Abstract
Introduction:
Maternal smoking places the child at risk during pregnancy and postpartum Most women who quit smoking do so early when they first learn of pregnancy. Few low-income women quit once they enter prenatal care. The purpose of this study is to test in a clinical prenatal care setting the effectiveness of the Smoke-Free Moms intervention that provides pregnant women a series of financial incentives for smoking cessation.
Study design:
A prospective nonrandomized controlled trial that collected control population data of smoking cessation rates at each clincal visit during pregnancy and postpartm with usual smoking counseling in 2013–2014. In 2015–2016 the same data was collected during the implementation of the Smoke-Free Moms intervention of financial incentives. Data analysis occurred in 2017.
Setting/participants:
Women who were smoking at the first prenatal visit at four federally qualified health centers in rural New Hampshire.
Intervention:
All women received 5A’s smoking counseling from clinic staff. At each clinic visit, with point-of-care confirmed negative urinary cotinine, intervention women received gift cards.
Main outcome measures:
Cotinine confirmed smoking cessation without relapse: (1) during pregnancy and (2) smoking cessation in both pregnancy and postpartum.
Results:
Of 175 eligible pregnant women enrolled, 134 women were followed to the postpartum visit (Intervention n=66, Control n=68). The quit rates during pregnancy did not differ between groups (Intervention 36.4%, Control 29.4%, p=0.46). However, significantly more intervention mothers quit and continued as nonsmokers postpartum (Intervention 31.8%, Control 16.2%, p=0.04). In a logistic regression model including baseline sociodemographic, depressed mood, stress, and readiness to quit items, confidence in being able to quit predicted both cessation outcomes. The financial incentive intervention was an independent predictor of cessation in pregnancy through postpartum.
Conclusions:
Financial incentives with existing smoking cessation counseling by staff in low-income clinical prenatal programs led to cessation that continued during the postpartum period. Further study in larger populations is indicated.
INTRODUCTION
Maternal smoking is the leading preventable cause of adverse pregnancy outcomes in the U.S.1 Smoking increases the risk of pregnancy complications including pre-eclampsia, placental abnormalities, and premature rupture of membranes.2 Smoking in pregnancy places the infant at greater risk because of prematurity and low birth weight, birth defects, and higher rates of sudden infant death.3 Ongoing exposure of the infant to secondhand smoke results in significant respiratory morbidity.4 Women’s smoking rates overall, and in pregnancy, have slowly declined and rural and lower-income women continue to have higher rates of smoking and lower quit rates in pregnancy.5–7 In addition national birth certificate data from 2014 shows 10.1% women smoked just prior to pregnancy and 8.4% reporting smoking in pregnancy. Of these smokers in pregnancy about one in five women had quit between the first and third trimester.6
Pregnancy can serve as a catalyst for mothers to change health behaviors to benefit their child.8 Mothers alter diet, alcohol, and tobacco use in response to being pregnant. Most women who quit tobacco do so early in pregnancy.9,10 Spontaneous rates of quitting upon first learning they are pregnant, before receiving prenatal care, averaged 28% in low-income populations.11 After presenting for prenatal care, cessation rates with smoking cessation interventions are very low.10 In a review of 72 intervention studies, smoking cessation by late pregnancy of women presenting as smokers occurred in only 3%–6%12 despite a variety of prenatal counseling approaches. The only approach that had better results was the use of financial incentives (24% cessation).12
Two different successful approaches have been used in the U.S. to financially incentivize pregnant women to quit. The first, contingency management, is based on success with this approach with alcohol and illicit drug users.13 Smokers were randomized to receive closely spaced reinforcement with increasing financial rewards for biochemically confirmed cessation. Intervals progress from daily to weekly after 12 weeks and then biweekly until delivery.14 A second approach randomly provided women at Women, Infant, and Child supplemental nutrition programs with monthly vouchers varying from $25 to $50 until the 2-month postpartum visit for biochemically confirmed cessation.15 Providing financial incentives to a supporter of the mother as well did not change results.15 These trials showing efficacy were conducted by outside research staff. More recently single arm and randomized trials in the United Kingdom using contingency16 or intermittent rewards17 with much larger incentives (up to 400–752 £), have had last trimester quit rates of 20%–22%. A large RCT in a U.S. Medicaid population intervened from early pregnancy through the first year postpartum. Financial incentives (up to $500), provided primarily for engagement in smoking treatment visits and calls, had mixed results.18 Smoking cessation by birth had not improved, but women who participated in more calls in either arm had greater smoking cessation at 6 months postpartum. All three of these studies referred patients from a clinical prenatal setting to well-established external programs that provided prenatal smoking cessation treatment. These programs had the resources for multiple home visits and phone calls that are not available in many clinical settings. To the best of the authors’ knowledge, there are no effectiveness studies where financial incentives were used as a complement to the clinical practice’s usual onsite prenatal smoking cessation counseling.
The objective of this study is to explore the effectiveness of a smoking cessation program with immediate financial incentives conducted in rural prenatal clinics serving low-income women.
METHODS
Study Population
Smoke-Free Moms, was a behavioral economics–based smoking cessation intervention19 designed to complement cessation counseling and be provided by clinical staff during routine prenatal and postpartum visits. It was an adjunct to current smoking cessation counseling already provided by staff at prenatal clinics. Smoke-Free Moms was directed at the population of pregnant smokers in clinic settings serving low-income populations who had not spontaneously quit on learning of their pregnancy and were still smoking at the time of their first prenatal visit.
Smoke-Free Moms was evaluated in a nonrandomized controlled prospective study in four rural New Hampshire federally qualified health centers. During the planning stage staff indicated it would be problematic to randomize women living in small communities where intervention women would be receiving financial incentives while control subjects seen at the same time would not receive rewards. Thus, first at each site in 2013 to 2014 at consecutive first prenatal visits, the control population was recruited and followed through to the postpartum visit. This was followed by the intervention phase in 2015–2016 with the same data collected. From the volume of prenatal care patients and known smoking rates, the goal was to recruit 100 women per study arm. Sample size calculations, based on the literature12 with quit rate of 6% spontaneously vs 24% with incentives, it was determined that 58 women per group would be needed in the final groups (α=0.05, β=0.80). The women recruited were still smoking at their first prenatal visit.
At each site usual care provided to all women included personalized smoking cessation counseling at their entry visit utilizing the best practice 5 A’s approach with referral to the state quit-line when desired. The 5A’s counseling framework includes: Ask about tobacco use, Advise to quit, Assess willingness to quit, Assist to quit, and Arrange follow-up and support. Pharmacologic treatment for smoking cessation in pregnant women was not recommended by the U.S. Preventive Services Task Force in 2009 or 201420 and nicotine replacement therapy was not used for pregnant women at these sites. Staff followed up about smoking status and encouraged women to quit at each visit. No other statewide or local community smoking cessation initiatives for pregnant women were initiated during data collection.
After completion of informed consent approved by the authors’ institutional committee for the protection of human subjects, subjects completed baseline surveys. If a woman stated she was not smoking, urinary cotinine testing was immediately completed at any prenatal visit and the 6-to 8-week postpartum visit. Urine cotinine was selected as the most accurate and practical assessment.21 It was determined using a rapid urinary cotinine screening test.22,23 Rooming staff were trained in interpretation using a color end point that was similar to other point-of-care clinical tests such as rapid streptococcal tests. This cotinine test detects urinary cotinine levels of ≥200 ng/mL reflecting smoking up to 2–3 days prior to the test.
Control subjects were paid $5 each visit for testing their urine but not informed of the results. During the intervention phase, women were enrolled in the Smoke-Free Moms program at their first visit and informed they would receive a $25 gift card to Walmart® at each visit if not smoking and their cotinine test was negative. In addition, at the postpartum visit at 6 to 8 weeks, they received $50 gift card if not smoking and their cotinine test was negative. Women who said they had quit but were cotinine positive were not confronted about the discrepancy but encouraged to stay in the program and quit longer to receive a gift card at a future visit.
Measures
At enrollment all women completed a baseline survey that assessed the sociodemographic factors and self-reported smoking status. The motivational factor of readiness to quit was assessed with three questions: (1) intent to quit during pregnancy, (2) plan to quit in next 30 days, and (3) confidence in being able to quit if decided to quit smoking in the next 30 days. Expected social support from friends, family, and coworkers if she tried to quit, assessment of depressed mood (how much of the time felt downhearted and blue in the past month), and perceived stress were assessed. The four-item abbreviated Perceived Stress Scale24 was scored for responses from 1 to 5 (never to very often). Baseline survey items were from the Robert Wood Johnson Foundation’s Smoke-Free Families multisite implementation and dissemination project.25,26 Smoking status was defined by a confirming negative rapid urinary cotinine test among women who said they had quit.
Statistical Analysis
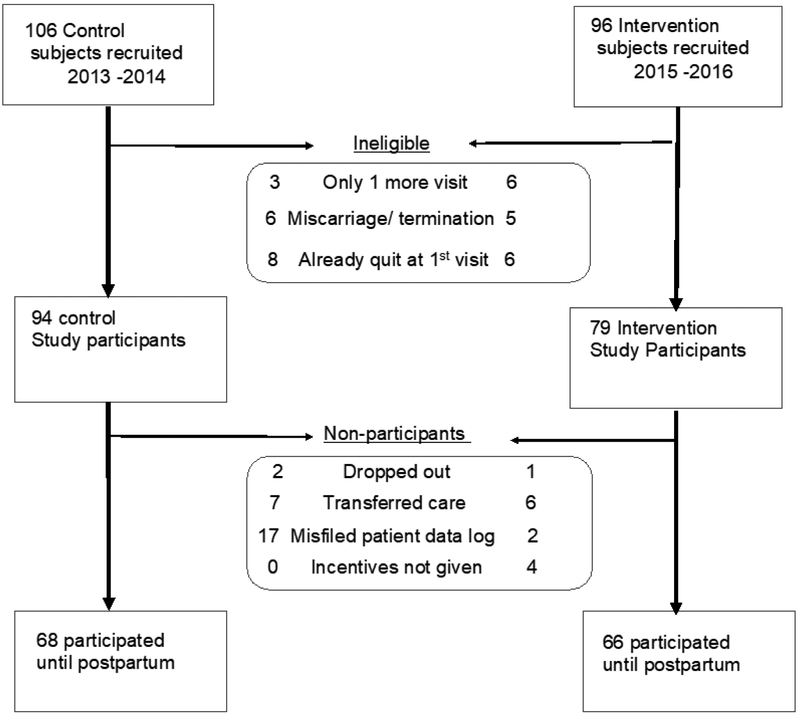
Data were tracked by subject identifier code only. Women were included in the analysis if they were smoking at their enrollment visit and had more than one follow-up prenatal visit before delivery. Initially clinic staff enrolled seven women who had already quit since learning of pregnancy and they were excluded. Nine women were seen only once after their enrollment visit. Cotinine logs were misfiled into medical records and could not be accessed for 19 subjects. Because of an interruption in the intervention protocol at one clinic during staff changes, four subjects were excluded because no financial incentives were provided. Three subjects dropped out. Thirteen women who transferred care and 14 with early miscarriage or termination of pregnancy were excluded. Details of which arm of the study these events occurred is provided in Figure 1. The final study population with data from first clinic visit through the postpartum visit was 64% (68/106) of consented control and 71% (66/92) of consented intervention subjects. Data were analyzed in 2017.
Figure 1.
CONSORT diagram.
The key outcomes assessed were: (1) quitting smoking without relapse during pregnancy and (2) smoking cessation in pregnancy that continued to the postpartum visit at 6–8 weeks. Baseline characteristics of each group for the key outcomes were first compared using chi-square and Fisher’s exact test, and Student’s t-test. The initial logistic regression analysis was conducted for both outcomes utilizing all baseline characteristics, practice site, and intervention status. Other than intervention status only variables in the initial logistic regression with p-values <0.2 were retained in the final multivariate adjusted logistic regression model.
For the above two key outcomes, an additional intention-to-treat analysis was conducted to confirm the findings. For this post-hoc analysis, mothers were also categorized as smokers with the following conditions: enrolled subjects with missing data, those who had quit upon learning of pregnancy prior to the first prenatal visit, those who withdrew, and those who had a miscarriage or termination or transferred care. Data were analyzed using SPSS, version 24.
RESULTS
At their first prenatal visit intervention and control subjects did not differ (Table 1). Maternal gestational age at this visit was similar (control 11.6 weeks [SD=6.6], intervention 12.6 weeks [SD=7], t-test, p=0.64). The baseline survey completed by 129 women showed that sociodemographic and insurance status, mood, and stress did not differ by intervention status. In both groups prior cigarette use was similar with reduced smoking frequency in the past week. Nearly all women reported planning to quit in the next month and nearly half reported being very confident they could quit in the next month. The number of prenatal visits did not differ between groups (Intervention 6.4 [SD=1.8], Control 5.9 [SD=2.3] visits, t-test, p=0.20).
Table 1.
Baseline Sociodemographic and Smoking-related Variables by Treatment Group (n=129)
| Characteristics | Control N (%) |
Intervention N (%) |
p-value |
|---|---|---|---|
| Births: none vs births at >20 weeks gestation | 29/64 (45.3) | 33/65 (50.8) | 0.48 |
| Years of education (mean/SD) | 11.6 ± 2.2 | 11.9 ± 2.2 | 0.38 |
| Gestational age (weeks) at first visit (mean/SD) | 11.6 ± 6.3 | 12.6 ± 7.0 | 0.37 |
| Caucasian race vs other | 62/64 (96.9) | 62/65 (95.4) | 0.56 |
| Private insurance vs Medicaid and uninsured | 7/64 (10.9) | 8/65 (12.3) | 0.81 |
| Married vs other | 9/64 (14.1) | 14/65 (21.5) | 0.45 |
| Depressed mood past montha | 9/64 (14.1) | 8/65 (12.3) | 0.80 |
| Perceived Stress Scoreb (mean/SD) | 9.62 ± 3.4 | 9.42 ± 2.7 | 0.71 |
| Support to quit from othersd | 51/64 (79.7) | 50/65 (76.9) | 0.83 |
| Cigarettes/day before pregnant (mean/SD) | 15.8 ± 7.5 | 17.2 ± 9.5 | 0.38 |
| Cigarettes/day in past 7 days (mean/SD) | 6.58 ± 5.8 | 7.68 ± 10.8 | 0.47 |
| Readiness to quit | |||
| Seriously thinking of quitting smoking during this Pregnancye | 63/64 (98.4) | 64/65 (98.5) | 0.37 |
| Plan to quit in the next 30 daysf | 61/64 (95.3) | 62/65 (95.3) | 0.98 |
| Confidence can quitc | 29/63 (46) | 28/65 (43.1) | 0.67 |
How much of the time, during the past month, have you felt downhearted and blue? (scoring: a lot, all the time, or a lot vs some or never).
Perceived Stress Scale (scoring: sum of four items range 4–20, scoring - high scores less stress).
If you decided to quit smoking during the next month, how confident are you that you could do it? (very vs somewhat, not very, or not at all).
If you tried to quit smoking, how much support or understanding do you think you would get from family, friends, and coworkers? (scoring; a lot vs some, not much, or none).
Are you seriously thinking about quitting smoking completely during this pregnancy (yes vs no).
Are you planning to quit smoking completely within the next 30 days (yes vs no).
Women’s smoking cessation patterns are summarized in Table 2. The average gestational age when women quit did not differ between the two groups (Control: 20.8 weeks [SD=9.8], Intervention: 18.9 weeks [SD=8.9], t-test, p=0.55). The proportion of mothers who quit smoking in pregnancy until delivery did not differ significantly (36.4% [24/66] Intervention vs 29.4% [20/68] Control, p=0.46). However, women in the intervention group were more likely to quit smoking and continue to not smoke to their postpartum visit (Intervention 32.3% [21/66] vs Control 16.2% [11/68], p=0.04). Examination of the impact of the intervention on smoking relapse rates in only the postpartum sample (n=44) showed similar results (Intervention 12.5% [3/24] vs Control 45% [9/20], p=0.016).
Table 2.
Smoking Cessation for Intervention Versus Control Pregnant Women (Control=68, Intervention=66)
| Smoking status | Control N (%) |
Intervention N (%) |
p-value Fisher’s exact test |
|---|---|---|---|
| During pregnancy | 0.46 | ||
| Quit until delivery | 20 (29.4) | 24 (36.4) | |
| Smokera | 48 (70.6) | 42 (63.6) | |
| Quit | 0.04 | ||
| During pregnancy until delivery | 9 (13.2) | 3 (4.5) | |
| During both pregnancy and postpartum | 11 (16.2) | 21 (32.3) |
Notes: Boldface indicates statistical significance (p<0.05). Percentages may not add to 100% due to rounding.
Includes: Quit but relapsed in pregnancy (control=1, Intervention=3, Relapse occurred after an average of 7.5 weeks), and Only quit at postpartum visit (control=1, Intervention=1).
The intention-to-treat analyses of all eligible subjects confirmed these findings. The proportion of women who quit during pregnancy did not differ significantly by intervention status (Intervention 30.4% [24/79] vs Control 21.3% [20/94], p=0.22). Women in the intervention were significantly more likely to quit in pregnancy through the postpartum visit (Intervention 26.9% [21/78] vs Control 11.7% [11/94], p=0.02).
The multivariate logistic regression final model (Table 3) shows the predictors for two outcomes: (1) quitting until delivery without relapse and (2) smoking cessation during pregnancy that continued to the postpartum visit. For every 1-point increase in the 4-point confidence score women were 2.47 times more likely to have quit smoking in pregnancy and 3.48 times more likely to have smoking cessation in both pregnancy and postpartum. Medicaid/uninsured at entry into care independently decreased the odds of quitting in pregnancy through postpartum (AOR=0.27) but not quit rates in pregnancy. In addition to these factors, the intervention group was an independent predictor (AOR=2.88) of quitting smoking in pregnancy to postpartum.
Table 3.
Final Logistic Regression Model of the Predictors of Smoking Cessation Outcomes (n=129)
| Variable | Quit in pregnancy without relapses |
Quit in pregnancy and through the postpartum visit |
||
|---|---|---|---|---|
| AOR (95% CI) | p-value | AOR (95% CI) | p-value | |
| Medicaid/uninsured ref: Private insurance | 0.54 (0.17, 1.69) | 0.29 | 0.27 (0.08, 0.92) | 0.037* |
| Education (years) | 1.14 (0.95, 1.38) | 0.16 | 1.21 (0.96, 1.53) | 0.10 |
| Prior births >20 weeks | 0.75 (0.48, 1.15) | 0.19 | 0.84 (0.51, 1.39) | 0.50 |
| Confidence can quita | 2.47 (1.27, 4.78) | 0.01* | 3.48 (1.58, 7.79) | 0.002** |
| Intervention ref: Control group | 1.36 (0.62, 2.98) | 0.44 | 2.88 (1.14, 7.3) | 0.026* |
Notes: Boldface indicates statistical significance (*p<0.05; **p<0.01). Variables in unadjusted model >0.2 that were removed in adjusted model: cigarettes pre-pregnancy, cigarettes in past 7 days at entry, thinking of quitting, plan to quit in next 30 days, support to quit, marital status, perceived stress, depressed mood in past month
AOR for each 1 point increase in confidence score from 1 to 4.
The program costs for cotinine testing and financial rewards were calculated for the intervention group. Point-of-care urinary cotinine tests ($2/test) were completed only if the mother said she had quit. The number of tests per mother averaged 5 (range, 2–9) for a cost of $10 per mother who said she had quit. The total cost of rewards was $3,700 (range, $50–$250) with an average of $148 per mother who had cotinine confirmed smoking cessation at any point in pregnancy or postpartum. These costs included mothers who quit but relapsed in pregnancy or quit postpartum. The number of women provided the incentive program for each woman who had smoking cessation that continued to the postpartum visit is 6.4 (number needed to treat). The total cost for an additional quit because of the intervention was $1,050 (number needed to treat x [average of rewards + tests]).
DISCUSSION
This study implemented financial incentives in addition to best practice smoking cessation counseling in low-income rural clinics. To the authors’ knowledge it is the first study using financial incentives, in addition to smoking cessation counseling, where the intervention occurred within the routine clinical prenatal care process without outside resources providing the incentives and counseling. This study found 5 A’s counseling and counseling with incentives had similar quit rates in pregnancy but that the addition of financial incentives resulted in more women whose smoking cessation persisted at 6–8 weeks postpartum. This study’s intervention cessation rates are consistent with other incentive studies with noncontingent vouchers14,15,17 where smoking cessation rates at delivery varied from 23% to 37% compared with this study’s 36% rate. Studies with postpartum follow-up at 2–3 months had cessation rates of 21%–33% compared with the current finding of 32%. In this study’s population, counseling alone resulted in better cessation rates (29%) than those found in earlier studies (9%–12%).
Since earlier perinatal smoking studies, there have been changing public policies and more public health messages about the risks of smoking. This may have resulted in nearly all smoking women in this study’s setting entering prenatal care intending to quit in the next 30 days compared with 64% in a similar population two decades ago.26 The impact of the intervention was strong after controlling for important baseline readiness to quit, social and psychosocial factors, whose impact has not been explored in previous incentive studies.14–18 It is important to note the key role of maternal confidence that she can quit, but not variation in supports from others to quit, on all smoking cessation outcomes. Unfortunately, the influence of age on the outcomes could not be explored because maternal age was not collected. In other studies intention to quit did not vary by age25 and the average age of women who both quit and relapsed was about 2 years younger, but age did not influence maintaining cessation postpartum.27,28
It was surprising to find in the regression model an independent strong impact of the incentives on sustained smoking cessation but not on quitting prior to delivery. The counseling provided to all patients may have a role. Psychosocial counseling interventions have some impact on quitting.29 Specifically the components of 5A’s have been shown to improve prenatal smoking cessation15,30,31 and endorsed as evidence-based best practices by the U.S. Preventive Services Task Force32 and the American College of Obstetrics and Gynecology.33 These efforts to widely implement 5A’s clinical counseling may be reflected in both the current results and in 2014 birth certificate data showing a 20% quit rate between the first and third trimester.34 Another possibility is that in this population, where all intended to quit, the clinical staff implementing the 5 A’s reinforced strategies to not smoke at all visits for both groups and this support was most effective in pregnancy for women with more confidence to quit. After having cessation rewarded in pregnancy, expectations of a financial reward may have then increased the motivation of intervention mothers to remain smoke free when new stressors occurred after birth. Because this was a pragmatic study where data collection was limited to not burden the clinical staff, the specifics of counseling session content at visits or a postpartum survey to explore these possible reasons is not available.
Limitations
These results were found in a rural white population. One of the sites cared for Hispanic patients, but most of these women were not smoking at their first prenatal visit. Although the quit rate in pregnancy favored the intervention (36.4% versus 29.4%), a larger randomized study is needed to determine if these differences were significant. In the future, this incentive approach could be studied in settings with ethnic and racial diversity and determine its applicability to urban settings. It may be helpful to vary incentives and determine if a greater incentive earlier in pregnancy could increase quitting in pregnancy. During immediate postpartum, relapse is common especially for women who did not spontaneously quit early.35 Although relapse postpartum was reduced, it was a limitation to not follow women further to see if it was sustained. Previously both maternal engagement in more postpartum counseling visits18 and a diaper incentive program for women who had quit have prevented relapse to 6 months postpartum.36 In the future, continuing postpartum incentives with counseling at well-child visits may prolong postpartum cessation.
It is important to consider these findings in light of the difficult challenge of prenatal and postpartum smoking. Despite extensive population-level primary prevention and cessation interventions addressing smoking in pregnancy, 9% of pregnant women smoke24,26 with higher rates in white low-income females. The prevalence of smoking in pregnancy is also higher in rural than urban women and did not decline from 2008 to 2016.6 Relapse in the postpartum period is not declining despite publicity about secondhand smoke.27 More widespread use of perinatal voucher incentives is possible in local hospital systems by partnership with local businesses and foundations. Although other studies with much larger incentives15–17 using external resources to deliver incentives and counseling can produce greater smoking cessation rates, this study has shown that with modest resources clinical practice sites can improve smoking cessation in pregnancy and beyond delivery.
CONCLUSIONS
This study demonstrates that clinical staff can incorporate cotinine testing and financial incentives into existing prenatal smoking cessation efforts and increase smoking cessation that lasts past delivery. The authors found that the program costs were reasonable. The average cost of incentives per woman who quit was similar to the cost of one clinical visit in the region. Counseling interventions may need to emphasize specific evidence-based approaches to enhance low confidence and provide extra support to women who present with low levels of confidence so that they can successfully quit smoking.
ACKNOWLEDGMENTS
Study data were managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Dartmouth College.37 REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources.
This publication was supported by The Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences of NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mund M, Louwen F, Klingelhoefer D, Gerber A. Smoking and pregnancy--a review on the first major environmental risk factor of the unborn Int J Environ Res Public Health. 2013;10(12):6485–6499. 10.3390/ijerph10126485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cnattinguis S The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(suppl 2):S125–S140. 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 3.Matthews TJ, MacDorman MF. Infant mortality statistics from the 2010 period linked birth/infant death data set. Natl Vital Stat Rep. 2013;62(8):1–26. [PubMed] [Google Scholar]
- 4.Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics. 1998;101(2):e8 10.1542/peds.101.2.e8. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Hansen AR, McGalliard Z, Gover L, Yan F, Zhang J. Trends in smoking and smoking cessation during pregnancy from 1985 to 2014, racial and ethnic disparity observed from multiple national studies. Matern Child Health J. 2018;22(5):685–693. 10.1007/s10995-018-2437-x. [DOI] [PubMed] [Google Scholar]
- 6.Nighbor TD, Doogan NJ, Roberts ME, et al. Smoking prevalence and trends among a U.S. national sample of women of reproductive age in rural versus urban settings. PLoS ONE. 2018;13(11):e0207818 10.1371/journal.pone.0207818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riaz M, Lewis S, Naughton F, Ussher M. Predictors of smoking cessation during pregnancy: a systematic review and meta-analysis. Addiction. 2017;113(4):610–622. 10.1111/add.14135. [DOI] [PubMed] [Google Scholar]
- 8.Davis AM, Wambach KA, Nelson EL, et al. Health behavior change in pregnant women: a two-phase study. Telemed J E Health. 2014;20(12):1165–1169. 10.1089/tmj.2013.0374. [DOI] [PubMed] [Google Scholar]
- 9.Heil SH, Herrmann ES, Badger GJ, Solomon LJ, Bernstein IM, Higgins ST. Examining the timing of changes in cigarette smoking upon learning of pregnancy. Prev Med. 2014;68:58–61. 10.1016/j.ypmed.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong VT, England LJ, Dietz PM, Asare LA. Smoking patterns and use of cessation interventions during pregnancy. Am J Prev Med. 2008;35(4):327–333. 10.1016/j.amepre.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Ockene JK, Ma YS, Zapka JG, Pbert LA, Goins KV, Stoddard AM. Spontaneous cessation of smoking and alcohol use among low-income pregnant women. Am J Prev Med. 2002;23(3):150–159. 10.1016/S0749-3797(02)00492-0. [DOI] [PubMed] [Google Scholar]
- 12.Lumley J, Chamberlian C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009;(3):CD001055 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 14.Higgins ST, Washio Y, Lopez AA, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med. 2014;68:51–57. 10.1016/j.ypmed.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donatelle R, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine Tob Res. 2004;6(suppl 2):S163–S179. 10.1080/14622200410001669196. [DOI] [PubMed] [Google Scholar]
- 16.Ierfino D, Mantzari E, Hirst J, Jones T, Aveyard P, Marteau TM. Financial incentives for smoking cessation in pregnancy: a single-arm intervention study assessing cessation and gaming. Addiction. 2015;110(4):680–688. 10.1111/add.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tappin D, Bauld L, Purves D, et al. Financial incentives for smoking cessation in pregnancy: randomised controlled trial. BMJ. 2015;350:h134 10.1136/bmj.h134. [DOI] [PubMed] [Google Scholar]
- 18.Baker TB, Fraser DF, Kobinsky K, et al. A randomized controlled trial of financial incentives to low income pregnant women to engage in smoking cessation treatment: effects on post-birth abstinence. J Consult Clin Psychol. 2018;86(5):464–473. 10.1037/ccp0000278. [DOI] [PubMed] [Google Scholar]
- 19.Sigmon SC, Patrick ME. The use of financial incentives in promoting smoking cessation. Prev Med. 2012;55(suppl):S24–S32. 10.1016/j.ypmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agency for Healthcare Research and Quality. Five major steps to intervention (The “5A’s”). www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.pdf. Published 2012. Accessed February 15, 2019.
- 21.Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health. 1987;77(11):1435–1438. 10.2105/AJPH.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MedicalDisposable.US. Nicotine – Cotinine Test. www.medicaldisposables.us/urine-nicotine-testing-kit-s/1830.htm. Accessed February 15, 2019.
- 23.Parker DR, Lasater TM, Windsor R, Wilkins J, Upegui DI, Heimdal J. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res. 2001;4(3):305–309. 10.1080/14622200210142715. [DOI] [PubMed] [Google Scholar]
- 24.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 25.The Robert Wood Johnson Foundation. Smoke-Free Families: State of the science capstone meeting. The Robert Wood Johnson Foundation; 2006. [Google Scholar]
- 26.Ershoff DH, Solomon LJ, Dolan-Mullen P. Predictors of intentions to stop smoking early in prenatal care. Tob Control. 2000;9(suppl III):iii41–iii45. 10.1136/tc.9.suppl_3.iii41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scherman A, Tolosa JE, McEvoy C. Smoking cecssation in pregnancy: a continuing challenge in the United States. Ther Adv Drug Saf. 2018;9(8):457–474. 10.1177/2042098618775366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockhill KM, Tong VT, Farr SL, Robbins CL, D’Angelo DV, England LJ. Postpartum smoking relapse after quitting during pregnancy: pregnancy risk assessment monitoring system, 2000–2011. J Womens Health (Larchmt). 2016;25(5):480–488. 10.1089/jwh.2015.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamberlain C, O’Mara-Eves A, Porter J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev. 2017;2:CD001055 10.1002/14651858,CD001055,pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobacco Use and Dependence Guideline Panel.. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: HHS; www.ncbi.nlm.nih.gov/books/NBK63952/. Published May 2008. Accessed February 15, 2019. [Google Scholar]
- 31.Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer-delivered brief intervention and low-intensity contigency managmement for smoking during pregnancy. Nicotine Tob Res. 2012;14(3):351–360. 10.1093/ntr/ntr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preventive US Services Task Force. Counseling and interventions to prevent tobacco use and tobacco caused disease in adults and pregnant women: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150(8):551–555. https://doi.org/10,7326/0003-4819-150-8-200904210-00009. [DOI] [PubMed] [Google Scholar]
- 33.Committee on Underserved Women, Committee on Obstetric Practice. Committee Opinion No. 721: Smoking cessation during pregnancy. Obstet Gynecol. 2017;130(4):e200–e204. 10.1097/AOG.0000000000002353. [DOI] [PubMed] [Google Scholar]
- 34.Curtin SC, Matthews TJ. Smoking prevalence and cessation before and during pregnancy: data from the birth certificate, 2014. Natl Vital Stat Rep. 2016;65(1):1–14. [PubMed] [Google Scholar]
- 35.Ma Y, Goins KV, Pbert L, Ockene JK. Predictors of smoking cessation in pregnancy and maintenance postpartum in low-income women. Matem Child Health J. 2005;9(4):393–402. 10.1007/s10995-005-0020-8. [DOI] [PubMed] [Google Scholar]
- 36.Gadomski A, Adams L, Tallman N, Krupa N, Jenkins P. Effectiveness of a combined prenatal and postpartum smoking cessation program. Matern Child Health J. 2011;15(2): 188–197. 10.1007/s10995-010-0568-9. [DOI] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]