Abstract
We present here a comparative planetology study of evolution of 14N/15N at Mars and Titan. Studies show that 14N/15N can evolve a great deal as a result of escape in the atmosphere of Mars, but not in Titan’s atmosphere. We explain this through the existence of an upper limit to the amount of fractionation allowed to occur due to escape that is a function of the escape flux and the column density of nitrogen.
Keywords: Atmospheres, evolution; Titan, atmosphere; Mars, atmosphere; Atmospheres, composition; Atmospheres, dynamics
1. Introduction
Stable isotope ratios measured in planetary atmospheres are a valuable tool for evaluating the history of that atmosphere due to escape and photochemistry. Escape is of particular interest because it preferentially removes the lighter isotope causing the ratio to become “heavier” over long time periods and can have a profound impact on atmospheric composition. As an example, the D/H ratio in the atmosphere of Venus has been used to determine the amount of water lost from Venus over the history of the Solar System (e.g. Donahue et al., 1997), while several isotope ratios in the atmosphere of Mars provide evidence for long-term erosion of the atmosphere (e.g. Jakosky, 1991).
The evolution of nitrogen in the atmospheres of Titan (e.g. Lunine et al., 1999; Mandt et al., 2009, 2012a, 2014) and Mars (e.g. Fox and Dalgarno, 1983; Fox, 1997) are of particular interest for evaluating the origin and evolution of nitrogen throughout the Solar System. Fig. 1 illustrates nitrogen isotope ratios (14N/15N) that have been measured in the terrestrial planets, Titan and comets. These ratios are designated as either primordial (triangles in Fig. 1), or evolved (circles in Fig. 1). Primordial ratios, such as those measured in comets, are believed to have retained the same value they had when the parent body formed during the early stages of the Solar System. These ratios are presumed to provide a direct record of conditions in the protosolar nebula (PSN). Evolved ratios are expected to have changed in value over the history of the Solar System due to processes such as atmospheric escape or photochemistry.
Fig. 1.
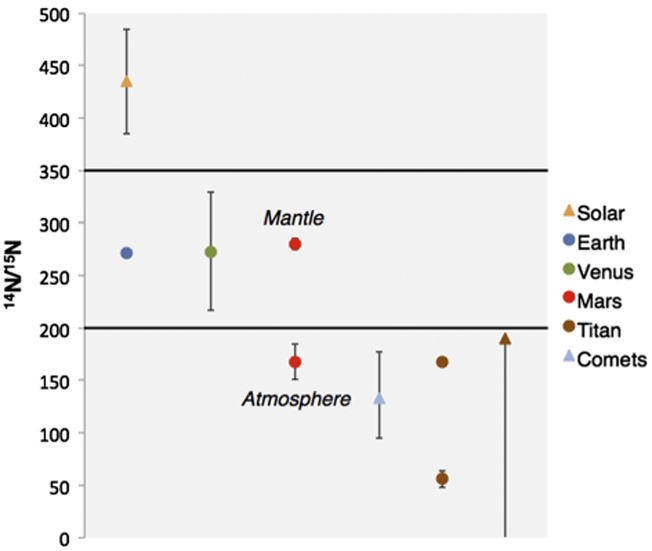
Measurements of nitrogen isotope ratios in the terrestrial planets, Titan and comets. Triangles are primordial values representing 14N/15N in the PSN. Circles are isotope ratios that have evolved over the 4.6 Billion year history of the Solar System. The primordial value for Titan is inferred from models of atmospheric evolution.
The primordial ratio that is most relevant to evaluating the evolution of Titan and Mars is 14N/15N is cometary NH3 (Rousselot et al., 2014; Shinnaka et al., 2014). This ratio is moderately enriched in 15N relative to the terrestrial value of 272 (Marty and Zimmermann, 1999), which is likely to be evolved (Pepin, 2006), and heavily enriched compared to a primordial solar ratio of ~435 based on measurements made in the solar wind (Marty et al., 2011) and Jupiter’s atmosphere (Owen et al., 2001). The cometary ratio represents 14N/15N in NH3 in the PSN, while the solar ratio represents 14N/15N in N2 in the PSN. The primordial ratio for both Titan and Mars, if properly constrained, can give an indication of the atmosphere’s source of nitrogen.
Two 14N/15N measurements have been made in Titan’s atmosphere: N2 of 167.7 ± 1.7 (Niemann et al., 2010) and HCN of 56 ± 8 (Vinatier et al., 2007). The much lower ratio in HCN is the result of self-shielding processes in the photodissociation of N2 (Liang et al., 2007). In Fig. 1 we also provide an upper limit of ~190 for Titan’s primordial 14N/15N due to demonstrated limitations in the degree to which Titan’s atmosphere can evolve due to escape (Mandt et al., 2014). The similarity between the primordial 14N/15N for Titan and 14N/15N in ammonia in comets (Rousselot et al., 2014; Shinnaka et al., 2014) suggests that the nitrogen in Titan’s atmosphere originated in the cold PSN where the building blocks of Titan are likely to have formed (Mousis et al., 2009; Mandt et al., 2014).
Fig. 1 also provides two measurements for 14N/15N at Mars. 14N/15N in the mantle of Mars has been determined to be 280 ± 5 based on 14N/15N measured in an SNC meteorite (Mathew and Marti, 2001). This is thought to represent the isotope ratio for nitrogen when the atmosphere of Mars formed. As Fig. 1 illustrates, the 14N/15N for the mantle of Mars is within the same range as 14N/15N on Earth and Venus. At this time, it is not clear how nitrogen was delivered to the terrestrial planets, but it appears that all three planets had a ratio within the range of 250–300 when their atmospheres formed (e.g. Pepin, 2006). Fig. 1 also shows the most recent value measured in the atmosphere of Mars, 173 ± 11 (Wong et al., 2013). Like Titan, this value is within the range of cometary NH3, but as previously stated atmospheric escape has been demonstrated to lower the ratio from the value determined for the mantle to the value found today in Mars’ atmosphere (e.g. Fox and Dalgarno, 1983; Fox, 1997).
The fact that Mars and Titan both have atmospheric 14N/15N in the range of cometary NH3, but vastly different reasons for this similarity is curious. It seems contradictory that the nitrogen isotopes are able to fractionate significantly through escape at Mars but not at Titan. However, the difference between the influence of escape on the nitrogen isotopes at Mars and Titan can be explained by a limitation in fractionation that is a function of the escape flux and the column density of the atmospheric constituent. We demonstrate here how it is possible for the limits of fractionation for Titan to be very different than the limits for Mars.
2. The upper limit for fractionation due to escape
2.1. Methodology
The Rayleigh distillation relationship is commonly used to describe fractionation of an isotope ratio due to a single process. This is determined to be a function of the initial, n0, and current, n, inventory of the constituent and a fractionation factor, f:
| (1) |
where R is the current ratio (heavy/light) and R0 is the initial ratio. The ratio R/R0 defines the degree of enrichment in the heavier isotope over the primordial value. When R/R0 is less than one, the current inventory is depleted in the heavy isotope compared to the initial inventory while a value greater than one demonstrates enrichment in the heavy isotope.
An upper limit for the initial inventory of the constituent can be determined based on the maximum escape flux and the amount of time the fractionating process has been in effect:
| (2) |
where ϕ is the maximum escape flux and t is time. Combining Eqs. (1) and (2) gives:
| (3) |
which can be rearranged to provide an upper limit for the enrichment in the heavier isotope relative to the primordial value:
| (4) |
Eq. (4) can be used to define an upper limit for the enrichment in the heavy isotope in a planet’s atmosphere when the fractionation factor, the maximum escape rate and the timescale are well understood.
Some consideration needs to be given to changing escape rates due to evolution of the Sun. In the early stages of the Solar System, the extreme ultraviolet (EUV) flux from the Sun was significantly greater than it is today (Ribas et al., 2005). As a result, the escape of particles from planetary atmospheres was likely to have been greater in the past. It is possible to evaluate the enrichment in the heavier isotope for a case where the escape flux varies with time by integrating the ratio of the escape flux to the column density with time as follows
| (5) |
2.2. Application to Mars and Titan
We can apply (Eqs. 4) and (5) to the evolution of 14N/15N in the atmospheres of Mars and Titan by evaluating what is known about escape processes for these two bodies. Table 1 gives input parameters based on research that has been done for each atmosphere.
Table 1.
Input parameters used to determine the upper limit for fractionation due to escape based on published studies of Mars and Titan.
| Escape type | Mars | Titan | |
|---|---|---|---|
| Atmospheric col. density (cm−2) | ~2 × 1023 | 2.37 × 1026 | |
| Nitrogen abundance | 0.02 | 0.98 | |
| n (cm−2) | ~4 × 1021 | 2 × 1026 | |
| ϕ (cm−2 s−1) | Non-thermal | 8.1 × 105 (now)a | 7 × 107c |
| 8.1–81.0 × 105 (past)b | |||
| Jeans | 3 × 109 (⩽40 Myr)d,e | ||
| Hydrodynamic | 4 × 1012 (⩽40 Myr)d | ||
| ϕ/n | Non-thermal | 2.0–20.0 × 10−16 | 3.5 × 10−19 |
| Jeans | 1.5 × 10−17 (⩽40 Myr) | ||
| Hydrodynamic | 2.0 × 10−14 (⩽40 Myr) | ||
| f | Non-thermal | 0.80 (now)f,g | 0.73e |
| 0.80 (past)b, f,g | 0.72e | ||
| Jeans | 0.82e | ||
| Hydrodynamic | 0.98e |
The results are illustrated in Fig. 2, where the degree of enrichment in the heavy isotope relative to the primordial value is provided as a function of time for different types of escape processes. For Mars, the thick red line in Fig. 2 represents enrichment due to non-thermal escape under current conditions as calculated with (Eq. 4). This is based on the average escape rate over the solar cycle (Bakalian and Hartle, 2006). According to the Monte Carlo computations made by these authors, the major non-thermal escape mechanism is photodissociation of N2, dissociative recombination of being 3 to 10 times less efficient depending on the level of solar activity. The fractionation factor for photodissociation escape is 0.82 based on the 15N14N/14N14N ratio at the exobase (Fox and Dalgarno, 1983 and references therein). The fractionation factor for dissociative recombination is in the range of 0.51–0.58 (Chassefière and Leblanc, 2004 and references therein). Due to the small contribution of dissociative recombination, the effective f value is in this case close to 0.8.
Fig. 2.
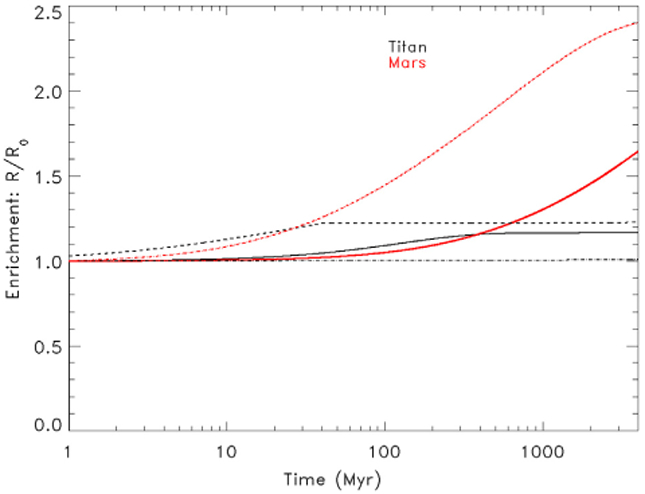
Upper limit for the enrichment of 14N/15N relative to the primordial value for Titan and Mars. For Titan, we compare the relative influence of sputtering (solid line), hydrodynamic escape (dashed line) and Jeans escape (dash-dot line). For Mars we illustrate the maximum enrichment based on current (solid line) and maximum historic (dashed line) non-thermal escape rates.
The dashed red line gives enrichment due to the maximum possible nonthermal escape rate under past conditions in the atmosphere of Mars (Chassefière and Leblanc, 2011). In order to take into account changes in escape over the history of the Solar System, we need to determine how the total escape rate has changed over time and the fractionation factor based on the dominant loss process. If dissociative recombination was the dominant loss process throughout the history of Mars, the greater abundance of nitrogen in the past would lead to a total past escape rate that is a factor of 2.51 greater than current escape rates and the fractionation factor would be 0.51 to 0.58 (Fox and Dalgarno, 1983). However, Bakalian and Hartle (2006) find that photodissociation is the dominant loss process. Changes in the solar EUV flux over the history of the Solar System would produce non-thermal escape fluxes that were an order of magnitude greater than current escape rates at the beginning of the Solar System (Ribas et al., 2005; Chassefière and Leblanc, 2011). The escape rate would then have decreased logarithmically over time to its current rate (see Chassefière and Leblanc, 2011, Fig. 3). In this case the fractionation factor would have been 0.80. We calculated the upper limit for fractionation at Mars using Eq. (5) and an escape flux that decreased with time based on the work of Chassefière and Leblanc (2011: Fig. 3) and f of 0.80.
Penz et al. (2005) and De la Haye et al. (2007) have evaluated current and past escape rates for nitrogen for Titan. Penz et al. (2005) found that thermal escape processes (hydrodynamic and Jeans escape) would have been effective for no more than 40 Myr based on changes in the EUV radiation of the Sun. In Fig. 2 we illustrate an upper limit of fractionation for Titan due to hydrodynamic (dashed black line) and Jeans escape (dot-dashed black line) of nitrogen based on this time limit. Fractionation due to sputtering (solid black line) is presented for the lifetime of the Solar System.
3. Discussion and conclusions
It is first clear from Fig. 2 that the longer an escape process is in effect, the greater the possible fractionation. It is also clear that, except in the case of hydrodynamic escape on Titan, the nitrogen isotopes on Mars will fractionate more rapidly than on Titan. We find that over the lifetime of the Solar System, sputtering on Mars would enrich the nitrogen isotopes by a factor of 1.45 to 2.40 relative to the primordial value, which gives a maximum initial 14N/15N of 415. The measured enrichment in 14N/15N on Mars is 1.6 times the presumed primordial value (Mathew and Marti, 2001; Wong et al., 2013), which is well within this range.
For Titan we find that neither sputtering nor Jeans escape is able to change the 14N/15N in Titan’s atmosphere by more than 1% as is illustrated in Fig. 2. This is because the column density, n, relative to the escape flux, ϕ, for these two processes is very low. In hydrodynamic escape of nitrogen, the escape flux relative to the column density is much higher, so total fractionation in this case is limited by time and the fractionation factor. For the hydrodynamic escape upper limit illustrated in Fig. 2, we use the most efficient combination of escape flux and fractionation factor as determined in Mandt et al. (2014). The upper limit based on (Eq. 4) for enrichment of 14N/15N on Titan is 1.23 times Titan’s primordial value. This gives an upper limit for primordial 14N/15N at Titan of 206. Therefore, it is possible for 14N/15N to fractionate more on Mars than on Titan due to the difference in escape rates relative to nitrogen column density. However, it is important to note that this evaluation does not consider late delivery of significant cometary NH3 to Titan after hydrodynamic escape has ended (e.g. Trigo-Rodriguez and Martin-Torres, 2012), which could reset the 14N/15N to the cometary value.
In this study we have not included fractionation due to past hydrodynamic escape of hydrogen that drags nitrogen out of the atmosphere for neither Mars nor Titan (e.g. Chassefière, 1996; Mandt et al., 2009). Pepin (1994) and Jakosky et al. (1994) concluded that nitrogen on Mars would not be fractionated by hydrodynamic escape of hydrogen based on the fractionation observed in the Xe and Kr isotopes. For Titan, we found in previous work (Mandt et al., 2009) that this type of fractionation is not efficient because too much of the heavy isotope is dragged out of the atmosphere.
The main limitation of this study is that it focuses only on escape and neglects photochemistry, which can also fractionate the isotopes. Photochemistry on Titan has been shown to preferentially remove the heavier isotope due to self-shielding in the atmosphere (Liang et al., 2007). This process will reduce the isotope enrichment described by (Eq. 4) and illustrated in Fig. 2 and will help to keep 14N/15N within the range determined for 14N/15N in cometary NH3.
Photochemical fractionation of nitrogen on Mars has been evaluated primarily for its role in producing odd nitrogen through photodissociation, which then escapes from the atmosphere (Fox and Dalgarno, 1983). Permanent loss of nitrogen through deposition of nitrate and nitrate minerals is estimated to be as much as 3 × 105 cm−2 s−1 (Yung et al., 1977), which is the same order of magnitude as the current nitrogen loss rates due to escape. However, we did a basic calculation of photodissociation rates for Mars by adapting our Ion-Neutral Thermal model (INT12 – Mandt et al., 2012b) for the atmospheric conditions of Mars (i.e. temperature, composition) and found that the column density of nitrogen is too low to provide self-shielding, so this process is not effective at Mars. The implication of this is that measurements of 14N/15N in surface nitrates on Mars would provide the opportunity to study the history of 14N/15N in the atmosphere of Mars.
Acknowledgments
K.E.M. acknowledges support from NASA Grant NNX13AQ99G. O.M. acknowledges support from CNES. The work contributed by O.M. was carried out thanks to the support of the A*MIDEX project (n° ANR-11-IDEX-0001-02) funded by the «Investissements d’Avenir» French Government program, managed by the French National Research Agency (ANR). We also thank two anonymous reviewers for their very helpful feedback.
References
- Bakalian F, Hartle RE, 2006. Monte Carlo computations of the escape of atomic nitrogen from Mars. Icarus 183, 55–68. [Google Scholar]
- Chassefière E, 1996. Hydrodynamic escape of oxygen from primitive atmospheres: Applications to the cases of Venus and Mars. Icarus 124, 537–552. [Google Scholar]
- Chassefière E, Leblanc F, 2004. Mars atmospheric escape and evolution; interaction with the solar wind. Planet. Space Sci 52,1039–1058. [Google Scholar]
- Chassefière E, Leblanc F, 2011. Methane release and the carbon cycle on Mars. Planet. Space Sci 59, 207–217. 10.1016/j.pss.2010.09.004. [DOI] [Google Scholar]
- de La Haye V et al. , 2007. Cassini Ion and Neutral Mass Spectrometer data in Titan’s upper atmosphere and exosphere: Observation of a suprathermal corona. J. Geophys. Res 112, A07309. [Google Scholar]
- Donahue TM et al. , 1997. In: Bougher SW, Hunten DM, Phillips RJ (Eds.), Venus II. University of Arizona Press, Tucson, pp. 385–414. [Google Scholar]
- Fox JL, 1997. Upper limits to the outflow of ions at Mars: Implications for atmospheric evolution. Geophys. Res. Lett 24, 2901 10.1029/97GL52842. [DOI] [Google Scholar]
- Fox JL, Dalgarno A, 1983. Nitrogen escape from Mars. J. Geophys. Res 88 (A11), 9027–9032. [Google Scholar]
- Jakosky BM, 1991. Mars volatile evolution – Evidence from stable isotopes. Icarus 94, 14–31. [Google Scholar]
- Jakosky BM et al. , 1994. Mars atmospheric loss and isotopic fractionation by solar-wind-induced sputtering and photochemical escape. Icarus 111, 271–288. [Google Scholar]
- Liang M-C et al. , 2007. Source of nitrogen isotope anomaly in HCN in the atmosphere of Titan. Astrophys. J 664, L115–L118. [Google Scholar]
- Lunine JI, Yung YL, Lorenz RD, 1999. On the volatile inventory of Titan from isotopic abundances in nitrogen and methane. Planet. Space Sci 47 (10–11), 1291–1303. [DOI] [PubMed] [Google Scholar]
- Mandt KE et al. , 2009. Isotopic evolution of the major constituents of Titan’s atmosphere based on Cassini data. Planet. Space Sci 57 (14–15), 1917–1930. 10.1016/j.pss.2009.06.005. [DOI] [Google Scholar]
- Mandt KE et al. , 2012a. The 12C/13C ratio on Titan from Cassini INMS measurements and implications for the evolution of methane. Astrophys. J 749 (2), 160 10.1088/0004-637X/749/2/160. [DOI] [Google Scholar]
- Mandt KE et al. , 2012b. Ion densities and composition of Titan’s upper atmosphere derived from the Cassini Ion Neutral Mass Spectrometer: Analysis methods and comparison of measured ion densities to photochemical model simulations. J. Geophys. Res 117 (E10), E10006 10.1029/2012JE004139. [DOI] [Google Scholar]
- Mandt KE et al. , 2014. Protosolar ammonia as the unique source of Titan’s nitrogen. Astrophys. J 788 (2), L24 10.1088/2041-8205/788/2/L24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty B, Zimmermann L, 1999. Volatiles (He, C, N, Ar) in mid-ocean ridge basalts: Assessment of shallow-level fractionation and characterization of source composition. Geochim. Cosmochim. Acta 63 (21), 3619–3633. [Google Scholar]
- Marty B et al. , 2011. A 15N-poor isotopic composition for the Solar System as shown by Genesis solar wind samples. Science 332, 1533–1536. [DOI] [PubMed] [Google Scholar]
- Mathew KJ, Marti K, 2001. Early evolution of martian volatiles: Nitrogen and noble gas components in ALH84001 and Chassigny. J. Geophys. Res 106 (E1), 1401 10.1029/2000JE001255. [DOI] [Google Scholar]
- Mousis O et al. , 2009. A primordial origin for the atmospheric methane of Saturn’s moon Titan. Icarus 204 (2), 749–751. 10.1016/j.icarus.2009.07.040. [DOI] [Google Scholar]
- Niemann HB et al. , 2010. Composition of Titan’s lower atmosphere and simple surface volatiles as measured by the Cassini–Huygens probe gas chromatograph mass spectrometer experiment. J. Geophys. Res 115 (E12), E12006 10.1029/2010JE003659. [DOI] [Google Scholar]
- Owen TC et al. , 2001. Protosolar nitrogen. Astrophys. J 553, L77–L79. [Google Scholar]
- Penz T et al. , 2005. The influence of the solar particle and radiation environment on Titan’s atmosphere evolution. Adv. Space Res 36 (2), 241–250. 10.1016/j.asr.2005.03.043 [DOI] [Google Scholar]
- Pepin RO, 1994. Evolution of the martian atmosphere. Icarus 111, 289–304. [Google Scholar]
- Pepin RO, 2006. Atmospheres on the terrestrial planets: Clues to origin and evolution. Earth Planet. Sci. Lett 252, 1–14. 10.1016/j.epsl.2006.09.014. [DOI] [Google Scholar]
- Ribas I et al. , 2005. Evolution of the solar activity over time and effects on planetary atmospheres. I. High-energy irradiances (1–1700 Å). Astrophys.J 622,680–694. [Google Scholar]
- Rousselot P et al. , 2014. Toward a unique nitrogen isotopic ratio in cometary ices. Astrophys.J 780 (2), L17 10.1088/2041-8205/780/2/L17. [DOI] [Google Scholar]
- Shinnaka Y et al. , 2014. 14NH2/15NH2 ratio in Comet C/2012 S1 (ISON) observed during its outburst in 2013 November. Astrophys. J 782 (2), L16 10.1088/2041-8205/782/2/L16. [DOI] [Google Scholar]
- Trigo-Rodriguez JM, Martin-Torres FJ, 2012. Clues on the importance of comets in the origin and evolution of the atmospheres of Titan and Earth. Planet. Space Sci 60, 3–9. [Google Scholar]
- Vinatier S, Bezard B, Nixon CA, 2007. The Titan 14N/15N and 12C/13C isotopic ratios in HCN from Cassini/CIRS. Icarus 191, 712–721. [Google Scholar]
- Wong MH et al. , 2013. Isotopes of nitrogen on Mars: Atmospheric measurements by Curiosity’s mass spectrometer. Geophys. Res. Lett 40, 1–5. 10.1002/2013GL057840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YL et al. , 1977. Photochemistry of nitrogen in the martian atmosphere. Icarus 30, 26–41. [Google Scholar]


