Consciousness may evade detection on bedside behavioral assessment. Due to impairments in self-expression, signs of consciousness are missed in up to 40% of patients with severe brain injury (Schnakers et al., 2009). Accurate diagnosis is particularly challenging in the intensive care unit (ICU), where pain, sedation, and medical illness may prevent purposeful responses. Given that early recovery of consciousness is associated with better long-term outcome (Giacino & Kalmar, 1997), misdiagnosis in the ICU could have devastating consequences, including early withdrawal of life-sustaining therapy or reduced access to rehabilitative care.
Comprehensive behavioral assessment with the Coma Recovery Scale-Revised (CRS-R) (Giacino, Kalmar, & Whyte, 2004) decreases the misdiagnosis rate (Schnakers et al., 2009). Nevertheless, approximately 20% of brain-injured patients who do not follow commands on the CRS-R (i.e. vegetative state [VS] or minimally conscious state without language function [MCS-]) demonstrate volitional brain responses on task-based functional magnetic resonance imaging (fMRI) (Edlow et al., 2017; Monti et al., 2010). This “covert consciousness,” recently labeled cognitive-motor dissociation (CMD) (Schiff, 2015), may have profound implications for decisions about life-sustaining therapy in the ICU. However, the neural networks that underlie covert consciousness have not been identified.
The default mode network (DMN) is believed to be essential for sustaining human consciousness (Bodien, Chatelle, & Edlow, 2017; Threlkeld et al., 2018). Patients with behavioral evidence of conscious awareness after severe brain injury have more intact DMNs (Threlkeld et al., 2018; Vanhaudenhuyse et al., 2010) and are more likely to appropriately deactivate the DMN during task-based fMRI (Crone et al., 2011). However, some patients without behavioral evidence of conscious awareness also have an intact DMN (Bodien et al., 2017; Boly et al., 2008; Norton et al., 2012). These observations suggest that the DMN may be necessary, but not sufficient, to sustain consciousness. Despite increasing evidence for the role of the DMN in consciousness, the DMN in covert consciousness has not been investigated. We tested the hypothesis that DMN properties in patients with covert consciousness (CMD) are more similar to those in patients with overt consciousness than to patients with coma, VS, or MCS-.
In accordance with the Transparency and Openness Promotion Guidelines, we report how we determined our sample size, all data exclusions (if any), all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. Sixteen patients with acute severe traumatic brain injury (TBI) admitted to the ICU and 16 age- and sex-matched healthy subjects participated in task-based and resting-state fMRI using methods described previously (Edlow et al., 2017; Threlkeld et al., 2018). Overt consciousness was defined by CRS-R diagnoses of MCS with language function [MCS+] and post-traumatic confusional state [PTCS]. Covert consciousness (CMD) was defined by fMRI activation in supplementary motor or premotor cortices during a hand-squeezing imagery task, in the absence of language function on the CRS-R (Schiff, 2015). Thus, in addition to coma and VS patients, MCS- patients who performed the fMRI task were also considered to be covertly conscious.
We analyzed three DMN properties: 1) resting blood-oxygen-level-dependent correlations within the DMN; 2) resting anticorrelations between DMN nodes and the whole brain; and 3) task-based DMN deactivations. Deactivations were analyzed in FSL 5.0.7 by applying a negative task-minus-rest contrast and extracting the percentage of voxels exceeding Z>3.1 and P=0.05 cluster-corrected thresholds in the DMN. A DMN property was considered intact if the Z-score (for correlations/anticorrelations) or percentage of suprathreshold voxels (for deactivations) was within a normal range defined by the 2.5th-to-97.5th percentile of healthy subjects. We categorized the DMN as being preserved, partially preserved, or absent if all three, one-to-two, or no DMN properties were intact, respectively. This novel categorization scheme comprises the most commonly reported DMN properties but has not been validated. We also analyzed the potential confounders of motion, time from injury to fMRI, and level of sedation among groups with different diagnoses and varying levels of DMN preservation (see Supplementary Materials). Finally, we report 6-month outcomes using standardized behavioral assessments (see Supplementary Materials).
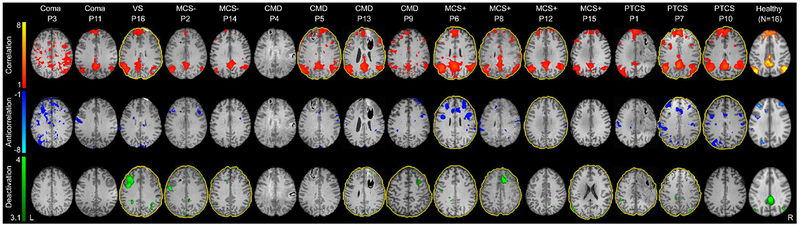
Data processing scripts, stimulus files, and region-of-interest files can be found at: github.com/comarecoverylab/DMN_CovertConsc/tree/v1.0. The conditions of our Institutional Review Board ethics approval do not permit public archiving of anonymized study data. Readers seeking access to the data should contact the senior author. No part of the study procedures or analysis was pre-registered prior to the research being conducted. Patient and injury characteristics are detailed in Supplementary Table 1. Four patients demonstrated covert consciousness. Level of consciousness was not related to sedation, time from injury to fMRI, or number of preserved DMN properties (see Supplementary Materials). Across all subjects, the degree of DMN correlation and DMN anticorrelation during rest, and DMN deactivation during task did not correlate with averaged motion parameters (see Supplementary Materials). Results are presented in Figure 1 and Supplementary Table 3. Among overtly conscious patients, the DMN was preserved in two (1 MCS+, 1 PTCS) and partially preserved in five (3 MCS+, 2 PTCS). Among covertly conscious patients, the DMN was partially preserved in three and absent in one. Among unconscious patients, the DMN was partially preserved in one (VS) and absent in two (coma). DMN deactivation during the motor imagery task was the most commonly preserved DMN property, observed in 10 patients, while DMN correlations were observed in eight and anticorrelations in four. All patients with DMN anticorrelations also demonstrated correlations. Three patients died in the ICU due to withdrawal of life-sustaining therapy. The remaining 13 patients recovered from a disorder of consciousness by six-months post-injury and had scores on the Glasgow Outcome Scale-Extended (Wilson, Pettigrew, & Teasdale, 1998) ranging from 3 to 7 (see Supplementary Materials).
Figure 1. Default mode network preservation in patients with acute traumatic disorders of consciousness.
Compared to healthy subjects, patients with acute severe traumatic brain injury diagnosed with coma, vegetative state (VS), minimally conscious state without language function (MCS−), and covert consciousness (CMD, cognitive-motor dissociation) had fewer intact default mode network (DMN) properties than did patients diagnosed with minimally conscious state with language function (MCS+) or post-traumatic confusional state (PTCS). Notably, DMN properties were completely absent in Patient 4 (P4), who had a behavioral diagnosis of VS but demonstrated covert consciousness on a functional magnetic resonance imaging (fMRI) motor imagery task. A yellow outline of the brain indicates an intact DMN property, defined by a Z-value (for correlations and anticorrelations) and percent of suprathreshold voxels (for deactivations) that is within the normal 2.5th-to-97.5th percentile of the healthy control group median. For example, P5 demonstrated intact DMN correlations while P6 demonstrated intact DMN correlations, anticorrelations, and deactivations. Color bars for correlations and anticorrelations represent Fisher-transformed Z values. The deactivation color bar represents the Z value for voxels surpassing a Z>3.1 and P=0.05 cluster-corrected threshold. All diagnoses are based on behavioral assessment with the Coma Recovery Scale-Revised, with the exception of the CMD diagnoses, which are based upon evidence of covert consciousness as described in Edlow et al., 2017 and Supplementary Materials.
Abbreviations: CMD cognitive motor dissociation, DMN default mode network, fMRI functional magnetic resonance imaging, L left, MCS− minimally conscious state without language function, MCS+ minimally conscious state with language function, P patient, PTCS post-traumatic confusional state, VS vegetative state
Contrary to our hypothesis, DMN preservation differed between patients with covert and overt consciousness in the ICU. Whereas all overtly conscious patients had at least one intact DMN property (range 1-3), covertly conscious patients had 0-2 intact DMN properties. Covertly conscious patients were, therefore, more similar to patients with a behavioral diagnosis of coma, VS, and MCS- than to overtly conscious patients. We also found that, like DMN correlations, DMN deactivations were present more frequently than anticorrelations, supporting prior evidence that anticorrelations are the last DMN property to reemerge (Threlkeld et al., 2018).
The presence of covert consciousness in the absence of intact DMN properties in one patient with a behavioral diagnosis of VS provides initial evidence that volitional modulation of brain activity may precede recovery of default brain networks. Elucidating the neuroanatomic basis for covert consciousness without an intact DMN requires further study in larger patient samples, but prior evidence suggests that differential thalamic connectivity may explain this phenomenon. Specifically, covert consciousness may be associated with disconnection of the thalamus from the primary motor cortex, which mediates overt behavioral output, but not from the supplementary motor and premotor cortices, which mediate covert motor imagery (Fernández-Espejo, Rossit, & Owen, 2015). This connectivity profile, in the context of thalamic disconnection from cortical DMN nodes (Soddu et al., 2012), could result in covert consciousness without an intact DMN. An alternative explanation is that the DMN was intact in patients with covert consciousness, but it remapped to different cortical locations in a manner not detected by our methods. Early remapping of default networks has not been demonstrated in patients with acute TBI, but such remapping has been reported in patients with chronic TBI (Lee, Polimeni, Price, Edlow, & McNab, 2018). Finally, because there is no ‘gold standard’ for detecting consciousness, it is possible that the diagnosis of covert consciousness was inaccurate. We took several measures to reduce the false positive rate for detection of covert consciousness, but misdiagnosis cannot be ruled out. In summary, although prior studies suggest a mechanistic link between consciousness and the DMN (Bodien et al., 2017; Norton et al., 2012, Threlkeld et al., 2018), our findings indicate that covert consciousness in the ICU may be dissociable from a preserved DMN.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by the NIH National Institute of Neurological Disorders and Stroke (K23NS094538), the James S. McDonnell Foundation, and National Institute on Disability, Independent Living and Rehabilitation Research (NIDILRR), Administration for Community Living (90DP0039, Spaulding-Harvard TBI Model System).
Role of the Funder/Sponsor: The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Additional Contributions: We thank the nursing staffs of the Massachusetts General Hospital Neurosciences ICU, Multidisciplinary ICU, and Surgical ICU. We are grateful to the patients and families in this study for their participation and support.
Abbreviations:
- CMD
cognitive motor dissociation
- CRS-R
Coma Recovery Scale-Revised
- DMN
default mode network
- ICU
intensive care unit
- IQR
interquartile range
- F
female
- fMRI
functional magnetic resonance imaging
- M
male
- MCS
minimally conscious state
- MCS−
minimally conscious state without language function
- MCS+
minimally conscious state with language function
- PTCS
post-traumatic confusional state
- TBI
traumatic brain injury
- VS
vegetative state
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None reported.
References
- Bodien YG, Chatelle C, & Edlow BL (2017). Functional networks in disorders of consciousness. Semin Neurol, 37(5), 485–502. doi: 10.1055/s-0037-1607310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu TT, … Laureys S. (2008). Intrinsic brain activity in altered states of consciousness: How conscious is the default mode of brain function? Ann N Y Acad Sci, 1129, 119–129. doi: 10.1196/annals.1417.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone JS, Ladurner G, Höller Y, Golaszewski S, Trinka E, & Kronbichler M (2011). Deactivation of the default mode network as a marker of impaired consciousness: An fmri study. PLoS One, 6(10), e26373. doi: 10.1371/journal.pone.0026373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, Chatelle C, Spencer CA, Chu CJ, Bodien YG, O’Connor KL, … Wu O. (2017). Early detection of consciousness in patients with acute severe traumatic brain injury. Brain, 140(9), 2399–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Espejo D, Rossit S, & Owen AM (2015). A thalamocortical mechanism for the absence of overt motor behavior in covertly aware patients. JAMA Neurol, 72(12), 1442–1450. doi: 10.1001/jamaneurol.2015.2614 [DOI] [PubMed] [Google Scholar]
- Giacino JT, & Kalmar K (1997). The vegetative and minimally conscious states: A comparison of clinical features and functional outcome. J Head Trauma Rehabil, 12(4), 36–51. [Google Scholar]
- Giacino JT, Kalmar K, & Whyte J (2004). The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch Phys Med Rehabil, 85(12), 2020–2029. [DOI] [PubMed] [Google Scholar]
- Lee S, Polimeni JR, Price CM, Edlow BL, & McNab JA (2018). Characterizing signals within lesions and mapping brain network connectivity after traumatic axonal injury: A 7 Tesla resting-state fMRI study. Brain Connect, 8(5), 288–298. doi: 10.1089/brain.2017.0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, . . Laureys S. (2010). Willful modulation of brain activity in disorders of consciousness. N Engl J Med, 362(7), 579–589. doi: 10.1056/NEJMoa0905370 [DOI] [PubMed] [Google Scholar]
- Norton L, Hutchison RM, Young GB, Lee DH, Sharpe MD, & Mirsattari SM (2012). Disruptions of functional connectivity in the default mode network of comatose patients. Neurology, 78(3), 175–181. doi: 10.1212/WNL.0b013e31823fcd61 [DOI] [PubMed] [Google Scholar]
- Schiff ND (2015). Cognitive motor dissociation following severe brain injuries. JAMA Neurology, 72(12), 1413. doi: 10.1001/jamaneurol.2015.2899 [DOI] [PubMed] [Google Scholar]
- Schnakers C, Vanhaudenhuyse A, Giacino J, Ventura M, Boly M, Majerus S, … Laureys S. (2009). Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol, 9, 35. doi: 10.1186/1471-2377-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soddu A, Vanhaudenhuyse A, Bahri MA, Bruno MA, Boly M, Demertzi A, … Noirhomme Q. (2012). Identifying the default-mode component in spatial IC analyses of patients with disorders of consciousness. Hum Brain Mapp, 33(4), 778–796. doi: 10.1002/hbm.21249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld ZD, Bodien YG, Rosenthal ES, Giacino JT, Nieto-Castanon A, Wu O, … Edlow BL (2018). Functional networks reemerge during recovery of consciousness after acute severe traumatic brain injury. Cortex. 106:299–308 doi: 10.1016/j.cortex.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, … Boly M. (2010). Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain, 133 161–171. doi: 10.1093/brain/awp313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JT, Pettigrew LE, & Teasdale GM (1998). Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: Guidelines for their use. J Neurotrauma, 15(8), 573–585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.