Abstract
Background
Individuals with chronic bronchitis or chronic obstructive pulmonary disease (COPD) may suffer recurrent exacerbations with an increase in volume or purulence of sputum, or both. Personal and healthcare costs associated with exacerbations indicate that therapies that reduce the occurrence of exacerbations are likely to be useful. Mucolytics are oral medicines that are believed to increase expectoration of sputum by reducing its viscosity, thus making it easier to cough it up. Improved expectoration of sputum may lead to a reduction in exacerbations of COPD.
Objectives
Primary objective
• To determine whether treatment with mucolytics reduces exacerbations and/or days of disability in patients with chronic bronchitis or COPD
Secondary objectives
• To assess whether mucolytics lead to improvement in lung function or quality of life
• To determine frequency of adverse effects associated with use of mucolytics
Search methods
We searched the Cochrane Airways Group Specialised Register and reference lists of articles on 12 separate occasions, most recently on 23 April 2019.
Selection criteria
We included randomised studies that compared oral mucolytic therapy versus placebo for at least two months in adults with chronic bronchitis or COPD. We excluded studies of people with asthma and cystic fibrosis.
Data collection and analysis
This review analysed summary data only, most derived from published studies. For earlier versions, one review author extracted data, which were rechecked in subsequent updates. In later versions, review authors double‐checked extracted data and then entered data into RevMan 5.3 for analysis.
Main results
We added four studies for the 2019 update. The review now includes 38 trials, recruiting a total of 10,377 participants. Studies lasted between two months and three years and investigated a range of mucolytics, including N‐acetylcysteine, carbocysteine, erdosteine, and ambroxol, given at least once daily. Many studies did not clearly describe allocation concealment, and we had concerns about blinding and high levels of attrition in some studies. The primary outcomes were exacerbations and number of days of disability.
Results of 28 studies including 6723 participants show that receiving mucolytics may be more likely to be exacerbation‐free during the study period compared to those given placebo (Peto odds ratio (OR) 1.73, 95% confidence interval (CI) 1.56 to 1.91; moderate‐certainty evidence). However, more recent studies show less benefit of treatment than was reported in earlier studies in this review. The overall number needed to treat with mucolytics for an average of nine months to keep an additional participant free from exacerbations was eight (NNTB 8, 95% CI 7 to 10). High heterogeneity was noted for this outcome (I² = 62%), so results need to be interpreted with caution. The type or dose of mucolytic did not seem to alter the effect size, nor did the severity of COPD, including exacerbation history. Longer studies showed smaller effects of mucolytics than were reported in shorter studies.
Mucolytic use was associated with a reduction of 0.43 days of disability per participant per month compared with use of placebo (95% CI ‐0.56 to ‐0.30; studies = 9; I² = 61%; moderate‐certainty evidence). With mucolytics, the number of people with one or more hospitalisations was reduced, but study results were not consistent (Peto OR 0.68, 95% CI 0.52 to 0.89; participants = 1788; studies = 4; I² = 58%; moderate‐certainty evidence). Investigators reported improved quality of life with mucolytics (mean difference (MD) ‐1.37, 95% CI ‐2.85 to 0.11; participants = 2721; studies = 7; I² = 64%; moderate‐certainty evidence). However, the mean difference did not reach the minimal clinically important difference of ‐4 units, and the confidence interval includes no difference. Mucolytic treatment was associated with a possible reduction in adverse events (OR 0.84, 95% CI 0.74 to 0.94; participants = 7264; studies = 24; I² = 46%; moderate‐certainty evidence), but the pooled effect includes no difference if a random‐effects model is used. Several studies that could not be included in the meta‐analysis reported high numbers of adverse events, up to a mean of five events per person during follow‐up. There was no clear difference between mucolytics and placebo for mortality, but the confidence interval is too wide to confirm that treatment has no effect on mortality (Peto OR 0.98, 95% CI 0.51 to 1.87; participants = 3527; studies = 11; I² = 0%; moderate‐certainty evidence).
Authors' conclusions
In participants with chronic bronchitis or COPD, we are moderately confident that treatment with mucolytics leads to a small reduction in the likelihood of having an acute exacerbation, in days of disability per month and possibly hospitalisations, but is not associated with an increase in adverse events. There appears to be limited impact on lung function or health‐related quality of life. Results are too imprecise to be certain whether or not there is an effect on mortality. Our confidence in the results is reduced by high levels of heterogeneity in many of the outcomes and the fact that effects on exacerbations shown in early trials were larger than those reported by more recent studies. This may be a result of greater risk of selection or publication bias in earlier trials, thus benefits of treatment may not be as great as was suggested by previous evidence.
Plain language summary
Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease
Background to the question
Chronic obstructive pulmonary disease (COPD) and chronic bronchitis are long‐term breathing conditions. They cause symptoms such as shortness of breath, cough, and excess sputum. People with COPD and chronic bronchitis may have flare‐ups (exacerbations) when their symptoms become worse.
Mucolytics are medicines taken orally that may loosen sputum, making it easier to cough it up. Mucolytics may have other beneficial effects on lung infection and inflammation and may reduce the number of flare‐ups that people with COPD and chronic bronchitis have. Mucolytics can also be inhaled, but we did not look at inhaled mucolytics in this review.
Study characteristics
We looked for studies lasting at least two months, in which it was decided at random whether a person received a mucolytic drug or a placebo. We did not include studies involving children or people with other breathing conditions such as asthma and cystic fibrosis.
We found 38 studies to include in our review. These studies included a total of 10,377 adults with COPD or chronic bronchitis. The studies used a variety of mucolytic drugs, including N‐acetylcysteine, carbocysteine, and erdosteine and lasted from two months to three years. Mucolytics were taken by mouth between one and three times per day. These studies measured several different outcomes to find out if the drug was useful, including flare‐ups, hospital admissions, quality of life, lung function, and side effects.
Key results
We found that people taking mucolytic drugs were less likely to experience a flare‐up compared to those taking placebo. Approximately eight people would need to take the drug for nine months for one extra person to avoid having a flare‐up. This result was based on 28 studies involving 6723 people. However, the studies carried out a longer time ago (1970s to 1990s) show greater benefit than those carried out more recently. Shorter studies also seemed to show more benefit than longer studies. This could be because the newer trials were larger and may be showing that mucolytics are less beneficial than the earlier studies showed. Or it could be that only studies that showed mucolytics as beneficial were published before the 2000s, when there was a push to report all trial results regardless of whether or not they showed benefit.
People taking mucolytics had fewer days of disability (i.e. days when they could not do their normal activities) every month, but this was quite a small difference ‐ less than half a day per person per month. They were also approximately one‐third less likely to be admitted to hospital, although this result is based on only five studies that provided this information.
Study results suggest that mucolytics do not have an important impact on quality of life or lung function. People taking mucolytics did not experience more unwanted side effects than those taking placebo. But we could not be sure about their impact on death during the study period because only 37 deaths occurred amongst the 3527 participants in studies where deaths were measured and reported.
Quality of the evidence
We are moderately confident about the results we have presented. Our confidence is reduced by the results from individual studies looking quite different from one another and the mix of older and newer studies that we found. Also, in some cases there were not enough data to be sure whether mucolytics were better or worse than, or the same as, placebo.
Conclusions
Mucolytics appear to be useful for reducing flare‐ups, days of disability, and hospital admissions in people with COPD or chronic bronchitis, and they do not appear to cause more side effects. However, they do not appear to have much impact on quality of life or lung function, and we could not be sure about their impact on death.
This plain language summary is current to April 2019.
Summary of findings
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a long‐term progressive condition primarily affecting the lungs, but with a wide range of extrapulmonary manifestations. Symptoms typically include shortness of breath (dyspnoea), impaired exercise tolerance, wheezing, cough, and sputum production. In more severe cases, COPD may progress to cor pulmonale, respiratory failure, and death (Qaseem 2011). It is estimated that COPD is the fourth most common single cause of death worldwide (WHO 2017). Few interventions have been demonstrated to convincingly reduce mortality, with the exception of smoking cessation, long‐term oxygen therapy in hypoxaemic patients and lung volume reduction surgery in selected patients (GOLD 2019; van Agteren 2016).
A diagnosis of COPD is usually made when a person who has symptoms of COPD is found to have airflow obstruction (post‐bronchodilator forced expiratory volume in one second (FEV₁)/forced vital capacity (FVC) < 0.70) in the absence of an alternative explanation for the symptoms (e.g. left ventricular failure) or the airflow obstruction (e.g. asthma) (Qaseem 2011). Many people with chronic bronchitis also have COPD. Smoking is the main risk factor for COPD; up to 50% of smokers will develop COPD, and most will have some breathing impairment (GOLD 2019; Rennard 2006). Chronic bronchitis and COPD are preventable and treatable diseases that are associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and the lung (GOLD 2019). Exacerbations and comorbidities contribute to overall severity in individual patients.
Exacerbations occur with increasing frequency as the disease becomes more severe. They are characterised by increased breathlessness or greater volume or purulence of sputum, or both. Exacerbations accelerate decline in lung function and are associated with worse quality of life and higher mortality. They are the largest contributor to healthcare costs in COPD (Criner 2015). Thus, treatments that reduce the frequency and duration of acute exacerbations will provide benefit for both individual patients and healthcare systems.
Description of the intervention
Mucolytics are oral medicines, given at least once daily, that are believed to increase expectoration of sputum by reducing its viscosity, thus making it easier to cough it up. There are several different types of mucolytic, including carbocysteine, acetylcysteine, erdosteine, and ambroxol (Yang 2018). They are given in combination with, rather than instead of, other COPD therapies, such as inhaled long‐acting beta₂‐agonists (LABAs) and long‐acting muscarinic antagonists (LAMAs).
Mucolytics are included as a treatment option for patients experiencing frequent exacerbations in several national and international management guidelines. International Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines state that mucolytics may reduce exacerbations and modestly improve health status, but there is currently a lack of evidence to precisely target the population most likely to benefit (GOLD 2019). COPD‐X guidelines, produced in Australia and New Zealand, give a stronger recommendation, stating "there is evidence to support the use of high dose oral N‐acetylcysteine in the reduction of COPD exacerbations and improvements in lung function" and "high dose (≥ 1200 mg/day) N‐acetylcysteine should be considered as an effective therapy for reducing exacerbations" (Yang 2018). UK National Institute for Health and Care Excellence (NICE) guidelines currently suggest that mucolytics should be considered for patients with chronic cough productive of sputum and continued if there is symptomatic improvement. However, the guidelines state they should not be routinely prescribed to prevent exacerbations (NICE 2018). Joint American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines for prevention of exacerbations make the following recommendation: "for patients who have COPD with moderate or severe airflow obstruction and exacerbations despite optimal inhaled therapy, we suggest treatment with an oral mucolytic agent to prevent future exacerbations" (Wedzicha 2017). However, this is qualified as being a conditional recommendation, based on low quality of evidence.
How the intervention might work
Mucus clearance is one of the most important tools the lung has to protect itself from pathogens (Rubin 2014). Mucus is a gel‐like material complete with glycoproteins called mucins, serum proteins, and water. In contrast, sputum refers to expectorated mucus with the addition of inflammatory cells and DNA. Mucus is removed from the lungs and airways via cilia hairs and airflow; however sputum is removed primarily by coughing (Rubin 2014).
Mucolytics work by changing the physical properties of the secretions themselves. They can work by degrading the mucin polymers, fibrin, or DNA in airway secretions, which makes them less viscous. This makes it easier for the body to clear them and reduces the risk of bacterial contamination. Classic mucolytics such as N‐acetylcysteine (NAC) exert their effects by depolymerising the mucin glycoproteins via a hydrolysis reaction (Rubin 2007). One study found that NAC may improve pulmonary function, but there was uncertainty as to whether or not this was in fact mediated by its antioxidant ability (Hansen 1994). Given that oxidative stress is thought to be an amplifying mechanism in COPD (Rahman 2005), this property of N‐acetylcysteine may be useful in chronic airways disease.
Lubricants and surfactant stimulators such as ambroxol can make the sputum less adhesive, making it easier for the cilia to clear and more likely that a cough will be able to transport it throughout the pharynx (Rubin 2007). In a chronic inflammatory process such as COPD, production of phospholipase A2 can cause destruction of the surfactant phospholipids, making the sputum incredibly adherent to the cilia and further causing airway obstruction (Rubin 2007). One study found that aerosolised surfactant was able to increase FEV₁ % predicted and FVC by up to 10% by reducing adherence of mucus in the airways (Anzueto 1997).
Why it is important to do this review
As illustrated by varied recommendations from guidelines, there is lack of international consensus on the place of mucolytics in the treatment of COPD. As theoretical reasons have been proposed to explain why mucolytics may work in both chronic bronchitis and COPD, and because treatments that reduce exacerbations are needed to reduce morbidity and costs, this review update will seek to determine the true effect of this class of medicines.
Objectives
Primary objective
To determine whether treatment with mucolytics reduces exacerbations and/or days of disability in patients with chronic bronchitis or COPD
Secondary objectives
To assess whether mucolytics lead to improvement in lung function or quality of life
To determine the frequency of adverse effects associated with use of mucolytics
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, placebo‐controlled trials.
Types of participants
We included studies of adults (over 20 years of age) with chronic bronchitis as defined by the British Medical Research Council (cough and sputum on most days during at least three consecutive months for longer than two successive years) or COPD as defined by the criteria of the American Thoracic Society, the Global Initiative for Chronic Obstructive Lung Disease (GOLD), the European Respiratory Society, or the World Health Organization (WHO). We excluded studies on patients with asthma or cystic fibrosis.
Types of interventions
Participants must have received regular treatment with oral mucolytics or placebo for at least two months. Oral mucolytics included the following compounds: N‐acetylcysteine (NAC), S‐carboxymethylcysteine, bromhexine, ambroxol, erdosteine, sobrerol, cithiolone, letosteine, iodinated glycerol, N‐isobutyrylcysteine, myrtol, and cineole.
We excluded studies of inhaled mucolytics and combinations of mucolytics with antibiotics and mucolytics with bronchodilators, as well as studies of deoxyribonuclease or proteases such as trypsin.
Types of outcome measures
Primary outcomes
Exacerbations, as measured by the number of participants with no exacerbations during the study period, as well as the total number of acute exacerbations per participant* and time to first exacerbation. Exacerbation was defined as an increase in cough and by volume and/or purulence of sputum
Number of days of disability variously defined as days in bed, days off work, or days on which the participant was unable to undertake normal activities. We also assessed days on antibiotics
*For the 2019 update, we removed exacerbations per patient per month analyses as these are not considered to be as statistically robust as the dichotomous exacerbation outcome, largely due to likely skew in this measure. Instead we present these data in tables.
Secondary outcomes
Measures of lung function, including forced expiratory volume in one second (FEV₁), forced vital capacity (FVC), and peak expiratory flow rate (PEFR)
Adverse effects of treatment
Hospitalisation and mortality
Quality of life as measured by a tool validated in patients with COPD
We had intended to use symptom scores as a secondary outcome measure, but it became clear that symptoms were not reported in a consistent fashion, and it was not possible to standardise symptom scores.
Adverse events were not usually reported in detail and generally were mild and self‐limiting, so we have entered only the total number of adverse events.
Search methods for identification of studies
Electronic searches
Search methods and search history for previous versions of this review are detailed in Appendix 1. The previously published version included searches up to July 2014. The search period for this update is July 2014 through April 2019.
We identified studies from the Cochrane Airways Group Trials Register (CAGR), which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies (CRS).
Weekly searches of MEDLINE Ovid.
Weekly searches of Embase Ovid.
Monthly searches of PsycINFO Ovid.
Monthly searches of Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO.
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings, are provided in Appendix 2. We searched for relevant trials in the Register using the search strategy presented in Appendix 3. We did not apply restrictions on language or type of publication.
Searching other resources
We checked the references of all papers and reviews for which we obtained the full text to identify other relevant articles. We asked other researchers in the field to provide additional references, and we remained open to unsolicited suggestions regarding potentially eligible studies. For the 2014 and 2019 updates, we searched these online clinical trials registers: ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO trials portal (www.who.int/ictrp/en/).
Data collection and analysis
Selection of studies
At least one review author (Peter Black and PP for original review; PP and Jimmy Chong for the 2001, 2006, and 2012 updates; and PP and RF for the 2019 update) assessed all abstracts obtained from the search of the CAGR. We obtained the full text for those that appeared to fit the criteria for inclusion (or if this was not clear from the abstract). Two review authors independently selected trials for inclusion in the original review and updates and resolved disagreements over inclusion by discussion. Six translators (two of whom were medically trained) assessed papers published in languages other than English. For the 2012 and 2014 updates, the review lead author (PP) was assisted by another Cochrane review author (Jimmy Chong) in extracting data. For the 2019 update, RF and KS extracted and entered data, with input from PP.
Data extraction and management
We extracted data onto worksheets before entering them into the Review Manager software (RevMan 5.3). We double‐checked all entries against the original paper. In the 1999 update, we rechecked all data from earlier studies. In the 2019 update, we rechecked lung function data from earlier studies to separate the analyses into FEV₁, percent predicted FEV₁, PEFR, and FVC, rather than a combined standardised mean difference analysis.
Assessment of risk of bias in included studies
We used the following to assess sources of bias in selection, allocation, performance, detection, attrition, or reporting (Higgins 2011).
Low risk of bias.
Unclear risk of bias: if insufficient information was available.
High risk of bias.
When assessing attrition bias, we used an approximate cut‐off of 20% dropout for high risk, although we also took into account the type of analysis performed (e.g. intention‐to‐treat), the balance between trial arms, and the reasons given for dropout.
Measures of treatment effect
We analysed continuous data using mean differences (MDs). We used Peto odds ratios (ORs) for dichotomous data and reported results with 95% confidence intervals (CIs).
Unit of analysis issues
We calculated exacerbation rates and days of disability by dividing the number of events by the number of participants and the number of months of the study (i.e. per participant per month). We scaled standard deviations for monthly rates in the same way. For the 2019 update, we archived the exacerbation rates analyses.
Dealing with missing data
If data were insufficient, we requested further information by writing to the study author or to the pharmaceutical company sponsoring the study.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. We reported cases of substantial heterogeneity and explored possible causes by performing prespecified subgroup analysis. As per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we considered the following ranges for assessing heterogeneity.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: may show considerable heterogeneity.
Assessment of reporting biases
When we were able to pool more than 10 trials, we created and examined a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We used summary statistics rather than individual patient data. We used a fixed‐effect model.
For the outcome of having 'no exacerbation in the study period', we calculated a number needed to treat for an additional beneficial outcome (NNTB) based on the pooled Peto odds ratio (Cates 2002), with baseline risk taken from the pooled control group event rate (total number of events divided by overall number of participants in the placebo group multiplied by 100).
Subgroup analysis and investigation of heterogeneity
From the outset, we planned a priori subgroup analyses based on type of mucolytic, dose, duration, country of study, disease severity, and whether or not participants were included, as they had a history of exacerbation.
Following publication of the BRONCUS study (Decramer 2005), which suggested a differential effect of mucolytics depending on concomitant treatment, we included an analysis on whether concomitant inhaled corticosteroids were permitted.
From 2012 onwards, we carried out a post hoc investigation of time trends in data for participants with one or more exacerbations by comparing results of trials published since 2000 versus those published earlier.
Sensitivity analysis
For 2012 onwards, we explored heterogeneity in results on exacerbations, and we conducted a sensitivity analysis using data from trials assessed as having low risk of selection bias (on the basis of allocation concealment). For the 2019 update, we conducted a sensitivity analysis removing studies judged to be at high risk of attrition bias.
Results
Description of studies
Results of the search
For details of the search history, see Appendix 1, and for the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) study flow diagram for this update, see Figure 3.
3.
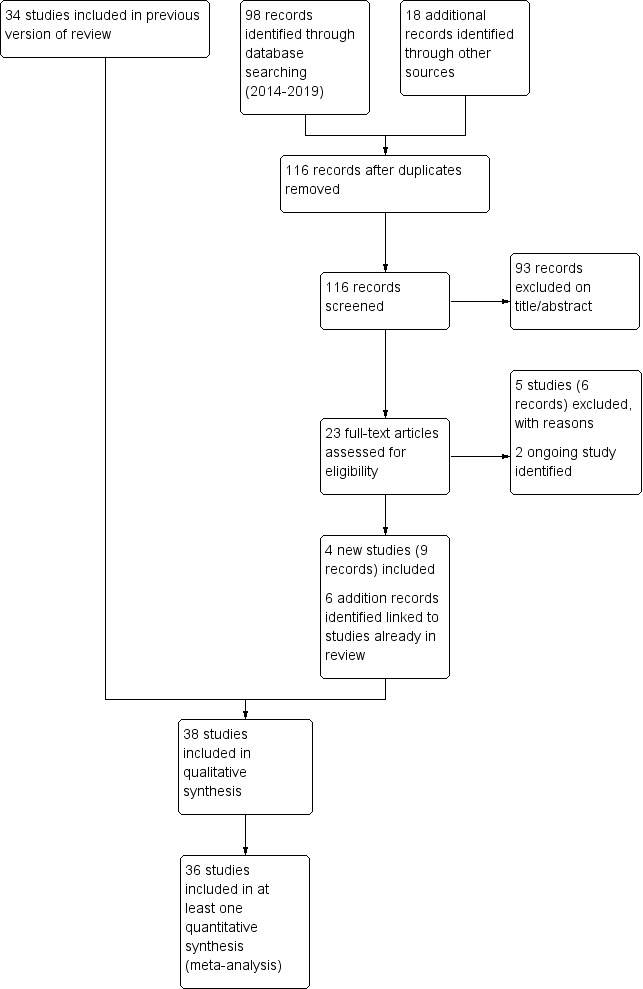
Study flow diagram: review update.
After de‐duplication, the database search run on 23 April 2019 yielded 98 references, and searches of clinical trial registries identified a further 18 records. We excluded 93 on the basis of title and abstract and reviewed 23 full texts for possible inclusion. We excluded a further six records (five unique studies) at this stage and identified two ongoing studies that meet the inclusion criteria for this review (Characteristics of ongoing studies). The remaining 15 records were eligible for inclusion. We added nine records, linked to four new unique studies, to the review (Dal Negro 2017; Fukuchi 2016; Johnson 2016; Xu 2014). We identified a further six records, which were additional references to studies already included in the review. We wrote to authors of all four newly included studies to request further information; we received a response from the authors of Dal Negro 2017, Fukuchi 2016, and Johnson 2016, and we are grateful to Professor Dal Negro, Professor Inoue, and Dr Niewoehner for the additional data/details they provided.
The 2014 search yielded 29 abstracts, as well as four new eligible studies ‐ all of NAC versus placebo. Four abstracts related to the eligible study of Zheng 2014, four to Tse 2013, three to De Backer 2013, and one to Roy 2014. We found a total of 17 reports of ineligible studies, including Moretti 2011, which in 2012 was awaiting classification. We found a further report of the Roy study while searching for study authors' contact details. Searches of online clinical trials databases yielded no further studies.
In the initial review in 1997, we wrote to the authors of 10 studies (Allegra 1996; Babolini 1980; Boman 1983; Castiglioni 1986; Christensen 1971; Grillage 1985; Jackson 1984; Nowak 1999; Parr 1987; Petty 1990) to request more information. We received further data for two studies (Allegra 1996; Nowak 1999). Dr Petty responded to our letter but could not supply data because they were held by a pharmaceutical company (the company has not replied to two letters). Dr Boman wrote to say that he was unable to supply us with additional data. This was also the case for Novartis Pharmaceuticals (UK), which responded on behalf of two study authors (Jackson 1984; Parr 1987), and Parke Davis Research Laboratories (Grillage 1985). We received no reply to our request for additional data related to the remaining three studies (Babolini 1980; Castiglioni 1986; Christensen 1971), although we sent two letters. We also wrote to the authors of Olivieri 1987 to clarify the error measurement used, but we received no reply. Pharmaceutical companies notified us of two studies (Meister 1986; Meister 1999); the former was unpublished. They also provided further information on four studies (Meister 1986; Meister 1999; Nowak 1999; Pela 1999). In 2008 we contacted an author of the COOPT study, 'A double‐blind placebo‐controlled trial comparing the efficacy and cost‐effectiveness of inhaled fluticasone propionate versus oral N‐acetylcysteine in the treatment of patients with COPD in general practice' (Clinical Trials identifier: NCT00184977), which was conducted from 1998 to 2003, to ascertain whether any data might be made available for this review. This study has now been published and is included in the review (Schermer 2009). In 2012, we contacted the lead author of Decramer 2005 to clarify conflicting information on quality of life in the published report; the lead author helpfully provided us with information derived from the St George's Respiratory Questionnaire (SGRQ).
In 2014, we wrote to Dr De Backer to request additional details on the secondary outcomes of spirometry and quality of life (De Backer 2013), but we received no response. As this was a small cross‐over study with few outcomes of relevance to this review, we have not pursued this. Dr Zheng provided the appendix to Zheng 2014, which contained further details on study design and outcomes. In response to another request, Dr Zheng provided standard deviations (SDs) of exacerbation rates and total SGRQ, as well as mean (SD) end of study FEV₁ and FVC values.
Included studies
By 2019, this review included 38 randomised controlled trials (RCTs), which had recruited a total of 10,377 participants. We provide full details of each study in Characteristics of included studies and an overview in Table 2.
1. Summary of study characteristics.
| Study ID | Total n | Study duration (weeks) | Mean age (years) | COPD severity | Country | Intervention | Control | Outcomes |
| Allegra 1996 | 440 | 26 | 60.0 | Moderate to severe | Italy | Carbocysteine‐lysine 2.7 g daily | Placebo | Diary of scores, exacerbations, time to first exacerbation, duration of exacerbation, days on antibiotics, AEs |
| Babolini 1980 | 744 | 26 | Not reported | Moderate to severe | Italy | NAC 200 mg twice daily | Placebo | Exacerbations, symptom scores, global assessments by patients and physicians, AEs, days on antibiotics |
| Bachh 2007 | 100 | 52 | 61.0 | Moderate to severe | India | NAC 600 mg once daily | Placebo | Exacerbations, hospital admissions, lung function, AEs |
| Boman 1983 | 259 | 26 | 51.9 | Severe to very severe | Sweden | NAC 200 mg twice daily | Placebo | Exacerbations, sick leave due to exacerbation, AEs |
| Bontognali 1991 | 60 | 13 | 57.0 | Italy | Cithiolone 400 mg twice daily | Placebo for 1 month followed by 400 mg once daily for a further 2 months | Exacerbations, duration of acute exacerbations, FEV₁, FVC, sputum viscosity, AEs | |
| Borgia 1981 | 21 | 26 | 45.3 | Moderate to severe | Italy | NAC 200 mg twice daily | Placebo | Exacerbations, lung function, symptom scores, clinical assessments, AEs |
| Castiglioni 1986 | 706 | 13 | 56.5 | Mild to moderate | Italy | Sobrerol 300 mg twice daily | Placebo | Exacerbation rate, consumption of antibiotics, clinical signs, laboratory data, lung function, global assessment by investigator and patient, AEs |
| Cegla 1988 | 180 | 104 | 51.1 | Germany | Ambroxol retard 75 mg | Placebo | Exacerbations, days sick (off work, in hospital), patient symptoms by diary card, lung function, extra medication use, assessment by investigator and patient, AEs | |
| Cremonini 1986 | 41 | 13 | 60.8 | Italian | Letosteine 50 mg 3 times daily | Placebo | Exacerbations, days off sick, lung function | |
| Dal Negro 2017 | 467 | 52 | 64.8 | Moderate to severe | 10 European countries | Erdosteine 300 mg twice daily | Placebo | Number of acute exacerbations, spirometry parameters, COPD symptoms, QoL, safety and tolerability of erdosteine |
| De Backer 2013 | 12 | 13 | 65.0 | Moderate to severe | Belgium | NAC 600 mg 3 times daily | Placebo | Spirometry, PEFR, raw, NO, specific airway resistance from plethysmography, CT to look at airway geometry, serum glutathione, enzymes, SGRQ, ABG |
| Decramer 2005 | 523 | 156 | 62.0 | Moderate to severe | Europe | NAC 600 mg daily | Placebo | Lung function, exacerbation rate, QoL, cost utility |
| Ekberg‐Jansson 1999 | 637 | 26 | 58.0 | Mild, moderate to severe | Europe | NIC 300 mg twice daily | Placebo | Time to first exacerbation, exacerbation rate, days sick (judged by patients and investigators), lung function, AEs |
| Fukuchi 2016 | 408 | 52 | Not reported | Moderate, severe to very severe | Japan | Lysozyme 90 mg 3 times daily | Placebo | Exacerbation rate, time to first exacerbation, lung function, CAT |
| Grassi 1976 | 80 | 26 | 60.9 | Italy | NAC 600 mg daily | Placebo | Exacerbations, clinical symptoms, sputum characteristics, AEs | |
| Grassi 1994 | 135 | 13 | 61.8 | Italy | Carbocysteine 1125 mg plus sobrerol 180 mg once daily | Placebo or alternating active‐placebo for 10 days each | Exacerbations, symptoms, sputum characteristics | |
| Grillage 1985 | 109 | 26 | Not reported | Britain | Carbocysteine 750 mg 3 times daily | Placebo | Exacerbations, lung function, AEs | |
| Hansen 1994 | 153 | 22 | 51.4 | Mild to moderate | Denmark | NAC 600 mg twice daily | Placebo | Exacerbations, subjective symptom scores, global well‐being, lung function, AEs |
| Jackson 1984 | 155 | 13 | 63.0 | Great Britiain | NAC 200 mg 3 times daily | Placebo | Exacerbations, subjective symptoms, clinical signs, radiological appearance, global well being, AEs | |
| Johnson 2016 | 51 | 8 | 70.0 | Mild to moderate | USA | NAC 1800 mg twice daily | Placebo | Change SGRQ, CBSAS, SF‐36; post‐bronchodilator lung function |
| Malerba 2004 | 242 | 52 | 60.0 | Moderate | Italy | Ambroxol 75 mg twice daily | Placebo | Exacerbation over first 6 months (winter period) and at 12 months, cough intensity and frequency, difficult expectoration, dyspnoea, days on antibiotics, number of working days lost |
| McGavin 1985 | 244 | 22 | 63.4 | Severe to very severe | Great Britain | NAC 200 mg 3 times daily | Placebo | Exacerbation, days of antibiotics, days in bed, FEV₁, VC, AEs |
| Meister 1986 | 252 | 26 | 57,2 | Germany | NAC 300 mg twice daily | Placebo | Exacerbation, days sick, concomitant treatment, AEs | |
| Meister 1999 | 246 | 26 | 57.0 | Mild to moderate | Germany | Myrtol 300 mg 3 times daily | Placebo | Exacerbation, number of exacerbations requiring antibiotics, well‐being, AEs |
| Moretti 2004 | 155 | 35 | 67.0 | Moderate, severe to very severe | Italy | Erdosteine 300 mg twice daily | Placebo | Exacerbation frequency, duration, hospitalisation, lung function, 6MWT, SGRQ, pharmacoeconomic analysis |
| Nowak 1999 | 313 | 35 | 57.0 | Europe | NAC 600 mg daily | Placebo | Exacerbation, severity of exacerbations, time to first exacerbation, days sick, lung function, patient symptoms, AEs | |
| Olivieri 1987 | 240 | 26 | Not reported | Mild, moderate to severe | Italy | Ambroxol retard 75 mg daily | Placebo | Exacerbation, course of antibiotics, days sick, FEV₁, VC, symptoms, auscultatory findings, physicians' and patients' global assessments, laboratory data, AEs |
| Parr 1987 | 526 | 26 | 63.0 | Great Britain | NAC 200 mg 3 times daily | Placebo | Exacerbation, days off work, AEs | |
| Pela 1999 | 169 | 26 | 66.0 | Moderate, severe to very severe | Italy | NAC 600 mg daily | Placebo | Exacerbation, exacerbation severity, days sick, patient preference, lung function |
| Petty 1990 | 367 | 8 | 65.0 | Moderate, severe to very severe | USA | Iodinated glycerol 30 mg 4 times daily | Placebo | Investigator assessment of symptoms, patient evaluation of symptoms and global assessment, frequency of bronchodilator use, number and duration of acute exacerbations, frequency of concomitant medications, AEs |
| Rasmussen 1988 | 116 | 26 | 58.9 | Sweden | NAC 300 mg twice daily | Placebo | Exacerbation, days sick evaluated by days on sick list and by patient diaries, AEs | |
| Roy 2014 | 80 | 26 | 61.0 | Mild to Moderate | India | NAC 600 mg twice daily | Placebo | Symptoms (cough, dyspnoea, sputum), lung function, haemoglobin levels, AEs |
| Schermer 2009 | 192 | 156 | 59.0 | Mild, moderate, severe to very severe | Netherlands | NAC 600 mg daily | Placebo | Rate of exacerbations, CRQ |
| Tse 2013 | 133 | 52 | 71.0 | Mild, moderate to severe | China | NAC 600 mg twice daily | Placebo | Small airways parameters FEF25‐75%, FOT, IC, spirometry, exacerbation rate, dyspnoea, SGRQ, 6MWD |
| Worth 2009 | 220 | 26 | 62.3 | Moderate to severe | Germany | Cineole 200 mg 3 times daily | Placebo | Exacerbations: number, severity, and duration, lung function, dyspnoea, SGRQ, AEs |
| Xu 2014 | 84 | 26 | Not reported | Moderate to severe | China | NAC 600 mg twice daily | Salmeterol/fluticasone propionate | FEV₁ %/FVC, FEV₁ % predicted, PEF% daily variation change, PaO₂, PaCO₂ |
| Zheng 2008 | 709 | 52 | 65.0 | Moderate, severe to very severe | China | Carbocysteine 500 mg 3 times daily | Placebo | Exacerbation rate, covariance‐adjusted exacerbation rate, QoL, lung function, arterial oxygen saturation |
| Zheng 2014 | 1006 | 52 | 66.0 | Moderate to severe | China | NAC 600 mg 3 times daily | Placebo | Exacerbation rate, exacerbation duration, time to first exacerbation, time to recurrent exacerbation, number of participants requiring systemic corticosteroids or antibiotics or SABA, SGRQ (Chinese version), lung function, AEs (including hospitalisation or death) |
6MWD: six‐minute walk distance; AEs: adverse events; CAT: COPD assessment test; CBSAS: Chronic Bronchitis Symptoms Assessment Scale; CRQ: chronic respiratory questionnaire; FEF25‐75%: forced expiratory flow at 25%‐75% of the pulmonary volume; FEV₁: forced expiratory volume in one second; FOT: forced oscillatory technique; FVC: forced vital capacity; IC: inspiratory capacity; NAC: N‐acetylcysteine; NIC: N‐isobutyrylcysteine; PaCO₂: partial pressure of carbon dioxide; PaO₂: partial pressure of oxygen; PEF: peak expiratory flow; QoL: quality of life; SABA: short‐acting beta‐agonist; SCMC‐Lys: carbocysteine lysine salt monohydrate; SF‐36:Short Form‐36 Health Survey; SGRQ: St. George's respiratory questionnaire; VC: vital capacity.
A total of 15 studies examined use of mucolytics in people with COPD only (Bachh 2007; Dal Negro 2017; De Backer 2013; Decramer 2005; Fukuchi 2016; Malerba 2004; Moretti 2004; Nowak 1999; Pela 1999; Roy 2014; Tse 2013; Worth 2009; Xu 2014; Zheng 2008; Zheng 2014). The other studies involved people with chronic bronchitis, COPD, or both.
All but four studies were randomised, double‐blind, and placebo‐controlled and used a parallel‐group design. Blinding was not described in Xu 2014. Study duration ranged from 2 to 36 months. Fourteen studies had a run‐in period (Allegra 1996; Boman 1983; Dal Negro 2017; Ekberg‐Jansson 1999; Fukuchi 2016; Malerba 2004; McGavin 1985; Meister 1999; Moretti 2004; Olivieri 1987; Schermer 2009; Tse 2013; Zheng 2008; Zheng 2014). Four studies were described as randomised and placebo‐controlled but not as double‐blind. One of these was labelled as 'open' (Pela 1999), and two (Bachh 2007; Roy 2014) were 'single‐blind' trials. The fourth was a randomised cross‐over trial (De Backer 2013). As a result of the potential for bias, these are reported separately in analyses of primary outcomes.
In one study conducted in primary care practices (Schermer 2009), investigators compared NAC 600 mg daily versus placebo as well as inhaled fluticasone 500 μg twice daily in a three‐arm study of double‐dummy design. This review used data from NAC and placebo arms only.
Inclusion and exclusion criteria
All studies indicated that participants fulfilled criteria for chronic bronchitis, COPD, or both (except Nowak 1999, which has been published in abstract form only). Exclusion criteria varied, and some studies did not report whether patients with other respiratory illnesses were excluded.
Lung function
All but two studies ‐ Grassi 1976 and Parr 1987 ‐ reported baseline lung function using PEFR, FEV₁ or FEV₁ % predicted. When studies reported pre‐bronchodilator and post‐bronchodilator lung function, we used the latter.
Age of participants
The mean age of participants ranged from 40 to 71 years. Most studies had an upper age limit for participants.
Gender of participants
All but three of the studies reported the proportion of males included in the study. This ranged from 44% to 93%. In another study, "almost all" of the participants were reported as male.
Smokers
All but five studies reported the percentage of current smokers or ex‐smokers, which ranged from 55% to 100%.
Mucolytics and dose
In 21 studies, the mucolytic used was N‐acetylcysteine (NAC). Other treatments studied included carbocysteine (N = 3), ambroxol (N = 3), erdosteine (N = 2), sobrerol (N = 1), carbocysteine‐sobrerol (N = 1), carbocysteine‐lysine (n = 1), letosteine (N = 1), cithiolone (N = 1), iodinated glycerol (N = 1), N‐isobutyrylcysteine (NIC) (N = 1), myrtol (N = 1), and cineole and lysozyme (N = 1).
Of the 21 studies of NAC, three used a total dose of 400 mg/day (Babolini 1980; Boman 1983; Borgia 1981); 11 used a total dose of 600 mg/day (Bachh 2007; Decramer 2005; Grassi 1976; Jackson 1984; McGavin 1985; Meister 1986; Nowak 1999; Parr 1987; Pela 1999; Rasmussen 1988; Schermer 2009); five used 1200 mg/day (Hansen 1994; Roy 2014; Tse 2013; Xu 2014; Zheng 2014); one used 1800 mg/day (De Backer 2013; ); and one used 3600 mg/day (Johnson 2016).
Size and duration
Study size ranged from 12 participants in De Backer 2013 to 1006 participants in Zheng 2014. Duration ranged from 2 months in Petty 1990 and Johnson 2016 to 36 months in Decramer 2005 and Schermer 2009. The mean duration of treatment, weighted by study size, was 9.4 months. Over a third of participants were enrolled in studies lasting 12 months or longer.
Countries
Twelve studies were conducted only in Italy, four in the United Kingdom, four in Germany, four in China, four in several European countries, three in Scandinavia, two in India, two in the United States, and one each in The Netherlands, Belgium, and Japan.
Funding
We have extracted and presented information on study funding since the 2014 update. A majority of studies included since 2014 report pharmaceutical sponsorship, with the exception of Johnson 2016,Roy 2014, and Xu 2014.
Excluded studies
We excluded 20 studies after scrutiny of the full text. See Characteristics of excluded studies for the reasons for exclusion.
Risk of bias in included studies
Details of our risk of bias judgements are presented in Characteristics of included studies and in an overview in Figure 4.
4.
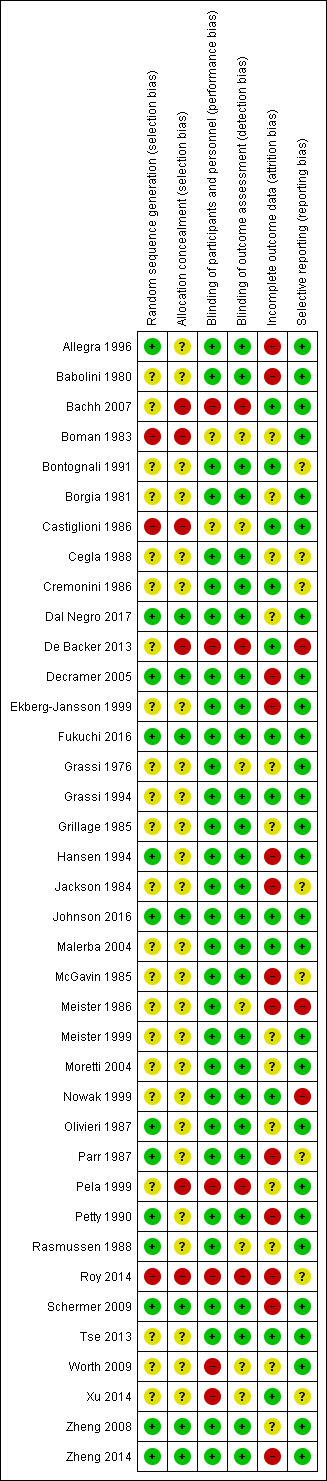
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Potential for bias in most studies was regarded as unclear, in that study authors stated that the study was randomised but did not indicate how this was achieved, where it was done, or how it was concealed. Seven studies were judged to be at low risk of bias for both random sequence generation and allocation concealment (Dal Negro 2017; Decramer 2005; Fukuchi 2016; Johnson 2016; Schermer 2009; Zheng 2008; Zheng 2014). Six studies were judged to be randomised but provided insufficient details about allocation concealment (Allegra 1996; Hansen 1994; Olivieri 1987; Parr 1987; Petty 1990; Rasmussen 1988). Six studies were judged to be at high risk of bias for one or both domains (Bachh 2007; Boman 1983; Castiglioni 1986; De Backer 2013; Pela 1999; Roy 2014).
Most studies reported baseline characteristics of treatment groups, which were well matched at baseline.
Blinding
Most studies (N = 30) reported that the placebo was identical in appearance to the active treatment and therefore were judged to be at low risk of performance bias. Six studies were regarded as high risk, which related largely to lack of blinding, although Xu 2014 provided no description of blinding, and so an open‐label policy must be assumed (Bachh 2007; De Backer 2013; Pela 1999; Roy 2014; Worth 2009; Xu 2014).
Blinding of outcome assessors was less well described, but 27 studies described adequate procedures, allowing us to judge these as having low risk of bias. Four studies were at high risk of bias and seven studies reported insufficient details about detection bias to permit a judgement.
Incomplete outcome data
Reported dropout ranged from 0% in Bachh 2007,Bontognali 1991,Cremonini 1986, and Xu 2014 to 37% in the three‐year BRONCUS study (Decramer 2005), and this rate was given as 43% in another three‐year study conducted in a general practice setting (Schermer 2009). When the rate exceeded 20%, we considered a high‐risk rating but also took into account whether numbers of dropouts were balanced between arms, and whether the reasons given for dropout were similar. We judged 13 studies to be at high risk (Allegra 1996; Babolini 1980; Decramer 2005; Ekberg‐Jansson 1999; Hansen 1994Jackson 1984; McGavin 1985; Meister 1986; Parr 1987; Petty 1990; Roy 2014; Schermer 2009; Zheng 2014). We judged 12 studies to be at low risk as dropout either was low or had been sufficiently well described that we were confident the results of the study were unlikely to be impacted (Bachh 2007; Bontognali 1991; Castiglioni 1986; Cremonini 1986; De Backer 2013; Fukuchi 2016; Grassi 1994; Johnson 2016; Malerba 2004; Nowak 1999; Tse 2013; Xu 2014). We judged the remaining studies to be at unclear risk because dropouts were not clearly reported or were sufficiently high to raise concern, even if numbers and reasons were balanced.
In most of the older studies and in Roy 2014, analyses were performed on participants who completed the study (per protocol), whereas in more recent studies, analyses tended to be performed on an intention‐to‐treat basis.
Selective reporting
Three studies were graded as high risk: two because they were unpublished (Meister 1986; Nowak 1999), and one because study authors did not report all study outcomes (De Backer 2013). Most of the other studies reported sufficient details that we could make a judgement of low risk of bias.
Other potential sources of bias
None were noted.
Effects of interventions
See: Table 1
Summary of findings for the main comparison. Mucolytic compared to placebo for chronic bronchitis or chronic obstructive pulmonary disease.
| Mucolytic compared to placebo for chronic bronchitis or chronic obstructive pulmonary disease | ||||||
| Patient or population: chronic bronchitis or COPD Setting: community Intervention: mucolytic Comparison: placebo | ||||||
| Outcomes* | Anticipated absolute effects† (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with mucolytic | |||||
|
Participants with no exacerbations in study period Follow‐up: 8.8 months |
386 per 1000 | 521 per 1000 (495 to 545) | Peto OR 1.73 (1.56 to 1.91) | 6723 (28 RCTs) | ⊕⊕⊕⊝ Moderatea | Generally larger effects in earlier studies of mucolytics in chronic bronchitis and smaller effects in more recent studies in COPD |
|
Days of disability per participant per month Follow‐up: 8.3 months |
Mean days of disability per participant per month was 1.57 days | MD 0.43 days lower (0.56 lower to 0.30 lower) | ‐ | 2259 (9 RCTs) | ⊕⊕⊕⊝ Moderatea,b | |
|
Health‐related quality of life (total score SGRQ) Scale from 1 to 100; lower scores indicate better quality of life Follow‐up: 14.1 months |
Mean SGRQ total score was 39.02 points | MD 1.37 lower (2.85 lower to 0.11 higher) | ‐ | 2721 (7 RCTs) | ⊕⊕⊕⊝ Moderatea,c | MCID for SGRQ is 4 points |
|
Hospitalisation during study period Follow‐up: 16.6 months |
188 per 1000 | 136 per 1000 (107 to 171) | Peto OR 0.68 (0.52 to 0.89) | 1833 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | |
|
FEV₁at end of study Follow‐up: 14.5 months |
Mean FEV₁ at end of study was 1.50 L | MD 0.04 L higher (0.01 higher to 0.07 higher) | ‐ | 3473 (14 RCTs) | ⊕⊕⊕⊝ Moderatea,b | MCID for FEV₁ in COPD is approximately 0.1 L (Jones 2013) |
|
Adverse effects Follow‐up: 8.2 months |
235 per 1000 | 205 per 1000 (185 to 224) | Peto OR 0.84 (0.74 to 0.94) | 7264 (24 RCTs) | ⊕⊕⊕⊝ Moderatea | |
|
Death during study period Follow‐up: 13.3 months |
11 per 1000 | 10 per 1000 (5 to 20) | Peto OR 0.98 (0.51 to 1.87) | 3527 (11 RCTs) | ⊕⊕⊕⊝ Moderated | 18 deaths on mucolytics and 19 on placebo |
| *Follow‐up was calculated as a weighted mean duration.
†The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease; FEV₁: forced expiratory volume in one second; MCID: minimally clinically important difference; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio; SGRQ: St. George's Respiartory Questionaire; WMD: weighted mean duration. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aStatistical and clinical heterogeneity identified. Downgraded once for inconsistency.
bFunnel plots suggest small negative trials under‐represented (Figure 1; Figure 2). However, removing the positive small trials from the analysis had little impact on the pooled estimate. No downgrade.
cConfidence interval includes possibility of no difference between groups, but both ends of confidence interval lie within MCID. No downgrade for imprecision.
dConfidence interval includes possibility of both an important increase or reduction in deaths. Downgraded once for imprecision.
Mucolytic versus control
Exacerbations
Patients with no exacerbations during study period
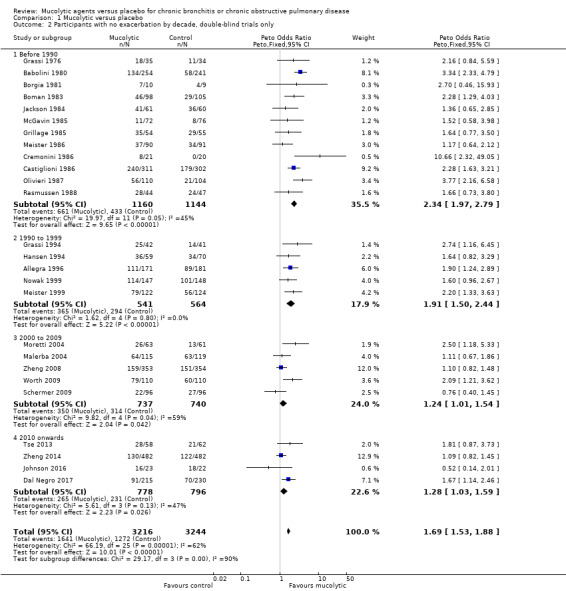
The odds ratio (OR) for having no exacerbations over the entire study period when treatment with mucolytics was provided in double‐blind trials was increased compared with placebo (Peto OR 1.69, 95% confidence interval (CI) 1.53 to 1.88; participants = 6460; studies = 26; I² = 62%; Figure 5; Analysis 1.1; moderate‐certainty evidence). This yielded a number needed to treat for an additional beneficial outcome (NNTB) of 8 (95% CI 7 to 10; Figure 6). Inclusion of single‐blind studies in the analysis had a minimal impact on the pooled effect (Peto OR 1.73, 95% CI 1.56 to 1.91; participants = 6723; studies = 28; I² = 62%). We also conducted a sensitivity analysis including only the studies judged to be at low risk of selection bias; this substantially reduced the number of studies in the meta‐analysis, and the effect was attenuated (Peto OR 1.15, 95% CI 0.96 to 1.37; participants = 2353; studies = 5; I² = 40%). However, removing the eight studies included in this analysis judged to be at high risk of attrition bias had little impact on the pooled effect estimate (Peto OR 1.84, 95% CI 1.62 to 2.09; participants = 4141; studies = 20; I² = 50%).
5.
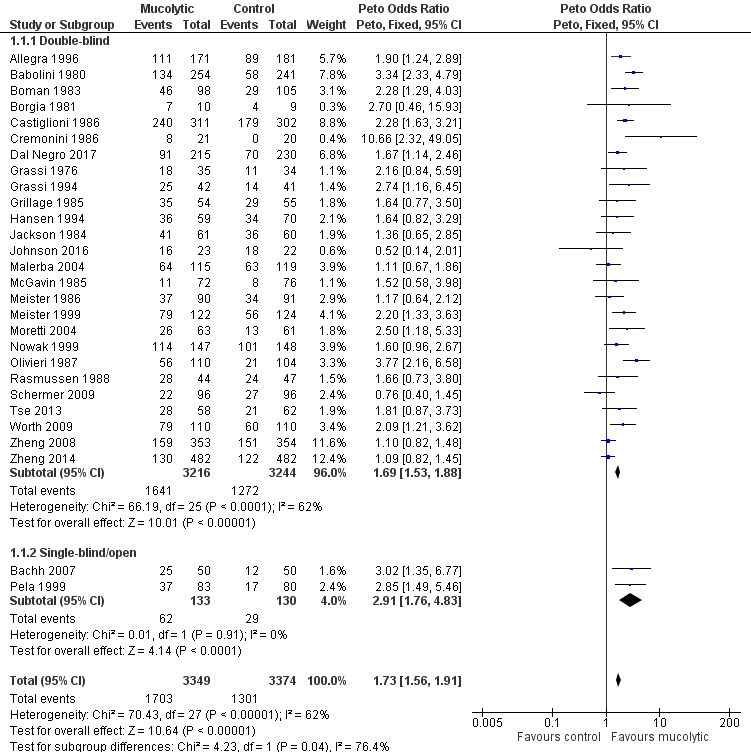
Forest plot of comparison: 1 Mucolytic versus placebo, outcome: 1.1 Participants with no exacerbations in study period.
1.1. Analysis.
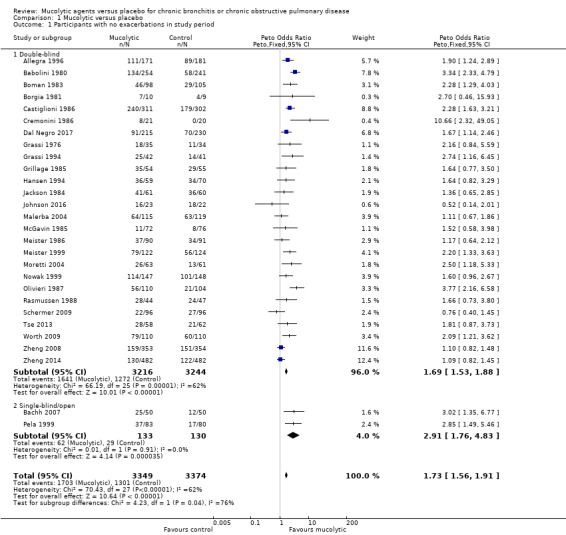
Comparison 1 Mucolytic versus placebo, Outcome 1 Participants with no exacerbations in study period.
6.
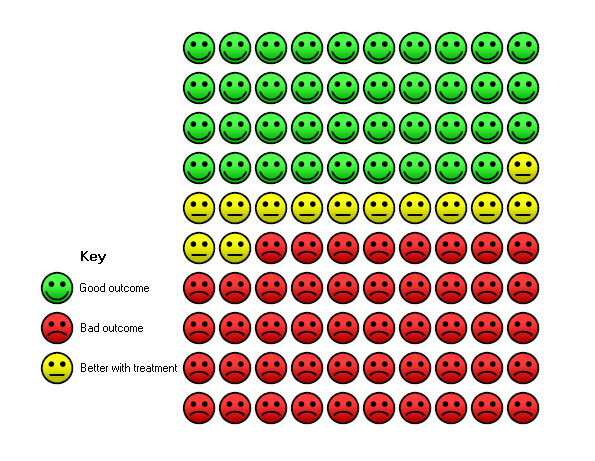
In the control group, 39 of 100 people were free from exacerbations over 9 months (represented by green faces) compared with 52 (95% CI 49 to 55) of 100 for the mucolytic group (represented by green plus yellow faces).
As heterogeneity in this result is high (I² = 62%), we carried out a post hoc subgroup analysis showing results of double‐blind trials ordered by year of publication and subgrouped by decade of publication (Analysis 1.2; Figure 7). This revealed a tendency for more recent studies to provide more conservative results: studies published before 1990 (Peto OR 2.34, 95% CI 1.97 to 2.79) and between 1990 and 1999 (Peto OR 1.91, 95% CI 1.50 to 2.44) have a greater effect size than those published between 2000 and 2009 (Peto OR 1.24, 95% CI 1.01 to 1.54) or since 2010 (Peto OR 1.28, 95% CI 1.03 to 1.59). It is also notable that of the six studies with adequate allocation concealment (Dal Negro 2017; Decramer 2005; Johnson 2016; Schermer 2009; Zheng 2008; Zheng 2014), only Dal Negro 2017 reported a notable benefit of treatment in preventing exacerbations.
1.2. Analysis.
Comparison 1 Mucolytic versus placebo, Outcome 2 Participants with no exacerbation by decade, double‐blind trials only.
7.
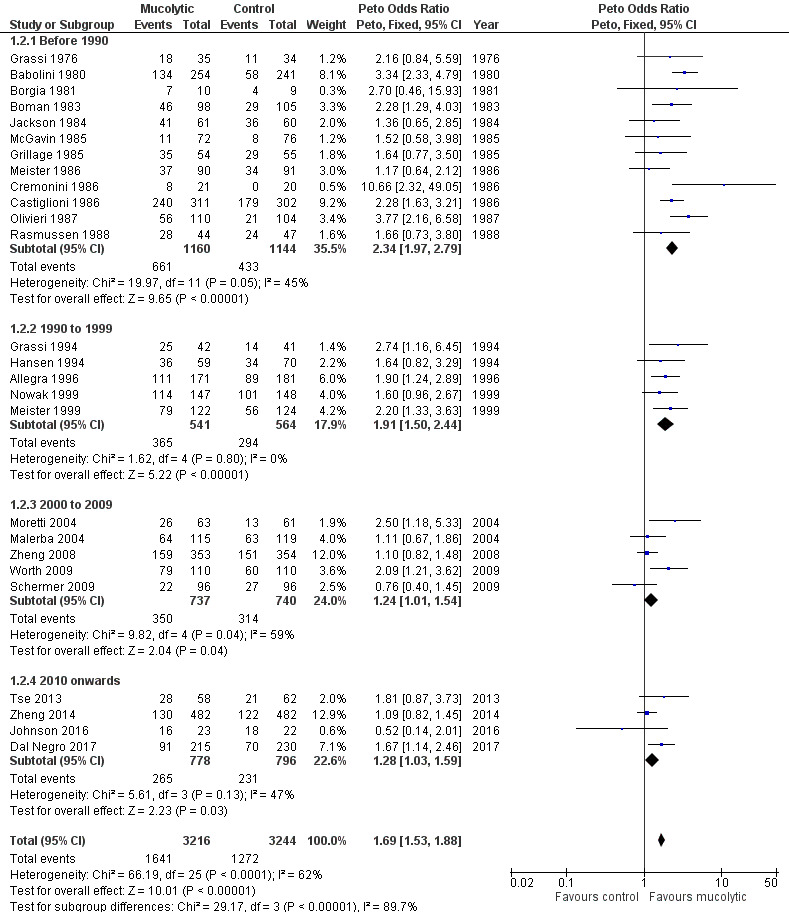
Forest plot of comparison: 1 Mucolytic versus placebo, outcome: 1.2 Participants with no exacerbation by decade; double‐blind trials only.
We carried out a separate analysis of studies conducted during winter months only and observed a larger effect size when compared to all studies (Peto OR 2.20, 95% CI 1.93 to 2.51; participants = 4007; studies = 21; I² = 19%; Analysis 1.3). When subgrouped by dose or type of mucolytic, we did not observe a consistent effect (I2 62%, Analysis 1.4). Overall we observed significant benefits over placebo for lower doses of NAC and carbocysteine,. The "other" mucolytic category also showed benefit compared to placebo; this category included studies of ambroxol (N = 2); erdosteine (N = 1); letosteine (N = 1); sobrerol (N = 1); myrtol (N = 1); and cineole (N = 1).
1.3. Analysis.
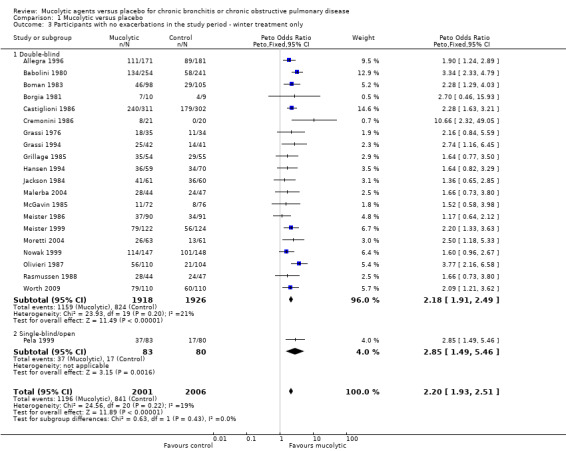
Comparison 1 Mucolytic versus placebo, Outcome 3 Participants with no exacerbations in the study period ‐ winter treatment only.
1.4. Analysis.
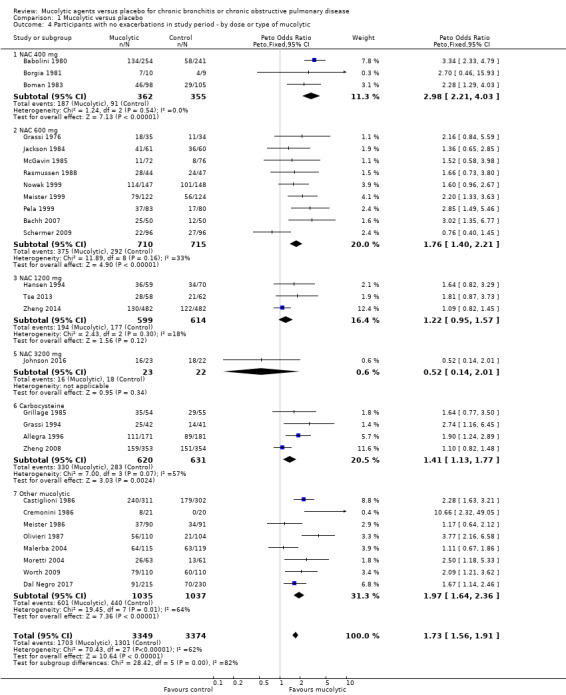
Comparison 1 Mucolytic versus placebo, Outcome 4 Participants with no exacerbations in study period ‐ by dose or type of mucolytic.
Studies with participants with on average better lung function at baseline found greater benefit from mucolytics when compared to those with on average poorer lung function (> 50% predicted vs ≤ 50% predicted; test for subgroup differences: Chi² = 4.14, df = 1 (P = 0.04; I² = 75.9%; Analysis 1.5). However, this result should be interpreted with caution as the poorer lung function subgroup contained only four studies.
1.5. Analysis.
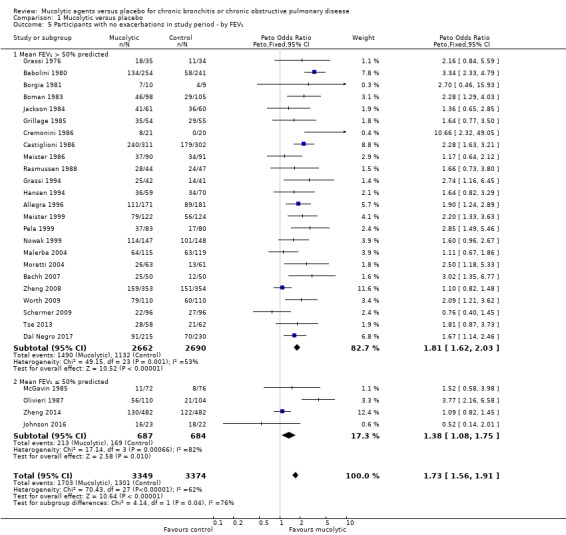
Comparison 1 Mucolytic versus placebo, Outcome 5 Participants with no exacerbations in study period ‐ by FEV₁.
Studies of greater duration on average had a lesser effect than those of shorter duration; the OR for studies ≥ 12 months was 1.16 (95% 0.98 to 1.37) compared to 2.14 (95% CI 1.62 to 2.82) and 2.20 (95% CI 1.91 to 2.54) for up to three months and three to 12 months, respectively (test for subgroup differences: Chi² = 35.72, df = 2 (P < 0.00001; Analysis 1.6).
1.6. Analysis.
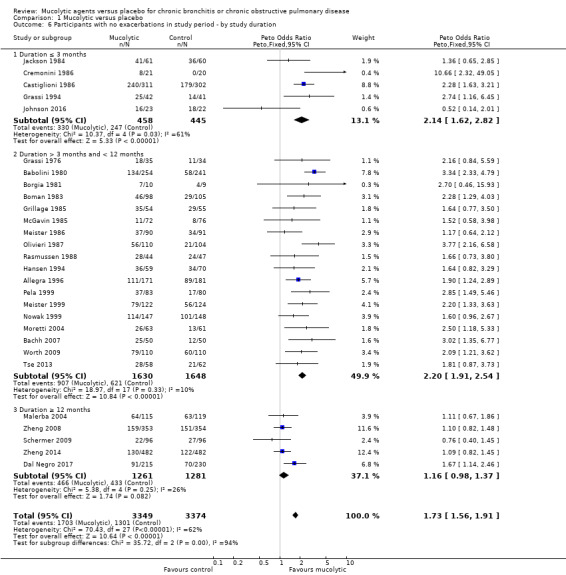
Comparison 1 Mucolytic versus placebo, Outcome 6 Participants with no exacerbations in study period ‐ by study duration.
We also observed a larger effect among studies conducted in Italy compared to those not conducted in Italy (test for subgroup differences: Chi² = 25.94, df = 1 (P < 0.00001; Analysis 1.7). This analysis was carried out as it has been noted in the past that some of the earlier trials of mucolytics in Italy were reporting greater effects than trials conducted elsewhere, We also noted a larger effect in those studies in which history of an exacerbation was not a requirement for study entry compared to those where it was (test for subgroup differences: Chi² = 12.51, df = 1 (P = 0.0004; Analysis 1.8).
1.7. Analysis.
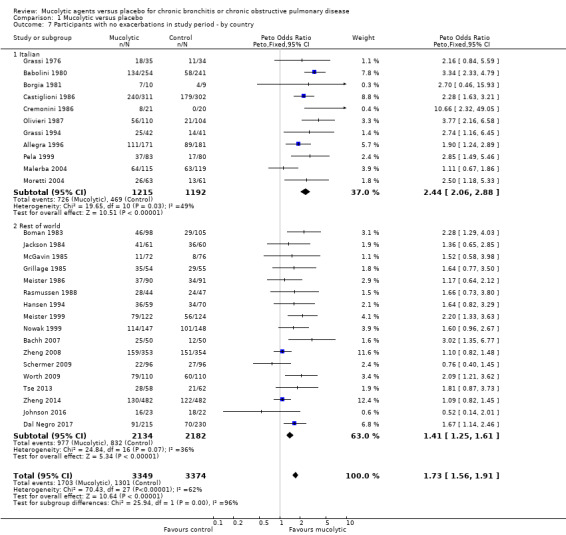
Comparison 1 Mucolytic versus placebo, Outcome 7 Participants with no exacerbations in study period ‐ by country.
1.8. Analysis.
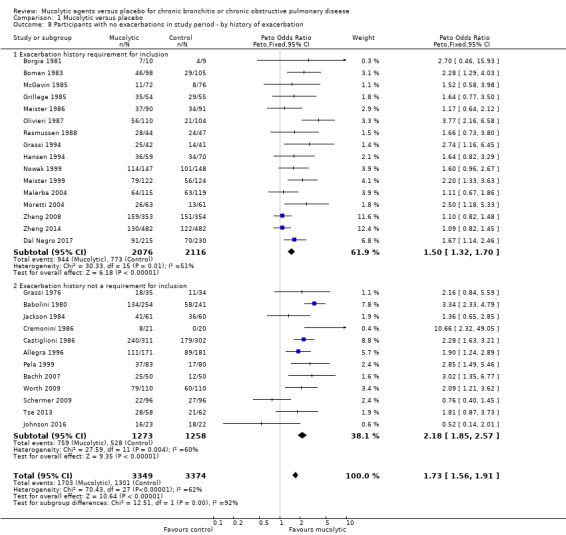
Comparison 1 Mucolytic versus placebo, Outcome 8 Participants with no exacerbations in study period ‐ by history of exacerbation.
A funnel plot for this outcome gave no clear indication of publication bias (Figure 8).
8.
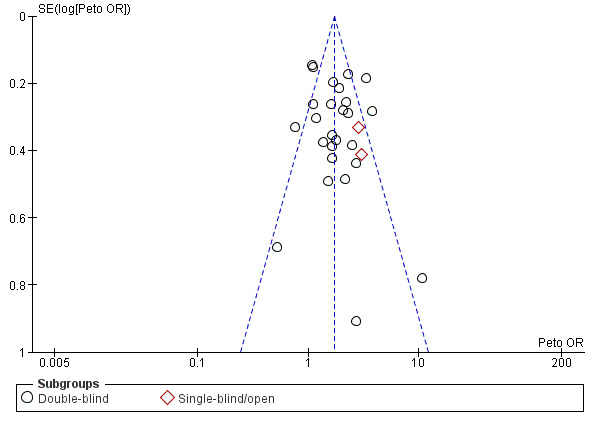
Funnel plot of comparison: 1 Mucolytic versus placebo, outcome: 1.1 Participants with no exacerbations in study period.
Exacerbations in patients by use of concomitant inhaled corticosteroids (ICS)
Subgrouping studies for this outcome according to whether ICS were or were not allowed (or unclear) did not suggest that this was an important effect modifier, and the test for subgroup differences was negative (test for subgroup differences: Chi² = 1.64, df = 2; (P = 0.44; Analysis 1.9).
1.9. Analysis.
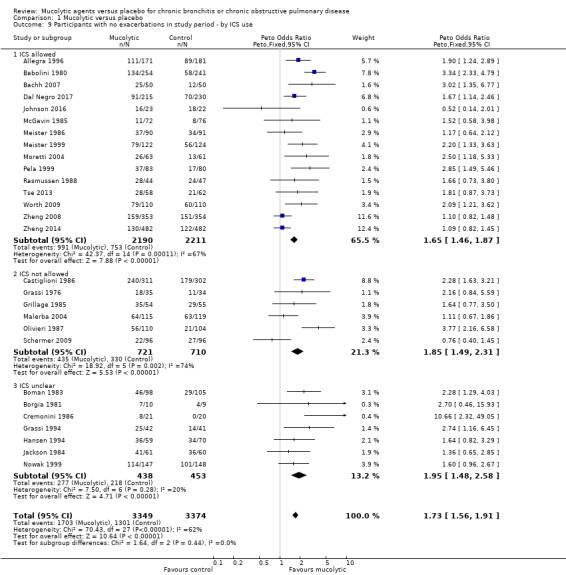
Comparison 1 Mucolytic versus placebo, Outcome 9 Participants with no exacerbations in study period ‐ by ICS use.
Time to first exacerbation
Sufficient data with which to perform a meta‐analysis are not yet available for this clinically relevant outcome. Post hoc analysis of the EQUALIFE study revealed that participants given erdosteine had a significantly longer time until their first exacerbation compared with those given placebo, with a hazard ratio of 0.639 (95% CI 0.416 to 0.981) (Ballabio 2008). Longer time to first exacerbation was also reported by Nowak 1999. In that study, participants with COPD treated with NAC had a mean of 139 days (SD 68) to first exacerbation versus 108 (SD 79) days for those given placebo (P < 0.05). More recently, Zheng 2014, Dal Negro 2017, and Fukuchi 2016 reported time to first exacerbation. Zheng 2014 reported no differences between time to first exacerbation in NAC‐ or placebo‐treated groups, but time to second and third exacerbations was shorter in the placebo group. Dal Negro 2017 reported increased time to first exacerbation in the erdosteine group compared to the placebo group, but this did not reach statistical significance (Kaplan–Meier plot of probability, P = 0.07). Fukuchi 2016 reported the median time to first exacerbation as 179 days in the lysozyme group and 210 days in the placebo group (hazard ratio 1.06; P = 0.626).
Number of exacerbations per patient per month
We calculated and meta‐analysed the number of exacerbations per patient per month as a primary outcome in previous versions of this review. For the 2019 update, we decided not to update these analyses due to concerns about high levels of heterogeneity, the need to impute much of the data, and the likely skew of this measure. Instead, we present the data in a table (Analysis 1.10). The mean difference in number of exacerbations per patient per month favoured the mucolytic intervention in most studies, but this finding should be interpreted with caution in light of the caveats already mentioned.
1.10. Analysis.
Comparison 1 Mucolytic versus placebo, Outcome 10 Number of exacerbations per participant per month.
| Number of exacerbations per participant per month | |||||||
|---|---|---|---|---|---|---|---|
| Study | Mean mucolytic group | SD | N | Mean control group | SD | N | Mean difference [95% CI] |
| Allegra 1996 | 0.07 | 0.11 | 223 | 0.11 | 0.14 | 218 | ‐0.04 [‐0.06, ‐0.02] |
| Babolini 1980 | 0.13 | 0.18 | 254 | 0.33 | 0.27 | 241 | ‐0.20 [‐0.24, ‐0.16] |
| Boman 1983 | 0.2 | 0.27 | 98 | 0.32 | 0.3 | 105 | ‐0.12 [‐0.20, ‐0.04] |
| Borgia 1981 | 0.05 | 0.08 | 10 | 0.15 | 0.17 | 9 | ‐0.10 [‐0.22, 0.02] |
| Castiglioni 1986 | 0.1 | 0.21 | 311 | 0.2 | 0.29 | 302 | ‐0.10 [‐0.14, ‐0.06] |
| Cremonini 1986 | 0.25 | 0.23 | 21 | 0.71 | 0.29 | 20 | ‐0.46 [‐0.62, ‐0.30] |
| Decramer 2005 | 0.1 | 0.11 | 256 | 0.11 | 0.16 | 267 | ‐0.01 [‐0.03, 0.01] |
| Fukuchi 2016 | 0.15 | 0.24 | 201 | 0.13 | 0.21 | 204 | 0.02 [‐0.02, 0.06] |
| Grassi 1976 | 0.14 | 0.15 | 35 | 0.27 | 0.21 | 34 | ‐0.13 [‐0.22, ‐0.04] |
| Grassi 1994 | 0.16 | 0.29 | 42 | 0.45 | 0.43 | 41 | ‐0.29 [‐0.45, ‐0.13] |
| Grillage 1985 | 0.1 | 0.12 | 54 | 0.12 | 0.15 | 55 | ‐0.02 [‐0.07, 0.03] |
| Hansen 1994 | 0.11 | 0.15 | 59 | 0.16 | 0.19 | 70 | ‐0.05 [‐0.11, 0.01] |
| Jackson 1984 | 0.11 | 0.14 | 61 | 0.13 | 0.16 | 60 | ‐0.02 [‐0.07, 0.03] |
| Malerba 2004 | 0.06 | 0.08 | 115 | 0.07 | 0.08 | 119 | ‐0.01 [‐0.03, 0.01] |
| McGavin 1985 | 0.42 | 0.34 | 72 | 0.52 | 0.35 | 76 | ‐0.10 [‐0.21, 0.01] |
| Meister 1986 | 0.15 | 0.15 | 90 | 0.2 | 0.19 | 91 | ‐0.05 [‐0.10, ‐0.00] |
| Meister 1999 | 0.06 | 0.15 | 122 | 0.1 | 0.15 | 124 | ‐0.04 [‐0.08, ‐0.00] |
| Moretti 2004 | 0.12 | 0.14 | 63 | 0.17 | 0.17 | 61 | ‐0.05 [‐0.10, 0.00] |
| Nowak 1999 | 0.03 | 0.06 | 147 | 0.06 | 0.12 | 148 | ‐0.03 [‐0.05, ‐0.01] |
| Olivieri 1987 | 0.18 | 0.31 | 110 | 0.33 | 0.41 | 104 | ‐0.15 [‐0.25, ‐0.05] |
| Parr 1987 | 0.18 | 0.21 | 243 | 0.21 | 0.21 | 210 | ‐0.03 [‐0.07, 0.01] |
| Pela 1999 | 0.14 | 0.15 | 35 | 0.27 | 0.21 | 34 | ‐0.13 [‐0.22, ‐0.04] |
| Rasmussen 1988 | 0.13 | 0.21 | 44 | 0.14 | 0.19 | 47 | ‐0.01 [‐0.09, 0.07] |
| Schermer 2009 | 0.08 | 0.1 | 96 | 0.06 | 0.05 | 96 | 0.02 [‐0.00, 0.04] |
| Tse 2013 | 0.08 | 0.24 | 58 | 0.14 | 0.24 | 62 | ‐0.06 [‐0.15, 0.03] |
| Worth 2009 | 0.067 | 0.136 | 110 | 0.15 | 0.24 | 110 | ‐0.08 [‐0.13, ‐0.03] |
| Zheng 2008 | 0.084 | 0.094 | 353 | 0.11 | 0.094 | 354 | ‐0.03 [‐0.04, ‐0.01] |
| Zheng 2014 | 0.1 | 0.15 | 482 | 0.13 | 0.17 | 482 | ‐0.03 [‐0.05, ‐0.01] |
Number of days of disability per participant per month ('sick days')
We were able to meta‐analyse data from nine studies, showing a significant reduction of 0.43 days of disability per participant per month with mucolytic therapy (95% CI ‐0.56 to ‐0.30; 9 studies, 2259 participants; Analysis 1.11; moderate‐certainty evidence) compared with placebo. This finding was associated with a high level of heterogeneity (I² = 61%).
1.11. Analysis.
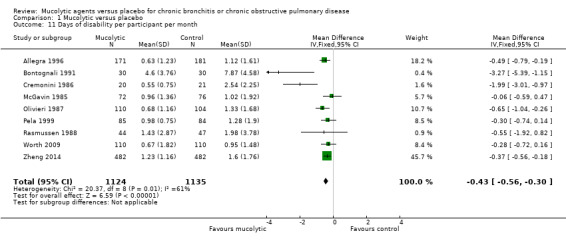
Comparison 1 Mucolytic versus placebo, Outcome 11 Days of disability per participant per month.
The following studies reported information that we were unable to meta‐analyse. Cegla 1988 reported total days off sick per group over the two years and noted that this did not differ significantly between the two treatments (1071 days in the ambroxol group and 979 in the placebo group over two years; participants = 180). Nowak 1999 reported the cumulative exacerbation days per group as 462 days in the NAC group and 776 days in the placebo group over eight months (participants = 295). Petty 1990 reported the mean duration in days of exacerbations between week 4 and week 8 of the trial. The mean duration in the iodinated glycerol group was 6.3 compared to 10.2 in the placebo group; the P value for the difference was reported as 0.029 (participants = 376). Moretti 2004 did not report total 'sick days'; however, investigators did report the numbers of individuals losing workdays: seven in the erdosteine group and 10 in the placebo group, for a mean number of days lost per person of 0.8 and 1.1, respectively.
In the three studies that reported it, a mean reduction of 0.53 days on antibiotics per participant per month was observed (95% CI ‐0.76 to ‐0.31; 3 studies; 714 participants; Analysis 1.12). These were older studies that included participants with chronic bronchitis. In the study of Meister 1999, 6/31 (52%) participants in the myrtol group with exacerbations needed antibiotics, compared with 30/49 (61%) in the placebo group. Courses of antibiotics were longer in the placebo group. The percentage of participants who needed antibiotics for longer than seven days was 37% in the myrtol group and 77% in the placebo group. Malerba 2004 reported no differences between ambroxol and placebo in terms of duration of courses of antibiotic treatment, working days lost, or number of days of hospitalisation (no data given). Moretti 2007 used post hoc analyses to report that compared with placebo, erdosteine use was associated with relatively fewer antibiotic courses (32%) and shorter durations of treatment (15%). The mean number of antibiotic courses per participant treated with erdosteine was also lower than for those given placebo (0.5 (SD 0.7) vs 0.7 (SD 0.7); P = 0.045).
1.12. Analysis.
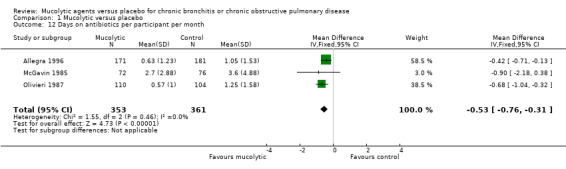
Comparison 1 Mucolytic versus placebo, Outcome 12 Days on antibiotics per participant per month.
Lung function
FEV₁
Fourteen studies reported FEV₁ in L at the end of the study. The pooled effect favours mucolytics over placebo, but the effect size is small (mean difference (MD) 0.04 L, 95% CI 0.01 to 0.07; participants = 3310; Analysis 1.13; moderate‐certainty evidence). We observed substantial heterogeneity in this outcome (I² = 70%), and so results should be interpreted with caution. Furthermore, this analysis includes data from the Moretti 2004 study, which reported a significant difference (> 300 mL) between mucolytic and placebo groups at the end of the study; however the mucolytic group had higher baseline lung function, and the net change was therefore closer to 200 mL. If this study is removed from the analysis, a significant difference between groups is no longer observed and heterogeneity is removed.
1.13. Analysis.
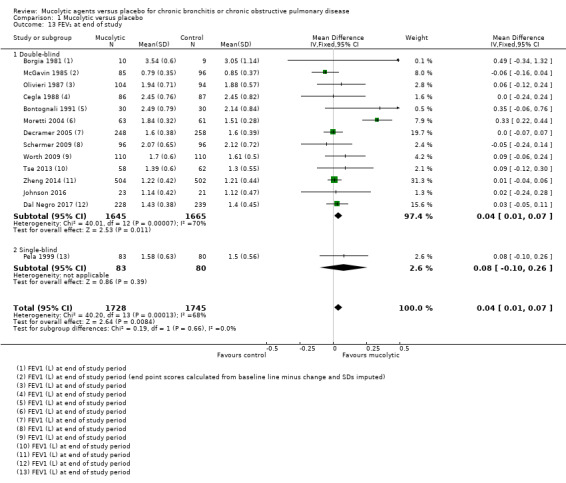
Comparison 1 Mucolytic versus placebo, Outcome 13 FEV₁ at end of study.
Nowak 1999 reported FEV₁ change from baseline in L but without variance, and so we were unable to include the results in the meta‐analysis. Trialists reported 0.225 L improvement in the NAC group (n = 33), compared to 0.062 L in the placebo group (n = 47).
Of note, two three‐year studies are included in this analysis. The BRONCUS study of Decramer 2005 found no differences between NAC‐treated and placebo‐treated groups over three years in terms of decline in FEV₁, FVC, or diffusing capacity of the lung for carbon monoxide (DLCO). FEV₁ declined by 54 mL and 47 mL, respectively, in the two groups. Study authors reported possible benefit of NAC for functional residual capacity (FRC), with a greater reduction in this measure. The difference was ‐0.374 L (SD 1.03; P < 0.01) for NAC‐treated participants, whereas for those treated with placebo, a decrease of only 0.008 L was reported. The other three‐year study found no differences between groups in lung function at the end of the study (Schermer 2009). In the NAC‐treated group, FEV₁ declined by 64 mL, and in the placebo group, by 60 mL. The decline in FVC was 79 mL and 65 mL, respectively.
In the HIACE study of Tse 2013, a significantly higher mean FEV₁ was reported for the NAC group at the end of the study, but this reflected differences at baseline, with no significant differences in the amount of change reported between groups. On the other hand, researchers reported significantly greater changes in the NAC group than in the placebo group for two measures of small airways function: forced expiratory flow at 25% to 50% (FEF25‐50) (P = 0.037) and forced oscillation technique (FOT) (P = 0.04), as well as for airways resistance (P = 0.01).
Percent predicted FEV₁
This outcome was reported by only four studies. Although the pooled effect favours mucolytics, this is driven by one study: Xu 2014, which was not blinded (MD 4.79, 95% CI 1.97 to 7.62; participants = 414; Analysis 1.14), and again, we detected substantial heterogeneity (I² = 89%).
1.14. Analysis.
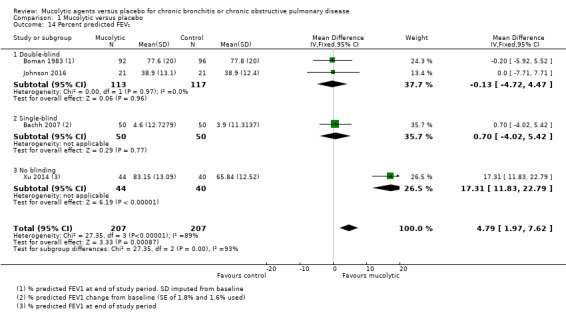
Comparison 1 Mucolytic versus placebo, Outcome 14 Percent predicted FEV₁.
Peak expiratory flow rate
Peak expiratory flow rate was reported by one study only (Grillage 1985). The result favours mucolytics but is very uncertain (MD 19.00, 95% CI ‐22.70 to 60.70; participants = 109; Analysis 1.15).
1.15. Analysis.
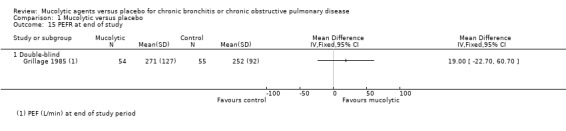
Comparison 1 Mucolytic versus placebo, Outcome 15 PEFR at end of study.
Forced vital capacity
Twelve studies reported this outcome, and the pooled effect revealed benefit of 50 mL of mucolytics over placebo (MD 0.05, 95% CI ‐0.00 to 0.10; participants = 3127; I² = 0%; Analysis 1.16), but the confidence interval includes no difference between groups.
1.16. Analysis.
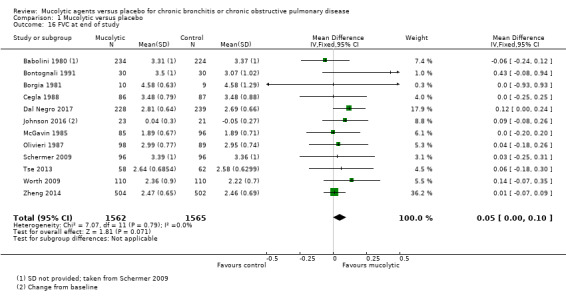
Comparison 1 Mucolytic versus placebo, Outcome 16 FVC at end of study.
In summary, it is likely that if mucolytics affect disease progression in chronic bronchitis or COPD, changes are very small and are confined to as‐yet small and undefined subgroups.
Adverse effects
The meta‐analysis of total numbers of adverse effects favours mucolytic treatment, but with some heterogeneity (OR 0.84, 95% CI 0.74 to 0.94; I² = 46% participants = 7264; studies = 24; Analysis 1.17; moderate‐certainty evidence). If a random‐effects model is used, this finding is less precise and the confidence interval includes no difference (OR 0.83, 95% CI 0.69 to 1.00).
1.17. Analysis.

Comparison 1 Mucolytic versus placebo, Outcome 17 Adverse effects.
Moreover, this analysis does not include data from several large studies. Parr 1987 reported 1263 events in 258 participants in the mucolytics group (mean 4.9 per participant) and 1202 events in 268 participants in the placebo group (mean 4.5 per participant). Decramer 2005 reported 1428 events in 256 participants in the mucolytics group (mean 5.58 per participant) and 1381 events among 267 participants in the placebo group (mean 5.17 per participant). None were thought to be drug‐related. Similar numbers in each group were admitted to hospital (55 and 69, respectively). Another study described 54 events in 59 participants in the mucolytic group and 66 events in 57 participants in the placebo group (Rasmussen 1988). Meister 1999 reported 201 adverse effects in 122 participants in the mucolytic group (1.65 per participant) and 170 adverse effects in 124 participants in the placebo group (1.37 per participant). These studies could not be included in the meta‐analysis because event rates exceeded numbers included in the treatment groups. Malerba 2004 also reported no greater risk of events and no greater severity of events with mucolytic treatment compared with placebo.
Hospitalisation
Comparative data were provided by five studies (Decramer 2005; Johnson 2016; Moretti 2004; Tse 2013; Zheng 2014). The Peto OR for hospitalisation with mucolytic treatment compared with placebo was 0.68 (95% CI 0.52 to 0.89; participants = 1833; Analysis 1.18; moderate‐certainty evidence); however, moderate heterogeneity in this result was observed (I² = 43%), and benefit was seen in only the two smaller studies (Moretti 2004; Tse 2013). Malerba 2004 reported no significant differences in hospitalisation rates but did not provide data. Bachh 2007 reported a significant reduction (P < 0.05) in hospitalisation when four months of NAC treatment was provided, with 55 hospitalisations reported for 50 participants in the control group but for only 37 of 50 in the treated group. As presented, these data cannot be included in the meta‐analysis because the number of events exceeds the number of participants in the control group. If a conservative estimate of hospitalisations in the control group is made by entering them as 50 (not 55), the beneficial effect of mucolytics for hospitalisation is greater (OR 0.62, 95% CI 0.48 to 0.80) but heterogeneity is increased (I² = 76%). Mucolytics may be associated with a small decrease in hospitalisations.
1.18. Analysis.
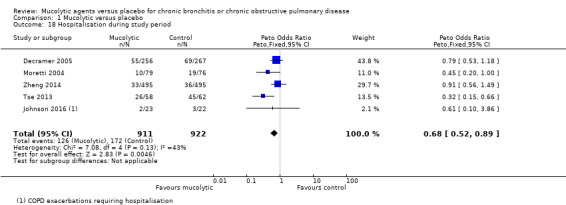
Comparison 1 Mucolytic versus placebo, Outcome 18 Hospitalisation during study period.
Days in hospital were reported by Moretti 2004. In this study, participants taking erdosteine spent 70 days in hospital, compared with 163 days for the placebo group (P = 0.04). This represented a mean of 1.1 days per treated participant compared with 2.7 days per control participant.
Mortality
Eleven studies reported on numbers of deaths in mucolytic‐treated and placebo groups, revealing no significant differences, but the confidence interval is wide (Peto OR 0.98, 95% CI 0.51 to 1.87; participants = 3527; Analysis 1.19; moderate‐certainty evidence). As no deaths were reported in either group in Johnson 2016, Xu 2014, or Zheng 2008, this information could not be incorporated into the meta‐analysis.
1.19. Analysis.
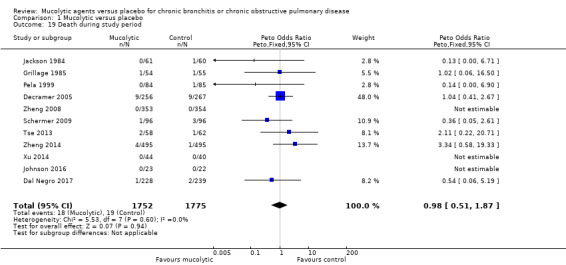
Comparison 1 Mucolytic versus placebo, Outcome 19 Death during study period.
Health‐related quality of life
Although many studies reported participant and/or physician global assessments of well‐being, only ten used validated tools to evaluate health‐related quality of life (HRQoL) among participants with COPD. In nine studies, investigators used the St George's Respiratory Questionnaire (SGRQ; Jones 1992) (Dal Negro 2017; De Backer 2013; Decramer 2005; Johnson 2016; Moretti 2004; Tse 2013; Worth 2009; Zheng 2008; Zheng 2014). Schermer 2009 used the Chronic Respiratory Questionnaire (CRQ; Guyatt 1987). In Johnson 2016, trialists reported the Short Form‐36 (SF‐36) as well as SGRQ, and in Fukuchi 2016, trialists reported the COPD Assessment Test (CAT).
The SGRQ total score is derived from scores on three subscales ‐ symptoms, activities, and impacts ‐ to yield a score out of 100 (Jones 1992). A well person has respiratory disease scores around 7 (Jones 1992). Lower scores indicate better quality of life.
We were able to combine total scores on the SGRQ for seven studies at the end of the treatment period. Although the pooled result favoured mucolytics over placebo, the confidence interval included no difference (MD ‐1.37, 95% CI ‐2.85 to 0.11; studies = 7, participants = 2721; Analysis 1.20; moderate‐certainty evidence). Considerable heterogeneity among studies was apparent (I² = 64%). This effect does not meet the minimum clinically important difference of ‐4 units on the SGRQ (Jones 2005). However it is not possible to assess the impact of mucolytics at a population level without performing a responder analysis, which we have been unable to do.
1.20. Analysis.
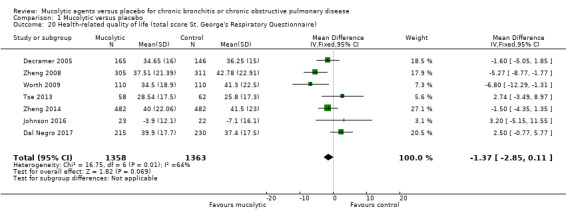
Comparison 1 Mucolytic versus placebo, Outcome 20 Health‐related quality of life (total score St. George's Respiratory Questionnaire).
The analysis includes data from the three‐year Decramer 2005 study of 600 mg NAC daily, in which participants were evaluated with the SGRQ, although for technical reasons only about 80% of participants completed the questionnaire. During the first year of the study, participants in both treatment and placebo groups showed significantly improved scores on both scales, with no significant differences between groups (‐3.76 units on NAC and ‐4.95 units on placebo; difference between groups 1.18; P = 0.358, as reported in the text of the paper). In the second year, this improvement tailed off again, with no differences noted between treatment groups. More participants given placebo withdrew from the trial, and dropouts had a worse SGRQ score than those who remained in the study. We have used data provided by study authors as obtained from the mixed‐effects model used in this study. In a one‐year study of a higher dose of NAC (600 mg twice daily; Tse 2013), no significant difference was observed between groups for SGRQ.
In Zheng 2008, baseline SGRQ scores were well matched among groups. After 12 months of treatment, changes in SGRQ total scores from baseline amounted to ‐4.06 units in the carbocysteine group and ‐0.05 in the placebo group, but these values did not represent a statistically significant difference between groups (P = 0.13). A very large difference in SGRQ symptom domain results between the carbocysteine group (‐11.34 units) and the placebo group (‐3.54 units; P = 0.004) remains unexplained. Results from the single measurement obtained at one year in this study contrast with multiple measurements taken in Decramer 2005, by which no significant differences in symptom scores between NAC and placebo were found over time.
In Worth 2009, the mean score change at six months from baseline was ‐4.3 in the placebo group and ‐9.9 in the cineole group (P = 0.06). However, we judged this study to be at high risk of selection bias.
In the recent one‐year Dal Negro 2017 study of erdosteine, trialists reported improvements in SGRQ in both intervention and control groups but no differences between groups. Similarly, in the eight‐month Moretti 2004 study of erdosteine, participants completed both SF‐36 and the SGRQ. The erdosteine‐treated group showed significant improvement in all domains of the SGRQ, as well as in total score, and no differences between treated and placebo groups were reported. Data from Moretti 2004 were not suitable for inclusion in Analysis 1.20.
In the three‐year study of NAC versus placebo (Schermer 2009), the CRQ was used. Groups were well matched at baseline, with evident improvement in both groups, particularly over the first year, but this never exceeded the 0.5 unit threshold regarded as clinically significant (Guyatt 1987). At the end of the study, no significant differences in CRQ total scores were reported between groups (P = 0.306).
In Fukuchi 2016, CAT scores were reported. Trialists reported that quality of life in both the lysozyme group and the placebo group improved according to this measure; improvement was greater in the lysozyme group at all time points, and the difference was significant at 24 weeks but did not remain so at 52 weeks (MD ‐0.90, 95% CI ‐2.22 to 0.42; participants = 340; studies = 1, Analysis 1.21).
1.21. Analysis.
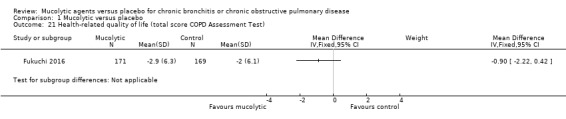
Comparison 1 Mucolytic versus placebo, Outcome 21 Health‐related quality of life (total score COPD Assessment Test).
Thus, considerable variation can be seen in evidence related to HRQoL, and we are not able to assess whether mucolytics had a clinically important effect on this outcome. Furthermore, and in keeping with the exacerbation outcome, more recent studies have tended to provide more conservative estimates of the impact of mucolytics on quality of life.
Systemic thiol donor versus control
One study investigated a systemic thiol donor, N‐isobutyrylcysteine (NIC), versus control (Ekberg‐Jansson 1999). This trial randomised more than 600 participants with chronic bronchitis. There was no clear difference between groups for the number of participants who remained exacerbation‐free (Peto OR 1.01, 95% CI 0.74 to 1.39; participants = 628; Analysis 2.1), the number of exacerbations per participant per month (MD 0.01; 95% CI ‐0.02 to 0.04), or days of disability per participant per month (MD ‐0.18, 95% CI ‐0.82 to 0.46; participants = 628; Analysis 2.3). Participants in the intervention group experienced more adverse events, but the confidence interval included no difference (Peto OR 1.39, 95% CI 0.98 to 1.95; participants = 628; Analysis 2.4).
2.1. Analysis.
Comparison 2 Systemic thiol donor versus placebo, Outcome 1 Participants with no exacerbations in the study period.
2.3. Analysis.
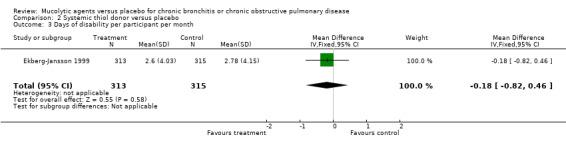
Comparison 2 Systemic thiol donor versus placebo, Outcome 3 Days of disability per participant per month.
2.4. Analysis.
Comparison 2 Systemic thiol donor versus placebo, Outcome 4 Adverse effects.
Discussion
Summary of main results
The previous update of this review was performed in 2015 (Poole 2015). Since that time, a further four studies that were eligible for inclusion have been conducted (Dal Negro 2017; Fukuchi 2016; Johnson 2016; Xu 2014). The present update strengthens findings from our previous reviews indicating that participants given a mucolytic agent for an average of nine months are more likely to be exacerbation‐free during that time (Peto odds ratio (OR) 1.73, 95% confidence interval (CI) 1.56 to 1.91). For one participant to be exacerbation‐free, eight (95% CI 7 to 10) had to be treated for at least nine months. Mucolytics may be associated with a reduction of approximately a half‐day of disability per participant per month, but the result is heterogeneous (mean difference (MD) ‐0.43, 95% CI ‐0.56 to ‐0.30; I² = 61%). Three studies reported days on antibiotics per participant per month, and the pooled result indicated benefit of mucolytics (MD ‐0.53, 95% CI ‐0.76 to ‐0.31).
Mucolytics may be associated with a decrease in hospitalisations, although this finding is based on data from only five studies (Peto OR 0.68, 95% CI 0.52 to 0.89). With the addition of newer studies, certainty that mucolytics do not have a substantial impact on lung function decline or mortality is increasing. Mucolytics may be associated with a reduction in all adverse events, but the effect estimate includes the possibility of no difference between groups (OR 0.83, 95% CI 0.69 to 1.00). The impact on quality of life as measured by the total St. George's Respiratory Questionnaire (SGRQ) score is smaller than the minimal clinically important difference (MCID) of 4 units and also includes the possibility of no differences between groups (MD ‐1.37, 95% CI ‐2.85 to 0.11). Furthermore, we cannot be certain about the population effect, as we were unable to carry out a responder analysis.
For many outcomes ‐ primary and secondary ‐ significant heterogeneity has been noted among studies; therefore the results do need to be interpreted with particular caution. The only outcomes for which heterogeneity among trials was not significant were days on antibiotics, forced vital capacity (FVC), and death during the study period. To explore causes of heterogeneity for the primary outcome of exacerbations, we performed subgroup analyses according to study date, baseline forced expiratory volume in one second (FEV₁) (as % predicted), type of mucolytic, dose of mucolytic, duration of therapy, whether participants were included because they had a history of exacerbations, whether concomitant inhaled steroids were used, and the country in which the study was conducted. Heterogeneity was generally less among trials with winter treatment only and those using the same dose of N‐acetylcysteine (NAC).
The tendency for participants given mucolytics to be more likely to be exacerbation‐free was seen in all studies except Schermer 2009 and Johnson 2016. Schermer 2009 was the first study that found an increased number of exacerbations in the mucolytic‐treated group compared with the placebo‐treated group; however, this difference was not statistically significant. The exacerbation rate was generally low in this study, and data were skewed by two participants in the NAC‐treated group who had very frequent exacerbations. Additionally, this study reported a high dropout rate (43%). Johnson 2016 also reported increased exacerbations in the mucolytic group, but the result was very imprecise and was reported in the context of a study stopped at eight weeks due to safety concerns after only 51 participants were recruited.
However, when we performed a post hoc investigation comparing more recent study results versus those from previous decades, we found a clear reduction in the effects of treatment in more recent studies (see Figure 7; I² = 89.7% between subgroups). Although all studies included in this analysis were placebo‐controlled, and most were double‐blind, the older studies were more difficult to judge in terms of bias (see Figure 4), and this may have led to an overestimation of treatment effect. Therefore we have a reduced level of confidence in the overall treatment effect estimate. On the other hand, internal consistency is evident in the findings, in that the effect on exacerbations rate is accompanied by a reduction in hospitalisations and a reduction in both days of disability and days on antibiotics.
Theoretical reasons have been proposed to explain why mucolytics may modify disease in ways other than by reducing exacerbations (i.e. through antioxidant and thiol donor effects). More recent studies have sought to explore whether the decline in FEV₁ over time is changed by mucolytics. NAC has been used at higher doses or for longer durations without providing additional benefits, although this may be due to insufficient power to detect a difference. The reduction in exacerbation rates seen with NAC was virtually identical to that observed with other mucolytics examined as a group. The mechanisms responsible for the benefits of mucolytic treatment for exacerbation rates and days of disability cannot be identified by this review. However, lack of effect of N‐isobutyrylcysteine (NIC) (a thiol donor with antioxidant properties) on exacerbation rates or days sick raises the possibility that the actions of NAC as a thiol donor are less important in the reduction of exacerbations.
We found no evidence to suggest that mucolytics are unsafe, and findings indicate that they do not adversely affect quality of life, even though medicines need to be taken at least once a day.
Overall completeness and applicability of evidence
This review has now been updated substantively seven times. Through the process of iterative searches, we are confident that we have identified almost all the major studies with mucolytics as the intervention.
Over time, with a steady increase in the numbers of studies published, even though a significant treatment effect of mucolytics on exacerbations has always been observed, the size of this effect has decreased from that described in the original report. This trend may be observed in Figure 7, where studies have been separated by decades of publication.
We have considered below two factors that may be contributing to this observation.
Improved study design, execution, and reporting over the years
Confidence intervals are narrower, and consequently greater weight is afforded to more recent studies. The forest plot in Figure 7 has been arranged in subgroups by date and shows this trend. Part of the explanation is that more recent studies, on average, have been larger than earlier ones. Another consideration is that publication bias may have influenced reporting of results of earlier trials. This is suggested by asymmetrical funnel plots in Analysis 1.11 and Analysis 1.13 (Figure 1 and Figure 2).
1.
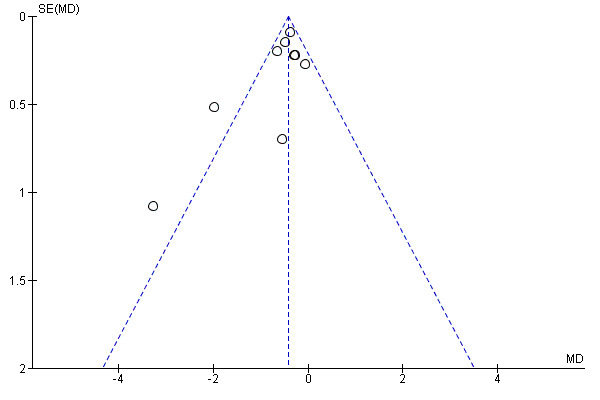
Funnel plot of comparison: 1 Mucolytic versus placebo, outcome: 1.11 Days of disability per participant per month.
2.
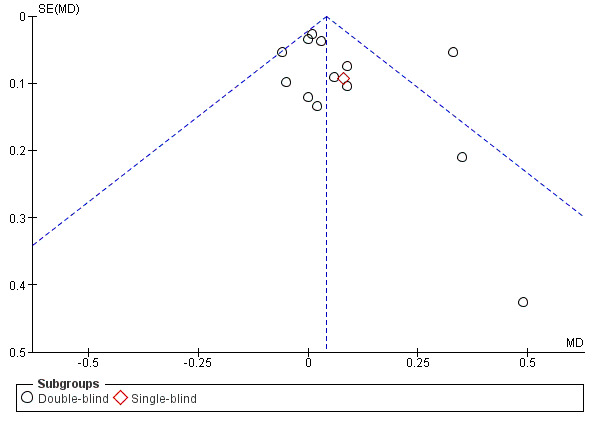
Funnel plot of comparison: 1 Mucolytic versus placebo, outcome: 1.13 FEV₁ at end of study.
Furthermore, tighter definitions of chronic obstructive pulmonary disease (COPD) have been used in later studies, which have generally included patients with, at most, moderate disease. To be included in earlier studies, patients needed only to have symptoms of chronic bronchitis, which is a clinical diagnosis. Furthermore, fewer dropouts in the intervention groups of longer studies might dilute any treatment effect, as those remaining in the study have a longer period over which to have an exacerbation recorded. Finally, as was mentioned previously, older studies may be at greater risk of selection bias, which may have inflated estimates of the treatment effect.
Improved COPD care
Comprehensive management of COPD now includes support for smoking cessation, vaccination, pulmonary rehabilitation, and use of inhaled corticosteroids (ICS), long‐acting beta‐agonists (LABAs), and anticholinergic agents (GOLD 2019), each of which may impact exacerbation frequency or severity.
Inhaled corticosteroids have been available for asthma since the late 1970s, but it is unlikely that they were used by participants with chronic bronchitis in trials before 1990. In most of the other studies, ICS treatment was allowed. In the present review, we divided the studies into whether cotreatment with ICS was allowed, not allowed, or unclear (Analysis 1.9). There was no significant subgroup difference (P = 0.44), suggesting that the effect of mucolytics is not affected by ICS use. The nature of reporting of the studies did not allow us to stratify by use of measures mentioned previously that may reduce exacerbations.
The trend in the likelihood that participants in control groups would be exacerbation‐free is 38% in pre‐1990 studies, 52% between 1990 and 2000, 42% from 2000 to 2009, and 29% from 2010 onwards (derived from Analysis 1.2). These findings suggest that up to 40% of study participants with COPD will have an exacerbation. One interpretation is that more recent studies show a trend toward improvement in overall care, but this needs follow‐up.
Quality of the evidence
We graded all pooled outcomes as moderate, indicating that the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different (Table 1). Our confidence in the pooled estimates was reduced by several considerations. Most outcomes were both clinically diverse and statistically heterogeneous.. Trials used a variety of types and doses of mucolytic, were conducted over two months to three years, and recruited populations with different baseline severity of COPD. Many were conducted in the 1980s and 1990s, at which time standard definitions and standard treatment for COPD differed from today. Subgroup analysis applied to our primary outcome of exacerbations explained some, but not all, the statistical heterogeneity. Therefore all outcomes, with the exception of mortality, were downgraded for inconsistency.
Although we had concerns about the conduct methods used for some of the included trials, including uncertainty about methods of allocation concealment and blinding, we did not downgrade any outcome for risk of bias, as the trials about which we had greatest concern were generally of low weight in the meta‐analyses. We also judged 13 studies to be at high risk of attrition bias due to high or unbalanced dropout. To explore this further, we conducted a post hoc sensitivity analysis on our primary outcome (participants with no exacerbations during follow‐up). Removal of studies considered to be at high risk of attrition bias had a minimal impact on the pooled effect estimate.
Funnel plots for days of disability and FEV₁ outcomes (Figure 1 and Figure 2) suggest a possible small‐study effect (i.e. missing small negative trials). Removing the small positive trials from the analyses had minimal impact on the pooled result; therefore we did not downgrade for this reason. Furthermore, a funnel plot for the primary outcome participants with no exacerbation appeared symmetrical, giving no indication of publication bias (Figure 8).
We considered a downgrade for imprecision for health‐related quality of life, but although the confidence interval includes no difference, both ends lie within the MCID for SGRQ, and thus we are reasonably confident that mucolytics do not have a substantial impact on quality of life. However, we did downgrade mortality for imprecision, as the confidence interval of the pooled effect estimate includes both important harm and benefit of the intervention.
Finally, although we considered indirectness on the basis of the age of some of the included studies, we did not judge our concerns to be sufficiently serious to warrant a downgrade.
Potential biases in the review process
The subgroup analysis by decade of publication is post hoc for updates from 2012 onward and should be interpreted with caution. In a few analyses, we have imputed standard deviations. When this has been done, it has been done conservatively and in accordance with accepted practices. This could have narrowed the confidence intervals for individual studies, thus increasing heterogeneity. Furthermore, the approach that we used may tend to overestimate the number of exacerbations per year in both groups, as more occur during the winter months, when many of these studies were performed.
Despite the use of a consistent approach, slight rounding errors may have been introduced by the calculation of exacerbation rates per participant per month from study data to fit into earlier versions of RevMan that allowed only two decimal points. Furthermore, we decided to remove the meta‐analysis for this outcome for the 2019 update, as we made a post hoc decision that this analysis is less robust than the dichotomous exacerbation outcome. Reasons include likely skew, high heterogeneity, and reliance on calculated/imputed data for this analysis.
Agreements and disagreements with other studies or reviews
In addition to this review, we have identified five other systematic reviews of the effects of NAC in chronic bronchitis/COPD. Our results are consistent with these findings. The largest of these reviews included 13 randomised controlled trials (RCTs) (Cazzola 2015). This meta‐analysis reported that patients treated with NAC had significantly and consistently fewer exacerbations of chronic bronchitis or COPD (risk ratio (RR) 0.75, 95% CI 0.66 to 0.84).
The second review demonstrated that individuals treated with NAC were more likely to remain exacerbation‐free (OR 1.56, 95% CI 1.37 to 1.77), with a number needed to treat for an additional beneficial outcome (NNTB) of 6 (95% CI 5 to 9) (Stey 2000). Participants were more likely to report improvement in symptoms with NAC (OR 1.78, 95% CI 1.54 to 2.05) than with placebo. The third review analysed nine trials that had been included in both Stey 2000 and this Cochrane review, and confirmed a significant effect on exacerbations (standardised mean difference (SMD) ‐1.37, 95% CI ‐1.5 to ‐1.25) (Grandjean 2000). A meta‐analysis published in 2017 investigating the effects of NAC on exacerbations of COPD showed that both high‐dose (RR 0.90, 95% CI 0.82 to 0.996) and low‐dose (RR 0.83, 95% CI 0.69 to 0.99) NAC reduced COPD exacerbations (Fowdar 2017). Therefore, the review concluded that long‐term therapy may reduce risk of COPD exacerbation, which is in agreement with our findings.
The fifth review investigated the use of mucolytics and antioxidants for COPD (Li 2015; abstract only available in English). The review includes 10 RCTs and reports that mucolytics and antioxidants reduce the number of exacerbations per patient per year compared to placebo, and that high‐dose NAC may be more effective than low‐dose, although a test for subgroup differences was not reported in the abstract.
The analyses in this review suggest that mucolytics might, in addition, have an effect on duration and severity of exacerbations that do occur, and on the likelihood of taking antibiotics. Data from four studies suggest that mucolytics are associated with decreased hospitalisation rates. It would be helpful if future studies looked at this outcome, as this is where most costs associated with more severe disease are incurred. Few other pharmacological treatments have been shown to reduce hospitalisation: an immunomodulatory agent OM‐85 BV, or Broncho‐Vaxom (Collet 1997), was shown to reduce the number of hospital admissions in COPD, even though it did not affect the number of exacerbations.
Researchers performed a retrospective cost‐effectiveness analysis of NAC in chronic bronchitis that was based on direct costs of NAC treatment, management of an acute exacerbation, and indirect costs of sick leave (Grandjean 2000a). Results suggest that costs of treatment and non‐treatment were equal at the point of a reduction of 0.6 exacerbations per six‐month period. In our review, a reduction of about 0.18 per six‐month period suggested that it would not be cost‐effective to treat everyone with COPD with mucolytics.
Bachh 2007 and colleagues from India estimated the cost of prophylactic NAC therapy to be INR 6000 (USD 120), whereas a short course of oral corticosteroids (OCSs) and antibiotics would cost INR 200 (USD 4). ICSs are also expensive. As the burden of COPD over coming decades is going to disproportionately affect developing nations, the relative costs of each strategy are important to determine.
Several national and international guidelines make recommendations about the use of mucolytics. In a recent North American guideline on treatments to prevent COPD exacerbations, NAC was suggested for patients with moderate or severe COPD and a history of two or more exacerbations in the previous two years (evidence grade 2B ‐ weak recommendation; moderate‐quality evidence; Criner 2015). Furthermore, carbocysteine was suggested (ungraded consensus‐based statement) for patients still having exacerbations in spite of maximal therapy provided to reduce exacerbations. This is consistent with the more recent Joint American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines for prevention of exacerbations, which gives a conditional recommendation for the use of mucolytics in patients with moderate to severe airflow obstruction and frequent exacerbations despite optimal therapy, based on low‐quality evidence (Wedzicha 2017). The 2019 update of the global COPD guidelines states that NAC may have a role in the treatment of patients with recurrent exacerbations (evidence grade B ‐ moderate‐quality evidence), and that carbocysteine or NAC may reduce exacerbations in patients not taking inhaled steroids (grade B) (GOLD 2019). COPD‐X guidelines give a stronger recommendation in favour of high‐dose oral NAC (Yang 2018), and UK National Institute for Health and Care Excellence (NICE) guidelines suggest use in patients with chronic cough productive of sputum and continued only if there is symptomatic improvement (NICE 2018).
Authors' conclusions
Implications for practice.
Mucolytics may reduce the number of exacerbations in people with chronic bronchitis or chronic obstructive pulmonary disease (COPD) by a small quantity, and do not appear to be associated with an increase in adverse events. Approximately one person in eight may avoid having an exacerbation, provided all take treatment every day for an average of nine months. Mucolytics are associated with a reduction in days of disability per month and a reduction in hospitalisations in the studies that reported this outcome. Mucolytics have not been shown to substantially slow the decline in lung function, and it is uncertain whether they improve quality of life. Results are too imprecise to be certain whether or not there is an effect on mortality. As reduction in exacerbations seems the main potential benefit, mucolytics might be considered (1) a treatment option for patients with frequent exacerbations who cannot take other therapies such as inhaled corticosteroids or long‐acting bronchodilators, which have a stronger evidence base for their effectiveness; or (2) as add‐on treatment once all other therapies to reduce exacerbations have been utilised.
Implications for research.
Future studies might address the value of mucolytic therapy:
in patients who have multiple exacerbations per year, or who have prolonged or severe exacerbations;
in patients already receiving current guideline‐based therapy; and
in patients with repeated admissions to hospital with exacerbations of COPD despite maximal therapy to reduce acute exacerbations of COPD.
Studies should stratify participants by (1) the new GOLD criteria (A‐D; GOLD 2019), which incorporate symptoms and exacerbations, as well as spirometry; and (2) use of concomitant medications (such as inhaled corticosteroids (ICSs), long‐acting bronchodilators, or macrolide antibiotics).
Outcomes of studies should include hospitalisations (COPD and all‐cause), mortality (COPD and all‐cause), numbers of days sick with exacerbations, and a validated measure of quality of life. A responder analysis for quality of life would add valuable information on the population effects of treatment.
Feedback
Incorrect dose reported in Zheng 2014 study, 13 February 2020
Summary
In reading the 2019 update to "Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease," I noticed that the N‐acetylcysteine (NAC) dose in Zheng 2014 was reported as 1800 mg. In reading the published PANTHEON study, the intervention of NAC was 600 mg twice daily which would put it in the 1200 mg per day subgroup. Could you please comment on the dose? Thank you.
Reference: Zheng JP, Wen FQ, Bai CX, et al.PANTHEON study group. Twice daily N‐acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, doubleblind placebo‐controlled trial. Lancet Respir Med. 2014 Mar;2(3):187‐94. doi: 10.1016/S2213‐2600(13)70286‐8
Reply
We thank the reader Kathy Grams very much for her interest in our review and for taking the time to give feedback, which in this case corrects an error.
We had indeed recorded the N‐acetylcysteine (NAC) dose in the PANTHEON study by Zheng et al. 2014 as 1800 mg. It is in fact 1200 mg, being 600 mg twice daily. As a result of this we have made the following changes:
Corrected the dose in the Characteristics of included studies table
Corrected text describing included studies
In Analysis 1.4 moved Zheng from the 1800 mg subgroup to the 1200 mg subgroup.
Minor change to text describing this result.
While there are no changes to the overall findings of the review, we appreciate getting the information in the review as correct as possible.
Contributors
Feedback contributor: Kathy Grams, PharmD, BCGP
Author contributor: Phillippa Poole on behalf of the author team.
What's new
| Date | Event | Description |
|---|---|---|
| 9 March 2020 | Feedback has been incorporated | Authors made changes to the reporting of one of the studies in the review. See Feedback 1. There were no changes to the overall findings of the review. |
| 9 March 2020 | Amended | Feedback added. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 23 April 2019 | New search has been performed |
|
| 23 April 2019 | New citation required and conclusions have changed |
|
| 3 July 2014 | New citation required but conclusions have not changed |
|
| 3 July 2014 | New search has been performed | New literature search |
| 5 July 2012 | New search has been performed | 2 new studies (Worth 2009 (cineole) and Schermer 2009 (N‐acetylcysteine (NAC)) included. Data from these studies and from Decramer 2005 included in a new analysis for SGRQ (St George Respiratory Questionnaire). 'Summary of findings' table added. Third review author (CC) added to the review. Potentially eligible abstract added to Studies awaiting classification |
| 5 July 2012 | New citation required and conclusions have changed | Conclusions similar, although smaller beneficial effects of mucolytics on exacerbations noted in more recent trials than in earlier trials |
| 1 November 2008 | New citation required but conclusions have not changed | Review updated to take account of 2 new studies |
| 15 September 2008 | New search has been performed | Search rerun |
| 8 August 2008 | Amended | Converted to new review format |
| 10 March 2006 | New citation required and conclusions have changed | 2005: search repeated, full update performed. Three new studies, including 3‐year BRONCHUS study of 600 mg NAC, included. Smaller effect size of all mucolytics combined than previously. Reasons for this discussed In the BRONCHUS study, significant effect of NAC on exacerbations noted among participants not using inhaled corticosteroids. New comparison added to address this Other new comparisons added: hospitalisations, deaths Otherwise, findings much the same as previously |
| 1 August 2002 | New search has been performed | 2002: no new studies found despite use of wider search strategy. Discussion expanded to include information on other recent meta‐analyses of NAC and a comparison of the effects of mucolytics and fluticasone on exacerbations. Jadad scores for studies now included Data and conclusions same as in 1999 |
| 31 August 1999 | New search has been performed | 1999: 2 studies in patients with COPD now included in the review, hence the title change. Data on 2 other agents ‐ myrtol and the thiol donor N‐isobutyrylcysteine ‐ also included. Eight additional studies and several new analyses included Correction made to reviewers' conclusions on the effects of mucolytics on the secondary endpoint of lung function. Our extracted data checked against original data and confirmed as correct. Small standard deviations in the Olivieri study noted; possibility that study authors reported standard errors. P values quoted in study analysis compatible with this conclusion. Until clarification, this trial removed from analysis. No significant change in lung function noted in data analysis (previously interpreted as favouring placebo). Changes made to relevant parts of Abstract, Results (Lung Function), and Discussion sections No change to overall conclusions of this review with respect to primary endpoint of exacerbation frequency and days of disability ('sick days'). High level of heterogeneity in the size of this effect between trials unclear; possibility that length of study is the cause of this should be examined in a future version of this review For adverse effects, Parr and Rasmussen data taken out of meta‐analysis and reported instead in text because event rates in these studies exceeded numbers in treatment groups. RevMan unable to manage dichotomous data when event rate exceeds 1. Possibility that adverse effects may be less frequent in the mucolytic‐treated group as suggested by meta‐analysis. In large study by Parr (n = 526), mean of 4.9 adverse effects reported per participant in the mucolytic group vs 4.5 adverse effects per participant in the placebo group. Therefore, no changes made to our original conclusion and no differences between treatments in terms of adverse effects |
Acknowledgements
This review is dedicated to the memory of Professor Peter Black, FRACP, who died in 2010. Peter made significant and broad contributions to asthma and COPD research, including work as a reviewer and editor for the Cochrane Airways Group.
We would like to acknowledge the contributions of the following.
Peter Black, who was the co‐author of the first four versions of this review.
Emma Dennett, Peter Gibson, Paul Jones, and Toby Lasserson of the Cochrane Airways Group for editorial support.
Emma Jackson, Anna Bara, Karen Blackhall, and Liz Stovold of the Cochrane Airways Group for searching and retrieving full‐text articles.
Translators Ms. Sharon Kramer, Dr Silvana Campanella, Dr Ruth Black, Dr Klaus Lehnert, Ms. Daniela Screnci, and Mr. Toby Lasserson.
Professor Marc Decramer, who provided information on SGRQ scores from Decramer 2005.
Dr Zheng, who gave us more information on Zheng 2008 and Tatsumi 2007a, as well as the appendix to Zheng 2014. For the latter study, Dr Zheng kindly provided further data on exacerbation rate, SGRQ, and spirometry.
Dompe Farmaceutici (Allegra 1996), which provided us with further data.
Zambon Group for providing the Meister 1986 study and for further information on Nowak 1999.
Douglas Pharmaceuticals and G. Pohl Boskamp for information on Meister 1999.
Professor Roberto Dal Negro, who provided additional information on Dal Negro 2017.
Dr Dennis Niewoehner, who provided additional information on Johnson 2016.
Professor Inoue, who provided additional information on Fukuchi 2016.
Other study authors and companies that took the trouble to write back, even though they could not provide further data (Dr Petty, Dr Boman, Novartis Pharmaceuticals).
Sally Spencer was the Editor for this review and commented critically on the review.
The review authors and Cochrane Airways are grateful to the following peer reviewers for their time and comments: Craig P. Hersh, Brigham and Women’s Hospital; Prof. Marc Decramer, University Hospital Gasthuisberg, Leuven, Belgium; and our consumer reviewer, Andy Coles, for assessing the plain language summary.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Search history
| Years | Search result detail |
| All years to January 1998 | We screened approximately 400 abstracts of papers identified by computer searches. After excluding studies that were clearly ineligible based on the abstract, we obtained the full text for 72 papers. 21 studies involved double‐blind, placebo‐controlled treatment with an oral mucolytic for at least 8 weeks. 3 were excluded because they did not provide information on the primary outcome (Edwards 1976; Maesen 1980; Rubin 1996). Three studies were excluded because they did not report the standard deviation for outcome measures of interest, and we could not obtain this information despite writing to study authors (Christensen 1971; Grillage 1985; Jackson 1984). 15 studies were included in the review |
| January 1998 to 1999 | For the 1999 update, one further study was identified that had been detected on the original search (Cegla 1988), but for which the full text had not been obtained in 1997. Grillage 1985 and Jackson 1984 were not included in the original review but were included in the update, as they had data on participants with no exacerbations ‐ an outcome measure that was added for the update. For this update, and until further clarification is obtained from study authors, we have assumed that error measurement reported in Olivieri 1987 is an SE rather than an SD (see Lung Function) |
| January 1999 to 2002 | In 2002, the search was widened to (chronic bronchitis or emphysema or chronic obstructive pulmonary disease or COPD) AND (mucolytics or mucoactive or N‐acetylcysteine or bromhexine or S‐carboxymethylcysteine or ambroxol or sobrerol or iodinated glycerol or N isobutyrylcysteine or myrtol or NAC or methylcysteine or carbocysteine or erdosteine or strepronin or gelsolin or MESNA). No further eligible studies were identified by this search |
| January 2002 to January 2003 | In 2003, a repeat search with the same terms yielded 44 titles, of which 18 abstracts were screened for eligibility and 5 full texts were retrieved; none were eligible |
| January 2003‐Sept 2005 | An update search conducted in 2005 yielded another 264 titles, of which 9 full texts were retrieved, yielding a further 3 studies for inclusion (Decramer 2005; Malerba 2004; Moretti 2004). |
| 2005‐2007 | A search in 2005 yielded another 16 titles, none of which were eligible; in 2006, a further 2 titles were found with the COOPT study eligible |
| 2008 | Searches in 2008 yielded 20 titles, with 2 more original studies for inclusion (Bachh 2007; Zheng 2008) |
| May 2011 | In 2011, 64 abstracts and papers were identified by the searches. Several reports were related to the PEACE study (Zheng 2008), and to the EQUALIFE study already included in this review (Moretti 2004). Of 7 full texts reviewed, 4 proved eligible: 2 related to the same study of cineole in COPD (Worth and Worth); another to a further study of cineole (Wilhelmi); one was a further post hoc analysis of EQUALIFE (Ballabio 2008a). One study (Lukas) of NAC in CB was excluded, as no data were available on outcomes in this review Furthermore, we were informed about studies of neltenexine, which is a mucolytic, and we considered the full texts of these, which were ineligible. Thus data from 2 new studies were added for the 2012 update (mucolytic* or "mucociliary clearance" or mucoactive or N‐acetylcysteine or bromhexine or S‐carboxymethylcysteine or ambroxol or sobrerol or "iodinated glycerol" or N isobutyrylcysteine or myrtol or NAC or methylcysteine or carbocysteine or erdosteine or strepronin* or gelsolin or MESNA) In 2011, the above search was run from 2008 to the present date, but with the addition of the term "cineole". We were notified about eligible studies of "neltenexine". This term should be included in the next search |
| July 2012 | In 2012, 8 abstracts and papers were identified. An abstract was added to "Studies awaiting classification" (Moretti 2011a) |
| July 2014 | A search in July 2014 using the terms below yielded 29 new references (The full search strategy used in this update is provided in Appendix 3) Full texts of studies that were possibly eligible were retrieved. The Moretti trial mentioned above was ineligible. Several studies had duplicate reports. A search was made of the bibliographies of eligible studies, as well as of online clinical trials. A duplicate paper on a trial already identified was found during a search for study author details. From these searches, 4 new eligible trials were identified for inclusion in this review (De Backer 2013; Roy 2014; Tse 2013; Zheng 2014). We wrote to Dr De Backer to request additional information on the secondary outcomes of SGRQ and spirometry alluded to in their paper, with no response. Dr Zheng provided further information on several outcomes (Zheng 2014) |
| July 2017 | A database search yielded 54 references, and searches of clinical trial registries identified a further 13 records. We excluded 50 on the basis of title and abstract and reviewed 17 full texts for possible inclusion. We excluded a further six records (5 unique studies) at this stage and identified 1 ongoing study that meets the inclusion criteria for this review. The remaining 10 records were eligible for inclusion. Six records, linked to 4 new unique studies, were added to the review (Dal Negro 2017; Fukuchi 2016; Johnson 2016; Xu 2014). A further 4 records identified were additional references to studies already included in the review |
Appendix 2. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Dates searched | Frequency of search |
| CENTRAL (via the Cochrane Register of Studies (CRS)) | From inception | Monthly |
| MEDLINE (Ovid) | 1946 onwards | Weekly |
| EMBASE (Ovid) | 1974 onwards | Weekly |
| PsycINFO (Ovid) | 1967 onwards | Monthly |
| CINAHL (EBSCO) | 1937 onwards | Monthly |
| AMED (EBSCO) | From inception | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the Cochrane Airways Trials Register
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 3. Search strategy to identify relevant trials from the Cochrane Airways Trials Register
Search platform: Cochrane Register of Studies (CRS)
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MeSH DESCRIPTOR Expectorants
#8 mucolytic*
#9 mucociliary* NEXT clearance*
#10 mucoactive
#11 *acetylcysteine
#12 bromhexine
#13 *carboxymethylcysteine
#14 ambroxol
#15 sobrerol
#16 "iodinated glycerol"
#17 isobutyrylcysteine
#18 myrtol
#19 NAC:ti,ab
#20 methylcysteine
#21 carbocysteine
#22 erdosteine
#23 strepronin*
#24 gelsolin
#25 mesna*
#26 cineole
#27 neltenexine
#28 eucalyptus
#29 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28
#30 #6 and #29
[Note: in search line #4, MISC1 denotes the field in which the reference has been coded for condition, in this case, COPD]
Data and analyses
Comparison 1. Mucolytic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with no exacerbations in study period | 28 | 6723 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.73 [1.56, 1.91] |
| 1.1 Double‐blind | 26 | 6460 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.53, 1.88] |
| 1.2 Single‐blind/open | 2 | 263 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.91 [1.76, 4.83] |
| 2 Participants with no exacerbation by decade, double‐blind trials only | 26 | 6460 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.53, 1.88] |
| 2.1 Before 1990 | 12 | 2304 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.34 [1.97, 2.79] |
| 2.2 1990 to 1999 | 5 | 1105 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.91 [1.50, 2.44] |
| 2.3 2000 to 2009 | 5 | 1477 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [1.01, 1.54] |
| 2.4 2010 onwards | 4 | 1574 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 3 Participants with no exacerbations in the study period ‐ winter treatment only | 21 | 4007 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.20 [1.93, 2.51] |
| 3.1 Double‐blind | 20 | 3844 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [1.91, 2.49] |
| 3.2 Single‐blind/open | 1 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.85 [1.49, 5.46] |
| 4 Participants with no exacerbations in study period ‐ by dose or type of mucolytic | 28 | 6723 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.73 [1.56, 1.91] |
| 4.1 NAC 400 mg | 3 | 717 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.98 [2.21, 4.03] |
| 4.2 NAC 600 mg | 9 | 1425 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.76 [1.40, 2.21] |
| 4.3 NAC 1200 mg | 3 | 1213 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.95, 1.57] |
| 4.5 NAC 3200 mg | 1 | 45 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.14, 2.01] |
| 4.6 Carbocysteine | 4 | 1251 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [1.13, 1.77] |
| 4.7 Other mucolytic | 8 | 2072 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.97 [1.64, 2.36] |
| 5 Participants with no exacerbations in study period ‐ by FEV₁ | 28 | 6723 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.73 [1.56, 1.91] |
| 5.1 Mean FEV₁ > 50% predicted | 24 | 5352 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.81 [1.62, 2.03] |
| 5.2 Mean FEV₁ ≤ 50% predicted | 4 | 1371 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [1.08, 1.75] |
| 6 Participants with no exacerbations in study period ‐ by study duration | 28 | 6723 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.73 [1.56, 1.91] |
| 6.1 Duration ≤ 3 months | 5 | 903 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.14 [1.62, 2.82] |
| 6.2 Duration > 3 months and < 12 months | 18 | 3278 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.20 [1.91, 2.54] |
| 6.3 Duration ≥ 12 months | 5 | 2542 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.98, 1.37] |
| 7 Participants with no exacerbations in study period ‐ by country | 28 | 6723 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.73 [1.56, 1.91] |
| 7.1 Italian | 11 | 2407 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.44 [2.06, 2.88] |
| 7.2 Rest of world | 17 | 4316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [1.25, 1.61] |
| 8 Participants with no exacerbations in study period ‐ by history of exacerbation | 28 | 6723 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.73 [1.56, 1.91] |
| 8.1 Exacerbation history requirement for inclusion | 16 | 4192 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.50 [1.32, 1.70] |
| 8.2 Exacerbation history not a requirement for inclusion | 12 | 2531 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [1.85, 2.57] |
| 9 Participants with no exacerbations in study period ‐ by ICS use | 28 | 6723 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.73 [1.56, 1.91] |
| 9.1 ICS allowed | 15 | 4401 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.46, 1.87] |
| 9.2 ICS not allowed | 6 | 1431 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.85 [1.49, 2.31] |
| 9.3 ICS unclear | 7 | 891 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [1.48, 2.58] |
| 10 Number of exacerbations per participant per month | Other data | No numeric data | ||
| 11 Days of disability per participant per month | 9 | 2259 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.56, ‐0.30] |
| 12 Days on antibiotics per participant per month | 3 | 714 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.76, ‐0.31] |
| 13 FEV₁ at end of study | 14 | 3473 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [0.01, 0.07] |
| 13.1 Double‐blind | 13 | 3310 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [0.01, 0.07] |
| 13.2 Single‐blind | 1 | 163 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.10, 0.26] |
| 14 Percent predicted FEV₁ | 4 | 414 | Mean Difference (IV, Fixed, 95% CI) | 4.79 [1.97, 7.62] |
| 14.1 Double‐blind | 2 | 230 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐4.72, 4.47] |
| 14.2 Single‐blind | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐4.02, 5.42] |
| 14.3 No blinding | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 17.31 [11.83, 22.79] |
| 15 PEFR at end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Double‐blind | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 FVC at end of study | 12 | 3127 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.00, 0.10] |
| 17 Adverse effects | 24 | 7264 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.94] |
| 18 Hospitalisation during study period | 5 | 1833 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.52, 0.89] |
| 19 Death during study period | 11 | 3527 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.51, 1.87] |
| 20 Health‐related quality of life (total score St. George's Respiratory Questionnaire) | 7 | 2721 | Mean Difference (IV, Fixed, 95% CI) | ‐1.37 [‐2.85, 0.11] |
| 21 Health‐related quality of life (total score COPD Assessment Test) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 2. Systemic thiol donor versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with no exacerbations in the study period | 1 | 628 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.74, 1.39] |
| 2 Number of exacerbations per participant per month | Other data | No numeric data | ||
| 3 Days of disability per participant per month | 1 | 628 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.82, 0.46] |
| 4 Adverse effects | 1 | 628 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.98, 1.95] |
2.2. Analysis.
Comparison 2 Systemic thiol donor versus placebo, Outcome 2 Number of exacerbations per participant per month.
| Number of exacerbations per participant per month | |||||||
|---|---|---|---|---|---|---|---|
| Study | Mean mucolytic group | SD | N | Mean control group | SD | N | Mean difference [95% CI] |
| Ekberg‐Jansson 1999 | 0.18 | 0.22 | 313 | 0.17 | 0.21 | 315 | 0.01 [‐0.02, 0.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allegra 1996.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre study, with 1 month run‐in before randomisation. Duration 6 months. ITT and PP analysis | |
| Participants | 440 participants with chronic bronchitis (MRC). Age 20 to 70; FEV₁ 40% to 70%; at least 2 exacerbations in previous 12 months
Exclusions: neoplastic disease, TB, asthma or uncompensated liver, kidney or heart disease, pregnancy Other mucoactive and anti‐cough agents, oral or inhaled corticosteroids not permitted Mean age 60 years; 75% had smoking history; FEV₁ 2.12 (SD 0.6) L; mean 2.7 (SD 1.3) exacerbations in past 12 months Dropouts: 89 (20%) |
|
| Interventions | 3 treatment arms. Carbocysteine lysine salt monohydrate (SCMC‐Lys) 2.7 g daily, placebo, and SCMC‐Lys 2.7 g daily alternating 1 week active, 1 week placebo. We assessed continuous vs placebo treatment only | |
| Outcomes | Diary scores of symptoms, exacerbations, time to first exacerbation, duration of exacerbation, days on antibiotics, adverse events | |
| Notes | Italian. Requested SD for exacerbations for per‐protocol and intention‐to‐treat analysis. Requested data were provided by sponsoring company. ITT analysis was done with an estimate of duration of treatment derived from the paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated; balanced per centre |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% dropout rate (89/440); dropout was higher in the intervention arm compared to the placebo arm (23% vs 16%), largely due to more participants in the intervention arm failing to comply with the trial protocol |
| Selective reporting (reporting bias) | Low risk | Reported main outcomes with ITT and PP analyses |
Babolini 1980.
| Methods | Double‐blind, placebo‐controlled, parallel, 36 centres. PP analysis. Duration 6 months | |
| Participants | 744 outpatients with chronic bronchitis defined by MRC. Excluded if too young, too sick, additional significant disease, history of peptic ulcer, on mucolytics. 60% were over the age of 50; 73.5% were male; mean FEV₁ 2.18 L; FEV₁ 40% to 70% predicted; 64.3% smokers. 249 dropouts. Baseline groups matched. Dropout groups matched | |
| Interventions | NAC 200 mg twice daily or placebo | |
| Outcomes | Exacerbations, symptom scores, global assessments by participants and physicians, adverse effects, days on antibiotics | |
| Notes | Italian. Same data also in Ferrari. SD calculated from graph. 5 or more exacerbations counted as 5. Further data requested but not yet provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Restricted' randomisation; balanced blocks |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; matching placebo, identified by code number |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 117/371 (32%) participants dropped out of the intervention arm and 132/373 (35%) participants dropped out of the placebo arm. More participants withdrew from the placebo arm due to lack of efficacy (6 vs 2) and adverse reactions (13 vs 6); other reasons were reasonably balanced |
| Selective reporting (reporting bias) | Low risk | None detected |
Bachh 2007.
| Methods | Randomised, single‐blind, placebo‐controlled, parallel, single‐centre. Follow‐up 12 months, although treatment given for only 4 months | |
| Participants | 100 outpatients with smoking‐related COPD. Age > 50 years; post‐bronchodilator FEV₁ 30% to 80% predicted; reversibility < 12%; FEV₁/FVC < 70%. Stable medications and ICS permitted at steady dose
Exclusions: intolerance of NAC, continuous treatment with OCS, NAC for 3/12 or more, asthma or atopy, other respiratory diseases, NYHA Class II or greater heart failure. Non‐compliance in taking medication Mean age: 61 (SD 7) years; 78% male. Mean duration of disease 6.4 years. Mean number of exacerbations in 2 years before study, 4.7. Mean FEV₁ 52% (SD 10) predicted and reversibility 6% (SD3). 18/100 (18%) were using ICS No dropouts recorded |
|
| Interventions | NAC 600 mg once daily or placebo for 4 months | |
| Outcomes | Exacerbations, hospital admissions, pulmonary function tests, adverse effects | |
| Notes | Indian study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | High risk | Single‐blind |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind; investigators not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts recorded |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
Boman 1983.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, run‐in, multi‐centre. Duration 6 months | |
| Participants | 259 outpatients with chronic bronchitis defined by MRC. Exclusion criteria: asthma, FEV₁ < 50%; other comorbidities; on antibiotics; women pregnant or trying for pregnancy. 56 dropouts. Mean age 51.9 years. FEV₁ 80% predicted. 100% smokers Exacerbations in past 12 months | |
| Interventions | NAC 200 mg twice daily or placebo | |
| Outcomes | Exacerbations, sick leave due to exacerbations, adverse effects | |
| Notes | Swedish. SD calculated from paper. 6 or more exacerbations counted as 6. Requested more information to calculate effect on sick days, but study authors unable to locate original material | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Done independently at each centre via a table of random numbers |
| Allocation concealment (selection bias) | High risk | Investigators aware of order of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, but may have been aware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind, but may have been aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High dropout rate (22%; 56/259), but numbers and reasons similar in both trial arms. All participants included in the safety analyses |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
Bontognali 1991.
| Methods | Randomised, double‐blind, placebo‐controlled. Duration 3 months | |
| Participants | 60 participants with chronic bronchitis recruited as inpatients; 63% male. Mean age 57 years. Admission criteria 20 mL sputum/day with history of 4 or more episodes of acute bronchitis in past 12 months and Tiffeneau index ≤ 40%. No loss to follow‐up | |
| Interventions | Cithiolone 400 mg twice daily or placebo for 1 month followed by 400 mg once daily for a further 2 months | |
| Outcomes | Exacerbations and duration of acute exacerbations, FEV₁ and FVC, sputum viscosity, adverse effects | |
| Notes | Italian. Surprising that no participants withdrew from the study. Huge confidence limits. Possible typographical error in paper, as SD for number of exacerbations per month is the same as for duration of exacerbations. We have used study authors' rates in comparison 01:02 and divided them by months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All completed study |
| Selective reporting (reporting bias) | Unclear risk | Main outcomes not stated viz "efficacy" |
Borgia 1981.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre. PP analysis. Duration 6 months | |
| Participants | 21 outpatients with chronic bronchitis defined by MRC and exacerbation in period before the study. Mean age 45.3 years and FEV₁ 3.82 L. Exclusions not stated except FEV₁ < 40%. 2 dropped out | |
| Interventions | NAC 200 mg twice daily or placebo | |
| Outcomes | Exacerbations, lung function, symptom scores, clinical assessment, adverse effects | |
| Notes | Italian. Published in Italian; therefore reliant on translation. Large differences in baseline rates for lung function | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9% dropout rate (2/21), both from the placebo arm. One participant failed to return for follow‐up and the other experienced diarrhoea |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
Castiglioni 1986.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (18). PP analysis. Duration 3 months | |
| Participants | 706 outpatients with chronic bronchitis defined by MRC. Mean age 56.5 years; 76% male; FEV₁ 73.3% predicted; 73.5% current or former smokers. Excluded were patients younger than 18 years or older than 75; FEV₁ < 60%; severe comorbidity; prior treatment with oral corticosteroids or antibiotics and > 2 other medications. 33 dropped out | |
| Interventions | Sobrerol 300 mg twice daily or placebo | |
| Outcomes | Exacerbation rate, consumption of antibiotics and other medicines, clinical signs, laboratory data, lung function, global assessment by investigator and participant, adverse effects | |
| Notes | Italian. Requested more information to allow determination of days on antibiotics; not yet provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Done independently at each centre with a table of random numbers to obtain balanced groups |
| Allocation concealment (selection bias) | High risk | Investigators aware of order of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind; matching placebo but may have been aware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind but may have been aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% dropout rate (33/706); numbers and reasons balanced between trial arms |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
Cegla 1988.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre. PP analysis. Duration 24 months | |
| Participants | 180 outpatients with chronic bronchitis defined by WHO Mean age 51.1 years; 64% male. Mean FEV₁ 2.15 L; 36% current smokers. Excluded were patients over 60 years of age and patients with asthma, cor pulmonale, pulmonary hypertension, or polycythaemia < 60%. 23 dropped out. 4 people died | |
| Interventions | Ambroxol retard 75 mg daily or placebo | |
| Outcomes | Exacerbations, days sick (off work, in hospital), participant symptoms by diary card, lung function, extra medication use, assessment by investigator and participant, adverse effects | |
| Notes | German. Written in German. Required translation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 23/180 (13%) participants did not complete follow‐up. 7/180 participants were excluded from the final analysis (4 in the intervention group and 3 in the placebo group). A further 16 participants were followed up for at least 6 months but dropped out before completing the trial. Reasons for loss to follow‐up are not given |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
Cremonini 1986.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel. Duration 3 months | |
| Participants | 41 outpatients with chronic bronchitis defined by ERS, all of whom completed the study. Exclusion criteria not stated. Mean age 60.8 years; FEV₁ 58.6% predicted | |
| Interventions | Letosteine 50 mg 3 times daily or placebo | |
| Outcomes | Exacerbations, days off work sick, lung function. Adverse effects not evaluated | |
| Notes | Italian. Written in Italian; therefore relying on translation. SD calculated from raw data in paper, but numbers in placebo and active groups vary (20/21 or 21/20 respectively) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All completed study |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
Dal Negro 2017.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (10). Duration 12 months | |
| Participants | 467 outpatients who were current or ex‐smokers aged 40 to 80 years with GOLD stage II/III and a stable therapeutic regimen for more than 8 weeks. Patients had to have experienced 2 or more acute COPD exacerbations requiring medical intervention in the previous 12 months Exclusions: pregnant, lactating mother; lack of efficient contraception in a subject with child‐bearing potential; acute exacerbation of COPD within 8 weeks before inclusion; treatment with antibiotics and/or systemic steroids and/or hospitalisations within 8 weeks before inclusion; change in therapeutic regimen for COPD in the last 8 weeks before inclusion; COPD stage IV; current or past diagnosis of asthma; FEV₁ reversibility test showing change in FEV₁ > 400 mL 30 minutes after inhalation of 400 µg of salbutamol pMDI; clinically significant or unstable concurrent disease or other significant renal impairment as indicated by creatinine clearance < 25 mL/ min; active peptic ulcer; liver cirrhosis Mean age: 64.8; 74% male Dropouts: 22% in erdosteine group; 22% in placebo group |
|
| Interventions | Erdosteine 300 mg twice daily or placebo | |
| Outcomes | Primary outcome: number of acute exacerbations Secondary outcomes: spirometry parameters, COPD symptoms, quality of life, safety and tolerability of erdosteine |
|
| Notes | RESTORE study: multi‐national study including 10 European countries funded by Edmond Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician generated a randomisation list of patient random numbers using a pseudo‐random number generator. Series of 4 patients for each of the 2 strata were assigned to study sites to achieve, within each centre, a balanced number of patients treated with erdosteine or placebo in each of the 2 strata |
| Allocation concealment (selection bias) | Low risk | Erdosteine and placebo capsules were manufactured and provided by the sponsor. Placebo was identical in composition, shape, colour, and size but did not contain any active ingredients. Erdosteine or placebo capsules were packed identically. The investigator or anyone at the study site was prevented from knowing the allocation sequence with code labelling |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was identical in composition, shape, colour, and size but did not contain any active ingredients. Erdosteine or placebo capsules were packed identically. The investigator or anyone at the study site was prevented from knowing the allocation sequence with code labelling. The sponsor and the clinical research associate were notified if there was a clinical reason for an individual’s treatment to be unmasked by the investigator |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specifically described in trial report, but in clinical trials, record outcome assessors described as blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Approx 20% withdrew from both arms (50/228 from the intervention arm and 52/239 from the placebo arm) for similar reasons, but ITT analysis conducted including over 90% of participants in both arms |
| Selective reporting (reporting bias) | Low risk | Several outcomes of interest reported narratively as 'no difference' in publication, but study authors supplied required information upon request |
De Backer 2013.
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over. Duration 3 months | |
| Participants | 12 outpatients with GOLD stage II or III COPD, age ≥ 40, smoking history at least 10 pack‐years but now smoke free, presence of COPD symptoms. 9 men and 3 women with mean age 65, 56 pack‐years, and FEV₁ 65%. All completed the study. Exclusions: recent exacerbation; allergy to or prior treatment with NAC; PKU; untreated peptic ulcer; organ insufficiency; ongoing treatment with oral, IV, or IM steroids; pregnancy or breastfeeding; treatment with oral cephalosporin | |
| Interventions | NAC 600 mg 3 times daily or placebo | |
| Outcomes | Measured at baseline and at end of each 3/12 treatment period: spirometry, PEFR, raw NO, specific airway resistance from plethysmography, CT to look at airway geometry, serum glutathione, enzymes, SGRQ, ABG | |
| Notes | Belgian. Funded by an imaging company and a pharmaceutical company Dr Backer works for FluidDA, a functional respiratory imaging company, contracted by Zambon, manufacturer of NAC Responder analysis. Did not report on spirometry or SGRQ results for treatment groups as a whole. These have been requested |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer‐generated randomisation list used; no further details |
| Allocation concealment (selection bias) | High risk | Cross‐over trial. Trial lasted from August 2009 to June 2012 for only 12 participants. No details on allocation or concealment procedures reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were their own controls. No information about similarity of NAC and placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Cross‐over trial with no washout period. Possible practice effects. Unsure how blinded investigators were |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Reported responder analysis. Did not report on spirometry or SGRQ results for treatment groups as a whole |
Decramer 2005.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre. ITT analysis. Duration 3 years | |
| Participants | 523 outpatients with smoking‐related COPD. Age 40 to 75 years; post‐bronchodilator FEV₁ 40% to 70% predicted; reversibility < 12% and 200 mL; FEV₁/FVC 88% for men and 89% for women; history of at least 2 exacerbations during 2 years before enrolment
Exclusions: intolerance of NAC, continuous treatment with oral steroids, NAC for 3/12 or longer, asthma or atopy, other respiratory diseases, NYHA Class II or greater heart failure, GI disease, likely LTOT or lung transplant, alpha 1 antitrypsin deficiency, enrolment in rehab or other study 3 months before this study. ICS permitted, although steady dose recommended Mean age 62 (SD 8) years; 79% male; FEV₁ 1.65 (SD 0.38) L; 57% (SD 9) predicted; 46% current smokers; 70% used ICS Yearly exacerbation rate (control group) 2.5 (SD 0.9) events Dropouts: 70 (27%) in NAC group and 99 (37%) in placebo group (P = 0.018) |
|
| Interventions | NAC 600 mg daily vs placebo | |
| Outcomes | Yearly reduction in lung function and exacerbation rate Secondary endpoints: quality of life (SGRQ), cost utility Planned subgroup analyses ‐ by baseline ICS dose and disease severity |
|
| Notes | European. BRONCUS study
Cost utility will be reported in another publication Data from mixed‐effects model used in this study have been provided by Professor De Cramer for total SGRQ scores. Change on NAC was ‐2.31 and on placebo ‐3.71 Add these to baseline (using baseline SD) 36.7 (16) and 36.3 (15) to get total SGRQ at end of study to enter into RevMan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Allocation concealed from study investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; identical placebo and active tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind; investigator unaware of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High and unbalanced dropout; 70/256 (27%) and 99/267 (37%) withdrew from mucolytics and placebo, respectively. A greater number of placebo participants withdrew consent (26 vs 13), experienced an adverse event leading to withdrawal (26 vs 19), or experienced worsening of disease/lack of efficacy (6 vs 2) |
| Selective reporting (reporting bias) | Low risk | None detected |
Ekberg‐Jansson 1999.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (41). PP analysis. Duration 6 months | |
| Participants | 637 outpatients with chronic bronchitis defined by MRC 1 exacerbation in previous winter. Average age 58 years; 61% male; mean FEV₁ 73% predicted; 100% current smokers or ex‐smokers. Exclusions: females of fertile age, FEV₁ < 40% predicted, significant reversibility, unstable non‐respiratory disease, other respiratory disease, atopy, peptic ulcer, lactose intolerance or daily purulent sputum. 134 dropped out | |
| Interventions | N‐isobutyrylcysteine (NIC) 300 mg twice daily or placebo | |
| Outcomes | Time to first exacerbation, exacerbation rate, days sick (judged by participants and investigators), lung function, adverse effects | |
| Notes | European including British. New agent‐free thiol donor derivative of NAC | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 72/316 (23%) dropped out of the intervention arm and 62/321 (19%) dropped out of the placebo arm. There were more adverse events leading to discontinuation in the intervention arm (42 vs 25), and more dropouts in the placebo arm were classified as "other reasons" (34 vs 22) |
| Selective reporting (reporting bias) | Low risk | Reported on main outcomes |
Fukuchi 2016.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel. Duration 12 months | |
| Participants | 408 outpatients between 20 and 85 years of age with smoking history, post‐bronchodilator ratio of FEV₁ to forced vital capacity (FVC) < 70%, and FEV₁ < 80% predicted in the screening Exclusions: history of COPD exacerbation within 7 days before the start of oral administration of study drugs; history of lung transplantation, pneumonectomy, or lung volume reduction surgery; clinically severe disease (e.g. pulmonary tuberculosis) Dropouts: 15% in lysozyme group; 17% in placebo group |
|
| Interventions | Lysozyme 90 mg 3 times daily or placebo | |
| Outcomes | Primary outcome: prevention of COPD exacerbation (as assessed by exacerbation rate and time to first exacerbation) Secondary outcomes: respiratory function assessed by spirometry, health status assessed by CAT | |
| Notes | Japanese. This study was conducted with funds from Aska Pharmaceutical Co., Ltd.; Nippon Shinyaku Co., Ltd.; and Eisai Co., Ltd. Two patients were withdrawn from the study before the start of oral administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After the screening period, patients were randomly assigned to lysozyme or placebo treatment in a ratio of 1:1. Correspondence with trial authors confirmed that "independent statisticians from sponsors made a randomized sequence. The randomization sequence was made by permuted block method with variable block size of block sizes 2 and 4 and equal randomization ratio, using SAS" |
| Allocation concealment (selection bias) | Low risk | Correspondence with trial authors confirmed "based on the randomized sequence, the study drug was placed in a box and sealed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study used a "matching placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Correspondence with trial authors confirmed that outcome assessors remained blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31/202 (15%) dropped out of the intervention arm and 35/204 (35%) dropped out of the placebo arm. Correspondence with trial authors confirmed that reasons for withdrawal were balanced between trial arms |
| Selective reporting (reporting bias) | Low risk | Quality of life score not reported numerically in published trial report but supplied by trial authors on request |
Grassi 1976.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (6). PP analysis. Duration 6 months | |
| Participants | 80 outpatients with chronic bronchitis defined by American and British criteria. 11 dropped out. Mean age 60.9 years; 80% male | |
| Interventions | NAC 600 mg daily or placebo for 3 days per week | |
| Outcomes | Exacerbations, clinical symptoms (3 months), sputum characteristics, adverse effects | |
| Notes | Italian. SD calculated from paper. 3 or more exacerbations counted as 3. 1 to 2 exacerbations counted as 1.5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/40 (13%) dropped out of intervention arm and 6/40 (15%) of placebo arm. A further 4 were excluded (3 placebo and 1 intervention) due to ineffectiveness of treatment. Reasons for 11 dropouts were not given |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
Grassi 1994.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre. PP analysis. Duration 3 months | |
| Participants | 135 outpatients with chronic bronchitis with at least 2 exacerbations previous winter randomly assigned to 1 of 3 treatments. Participants aged 40 and 75, mean age 61.8 years; chronic bronchitis for at least 5 years; FEV₁ 56.7% predicted; 76% smokers For this analysis, n = 87. 4 dropped out | |
| Interventions | Carbocysteine‐sobrerol 1 dose daily, placebo 1 dose daily, or alternating active‐placebo for 10 days each, for 3 months. 1 treatment group was intermittent; this is not included in the analysis | |
| Outcomes | Exacerbations, symptoms, sputum characteristics | |
| Notes | Italian. Published in Italian; therefore relying on translation. SD calculated from paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/45 (7%) from the intervention group and 1/42 (2%) from the placebo group dropped out. Reasons for withdrawal from the intervention group included refusal of treatment, non‐attendance at follow‐up, and an adverse event. The only participant who dropped out of the placebo group refused treatment |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
Grillage 1985.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (17). PP analysis. Duration 6 months | |
| Participants | 109 general practice patients with chronic bronchitis defined by MRC, reversibility < 20%. Exclusions: severe hepatic or renal impairment or peptic ulcer; taking mucolytics or steroids. Participants were over 40 years of age; mean PEFR 232 L/min, with episodes of bronchitis in previous winters. 11 dropped out, including 2 who died | |
| Interventions | Carbocysteine 750 mg 3 times daily or placebo | |
| Outcomes | Exacerbations, lung function, adverse effects | |
| Notes | British. Excluded from original review, but with new comparison, "pts with no exacerbations" can now be included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/54 (11%) dropped out of the intervention arm: 3 due to adverse events, 2 due to non‐compliance, and 1 moved to another area. 3/55 (5%) dropped out of the placebo arm: 2 due to adverse events, 1 due to inefficacy of the trial medication |
| Selective reporting (reporting bias) | Low risk | Reported on main outcomes |
Hansen 1994.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (6). 4‐week run‐in. PP analysis. Duration 5 months | |
| Participants | 153 outpatients with chronic bronchitis defined by MRC. At least 2 exacerbations in past year; FEV₁ ≥ 50% predicted; < 20% reversibility. 100% had smoked. Exclusions were those with atopy or heart disease and on long‐term antibiotics. Mean age 51.4 years; 43% male. Mean FEV₁ 2.34 L; 24 dropped out | |
| Interventions | NAC 600 mg twice daily or placebo | |
| Outcomes | Exacerbations, subjective symptom scores, global well‐being, lung function, adverse effects. Sick days not assessed | |
| Notes | Danish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in blocks of 4 provided by third party |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/75 (21%) dropped out of the intervention arm and 8/78 (10%) from the placebo arm. Reasons for dropout not reported |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
Jackson 1984.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (16). PP analysis. Duration 3 months | |
| Participants | 155 general practice patients with chronic bronchitis defined by MRC. 88% had smoked. Exclusions were those with other serious respiratory disease or peptic ulcer and those on long‐term antibiotics or requiring mucolytics. Mean age 63 years; 67% male. 34 dropped out | |
| Interventions | NAC 200 mg 3 times daily or placebo | |
| Outcomes | Exacerbations, subjective symptom scores, clinical signs, radiological appearance, global well‐being, adverse effects | |
| Notes | British. Excluded from original review, but with new comparison, "pts with no exacerbations" can now be included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22% overall dropout rate (34/155). 4 participants withdrew from the intervention arm due to adverse events and 5 from the placebo arm. Other reasons for withdrawal from each arm not given |
| Selective reporting (reporting bias) | Unclear risk | None detected |
Johnson 2016.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel. Duration 8 weeks | |
| Participants | 51 outpatients with chronic cough and sputum production. Principal eligibility criteria were as follows: (1) ratio of post‐bronchodilator FEV₁/FVC < 0.70 along with FEV₁ < 65% predicted; (2) age > 40 years and < 85 years; (3) current or past history of cigarette smoking of at least 10 pack‐years; (4) no COPD exacerbation in the last 4 weeks; (5) presence of chronic bronchitis Exclusions: (1) primary clinical diagnosis of asthma; (2) uncompensated heart failure; (3) cirrhosis with ascites and edema; (4) estimated glomerular filtration rate 30 mL/min/1.73 m²; (5) use of long‐acting nitrates; (6) inability to provide informed consent Mean age 70 years; average FEV₁ 40% predicted Dropouts: 15% in NAC group; 8% in placebo group |
|
| Interventions | NAC 1800 mg twice daily or placebo | |
| Outcomes | Primary outcome: change in total score of the SGRQ Secondary outcomes: changes in the 3 domains of the SGRQ, CBSAS, SF‐36, lung function with post‐bronchodilator spirometry |
|
| Notes | American. Funded by the Minnesota Veterans Medical Research and Education Foundation, the HealthPartners Institute of Education and Research, and the University of Minnesota Graduate School. Trial terminated due to safety concerns before enrolment completed. Unclear what impact this had on results reported. Study authors conducted an analysis to determine the probability of a statistically significant difference in SGRQ had the trial continued, and concluded that had a mid‐study futility analysis been incorporated into the protocol, this result in itself would have terminated the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At each site, patients were randomized 1:1 to active drug or placebo in permuted blocks of size 2. Research pharmacists at each site were the only study personnel with access to the randomisation list |
| Allocation concealment (selection bias) | Low risk | Research pharmacists at each site were the only study personnel with access to the randomisation list; they assigned treatment accordingly. All other study personnel and study patients were fully blinded to the allocation arm |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo tablets were indistinguishable from active drug in terms of appearance, effervescence, taste, and odour |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel and study patients were fully blinded to the allocation arm. The study team was unblinded to efficacy outcomes only after the decision had been made to terminate the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/27 (15%) participants in the intervention arm did not complete the trial; 2/24 (8%) participants in the placebo arm did not complete. One placebo participant was unable to make the follow‐up visit; the remainder did not complete due to early trial termination |
| Selective reporting (reporting bias) | Low risk | All planned outcomes of interest in this review reported fully. SGRQ only outcome listed on clinical trials record |
Malerba 2004.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (26). ITT and OT. Duration 12 months | |
| Participants | 242 participants with COPD (ATS definition) and chronic bronchitis. Age 40 to 75; FEV₁ 60% to 80% (GOLD stage IIA); pathological chest auscultatory findings; at least 1 exacerbation in previous 12 months
Exclusions: CF, bronchiectasis, asthma, centrilobular emphysema, peptic ulcer or liver, kidney or heart insufficiency Other mucoactive and anti‐cough agents, OCS, or ICS not permitted. ICS withdrawn at least 4 weeks before study Mean age 60 years; 75% had smoking history; FEV₁ 2.12 (SD 0.6) L; mean 2.7 (SD 1.3) exacerbations in past 12 months Dropouts: 34 (16%) |
|
| Interventions | Ambroxol 75 mg twice daily or placebo | |
| Outcomes | Exacerbations over first 6 months (winter period) and at 12 months Secondary: cough intensity and frequency, difficult expectoration, dyspnoea, days on antibiotics, number of working days lost, number of days of hospitalisation |
|
| Notes | Italian. AMETHIST study Post hoc analysis on participants with more severe condition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14% dropout rate (34/242), but only 3% excluded from intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported; some post hoc analysis |
McGavin 1985.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (26). PP analysis. Duration 5 months | |
| Participants | 244 participants entered the study, with 200 participants randomly assigned. 181 randomly assigned appropriately (others ineligible or untraceable). Chronic bronchitis defined by MRC; 1 or more exacerbations per year for the past 3 years; FEV₁ < 50% and FEV₁/FVC < 70% predicted. Mean FEV₁ 0.86 L. Mean age 63.4 years; 85% male. 99% current smokers or ex‐smokers. 148 completed 5 months of treatment | |
| Interventions | NAC 200 mg 3 times daily or placebo | |
| Outcomes | Exacerbations, days of antibiotics, days in bed, FEV₁ and VC, adverse effects | |
| Notes | British. BTS research committee. Mean exacerbation rate given by study authors does not agree with what we calculated from their raw data. Have used authors' rates. Have used SE from body of text (same value reported in abstract as SD). For post‐treatment FEV₁, SD estimated from baseline data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52/200 (26%) randomised participants did not complete the trial. 14 were found to be 'ineligible' (10 in the intervention group and 4 in the placebo group), and a further 5 were lost from the intervention group through "administrative error". Of the remaining eligible participants, 13 dropped out from the intervention group and 20 from the placebo group. The imbalance in numbers is largely due to more participants in the placebo group dropping out due to being "too ill" |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not stated clearly, viz "the effect" of ... |
Meister 1986.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (54). Duration 6 months | |
| Participants | 252 outpatients with chronic bronchitis defined by WHO. At least 1 exacerbation in the past winter. 10 patients with asthma and chronic bronchitis were included. Exclusions: those who had received at least 14 days of antibiotics for chronic bronchitis in the past 6 months; pregnancy. Average age 57.2 years; 59% male. Average PEFR 303 L/min. 88% had smoked. 71 dropped out | |
| Interventions | NAC 300 mg twice daily or placebo | |
| Outcomes | Exacerbations, days sick, concomitant treatment, adverse effects | |
| Notes | German. Provided by Zambon. Not published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 38/128 (30%) dropped out of the intervention group and 33/124 (27%) dropped out of the placebo group. Reasons were given and were balanced between arms; the trialist reported that sensitivity analysis suggested no important differences between those who dropped out and those who remained in the study. Results are reported for those who completed, rather than results of an intention‐to‐treat analysis. High attrition for a trial of 6 months' duration, so judged to be at high risk |
| Selective reporting (reporting bias) | High risk | Not published |
Meister 1999.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (19). PP and ITT analyses reported. Duration 6 months | |
| Participants | 246 outpatients with chronic bronchitis as defined by WHO and FEV₁ > 50% predicted. 215 completed 6 months. At least 1 exacerbation in the past winter. Exclusions: those who had antibiotics in past 2 months, peptic ulcer disease, neoplasia, allergy to essential oils, pregnancy, lactation, severe concomitant disease. AveraMoretti 2004: age 57 years, 44% male. Mean FEV₁ 78% predicted. 55% had smoked. 42 dropped out | |
| Interventions | Myrtol 300 mg 3 times daily or placebo | |
| Outcomes | Exacerbations, number of exacerbations requiring antibiotics, well‐being, adverse effects | |
| Notes | German. Abstract provided by Douglas Pharmaceuticals. Full paper (English) provided by Pohl‐Boskamp. PP analysis used in review (participants completing 6 months). Results of ITT analysis consistent with PP analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; matched placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 260 participants received study medication at least once, of whom 42 (16%) discontinued the study prematurely. The ITT population comprised those who has received study medication for at least 1 month. 12/122 (10%) dropped out of the intervention ITT group and 19/124 (15%) from the placebo ITT arm. Reasons were balanced |
| Selective reporting (reporting bias) | Low risk | Reported on main outcomes: both PP and ITT |
Moretti 2004.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (9). PP analysis reported. Duration 8 months | |
| Participants | 155 outpatients with COPD defined by ERS. Age 25 to 85 years; 1 or more exacerbations in previous winter; FEV₁ < 70% predicted; CXR no acute lung disease; smoking history > 20 pack‐years; stable and at least 4 weeks since last exacerbation Exclusions: continuous treatment with oral steroids or expectorants; rapidly progressive bronchial disease; serious comorbidity; asthma; known poor compliance Mean age 67 years; 80% male; 33% smokers; FEV₁ after salbutamol 1.68 L (SD 0.31) in erdosteine group and 1.59 L (0.29) in placebo group Dropouts: 31/155 (20%). Equal in both groups and similar reasons. 63 in mucolytic group and 61 in placebo group completed |
|
| Interventions | Erdosteine 300 mg twice daily or placebo | |
| Outcomes | Exacerbation frequency, duration, hospitalisation, lung function, 6‐minute walk test, quality of life (SGRQ), pharmacoeconomic analysis | |
| Notes | Italian. EQUALIFE study Mucolytic group had (insignificantly) more males and better lung function at baseline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16/79 (20%) dropped out of the intervention group and 15/76 (20%) dropped out of the placebo group. Reasons were balanced |
| Selective reporting (reporting bias) | Low risk | Reported all primary outcomes |
Nowak 1999.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (10 centres). PP analysis. Duration "long term" means 8 months | |
| Participants | 313 outpatients with COPD (diagnostic criteria not clear). Mean age 57 years; 60% male. Mean FEV₁ 60% predicted. 18 dropped out | |
| Interventions | NAC 600 mg daily or placebo | |
| Outcomes | Exacerbations, severity of exacerbations, time to first exacerbation, days sick, lung function, participant symptoms, adverse effects | |
| Notes | European. COPD, not chronic bronchitis. BREATHE study. Published in abstract form only. Zambon provided more information. Study never published in full | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12/159 (8%) dropped out of the intervention arm and 6/154 (4%) dropped out of the placebo arm. Reasons for dropout not reported, but overall low rate of attrition |
| Selective reporting (reporting bias) | High risk | Information not available |
Olivieri 1987.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (13). PP analysis. Duration 6 months | |
| Participants | 240 outpatients with chronic bronchitis defined by MRC. At least 3 exacerbations in previous year or pathological auscultatory assessment or reduction of 15% to 40% in FEV₁. Exclusions: patients with asthma, FEV₁ < 40% predicted, peptic ulcer or other serious comorbidity, pregnancy, long‐term antibiotics or mucolytics. 26 dropped out | |
| Interventions | Ambroxol retard 75 mg or placebo daily | |
| Outcomes | Exacerbations, courses of antibiotics, days sick, FEV₁, VC, symptoms, auscultatory findings, physician and participant global assessments, laboratory data, adverse effects | |
| Notes | Italian. We suspect that what is reported as SD in the paper is in fact SE (using t statistic and P values). We wrote to study authors for clarification. We received no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐randomised |
| Allocation concealment (selection bias) | Unclear risk | Each centre provided with a list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11/121 (9%) dropped out of the intervention arm and 15/119 (13%) dropped out of the placebo arm. More participants in the placebo group "failed to return" (9 vs 3) or experienced "inefficacy" (3 vs 1) or an adverse reaction leading to withdrawal (2 vs 0). More participants in the intervention group withdrew due to "poor collaboration" (2 vs 0) or leaving the department (3 vs 1) |
| Selective reporting (reporting bias) | Low risk | PP and ITT analyses of all main outcomes |
Parr 1987.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre. PP analysis. Duration 6 months | |
| Participants | 526 general practice patients with chronic bronchitis defined by MRC, with at least 1 exacerbation in past 12 months. Exclusions: other significant respiratory disease, active peptic ulceration, severe heart failure, continuous therapy with antibiotics or mucolytics. 204 dropouts. Mean age 63 years; 66% male; 86% had smoked | |
| Interventions | NAC 200 mg 3 times daily or placebo | |
| Outcomes | Exacerbations, days off work, adverse effects | |
| Notes | British. Pharmaceutical company trial. Large number of dropouts, although seemed matched. SD calculated from raw data in paper. More data needed to calculate days sick | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned in blocks of 4 |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; interventions identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 204/526 (39%) did not complete all follow‐up over the 6‐month follow up period. 49 missed 1 or more assessment but were subsequently followed up. Two participants remained lost to follow‐up. 153 dropped out; 79/258 (31%) dropped out of the intervention group and 75/268 (28%) dropped out of the placebo group. Reasons for dropout were reasonably balanced, although more participants withdrew from the intervention arm due to "lack of efficacy" (15 vs 6) |
| Selective reporting (reporting bias) | Unclear risk | No specific outcomes stated |
Pela 1999.
| Methods | Randomised, open, placebo‐controlled, parallel, multi‐centre (5). Duration 6 months. PP analysis | |
| Participants | 169 outpatients with COPD (defined by ATS and ERS); aged 40 to 75 years; FEV₁ < 70% predicted; reversibility < 12% Exclusions: lung cancer, cardiomyopathy, metabolic disease, renal failure, other severe disease. Mean age 66 years; 76% male; mean FEV₁ 1.49 L; 58% predicted; 28% current smokers. 6 dropped out | |
| Interventions | NAC 600 mg daily or placebo | |
| Outcomes | Exacerbations, exacerbation severity, days sick, participant preference, lung function | |
| Notes | Italian study. Open study. COPD, not chronic bronchitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | High risk | Investigators aware of order of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2/85 (2%) dropped out of the intervention group and 4/84 (5%) from the standard care group. Reasons were balanced |
| Selective reporting (reporting bias) | Low risk | Reported on main outcomes |
Petty 1990.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre. Duration 2 months. ITT analysis | |
| Participants | 367 outpatients with stable chronic bronchitis defined by ATS were randomly assigned. Required pre‐bronchodilator FEV₁ < 75% predicted. 79 dropouts (33 in mucolytic group and 46 in placebo group). Mean age 65 years; 70% male; mean FEV₁ 44.5% predicted. Exclusions: pregnant or lactating, allergic to iodine, comorbidity that would confound response or compliance, asthma, exacerbation in past month, using antibiotics or anticholinergics | |
| Interventions | Iodinated glycerol 30 mg, 2 tabs 4 times a day, or identical‐looking placebo | |
| Outcomes | Investigator assessment of symptoms; participant evaluation of symptoms; global assessment at weeks 0, 4, and 8; frequency of bronchodilator use; number and duration of acute exacerbations; frequency of concomitant medications; adverse experiences Dropouts assessed at weeks 4 and 8 | |
| Notes | American. Requested more information from study author, but study author was unable to provide. Pharmaceutical company (Wallace) approached. No reply. No significant differences (reported) between groups in exacerbation rates; however, significantly fewer days sick in treatment group. We estimated sample SD from t statistic and pooled t formula and assumed equal variances to arrive at an estimate for SD of 18.8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; matched placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 35/182 (19%) dropped out of the intervention arm and 50/185 (27%) from the placebo arm. Reasons were relatively balanced, with the exception of withdrawals due to adverse events (10 in the intervention group vs 24 in the placebo group), which accounts for the imbalance in numbers |
| Selective reporting (reporting bias) | Low risk | None detected |
Rasmussen 1988.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (9). PP analysis. Duration 6 months | |
| Participants | 116 outpatients with chronic bronchitis defined by MRC. At least 1 exacerbation previous winter. 100% had smoked. Mean age 58.9 years; 57% male; average PEFR 305 L/min. 25 dropped out | |
| Interventions | NAC 300 mg twice daily or placebo | |
| Outcomes | Exacerbations, days sick evaluated by days on sick list and by participant diaries, adverse effects | |
| Notes | Swedish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in blocks of 4 |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15/59 (25%) dropped out of the intervention group and 10/57 (18%) from the placebo group. Reasons were reasonably balanced between arms |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
Roy 2014.
| Methods | Randomised, single‐blind, placebo‐controlled, parallel, single‐centre. PP analysis. Duration 6 months Followed up every month |
|
| Participants | 80 outpatients age > 40, stable mild to moderate COPD, smoking history at least 10 pack‐years. Exclusions: those with asthma, lung cancer, cardiomyopathy, LVRS or transplant, or on LTOT or corticosteroids. Mean age 61; 89% male. Total 20 dropouts, evenly matched between groups | |
| Interventions | NAC 600 mg twice daily or placebo. Both groups received a bronchodilator Deriphylline Retard 150 mg in addition | |
| Outcomes | Symptoms (cough, dyspnoea, sputum), spirometry, Hb, adverse events | |
| Notes | Indian Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No details on this, except it was a "simple method" |
| Allocation concealment (selection bias) | High risk | Single‐blind study; few details on allocation or concealment of sequence given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details on match between placebo and NAC, or on who performed measurements; sIngle‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | SIngle‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% dropout rate (20/80); numbers and reasons per arm not given |
| Selective reporting (reporting bias) | Unclear risk | Spirometric data reported in units that read "total count" |
Schermer 2009.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (44 general practices). Duration 3 years. ITT and PP analyses | |
| Participants | 192 (in study arms NAC and placebo, each n = 96) GP outpatients with chronic bronchitis or stable COPD between ages of 35 and 75. Current or former smokers with chronic dyspnoea, sputum, and cough for at least 3 consecutive months in previous 2 years; post‐bronchodilator FEV₁ < 90% and/or post‐bronchodilator FEV₁/FVC ratio < 0.88 for men and < 0.89 for women Exclusions: FEV₁/FVC ratio < 0.4 and/or history of asthma, allergic rhinitis, or eczema 84 dropouts (44 in mucolytic group and 40 in placebo group). Mean age 59 years; 73% male. Mean post‐bronchodilator FEV₁ 2.15 L (62% predicted). 53% were still smoking. 22% had chronic bronchitis with no obstruction: 14% mild, 47% moderate, and 17% severe COPD. Mean CRQ score 4.84; baseline exacerbation rate mean 0.88 per year/median 0.5 Participants well matched at baseline. High dropout rate. Generally low exacerbation rates, except small number of participants who experienced very frequent exacerbations |
|
| Interventions | 3 arms, double‐dummy (tablet and inhaler). NAC 600 mg effervescent tablet daily vs fluticasone 500 μg twice daily vs placebo. This review included only NAC vs placebo arms. 2 weeks of pretreatment with prednisone 30 mg daily | |
| Outcomes | Primary outcomes: rates of exacerbation and disease‐specific quality of life, as measured by CRQ Other outcomes: lung function, hospitalisation |
|
| Notes | Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List generated by independent statistician |
| Allocation concealment (selection bias) | Low risk | Neither participants nor investigators aware of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 44% dropout rate (44/96 and 40/97 dropped out on mucolytics and placebo, respectively). Reasons and numbers are balanced but high rate overall leads to judgement of high risk |
| Selective reporting (reporting bias) | Low risk | None detected |
Tse 2013.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel. 1 hospital centre. Duration 1 year 4‐week run‐in period; randomisation, then follow‐up at 16, 32, and 48 weeks Analysis ITT |
|
| Participants | 133 outpatients aged 50 to 80 with stable COPD (FEV₁/FVC < 0.7) recruited, 120 randomised. Exclusions: co‐existent pulmonary disease, LTOT, BiPAP, severe dyspnoea, poor reliability or compliance. Mean age 71; 93% male; 23% current smokers 18% GOLD 1, 40% GOLD 2, 34% GOLD 3, 8% GOLD 4. Median of 2 exacerbations in past year. Groups well matched at baseline. 12 dropouts ‐ 6 in each group |
|
| Interventions | NAC 600 mg twice daily or placebo | |
| Outcomes | Primary: small airways parameters FEF25%‐75%, FOT, IC, spirometry Secondary: exacerbation rate, mMRC dyspnoea scale, SGRQ, 6MWD |
|
| Notes | Chinese (Hong Kong). HIACE study. Funded by pharmaceutical company Funding from local hospital research fund. Zambon provided NAC and placebo. 1 study author (Dr Ratieri) employed by Zambon |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail on this |
| Allocation concealment (selection bias) | Unclear risk | Not well described: "randomisation and allocation details known only to a third party" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | NAC and placebo "identical in appearance"; "patients and investigators blinded to treatment allocation during the study". Compliance assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients and investigators blinded to treatment allocation during the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% dropout rate (12/120) after randomisation. Flow chart of dropout numbers provided and reasons relatively balanced |
| Selective reporting (reporting bias) | Low risk | All major outcomes reported in detail |
Worth 2009.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (11 centres; 4 GPs and 7 specialists). ITT analysis Duration 6 months over winter | |
| Participants | 220 outpatients aged 40 to 80 with moderate or severe COPD defined by GOLD. 30% > FEV₁ < 70%, with reversibility below 15%. All were smokers or ex‐smokers. Mean age 62.3 years; 64% were male. Mean FEV₁ 1.61 L (54.7% predicted). Exclusions: severe medical conditions such as bronchial carcinoma, MI, alcoholism, or heart failure. Unclear how many participants finished the study Groups well matched at baseline. Compliance said to be 'good' in all participants |
|
| Interventions | Cineole 2 × 100 mg 3 times daily (total 600 mg) or placebo | |
| Outcomes | Primary outcome: exacerbations ‐ number, severity, duration Secondary outcomes: lung function, dyspnoea, quality of life (SGRQ), adverse effects Primary outcomes, dyspnoea, and adverse effects assessed at each visit. Lung function assessed at 0, 3, and 6 months. Quality of life assessed at 0 and 6 months |
|
| Notes | German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Apart from an indication of stratification by site, no details given on randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants instructed to take medication a half hour before meals to avoid the smell of cineole. Active and placebo capsules looked identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details on dropouts |
| Selective reporting (reporting bias) | Low risk | None apparent |
Xu 2014.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel. Duration 6 months | |
| Participants | 84 outpatients over 20 years of age with chronic bronchitis as defined by the MRC, or COPD as defined by criteria of the ATS, GOLD, ERS, or WHO Exclusion criteria: not known Dropouts: no dropouts in either arm |
|
| Interventions | NAC 600 mg twice daily or salmeterol/fluticasone propionate alone | |
| Outcomes | FEV₁/FVC, FEV₁ % predicted, PEF% daily variation change, arterial blood gas analysis index (PaO₂ and PaCO₂) | |
| Notes | Study published in Chinese. Funded by Jilin provincial science and technology department | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised"; no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description of placebo or blinding; assume open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of placebo or blinding; assume open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | All stated outcomes of interest to this review reported numerically, but no published protocol or trial registration identified |
Zheng 2008.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (22 centres). Duration 1 year. ITT analysis | |
| Participants | 709 outpatients with stable COPD defined by GOLD criteria with post‐bronchodilator FEV₁/FVC ratio < 0.7 and FEV₁ between 25% and 79% predicted. Patients between ages of 40 and 80 with history of at least 2 COPD exacerbations in previous 2 years. Clinically stable in past 4 weeks. 91 dropouts (48 in mucolytic group and 43 in placebo group). Mean age 65 years; 78% male; mean FEV₁ 1.09 L (44.5% predicted). 75% had ever smoked. 49% were GOLD 2, 39% GOLD 3, and 12% GOLD 4. Mean SGRQ was 42. Exclusions: asthma, non‐COPD respiratory disorders, LVRS or transplant or other conditions that would interfere with the study, those on LTOT or pulmonary rehabilitation or on OCS, pregnancy or lactating. Patients involved in another investigational drug trial in past 12 weeks were also excluded 18% of intervention group and 15% of placebo group were on ICS |
|
| Interventions | Carbocysteine 1500 mg daily (2 × 250 mg 3 times daily) orally or placebo | |
| Outcomes | Primary endpoint: exacerbation rate (defined by Anthonisen) Secondary endpoints: co‐variance‐adjusted exacerbation rate, quality of life (SGRQ), lung function, arterial oxygen saturation |
|
| Notes | Chinese. Main PEACE study. Financial support from Kyron Pharmaceutical, Japan Lancet report for main PEACE study describes 709 participants from 22 centres in China. Another 2 references to PEACE study from Japan (Tatsumi 2007a; Tatsumi 2007b). Both refer to same sample of 142 patients ‐ 70 in control group and 72 in study group. Have written to Dr Zhong to ask if a substudy of main PEACE study was a different study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Low risk | "Neither the investigator nor the patient knew the group allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo was identical to the drug in appearance labelling and packaging" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Statistical analysis done without awareness of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13% dropout rate (48/353 and 43/354 withdrew from mucolytics and placebo, respectively). Some imbalance noted in the reasons for dropout; a greater number of intervention participants dropped out due to "no compliance or lack of consent" (30 vs 16), whereas more placebo participants were lost to follow‐up (21 vs 10). Analyses performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | None apparent |
Zheng 2014.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel, multi‐centre (34 centres). Duration 1 year 2 week run‐in period; then randomisation and visits at 1, 2, 6, 9, and 12 months. Analysis conducted on "patients who received at one dose of study drug, and had at least one visit assessment after randomisation" This ended up being 482 in each group (total 964). Completers totaled 763. Methods for handling missing data not outlined |
|
| Participants | From 1297 screened, investigators enrolled 1006 outpatients aged 40 to 80 with moderate to severe COPD (FEV₁ < 30% to 70% predicted and ratio < 0.7). These were stratified by previous regular use of ICS at baseline (500 to 2000 μg/day of beclomethasone or equivalent). Exclusions: bronchial asthma, LTOT ≥ 12 hours per day or pulmonary rehabilitation, major comorbidity, poor reliability or compliance. Ratio of ICS users to ICS naïve participants was set at about 4:6 Groups were well matched at baseline. Mean age 66 years; 82% male; 76% ever smokers; mean FEV₁ 49% predicted. 46% GOLD 2, 53% GOLD 3, and 1% GOLD 4. 243 dropouts ‐ 124 in treatment group and 119 in placebo group ‐ with main reasons being loss to follow‐up and adverse events. Provided analysis of dropouts (N = 243) vs completers (N = 763) ‐ similar among the 2 groups |
|
| Interventions | NAC 600 mg twice daily or placebo | |
| Outcomes | Primary: exacerbation rate in 1 year, exacerbation duration Secondary: time to first exacerbation, time to recurrent exacerbation, number of participants requiring systemic corticosteroids or antibiotics or use of SABA rescue medication, SGRQ (Chinese version), spirometry, adverse events (including hospitalisation or death) |
|
| Notes | Chinese. PANTHEON study. Funded by a pharmaceutical company (Hainan Zambon Pharmaceutical). Study authors had full access to all data and were involved in data interpretation and preparation of manuscript in collaboration with sponsor. Corresponding authors had final responsibility for decision to submit for publication Dr Zheng provided Appendix, as well as further data on exacerbation rates, SQRG scores, and spirometry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation conducted using a pre‐determined computer‐generated randomisation list provided by a statistician from a third party not involved in the study. This third party was exclusively responsible for randomisation, data management, data analysis, and data quality control |
| Allocation concealment (selection bias) | Low risk | Supplies of tablets for every participant were identified by a 4‐digit number. A sealed envelope containing the randomisation code for each participant was kept by the investigator and was not to be opened during the study, unless a serious life‐threatening adverse event occurred |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both NAC and placebo tablets were provided by Hainan Zambon Pharmaceutical Co., Ltd. The placebo was identical in composition, shape, colour, and size but did not contain any active ingredients. NAC and placebo tablets were packaged and labelled in such a way that they could not be distinguished from each other |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators were trained before the trial to ensure reliable study quality, with special emphasis on understanding the protocol, performing spirometry tests, blinding to allocation, managing the drug supply, and maintaining compliance with Good Clinical Practice (GCP). Details of study design were published ahead of the study results |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24% dropout rate (243/1006); 124/504 (25%) in the intervention group and 119/502 (24%) in the placebo group. Some imbalance noted in the reasons for withdrawal; in the intervention group, more participants withdrew due to adverse events (32 vs 24), whereas in the placebo group, more participants were lost to follow‐up (56 vs 48) and withdrew due to lack of efficacy 21 vs 17) |
| Selective reporting (reporting bias) | Low risk | CONSORT statement was followed to ensure proper reporting of this study |
6MWD: six‐minute walk distance; ABG: arterial blood gas; ATS: American Thoracic Society; BiPAP: bi‐level non‐invasive ventilation; BTS: British Thoracic Society; CBSAS: Chronic Bronchitis Symptoms Assessment Scale; CF: cystic fibrosis; COPD: chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Questionnaire; CXR: chest X‐ray; ERS: European Respiratory Society; FEF25%‐75%: forced expiratory flow at 25‐75% of the pulmonary volume; FEV₁: forced expiratory volume in one second; FOT: forced oscillation technique; FVC: forced vital capacity; GI: gastrointestinal; GOLD: Global Initiative for Obstructive Lung Disease; IC: inspiratory capacity; ICS: inhaled corticosteroids; ITT: intention‐to‐treat; LTOT: long‐term oxygen therapy; LVRS: lung volume reduction surgery; MI: myocardial infarction; MRC: Medical Research Council; NAC: N‐acetylcysteine; NYHA: New York Heart Association; OCS: oral corticosteroids; OT: on treatment; PaCO₂: partial pressure of carbon dioxide; PaO₂: partial pressure of oxygen; PEFR: peak expiratory flow rate; PKU: phenylketonuria; pMDI: pressurised metered‐dose inhaler; PP: per protocol; SABA: short‐acting beta‐agonist; SCMC‐Lys: carbocysteine lysine salt monohydrate; SD: standard deviation; SE: standard error; SF‐36: Short Form‐36; SGRQ: St. George's Respiratory Questionnaire; TB: tuberculosis ; VC: vital capacity; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baglioni 2001 | Preliminary, small, open RCT of NAC vs placebo in patients on LTOT, published in abstract form only, with no numerical data on clinical outcomes |
| Cattaneo 2001 | Only 20 days long |
| Christensen 1971 | No response to 2 letters requesting more data. Old study ‐ unlikely to be successful with further attempts. Did not evaluate primary outcome, although did evaluate days sick |
| Edwards 1976 | Did not evaluate primary outcome |
| Habich 1994 | Included both patients with asthma and patients with COPD |
| Kasielski 2001 | Did not evaluate clinical outcomes |
| Lukas 2005 | Translated from German. Patients with chronic bronchitis given NAC, placebo, Vit C or NAC + Vit C for 3 months. Did not evaluate primary outcome. Outcomes were lung function, symptoms, neutrophils, and other blood outcomes such as oxidising ability. No numerical data presented on lung function or symptoms, although study authors reported no differences for either of these |
| Maesen 1980 | Did not evaluate primary outcome |
| Michnar 1996 | Did not evaluate primary outcome |
| Moretti 2011 | Acute setting; 10 days of treatment with erdosteine |
| Moretti 2014 | Acute setting; 10 days of treatment with erdosteine |
| Pirabbasi 2016 | Four‐arm study including NAC and placebo; focus on nutritional and antioxidant status |
| Rubin 1996 | Did not evaluate primary outcome |
| Saibene 2016 | Trial not an RCT. Used a before and after design with all participants taking carbocysteine |
| Salve 2016 | Randomised trial of combined effect of NAC and daily physical activity in stable COPD; thus impossible to determine NAC effect |
| Sushko 2015 | Study specifically in people with COPD post‐Chernobyl, so not a typical, stable COPD population |
| Tatsumi 2007a | Even though randomised, not placebo‐controlled |
| Tatsumi 2007b | Even though randomised, not placebo‐controlled |
| Velazquez 2001 | Only 4 weeks long |
| Wilhelmi 2010 | Has been translated from German. Patients with COPD given cineole or placebo for 6 months. Evaluated primary outcome of exacerbations; although P values given for a significant reduction in exacerbations with cineole compared with placebo, no data supplied for event rates. Appears to be a short report summarising original trial |
COPD: chronic obstructive pulmonary disease; LTOT: long‐term oxygen therapy; NAC: N‐acetylcysteine; RCT: randomised controlled trial; vs: versus.
Characteristics of studies awaiting assessment [ordered by study ID]
CTRI/2015/01/005432.
| Methods | Randomised, parallel‐group, multiple‐arm trial |
| Participants | Included people with COPD, diagnosed clinically and spirometrically, symptoms of breathlessness, chest tightness and cough with or without sputum, GOLD classification I to III Excluded people with respiratory failure or bronchial asthma; pregnant and lactating mothers; people with clinically relevant, abnormal laboratory values suggesting an unknown disease requiring further investigation; people with psychotic; people with HIV/HBsAg/Anti‐HCV‐positive serology and immune compromised patients |
| Interventions | • Standard therapy + oral Superoxide Dismutase (SOD) 1 tab once daily (140 IU) for 12 weeks • Standard therapy + oral N‐acetyl‐L‐cysteine (NAC) 600 mg once daily for 12 weeks • Standard therapy for 12 weeks |
| Outcomes | Lung function tests, haemogram with ESR, blood sugar, ECG, X‐ray chest P/A view, liver function tests, renal function tests |
| Notes | Study authors contacted 18/12/17, and again 11/01/18, for further information. To date, no response received Contact details: Dr Waseem Rizvi, Associate Professor Department of Pharmacology Jawaharlal Nehru Medical College, AMU Aligarh Tuuar Pradesh 202002 India Email: waseemnakhat@gmail.com |
COPD: chronic obstructive pulmonary disease; ECG: electrocardiogram; ESR: erythrocyte sedimentation rate; GOLD: Global Initiative for Obstructive Lung Disease; HBsAg: surface antigen of hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; NAC: N‐acetylcysteine; P/A: posteroanterior.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IIR‐17012604.
| Trial name or title | Long‐term regular treatment of early COPD with randomised, double‐blind, placebo‐controlled multi‐centre clinical study with acetylcysteine effervescent tablets |
| Methods | Parallel randomised double‐blind placebo‐controlled trial |
| Participants | Aged 40 to 80 years, male or female, community or outpatient; with respiratory symptoms (chronic cough, sputum, shortness of breath) and/or chronic obstructive pulmonary exposure risk factors (smoking, occupational exposure, indoor and outdoor air pollution, family history of COPD, recurrent respiratory tract infection, low birth weight, and genetic factors, etc.); GOLD stage I to II COPD: FEV₁/FVC < 70%; and FEV₁ ≥ 50% predicted after 20 minutes with 400 μg of salbutamol inhalation; patients in a stable period, that is, nearly 4 weeks without COPD acute exacerbations; patient is able to communicate in words, agrees, and has the ability to complete the test‐related auxiliary examination. Signs informed consent |
| Interventions | Acetylcysteine effervescent tablets vs placebo |
| Outcomes | Lung function, number of acute exacerbations of COPD, quality of life (CAT), symptom score, COPD acute exacerbation severity, adverse events, attrition |
| Starting date | 2017‐09‐06 |
| Contact information | Yumin Zhou: zhouyumin410@126.com The First Affiliated Hospital of Guangzhou Medical University 151 Yanjiang Road Guangzhou Guangdong China |
| Notes |
ChiCTR1800016712.
| Trial name or title | Early intervention with carbocysteine and low‐dose theophylline in Chinese patients with chronic obstructive pulmonary disease |
| Methods | Multi‐centre clinical study screening for effective drugs for early‐stage COPD. Parallel RCT |
| Participants | Community or clinic COPD patients, between 40 and 80 years of age, male or female; FEV₁/FVC < 70% after inhaled bronchodilator; FEV₁ 50% predicted (Gold stage I to II); no acute exacerbation of COPD in the last 4 weeks; ability to communicate in languages or words; ability to voluntarily participate in the study and sign informed consent |
| Interventions | Carbocysteine tablets 500 mg, 3 times daily; theophylline sustained‐release tablets 100 mg, 2 times daily; carbocysteine‐placebo group: carbocysteine‐placebo tablets 500 mg 3 times daily; theophylline‐placebo group: theophylline sustained‐release placebo tablets 100 mg, 2 times daily |
| Outcomes | Lung function; number of COPD exacerbations; symptom score; time to first acute exacerbation of COPD; severity, interval, and duration of acute exacerbations of COPD; dropout rate; administration of rescue medication; cost‐effectiveness analysis |
| Starting date | 2018‐06‐25 |
| Contact information | Wang Qiuyue: qywngcmu@163.com The First Hospital of China Medical University 155 Nanjing Street North Heping District Shenyang, Liaoning China +86 13998892756 |
| Notes |
CAT: COPD Assessment Test; COPD: chronic obstructive pulmonary disease; FEV₁: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global Initiative for Obstructive Lung Disease; RCT: randomised controlled trial.
Differences between protocol and review
We searched trial registries for the update.
This review has used a modified version of the full 'Risk of bias' tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions. The protocol and initial review versions used Jadad scores to assess trial quality. We have updated the 'Risk of bias' assessment to use the latest version of the Cochrane 'Risk of bias' tool.
Additional outcomes were added for updates from 2006 to 2012.
Hospitalisation and mortality (added as outcomes for the 2006 and 2008 updates).
Quality of life (added for the 2008 update, with a meta‐analysis of SGRQ scores included for the 2012 update).
Double‐blinding was not an inclusion criterion.
For the 2019 update, we removed the exacerbations per patient per month analyses, as these are not considered to be as statistically robust as the dichotomous exacerbation outcome, largely due to likely skew in this measure. In addition, we reviewed the Bontognali 1991 data for this outcome and removed them due to discrepancies in Table II of the publication, leading us to believe there are mistakes in the reported exacerbation data. Furthermore, following editorial advice, we conducted a sensitivity analysis while removing those studies judged to be at high risk of attrition bias.
Contributions of authors
Dr Phillippa Poole has had the primary overall responsibility for this review throughout its iterations. Until his death in 2010, Dr Black contributed to all aspects of the review, including approval of the final version of the substantive updates in 1999, 2002, 2005, 2006, and 2008. Dr Chris Cates has provided support for the review from inception. He has assisted with analysis, interpretation, data‐checking, and write‐up of the 2012 and 2014/15 updates. Dr Jimmy Chong assisted with determining study eligibility, checking data, and writing up the 2012 and 2014/15 updates. Dr Rebecca Fortescue and Kavin Sathananthan joined the team for the 2019 update and contributed to data extraction and entry and write‐up. Dr Jimmy Chong and Dr Chris Cates stepped down from the author line for this most recent update.
Contributions of editorial team
Chris Cates (Co‐ordinating Editor) checked the data entry before the full write‐up of the review. Sally Spencer (Editor) edited the review; advised on methodology, interpretation, and content; and approved changes after peer review. Emma Dennett (Managing Editor) co‐ordinated the editorial process; advised on interpretation and content; and edited the review. Emma Jackson (Assistant Managing Editor) conducted peer review; and edited the plain language summary and reference sections of the protocol and the review. Elizabeth Stovold (Information Specialist) designed the search strategy; ran the searches; and edited the search methods section. Sarah Hodgkinson (Associate Editor, Cochrane Circulation and Breathing Network) screened the review and provided feedback.
Sources of support
Internal sources
No support received, Other.
The authors declare that no such funding was received for this systematic review, Other.
External sources
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
PP: none known. I am an editor with Cochrane Airways.
KS: none known.
RF: none known. I am Joint Co‐ordinating Editor of Cochrane Airways, employed by an NIHR grant, and a qualified general practitioner.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Allegra 1996 {published and unpublished data}
- Allegra L, Cordaro CI, Grassi C. Prevention of acute exacerbations of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: a multicenter, double‐blind, placebo‐controlled trial. Respiration 1996;63:174‐80. [DOI] [PubMed] [Google Scholar]
Babolini 1980 {published data only}
- Multicenter Study Group. Long‐term oral acetylcysteine in chronic bronchitis. A double‐blind controlled study. European Journal of Respiratory Disease 1980;61(111 Suppl):93‐108. [PubMed] [Google Scholar]
Bachh 2007 {published data only}
- Bachh AA, Shah NN, Bhargava R, Ahmad Z, Pandey DK, Dar KA. Effect of oral N‐acetylcysteine in COPD ‐ a randomised controlled trial. JK‐Practitioner 2007;14(1):12‐6. [Google Scholar]
Boman 1983 {published data only}
- Boman G, Backer U, Larsson S, Melander B, Wahlander L. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis; report of a trial organized by the Swedish Society for Pulmonary Diseases. European Journal of Respiratory Disease 1983;64:405‐15. [PubMed] [Google Scholar]
Bontognali 1991 {published data only}
- Bontognali E. Clinical effectiveness and tolerance of cithiolone in the prophylaxis of acute infective exacerbations in patients suffering from chronic bronchitis. Acta Therapeutica 1991;17:155‐62. [Google Scholar]
Borgia 1981 {published data only}
- Borgia M, Sepe N, Ori‐Belometti M, Borgia R. Comparison between acetylcysteine and placebo in the long term treatment of chronic bronchitis [Confronto tra acetilcisteina e placebo nel trattamento a lungo termine della bronchite cronica]. Gazzetta Medica Italiana 1981;140:467‐72. [Google Scholar]
Castiglioni 1986 {published data only}
- Castiglioni CL, Gramolini C. Effect of long‐term treatment with sobrerol on the exacerbations of chronic bronchitis. Respiration 1986;50:202‐17. [DOI] [PubMed] [Google Scholar]
Cegla 1988 {published data only}
- Cegla UH. Long‐term treatment of chronic bronchitis for two years with ambroxol (Mucosolvan) retard capsules. Results of a double blind trial including 180 patients [Langzeittherapie uber 2 Jahre mit Ambroxol (Mucosolvan) Retardkapseln bei Patienten mit chronischer Bronchitis. Ergebnisse einer Doppelblindstudie an 180 Patienten]. Praxis Klinik der Pneumologie 1988;42:715‐21. [PubMed] [Google Scholar]
Cremonini 1986 {published data only}
- Cremonini C, Spada E, Cellini F, Cioni R, Giovannini M, Perri G, et al. I farmaci attivi sul muco nel trattamento di fondo della bronchite cronica [Pharmacotherapy which acts on mucus in long‐term treatment of chronic bronchitis]. La Clinica Terapeutica 1986;116:121‐9. [PubMed] [Google Scholar]
Dal Negro 2017 {published data only}
- Calverley PMA, Page C, Dal Negro RW, Fontana G, Iversen M, Cicero AF, et al. Effect of erdosteine in moderately severe COPD patients. European Respiratory Journal. 2018; Vol. 52:PA776.
- Calverley PMA, Page C, Dal Negro RW, Fontana G, Iversen M, Cicero AF, et al. Effect of erdosteine on the rate of COPD exacerbations in spirometrically defined Gold II patients. Respirology. 2017; Vol. 2:49.
- Dal Negro RW, Iversen M, Calverly PM. Efficacy and safety of erdostein in COPD: results of a 12‐month prospective, multinational study. European Respiratory Journal 2015;46:PA1495. [Google Scholar]
- Dal Negro RW, Wedzicha JA, Iversen M, Fontana G, Page C, Cicero AF, et al. Effect of erdosteine on the rate and duration of COPD exacerbations: the RESTORE study. European Respiratory Journal 2017;50(4):1700711. [DOI: 10.1183/13993003.00711-2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Negro RW, Wedzicha JA, Iversen MI, Fontana G, Page C, Cicero AF, et al. Effect of erdosteine on the rate and duration of COPD exacerbations: the RESTORE study (Reducing Exacerbations and Symptoms by Treatment with ORal Erdosteine). European Respiratory Journal. 2017; Vol. 50:PA675. [DOI] [PMC free article] [PubMed]
De Backer 2013 {published data only}
- Backer J, Vos W, Holsbeke C, Vinchurkar S, Claes R, Parizel P, et al. Double blind, randomized, two‐way crossover, pilot study to assess the effect of high dose N‐acetylcysteine on airway geometry, inflammation and oxidative stress in COPD patients using functional respiratory imaging. American Journal of Respiratory and Critical Care Medicine. 2013; Vol. 187, issue A2447.
- Backer J, Vos W, Holsbeke C, Vinchurkar S, Claes R, Parizel PM, et al. Effect of high‐dose N‐acetylcysteine on airway geometry, inflammation, and oxidative stress in COPD patients. International Journal of Chronic Obstructive Pulmonary Disease 2013;8:569‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Decramer 2005 {published data only}
- Decramer M, Rutten‐van Molken M, Dekhuijzen PN, Troosters T, Herwaarden C, Pellegrino R, et al. Effects of N‐acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost‐Utility Study, BRONCUS): a randomised placebo‐controlled trial. Lancet 2005;365(9470):1552‐60. [DOI] [PubMed] [Google Scholar]
Ekberg‐Jansson 1999 {published data only}
- Ekberg‐Jansson A, Larson M, MacNee W, Tunek A, Wahlgren L, Wouters EFM, et al. N isobutyrylcysteine, a donor of systemic thiols, does not reduce the exacerbation rate in chronic bronchitis. European Respiratory Journal 1999;13(4):829‐34. [DOI] [PubMed] [Google Scholar]
Fukuchi 2016 {published data only}
- Fukuchi Y, Tatsumi K, Inoue H, Sakata Y, Shibata K, Miyagishi H, et al. Prevention of COPD exacerbation by lysozyme: a double‐blind, randomized, placebo‐controlled study. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:831‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M, Fukuchi Y, Tatsumi K, Inoue H, Arai T, Shibata K, et al. Prevention of COPD exacerbation by lysozyme: a double‐blind, randomized, placebo‐controlled study. European Respiratory Journal 2015;46:PA4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grassi 1976 {published data only}
- Grassi C, Morandini GC. A controlled trial of intermittent oral acetylcysteine in the long‐term treatment of chronic bronchitis. European Journal of Clinical Pharmacology 1976;9:393‐6. [DOI] [PubMed] [Google Scholar]
Grassi 1994 {published data only}
- Grassi C, Casali L, Ciaccia A, et al. [Terapia intervallare con l'associazione carbocisteina‐sobrerolo nella profilassi delle riacutizzazioni della bronchite cronica]. Italian Journal of Chest Disease 1994;48:17‐26. [Google Scholar]
Grillage 1985 {published data only}
- Grillage M, Barnard‐Jones K. Long‐term oral carbocisteine therapy in patients with chronic bronchitis. A double blind trial with placebo control. British Journal of Clinical Practice 1985;39(10):395‐8. [PubMed] [Google Scholar]
Hansen 1994 {published data only}
- Hansen NC, Skriver A, Brorsen‐Riis L, Balsløv S, Evald T, Maltbaek N, et al. Orally administered N‐acetylcysteine may improve general well‐being in patients with mild chronic bronchitis. Respiratory Medicine 1994;88:531‐5. [DOI] [PubMed] [Google Scholar]
Jackson 1984 {published data only}
- Jackson IM, Barnes J, Cooksey P. Efficacy and tolerability of oral acetylcysteine (fabrol) in chronic bronchitis: a double‐blind placebo controlled trial. Journal of International Medical Research 1984;12:198‐206. [DOI] [PubMed] [Google Scholar]
Johnson 2016 {published data only}
- Johnson K, McEvoy CE, Naqvi S, Wendt C, Reilkoff RA, Kunisaki KM, et al. High‐dose oral N‐acetylcysteine fails to improve respiratory health status in patients with chronic obstructive pulmonary disease and chronic bronchitis: a randomized, placebo‐controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:799‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malerba 2004 {published data only}
- Malerba M, Ponticello A, Radaeli A, Bensi G, Grassi V. Effect of twelve‐months therapy with oral ambroxol in preventing exacerbations in patients with COPD. Double‐blind, randomized, multi‐center, placebo‐controlled study (the AMETHIST trial). Pulmonary Pharmacology and Therapeutics 2004;17(1):27‐34. [DOI] [PubMed] [Google Scholar]
McGavin 1985 {published data only}
- British Thoracic Society Research Committee. Oral N‐acetylcysteine and exacerbation rates in patients with chronic bronchitis and severe airways obstruction. Thorax 1985;40(11):832‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meister 1986 {unpublished data only}
- Meister R. Long‐term therapy with acetylcysteine retard tablets in patients with chronic bronchitis: a double‐blind, placebo controlled study. Unpublished source 1986.
Meister 1999 {published data only}
- Beeh K‐M, Beier J, Candler H, Wittig T. Effect of ELOM‐080 on exacerbations and symptoms in COPD patients with a chronic bronchitis phenotype ‐ a post‐hoc analysis of a randomized, double‐blind, placebo‐controlled clinical trial. International Journal of Chronic Obstructive Pulmonary Disease 2016;11(1):2877‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister R, Wittig T, Beuscher N, Mey C. Efficacy and tolerability of myrtol standardized in long‐term treatment of chronic bronchitis. A double‐blind, placebo‐controlled study. Study Group Investigators. Arzneimittelforschung 1999;49(4):351‐8. [DOI] [PubMed] [Google Scholar]
Moretti 2004 {published data only}
- Moretti M, Bottrighi P, Dallari R, Porto R, Dolcetti A, Grandi P, et al. The effect of long‐term treatment with erdosteine on chronic obstructive pulmonary disease: the EQUALIFE study. Drugs Under Experimental and Clinical Research 2004;30(4):143‐52. [PubMed] [Google Scholar]
Nowak 1999 {published data only}
- Nowak D, Carati L, Pirozynski M. Long‐term administration of N‐acetylcysteine reduces the number of acute exacerbation episodes in subjects with chronic obstructive pulmonary disease:report of the BREATHE study. European Respiratory Journal 1999;14 Suppl:381S‐2S. [Google Scholar]
Olivieri 1987 {published data only}
- Olivieri D, Zavattini G, Tomasini G, Daniotti S, Bonsignore G, Ferrara G, et al. Ambroxol for the prevention of chronic bronchitis exacerbations: long‐term multicenter trial. Respiration 1987;51 Suppl 1:42‐51. [DOI] [PubMed] [Google Scholar]
Parr 1987 {published data only}
- Parr GD, Huitson A. Oral fabrol (oral n‐acetyl‐cysteine) in chronic bronchitis. British Journal of Diseases of the Chest 1987;81(4):341‐8. [DOI] [PubMed] [Google Scholar]
Pela 1999 {published data only}
- Pela R, Calcagni AM, Subiaco S, Isidori P, Tubaldi A, Sanguinetti CM. N‐acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD. Respiration 1999;66(6):495‐500. [DOI] [PubMed] [Google Scholar]
Petty 1990 {published data only}
- Morgan EJ, Petty TL. Summary of the national mucolytic study. Chest 1990;97(Suppl):24S‐7S. [DOI] [PubMed] [Google Scholar]
- Petty TL. The national mucolytic study: results of a randomized, double‐blind, placebo‐controlled study of iodinated glycerol in chronic obstructive bronchitis. Chest 1990;97(1):75‐83. [DOI] [PubMed] [Google Scholar]
Rasmussen 1988 {published data only}
- Rasmussen JB, Glennow C. Reduction in days of illness after long‐term treatment with N‐acetylcysteine controlled‐release tablets in patients with chronic bronchitis. European Respiratory Journal 1988;1:351‐5. [PubMed] [Google Scholar]
Roy 2014 {published data only}
- Roy P, Haran A, Srinivas BN. Role of n‐acetylcysteine as an adjuvant to mainstay bronchodilator therapy in chronic obstructive pulmonary disease. Indian Journal of Pharmacology Conference Publication 2013;45:S107. [Google Scholar]
- Roy P, Haran A, Srinivas BN, Mjumdar P. Evaluation of effect of N‐acetylcysteine as an adjuvant to mainstay bronchodilator therapy in mild to moderate cases of chronic obstructive pulmonary disease. International Journal of Medical and Applied Sciences 2014;3(1):95‐105. [Google Scholar]
Schermer 2009 {published data only}
- Chavannes N, Schermer T, Wouters EFM, Folgering H. Demographic and clinical determinants of response to N‐acetylcysteine versus fluticasone in mild to moderate COPD in primary care: the COOPT study. European Respiratory Journal 2005;26 (S49):1356. [Google Scholar]
- Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N‐acetylcysteine in primary care patients with COPD or chronic bronchitis. Respiratory Medicine 2009;103:542‐51. [DOI] [PubMed] [Google Scholar]
Tse 2013 {published data only}
- Tse HN, Raiteri L, Wong KY, Ng LY, Yee Ks, Tseng CZS. Benefits of high‐dose N‐acetylcysteine to exacerbation‐prone patients with COPD. Chest 2014;146(3):611‐23. [DOI] [PubMed] [Google Scholar]
- Tse HN, Raiteri L, Wong KY, Yee KS, Ng LY, Wai KY, et al. High‐dose N‐acetylcysteine in stable COPD: the 1‐year, double‐blind, randomized, placebo‐controlled HIACE study. Chest 2013;144:106‐18. [DOI] [PubMed] [Google Scholar]
- Tse HN, Wong KY, Yee KS, Ng LY. The effect of high dose N‐acetylcysteine (1200mg daily) on airway function and airway trapping in COPD patients: a double blinded randomized placebo controlled trial. European Respiratory Journal 2012;40 Suppl 56:P2105. [Google Scholar]
- Tse HN, Wong KY, Yee KS, Ng LY, Wai KY, Loo CK, et al. The effect of high dose N‐acetylcysteine (600 mg twice daily) in patients with stable chronic obstructive pulmonary disease ‐ a one‐year, double blind, randomized, placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2013;187:A2448. [Google Scholar]
Worth 2009 {published data only}
- Worth H, Schacher C, Dethlefsen U. Concomitant therapy with cineole (eucalyptole) reduces exacerbations in COPD: a placebo‐controlled double‐blind trial. Respiratory Research 2009;10(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth H, Schacher C, Dethlefsen U. Effects of concomitant therapy with Cineole (Eucalyptole) on exacerbation rate in COPD: a placebo‐controlled double‐blind trial. European Respiratory Society 20th Annual Congress; 2010 Sep 18‐22; Barcelona. 2010; Vol. P538.
Xu 2014 {published data only}
- Xu X‐G, Jiang Z‐Y, Du M‐J, Yang Y‐Q, Jiang Y‐C. Evaluation on effectiveness of salmeterol/fluticasone propionate combined with N‐acetylcysteine in treatment of chronic obstructive pulmonary disease. [Chinese]. Journal of Jilin University Medicine Edition 2014;40(40):870‐4. [Google Scholar]
Zheng 2008 {published data only}
- Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, et al. Effect of carbocisteine on acute exacerbations of chronic obstructive pulmonary disease (PEACE study): a randomised placebo‐controlled trial. Lancet 2008;371(9629):2013‐8. [DOI] [PubMed] [Google Scholar]
Zheng 2014 {published data only}
- Papi A, Brusselle G, Sergio F, Pannacci M, Zheng J, Criner G. Blood eosinophils and smoking history affect outcome with high dose N‐acetylcysteine treatment in COPD. European Respiratory Journal. 2017; Vol. 50:PA1066.
- Papi A, Zheng J, Criner GJ, Fabbri LM, Calverley PMA. Impact of smoking status and concomitant medications on the effect of high‐dose N‐acetylcysteine on chronic obstructive pulmonary disease exacerbations: a post‐hoc analysis of the PANTHEON study. Respiratory Medicine 2019;147:37‐43. [DOI] [PubMed] [Google Scholar]
- Sergio F, Papi A, Criner G, Brusselle G, Calverley P, Fabbri L, et al. Long term treatment with n‐acetylcysteine reduces moderate‐severe exacerbations of chronic obstructive pulmonary disease. Respirology 2016;21:186. [Google Scholar]
- Zheng JP, Wen FQ, Bai CX, Wan HY, Kang J, Chen P, et al. High‐dose N‐acetylcysteine in the prevention of COPD exacerbations: rationale and design of the PANTHEON study. COPD: Journal of Chronic Obstructive Pulmonary Disease 2013;10:164‐71. [DOI] [PubMed] [Google Scholar]
- Zheng JP, Wen FQ, Bai CX, Wan HY, Kang J, Chen P, et al. High‐dose N‐acetylcysteine in the prevention of COPD exacerbations: results of the PANTHEON study. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013.
- Zheng JP, Wen FQ, Bai CX, Wan HY, Kang J, Chen P, et al. Twice daily N‐acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double‐blind placebo‐controlled trial. Lancet Respiratory Medicine 2014;2:187‐94. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baglioni 2001 {published data only}
- Baglioni S, Tazza R, Rossi S, Eslami A, Ferranti P, Dottorini M. Effects of N‐acetylcysteine treatment during long term oxygen therapy (LTOT) in COPD patients. An open parallel‐group study. European Respiratory Journal 2001;18(33 Suppl):58S. [Google Scholar]
Cattaneo 2001 {published data only}
- Cattaneo C. Neltenexine tablets in smoking and non‐smoking patients with COPD. A double‐blind, randomised, controlled study versus placebo. Minerva Medica 2001;92(4):277‐84. [PubMed] [Google Scholar]
Christensen 1971 {published data only}
- Andreasen T. Mucolytic treatment of chronic bronchitis during two winter periods. Scandinavian Journal of Respiratory Disease 1974;90:69‐70. [PubMed] [Google Scholar]
- Christensen SB, Kjer J, Ryskjaer S, Arseth‐Hansen P, Christensen F. Mucolytic treatment of chronic bronchitis during two winter periods. Scandinavian Journal of Respiratory Disease 1971;52(1):48‐57. [PubMed] [Google Scholar]
Edwards 1976 {published data only}
- Edwards GF, Steel AE, Scott JK, Jordan JW. S‐carboxymethylcysteine in the fluidification of sputum and treatment of chronic airway obstruction. Chest 1976;70(4):506‐13. [DOI] [PubMed] [Google Scholar]
Habich 1994 {published data only}
- Habich G, Repges R. Chronic obstructive lung diseases: the efficacy of cineole [Cineol als medikation sinnvoll und bewart]. Therapiewoche 1994;44(6):356‐65. [Google Scholar]
Kasielski 2001 {published data only}
- Kasielski M, Nowak D. Long‐term administration of N‐acetylcysteine decreases hydrogen peroxide exhalation in subjects with chronic obstructive pulmonary disease. Respiratory Medicine 2001;95(6):448‐56. [DOI] [PubMed] [Google Scholar]
Lukas 2005 {published data only}
- Lukas R, Scharling B, Schultze‐Werninghaus G, Gillisen A. Administration of N‐acetylcysteine and vitamin C to augment antioxidant properties in patients with chronic bronchitis. Deutsche Medizinishe Wochenscrift 2005;130:563‐7. [DOI] [PubMed] [Google Scholar]
Maesen 1980 {published data only}
- Maesen FP, Brombacher PJ. Treatment of chronic bronchitis with oral acetylcysteine, a double‐blind study. European Journal of Respiratory Disease 1980;61:110. [Google Scholar]
Michnar 1996 {published data only}
- Michnar M, Milanowski J. Assessment of efficacy and tolerability of the oral treatment of ambrosol in patients with chronic bronchitis [Ocena kliniczna skutecznosci i tolerancji doustnego leczenia ambrosolem u chorych na przewlekle zapalenie oskrzeli]. Pneumonologia i Alergologia Polska 1996;64(Suppl 1):90‐6. [PubMed] [Google Scholar]
Moretti 2011 {unpublished data only}
- Moretti M, Ballabio M. Effect of erdosteine on airflow obstruction and symptom recovery in severe COPD exacerbations [Abstract]. European Respiratory Society 21st Annual Congress; 2011 Sep 24‐28; Amsterdam. 2011; Vol. 38 (55):76s [P536].
Moretti 2014 {published data only}
- Moretti M. Erdosteine oral treatment of hospitalised COPD exacerbation prolongs time to first re‐exacerbation [Abstract]. European Respiratory Journal 2014;44:P293. [Google Scholar]
Pirabbasi 2016 {published data only}
- Pirabbasi E, Shahar S, Manaf ZA, Rajab NF, Manap RA. Efficacy of ascorbic acid (vitamin C) and/N‐acetylcysteine (NAC) supplementation on nutritional and antioxidant status of male chronic obstructive pulmonary disease (COPD) patients. Journal of Nutritional Science and Vitaminology 2016;62(1):54‐61. [DOI] [PubMed] [Google Scholar]
Rubin 1996 {published data only}
- Rubin BK, Ramirez O, Ohar JA. Iodinated glycerol has no effect on pulmonary function, symptom score, or sputum properties in patients with stable chronic bronchitis. Chest 1996;109(2):348‐52. [DOI] [PubMed] [Google Scholar]
Saibene 2016 {published data only}
- Saibene F, Paone G, Lanata L, Puglisi G, Moscatelli B, Colli RD. Effect of carbocysteine lysine salt on frequency of exacerbations in COPD patient treated with or without inhaled steroids. European Respiratory Journal 2016;48:PA544. [DOI] [PubMed] [Google Scholar]
Salve 2016 {published data only}
- Salve VT, Atram JS. N‐Acetylcysteine combined with home based physical activity: effect on health related quality of life in stable COPD patients ‐ a randomised controlled trial. Journal of Clinical and Diagnostic Research 2016;10(12):OC16‐OC19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sushko 2015 {published data only}
- Sushko V, Shvaiko L, Bazyka K, Riazhska A. Treatment of chronic obstructive pulmonary disease in clean‐up workers of the Chornobyl NPP accident in the remote period after irradiation with additional 6 month prescription combination of ambroxol and essential phospholipids. European Respiratory Journal 2016;48:PA4299. [Google Scholar]
- Sushko VO, Shvaiko LI, Bazyka KD, Riazhska AS. Optimization of chronic obstructive pulmonary disease treatment in clean‐up workers of the Chornobyl NPP accident in the remote period after irradiation. Problemy Radiatsiinoi Medytsyny Ta Radiobiolohii 2015;20:457‐66. [PubMed] [Google Scholar]
Tatsumi 2007a {published data only}
- Tatsumi K, Fukuchi Y. Carbocisteine improves quality of life in patients with chronic obstructive pulmonary disease. Journal of the American Geriatrics Society 2007;55(11):1884‐5. [DOI] [PubMed] [Google Scholar]
Tatsumi 2007b {published data only}
- Tatsumi K, Fukuchi Y. Carbocisteine reduces the frequency of exacerbations in patients with COPD: findings from the PEACE study. American Thoracic Society International Conference; 2007; May 18‐23; San Francisco. 2007:C97.
Velazquez 2001 {published data only}
- Velazquez A, Aguilar G, Sanchez C, Ochoa L, Sansores R, Remirez‐Venegas A. The mucolytic effect on quality of life in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A57. [Google Scholar]
Wilhelmi 2010 {published data only}
- Wilhelmi E. Treatment of COPD: benefit of 1.8 cineole as co medication confirmed [Behandlung der COPD: nutzen voan 1.8‐cineol als zusatztherapie bestatigt]. Journal Pharmakol U Ther 2010;2:554‐5. [Google Scholar]
References to studies awaiting assessment
CTRI/2015/01/005432 {published data only}
- Hussain A, Rizvi W. Comparative Evaluation of Efficacy and Safety Profile of Antioxidants‐N‐Acetyl‐L‐Cysteine(NAC) and Superoxide Dismutase (SOD) Supplementation in Patients of COPD. Clinical Trials Registry ‐ India 2015.
References to ongoing studies
ChiCTR1800016712 {published data only}
- Early Intervention With Carbocysteine and Low Dose Theophylline in Chinese Patients With Chronic Obstructive Pulmonary Disease. Chinese Clinical Trials Registry 2018. [ChiCTR1800016712]
ChiCTR‐IIR‐17012604 {published data only}
- Zhou Y, Ran P. Long‐Term Regular Treatment of Early COPD With Randomized, Double‐Blind, Placebo‐Controlled Multicenter Clinical Study With Acetylcysteine Effervescent Tablets. Chinese Clinical Trial Registry 2017. [ChiCTR‐IIR‐17012604 ]
Additional references
Anzueto 1997
- Anzueto A, Jubran A, Ohar JA, Piquette CA, Rennard SI, Colice G. Effects of aerosolized surfactant in patients with stable chronic bronchitis: a prospective randomized controlled trial. Journal of American Medical Association 1997;278(17):1426–31. [PubMed] [Google Scholar]
Ballabio 2008
- Ballabio M, Nicola M, Moretti M. Long‐term use of antioxidant mucolytic erdosteine is especially beneficial in patient with more severe COPD. European Respiratory Society 18th Annual Congress; 2008 Oct 3‐7; Berlin. 2008:abstract ERS08L1_678.
Cates 2002
- Cates CJ. Simpson's paradox and calculation of number needed to treat from meta‐analysis. BMC Medical Research Methodology 2002;2:1. [DOI: 10.1186/1471-2288-2-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cazzola 2015
- Cazzola M, Calzetta L, Page C, Jardim J, Chuchalin AG, Rogliani P, et al. Influence of N‐acetylcysteine on chronic bronchitis or COPD exacerbations: a meta‐analysis. European Respiratory Review 2015;24(137):451‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collet 1997
- Collet JP, Shapiro P, Ernst P, Renzi T, Ducruet T, Robinson A. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;156(6):1719‐24. [DOI] [PubMed] [Google Scholar]
Criner 2015
- Criner GJ, Bourbeau J, Diekemper RL, Ouellette DR, Goodridge D, Hernandez P, et al. Prevention of acute exacerbation of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015;147(4):883‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fowdar 2017
- Fowdar K, Chen H, He Z, Zhang J, Zhong X, Zhang J, et al. The effect of N‐acetylcysteine on exacerbations of chronic obstructive pulmonary disease: a meta‐analysis and systematic review. Heart and Lung 2017;46(2):120‐8. [DOI] [PubMed] [Google Scholar]
GOLD 2019
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019. goldcopd.org/wp‐content/uploads/2018/11/GOLD‐2019‐v1.7‐FINAL‐14Nov2018‐WMS.pdf (accessed before 14 December 2018).
Grandjean 2000
- Grandjean EM, Berthet PH, Ruffman R, Leuenberger PH. Efficacy of oral long‐term N‐acetylcysteine in chronic bronchopulmonary disease: a meta‐analysis of published double‐blind, placebo‐controlled clinical trials. Clinical Therapeutics 2000;22(2):209‐20. [DOI] [PubMed] [Google Scholar]
Grandjean 2000a
- Grandjean EM, Berthet PH, Ruffman R, Leuenberger PH. Cost‐effectiveness analysis of oral N‐acetylcysteine as a preventive treatment in chronic bronchitis. Pharmacology Research 2000;42(1):39‐50. [DOI] [PubMed] [Google Scholar]
Guyatt 1987
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42(10):773‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Available from www.handbook.cochrane.org]
Jones 1992
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self‐complete measure of health status for chronic airflow limitation: the St. George's Respiratory Questionnaire. American Review of Respiratory Disease 1992;145(6):1321‐7. [DOI] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005;2(1):75‐9. [DOI] [PubMed] [Google Scholar]
Jones 2013
- Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. American Journal of Respiratory and Critical Care Medicine 2013;189(3):250‐5. [DOI] [PubMed] [Google Scholar]
Li 2015
- Li X, Sun H, Liu C, Kang J. Mucolytic and antioxidant agents for exacerbations of chronic obstructive pulmonary disease: a meta‐analysis. Chinese Journal of Tuberculosis and Respiratory Diseases 2015;38(8):600‐6. [PubMed] [Google Scholar]
Moretti 2007
- Moretti M, Nicola M. Prevention of COPD exacerbations and reduction of health care utilization with erdosteine. European Respiratory Journal 2007;30(Suppl 51):557s. [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. www.nice.org.uk/guidance/ng115 (accessed before 14 December 2018).
Qaseem 2011
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179‐91. [DOI] [PubMed] [Google Scholar]
Rahman 2005
- Rahman I. Oxidative stress in the pathogenesis of chronic obstructive pulmonary disease; cellular and molecular mechanisms. Cell Biochemistry and Biophysics 2005;43(1):167‐88. [DOI] [PubMed] [Google Scholar]
Rennard 2006
- Rennard SI, Vestbo J. COPD: the dangerous underestimate of 15%. Lancet 2006;367(9518):1216‐9. [DOI] [PubMed] [Google Scholar]
Rubin 2007
- Rubin BK. Mucolytics, expectorants, and mucokinetic medications. Respiratory Care 2007;52:859–65. [PubMed] [Google Scholar]
Rubin 2014
- Rubin BK. Secretion properties, clearance, and therapy in airway disease. Translational Respiratory Medicine 2014;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stey 2000
- Stey C, Steurer J, Bachmann S, Medici TC, Tramer MR. The effect of oral N‐acetylcysteine in chronic bronchitis: a quantitative systematic review. European Respiratory Journal 2000;16(2):253‐62. [DOI] [PubMed] [Google Scholar]
van Agteren 2016
- Agteren JEM, Carson KV, Tiong LU, Smith BJ. Lung volume reduction surgery for diffuse emphysema. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD001001.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wedzicha 2017
- Wedzicha JA, Calverley PMA, Albert RK, Anzueto A, Criner GJ, Hurst JR, et al. Prevention of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. European Respiratory Journal 2017;50(3):pii: 1602265. [DOI] [PubMed] [Google Scholar]
WHO 2017
- World Health Organization. The top 10 causes of death. www.who.int/mediacentre/factsheets/fs310/en/ (accessed before 14 December 2018).
Yang 2018
- Yang IA, Brown JL, George J, Jenkins S, McDonald CF, McDonald V, et al. The COPD‐X Plan: Australian and New Zealand Guidelines for the Management of Chronic Obstructive Pulmonary Disease. Version 2.53. copdx.org.au/wp‐content/uploads/2018/11/COPDX‐V2‐55‐Aug‐2018.pdf (accessed before 13 December 2018).
References to other published versions of this review
Poole 1998
- Poole PJ, Black PN. The effect of mucolytic agents on exacerbation frequency in chronic bronchitis. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD001287] [DOI] [Google Scholar]
Poole 1999
- Poole PJ, Black PN. Mucolytic agents for chronic bronchitis. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001287] [DOI] [Google Scholar]
Poole 2001
- Poole PJ, Black PN. Oral mucolytic drugs for exacerbations of chronic obstructive pulmonary disease: systematic review. BMJ 2001;322:1271‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poole 2006
- Poole PJ, Black PN. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001287.pub2] [DOI] [PubMed] [Google Scholar]
Poole 2012
- Poole PJ, Black PN, Cates CJ. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD001287] [DOI] [PubMed] [Google Scholar]
Poole 2015
- Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD001287.pub5] [DOI] [PubMed] [Google Scholar]


