum -- more consistent with pan-colonic ulcerative colitis, and a high proportion of lymphocytes and apoptotic epithelial cells) [49]. Considering the complexity of autoimmunity, deeper profiling (e.g. transcriptional, proteomic, metabolomics, etc.) may help define similarities between autoimmune diseases and irAEs, and clarify how treatment modalities for autoimmune diseases might be used to manage irAEs in cancer patients.
IrAE management and impact on clinical practice and drug development.
The need to manage irAEs has complicated administration of cancer immunotherapies and the subsequent course of cancer treatment. With the large number of new clinical syndromes, cancer centers have had to develop new expertise within other medicine subspecialties to diagnose and manage these irAEs. Specific recommendations on the management of various grades of irAEs have been reviewed elsewhere [6, 37, 38]. Generally, high dose corticosteroids are the first line for managing irAEs, and, often, effective in mitigating symptoms. For severe irAEs, immunotherapy may be halted while these events are managed. While these treatment options have largely been effective in managing irAE-driven inflammation, high dose corticosteroids and/or discontinuous immunotherapy regimens may be detrimental to the development of host immune responses [39, 40]. In one study, glioblastoma patients received 20 mg of the steroid dexamethasone, and expression of the co-inhibitory receptors PD-1, Tim-3, and CTLA-4 was higher than in patients who did not receive steroids [41]. Additionally, in a retrospective study of NSCLC patients receiving PD-1 checkpoint blockade, patients receiving greater than 10 mg/day of the steroid prednisone showed poorer outcomes (decreased progression-free survival and overall survival) than patients taking less than 10 mg/day of prednisone [42]. By extension, for lower grade irAEs, the deleterious effects of steroids on anti-tumor immunity might outweigh the benefits of irAE management; however, further work is needed to fully understand the impact of steroids on immunotherapy. In severe cases when death is a possibility following irAEs, cessation of tumor therapy and high dose steroid therapy or other immunosuppressive measures are necessary. The high rate of severe irAEs is a major limitation of combination therapy with nivolumab and ipilimumab, reducing its use as front-line therapy for most patients with melanoma. Moreover, the effects of this combination relative to nivolumab alone on long-term survival in melanoma patients have been relatively modest (at 36 months, progression-free survival = 32% for nivolumab and 39% for nivolumab plus ipilimumab, and overall survival = 52% for nivolumab and 58% nivolumab plus ipilimumab) [32]. This highlights the need to determine whether the cost:benefit ratio outweighs the increased toxicity. Whether more nuanced management of these toxicities could improve tolerability of this combination, or whether reduced use of steroids could improve long-term survival, is presently unknown. The dose of ipilimumab used in combination clearly influences the risk of irAEs. At 3 mg/kg ipilimumab, the combination with nivolumab leads to dose-limiting toxicity in more than half of melanoma patients [32, 43, 44]. Efforts to reduce toxicity have included decreasing the dose of ipilimumab to 1 mg/kg, or altering the sequence of checkpoint administration (e.g. ipilimumab followed by nivolumab vs concomitant administration) [45–48]. However, whether this less toxic dosing provides a significant therapeutic advantage over anti-PD1 therapy alone is still unclear. Further refinement of methods to mitigate toxicity without compromising anti-tumor efficacy will likely benefit the clinical development of combination therapies.
An important question in the field is the degree of similarity between checkpoint blockade-induced loss of tolerance (e.g. irAEs) and spontaneous loss of tolerance (e.g. autoimmune diseases) (Box 2, Key Figure, Figure 1). Depending on the degree of similarity between irAEs and autoimmune disorders, empiric treatment using therapies developed for autoimmunity may improve our ability to manage irAEs. This may likely be the case for most endocrine diseases since management of these focuses on hormone replacement; this may be a similar approach regardless of whether the endocrine organ was damaged by immunotherapy, or by naturally arising autoimmune disorders (e.g. insulin injections to manage glucose levels for checkpoint-induced diabetes and autoimmune, Type 1 Diabetes (T1D)). In another example, anti-TNFα antibodies are frequently used to treat steroid-refractory inflammatory bowel disease, and this strategy has also been highly effective in managing checkpoint-induced colitis [49]. Increasing input from specialists in autoimmune diseases (e.g. rheumatologists, cardiologists, endocrinologists) will be critical to continue shaping treatment options for cancer immunotherapy patients.
Investigating and reporting on irAEs.
Many factors have complicated reporting irAEs in cancer patients receiving immunotherapy. The rate of irAEs following immunotherapy is likely underestimated because: (i) for some symptoms, the etiology is unclear (e.g. lethargy, flu-like symptoms); (ii) clinical characterization is often insufficient, making definitive diagnoses challenging; (iii) the time of onset can be extremely variable, and events that arise late may not be attributed to the immunotherapy; and (iv) it can be difficult to accurately diagnose autoimmune diseases in the clinic, especially during early stages of disease. In addition to challenges in diagnosing irAEs, there have been major obstacles to performing deep mechanistic studies on these events. The low frequency of irAEs of a given subtype, particularly for some of the rare, life-threatening events (e.g. neurological, cardiac, and hematologic toxicities), makes it difficult for individual institutions to acquire sample sets large enough for deep correlative studies. The heterogeneity of patients (people receiving the same immunotherapy but with distinct underlying malignancies), and co-treatments that are often tumor-specific (chemotherapy, radiation therapy, targeted therapy) adds another layer of complexity, further reducing the size of the patient population with any given irAE. Thus, multi-institutional and multi-specialty studies are needed to increase sample size for studies of specific irAEs. The National Cancer Institute has put forth Common Terminology Criteria for Adverse Events (CTCAE) (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50), which is a useful resource for developing a shared terminology for discussing irAEs in patients across different centers.
Developing methods to dissociate anti-tumor immunity from inflammation-driven irAEs.
One of the most fundamental questions for managing irAEs is whether we can therapeutically dissociate beneficial anti-tumor immunity from irAE-associated inflammation. Since checkpoint blockade inhibits molecules that promote tolerance and the resolution of inflammation, dissociating the anti-tumor effect from irAEs may be challenging. Ultimately, this will likely depend on specific toxicities, cancer type, and mechanisms underlying anti-tumor and irAE-driven responses. The answers to this question will likely impact many aspects of patient care, including the range of immunotherapies that have an acceptable risk:benefit ratio, the percentage of patients who can benefit from these therapies, as well as morbidity and mortality.
One illustrative example of how dissociating these two events has been transformative is the use of anti-interleukin (IL)-6 receptor (tocilizumab) antibodies in Chimeric Antigen Receptor (CAR) T cell therapy [50] (Box 3). The IL-6-induced cytokine storm that can occur following CAR T cell infusion can be lethal, potentially severely limiting the therapeutic window for CAR T cells. However, blockade of the IL-6 receptor is highly effective in treating this life-threatening toxicity without interfering with the anti-tumor activity of CAR T cells [50]. CAR T cells have been extremely effective at providing cures for B cell malignancies including B-cell acute lymphoblastic leukemia (ALL) and non-Hodgkin’s lymphoma (CD19 CAR T cells); this has been possible because the dangerous side effects (cytokine storm) can be managed before they can become life-threatening [50] (Box 3). Thus, developing similar strategies for checkpoint blockade might increase the number of patients who can benefit from these therapies. One possibility for managing checkpoint blockade-induced irAEs is to block homing molecules that are involved in facilitating recruitment of pathogenic lymphocytes into non-tumor bearing target organs. Because T cells must contact peptide:MHC expressing cells to execute effector functions, T cells cannot induce damage if they cannot traffic to a given tissue. This approach may be useful for irAEs associated with the gut or skin, each of which have a unique set of chemokine receptors, selectins, and/or integrins that are involved in homing of T cells into a specific organ (integrin α4β7 and chemokine receptor CCR9 for the gut; E-selectin and chemokine receptors CCR4 and CCR10 for the skin, in both mice and humans) [51]. In humans, the α4β7 inhibitor vedolizumab has been used to block T cell trafficking to the gut, with up to 95% efficacy in treating steroid-refractory colitis in a small cohort of cancer patients (28 patients with melanoma, RCC, prostate, or urothelial cancer) receiving checkpoint inhibitors (either anti-CTLA-4 or anti-PD-1/L1 alone or combination anti-PD-1/anti-CTLA-4) [52]. However, this approach may be less effective for other tissues (e.g. pancreas, thyroid, pituitary gland, etc.) where a set of tissue-specific integrins, selectins, and chemokine receptors have not been described (redundant receptors shared between tissues are used to recruit cells in context of inflammation) [51]. Another option may be to modify these inhibitors so that they are only active within the tumor microenvironment, or to restrict their delivery to the tumor microenvironment (Box 4). Collectively, these approaches may be useful to limiting toxicities following checkpoint blockade, but extensive preclinical and clinical work is needed to determine the safety and efficacy of these strategies.
Box 3: IrAEs caused by CAR T cell therapy.
Unlike checkpoint blockade which aims to alleviate immunosuppression of naturally arising anti-tumor responses, adoptive T cell transfer (ACT) therapies, including CAR T cells, use ex vivo activation and expansion of cells to generate effector populations to combat the tumor. The types of irAEs associated with CAR T cells are very different from those associated with checkpoint blockade. The most common irAE associated with CAR T cells is a cytokine storm or cytokine release syndrome (CRS). Currently, CRS is managed by administering tocilizumab, siltuximab, Janus kinase [82] inhibitors, and/or corticosteroids to block the effects of IL-6, reducing fevers, hypotension and hypoxia [50].
Neurotoxicity has been observed as well [83, 84]. Neurotoxicity resulting from CAR T cells may be caused by CRS directly, or by CRS-independent mechanisms (e.g., elevated concentrations of excitatory neurotransmitters such as glutamate and quinolinic acid). The severity of neurotoxicity can correlate with the severity of CRS, and CRS-associated cytokines including IL-6 can be enriched in the cerebral spinal fluid (CSF) during these events [84]. Development of CRS generally correlates with high tumor burden [85, 86]. In one study of ALL patients receiving CTL019 (a CD19-specific CAR T cell), out of 43 cytokines assessed in the serum, 24 were highly associated with critical illness due to CRS relative to patients not developing CRS [87]. The presence of only two cytokines (interferon (IFN)γ and soluble glycoprotein (sgp)130) could predict the majority of patients who would become ill with severe CRS; and the presence of IFNγ, spg130, and macrophage inflammatory protein (MIP)1α together could predict 100% of cases [87]. Overall, management strategies for CRS have been effective for most patients, limiting issues associated with the irAE [50].
On-target off-tumor complications continue to be a consideration with CAR T cells as new tumor targets are tested. The first cancer patient (colon cancer metastatic to the lungs and liver) to receive ERBB2/HER2-targeted CAR T cells presented with fatal toxicity likely due to expression of ERBB2 on lung epithelial cells, leading to CAR T cell-mediated pulmonary failure [88]. Additionally, CD19 CAR T cells can deplete normal B cells, though adverse side effects of B cell aplasia are generally manageable [89]. Thus, identifying targets that are uniquely expressed by cancer cells, or expressed by cancer cells and non-essential cell types (e.g. CD19 and B cells) will be essential for designing safe CAR T cell therapies in increasingly diverse cancer types.
Box 4: Examples of Targeted Drug Delivery in the Tumor Microenvironment.
A recent mouse study selectively targeted the delivery of anti-PD-1 to Her2/neu-expressing RENCA tumors upon infection with an adenovirus expressing anti-PD-1 [53]. Her2/neu is the entry receptor for adenovirus, so the virus selectively entered tumor cells, which began secreting anti-PD-1 into the tumor microenvironment [53].
A checkpoint inhibitor can also be targeted to the tumor using a bispecific antibody, where one arm targets the checkpoint and the other targets the tumor. A proof-of-concept in vitro study used both primary human cells and cell lines to show that a bispecific antibody targeting PD-L1 and epidermal growth factor receptor (EGFR) successfully targeted the anti-PD-L1 antibody to EGFR-expressing tumor cells [54]. In another study, an anti-CD3-scfv (short chain variable fragment) was developed and conjugated to either Trop-2 and CEACAM5 to target the anti-CD3 to Trop-2 and CEACAM5-expressing tumor cells in humanized mice [55]. This bispecific antibody caused growth inhibition and subsequent lysis of tumor cells [55].
Molecular “shields” have also been developed to restrict activity of the checkpoint inhibitor until it reaches the tumor microenvironment [56]. One study combined bispecific antibody and molecular shield approaches to target bioactive anti-CTLA-4 selectively to the tumor [56]. The outer portion of the antibody specific for prostate stem cell antigen (PSCA) was attached by a cleavable linker to the anti-CTLA-4 domain, blocking the activity of anti-CTLA-4. The anti-PSCA portion targeted the antibody to the tumor, and once within the tumor, an enzyme enriched in the tumor microenvironment (MT-SP1) cleaved the linker, releasing the anti-PSCA portion of the antibody, thus allowing anti-CTLA-4 to be biologically active [56].
Concluding Remarks
Cancer immunotherapy is now considered a pillar of cancer treatment alongside chemotherapies, radiation therapies, targeted therapies and surgery. Despite the early successes of cancer immunotherapy, only a subset of patients benefit from these treatments, and irAEs can be an important limitation to the reach of cancer immunotherapy. Understanding and managing these inflammatory toxicities represents a critical challenge for the field. Moving forward, determining the mechanisms underlying these iatrogenic diseases will be essential to (i) better predict who is at risk of developing irAEs, (ii) best manage the clinical symptoms of irAEs with as little disruption to the anti-tumor response as possible, and (iii) safely develop novel combination therapies while minimizing toxicities (Outstanding Questions Box).
Outstanding Questions Box.
How much of the anti-tumor response targets self proteins? Since many cancer immunotherapies target pathways that inhibit autoreactive lymphocytes, autoimmunity following cancer immunotherapy is somewhat expected. The high degree of overlap between tumor antigens and self-antigens makes cross-reactive T cell responses possible, though the degree to which this occurs clinically remains unknown.
Can we predict who will develop irAEs? Can use of known polymorphisms or other genetic risk factors in human autoimmune diseases and inflammatory diseases (e.g. HLA haplotypes, PTPN22, IL23R, TNFAIP3, IL2RA, CTLA4) serve as predictive biomarkers of the risk of a pathogenic autoimmune outcome during cancer immunotherapy? Carefully designed prospective trials are needed to answer this question.
Can immunotherapy help us understand the early events driving autoimmunity? Are the underlying mechanisms and disease pathologies of irAEs similar to spontaneous autoimmune disorders? To the extent that these diseases overlap, can we use the carefully controlled setting of immunotherapy to uncover information about the pathogenesis of human autoimmune diseases?
How can we streamline resources to better manage irAEs? Clinicians who specialize in irAE diagnosis and management should be involved in the development of clinical trials, and trials should be designed to address irAEs instead of passively managing these events as they arise. Such a strategy could have widespread ramifications, potentially enabling continued development of drugs that may otherwise fail in early clinical assessment due to toxicity.
Can a mechanistic understanding of pathways involved in irAEs lead to new therapeutic targets? This question applies not only to immunotherapy for cancer, but also to treatment of autoimmune diseases. In addition to using human data to compare pathogenic immune responses driving irAEs to productive anti-tumor responses, generation of better mouse models to investigate irAEs would be useful for dissecting mechanisms.
These inflammatory toxicities from immunotherapy are more than just side-effects to be treated: They are a window into immune regulation and homeostasis in humans. These irAEs provide an opportunity to learn more about how perturbing immune homeostasis through checkpoint blockade leads to breakdown of tolerance, the induction and progression of autoimmunity in patients, and the relationship to the specific receptors targeted (Key Figure, Figure 1). These cases share a common feature that is rare in medicine: There is a known “time zero” that marks the initiation of the immune perturbation. The immune system can be studied pre-treatment, on-treatment before the onset of an irAE, and after irAE onset. There is also extensive clinical information associated with each patient as part of oncology care, which will include concomitant medications, infections, or other underlying diseases. Now is a critical time for the field to come together, pairing expertise from basic and clinical arenas with cutting edge technologies. It will propel our fundamental understanding of the immune system forward and provide the best patient care options possible. Furthermore, this knowledge has important implications not only for the treatment of malignancy, but also for the treatment of autoimmune and other immune-mediated diseases.
Highlights.
Immune-related adverse events (IrAE) represent a major obstacle for safely administering checkpoint blockade in cancer patients. Developing methods to minimize irAEs while maintaining effective anti-tumor immunity could make immunotherapy safer for more cancer patients
Diverse mechanisms likely drive irAEs, which may share varying degrees of similarities and differences with spontaneous autoimmunity.
IrAEs that develop following checkpoint blockade in cancer provide an opportunity to better understand spontaneous autoimmune and inflammatory disorders.
A deeper understanding of why some patients develop irAEs and others do not is needed to determine who is at highest risk for developing life threatening irAEs and to develop biomarkers to identify these individuals.
Improvements in managing, investigating, and reporting on these irAEs are needed to reduce clinical challenges and maximize opportunities to learn from irAEs.
Acknowledgements
This article was inspired by a colloquium hosted by the American Autoimmune Disease Related Association (AARDA) on April 22, 2017 in Washington D.C. This meeting brought together a group of basic scientists and clinicians specializing in oncology, autoimmunity, and immunology to discuss issues related to irAEs, autoimmune disease and cancer immunotherapy. Drs. Yi-Chi Kong, Jeffery Sosman, Arlene Sharpe, Benjamin Olenchock, Siwen Hu-Lieskovan, Justin Balko, Javid Moslehi, Andrew Lichtman, Patrizio Caturegli, Alexander Faje, Kevan Herold, Adil Daud, Patrick Ott, David Teachey, Elad Sharon, Michael Dougan, and Le Min presented at this meeting. We thank the meeting participants for their thoughtful discussions, which motivated this article. The authors apologize to colleagues whose work was not cited due to space constraints. This work was supported by grants from the US National Institutes of Health Mentored Clinical Scientist Development Award 1K08DK114563–01 and the American Gastroenterological Association Research Scholars Award (M.D.), National Institutes of Health grants HL121363 and HL13186 (A.H.L.), and National Institutes of Health grants R01 AI040614 and P01 AI056299 and AI108545 (A.H.S.). M.D. has research funding from Novartis and consulting from Genentech. A.H.S. holds patents on the PD-1 pathway related to cancer immunotherapy, has research funding from Ipsen, Novartis, Roche, Quark Ventures and UCB, and consults for Novartis. The authors declare no additional competing financial conflicts of interest.
Glossary
- ACT therapies
class of immunotherapies where cells are manipulated ex vivo to express genetically-modified receptors endowing cancer specificity; the cells are then transfused back into the patient
- A1C concentrations
measure of glycated hemoglobin in the blood, which correlates with blood glucose concentrations
- Anergy
state of T cell dysfunction caused by inadequate activation signals at priming. These cells are functionally inert, but effector functions can be restored if the proper signals are provided
- CAR T cell therapy
a patient’s T cells are isolated, expanded, engineered to express a chimeric antigen receptor (CAR) T cell that is specific for the patient’s cancer; the cells are then transferred back into the patient
- Cancer immunotherapy
Drugs aiming to target the anti-tumor immune response rather than the cancer cells themselves
- Cellular Immune Responses
Adaptive immune responses mediated by cells, predominantly antigen-specific CD4+ and/or CD8+ T cells
- Central tolerance
Tolerance mechanisms occurring during lymphocyte development (bone marrow for B cells; thymus for T cells), including deletion
- Checkpoint
A term used to describe regulatory nodes/pathways during immune responses, such as PD-1 and CTLA-4
- Cytokine storm
condition occurring with severe overproduction of inflammatory cytokines by the immune system
- Hypophysitis
A condition which involves inflammation of the pituitary gland. This can lead to pituitary gland failure, including adrenal insufficiency and hypothyroidism
- ILC
innate population of cells with relatively similar functions to T cells
- Immunological tolerance
Mechanisms that limit activation of self-reactive T cells and B cells, including central tolerance (during lymphocyte development), and peripheral tolerance (after lymphocyte development)
- T-bet+ iTreg
A population of inducible regulatory T cells (Treg) that also expresses T-bet
- Humoral Immune Responses
Adaptive immune responses mediated by soluble effector molecules, predominantly antibodies
- Inducible Treg
or “adaptive” Tregs, arise from conventional CD4+ T cells induced to express Foxp3
- Off-tumor inflammatory responses
Events leading to inflammation caused by an anti-cancer agent, and impacting host cells that are not the malignant cells
- NKT cells
innate-like lymphocyte population that rapidly produces inflammatory cytokines and lyses target cells after recognizing antigen presented in CD1d
- Peripheral Tolerance
Tolerance mechanisms occurring in the secondary lymphoid organs or peripheral tissues, including anergy, suppression by Treg cells, and inhibition of effector functions by PD-1 and/or CTLA-4
- T follicular regulatory cells (TFR)
subset of Treg cells suppressing T follicular helper cells (TFH) and humoral immune responses
- T cell exhaustion
state of T cell dysfunction caused by chronic antigen exposure and inflammation. It occurs after full effector differentiation
- Treg
regulatory subset of CD4+ T cells, generally defined by the expression of Foxp3 (although some IL-10 expressing Tregs, e.g. Tr1 cells, do not express Foxp3)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ribas A and Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359 (6382), 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen DS and Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 541 (7637), 321–330. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe AH and Pauken KE (2017) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. [DOI] [PubMed] [Google Scholar]
- 4.Schildberg FA, et al. (2016) Coinhibitory Pathways in the B7-CD28 Ligand- Receptor Family. Immunity 44 (5), 955–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang DY, et al. (2018) Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 4 (12), 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haanen J, et al. (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28 (suppl_4), iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 7.Postow MA, et al. (2018) Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 378 (2), 158–168. [DOI] [PubMed] [Google Scholar]
- 8.Boutros C, et al. (2016) Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 13 (8), 473–86. [DOI] [PubMed] [Google Scholar]
- 9.Weber JS, et al. (2012) Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 30 (21), 2691–7. [DOI] [PubMed] [Google Scholar]
- 10.Barber DL, et al. (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439 (7077), 682–7. [DOI] [PubMed] [Google Scholar]
- 11.Pauken KE and Wherry EJ (2015) Overcoming T cell exhaustion in infection and cancer. Trends Immunol 36 (4), 265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei SC, et al. (2017) Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 170 (6), 1120–1133 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twyman-Saint Victor C, et al. (2015) Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520 (7547), 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sotomayor EM, et al. (1999) In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci U S A 96 (20), 11476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krummel MF and Allison JP (1995) CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182 (2), 459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P and Allison JP (2015) The future of immune checkpoint therapy. Science 348 (6230), 56–61. [DOI] [PubMed] [Google Scholar]
- 17.Robert L, et al. (2014) Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes. Oncoimmunology 3, e29244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das R, et al. (2015) Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 194 (3), 950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvistborg P, et al. (2014) Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med 6 (254), 254ra128. [DOI] [PubMed] [Google Scholar]
- 20.Tumeh PC, et al. (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515 (7528), 568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wing K, et al. (2008) CTLA-4 control over Foxp3+ regulatory T cell function. Science 322 (5899), 271–5. [DOI] [PubMed] [Google Scholar]
- 22.Paterson AM, et al. (2015) Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J Exp Med 212 (10), 1603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sage PT and Sharpe AH (2016) T follicular regulatory cells. Immunol Rev 271 (1), 246–59. [DOI] [PubMed] [Google Scholar]
- 24.Sage PT, et al. (2013) The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol 14 (2), 152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francisco LM, et al. (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 206 (13), 3015–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amarnath S, et al. (2011) The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med 3 (111), 111ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stathopoulou C, et al. (2018) PD-1 Inhibitory Receptor Downregulates Asparaginyl Endopeptidase and Maintains Foxp3 Transcription Factor Stability in Induced Regulatory T Cells. Immunity 49 (2), 247–263 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson JJ, et al. (2012) Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J Clin Invest 122 (8), 2967–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggermont AM, et al. (2016) Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med 375 (19), 1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robert C, et al. (2015) Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 372 (26), 2521–32. [DOI] [PubMed] [Google Scholar]
- 31.Hodi FS, et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363 (8), 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolchok JD, et al. (2017) Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 377 (14), 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoushtari AN, et al. (2018) Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol 4 (1), 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodi FS, et al. (2018) Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 19 (11), 1480–1492. [DOI] [PubMed] [Google Scholar]
- 35.Johnson DB, et al. (2016) Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol 2 (2), 234–40. [DOI] [PubMed] [Google Scholar]
- 36.Menzies AM, et al. (2017) Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28 (2), 368–376. [DOI] [PubMed] [Google Scholar]
- 37.Brahmer JR, et al. (2018) Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36 (17), 1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puzanov I, et al. (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 5 (1), 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu X, et al. (2003) Inhibition of IFN-gamma signaling by glucocorticoids. J Immunol 170 (9), 4833–9. [DOI] [PubMed] [Google Scholar]
- 40.Bereshchenko O, et al. (2014) GILZ promotes production of peripherally induced Treg cells and mediates the crosstalk between glucocorticoids and TGF-beta signaling. Cell Rep 7 (2), 464–475. [DOI] [PubMed] [Google Scholar]
- 41.Park J, et al. (2019) Immune Checkpoint Inhibitor-induced Reinvigoration of Tumor-infiltrating CD8(+) T Cells is Determined by Their Differentiation Status in Glioblastoma. Clin Cancer Res. [DOI] [PubMed] [Google Scholar]
- 42.Arbour KC, et al. (2018) Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 36 (28), 2872–2878. [DOI] [PubMed] [Google Scholar]
- 43.Hodi FS, et al. (2016) Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 17 (11), 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larkin J, et al. (2015) Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 373 (1), 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirchberger MC, et al. (2018) Real world experience in low-dose ipilimumab in combination with PD-1 blockade in advanced melanoma patients. Oncotarget 9 (48), 28903–28909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammers HJ, et al. (2017) Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J Clin Oncol 35 (34), 3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long GV, et al. (2017) Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol 18 (9), 1202–1210. [DOI] [PubMed] [Google Scholar]
- 48.Weber JS, et al. (2016) Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 17 (7), 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dougan M (2017) Checkpoint Blockade Toxicity and Immune Homeostasis in the Gastrointestinal Tract. Front Immunol 8, 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.June CH, et al. (2018) CAR T cell immunotherapy for human cancer. Science 359 (6382), 1361–1365. [DOI] [PubMed] [Google Scholar]
- 51.Masopust D and Schenkel JM (2013) The integration of T cell migration, differentiation and function. Nat Rev Immunol 13 (5), 309–20. [DOI] [PubMed] [Google Scholar]
- 52.Abu-Sbeih H, et al. (2018) Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J Immunother Cancer 6 (1), 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reul J, et al. (2019) Tumor-Specific Delivery of Immune Checkpoint Inhibitors by Engineered AAV Vectors. Front Oncol 9, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koopmans I, et al. (2018) A novel bispecific antibody for EGFR-directed blockade of the PD-1/PD-L1 immune checkpoint. Oncoimmunology 7 (8), e1466016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang CH, et al. (2017) Combination Therapy with Bispecific Antibodies and PD-1 Blockade Enhances the Antitumor Potency of T Cells. Cancer Res 77 (19), 5384–5394. [DOI] [PubMed] [Google Scholar]
- 56.Pai CS, et al. (2019) Tumor-conditional anti-CTLA4 uncouples antitumor efficacy from immunotherapy-related toxicity. J Clin Invest 129 (1), 349–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishino M, et al. (2017) Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 14 (11), 655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Topalian SL, et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366 (26), 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brahmer JR, et al. (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28 (19), 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garon EB, et al. (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372 (21), 2018–28. [DOI] [PubMed] [Google Scholar]
- 61.Herbst RS, et al. (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515 (7528), 563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le DT, et al. (2015) PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372 (26), 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Llosa NJ, et al. (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5 (1), 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daud AI, et al. (2016) Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest 126 (9), 3447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller BC, et al. (2019) Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 20 (3), 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang AC, et al. (2017) T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545 (7652), 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sade-Feldman M, et al. (2018) Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 175 (4), 998–1013 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thommen DS, et al. (2018) A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 24 (7), 994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siddiqui I, et al. (2019) Intratumoral Tcf1(+)PD-1(+)CD8(+) T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 50 (1), 195–211 e10. [DOI] [PubMed] [Google Scholar]
- 70.Kurtulus S, et al. (2019) Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1(−)CD8(+) Tumor-Infiltrating T Cells. Immunity 50 (1), 181–194 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12 (4), 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taube JM, et al. (2012) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4 (127), 127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riaz N, et al. (2017) Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 171 (4), 934–949 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma P and Allison JP (2015) Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161 (2), 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sockolosky JT, et al. (2016) Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A 113 (19), E2646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yanagita T, et al. (2017) Anti-SIRPalpha antibodies as a potential new tool for cancer immunotherapy. JCI Insight 2 (1), e89140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Advani R, et al. (2018) CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med 379 (18), 1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rioux JD and Abbas AK (2005) Paths to understanding the genetic basis of autoimmune disease. Nature 435 (7042), 584–9. [DOI] [PubMed] [Google Scholar]
- 79.Lo B, et al. (2016) CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency. Blood 128 (8), 1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kleffel S, et al. (2015) Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell 162 (6), 1242–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stamatouli AM, et al. (2018) Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 67 (8), 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cameron BJ, et al. (2013) Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 5 (197), 197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morgan RA, et al. (2013) Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36 (2), 133–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santomasso BD, et al. (2018) Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov 8 (8), 958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brentjens RJ, et al. (2013) CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5 (177), 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maude SL, et al. (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371 (16), 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teachey DT, et al. (2016) Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov 6 (6), 664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morgan RA, et al. (2010) Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18 (4), 843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Porter DL, et al. (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365 (8), 725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]