ABSTRACT
Neutrophils release branched-chain (valine, isoleucine, leucine), aromatic (tyrosine, phenylalanine) and positively charged free amino acids (arginine, ornithine, lysine, hydroxylysine, histidine) when adhere and spread onto fibronectin. In the presence of agents that impair cell spreading or adhesion (cytochalasin D, fMLP, nonadhesive substrate), neutrophils release the same amino acids, except for a sharp decrease in hydroxylysine and an increase in phenylalanine, indicating their special connection with cell adhesion. Plasma of patients with diabetes is characterized by an increased content of branched-chain and aromatic amino acids and a reduced ratio of arginine/ornithine compared to healthy human plasma. Our data showed that the secretion of neutrophils, regardless of their adhesion state, can contribute to this shift in the amino acid content.
Abbreviations: BCAAs: branched-chain amino acids; Е2: 17β-estradiol; LPS: lipopolysaccharide from Salmonella enterica serovar Typhimurium; fMLP: N-formylmethionyl-leucyl-phenylalanine.
KEYWORDS: Neutrophil, adhesion, secretion, free amino acids, diabetes, cardiovascular disease
Introduction
In experimental diabetes mellitus in rats, as well as in the study of autopsy material of patients with diabetes mellitus, histological sections showed that the retinal capillaries are filled with monocytes and neutrophils [1,2]. It was also established that in neutrophils isolated from the blood of patients with diabetes mellitus, the expression of integrins was increased [3]. The authors of many modern studies consider the integrin-dependent adhesion of neutrophils to the vessel walls and the associated secretion of aggressive bactericidal agents as a cause of the development of early stages of retinopathies [4–6] and nephropathies [7,8] in patients with diabetes mellitus. Vascular complications upon reperfusion after ischemia develop also as a consequence of pathological adhesion of neutrophils to the vessel walls [9].
Proteome analysis revealed that control neutrophils upon adhesion to fibronectin, which was used as a model of integrin-dependent adhesion, secreted lactoferrin, myeloperoxidase, albumin, neutrophil gelatinase associated lipocalin, S100A8 and S100A9 proteins and lysozyme [10]. In the presence of classical secretory stimuli such as peptide fMLP or LPS neutrophils released metalloproteinases MMP-8 and MMP-9 in addition to proteins secreted by control cells. LPS stimulated also secretion of primary granule bactericides cathepsin D and defensins [11]. The composition of neutrophil protein secretion in the attachment to fibronectin is controlled by hormones [12]. Insulin, a hormone that reduces vascular complications in metabolic disorders [13–15], initiated the secretion of metalloproteinases MMP-8 and MMP-9. Glucagon, a hormone that plays a significant role in the pathophysiology of diabetes [16–18], caused the release of the aggressive bactericidal agent cathepsin G.
In the present work, we studied the secretion of free amino acids by human neutrophils. The development of diabetes is accompanied by a significant change in the levels of free amino acids in the plasma of patients. It was shown that a wide range of amino acids, including leucine, isoleucine, valine, phenylalanine, tyrosine, is significantly increased in the blood of patients with type 1 [19] or type 2 [20] diabetes mellitus. Recent epidemiological studies consider the elevated level of branched-chain (valine, isoleucine and leucine) and aromatic (phenylalanine and tyrosine) amino acids in the blood of patients as highly significant predictors of future diabetes [21,22]. However, the physiological causes that initiate these shifts in the composition of amino acids are unknown. Using amino acid analysis, we studied the secretion of free amino acids by human neutrophils incubated over fibronectin-coated substrata under the control conditions, in the presence of hormones (insulin, glucagon or E2), inflammatory stimuli (LPS, fMLP), actin depolymerizing mold alkaloid cytochalasin D or during incubation over nonadhesive substrata.
Materials and methods
Materials
Ficoll-Paque was obtained from Pharmacia (Uppsala, Sweden). Fibronectin was from Calbiochem (La Jolla, USA). Bicarbonate-free Hank’s solution, Ca2+-free Dulbecco PBS, insulin, glucagon, 17ß-estradiol (E2), cytochalasin D, fMLP (N-formylmethionyl-leucyl-phenylalanine), LPS (lipopolysaccharide from Salmonella enterica serovar Typhimurium) and cytochalasin D were obtained from Sigma (Steinheim, Germany). Analytical chromatography conditions: eluent MCI Buffer L-8800-PH-1–4 and ninhydrin coloring solution kit for Hitachi 29970501 (Wako Chemicals GmbH, USA).
Neutrophil isolation
Neutrophils were obtained from the blood of healthy volunteers. All experimental procedures were approved by the Ethics Committee of the A. N. Belozersky Institute. Erythrocytes were precipitated with 3 % dextran T-500 at 20º C. Neutrophils were isolated from the plasma by centrifugation via Ficoll-Paque (density 1,077 g/ml). Hypotonic lysis was used to eliminate the remaining red blood cells. Neutrophils were washed and stored prior to the experiment in Dulbecco’s PBS containing 1 mg/ml glucose (without CaCl2). The purity of the neutrophil fraction was 96–97 %, viability 98–99 %.
Adhesion of neutrophils to fibronectin-coated plates and incubation over a nonadhesive substrate
Six-well culture plates were coated with fibronectin for 2 hours of incubation in Hank’s solution containing 5 μg/mL fibronectin at room temperature and washed. Neutrophils adhered to the protein-coated wells (3 × 106 cells in 1.3 mL per well) in Hank’s solution containing 10 mM HEPES (pH 7.35) for 20 minutes at 37º C. Insulin (0.1 μM), glucagon (0.1 μM), or 17β-estradiol (0.1 μM), cytochalasin D (10 μg/mL), fMLP (1 μM), or LPS (10 μg/mL) were added to the cells prior to plating. After the incubation, the extracellular medium was sampled. Aliquots from three similar wells combined.
Neutrophils were also incubated in Hanks’ solution at the same temperature and at the same time over the non-adhesive substrate, polypropylene tubes that usually used for the isolation of neutrophils. After incubation, the neutrophils were removed by centrifugation for 5 minutes at 1000 rpm and extracellular medium was sampled.
Preparation of extracellular media samples for amino acid analysis
Inhibitors of metalloproteinase (EDTA, 5 mM), serine (PMSF, 200 μM), cysteine (E64, 10 μM) and myeloperoxidase (sodium azide, 0.025%) were immediately added to all extracellular media samples, which were then centrifuged at 2000 for complete removal of nonaherent neutrophils. After concentrating the extracellular media samples with Centrivap Concentrator Labconco (USA), the proteins were precipitated with sulfosalicylic acid (4.4%) and removed by centrifugation for 30 min at 18,000 g. The supernatants were collected and centrifuged through Vivaspin 500 membrane ultrafilters Membrane 3000 PES MWCO (Sartorius, Germany).
Preparing of samples of donor plasma for amino acid analysis
Plasma was obtained from citrate-anticoagulated freshly prepared donor blood. Blood cells were removed by centrifugation for 10 minutes at 2000 g. Proteins were precipitated with sulfosalicylic acid (4.4%) and removed by centrifugation for 10 min at 10,000 g.
Amino acid analysis of samples
The amino acid analysis was conducted on an L-8800 amino acid analyzer (Hitachi, Tokyo, Japan) with an electronic heating bath and two single-channel colorimeters according to the manufacturer’s user manual (Hitachi High-Technologies Corporation, Japan, 1998). The prepared samples of extracellular medium or plasma were separated on an 2622SC-PH ion-exchange column (Hitachi, Ltd., P/N 855–4506, 4.6*60 mm) by step gradient of four sodium-acetate buffers at an elution rate 0.4 ml/min and a thermostating column at 57° C. To calibrate the system, the Amino Acid Standard (AA-S-18 −5ML analytical standard, SIGMA, product code 1001357972), 2 nmol of each, were used. Post column derivatization (136° C, flow rate 0.35 mL/min) was performed using a mix of equal volumes of ninhydrin buffer R2 and ninhydrin solution R1 (Wako Pure Chemical Industries, P/N 298–69601). The stained products were detected by measuring the absorbance at 570 nm for all amino acids except proline and at 440 nm for proline. MultiChrom for Windows software (Ampersand Ltd., Moscow, Russia) was used for processing the chromatographic data.
Statistics
Results are reported as mean ± SEM. Analysis of the statistical significance was evaluated using a two-way ANOVA with a Tukey’s multiple comparisons test using GraphPadPrism7 software. *- p values of less than 0.05 were considered significant.
Scanning electron microscopy
The coverslips were covered with fibronectin for 2 hours incubation at room temperature in a buffer containing 5 μg/ml fibronectin and washed. Neutrophils adhered to the fibronectin-coated cover slip (3 × 106 cells in 2 ml per well) during 20 minutes incubation in a Hanks solution containing 10 mM HEPES (pH 7.35) at 37° C. LPS, fMLP and cytochalasin D were added before plating. The cells were then fixed in 2.5 % glutaraldehyde in Hanks buffer without Ca2+ or Mg2+ ions, but containing metalloproteinase (5 mM EDTA) and serine proteases (0.5 mM PMSF) inhibitors and 10 mM HEPES at pH 7.3. The cells were additionally fixed with a 1 % solution of osmium tetroxide in 0.1 M sodium cacodylate containing 0.1 M sucrose at pH 7.3. After this, the cells were dehydrated in a series of acetones (10–100 %) and dried in a Balzer apparatus at a critical point with liquid CO2 as the transition liquid. The samples were coated with gold/palladium sputter and examined at 15 KV using a Scanning Electron Microscope Camscan S-2.
Results and discussion
The control neutrophils were attached and flattened on fibronectin for 20 minutes of incubation. Amino acid analysis showed that the neutrophils secretion during this time is characterized by a stable profile of free amino acids (Figure 1(a)), which includes eight standard amino acids such as branched chain (valine, isoleucine and leucine), aromatic (tyrosine and phenylalanine) and positively charged amino acids (arginine and its metabolite ornithine, lysine and its metabolites hydroxylysine and histidine). The number of free amino acids secreted by neutrophils is limited in comparison to the number of free amino acids found in the blood plasma (Figure 1(b)). Human plasma included 20 standard amino acids and was enriched with glutamate, alanine, valine and lysine. The profile of amino acids in human plasma, presented in our work, basically coincided with the profile of amino acids in the plasma of healthy donors determined by tandem mass spectrometry with liquid chromatography (LC-MS/MS) [19]. In this work Lanza and coauthors demonstrated that in insulin-deprived individuals with diabetes type 1the level of 5 free amino acids in plasma was significantly increased (leucine, 2 fold, isoleucine, 2 fold, valine, 1.6 fold, phenylalanine, 1.2 fold, tyrosine, 1.1 fold). The elevated levels of these amino acids in the blood of patients are now seen as precursors of the development of diabetes in the future [21,22].
Figure 1.
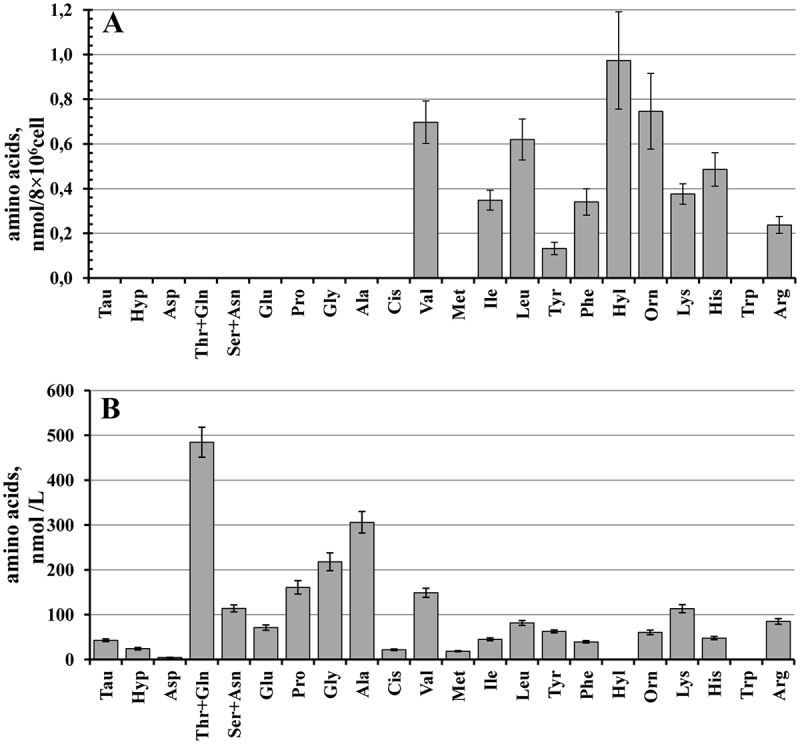
Comparison of free amino acid profiles of neutrophil secretion upon adhesion to fibronectin and human plasma. (a) Human neutrophils were attached to fibronectin-coated substrata for 20 min under control conditions. The number of amino acids detected (mean ± SEM) is presented as nanomoles per cell number. Amino acid profiles were obtained by summing the results of seven independent experiments. (b) The profile of free amino acids in human plasma. The amount of detected amino acids (mean ± SEM) is presented as nanomoles per liter. Amino acid profiles were obtained by summing the results of twenty independent experiments.
Neutrophils release positively charged arginine and its metabolite ornithine, histidine, lysine and its metabolite hydroxylysine during adhesion to fibronectin (Figure 1(a)). Arginine is the common substrate for both nitric oxide (NO) synthases and arginases [23]. NO synthases produces NO and citrulline from arginine. Arginase converts the amino acid to ornithine. Increase in arginase activity has been reported in a variety of disease conditions characterized by vascular dysfunctions such as diabetes [24]. Because NO synthase and arginase compete for the same substrate (L-arginine), excessive arginase can affect the synthesis of NO, an important vasoactive substance derived from the endothelium, which is important for vascular health and homeostasis [25]. Recently L-arginine divided by L-ornithine and L-citrulline (global arginine bioavailability ratio), as well as L-arginine/L-ornithine ratio has been introduced as a potential parameter that is reduced in subjects with diabetes or cardiovascular diseases [26,27]. In our experiments, the arginine/ornithine ratio in healthy human plasma was 1.54 ± 0.13 (n = 20) (Figure 1 B), while in neutrophil secretion it was 0.39 ± 0.07 (n = 7) (Figure 1(a)). These data suggest that neutrophil secretion products сan contribute to the reduction of the arginine/ornithine ratio in plasma of patients with metabolic disorders.
Positively charged histidine is also present in the amino acid composition of neutrophil secretion (Figure 1(a)). Neutrophils have some ability to produce histamine from histidine [28]. Histamine, a vital inflammatory agent in immune responses, can initiate the development of vascular complications arising from the secretion of neutrophils.
The amount of hydroxylysine, lysine metabolite, was two to three times higher than the amount of lysine in the secretion of neutrophils (Figure 1(a)). Hydroxylysine was not detected in the healthy human plasma in our experiments (Figure 1(b)) or was observed in trace amounts in the experiments of Lanza and co-authors [19]. Lysyl hydroxylase is a multifunctional protein that localizes to the endoplasmic reticulum. This enzyme plays an essential role in the extracellular matrix remodeling mainly via the hydroxylation of lysine residues of fibrillary collagens. Lysyl hydroxylase is also secreted into the extracellular space and there modifies extracellular matrix proteins thus affecting the adhesion and metastatic properties of cells [29,30]. All these data point to the special role of lysine metabolism, mediated by lysyl hydroxylase, in the interactions of neutrophils with extracellular matrix.
To determine the factors controlling the secretion of amino acids by neutrophils, we studied the effect of hormones (insulin, E2 and glucagon), natural neutrophil activators such as inflammatory stimuli LPS and fMLP, and microbial alkaloid cytochalasin D on the profile of amino acids released by neutrophils in incubation over fibronectin-coated substrata. Insulin significantly stimulated the amount of secreted hydroxylysine, but had no significant effect on the release of other amino acids (Figure 2). The steroid hormone E2 also supports vascular health and reduces the infiltration of neutrophils into the myocardium during reperfusion after ischemia, presumably through a fast, non-nuclear pathway [31,32]. Neither E2 nor glucagon, an insulin antagonist, altered the amino acid profile, and initiated only minor changes in the number of individual amino acids (Figure 2). LPS also had no significant effect on the profile of amino acid secretion by neutrophils in adhesion to fibronectin (Figure 3). Neither LPS (Figure 4), nor the tested hormones [12] notably affected the morphology of the neutrophils that were attached and spread onto fibronectin during incubation.
Figure 2.
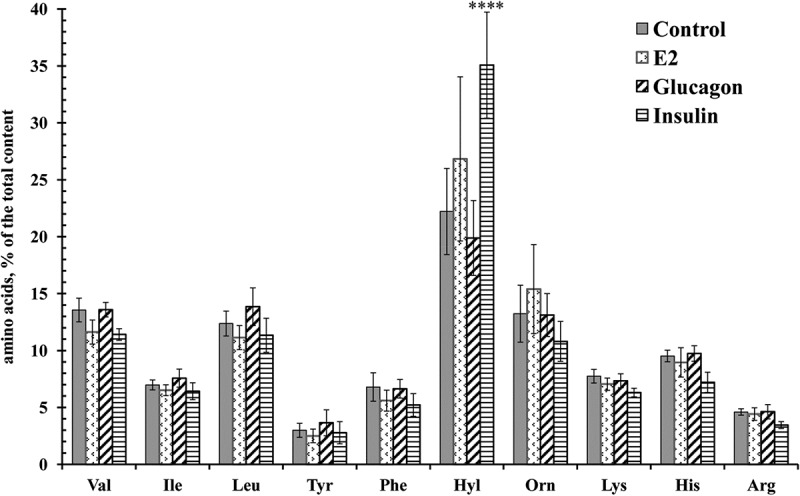
Free amino acid composition of secretion of the neutrophils during adhesion to fibronectin in the presence of hormones. Human neutrophils were attached to fibronectin-coated substrata for 20 min under control conditions or in the presence of 0.1 μM E2, insulin, or glucagon. The amount of amino acid is represented as a percentage of the total content of the detected free amino acids (mean ± SEM). Amino acid profiles were obtained by summing the results of four independent experiments. ****- significant differences when compared to the value for the same amino acid in the control cells (P < 0.0001).
Figure 3.
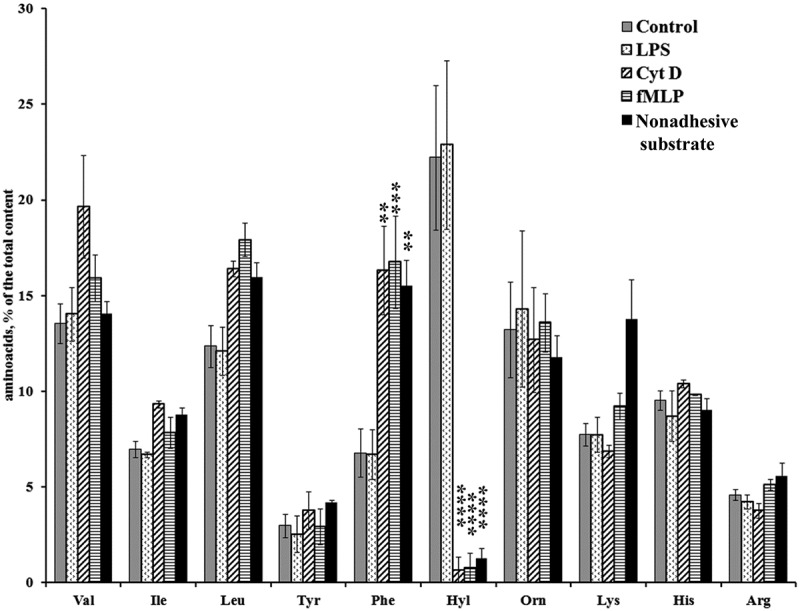
Free amino acid composition of secretion of the neutrophils during incubation over fibronectin-coated substrata in the presence of LPS, fMLP, cytochalasin D and during incubation over nonadhesive substrata. Human neutrophils were incubated over fibronectin-coated substrata for 20 min under control conditions or in the presence of LPS (10 μg/mL), fMLP (1 μM), and cytochalasin D (10 μg/mL) or incubated at the control conditions for the same time over polypropylene plastic. The amount of amino acid is represented as a percentage of the total content of the detected free amino acids (mean ± SEM). Amino acid profiles were obtained by summing the results of three independent experiments. *- significant differences when compared to the value for the same amino acid in the control cells (** – P < 0.001; *** – P < 0.0006; ****- P < 0.0001).
Figure 4.
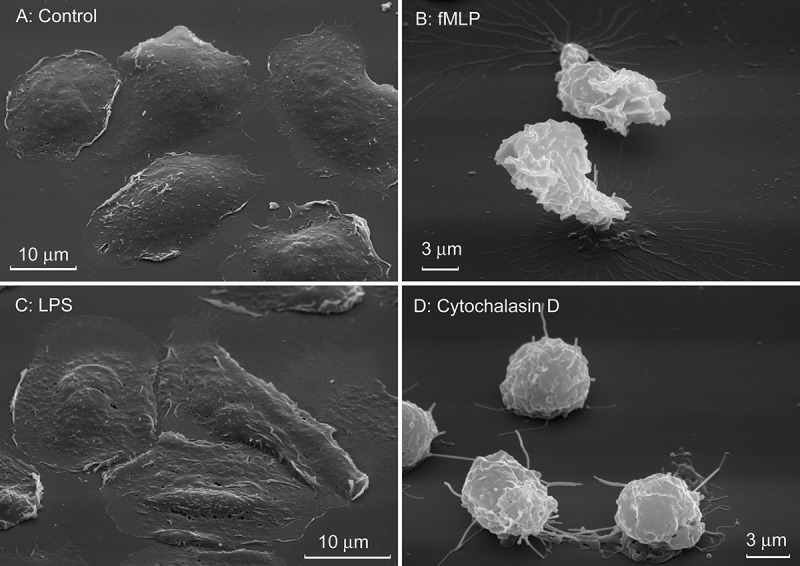
Effect of LPS, fMLP and cytochalasin D on the morphology of human neutrophils attached to fibronectin. Scanning electron microscopy images of neutrophils that were attached to fibronectin for 20 min in control conditions or in the presence of LPS (10 μg/mL), fMLP (1 μM), and cytochalasin D (10 μg/mL). Pictures represent typical images observed in two independent experiments.
The peptide fMLP and cytochalasin D, the agent depolymerizing actin, caused a strong decrease in the hydroxylysine content and increased the content of phenylalanine (Figure 3). Previously fMLP and cytochalasin D have been shown to affect protein secretion by neutrophils during incubation over fibronectin in the opposite way: fMLP stimulates the secretion of MMP-9, and cytochalasin D inhibits the secretion of MMP-9 and initiates the secretion of the primary bactericidal granule, such as cathepsin G and defensins [11,33]. Due to what general property, these two agents can similarly change the profile of amino acid secretion by neutrophils?
Scanning electron microscopy showed that fMLP and cytochalasin D impaired attachment and blocked the spreading of neutrophils on fibronectin (Figure 4), indicating that secretion of hydroxylysine and adhesion and spreading of the cells are closely related events. Moreover, the composition of secretion of neutrophils incubated over polypropylene plastic, nonadhesive instead of fibronectin-coated substrate, at the same conditions mainly coincided with secretion of fMLP- or cytochalasin D-treated neutrophils (Figure 3). These data indicate, at first, that the secretion of branched-chain, aromatic and positively charged amino acids (except of hydroxylysine) by neutrophils did not depend on adhesion to fibronectin, but is a characteristic property of neutrophils. Secondly, cell adhesion, which includes both cell attachment and spreading, specifically initiates secretion of hydroxylysine, metabolite of lysine produced by lysyl hydroxylase. The production of hydroxylysine rises in the presence of insulin (Figure 2). The specific role of lysyl hydroxylase in neutrophil adhesion and spreading onto solid substrata is confirmed by inhibition of cellular spreading by minoxidil, an inhibitor of lysyl hydroxylase [34].
Neutrophils constitute the majority (60%) of leukocytes in the blood. The profile of free amino acid secretion of neutrophils includes and is limited by branched-chain, aromatic and positively charged amino acids. Branched-chain and aromatic amino acids are considered now as the precursors of diabetes. In the secretion of neutrophils, positively charged ornithine predominated in comparison with arginine. This can contribute to the reduction of the arginine/ornithine ratio in plasma, which is typical for patients with diabetes or cardiovascular diseases. Taken together our data indicate that secretion of neutrophils can contribute to the shift of amino acid content in the plasma of patients with metabolic disorders. The role of hydroxylysine secreted by neutrophil upon adhesion in development of vessel complications remains to be elucidated.
Funding Statement
This work was supported by a grant from the Russian Foundation of Basic Research (16-04-00670).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].McLeod DS, Lefer DJ, Merges C, et al. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol. 1995;147:642–653. [PMC free article] [PubMed] [Google Scholar]
- [2].Schroder S, Palinski W, Schmid-Schonbein GW.. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991;139:81–100. [PMC free article] [PubMed] [Google Scholar]
- [3].Barouch FC, Miyamoto K, Allport JR, et al. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci. 2000;41:1153–1158. [PubMed] [Google Scholar]
- [4].Hirata F, Yoshida M, Niwa Y, et al. Insulin enhances leukocyte-endothelial cell adhesion in the retinal microcirculation through surface expression of intercellular adhesion molecule-1. Microvasc Res. 2005;69:135–141. [DOI] [PubMed] [Google Scholar]
- [5].Mastej K, Adamiec R. Neutrophil surface expression of CD11b and CD62L in diabetic microangiopathy. Acta Diabetol. 2008;45:183–190. [DOI] [PubMed] [Google Scholar]
- [6].Patel N. Targeting leukostasis for the treatment of early diabetic retinopathy. Cardiovasc Hematol Disord Drug Targets. 2009;9:222–229. [DOI] [PubMed] [Google Scholar]
- [7].Takahashi T, Hato F, Yamane T, et al. Increased spontaneous adherence of neutrophils from type 2 diabetic patients with overt proteinuria: possible role of the progression of diabetic nephropathy. Diabetes Care. 2000;23:417–418. [DOI] [PubMed] [Google Scholar]
- [8].Fardon NJ, Wilkinson R, Thomas TH. Abnormalities in primary granule exocytosis in neutrophils from Type I diabetic patients with nephropathy. Clin Sci. 2002;102:69–75. [PubMed] [Google Scholar]
- [9].Schofield ZV, Woodruff TM, Halai R, et al. Neutrophils - A key component of ischemia reperfusion injury. Shock. 2013;40:463–470. [DOI] [PubMed] [Google Scholar]
- [10].Galkina SI, Fedorova NV, Serebryakova MV, et al. Proteome analysis identified human neutrophil membrane tubulovesicular extensions (cytonemes, membrane tethers) as bactericide trafficking. Biochim Biophys Acta. 2012;1820:1705–1714. [DOI] [PubMed] [Google Scholar]
- [11].Galkina SI, Fedorova NV, Serebryakova MV, et al. Mold alkaloid cytochalasin D modifies the morphology and secretion of fMLP-, LPS-, or PMA-stimulated neutrophils upon adhesion to fibronectin. Mediators Inflamm. 2017;2017:4308684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fedorova NV, Ksenofontov AL, Serebryakova MV, et al. Neutrophils release metalloproteinases during adhesion in the presence of insulin, but cathepsin G in the presence of glucagon. Mediators Inflamm. 2018;2018:1574928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Langouche L, Vanhorebeek I, Vlasselaers D, et al. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115:2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nathan DM, Cleary PA, Backlund JY, et al. Complications trial/epidemiology of diabetes, G. complications study research, intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li J, Wu F, Zhang H, et al. Insulin inhibits leukocyte-endothelium adherence via an Akt-NO-dependent mechanism in myocardial ischemia/reperfusion. J Mol Cell Cardiol. 2009;47:512–519. [DOI] [PubMed] [Google Scholar]
- [16].Lefebvre PJ, Paquot N, Scheen AJ. Inhibiting or antagonizing glucagon: making progress in diabetes care. Diabetes Obes Metab. 2015;17:720–725. [DOI] [PubMed] [Google Scholar]
- [17].Lee Y, Wang MY, Du XQ, et al. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang MY, Yan H, Shi Z, et al. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc Natl Acad Sci U S A. 2015;112:2503–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lanza IR, Zhang S, Ward LE, et al. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PloS one. 2010;5:e10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Suhre K, Meisinger C, Doring A, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PloS one. 2010;5:e13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Friedrich N. Metabolomics in diabetes research. J Endocrinol. 2012;215:29–42. [DOI] [PubMed] [Google Scholar]
- [23].Huynh NN, Chin-Dusting J. Amino acids, arginase and nitric oxide in vascular health. Clin Exp Pharmacol Physiol. 2006;33:1–8. [DOI] [PubMed] [Google Scholar]
- [24].Romero MJ, Platt DH, Tawfik HE, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tang WH, Wang Z, Cho L, et al. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53:2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tripolt NJ, Meinitzer A, Eder M, et al. Multifactorial risk factor intervention in patients with Type 2 diabetes improves arginine bioavailability ratios. Diabetic Med: J British Diabetic Assoc. 2012;29:e365–368. [DOI] [PubMed] [Google Scholar]
- [28].Xu X, Zhang H, Song Y, et al. Strain-dependent induction of neutrophil histamine production and cell death by Pseudomonas aeruginosa. J Leukoc Biol. 2012;91:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Salo AM, Wang C, Sipila L, et al. Lysyl hydroxylase 3 (LH3) modifies proteins in the extracellular space, a novel mechanism for matrix remodeling. J Cell Physiol. 2006;207:644–653. [DOI] [PubMed] [Google Scholar]
- [30].Chen Y, Guo H, Terajima M, et al. 2 is secreted by tumor cells and can modify collagen in the extracellular space. J Biol Chem. 2016;291:25799–25808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Booth EA, Lucchesi BR. Estrogen-mediated protection in myocardial ischemia-reperfusion injury. Cardiovasc Toxicol. 2008;8:101–113. [DOI] [PubMed] [Google Scholar]
- [32].Ueda K, Karas RH. Emerging evidence of the importance of rapid, non-nuclear estrogen receptor signaling in the cardiovascular system. Steroids. 2013;78:589–596. [DOI] [PubMed] [Google Scholar]
- [33].Galkina SI, Fedorova NV, Serebryakova MV, et al. Inhibition of the GTPase dynamin or actin depolymerisation initiates outward plasma membrane tubulation/vesiculation (cytoneme formation) in neutrophils. Biol Cell/Under Auspices Eur Biol Organ. 2015;107:144–158. [DOI] [PubMed] [Google Scholar]
- [34].Saika S, Hashizume N, Okada Y, et al. Prolyl hydroxylase inhibitor and lysyl hydroxylase inhibitor inhibit spreading of corneal epithelium. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie. 1994;232:499–502. [DOI] [PubMed] [Google Scholar]


