Abstract
Background
Patients undergoing deep vein thrombosis (VT) have over 30% recurrence, directly increasing their risk of post-thrombotic syndrome. Current murine models of inferior vena cava (IVC) VT model host one thrombosis event.
Objective
We aimed to develop a murine model to study IVC recurrent VT in mice.
Materials and Methods
An initial VT was induced using the electrolytic IVC model (EIM) with constant blood flow. This approach takes advantage of the restored vein lumen 21 days after a single VT event in the EIM demonstrated by ultrasound.We then induced a second VT 21 days later, using either EIM or an IVC ligation model for comparison. The control groups were a sham surgery and, 21 days later, either EIM or IVC ligation. IVC wall and thrombus were harvested 2 days after the second insult and analysed for IVC and thrombus size, gene expression of fibrotic markers, histology for collagen and Western blot for citrullinated histone 3 (Cit-H3) and fibrin.
Results
Ultrasound confirmed the first VT and its progressive resolution with an anatomical channel allowing room for the second thrombus by day 21. As compared with a primary VT, recurrent VT has heavier walls with significant up-regulation of transforming growth factor-β (TGF-β), elastin, interleukin (IL)-6, matrix metallopeptidase 9 (MMP9), MMP2 and a thrombus with high citrullinated histone-3 and fibrin content.
Conclusion
Experimental recurrent thrombi are structurally and compositionally different from the primary VT, with a greater pro-fibrotic remodelling vein wall profile. This work provides a VT recurrence IVC model that will help to improve the current understanding of the biological mechanisms and directed treatment of recurrent VT.
Keywords: animal model, disease models, recurrence, venous thrombosis, venous thromboembolism
Introduction
Venous thromboembolism (VTE) is a major clinical problem, resulting in significant morbidity and mortality. In the United States alone, it has been estimated that there are 900,000 cases of pulmonary embolism (PE) and deep vein thrombosis (DVT) every year, resulting in up to 300,000 deaths.1 DVT often becomes a chronic disease, with 25% of patients developing recurrent ipsilateral DVTwithin 10 years of initial DVT. The highest incidence for recurrence is in the first 6 to 12 months following an acute DVT.2 Up to 50% of patients with DVT develop post-thrombotic syndrome (PTS) within 2 years, including pain, heaviness and swelling; this can result in venous ulceration in 3% of cases.3 PTS is associated with significant morbidity and impacts quality of life measures, with these measures being comparable to those with angina, cancer and congestive heart failure.4
Few strategies are available to prevent PTS. For example, in a randomized placebo control trial measuring the effectiveness of compression stockings, SOX trial investigators found no difference in the incidence of PTS after 2 years in those who wore compression stockings compared with those who wore placebo-controlled stockings.5 Additionally, in an open-label randomized control trial, CaVenTstudy investigators found that while the addition of thrombolysis to conventional anticoagulation did significantly reduce PTS compared withanticoagulationalone,therewasstilla41%incidenceofPTSat24monthsin the treatment group.6 These studies suggest that there is clinical incentive for understanding the risk factors of PTS.
Recurrent DVT can increase the risk of PTS by 10-fold, but the pathophysiology is not well understood.7 Certainly, a fully occlusive DVT is associated with significant disability.8 Several murine models are well established in studying acute and chronic venous thrombosis (VT), but all represent single VT episodes and there is no validated mouse model for studying recurrent VT in the mouse inferior vena cava (IVC). Hara et al demonstrated the first murine model of recurrent VT in the jugular vein using a recurrent stasis model.9 While this technique generated a successful model to study recurrent VT, the jugular vein provides a tissue sample that may be insufficient for consistent tissue analysis and is not comparable with current IVC VT models.
In this context, we present a novel model of recurrent VT combining two single VT models consecutively performed in a single animal in the same vein. We also confirm the validity of the model and characterize the differences in thrombus and vein wall responses between single VT and recurrent VT.
Materials and Methods
Mice
Male C57BL/6 mice, age 8 to 12 weeks (mean body weight = 22.7 g), were purchased from Jackson Laboratories (Bar Harbor, Maine, United States). Seventy-five mice were used for model development. The study was approved by the University of Michigan Committee on Use and Care of Animals and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NRC 2011). The University of Michigan’s Unit for Laboratory Animal Medicine (ULAM) program and facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC). All animals used in this study were healthy and specific pathogen free.
Surgical Technique
To develop the IVC recurrent model of VT, we combined two previously described single VT models. A first insult was performed using the electrolytic IVC model (EIM) as previously described in the literature.10–14 In brief, a midline laparotomy incision was made to access the IVC and all venous side branches caudal to the left renal vein were ligated using 7–0 Prolene suture (Ethicon, Inc, Somerville, New Jersey, United States). A 25-G stainless-steel needle attached to a silver-coated copper wire (KY-30–1-GRN, Electrospec, Dover, New Jersey, United States) was introduced into the caudal IVC (anode) and another needle was implanted subcutaneously (cathode). Note that 250 μA of current was applied to the IVC over a time frame of 15 minutes. After 15 minutes, the needle was removed from the IVC and haemostasis was achieved. The abdominal wall was closed with 5–0 Vicryl (Ethicon, Inc) and the skin was closed using Nexaband glue (3M Animal Care Products, St. Paul, Minnesota, United States).
Twenty-one days after the primary VT insult, a secondary VT was created using either the EIM or the stasis model, named from now on the ligation model, as described previously.13,15 The ligation model procedure involves cauterizing all the lumbar vein branches ligating side branches draining into the IVC between the left renal vein and iliac bifurcation, and completely tying off the IVC immediately distal to the left renal vein using a 7–0 Prolene suture (Ethicon, Inc). The EIM used to create the secondary VT is performed in the same manner as the primary VT except for needle placement and duration of current. The needle is placed in a different location (in the first insult, the needle entry point is placed towards the right, and in the second insult it is placed towards the left) and the duration was changed to 10 minutes. The animal’s incision is closed in the same manner as the primary surgery.
Sham groups were also studied with the primary VT performed as a sham EIM surgery. This procedure is done in the same manner as an EIM procedure but with no electrical current applied as was previously described.12 The secondary EIM and ligation procedure is done in the same manner as described above. It is important to mention that the equipment originally described for implementing the EIM has been discontinued. This issue can be overcome by using a voltage to current converter, described by Palmer et al.14
Experimental Design
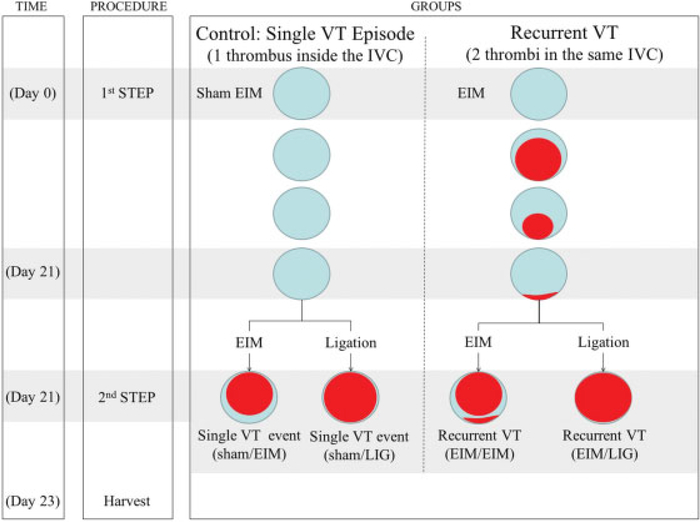
The timeline and surgeries of each group studied are shown in Fig. 1. Control groups simulated a single VT (sham/EIM; sham/LIG) while the experimental groups represented recurrent VT (EIM/EIM; EIM/LIG). Specifically, on day 0, mice in the control group received a sham surgery while experimental (recurrent) mice had an EIM surgery. On day 21, all mice had either EIM or ligation surgery, identified as a single VT for controls, and a second, recurrent VT for experimental groups. In all groups, VTs were harvested 2 days after the secondary VT. Experimentally, this secondary 2-day time point represents an acute thrombus in both groups, as opposed to a chronic thrombus.
Fig. 1.
Experimental design: Schematic representation. Control group: Sham EIM followed by either EIM or ligation models. Recurrent model: EIM as primary insult followed by either EIM or ligation models as secondary insults. Of note, the EIM is the only IVC VT model available that recuperates vein lumen (almost 100%) after 21 days post-thrombosis. We took advantage of this condition to host a secondary thrombus in the recurrent model. Abbreviations: EIM, electrolytic IVC model; IVC, inferior vena cava; VT, venous thrombosis.
Ultrasound Protocol
Mice were anaesthetized via isoflurane gas inhalation (2%). Warm ultrasound gel and heating pads were used to maintain normal body temperature. IVC images were obtained from the anterior/right side of the mouse abdomen using a Vevo 2100 (Visualsonics, Toronto, ON, Canada) equipped with a linear array MicroScan transducer (MS 550D, frequency 22–55 MHz). Duplex ultrasonography and tissue Doppler imaging were performed at baseline (T0) and days 21 and 23.
Thrombus Weight
Two days after the secondary insult (day 23), the IVC containing the acute thrombus was carefully removed. Samples from both groups were measured and analysed for thrombus weight (g), IVC wall weight (g), thrombus weight to length (g/cm), IVC wall weight to length (g/cm) and total weight to length (g/cm).
Histology/Immunohistochemical/Collagen/Elastin Staining
Fresh tissue (IVC with intact thrombus) was fixed in 10% buffered formalin for 2 hours and then transferred to 70% ethanol for subsequent paraffin embedding and slide mounting (5 μm thickness) as previously described.16 Antigen retrieval was performed using the heat-mediated sodium citrate method; 10 mM sodium citrate solution, pH 6.0 at 95°C for 10 minutes and then allowed to cool for 20 minutes. Nonspecific antigen-binding sites were blocked with normal serum, and sections were incubated with primary antibodies to Ly6G (551459, 0.5 ug/mL, BD Biosciences, San Jose, California, United States) and citrullinated histone3 (Cit-H3)(ab5103, 2 ug/mL, Abcam, Cambridge, Massachusetts, United States). A species-specific peroxidase kit (Vector Laboratories Inc., Burlingame, California, United States) was used according to the manufacturer’s instructions for the corresponding secondary antibody and 3,3′-diaminobenzidine (DAB) substrate application. The slides were counterstained with haematoxylin. In a blinded fashion, positive cells were counted and totaled in five high-power fields (hpf, 1,000 × ) radially around the IVC wall.
Picrosirius red staining to quantify collagen content was performed as previously described.16 These sections were then analysed in crossed-plane polarized light from a monochromatic source to assess cross-linked collagen. Six images for each were obtained using a Zeiss Axio Imager M1 microscope and Zeiss Zen 2 (blue edition) software (Carl Zeiss Microimaging GmbH, Göttingen, Germany) at 0 and 90° to the plane of polarization, to capture the birefringence of fibres extinguished in one direction. The images were analysed blindly utilizing the National Institutes of Health (NIH) Image J software. The area corresponding to the vein wall was selected as a region of interest, and then the image underwent threshold segmentation to differentiate collagen from other (mainly cellular and empty space) components of the vein wall. A vein wall collagen score was assigned by the formula [(%birefringent area) × (measured vein wall area)] / (total specimen area). Haematoxylin and eosin stain for vein wall thickness score, as previously reported,12 and Verhoeff’s elastic stain for elastin visualization were performed in the University of Michigan Dental School Histology Core.
Western Blot Analysis
Protein was isolated from IVC segments or thrombus using RIPA buffer (ThermoScientific, Rockford, Illinois, United States) with cOmplete ULTRA Tablets, Mini (Roche, Mannheim, Germany). Proteins were electrophoretically separated on NuPAGE 4 to 12% Bis Tris gels (Invitrogen, Carlsbad, California, United States) and blotted onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, Massachusetts, United States). Non-specific binding was blocked with starting block (tris-bufferedsaline [TBS]) buffer (ThermoScientific). Antibodies used included: anti-Cit-H3 (a5103, 2ug/mL, Abcam), anti-fibrin (clone 59D8, 1 ug/mL, a gift from Dr. Charles Esmon, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, United States) and β-actin (sc-47778, 40 ug/mL, Santa Cruz, Dallas, Texas, United States).
Quantitative (Real-Time) Polymerase Chain Reaction
The vein wall was separated from its associated thrombus, placed in a 15-mL conical tube filled with 1.5 mL of TRIzol (Invitrogen), and homogenized using the standard homogenization protocol. The ribonucleic acid (RNA) was cleaned using the Direct-zol RNA mini-prep kit according to the manufacturer’s protocol (Zymo ResearchTotal, Irvine, California, United States). For this procedure, RNA concentrations were verified using the Nanodrop spectrophotometer (Thermo Fischer Scientific Inc., Wilmington, Delaware, United States). Reverse transcription of the RNA (450 ng) into complementary deoxyribonucleic acid (cDNA) was done using SABiosciences’ RT 2 First Strand kit and the cDNA concentration was determined using a Nanodrop 2000c spectrophotometer. All of the samples were diluted to 22 ng/μL in this protocol. Real-time polymerase chain reaction (PCR) was run on the cDNA template according to the manufacturer’s protocol for β-Actin (mouse β-actin: PPM02945B), Collagen Ia2 (PPM04448F), Collagen III (PPM04784B), TGF-β (PPM02991B), Elastin (PPM36834B), MMP2 (PPM03642C) and MMP9 (PPM03661C) mouse primers using a SybrGreen Master Mix (SABiosciences-Qiagen, Valencia, California, United States). Samples were run in a RotorGene 6000 Thermocycler using a two-step/melt protocol for 10 minute at 95°C, 40 cycles of 95°C for 15 seconds, and 60°C for 60 seconds. Cycle threshold values were evaluated with the delta cycle threshold (CT) method to determine increases or decreases in gene expression between the animal groups.
Statistical Analysis
Statistical analysis and methods included mean standard deviation. Statistical significance was calculated using an unpaired t-test with Welch’s correction (GraphPad Software, Inc., La Jolla, California, United States) and was defined as p-value of ≤ 0.05. Direct comparisons were made between the control and experimental groups for weight-to-length data, western blot data and real-time PCR values.
Results
Ultrasound and Histology Characteristics of the IVC Recurrent VT Model
Mice scanned with duplex colour ultrasound demonstrated that all IVCs were patent before performing any surgery. For the control mice (those that received sham EIM as a first step), ultrasonography demonstrated absence of a thrombus before the second insult (Fig. 2A). For the recurrent model, ultrasonography performed before the second surgery demonstrated the presence of a patent lumen prior to the second thrombus induction. In addition, ultrasonography provided information regarding the ‘chronic’ thrombus, observed to be a part of the vein wall. This was confirmed by histologyat harvest (Fig. 2B and C). An echo-dense area in the peri-lumenal region is consistent with the resolving primary VT.
Fig. 2.
Ultrasonography and histology to validate the experimental design principles of the IVC recurrent model. Ultrasonography was performed in all animals prior to second insult (A) and post-second insult (B and C); (A) Ultrasound showing IVC patency to host the secondary thrombi; (B) Ultrasonography performed after the second insult and histology showing the primary thrombus incorporated to the vein wall co-existing with the new (EIM) thrombus and its lumen within the IVC. (C) Ultrasonography post-second insult and histology showing the primary thrombus incorporated to the vein wall and the new thrombus totally occluded in the IVC secondary to the ligation model. White arrow: lumen; black arrows: primary thrombus incorporated to the vein wall. Abbreviations: EIM, electrolytic IVC model; IVC, inferior vena cava.
Vein Wall Characteristics of the Primaryand Recurrent VT
Fibrosis is the end stage of chronic VT and vein wall injury. We analysed expression of several fibrotic markers to assess the impact of recurrent VT on fibrosis as compared with controls. IVC wall size was standardized using wall weightto-length ratio, and is reflective of the initial VT and its incorporation into the vein wall as scar tissue. Recurrent VT resulted in a heavier IVC wall than the sham/primary VT by both the EIM/EIM and the EIM/LIG mechanism (Fig. 3A), with increased thickness as demonstrated by histology (Fig. 3B). Inflammatory cells were non-significantly increased in the recurrent VT groups (Fig. 3C, D).
Fig. 3.
Comparative vein wall analysis between recurrent VT ad single VT. (A) The vein wall weight-to-length ratio demonstrated heavier vein walls in mice with recurrent VT compared with single VT. (B) Vein wall thickness score. Histology analysis on H&E stained slides was performed to determine wall thickness of the IVC area where the thrombi were located. (C) Vein wall neutrophils count and (D) monocyte counts were performed to measure inflammatory component of the vein wall after recurrent VT and compared with single VT. (E) Gross anatomy representative pictures of the vein walls. Of note, the transparency of the vein wall on the single VT and the contrasted opaque vein wall on the recurrent VT was repeatedly observed through all the investigation. Significance: p < 0.05, n = 6–8 per group. Abbreviations: EIM, electrolytic IVC model; H&E, haematoxylin and eosin; VT, venous thrombosis.
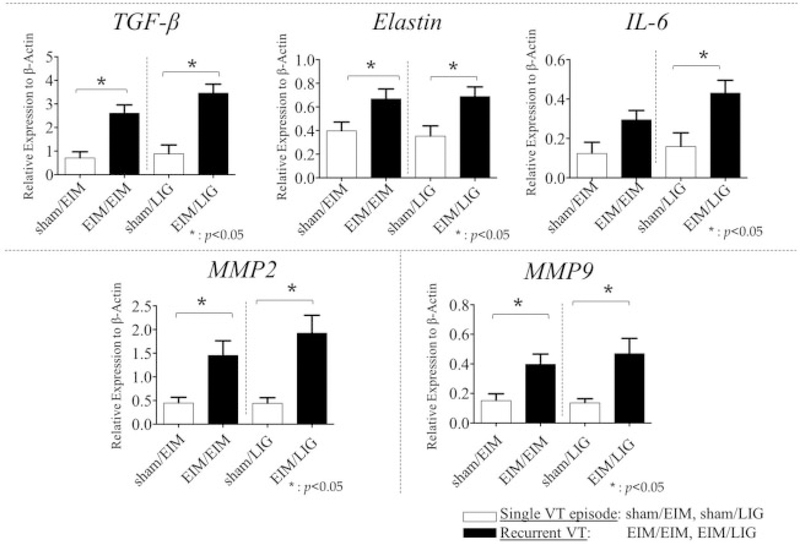
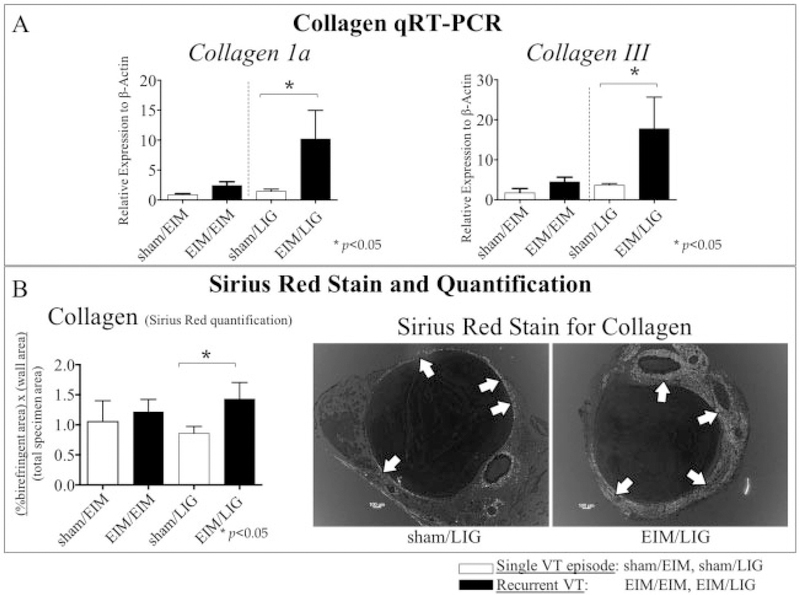
The recurrent VT vein walls had several markers suggesting greater fibrosis than a primary VT (Fig. 4). First, the vein walls subjected to a recurrent VT had significantly more transforming growth factor-β (TGF-β) gene expression as compared with controls, in both EIM/LIG and EIM/EIM. Similarly, matrix metallopeptidase 2 (MMP2) gene expression was significantly increased in the EIM/EIM and EIM/LIG groups. MMP9 gene expression was significantly increased in mice receiving EIM/EIM and EIM/LIG as compared with control. Elastin gene expression was also significantly increased in the vein walls of our recurrent groups by both mechanisms. Interleukin (IL)-6 was significantly increased only in the EIM/LIG group. By picrosirius red staining analysis, collagen content was not significantly different in the EIM/EIM compared with controls. However, in the EIM/LIG setting, collagen deposition was significantly higher in the recurrent VT groups as compared with controls (Fig. 5).
Fig. 4.
Vein wall markers of fibrosis, qRT-PCR analysis. Fibrosis is a landmark on chronic venous thrombosis. We analysed the expression of fibrotic markers to investigate the impact of recurrent VT on fibrosis and compared with single VT. TGF-β, elastin, IL-6, MMP2 and MMP9 gene expression were determined using qRT-PCR showing the pro-fibrotic condition of the recurrent VT compared with single VT. Significance: p < 0.05, n = 5–7 per group. Abbreviations: EIM, electrolytic IVC model; IL-6, interleukin-6; LIG, ligation; MMP, matrix metallopeptidase; qRT-PCR, quantitative real-time polymerase chain reaction; TGF- β, transforming growth factor-β; VT, venous thrombosis.
Fig. 5.
The major biological element that represents tissue fibrosis is collagen. Collagen analysis was performed and compared between recurrent VT and single VT. (A) Collagen 1a and III gene expression were determined using qRT-PCR. (B) Quantification of the collagen deposition and representative pictures of the picosirius red stain in the sham/LIG group versus the EIM/LIG group. White arrows: vein wall collagen. Significance: p < 0.05, n = 5–6 per group. Abbreviations: EIM, electrolytic IVC model; LIG, ligation; qRT-PCR, quantitative real-time polymerase chain reaction; VT, venous thrombosis.
Thrombus Characteristics of Recurrent VT
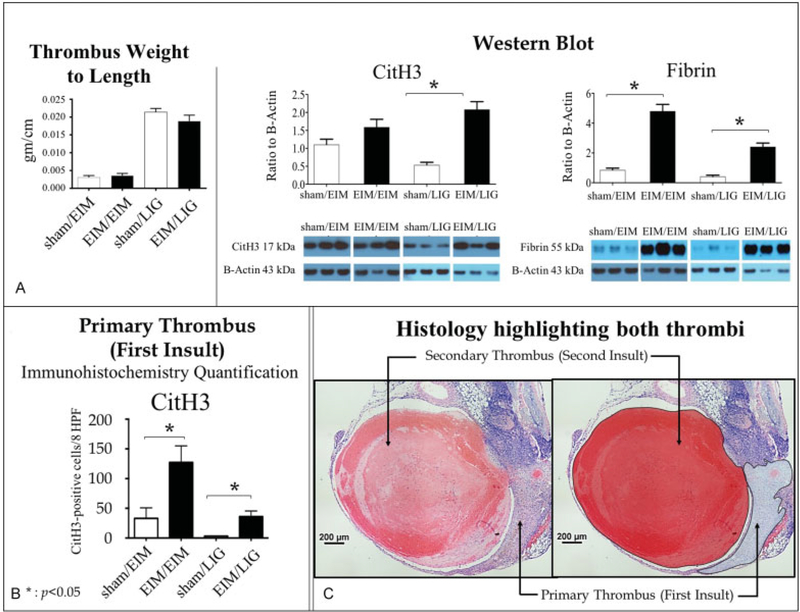
Thrombus weight-to-length ratio is a simple way to directly measure thrombus resolution.16 There was no significant difference in the 2-day acute thrombus size between the sham and the EIM or ligation secondary injuries (data not shown); however, the thrombus composition was different between recurrent VT and controls. First, the recurrent VT had a relative increase in fibrin amount when compared with primary VT. Mice receiving EIM/LIG surgery had a nearly sixfold increase in fibrin deposition compared with control (Fig. 6). Similarly, EIM/EIM mice showed significantly higher thrombus fibrin as compared with control.
Fig.6.
Analysis of the first and secondary thrombi, using IHC and western blot. (A) Secondary thrombus analysis. Left panel: Thrombus weight to length was analysed on the secondary thrombus. No significant differences were observed between sham/EIM and EIM/EIM or sham/LIG and EIM/ LIG. Central and right panel: Western blot. Cit-H3 and fibrin western blot were performed on the fresh secondary thrombus identifiable and removable. Bars represent the quantification expressed as ratio to β-actin and show the effect of recurrent VT on those markers. (B) Primary thrombus analysis: Cit-H3 IHC quantification was performed on the first thrombus, which had been incorporated into the vein wall. This thrombus is identifiable but un-removable. The quantification showed the effect of recurrent VT. (C) Representative H&E pictures highlighting the primary and secondary thrombus. These representative pictures are showing the location of both thrombi, highlighted in the second panel: the thrombus incorporated to the vein wall after the first insult (inverted C shape) and the fresh secondary thrombus located in the vein lumen (round shape). Significance: p < 0.05, n = 5–6 per group. Abbreviations: Cit-H3, citrullinated histone H3; EIM, electrolytic IVC model; H&E, haematoxylin and eosin; IHC, immunohistochemistry; LIG, ligation; Sham, sham surgery; VT, venous thrombosis.
Second, we tested if neutrophil extracellular traps (NETs) are associated with thrombus initiation using Cit-H3, a marker of NETosis. EIM/LIG mice had significantly higher levels of Cit-H3 within the thrombus compared with control, indicating increased extracellular DNA deposition (Fig. 6). However, EIM/EIM did not have statistically higher levels of Cit-H3 compared with control.
Additionally, we examined the thrombus incorporated to the vein wall as a consequence of the first insult. Cit-H3 was significantly increased when EIM/EIM and EIM/LIG were compared (Fig. 6).
Discussion
Clinically, 25% of VT patients develop a recurrent ipsilateral VTwithin 10 years of the initial thrombotic process, with the highest incidence within the first 6 to 12 months.2 Recurrent VT is the most important risk factor for PTS, which can increase the risk of developing PTS up to 10-fold.7 Thus, understanding the mechanisms of recurrent VT may illustrate new therapeutic avenues. However, there is a need for pre-clinical animal models to study VT recurrence in the IVC. In this work, we present a novel mouse model that produces two consecutive thrombotic events within the same mouse vein. We successfully induced two thrombotic episodes within a single mouse IVC in all mice that underwent this procedure, with no mortality or overt morbidity observed. Although the purpose of this article is to introduce a novel model to the field, initial results on its characterization led us to identify biological differences between primary and recurrent VT that may underscore why a recurrent VT in humans increases the risk of PTS.
This recurrent VT model was possible due to the use of the well-characterized flow kinetics of the EIM, which produces thrombi that are consistent in size and are developed in the constant presence of blood flow.10,12,14 As shown in Fig. 1, we used the EIM to induce the first insult since it is the only model that, after 21 days, allows remodelling of the IVC wall to accommodate blood flow in the IVC lumen. This strategy allowed the vein to be re-accessed for a second thrombogenic insult by two methods, namely, a second EIM or the ligation model (Fig. 2). The ligation model is typically less technically challenging because dissection involves a non-surgically dissected vein segment of the infrarenal IVC. The second EIM technique requires another dissection and manipulation of the distal IVC and is more technically challenging.
This approach allows for a thrombus to develop and resolve without narrowing a segment of the lumen as seen in other IVC models.13,14 Thus, the IVC lumen that remains patent after the first insult (Fig. 1)—demonstrated here by ultrasound (Fig. 2) and in previous work by magnetic resonance imaging (MRI)14—hosts a second thrombus within the vein that already sustained a primary thrombus. Prior works have confirmed the near 3:1 thrombus:lumen ratio in the EIM at day 2 when the thrombus burden is at its maximum, which then resolves with the thrombus–lumen relationship inverted at chronic time points12,14 due to the fact that the thrombus is progressively incorporated into the vein wall. Taking advantage of the persistent lumen, we were able to introduce a second thrombus, providing a platform that will allow research to have two consecutive thrombi within the same vein in an attempt to model VTrecurrence in two secondary conditions: persistent flow in the EIM/EIM setting and non-flow in the EIM/LIG.
With this IVC recurrent VT model, we performed analysis of the vein wall, the acute secondary thrombus and the chronic primary thrombus (incorporated into the vein wall) to test whether the model was capable of showing differences between a single VT episode and a recurrent VT (Fig. 1). Indeed, we detected differences between a single and recurrent VT by tissue analysis.
Vein wall analysis:
The vein walls were heavier and thicker (Fig. 3), had more collagen and altered growth factor and MMP gene expression in mice with a recurrent VT as compared with the control group (Figs. 4 and 5). The heavier walls in both EIM/EIM and EIM/LIG is not surprising given that the initial thrombus incorporates intothe wall as it resolves, but represents a different biological response compared with a single thrombotic injury (Fig. 3).
Fibrotic markers are up-regulated in chronic VT, and we tested whether those markers would be affected by the second VT event in this new model. First, TGF-β is a common mediator involved in tissue remodelling and fibrotic change in tissues undergoing repair.17 We found significantly increased TGF-β gene expression in the vein walls of recurrent VT as compared with a primary VT (Fig. 4). In addition, TGF-β also regulates elastin gene expression,17 which is an interstitial matrix protein and a surrogate marker for vein wall compliance.18 We found increased MMP2 and 9, as well as elastin gene expression, elevated in recurrent VT as compared with primary VT (Fig. 4). These explored factors are consistent with an increase in matrix turnover in the vein wall and we can speculate that this may be due to the preceding thrombus resolution mechanisms. However, investigations of chronic secondary time points are needed to certify whether the vein wall parameters explored in this work are primed for greater fibrotic injury given the preceding VT. Such investigations are now possible with the availability of this new model.
IL-6 is a pro-inflammatory and pro-fibrotic cytokine that participates in VT19 and has been linked to fibrosis in the stasis model of VT.20 IL-6 gene expression levels trended higher in the EIM/EIM model but were significantly elevated in the EIM/LIG model (Fig. 4). Certainly, IL-6 has profibrotic effects and a link between inflammation and experimental VT was previously demonstrated in a single VT episode using the ligation model.20 Interestingly, only the EIM/LIG stasis mechanism of thrombosis (as compared with EIM/EIM) was associated with increased collagen by gene expression and phenotypically by histological assessment. This suggests that IL-6 participates in recurrent VT, driving the fibrotic vein wall response in a non-flow condition. We can speculate that flow (produced here by EIM) versus nonflow (produced here by LIG) conditions may alter the biological impact of IL-6 in the fibrotic response and further studies should focus on the effects of fluid dynamics in thrombogenesis. Non-surprisingly, collagen deposition results were aligned with the IL-6 results, with statistical differences only in the non-flow condition.
Thrombus analysis:
The secondary thrombi were similar in size compared with controls (Fig. 6A). Extracellular DNA, in the form of NETs, has been linked to VT in preclinical and clinical settings.21,22 Our results showed increased Cit-H3 in the recurrent acute VT as compared with controls in the EIM/LIG model (Fig. 6A). Interestingly, we found Cit-H3 positive staining in the primary thrombus that was incorporated into the vein wall at 23 days (Fig. 6B). This may be due to increased polymorphonuclear leukocyte (PMN) or other leukocyte cell death in the recurrent VT as compared with primary VT. Further work needs to be done to determine the role of extracellular DNA during thrombogenesis in recurrent VT.
Fibrin deposition was also quantified within the thrombus (Fig. 6A). The initial haemostatic reaction of the vessel wall following damage involves fibrin deposition, which participates in thrombogenesis during VT.23,24 Recurrent VT showed increased fibrin content as compared with single VT (Fig. 6A). Both recurrent VT conditions showed high fibrin content, which demonstrates that recurrent VT facilitates a pro-thrombotic environment for the secondary VT. While not directly assessed, this could also be driven by greater thrombin activity in the residual VT.
In conclusion, we present a straightforward and useful mouse model to study recurrent VT within the murine IVC. The model is feasible, consistent and initially reveals differences between single and recurrent VT. Our aim was to fill a gap in the field by developing a new in vivo pre-clinical tool to allow the study of recurrent VT within the IVC; we do not intend to fully characterize recurrent VT in this initial communication. There are limitations in comparing mice and humans at this stage of the disease. Obtaining human tissue samples at this stage of the disease is very rare. There were some attempts by our group to biopsy thrombi during endovascular interventional procedures, but results were difficult to interpret. In the past, open surgeries were performed on patients with severe symptoms and we learned that fibrosis is a key player in recurrent VT. However, the in vivo setting in mice is a great tool that allowed us to identify several pro-fibrotic and pro-thrombotic factors that are up-regulated in recurrent VT as compared with single acute VT, indicating that the biology of recurrent VT may differ from single VT. Further work using chronic time points for the secondary thrombi will delineate the potential mechanisms of injury after two thrombotic episodes, as well as investigate therapeutic efficacy in recurrent VT.
What is known about this topic?
Patients with deep vein thrombosis (VT) have over 30% recurrence, regardless of the time elapsed since the first event, which directly increases their risk of post-thrombotic syndrome.
Biology behind recurrent venous thrombosis (RVT) is under-studied and clinical outcomes suggest a more complicated biology than single VT. Also, there is a lack of in vivo models to study RVT in the mouse vena cava.
Currently, there is a lack of recurrent pre-clinical models to study recurrent VT in the inferior vena cava (IVC).
What does this paper add?
We have provided the first IVC preclinical model to study the biology of RVT. This novel in vivo model introduces two consecutive clots within the same vein to mimic recurrence.
Results in this article establish the ability of the model to study RVT biology, and suggest that there are biological differences between single VT and RVT.
Funding
This work was supported by the University of Michigan Research Advisory Committee (RAC) Grant: “Developing the First IVC Animal Model to Study Recurrent Venous Thrombosis” (J.A.D.) and RO1-HL 132988 (P.K.H.).
Footnotes
Conflict of Interest
J.A.D. is on the Board of Directors of the American Venous Forum, as a Research Council Chair and Member of the ISTH-SSC Board. All the other authors report no conflict of interest.
This work was partially presented at the American Venous Forum Annual Meeting, Palm Springs, CA, February 25, 2015, as an oral presentation and awarded as Best Basic Science Abstract.
References
- 1.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008;28(03):370–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulman S, Lindmarker P, Holmström M, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost 2006;4(04):734–742 [DOI] [PubMed] [Google Scholar]
- 3.Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg 2011;53(02): 500–509 [DOI] [PubMed] [Google Scholar]
- 4.Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost 2008;6(07):1105–1112 [DOI] [PubMed] [Google Scholar]
- 5.Kahn SR, Shapiro S, Wells PS, et al. ; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet 2014;383(9920):880–888 [DOI] [PubMed] [Google Scholar]
- 6.Enden T, Haig Y, Kløw NE, et al. ; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379(9810):31–38 [DOI] [PubMed] [Google Scholar]
- 7.Kahn SR. The post-thrombotic syndrome: progress and pitfalls. Br J Haematol 2006;134(04):357–365 [DOI] [PubMed] [Google Scholar]
- 8.Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg 2004;239(01):118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara T, Truelove J, Tawakol A, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography enables the detection of recurrent same-site deep vein thrombosis by illuminating recently formed, neutrophil-rich thrombus. Circulation 2014;130(13):1044–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz JA, Hawley AE, Alvarado CM, et al. Thrombogenesis with continuous blood flow in the inferior vena cava. A novel mouse model. Thromb Haemost 2010;104(02):366–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz JA, Wrobleski SK, Hawley AE, Lucchesi BR, Wakefield TW, Myers DD Jr. Electrolytic inferior vena cava model (EIM) of venous thrombosis. J Vis Exp 2011;(53):e2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz JA, Alvarado CM, Wrobleski SK, et al. The electrolytic inferior vena cava model (EIM) to study thrombogenesis and thrombus resolution with continuous blood flow in the mouse. Thromb Haemost 2013;109(06):1158–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz JA, Obi AT, Myers DD Jr, et al. Critical review of mouse models of venous thrombosis. Arterioscler Thromb Vasc Biol 2012;32 (03):556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pamer OR, Shaydakov ME, Rainey JP, Lawrence DA, Greve JM, Diaz JA. Update on the electrolytic IVC model for pre-clinical studies of venous thrombosis. Res Pract Thromb Haemost 2018;2(02):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wrobleski SK, Farris DM, Diaz JA, Myers DD Jr, Wakefield TW. Mouse complete stasis model of inferior vena cava thrombosis. J Vis Exp 2011;52(52):2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deatrick KB, Obi A, Luke CE, et al. Matrix metalloproteinase-9 deletion is associated with decreased mid-term vein wall fibrosis in experimental stasis DVT. Thromb Res 2013;132(03):360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kähäri VM, Olsen DR, Rhudy RW, Carrillo P, Chen YQ, Uitto J. Transforming growth factor-beta up-regulates elastin gene expression in human skin fibroblasts. Evidence for post-transcriptional modulation. Lab Invest 1992;66(05):580–588 [PubMed] [Google Scholar]
- 18.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001;12(09):2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeRoo EP, Wrobleski SK, Shea EM, et al. The role of galectin-3 and galectin-3-binding protein in venous thrombosis. Blood 2015;125 (11):1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojcik BM, Wrobleski SK, Hawley AE, Wakefield TW, Myers DD Jr, Diaz JA. Interleukin-6: a potential target for post-thrombotic syndrome. Ann Vasc Surg 2011;25(02):229–239 [DOI] [PubMed] [Google Scholar]
- 21.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol 2012;32(08):1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz JA, Fuchs TA, Jackson TO, et al. ; for the Michigan Research Venous Group. Plasma DNA is elevated in patients with deep vein thrombosis. J Vasc Surg Venous Lymphat Disord 2013;1(04): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz JA, Wrobleski SK, Alvarado CM, et al. P-selectin inhibition therapeutically promotes thrombus resolution and prevents vein wall fibrosis better than enoxaparin and an inhibitor to von Willebrand factor. Arterioscler Thromb Vasc Biol 2015;35(04):829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara T, Bhayana B, Thompson B, et al. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC Cardiovasc Imaging 2012;5(06):607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]