Abstract
Background
Although cardiology organizations recommend early introduction of palliative care for patients with heart failure (HF), integration has remained challenging, particularly in patients with cardiac devices such as cardiac implantable electronic devices and left ventricular assist devices. Study authors suggest that patients often have limited and erroneous understanding of these devices and their implications for future care.
Objective
The aim of this study was to assess perceptions of cardiac devices in patients with HF and how these perceptions impacted advance care planning and future expectations.
Methods
This study used qualitative semistructured interviews with 18 community-dwelling patients with New York Heart Association stage II to IV HF.
Results
We interviewed 18 patients (mean ejection fraction, 38%; mean age, 64 years; 33% female; 83% white; 39% New York Heart Association class II, 39% class III, and 22% class IV). All had a cardiac implantable electronic device (6% permanent pacemaker, 56% implantable cardioverter-defibrillator, 28% biventricular implantable cardioverter-defibrillator); 11% had left ventricular assist devices. Patients with devices frequently misunderstood the impact of their device on cardiac function. A majority expressed the belief that the device would forestall further deterioration, regardless of whether this was the case. This anticipation of stability was often accompanied by the expectation that emerging technologies would continue to preempt decline. Citing this faith in technology, these patients frequently saw limited value in advance care planning.
Conclusions
In our sample, patients with cardiac devices overestimated the impact of their devices on preventing disease progression and death and deprioritized advance care planning as a result.
Keywords: advance care planning, cardiac devices, heart failure, implantable cardioverter-defibrillator, palliative care, permanent pacemaker, ventricular assist device
Heart failure (HF) is a common, chronic, and life-limiting condition affecting more than 6 million adults in the United States.1 As patients experience the numerous symptoms associated with this progressive disease, they may be offered a variety of technological interventions to try to extend quantity and quality of life. Device-driven interventions include permanent pacemakers (PPMs), implantable cardioverter-defibrillators (ICDs), cardiac resynchronization therapy (also known as biventricular pacing, which can be delivered via PPM or ICD), and left ventricular assist devices. Although these devices have a significant positive effect on quality of life, function, and survival,2–6 they are not without risks, such as inappropriate shocks and infection,7,8 and frequently require interventions to maintain device function over time. As such, patient education and engagement regarding the role of the device in current and future care is recommended as an essential component of the preimplantation decision-making process.9 The Centers for Medicare and Medicaid Services recently updated guidelines to mandate a preimplantation shared decision-making encounter between patient and provider.10
Research authors suggest that patients have limited understanding of how cardiac devices impact overall disease management, often overestimating the potential benefits of the device.11,12 Patients and families are then predictably reluctant to consider deactivation of ICDs, although, by failing to do so, they may increase suffering at the end of life. This problem is compounded by poor physician adherence to recommendations regarding the need for advance care planning before device implantation.13–15 Less clearly delineated is the impact of the device implantation process on patient expectations for their future care, including possible future palliative care needs. In this study, we explored how successful technological intervention may impact patient perceptions of prognosis and willingness to engage in advance care planning.
Methods
Design
We conducted semistructured qualitative interviews to allow for in-depth exploration of topics related to a parent study looking at attitudes and preferences regarding palliative care delivery models among individuals with HF. This article reflects the results of a secondary analysis about attitudes toward devices in this population. The University of Pittsburgh Institutional Review Board approved this study.
Sample and Recruitment
Candidate patients were identified by staff at an advanced HF clinic, or from general medicine inpatient wards, both at an American academic tertiary care hospital. Potential respondents were approached in person with a description of the study and, if interested, asked to provide informed consent to participate. Recruitment criteria included (1) New York Heart Association class II to IV HF, (2) ability to speak and understand English, and (3) no significant hearing or cognitive impediments prohibiting participation in a telephone interview.
Data Collection
Interviews were conducted by a single medical anthropologist with extensive qualitative training. Respondents were engaged in a semistructured discussion exploring palliative domains, including unmet symptom and emotional management needs, disease understanding and prognostic awareness, goals of care, and advance care planning (Appendix 1). The interview guide was informed by the National Consensus Project for Quality Palliative Care’s 2013 Clinical Guidelines16 and was developed with input from cardiology and palliative care clinicians and researchers. Demographic and relevant medical information was collected from patients and supplemented by chart review upon study enrollment. Interviews were audiotaped and transcribed verbatim. Responses from the subgroup of 18 patients (66% of the study population) identified as having cardiac devices were further analyzed for this article.
Analysis
We used template analysis, a qualitative analytic technique that combines content analysis and grounded theory.17 Template analysis yields a hybrid inductive/ deductive approach to theme identification. Data analysis was performed iteratively, with the initial interview guide developed through a literature review. Two investigators independently coded all transcripts iteratively with multiple intermediate consensus meetings to discuss and arbitrate discrepancies. Using the constant comparative method, newly coded text units were compared with previously coded data to ensure stability of identified themes.18 We achieved thematic saturation when repetition of themes was noted. A minimum of 12 interviews are recommended to achieve thematic saturation, which we surpassed.19 NVivo software (version 11, QSR International) was used to manage coding and analysis.
We used a variety of techniques to strive for qualitative trustworthiness.20,21 Regarding credibility, we used deliberate probes to richly and accurately understand participants’ lived experiences, and we frequently debriefed among the research team to ensure that our own biases were explicated and managed (ie, bracketing). Regarding dependability, we retained an extensive audit trail of analytic decision rules and memos. Finally, regarding confirmability, we triangulated among investigators in our team, each of which contributed unique perspectives based on education, specialization, and experience with HF or palliative care.
Results
Of 35 individuals with HF approached, 27 (77%) agreed to participate and were interviewed. Eighteen of these (66%) reported at least 1 cardiac device and were included in this analysis. This sample had a mean age of 64 years and was predominantly male and white (Table). Eleven (61%) had marked or severe physical limitation or HF symptoms (New York Heart Association class III/IV). Single- and dual-chamber ICDs predominated; only 6% of patients had pacemakers without defibrillator function. Two respondents had left ventricular assist devices. Interviews lasted an average of 47 minutes (range, 29–82 minutes). During analysis, 3 distinct themes emerged among those patients with devices: (1) poor understanding of HF trajectory and of how devices and novel technologies impact disease management, (2) minimal discussion about prognosis and how it might be impacted by devices, and (3) the expectation that ongoing technological advances would indefinitely improve survival and quality of life (Figure).
TABLE.
Participant Demographic and Medical Characteristics by Heart Failure Stage
| Characteristic | Full Sample | Stage II | Stage III | Stage IV |
|---|---|---|---|---|
| n (%) | 18 | 7 (39) | 7 (39) | 4 (22) |
| Demographic data | ||||
| Age, y | 64 | 69 | 62 | 60 |
| Race: white | 15 (83) | 5 (71) | 6 (86) | 4 (100) |
| Race: black | 3 (17) | 2 (29) | 1 (14) | 0 |
| Sex: female | 6 (33) | 4 (57) | 1 (14) | 1 (25) |
| Medical characteristics | ||||
| No additional comorbidities (HTN, DM, afib, stroke, COPD, renal disease) | 2 (11) | 1 (14) | 1 (14) | 0 (0) |
| 1 additional comorbidity | 3 (17) | 1 (14) | 1 (14) | 1 (25) |
| 2 additional comorbidities | 6 (33) | 4 (57) | 1 (14) | 1 (25) |
| At least 3 additional comorbidities | 7 (39) | 1 (14) | 4 (57) | 2 (50) |
| Mean LVEF (range) | 38 (10–75) | 41 (12–75) | 34 (15–65) | 41 (10–75) |
| Devices | ||||
| PPM | 1 (6) | 1 (14) | 0 | 1 (25) |
| ICD | 10 (56) | 5 (72) | 4 (57) | 1 (25) |
| BiV ICD | 5 (28) | 1 (14) | 3 (43) | 1a (25) |
| PPM/ICD | 1 (11) | 0 | 0 | 1a (25) |
| LVAD | 2 (11) | 0 | 0 | 2 (50) |
Abbreviations: afib, atrial fibrillation; BiV ICD, biventricular implantable cardioverter-defibrillator; CIED, cardiac implantable electronic device; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; ICD, implantable cardioverter-defibrillator; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; PPM, permanent pacemaker.
Respondent also reported LVAD.
FIGURE.
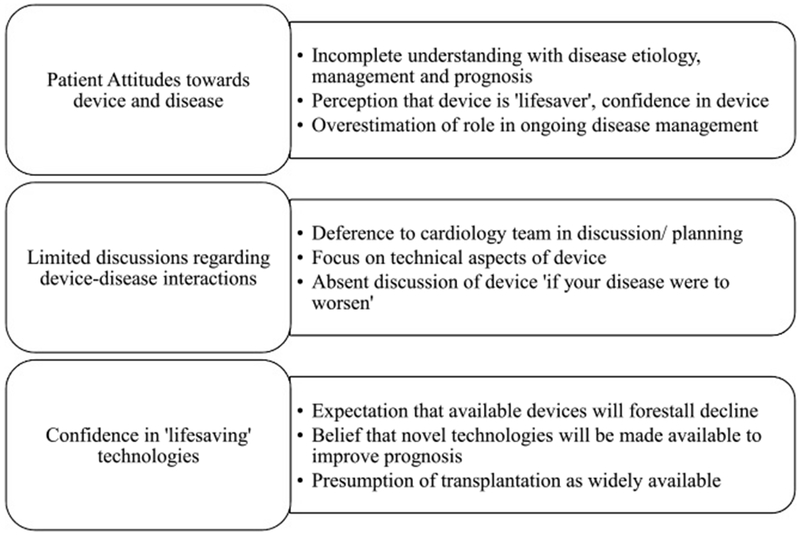
Themes elicited from participants regarding their devices.
Theme 1: Limited Disease Understanding and Overestimation of Device Function
Although respondents were able to cite specific details about cardiac function, their overall understanding of prognosis was ambiguous. Some hoped that their clinical status would improve spontaneously. Others expressed frustration with their limited understanding of etiology and trajectory, which left their futures uncertain:
Respondent (40M, class III, BiV ICD): I’d like to know what caused [my HF…]. And I’d like to know if it’s possible to fix it […]Generally speaking, where I’m at right now, is I kind of feel like I’m just in…limbo.
Participants frequently wished for improved symptom control without an in-depth understanding of what that might entail:
Respondent (55F, class III, ICD): No. There’s nothing really I can do [to manage my symptoms] at home. I don’t have to do really anything there. I just know I usually just go to the hospital. I know where it’s at and what they do to me.
This confusion regarding disease management did not extend to respondents’ devices, which they viewed with enthusiasm. Several had previously been defibrillated and were quick to note that they might not be alive had they not received the shock. When asked whether they experienced any anxiety or concerns regarding their devices, participants generally responded positively, with comments such as “I have faith in the defibrillator” and “[It’s] done its job and saved my life.”
Respondent (65M, class IV, ICD): […] the way I look at it, [the defibrillator] saved my life…even though it didn’t feel good getting shocked … If I would have had [a pacemaker], I would have probably been dead. But with the defibrillator kicking in, I understand it brought my heart back into rhythm. So it did save my life.
The conviction that the device provided a concrete and substantial benefit was often accompanied by confidence on the part of the participants about their understanding of the device and its functionality, even when inaccurate:
Respondent (82M, class III, BiV ICD): [I feel wonderful about my defibrillator] …I have my PhD in defibrillators going on.
Misconceptions about device role in overall disease management were pervasive. Respondents with ICDs in particular viewed their device’s role as much broader than its clinical definition would suggest. When asked about possible deactivation of his single-chamber ICD in the setting of eventual terminal progression, this patient (81M, class II) believed incorrectly that his device’s functionality could be increased:
Respondent (81M, class II, ICD): …No, no, no, not deactivate. If anything, I’d want them to activate it more. Besides if they deactivate the device what will happen in, heart surgery? I don’t think—I know people just got a defibrillator and had by-pass heart surgery.
Theme 2: Inadequate Discussion About Device Role, Limitations, and Advance Care Planning
Further questioning revealed that many respondents, particularly those with cardiac implantable electronic devices, had experienced little counseling regarding the role of the device as their disease progressed, a factor that likely contributed to their misperceptions. Many respondents with devices denied any discussion with their cardiologist about their choices regarding their device if their disease were to progress. Of the 18 participants with cardiac implantable electronic devices included in our study, 14 reported having had no conversation about possible device deactivation in the future; only 1 participant had discussed turning off his defibrillator in the event of further decline. Education about devices frequently centered around technical aspects of management, specifically device maintenance:
Respondent (79M, class III, ICD): …I’ve already had [the generator] changed three times.
Respondents deferred to their cardiologists regarding device management, attributing the same lifesaving properties to the provider as to the device. The cardiologist drove discussion, and the patient deferred to their recommendations:
Respondent (53M, class III, ICD): If my mechanic says, “You need a new spark plug,” I’m like, “Okay, go ahead.” Doctor says, “You need a new heart. “Okay, go ahead.” I don’t want to know what they do; I just want them to do it.
Respondent (60M, class III, BiV ICD): The defibrillator, what’s going to happen, if my heart goes—it’s just like being in an ambulance. They’re going to shock me and that’s going to be it. As a matter of fact, I was just at my other doctor the other day, and he checked it out and he said I’m doing great. He says, “Your battery’s going to last you for about eight more years.” I said, “That’s good.”
This focus on technical features of devices, but not their implications, left respondents unprepared to talk about the broader implications of their disease.
Theme 3: Confidence in Novel Technologies and Ongoing Advances
Despite a lack of clarity regarding device function or outcome, respondents continued to voice the expectation that novel devices and therapies would continue to drive their disease management.
Participants with a device frequently expressed the expectation that other novel interventions would continue to improve their prognosis:
Respondent (60M, class III, BiV ICD): […My doctors] have already talked about a heart pump, although I’m not ready for that yet. […] The heart pump will go on before the transplant.
These interventions frequently culminated in planning for transplantation, which was taken for granted:
Respondent (61M, class IV, PPM, BiV ICD, left ventricular assist device): …I’ve faced my mortality and the left ventricular assist device and the prospect of what if that didn’t exist, where would I be today? And I’m looking forward to a heart transplant.
Other respondents described a spectrum of novel innovations in what they viewed as a rapidly evolving care landscape:
Respondent (83F, class II, PPM): But I still believe that it’s not over until it’s over. There’s so much today that can turn things around technology-wise that there is more hope, I think, than there used to be.
Respondent (55M, class IV, BiV ICD, left ventricular assist device): Do you remember the first heart pumps to come out? […] They had to carry a suitcase with batteries in it, with wheels. And now look at it, I can put it on a belt. It’s just a matter of time…[before] you just go on, you just buy a heart.
Respondent (60M, class III, BiV ICD): I have been doing a lot of research on [stem cell transplantation]. They are actually growing livers, kidneys and hearts…
Even patients who did not have implantable devices referenced a device-driven future:
Respondent (75M, class III, no device currently): Yeah. As I said before, [my cardiologist is] going to put in one more stent and they’re going to put in [an ICD]. That’s going to be at the end of this month. And hopefully they can get—they have to get the heart more than 15% efficiency, or they said you’ll just be tired. So hopefully they can do that.
These perceptions of a limitless future, maintained by ongoing interventions, conflicted with other planning strategies, including consideration of palliative care consultation and advance care planning.
Respondent (61M, class IV, PPM, BiV ICD, left ventricular assist device): I haven’t [participated in advance care planning] because I don’t think I need any. My wife is my caregiver. left ventricular assist device has given me a very good quality of life, and I’m listed for transplant and the next step for me is transplant.
Discussion
Our findings suggest that the barriers to advance care planning in patients with cardiac devices are multifactorial and are impacted not only by patient-provider communication and education but also by our societal relationship with novel technologies, a phenomenon described as the “biotechnical embrace.”22 Not only do these findings reinforce the limitations of existing preimplantation shared decision-making paradigms; they also suggest that societal appetites for novel technological development may contribute to the belief that ever-more complex innovations will be available to forestall death. These results lend nuance to previous studies indicating that cardiac devices are poorly understood by patients with HF11,23–25 and suggest that approaches to improve shared decision making in this population may need to address cultural, as well as medical, factors.
The exclusion of devices from advance care planning for patients with HF has been extensively demon- strated.13,15,26,27 When combined with the frequent overestimation of device utility among both patients and providers,23,25 this discrepancy creates a gap in the care dialogue that may have far-reaching impact upon patient approaches to their disease and therapy. Advance directives for patients with HF rarely address device management, and few hospitals have implemented formal policies for device deactivation at the end of life.28 Despite evidence suggesting that, by 5 years postimplantation, 50% of patients with ICDs will be either enrolled in hospice or deceased,29 providers and institutions have been slow to discuss device management at the end of life. A majority of providers agree that deactivation of ICDs and left ventricular assist devices with family consent is ethical at the end of life,30 but many express discomfort discussing these issues with their patients.14,31,32
Patients navigating HF treatment are expected to understand and incorporate complex information into their decision making regarding cardiac devices. Our respondents did demonstrate some understanding of their disease trajectory and the role of the device therein, although it is unclear the extent to which this knowledge enabled them to truly participate in shared decision making. Many had already been part to a successful intervention (device placement and, on occasion, defibrillation) and correctly highlighted the role of their device and future technology in disease management. However, those respondents with end-stage disease, whose care trajectory should theoretically have already included discussions of both advance care planning and involvement of specialty palliative care, remained fixated on future procedural interventions for which their candidacy was questionable. These findings are corroborated by studies demonstrating that patients who have experienced ICD-mediated defibrillation often display greater confidence in and less willingness to discontinue their device.12,33 Although the desire to continually search for therapies to extend life is understandable, it is unclear that patients fully appreciate the nuanced implications of device therapy.
Our findings suggest that cardiac devices, and specifically the technological developments that they represent, may act as an additional confounder to establishing goals of care for patients with advanced HF. The anticipation of device-mediated stability expressed by our respondents, accompanied by the expectation that emerging technologies will continue to preempt decline, may complicate delivery of context-appropriate advance care planning. Further research is needed to determine whether current practice regarding shared decision making for devices is adequate and, if not, how better to provide patients with a complete understanding of their options and potential future decision-making scenarios before device implantation. Our findings suggest that explicit discussion of device roles and their limitations, as well as the availability of viable “next steps,” may promote more realistic expectations. Specialist palliative care may have a role to play in these discussions similar to that played in advance care planning before left ventricular assist device implantation, although all cardiology providers should possess fundamental competency in eliciting goals of care.34,35
As with any study, several limitations are worth noting. Respondents were drawn from a single institution, a large academic quaternary care medical center, and were predominantly male and white; all of these factors may limit generalizability of our findings. In addition, although the overall proportion of respondents with a cardiac device was high, representation of advanced therapies, particularly left ventricular assist devices, was limited.
Conclusion
Misconceptions about cardiac devices and their role in disease management may be related to unrealistic expectations about the future for patients with HF. Focusing on technological interventions may limit patient receptiveness to discussion about prognosis and advance care planning. Further research is indicated to develop and evaluate initiatives educating patients with HF about their devices and facilitating meaningful discussion of advance care planning and device deactivation into the device implantation process.
What’s New and Important.
-
■
Authors of previous studies have demonstrated that patients with HF have limited understanding of cardiac devices and their impact on prognosis.
-
■
In this study, we find that the experience of having a device implanted generates expectations that future technological interventions will be made available to preempt clinical decline.
-
■
This embrace of technology seems to act as a barrier to advance care planning in patients with HF.
Acknowledgments
The authors sincerely thank the following individuals for their assistance in conducting the study: Megan Hamm, PhD; Adelina Malito, MSW; Laura Obregon, BS; Ramy Khalil, MD; and Zachariah Hoydich, BS. We also thank the participants who offered their time and perspectives, without which this study would have been impossible.
This study was supported by the Agency for Healthcare Research and Quality (K12HS022989) and the National Palliative Care Research Center. Dr Kavalieratos also received research support from the National Heart Lung and Blood Institute (K01HL133466).
APPENDIX 1: Semistructured Interview Guide
| Domain | Primary Palliative Care Skillset | Question |
|---|---|---|
| Introduction | ||
| Physical aspects of care | Symptom management | Thinking about your heart failure (HF), which symptoms most affect your quality of life? |
| Psychological aspects of care | Psychological management | Many people living with HF say they experience depression, sadness, or anxiety. I’m curious about your experiences with depression or anxiety since you were diagnosed with HF. Have you had any feelings of sadness or depression? What about feelings of anxiety? |
| Social aspects of care | How do you feel that your HF has impacted your relationships with your loved ones? | |
| Spiritual aspects of care | What role does spirituality play in your life? How do your spiritual practices impact how you deal with having HF? | |
| Ethical and legal aspects of care | Prognostication, communication preferences, and advance care planning | How much do you want to know about the future with heart failure? Could you tell me about your experiences in making preparations for healthcare if your heart failure gets worse? Have you made an advance directive or living will? |
| Device-specific preferences | Do you have a cardiac device, like a defibrillator, or a pump? What kind of discussions have you and your cardiologist had about that device if your HF gets worse? | |
| Outcomes that matter to patients with HF | Goals of treatment | What are you hoping to get out of your healthcare regarding HF? |
| Perceptions of supportive care | How familiar are you with the term “supportive care”? Can you please tell me what you know or what you’ve heard about it? | |
| Perceptions of palliative care | Do you think there will come a point in the progression of your HF where you’d like to see a palliative care specialist? What would that/those points be? | |
| Closing | Given everything we’ve talked about today, what does/would high-quality HF care look like to you? |
Footnotes
The authors have no conflicts of interest to disclose.
Contributor Information
Rachel A. Hadler, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Nathan E. Goldstein, Brookdale Department of Geriatrics and Palliative Medicine, Icahn School of Medicine at Mount Sinai, New York, New York.
David B. Bekelman, Division of General Internal Medicine, Department of Medicine, University of Colorado School of Medicine, Aurora.
Barbara Riegel, School of Nursing, University of Pennsylvania, Philadelphia.
Larry A. Allen, Division of Cardiology, Department of Medicine, University of Colorado School of Medicine, University of Colorado Hospital Cardiac and Vascular Center, Aurora.
Robert M. Arnold, Section of Palliative Care and Medical Ethics, Department of Medicine, University of Pittsburgh, Pennsylvania.
Matthew E. Harinstein, Division of Cardiology, Department of Medicine, University of Pittsburgh Medical Center, Pennsylvania.
Dio Kavalieratos, Section of Palliative Care and Medical Ethics, Department of Medicine, University of Pittsburgh, Pennsylvania.
REFERENCES
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Bradley DJ, Bradley EA, Baughman KL, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003; 289:730–740. [DOI] [PubMed] [Google Scholar]
- 3.Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA. 2004;292:2874–2879. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann KE, Orav EJ, Lamas GA, et al. Pacemaker implantation and quality of life in the Mode Selection Trial (MOST). Heart Rhythm. 2006;3:653–659. [DOI] [PubMed] [Google Scholar]
- 5.Linde C, Braunschweig F, Gadler F, Bailleul C, Daubert J-C. Long-term improvements in quality of life by biventricular pacing in patients with chronic heart failure: results from the MUltisite STimulation In Cardiomyopathy Study (MUSTIC). American Journal of Cardiology. 2003;91:1090–1095. [DOI] [PubMed] [Google Scholar]
- 6.Udo EO, van Hemel NM, Zuithoff NP, et al. Long term quality-of-life in patients with bradycardia pacemaker implantation. Int J Cardiol. 2013;168:2159–2163. [DOI] [PubMed] [Google Scholar]
- 7.Calvagna GM, Torrisi G, Giuffrida C, Patanè S. Pacemaker, implantable cardioverter defibrillator, CRT, CRT-D, psychological difficulties and quality of life. Int J Cardiol. 2014;174:378–380. [DOI] [PubMed] [Google Scholar]
- 8.Sears SF Jr., Conti JB. Quality of life and psychological functioning of ICD patients. Heart. 2002;87:488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure a scientific statement from the American Heart Association: endorsed by Heart Failure Society of America and American Association of Heart Failure Nurses. Circulation. 2012;125:1928–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Medicare and Medicaid Services. Decision memo for implantable cardioverter-defibrillators. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=288. Updated February 15, 2018. Accessed September 30, 2018.
- 11.Goldstein NE, Mehta D, Siddiqui S, et al. “That’s like an act of suicide” patients’ attitudes toward deactivation of implantable defibrillators. J Gen Intern Med. 2008;23:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svanholm JR, Nielsen JC, Mortensen P, Christensen CF, Birkelund R. Refusing implantable cardioverter defibrillator (ICD) replacement in elderly persons—the same as giving up life: a qualitative study. Pacing and Clinical Electrophysiology. 2015;38:1275–1286. [DOI] [PubMed] [Google Scholar]
- 13.Merchant FM, Binney Z, Patel A, et al. Prevalence, predictors, and outcomes of advance directives in implantable cardioverter-defibrillator recipients. Heart Rhythm. 2017;14:830–836. [DOI] [PubMed] [Google Scholar]
- 14.Waterhouse E, Ahmad F. Do implantable cardioverter defibrillators complicate end-of-life care for those with heart failure? Curr Opin Support Palliat Care. 2011;5:307–311. [DOI] [PubMed] [Google Scholar]
- 15.Niewald A, Broxterman J, Rosell T, Rigler S. Documented consent process for implantable cardioverter-defibrillators and implications for end-of-life care in older adults. J Med Ethics. 2013;39:94–97. [DOI] [PubMed] [Google Scholar]
- 16.National Consensus Project for Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. Pittsburgh, PA: National Consensus Project for Quality Palliative Care; 2013. [Google Scholar]
- 17.King N Template analysis In: Qualitative methods and analysis in organizational research: A practical guide. Thousand Oaks, CA: Sage Publications Ltd; 1998:118–134. [Google Scholar]
- 18.Sandelowski M Qualitative analysis: what it is and how to begin. Res Nurs Health. 1995;18:371–375. [DOI] [PubMed] [Google Scholar]
- 19.Guest G, Bunce A, Johnson L. How many interviews are enough?: an experiment with data saturation and variability. Field Methods. 2006;18:59–82. [Google Scholar]
- 20.Nowell LS, Norris JM, White DE, Moules NJ. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16:1609406917733847. [Google Scholar]
- 21.Kavalieratos D, Mitchell EM, Carey TS, et al. “Not the ‘grim reaper service’”: an assessment of provider knowledge, attitudes, and perceptions regarding palliative care referral barriers in heart failure. J Am Heart Assoc. 2014;3:e000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Good MJ. The biotechnical embrace. Cult Med Psychiatry. 2001;25:395–410. [DOI] [PubMed] [Google Scholar]
- 23.Groarke J, Beirne A, Buckley U, et al. Deficiencies in patients’ comprehension of implantable cardioverter defibrillator therapy. Pacing and Clinical Electrophysiology. 2012;35:1097–1102. [DOI] [PubMed] [Google Scholar]
- 24.Matlock DD, Nowels CT, Masoudi FA, et al. Patient and cardiologist perceptions on decision making for implantable cardioverter-defibrillators: a qualitative study. Pacing Clin Electrophysiol. 2011;34:1634–1644. [DOI] [PubMed] [Google Scholar]
- 25.Stewart GC, Weintraub JR, Pratibhu PP, et al. Patient expectations from implantable defibrillators to prevent death in heart failure. J Card Fail. 2010;16:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tajouri TH, Ottenberg AL, Hayes DL, Mueller PS. The use of advance directives among patients with implantable cardioverter defibrillators. Pacing and Clinical Electrophysiology. 2012;35:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkpatrick JN, Gottlieb M, Sehgal P, Patel R, Verdino RJ. Deactivation of implantable cardioverter defibrillators in terminal illness and end of life care. Am J Cardiol. 2012;109:91–94. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med. 2010;152:296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer DB, Reynolds MR, Normand SL, et al. Hospice use following implantable cardioverter-defibrillator implantation in older patients: results from the National Cardiovascular Data Registry. Circulation. 2016;133:2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daeschler M, Verdino RJ, Caplan AL, Kirkpatrick JN. Defibrillator deactivation against a patient’s wishes: perspectives of electrophysiology practitioners. Pacing and Clinical Electrophysiology. 2015;38:917–924. [DOI] [PubMed] [Google Scholar]
- 31.Kraynik SE, Casarett DJ, Corcoran AM. Implantable cardioverter defibrillator deactivation: a hospice quality improvement initiative. J Pain Symptom Manage. 2014;48:471–477. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein NE, Mehta D, Teitelbaum E, Bradley EH, Morrison RS. “It’s like crossing a bridge” complexities preventing physicians from discussing deactivation of implantable defibrillators at the end of life. Journal of General Internal Medicine. 2008;23:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill L, McIlfatrick S, Taylor BJ, et al. Implantable cardioverter defibrillator (ICD) deactivation discussions: reality versus recommendations. Eur J Cardiovasc Nurs. 2016;15:20–29. [DOI] [PubMed] [Google Scholar]
- 34.Kavalieratos D, Gelfman LP, Tycon LE, et al. Palliative care in heart failure: rationale, evidence, and future priorities. J Am Coll Cardiol. 2017;70:1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. New England Journal of Medicine. 2013;368:1173–1175. [DOI] [PubMed] [Google Scholar]


