Abstract
Background:
Many aging adults undergo progressive loss of autonomy, develop increasingly complex medical needs and experience multiple care transitions. We sought to determine the degree of variation in rates of transfer from home care services and long-term care in several Canadian jurisdictions.
Methods:
In this retrospective cohort study, we examined transitions from home care services and long-term care to different possible end states: change in health stability (getting better or worse), transfer to hospital, transfer to another care setting or death. We used standardized interRAI assessments from long-term care and home care linked to hospital records (data from the Discharge Abstract Database and National Ambulatory Care Reporting System) from 2010 to 2016. Multistate modelling was used to adjust for patients with complex health status and transitions in care.
Results:
We report data for 254 664 patients in home care programs and 162 045 residents in long-term care. Compared with patients in Ontario, patients requiring home care services in Alberta and British Columbia had increased odds of being admitted to hospital regardless of the underlying severity of illness (the adjusted odds ratios [OR] ranged from 2.08 to 3.77 in Alberta and from 1.28 to 1.46 in BC). Residents in long-term care in Alberta and BC had less than half the odds of being transferred to hospital, independent of all other factors, when compared with long-term care residents in Ontario (the adjusted OR ranged from 0.38 to 0.39 in Alberta and from 0.33 to 0.44 in BC).
Interpretation:
Significant variations in transfer rates were observed between provinces, even after controlling for individual patient characteristics. These results suggest that transfers to hospital are largely driven by health care policies, health care professional practice patterns and available infrastructure rather than individual patient needs.
Many older adults embark on a journey of increasing ill health because of comorbid illnesses, progressive frailty and functional decline that takes them from full independence to receiving assistance in the community, followed by full care then eventual palliation and death (often in institutional settings). Such trajectories are quite variable from patient to patient. They will also result in patients needing many caregivers and health professionals in a variety of settings. Even under the best of circumstances, most patients will undoubtedly go through many transitions in care. However, poorly executed transitions, inconsistencies in assessments among practitioners, and interventions that are often poorly tailored to meet the person’s needs and expressed goals, available resources and health care settings may result in frail older adults being put at risk of adverse outcomes.1 Indeed, poor transitions can lead to deleterious consequences such as medical errors and loss of critical health information, premature transfers from home care to nursing homes, unnecessary transfers to hospital emergency departments and inadequate end-of-life care planning.
Hospital admissions from home care or long-term care, even if judged necessary, can lead to deteriorations in overall health during the acute care hospital stay.2 Even though prognoses, care needs, values and expectations may vary substantially from patient to patient, the use of emergency care and acute care facilities remains elevated, often for potentially avoidable reasons3 and often in disaccord with patient wishes. Indeed, older adults use emergency departments at higher rates than younger people,4,5 representing as much as 21% of all emergency department visits.6 In addition, the proportion of emergency department visits by older adults has substantially increased over the last decade.6,7 For elderly people who make use of emergency care, outcomes may be guarded, with high admission rates to hospital wards or the intensive care unit often followed by deterioration in symptoms and overall function. 8 Thus, emergency services seem to be the default means of service delivery in many jurisdictions. The overall use of acute care resources should also be similar from jurisdiction to jurisdiction. Nonetheless, jurisdictional differences in care programs, resources and types of services provided, as well as ready access to reliable, timely health information, may result in distinct patterns of care. These patterns of care may result in unwarranted variations that are not explained by individual patients’ characteristics, their illness or their needs.
Given that health care is under provincial jurisdiction, we sought to determine the degree of variation in rates of transfer from home care services and long-term care to acute care between jurisdictions in Canada.
Methods
Source of data
In this retrospective cohort study, we obtained linked data sets from the Canadian Institute for Health Information and combined them with the following data sets: (a) interRAI data Resident Assessment Instrument-Minimum Data Set (RAI-MDS) 2.0 for long-term care and RAI-Home Care (RAI-HC), (b) the Discharge Abstract Database (DAD) to track acute hospital admissions and (c) the National Ambulatory Care Reporting System (NACRS) to track emergency department visits. We included linked patients (i.e., patients whose information was contained in multiple data sources) if they had (a) at least 1 consecutive follow-up assessment within the same admission episode or (b) a date of discharge or death. These data were all obtained from Canadian Institute for Health Information, which exerts strict controls over missing values in data. Records with missing data are rejected until appropriate codes are applied. Also, none of the covariates allowed for “structural” missing categories. These data sets have been previously validated for quality in home care9 and long-term care.10 Exclusion criteria are outlined in Appendix 1, available at www.cmajopen.ca/content/7/2/E341/suppl/DC1. Baseline assessments were defined as occurring within 14 days of admission to long-term care or home care. As mandated, follow-up RAI-MDS 2.0 and RAI-HC assessments are completed every 90 days or 6 months, respectively, or earlier in the event of major clinical changes. During the study period, some patients had multiple admissions to the home care system; however, only their first episode of care was used for the present analyses.
We used data from 2010 to 2016 for home care clients with a RAI-HC in Ontario, Alberta and British Columbia (all available data) and long-term care residents with a RAI 2.0 in these provinces and the Yukon (all available data). Ontario, Alberta and BC mandate the assessment of home care patients expected to require services for more than 60 days and long-term care residents with stays of 14 days or more. Each assessment includes measuring cognition, mood and behaviour, informal support services, physical functioning and other patient characteristics. The assessments also have multiple embedded scales such as the Activities of Daily Living Hierarchy Scale, the Instrumental Activities of Daily Living Scale,11 the Changes in Health, End-Stage Disease, Signs, and Symptoms Scale (CHESS),12,13 the Depression Rating Scale14,15 and the Cognitive Performance Scale.9,16,17
Assessment times are reported as Time 1 (T1) for baseline assessment and Time 2 (T2) for the follow-up assessment. The CHESS score is a measure of health instability and ranges from 0 to 5, with 0 denoting low instability and 5 denoting high instability/disease severity and greater risk of death. This health instability scale was simplified to 3 different categories corresponding to low, medium and high health instability/disease severity: 0, 1–2, and 3 or more, respectively. 9 We completed sensitivity analyses to test alternative cut-points (0–1, 2–3, ≥ 4), but these resulted in few substantively meaningful changes in the associations reported here. We opted to use 0 as a distinctive first state (rather than 0–1) because 0 designates the absence of any indicators of health instability, a clinically relevant break point. Putting 0 and 1 together mixes residents with no symptoms with those with 1 important symptom.
Statistical methods
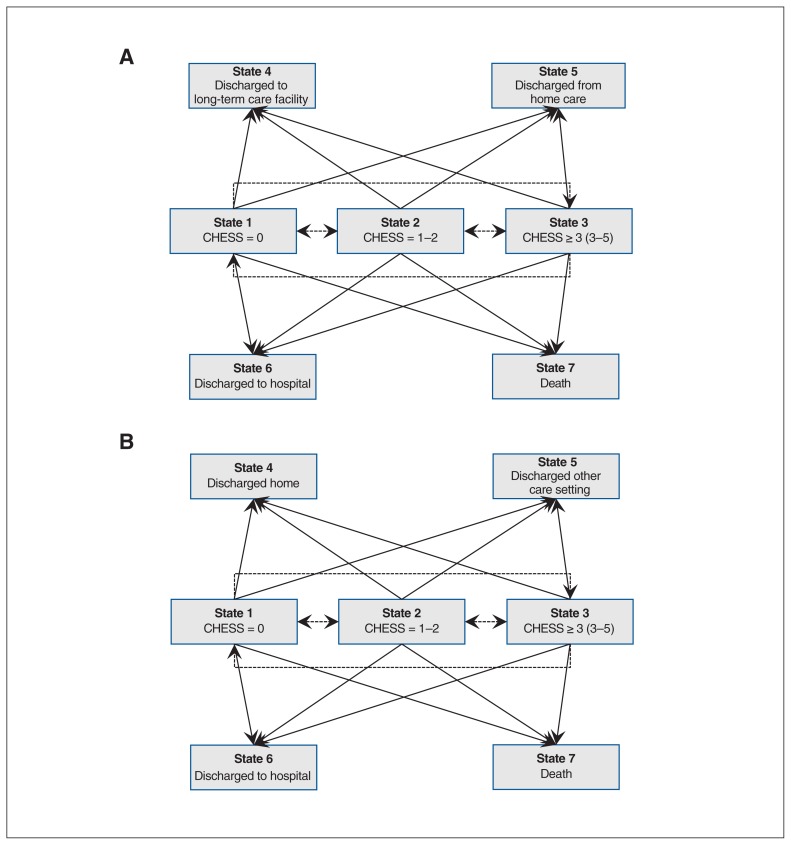
We used methods previously applied by Cook and colleagues18 and summarize them briefly here. Multistate processes are a powerful tool to examine longitudinal changes in multiple health status outcomes over time and identify factors that influence these changes. This allows for the examination of competing risks in models where different outcomes (e.g., death and hospital admission) may be affected by similar risk factors. Figures 1A and 1B are state-space diagrams for home care and long-term care, respectively. Patients in home care or long-term care are initially categorized according to 1 of 3 health instability levels. They can then transition to 1 of 7 possible states: 3 possible health instability states if the patient remains in home care or at a long-term care facility and 4 possible discharge possibilities. From home care, the 4 possible discharge possibilities are discharge to hospital, death, discharge to a long-term care facility and discharge from service, whereas from a long-term care facility the discharge possibilities are discharge to hospital, death, discharge to another setting and discharge to home care. Discharge destinations are so-called absorbing states, because transitioning to 1 of these states defines the end of the particular care episode with home care or the long-term care facility.
Figure 1:
State-space diagram for possible transitions from home care (A) and long-term (B) care in multistate Markov model. At admission to home care (A) or long-term care (B), clients can be in State 1 (CHESS score = 0), State 2 (CHESS score = 1 or 2) or State 3 (CHESS score ≥ 3), with State 3 representing the greatest health instability and State 1 the least health instability. From this initial state, clients who remain in home care (A) or long-term care (B) can improve (e.g., a transition from State 2 to State 1, or a transition from State 3 to State 1 or 2) or can worsen (e.g., transition from State 1 to State 2 or 3, or transition from State 2 to State 3). A client can also transition out of home care (A) from 1 of the 3 initial admission states (State 1, 2 or 3) to 1 of 4 possible discharge possibilities: discharge to a long-term care facility (State 4), discharge from home care (no longer requiring services, State 5), discharge to hospital (State 6) or death (State 7). A long-term care resident (B) can transition out of long-term care from 1 of the 3 initial admission states (State 1, 2 and 3) to 1 of 4 possible discharge possibilities: discharge home (State 4), discharge to another care setting (State 5), discharge to hospital (State 6) or death (State 7). In Figure 1A the broken lines reflect transitions between health states for those remaining in home care. The solid lines reflect transitions to “absorbing states” outside of the home care. In Figure 1B the broken lines reflect transitions between health states within the long-term care facility. The solid lines reflect transitions to “absorbing states” outside of the long-term care facility.
To contribute to the analysis, all cases must have had complete baseline covariate information at the time of entering into home care or a long-term care facility and a completed assessment as per the required schedule (180 d for home care, 90 d for long-term care facilities) until discharge (the 4 absorbing states). In the analyses for recipients of home care we adjusted for home nursing visits, age, sex, marital status, Activities of Daily Living Hierarchy Scale score, Cognitive Performance Scale score, diagnosis (binary variables for chronic obstructive pulmonary disease, pneumonia, diabetes, arthritis, renal failure, urinary tract infection, Alzheimer’s dementia and related dementias, heart failure, cancer and depression), day of stay and functional improvement potential. For long-term care residents, we adjusted for the same covariates as well as facility size and advanced directives (i.e., do not resuscitate, do not hospitalize). We chose covariates on the basis of their expected associations with 1 or more of the outcome states we modelled. We only retained covariates that had significant associations with at least 1 outcome of interest in the models we used.
The statistical analyses, aimed at modelling changes in states, were based on a discrete time nonhomogeneous Markov chain model, using a previously detailed method.18 Patients remained in the longitudinal model until the end of the study period or their first transition out of the care setting they began in. Once they transitioned to an “absorbing state” (e.g., death, hospital admission, discharge home, admission to another care setting) they were dropped from the analyses. Transitions after the first transition to another setting were not considered (e.g., death after discharge to hospital) in these models, which focused on the first transition.
Ethics approval
We obtained research ethics approval from the University of Waterloo’s Office of Research Ethics (ORE no. 18228).
Results
Baseline characteristics in home care and long-term care
Among the 416 709 elderly patients in this study, 254 664 received home care services and 162 045 lived in long-term care facilities between 2010 and 2016 (Table 1, Table 2). Overall, about 60% were women, and about 80% of patients were older than 75 years. Across Canada, 71% of residents in long-term facilities had “do not resuscitate” advanced directives, with the greatest proportion in Alberta at 83% and the lowest in Ontario at 70% (Table 2). Over a quarter of residents in long-term care facilities had “do not hospitalize” directives (Table 2).
Table 1:
Baseline characteristics of 254 664 patients who received home care services
| Covariate/domain | No. (%) of patients; region | ||||
|---|---|---|---|---|---|
| Ontario n = 194 094 |
British Columbia n = 46 359 |
Alberta n = 13 983 |
Yukon n = 228 |
Overall n = 254 664* |
|
| Age group, yr | |||||
| 65–74 | 43 941 (23) | 6592 (14) | 2838 (20) | 69 (30) | 53 440 (21) |
| 75–84 | 83 866 (43) | 18 767 (40) | 5925 (42) | 107 (47) | 108 665 (43) |
| 85–94 | 61 763 (33) | 19 181 (41) | 4885 (35) | 50 (22) | 85 879 (34) |
| ≥ 95 | 4524 (2) | 1819 (4) | 335 (2) | 2 (1) | 6680 (3) |
| Sex | |||||
| Female | 115 723 (60) | 27 749 (60) | 8331 (60) | 122 (54) | 151 925 (60) |
| Marital status | |||||
| Married† | 88 506 (46) | 16 049 (35) | NA | 70 (31) | 104 625 (43) |
| CHESS score | |||||
| 0 | 32 708 (17) | 10 842 (23) | 4642 (33) | 79 (35) | 48 271 (19) |
| 1 | 60 761 (31) | 14 031 (30) | 4186 (30) | 71 (31) | 79 049 (31) |
| 2 | 57 666 (30) | 12 781 (28) | 3111 (23) | 44 (19) | 73 602 (29) |
| 3 | 33 266 (17) | 6284 (14) | 1547 (11) | 24 (11) | 41 121 (16) |
| 4 | 9030 (5) | 2165 (5) | 456 (3) | 9 (4) | 11 660 (5) |
| 5 | 663 (0) | 256 (1) | 41 (0) | 1 (0) | 961 (0) |
| Diagnoses | |||||
| Congestive heart failure | 22 860 (12) | 6763 (15) | 2026 (14) | 18 (8) | 31 667 (12) |
| Chronic obstructive pulmonary disease | 33 603 (17) | 8177 (18) | 2850 (20) | 53 (23) | 44 683 (18) |
| Pneumonia | 6566 (3) | 835 (2) | 447 (3) | 6 (3) | 7854 (3) |
| Diabetes | 51 006 (26) | 10 172 (22) | 3408 (24) | 54 (24) | 64 640 (25) |
| Arthritis | 89 113 (46) | 17 804 (38) | 6617 (47) | 112 (49) | 113 646 (45) |
| Renal infection | 13 803 (7) | 5290 (11) | 1099 (8) | 6 (3) | 20 198 (8) |
| Urinary tract infection | 10 724 (6) | 2322 (5) | 903 (6) | 8 (4) | 13 957 (5) |
| Dementia | 41 128 (21) | 17 234 (37) | 3620 (26) | 51 (22) | 62 033 (24) |
| Depression | 22 388 (12) | 7237 (16) | 1906 (14) | 16 (7) | 31 547 (12) |
| Cancer | 34 531 (18) | 5593 (12) | 1971 (14) | 37 (16) | 42 132 (17) |
| Nurse visits in the last 7 d | 54 906 (28) | 5942 (13) | 4043 (29) | 46 (20) | 64 937 (25) |
| Cognitive Performance Scale score | |||||
| 0 | 75 913 (39) | 11 198 (24) | 6241 (45) | 98 (43) | 93 450 (37) |
| 1 or 2 | 98 933 (51) | 26 766 (58) | 6390 (46) | 108 (47) | 132 197 (52) |
| 3 or 4 | 14 340 (7) | 6612 (14) | 1010 (7) | 21 (9) | 21 983 (9) |
| 5 or 6 | 4908 (3) | 1783 (4) | 342 (2) | 1 (0) | 7034 (3) |
| Activities of Daily Living Hierarchy Scale score | |||||
| 0 | 119 003 (61) | 27 841 (60) | 10 759 (77) | 190 (83) | 157 793 (62) |
| 1 or 2 | 53 350 (27) | 12 647 (27) | 2343 (17) | 28 (12) | 68 368 (27) |
| ≥ 3 | 21 741 (11) | 5871 (13) | 881 (6) | 10 (4) | 28 503 (11) |
| Functional improvements in activities of daily living | |||||
| Yes | 61 273 (32) | 11 045 (24) | 3869 (28) | 73 (32) | 76 260 (30) |
Note: CHESS = Changes in Health, End-Stage Disease, Signs, and Symptoms Scale; NA = not applicable.
Unless indicated otherwise.
n = 240 680.
Table 2:
Baseline characteristics of 162 045 patients living in long-term care facilities
| Covariate/domain | No. (%) of patients; region | |||
|---|---|---|---|---|
| Ontario n = 113 552 |
British Columbia n = 22 732 |
Alberta n = 25 761 |
Overall n = 162 045* |
|
| Age group, yr | ||||
| 65–74 | 12 317 (11) | 2409 (11) | 2980 (12) | 17 706 (11) |
| 75–84 | 41 164 (36) | 7783 (34) | 8874 (34) | 57 821 (36) |
| 85–94 | 52 842 (47) | 10 771 (47) | 11 844 (46) | 75 457 (47) |
| ≥ 95 | 7229 (6.4) | 1769 (8) | 2063 (8) | 11 061 (7) |
| Sex | ||||
| Female | 74 023 (65) | 14 407 (63) | 15 966 (62) | 104 396 (64) |
| Marital status | ||||
| Married† | 35 651 (31) | 6666 (29) | NA | 42 317 (31) |
| Diagnoses | ||||
| Congestive heart failure | 16 504 (15) | 3626 (16) | 4701 (18) | 24 831 (15) |
| Chronic obstructive pulmonary disease | 18 375 (16) | 3374 (15) | 5291 (21) | 27 040 (17) |
| Pneumonia | 2103 (2) | 347 (2) | 566 (2) | 3016 (2) |
| Diabetes | 29 677 (26) | 4739 (21) | 6079 (24) | 40 495 (25) |
| Arthritis | 46 807 (41) | 6753 (30) | 9371 (36) | 62 931 (39) |
| Renal infection | 11 791 (10) | 2769 (12) | 2755 (11) | 17 315 (11) |
| Urinary tract infection | 9758 (9) | 1883 (8) | 3108 (12) | 14 749 (9) |
| Dementia | 70 244 (62) | 14 521 (64) | 15 597 (61) | 100 362 (62) |
| Depression | 25 913 (23) | 4308 (19) | 7223 (28) | 37 444 (23) |
| Cancer | 12 060 (11) | 2246 (10) | 2666 (10) | 16 972 (11) |
| CHESS score | ||||
| 0 | 55 901 (49) | 13 431 (59) | 9081 (35) | 78 413 (48) |
| 1 | 36 206 (32) | 5882 (26) | 8034 (31) | 50 122 (31) |
| 2 | 15 305 (13) | 2431 (11) | 5425 (21) | 23 161 (14) |
| 3 | 4552 (4) | 735 (3) | 2257 (9) | 7544 (5) |
| 4 | 1363 (1) | 213 (1) | 877 (3) | 2453 (2) |
| 5 | 225 (0) | 40 (0) | 87 (0) | 352 (0) |
| Physician examination in last 14 d | 96 057 (85) | 11 708 (52) | 21 038 (82) | 128 803 (80) |
| Cognitive Performance Scale score | ||||
| 0 | 14 444 (13) | 2134 (9) | 1930 (7) | 18 508 (11) |
| 1 or 2 | 41 830 (37) | 8100 (36) | 8214 (33) | 58 144 (36) |
| 3 or 4 | 46 466 (41) | 9464 (42) | 11 640 (45) | 67 570 (42) |
| 5 or 6 | 10 812 (10) | 3034 (13) | 3977 (15) | 17 823 (11) |
| Activities of Daily Living Hierarchy Scale score | ||||
| 0 | 5238 (4.6) | 2298 (10.1) | 517 (2) | 8053 (5) |
| 1 or 2 | 29 518 (26) | 8157 (36) | 5441 (21) | 43 116 (27) |
| ≥ 3 | 78 796 (69) | 12 277 (54) | 19 803 (77) | 110 876 (68) |
| Functional improvements in activities of daily living | ||||
| Yes | 27 578 (24) | 5930 (26) | 6890 (27) | 40 398 (25) |
| Advanced directive | ||||
| Do not hospitalize | 26 679 (25) | 4557 (24) | 4664 (31) | 35 900 (26) |
| Do not resuscitate | 74 464 (70) | 13 982 (72) | 12 360 (83) | 100 806 (71) |
| Facility size, no. of beds | ||||
| 1–49 | 3764 (3) | 1426 (6) | 2468 (10) | 7658 (5) |
| 50–99 | 27 656 (24) | 7046 (31) | 6363 (25) | 41 065 (25) |
| 100–149 | 57 147 (50) | 6559 (29) | 11 123 (43) | 74 829 (46) |
| ≥ 150 | 24 985 (22) | 7701 (34) | 5807 (23) | 38 493 (24) |
Note: CHESS = Changes in Health, End-Stage Disease, Signs, and Symptoms Scale; NA = not applicable.
Unless indicated otherwise.
n = 136 284.
Characteristics influencing mortality and hospital admissions
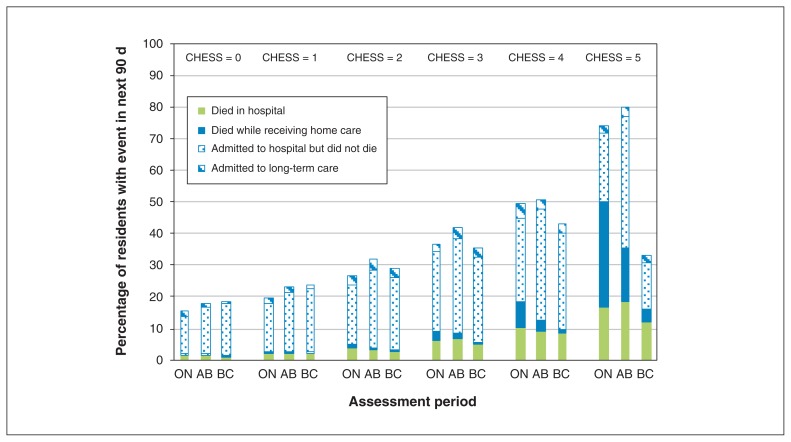
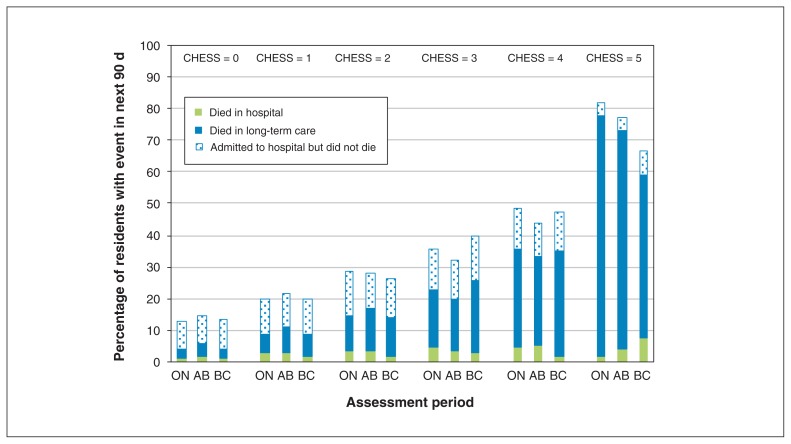
Figures 2 and 3 show the unadjusted 6-month rates of hospital admission, death and long-term care placement for home care clients and the unadjusted 90-day hospital admission and death rates for patients in long-term care, by health instability score and province. In both settings, greater health instability was consistently associated with higher hospital admission and mortality rates (except for the highest health instability score in home care in BC). However, there were regional variations in these rates within CHESS scores. The results were remarkably consistent regardless of the cutpoints chosen. Indeed, we tested the effects of alternative cutpoints (0–1, 2–3, ≥ 4) but found only minor differences in interpretation. Out of the 36 associations we examined, 30 did not change meaningfully in direction or magnitude. Only 6 changed meaningfully in magnitude but 4 of these became nonsignificant. Two associations went from nonsignificant to significant and were protective.
Figure 2:
Unadjusted rates of transitions from home care by CHESS score and by province. The figure depicts the percentage of home care clients who were admitted to long-term care, who died (at home or in hospital) or who were admitted to hospital but did not die there within 6 months of intake assessment, by CHESS score at intake, in Ontario (ON), Alberta (AB) and British Columbia (BC). Note: CHESS = Changes in Health, End-Stage Disease, Signs, and Symptoms Scale.
Figure 3:
Unadjusted rates of transitions from long-term care by CHESS score and by province. The figure depicts the percentage of residents who died (in a long-term care facility or hospital) or were admitted to hospital but did not die there within 90 days of admission assessment, by CHESS score at admission, in Ontario (ON), Alberta (AB) and British Columbia (BC). Note: CHESS = Changes in Health, End-Stage Disease, Signs, and Symptoms Scale.
We initially examined individual characteristics to determine if they explained some of the differences observed in transitions between health instability states, as well as discharges including hospital admission and mortality. Several of the covariates were associated with differential transition rates. For example, increased age consistently increased the risk of death, irrespective of disease severity, in both long-term care (from 1.4 to 4.0 at the lowest health instability, when compared with the lowest age range) and home care services (from 1.0 to 2.8 at the lowest health instability, when compared with the lowest age range; Supplemental Table 1 in Appendix 1). Diagnoses of chronic obstructive pulmonary disease (at the lower health instabilities) and pneumonia (especially in long-term care) also increased the risk of death and transfer to hospital (Supplemental Table 1 in Appendix 1).
Provincial variations in home care and long-term care
We next examined whether we could observe differences in care patterns across the provinces, with emphasis on mortality and hospital admissions. We compared care patterns in Alberta and BC with those in Ontario. Irrespective of severity of illness, patients in home care in Alberta had greater odds of being admitted to hospital than patients in Ontario (Table 3). Indeed, at the lowest health instability, the adjusted odds ratio (OR) was 2.08 (95% confidence interval [CI] 1.92–2.24) while at high health instability the adjusted OR was 3.77 (95% CI 3.24–4.40) in Alberta compared with Ontario (Table 3). Similarly, in BC, patients in home care had increased odds of being admitted to a hospital irrespective of the initial severity of illness (Table 3); at the lowest health instability, the adjusted OR was 1.46 (95% CI 1.39–1.54). Adjusted ORs for mortality were higher in Alberta and lower in BC, when compared with Ontario (Table 3). As noted, these effects were adjusted for about 20 other covariates including demographic, diagnostic and clinical indicators. In both Alberta and BC, the odds ratios for long-term care admissions from home care were considerably lower than in Ontario.
Table 3:
Effect of province on home care transitions: odds of transition from baseline health instability score in home care to another health instability score (if the patient stayed in home care) or to hospital, death, long-term care or other setting at 6-mo follow-up
| Region | Adjusted odds ratio (95% CI) for transition at 6-mo follow-up (T2)* | ||||||
|---|---|---|---|---|---|---|---|
| Remained in home care; health instability score† | Admitted to hospital | Died | Admitted to long-term care | Discharged to other setting‡ | |||
| Low (0) | Medium (1, 2) | High (≥ 3) | |||||
| Alberta (Ref. = Ontario); health instability score at baseline (T1)† | |||||||
| Low (0) | – | 0.82 (0.75–0.90) | NS | 2.08 (1.92–2.24) | 1.80 (1.48–2.20) | 0.26 (0.18–0.36) | 0.67 (0.62–0.72) |
| Medium (1, 2) | 1.85 (1.71–2.00) | – | NS | 2.44 (2.30–2.59) | 2.11 (1.84–2.42) | 0.42 (0.35–0.49) | 1.14 (1.07–1.21) |
| High (≥ 3) | 4.83 (3.82–6.12) | 1.80 (1.51–2.16) | – | 3.77 (3.24–4.40) | 2.63 (2.09–3.32) | NS | 2.67 (2.26–3.16) |
| British Columbia (Ref. = Ontario); health instability score at baseline (T1)† | |||||||
| Low (0) | – | 1.44 (1.38–1.51) | 1.98 (1.80–2.18) | 1.46 (1.39–1.54) | 0.46 (0.37–0.56) | 0.55 (0.48–0.62) | 0.31 (0.30–0.33) |
| Medium (1, 2) | 1.67 (1.60–1.73) | – | 1.45 (1.39–1.52) | 1.39 (1.35–1.43) | 0.54 (0.49–0.60) | 0.76 (0.72–0.81) | 0.62 (0.60–0.64) |
| High (≥ 3) | 3.13 (2.81–3.48 | 1.39 (1.30–1.48) | – | 1.28 (1.21–1.35) | 0.39 (0.34–0.45) | 0.85 (0.78–0.93) | 1.53 (1.44–1.63) |
Note: CI = confidence interval, NS = not significant, Ref. = reference category.
Multistate transition models were adjusted for home nursing visits, age, sex, marital status, Activities of Daily Living Hierarchy Scale score, Cognitive Performance Scale score, diagnosis (binary variables for chronic obstructive pulmonary disease, pneumonia, diabetes, arthritis, renal failure, urinary tract infection, Alzheimer’s dementia and related dementias, heart failure, cancer, depression), day of stay and functional improvement potential.
Health instability was measured with the Changes in Health, End-Stage Disease, Signs, and Symptoms Scale (CHESS); higher scores indicate greater instability.
Other settings typically involved discontinuation of home care services (i.e., discharge from the program).
In long-term care (Table 4), patients in Alberta had less than half the odds of going to hospital compared with patients in Ontario, regardless of baseline severity of illness. For example, at high health instability, the adjusted OR was 0.39 (95% CI 0.34–0.43). The situation was very similar in BC, with an adjusted OR of 0.33 (95% CI 0.29–0.37) of being admitted to hospital when compared with Ontario (Table 4). Mortality rates were more nuanced, with higher mortality at low health instability in Alberta and BC but lower adjusted ORs at high health instability scores (Table 4).
Table 4:
Effect of province on long-term care facility transitions (ref = Ontario): odds of transition from baseline health instability score in long-term care to another health instability score (if the patient remained in the same long-term care facility) or to hospital, death, another setting or home at 90-d follow-up
| Region | Adjusted odds ratio (95% CI) for transition at 90-d follow-up (T2)* | ||||||
|---|---|---|---|---|---|---|---|
| Remained in the same long-term care facility; health instability score† | Admitted to hospital | Died | Discharged to other setting‡ | Discharged home | |||
| Low (0) | Medium (1, 2) | High (≥ 3) | |||||
| Alberta (Ref. = Ontario); health instability score at baseline (T1)† | |||||||
| Low (0) | – | 1.43 (1.37–1.48) | 2.02 (1.83–2.23) | 0.38 (0.35–0.40) | 1.21 (1.09–1.36) | 2.31 (1.93–2.77) | NS |
| Medium (1, 2) | 0.96 (0.92–0.99) | – | 1.46 (1.38–1.54) | 0.39 (0.37–0.41) | 0.93 (0.87–0.98) | 1.46 (1.24–1.71) | NS |
| High (≥ 3) | 0.76 (0.66–0.87) | 0.77 (0.71–0.85) | – | 0.39 (0.34–0.43) | 0.52 (0.47–0.58) | NS | NS |
| British Columbia (Ref. = Ontario); health instability score at baseline (T1)† | |||||||
| Low (0) | – | 0.84 (0.77–0.93) | 0.84 (0.77–0.93) | 0.44 (0.42–0.46) | 1.39 (1.28–1.51) | 0.74 (0.62–0.90) | 0.50 (0.42–0.60) |
| Medium (1, 2) | NS | – | 1.15 (1.08–1.22) | 0.51 (0.48–0.53) | 1.35 (1.27–1.43) | NS | 0.55 (0.43–0.70) |
| High (≥ 3) | 0.40 (0.34–0.48) | 0.55 (0.50–0.61) | – | 0.33 (0.29–0.37) | 0.58 (0.52–0.65) | 0.51 (0.31–0.83) | 0.52 (0.27–0.99) |
Note: CI = confidence interval, NS = not significant, Ref. = reference category.
Multistate transition models adjusted for physician visits, age, gender, marital status, ADL Hierarchy scale score, Cognitive Performance Scale score, diagnosis (binary variables for Chronic Obstructive Pulmonary Disease, pneumonia, diabetes, arthritis, renal failure, Urinary tract infection, Alzheimer’s Dementia and Related Dementias, heart failure, cancer, depression), facility size, Advanced directives (i.e., do not resuscitate, do not hospitalize), day of stay, functional improvement potential.
Health instability was measured with the Changes in Health, End-Stage Disease, Signs, and Symptoms Scale (CHESS); higher scores indicate greater instability.
Other settings for transitions from nursing homes included discharges to other nursing homes, assisted living or retirement homes.
In Alberta, we noted considerably greater odds of transfers to other care settings (2.31 [95% CI 1.93–2.77] at low health instability; Table 4) compared with Ontario. Those odds were generally lower in BC. Finally, being discharged home from long-term care was a rare event, but it was least likely to occur in BC after adjusting for other covariates (Table 4).
Interpretation
We identified substantial interprovincial variations in hospital admissions for patients using home care services or living in long-term care. In Ontario, long-term care residents had more than twice the odds of being transferred to hospital, independent of all other factors, compared with those in Alberta and BC. In contrast, people making use of home care services in Alberta and BC were more likely than those in Ontario to be admitted to hospital regardless of their underlying severity of illness and other factors. In both Alberta and BC, home care clients were also less likely to be admitted to long-term care facilities than in Ontario.
Our multistate model approach allowed us to simultaneously consider patients who transition from one health state at baseline to another state (better or worse in care setting, transferred to another care setting, or deceased), all the while considering a number of important individual, facility and system characteristics. Although certain conditions, such pneumonia, were associated with increased transfer to hospital from either long-term care or home care services, these differences did not explain the large regional variations. Indeed, the health system itself, that is the province, remained one of the most important drivers of decisions to transfer patients. Local practice patterns played an important role and suggest that system-based considerations such as the distribution of resources were dominant factors in determining care and access to services rather than diagnostic and clinical factors or patient desires. Indeed, Alberta has substantially increased its emphasis on assisted living as a form of residential care so it is not surprising that we noted more transfers to other care settings from long-term care. Our results are in agreement with previous work suggesting that care decisions such as hospital admissions are being driven by differing care patterns and resources rather than aligning services with the care needs of patients.19,20 This result is also consistent with previous reports that state Medicaid policies influence transfers to hospitals from long-term care facilities.21 Provincial differences in nursing home transfer rates have been previously reported in Ontario.22 However, transfers to acute care institutions have not been widely evaluated across major health systems. We were not able to document comparisons across systems that considered adjusted comparisons to ensure that any number of patient-related variables did not explain the large variations.
One of the strengths of this study was the use of multistage modelling to examine a complex system-wide process. Our approach may be used to monitor the implementation of system-wide initiatives using transfer rates and mortality as outcomes. Indeed, the analytic approach may be adapted to facilitate stepwise comparisons of interventions. It also demonstrates the importance of taking into account confounding variables related to differences in patient characteristics that may mask the true magnitude of regional differences.
Limitations
Despite the use of advanced modelling techniques, our approach may not have fully captured the nuances of complex care and systems. The use of administrative data on hospital admissions may have resulted in misclassifications of diagnoses or transitions. However, the interRAI assessments are based on direct clinical observations by trained health professionals done at the point of care. Further, data are not continuous in this study and represent snapshots at different points in time. These snapshots may not be an accurate reflection of patient status at the moment that transfer decisions were made. However, we do not expect that these limitations could have accounted for the large differences we observed between provinces in rates of transfer to hospitals or mortality.
Conclusion
Our study highlights substantial variations in transfer rates, suggesting organizational concerns or care gaps. At a population level, a doubling in the odds of transfers in 1 jurisdiction compared with another represents sending a large number of patients to hospital. Inappropriate transfers to acute care settings can be very costly monetarily and can strain limited health care resources. For long-term care residents who are known to have a limited life expectancy, aggressive care may be completely inappropriate and not even respect their wishes. Iatrogenic complications from the acute care episode may also reduce quality of life. On the other hand, it is possible that provinces with half the number of transfers are not providing acute medical services to patients who would benefit from such care, particularly patients with lower severity of illness. In either scenario, services could be misaligned with patients’ wishes or needs. Future work should focus on the reasons underlying these differences. Health systems should strive to ensure that health care delivery meets the actual needs and wishes of vulnerable older adults.
Supplementary Material
Acknowledgements
The authors thank Jonathan Chen, Micaela Jantzi and Veronica Jung for their work on the data analysis as well as Julie Koreck and Marie-Noël Nadeau for administrative assistance.
Footnotes
Competing interests: Anne Morinville became an employee of Novartis after this article was submitted for publication but before revisions were undertaken. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Paul Hébert, John Hirdes and George Heckman contributed to the initial conceptualization and design of the study. John Hirdes oversaw data analysis. All authors contributed equally to data interpretation. Paul Hébert and Anne Morinville drafted the manuscript; Andrew Costa, George Heckman and John Hirdes revised it critically for important intellectual content. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by a Strategic Impact Grant from the Canadian Frailty Network (SIG2014F-31). George Heckman is supported by the Schlegel Chair in Geriatric Medicine at McMaster University. Andrew Costa is supported by the Schlegel Chair in Clinical Epidemiology and Aging and McMaster University.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/7/2/E341/suppl/DC1.
References
- 1.Heckman GA, Hillier L, Manderson B, et al. Developing an integrated system of care for frail seniors. Healthc Manage Forum. 2013;26:200–8. doi: 10.1016/j.hcmf.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Adams LY, Koop P, Quan H, et al. A population-based comparison of the use of acute healthcare services by older adults with and without mental illness diagnoses. J Psychiatr Ment Health Nurs. 2015;22:39–46. doi: 10.1111/jpm.12169. [DOI] [PubMed] [Google Scholar]
- 3.Gruneir A, Bell CM, Bronskill SE, et al. Frequency and pattern of emergency department visits by long-term care residents — a population-based study. J Am Geriatr Soc. 2010;58:510–7. doi: 10.1111/j.1532-5415.2010.02736.x. [DOI] [PubMed] [Google Scholar]
- 4.Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med. 2002;39:238–47. doi: 10.1067/mem.2002.121523. [DOI] [PubMed] [Google Scholar]
- 5.Gruneir A, Silver MJ, Rochon PA. Emergency department use by older adults: a literature review on trends, appropriateness, and consequences of unmet health care needs. Med Care Res Rev. 2011;68:131–55. doi: 10.1177/1077558710379422. [DOI] [PubMed] [Google Scholar]
- 6.Pines JM, Mullins PM, Cooper JK, et al. National trends in emergency department use, care patterns, and quality of care of older adults in the United States. J Am Geriatr Soc. 2013;61:12–7. doi: 10.1111/jgs.12072. [DOI] [PubMed] [Google Scholar]
- 7.Chan BTB, Schull MJ, Schultz SE. Emergency department services in Ontario 1993–2000. Toronto: Institute for Clinical Evaluative Sciences; 2001. [Google Scholar]
- 8.Fayyaz J, Khursheed M, Umer Mir M, et al. Pattern of emergency department visits by elderly patients: study from a tertiary care hospital, Karachi. BMC Geriatr. 2013;13:83. doi: 10.1186/1471-2318-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landi F, Tua E, Onder G, et al. Minimum data set for home care: a valid instrument to assess frail older people living in the community. Med Care. 2000;38:1184–90. doi: 10.1097/00005650-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Hirdes JP, Poss JW, Caldarelli H, et al. An evaluation of data quality in Canada’s Continuing Care Reporting System (CCRS): secondary analyses of Ontario data submitted between 1996 and 2011. BMC Med Inform Decis Mak. 2013;13:27. doi: 10.1186/1472-6947-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris JN, Caldarelli H, Berg K, et al. Outcome measures for use with home care clients. Can J Aging. 2000;19:87–105. [Google Scholar]
- 12.Hirdes JP, Frijters DH, Teare GF. The MDS-CHESS scale: a new measure to predict mortality in institutionalized older people. J Am Geriatr Soc. 2003;51:96–100. doi: 10.1034/j.1601-5215.2002.51017.x. [DOI] [PubMed] [Google Scholar]
- 13.Hirdes JP, Poss JW, Mitchell L, et al. Use of the interRAI CHESS scale to predict mortality among persons with neurological conditions in three care settings. PLoS One. 2014;9:e99066. doi: 10.1371/journal.pone.0099066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrows AB, Morris JN, Simon SE, et al. Development of a minimum data set-based depression rating scale for use in nursing homes. Age Ageing. 2000;29:165–72. doi: 10.1093/ageing/29.2.165. [DOI] [PubMed] [Google Scholar]
- 15.Szczerbińska K, Hirdes JP, Zyczkowska J. Good news and bad news: depressive symptoms decline and undertreatment increases with age in home care and institutional settings. Am J Geriatr Psychiatry. 2012;20:1045–56. doi: 10.1097/JGP.0b013e3182331702. [DOI] [PubMed] [Google Scholar]
- 16.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49:M174–82. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 17.Morris JN, Howard EP, Steel K, et al. Updating the Cognitive Performance Scale. J Geriatr Psychiatry Neurol. 2016;29:47–55. doi: 10.1177/0891988715598231. [DOI] [PubMed] [Google Scholar]
- 18.Cook RJ, Berg K, Lee KA, et al. Rehabilitation in home care is associated with functional improvement and preferred discharge. Arch Phys Med Rehabil. 2013;94:1038–47. doi: 10.1016/j.apmr.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Ouslander JG, Berenson RA. Reducing unnecessary hospitalizations of nursing home residents. N Engl J Med. 2011;365:1165–7. doi: 10.1056/NEJMp1105449. [DOI] [PubMed] [Google Scholar]
- 20.Ackerly DC, Grabowski DC. Post-acute care reform — beyond the ACA. N Engl J Med. 2014;370:689–91. doi: 10.1056/NEJMp1315350. [DOI] [PubMed] [Google Scholar]
- 21.Grabowski DC, Stewart KA, Broderick SM, et al. Predictors of nursing home hospitalization: a review of the literature. Med Care Res Rev. 2008;65:3–39. doi: 10.1177/1077558707308754. [DOI] [PubMed] [Google Scholar]
- 22.Gruneir A, Bronskill SE, Newman A, et al. Variation in emergency department transfer rates from nursing homes in Ontario, Canada. Healthc Policy. 2016;12:76–88. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.