Abstract
As average life expectancy continues to rise in the developed world, age associated pathologies are increasing in prevalence. The hallmarks of cardiac aging include cardiomyocyte loss, fibrosis and hypertrophy, all of which contribute to an increased incidence of cardiac disease. At the molecular level, cellular aging is characterized by increased ROS production, mitochondrial dysfunction and the accumulation of damaged proteins and organelles. Cardiomyocytes and other senescent cell types rely upon autophagy, a lysosome-mediated degradation pathway, to remove potentially toxic protein aggregates and damaged organelles from the cellular milieu. However, increasing lines of evidence point to an age-associated decrease in cardiomyocyte autophagy, with predictably negative consequences for cardiac functionand health. Conversely, stimulation of autophagy has been shown to improve cellular health and cardiac function, and to increase lifespan in numerous model organisms. Clearly, autophagy represents a critical pathway for cellular vitality, as well as a promising therapeutic target for the treatment of age related cardiac pathologies. In this review, we will discuss the mechanism of autophagy and its regulation in the cell, the role of autophagy in the aging heart, and how the autophagy pathway might be targeted to improve cardiac health.
The Effect of Aging on Cardiac Structure and Function
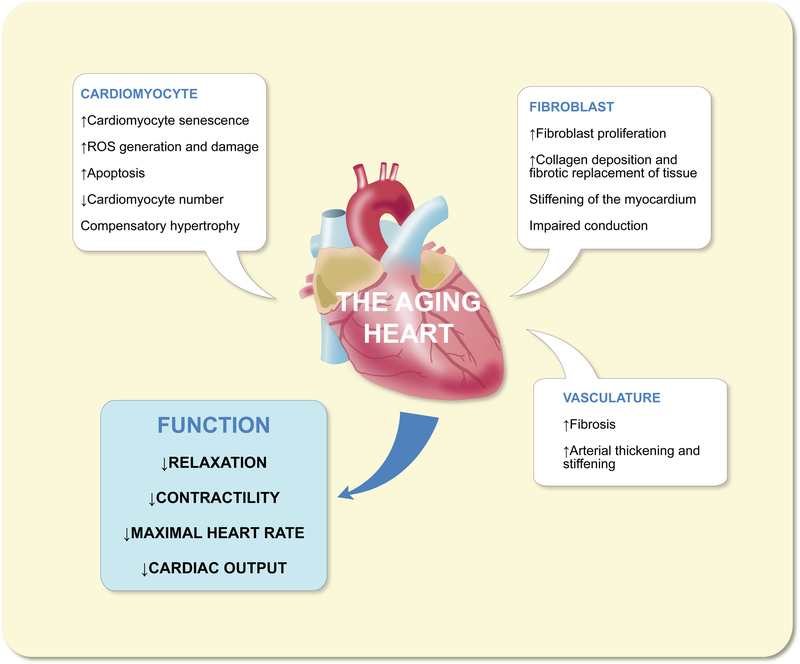
With the onset of advanced age, both the heart and vasculature are subject to significant changes in composition and structure, to the detriment of cardiovascular function (Fig. 1). The structural hallmarks of cardiac aging include left ventricularhypertrophy, decline in cardiomyocyte number and fibroblast proliferation, resulting in increased fibrotic tissue area.1,2 The net result of these changes is a decrease in LV relaxation and diastolic function, in addition to diminished contractility and ejection fraction. Furthermore, reduced activity of sinoatrial node myocytes coupled with impaired conduction of electrical impulses due to fibrosis produces a decrease in maximal heart rate, further diminishing cardiac output in the aging heart.3
Figure 1 –
Structural and functional characteristics of the aging heart
The age-associated decline in cardiac myocyte number is precipitated by multiple factors. Foremost, cardiomyocyte senescence increases with age, with annual rates of turnover decreasing from just 1% in early age, to 0.45% in advanced age.4 With such limited capacity for self-renewal, cardiomyocytes lost through standard wear and tear are difficult to replace, making a decrease in number over time inevitable. This is further compounded in advanced age as cardiomyocytes become more sensitive to stressors, including ROS produced by the mitochondria, leading to increased rates of apoptosis.5 In response to the loss of cardiomyocytes, fibroblasts proliferate to fill vacant space with collagen, resulting in fibrosis and a progressive stiffening of the myocardium.
The vasculature is not immune from the effects of aging. Notably, aging arteries increase in both thickness and stiffness, elevating systolic pressure. This is significant, because the health and vitality of the myocardium is inextricably linked to that of the vasculature.6
The cumulative effect of the above-listed molecular and structural changes brought about by age on the cardiovascular system is to diminish both systolic and diastolic function and ultimately to decrease cardiac output. Under these circumstances, the heart seeks to make up for the deficit in function via compensatory hypertrophy, with individual cardiomyocytes increasing in size to increase muscle mass as a whole. While this process is initially adaptive, temporarily increasing cardiac output, hypertrophy is not viable in the long term, becoming maladaptive overtime, eventually leading to decompensation and heart failure.7
Molecular Mechanisms of Aging in the Heart
The human heart is an extremely hard working organ, consuming in the order of 5–6kg of ATP per day to supply the body with sufficient quantities of oxygenated blood.8 Around 95% of the ATP used by the heart is generated by mitochondrial oxidative phosphorylation; for this reason, around 40% of the cytosolic volume in a cardiomyocyte is occupied by mitochondria.9 Since mitochondria are the major source of ROS in the cell, cardiomyocytes are at unique risk of ROS accumulation and damage compared to most other cells of the body. The amount of ROS produced by individual mitochondria is inversely linked to the health of the organelle, which declines with age. Mitochondria with defects in the electron transport chain machinery will produce significantly more ROS as a consequence of electron leakage, than their healthier counterparts. Imbalances in levels of ROS producing proteins and antioxidant enzymes further increase ROS levels in aging mitochondria.10 Accumulated ROS promotes a negative feedback loop in the mitochondria, whereby ROS induced mtDNA damage precipitates mtDNA mutations in key OXPHOS components, which in turn increase ROS generation.11 As a result ROS accumulation is greatly enhanced in aging cells and tissues of the body.
Studies using the mitochondria targeted catalase (mCAT) transgenic mouse model convincingly demonstrate the contribution of mitochondrial-derived ROS to the agingprocess. Mitochondrial oxidative damage, as measured by mitochondrial protein carbonyls and mtDNA deletions were attenuated in the skeletal muscle and hearts of aged mCAT mice, relative to WT controls.12 Consequently, cardiac function was vastly improved in aged mCAT mice compared to WT. Left ventricular hypertrophy, fibrosis, and decline in systolic and diastolic function observed in aged WT mice were assuaged by mCAT overexpression in the transgenic group.5 Overall, these effects contributed to a 17 21% increase in lifespan in mCAT mice compared to WT.12
The contribution of mtDNA damage to the propagation of negative cycles of mitochondrial ROS damage has been demonstrated by the ‘mtDNA-mutator’, or ‘Polgm/m’ mouse model. These mice harbor a homozygous mutation in mitochondrial polymerase gamma, rendering it proofreading deficient and prone to generating point mutations and deletions each time the mitochondrial genome is copied.13,14 The lifespan of Polgm/m mice is around half that of WT littermates. The increased mutational burden in Polgm/m mice is responsible for a host of age-associated phenotypes beginning around 9 months, including weight loss, hair loss, kyphosis, and osteoporosis. Mechanistically, one explanation for the advanced aging of tissues and organs in the POLG mice is an increase in apoptosis, driven by mutation of the mitochondrial genome. This would explain why tissues and organs with low rates of cellular renewal, such as the heart, are particularly afflicted in the Polgm/m mouse. Following an early stage compensatory LV hypertrophy, Polgm/m mice progress to cardiomyopathy by 13–14 months of age.15 This cardiac phenotype was partially rescued by crossing Polgm/m mice with mCAT transgenic mice; once again emphasizing the negative feedback loop between mtDNA mutation and ROS generation.15 It should be noted, however, that one key shortcoming of both the Polgm/mand mCAT transgenic models, at least for the study of cardiac aging, is that neither is cardiac specific. It is therefore difficult to discount the contribution of mtDNA damage and ROS in other tissues, especially the vasculature, to the cardiac phenotype observed in these mice.
Autophagy and Mitophagy
The efficient removal and replacement of damaged proteins and organelles is essential to maintain cellular homeostasis and to avoid cell death. This is especially true for long-lived cells such as neurons and cardiomyocytes. Additionally, cardiomyocytes are at a greater risk of incurring oxidative damage to their proteins and organelles due to elevated levels of mitochondria derived ROS. Autophagy is an evolutionarily conserved process, by which proteins and organelles are removed from the cell via degradation. This mechanism serves two key purposes; firstly to remove potentially toxic molecules and organelles from the cell. Secondly, autophagy acts as a cellular recycling program during conditions of nutrient deprivation, reclaiming amino acids, lipids and other molecular building blocks liberated from substrates. Autophagy substrates are delivered to the ysosome, wherein lysosomal acidic hydrolases conduct the task of degradation. Three types of autophagy have been classified: macroautophagy, microautophagy and chaperone-mediated autophagy. The finer points of each of these autophagy mechanisms have been reviewed in great depth elsewhere.16–18 For the purposes of this review we will primarily focus on macroautophagy, hereafter referred to as ‘autophagy’.
The Mechanism of Autophagy
The following is a deliberately simplistic overview of autophagy. Autophagy is a complex process involving more than 30 genes; while a fine-grained mechanistic description of autophagy is beyond the scope of this review, such information can be found in a number of excellent reviews.16–19
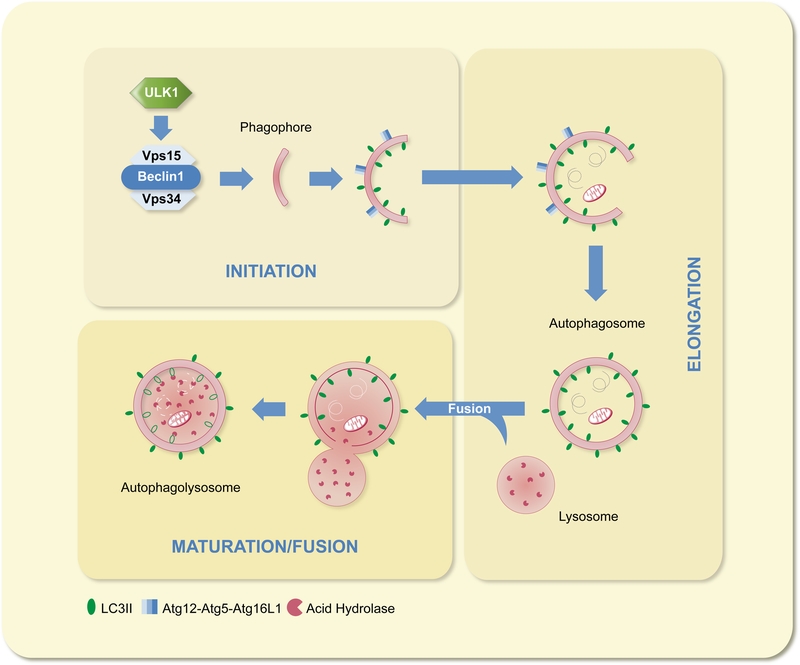
Autophagy consists of three discrete steps: initiation, elongation and maturation/fusion (Fig. 2). Initiation involves the nucleation and formation of a vesicular sac, known as the phagophore. The membrane source from which the phagophore is derived is yet to be determined and a matter of contention in the field. Current evidence points to the ER, mitochondria and plasma membrane as potential contributors.20–22 It is also possible that the membrane source may vary depending on the site of autophagosome formation in the cell in addition to the target substrate. Initiation begins with the de-repression of Unc-51 like kinase 1 (ULK1), which is maintained in the inactive, phosphorylated state (pULKI-Ser-757) by mammalian target of rapamycin (mTOR).23 Inhibition of mTOR allows ULK1 to activate a Class III PI3K complex, consisting of Beclin-1, Vps15 and Vps34. Once active, the PI3K complex initiates phagophore nucleation with assistance from other autophagy proteins.
Figure 2 –
Summary of the stages of autophagy
Elongation, the second step of autophagy, is reliant upon ubiquitin-like conjugation systems. The first of which is the formation of the Atg12-Atg5-Atg16L1 complex. This complex is essential for pre-autophagosomal membrane elongation, and also takes part in LC3-II formation, prior to dissociation from the fully formed autophagosome.24 The second ubiquitin-like reaction facilitates the two-step conversion of LC3 into LC3-II. In the first step, Atg4 cleaves LC3 at its C-terminus to generate LC3-I. Atg3 and Atg7 then promote the lipidation of LC3-I to produce LC3-II.17 LC3-II is incorporated into both facesof the autophagosomal membrane and is essential for pre-autophagosome elongation and cargo recognition.25,26
Once the pre-autophagic membrane has fused to form an autophagosome, engulfing its cargo in the process, the maturation/fusion phase can begin. Microtubules play a key role here by transporting autophagosomes towards their final destination at the lysosome. In fact, autophagy can be blocked via the disruption of microtubules or by inhibiting dynein.27 Autophagosomes can also fuse with late endosomes on their way towards the lysosome, a process requiring numerous accessory proteins including ESCRT, SNAREs and Rabs.28 However, why endosomal fusion occurs with only some autophagosomes and not others is currently unknown. Finally, the fully mature autophagosomes fuse with the lysosome resulting in the formation of autophagolysosomes. Upon fusion, the contents of the autophagosome, in addition to the inner membrane leaflet will be degraded by lysosomal acidic hydrolases.19
Mitophagy
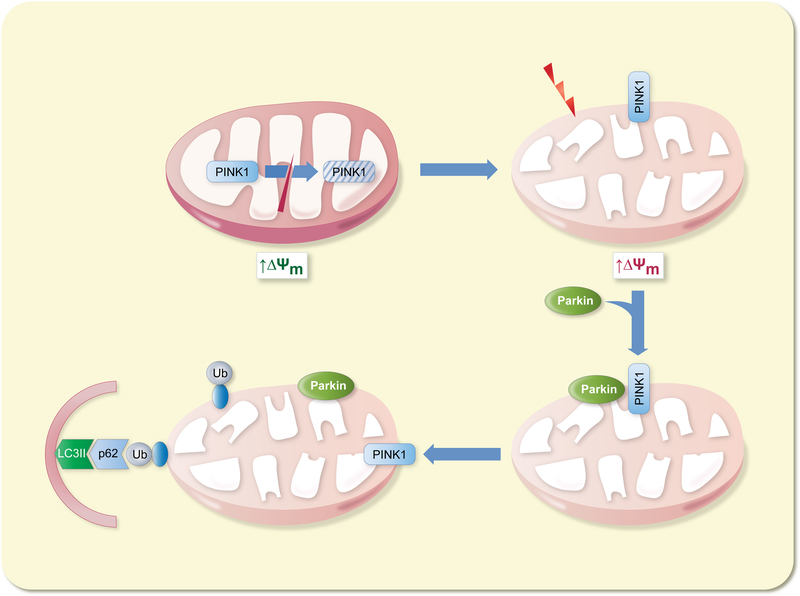
Mitophagy is a specialized form of autophagy, dealing in the select removal of unwanted or dysfunctional mitochondria from the cell (Fig. 3). The PINK1 kinase plays a key role in the identification of damaged mitochondria. Under normal conditions, newly synthesized PINK1 is imported into mitochondria where it is subjected to proteolytic cleavage and subsequent degradation.29 Following loss of mitochondrial membrane potential (ΔΨm), PINK1 import is abrogated, accumulating on the outer membrane instead.30 Here, PINK1 recruits the E3 ubiquitin ligase Parkin to the mitochondrial surface, resulting in the ubiquitination of numerous outer membrane protein substrates.31 Variousadaptor proteins then interact with the ubiquitinated outer membrane proteins, facilitating autophagosomal engulfment of the damaged mitochondria, and degradation at the lysosome.32
Figure 3 – Summary of the initiation of mitophagy.
Under basal conditions, PINK1 is subjected to proteolytic cleavage inside the mitochondria. Following a drop in mitochondrial membrane potential, PINK1 accumulates on the outer mitochondrial membrane. Parkin, recruited to the outer membrane by PINK1, ubiquitinates numerous outer membrane target proteins. Adaptor proteins, such as p62, bind to ubiquitinated mitochondrial proteins, facilitating autophagosomal engulfment of the mitochondria.
Autophagy in the Aging Heart
There is strong evidence that both autophagy and mitophagy decrease with age in numerous tissues, including the heart.33–36 However, the mechanistic underpinnings for the age related decline in autophagy remain unclear and likely complex. One simple yet plausible explanation is that the autophagy machinery is simply overburdened in the aging cell, struggling to keep pace with increased ROS generation and oxidative damage. For instance, a similar situation has been observed in sepsis, in which the rate of autophagic clearance of damaged organelles was outpaced by the extent of sustained insult to hepatocytes.37 Conversely, it is possible that the increase in oxidative stress impairs autophagic activity in cells.38,39
Metabolic and hormonal changes accompanying aging may contribute to lower levels of autophagy. Age-related activation of autophagy suppressors, in addition to inhibition of autophagy activators may also play a role here. For instance, hyperactivation of mTOR, may lead to inhibition of autophagy through mechanisms outlined earlier.40 Perhaps the best-known example is the age-related dysregulation of the IGF-1/Akt signaling axis.41 Mechanistically, this appears to be, in large part, dependent upon members of the FoxO transcription factor family, which are key activators of autophagy gene expression.41 Aberrant Akt-mediated phosphorylation of FoxO1/3, downstream of IGF-1 receptor activation, promotes nuclear exclusion of FoxO1/3 and thus suppression of autophagy gene expression.42,43 Loss of function mutation or inhibition of the IGF-1/Aktpathway significantly increases lifespan in numerous animal models.44–46
Any decrease in the rate of mitophagy is of pressing concern for cardiomyocytes, which are unable to dilute toxic proteins and organelles through cell division. The functional importance of mitophagy has been demonstrated in Parkin-deficient mouse hearts, which exhibit abnormal mitochondrial network morphology at baseline, characterized by fragmented clusters of mitochondria. Following myocardial infarction injury, swollen mitochondria with disrupted cristae accumulate in the infarct border zone of Parkin deficient hearts, contributing to a higher mortality rate in these animals.47, 48
Interestingly, enlarged mitochondria with disrupted cristae are characteristic of both aged and diseased tissues, leading some to hypothesize that the size of these mitochondria may be directly related to their persistence in the cell.49–51 Studies have found that mitochondrial fission precedes mitophagic clearance and that elongated mitochondria are protected from degradation.52,53 Thus, in this theory, it is assumed that giant mitochondria exceed the engulfment capacity of autophagosomes, whereas normal sized mitochondria continue to be autophagocytosed at the standard rate, resulting in the enrichment of enlarged, dysfunctional mitochondria overtime.54 Enlarged mitochondria typically exhibit reduced respiratory function, ATP production and ROS generation. Consequently, in the ‘survival of the slowest’ hypothesis, it is theorized that these mitochondria may be less exposed to sustained ROS induced membrane damage, and therefore less likely to be targeted for removal via PINKI/Parkin-mediated mitophagy.55 These two theories are not mutually exclusive, and it’s likely that both contribute to the age associated decline in mitophagy.
The mitochondrial-lysosomal axis theory of aging offers yet another explanation for the decline in autophagy/mitophagy with age. This theory describes the impairment of lysosomal function by the intralysosomal accumulation of lipofuscin, a toxic yellow aggregate, commonly referred to as ‘age-pigment’.56 Presence of lipofuscin, which can be observed under the microscope by virtue of its autofluorescence, is a hallmark of aging cells. Lipofuscin is formed when ROS-damaged macromolecules, namely proteins, lipids and carbohydrates are cross-linked to produce large aggregates, which are highly resistant to degradation.57 The presence of such aggregates strains the degradative resources of the lysosome, impairing autophagosomal/lysosomal function, leading to the accumulation of autophagy substrates including damaged mitochondria. This, in turn, generates a negative feedback loop in which the lysosomal retention of damaged mitochondria contributes to higher levels of oxidative stress and enhanced generation of lipofuscin. Ultimately, these events culminate in a loss of autophagic flux and an inability to maintain cellular homeostasis, compromising cell survival.58–60 Accumulation of lipofuscin is especially prevalent in senescent cell types, such as cardiomyocytes, and therefore represents a potential threat to myocardial integrity and function.
Regulation of Autophagy
Sirtuins
Sirtuin 1 (Sirt1) is the mammalian orthologue of silent information regulator 2, an NAD+ dependent deacetylase in yeast and drosophila. Numerous studies demonstrate Sirt1, and its orthologues, to be a promoter of both autophagy and longevity in multiple model organisms.61 The mechanism by which Sirt1 mediates autophagy is not entirelyclear. Sirt1 acts in both the nucleus and the cytosol; however, studies using a cytoplasmic restricted Sirt1 mutant suggest that the pro-autophagy effects of Sirt1 are primarily non nuclear.62 Sirt1 deacetylates numerous cytosolic targets, including autophagy proteins ATG5, ATG7, and ATG8, favoring an increase in autophagy.63 Importantly, Sirt1 has been shown to retard aging in the myocardium.64 Sirt1 stimulates autophagy in fasting hearts via deacetylation of FoxOI and subsequent upregulation of autophagy mediators.65 Furthermore, Sirt1 is protective against ischemia/reperfusion injury of the heart.66
Caloric restriction and resveratrol, two long-known promoters of longevity are activators of Sirt1 (Table 1). Caloric restriction upregulates Sirt1 expression in a variety of rodent tissues including brain, liver, kidney and adipose.67 Multiple lines of evidence suggest that Sirt1 is in large part responsible for many of the beneficial effects of caloric restriction and resveratrol treatment. Mice deficient in Sirt1 do not show enhanced life span in response to caloric restriction.68 Furthermore, in yeast and C. elegans, the longevity enhancing effect of CR or resveratrol is abolished following knockdown of atg genes.69 Taken together, these findings point to the fact that Sirt1 is required for the life span prolonging effects of CR and resveratrol, and that autophagy is key to this process. Sirt1, therefore, represents a promising therapeutic target to increase autophagy and longevity in the myocardium. In addition resveratrol and CR are both well-researched and relatively safe treatment modalities, further favoring Sirt1 as a therapeutic target.
Table 1.
Summary of autophagy targets and effects
| Target | Treatment | Effect | Refs |
|---|---|---|---|
| Sirt1 | Cardiac specific overexpression | Increased autophagy, protection against I/R injury in mice | 63 |
| Sirt1 deletion | Elevated acetylation of autophagy proteins, decreased autophagy | 68 | |
| Caloric restriction | Upregulation of Sirti expression, increased autophagy, enhanced lifespan | 65–67 | |
| Resveratrol | Increased autophagy, enhanced lifespan | 69, 96 | |
| NAD+ | Cardiac specific overexpression of Nampt | Increased autophagy, protective against I/R injury | 71 |
| Nicotinamide Mononucleotide (NMN) | Increased autophagy, protective against I/R injury | 73 | |
| AMPK | Metformin | Enhaced lifespan, cardioprotection | 78, 79, 98–100 |
| mTOR | Rapamycin | Increased autophagy, enhanced lifespan, cardioprotection | 84–88 |
NAD+
Sirtuin-mediated decetylation is an NAD+ dependent process; therefore, the cellular availability of NAD+ is closely linked to the level of protein acetylation, throughregulation of sirtuins, as well as autophagic flux and cellular longevity. The cellular concentration of NAD+ is a balance between consumption of NAD+ by NAD+ dependent enzymes and synthesis of NAD+ by designated de novo and salvage pathways. NAD+ levels decrease with age in many organisms, to the detriment of sirtuin activity and downstream activation of autophagy.70, 71 Decreased NAD+ levels also impair lysosomal function, further diminishing autophagic flux in the cell.71,72 Cardiac specific upregulation of nicotinamide phosphoribosyltransferase (Nampt), a rate-limiting enzyme in the NAD+ salvage pathway, increased NAD+ content in the heart and was protective against ischemia reperfusion injury.71 Furthermore, exogenous delivery of nicotinamide mononucleotide (NMN), the product of Nampt in the NAD+ salvage pathway, had a similar effect to upregulating Nampt itself.73 NAD+ levels were increased in the hearts of NMN treated animals, leading to increased Sirt1 activity and protection against ischemia reperfusion injury.73 Thus NMN treatment represents a promising way to activate autophagy in the heart and improve cellular health and longevity.
AMPK
AMP activated protein kinase (AMPK) is a key nutrient sensor in the cell, as well as a master regulator of metabolic homeostasis and mitochondrial biogenesis.74 AMPK is also an important regulator of autophagy in the cell and achieves this via several mechanisms, including inhibitory phosphorylation of mTOR and activation of TSC1/2.75 Additionally, AMPK stimulates autophagy through activating phosphorylation of ULK1.76 Finally, AMPK enhances NAD+ levels in the cell, leading to the activation of Sirt1 and the subsequent deacetylation of Sirt1 targets involved in the activation of autophagy.77 Administration of metformin, an AMPK activator, increases lifespan in mice.78 In addition, AMPK is activated by thiazolidinediones, exercise and caloric restriction, and is believed to be a key contributor to the therapeutic benefits of these treatments.79,80
mTOR
Mammalian target of rapamycin (mTOR), a serine/threonine protein kinase is an important regulator of nutrient homeostasis. Sitting downstream of IGF-1, sirtuin and AMPK signaling, mTOR integrates inputs from these pathways, thereby acting as a central, nutrient-sensing signaling hub.61, 74 mTOR inhibits autophagy through phosphorylation of ULK1 at Ser 757.23 Either deletion of mTOR, or inhibition with rapamycin extends lifespan in several animal models, and ameliorates age-related damage to the heart.81–87 mTOR has a wide variety of cellular functions; however, activation of autophagy is believed to be at least partly responsible for the life extending benefits observed, following the inhibition of mTOR.87, 88 IGF-1 mediated stimulation of Akt activates mTOR, thereby inhibiting autophagy. Perhaps unsurprisingly then, loss of IGF-1 in C elegans promotes lifespan extension.89 mTOR is readily inhibited by caloric restriction (via AMPK activation), in addition to treatment with rapamycin, the FDA- approved namesake inhibitor of mTOR. mTOR therefore represents an attractive therapeutic target for the promotion of autophagy and extension of lifespan.
Targeting Autophagy
Life-style modification may offer one of the simplest, yet also one of the more effective ways to stimulate autophagy. CR has been shown to extend lifespan and reduce age-related pathologies in multiple animal models. In human trials, a relatively modest 25% reduction in calorie intake significantly improved cardiovascular disease risk, as well as mitochondrial function and health.90, 91 While increased autophagy is believed to play a role in these beneficial effects, further research is required to determine the exact contribution. CR has the distinct advantage of simultaneously stimulating multiple upstream activators of autophagy including Sirt1, AMPK and mTOR, making it a particularly attractive and efficacious treatment option. However, a recent study found thatneither lifespan nor health of Polgm/m mice were improved by CR, suggesting that this treatment may be rendered ineffective by mitochondrial DNA mutations.92
In addition, voluntary exercise, which has conclusively been shown to reduce the risk of cardiovascular disease in numerous studies, is also an activator of autophagy.93 As with CR, the benefits conferred by exercise to an organism are vast and multifactorial, making it somewhat difficult to pin down the specific contribution of enhanced autophagy. However, one study confirmed that exercise increased autophagic flux in the heart, reducing levels of protein aggregates and alleviating desmin related cardiomyopathy.94 CR and exercise share the benefit of being well tolerated in suitable individuals and lack the off-target effects commonly associated with pharmaceutical treatments. Unfortunately, CR or voluntary exercise are viable options for some patients, particularly for those with diminished cardiovascular function. In such cases, pharmacological intervention is a more suitable approach. Furthermore, even in suitable candidates, lifestyle modifications are successful only in the short term for the vast majority of cases, with roughly 95% of individuals unable to sustain these changes beyond three years.95
One of the major effects of CR, in relation to autophagy, is to increase the expression and activity levels of Sirt1; therefore, pharmacological activators of Sirt1 may represent a suitable alternative to CR. Resveratrol is one such activator and mimics important outcomes of CR, including protective effects on the myocardium and extension of lifespan, and thus warrants further study.69,96
AMPK activation is also implicated in the beneficial effects of CR. In fact, AMPK may be a particularly appealing target since it also activates Sirt1 via increases in NAD+ levels. Furthermore, AMPK also inhibits mTOR, relieving mTOR mediated inhibition ofautophagy. The AMPK activator metformin has shown great therapeutic promise in several cardiac disease models, including aging-induced myocardial contractile dysfunction.97–100 Since AMPK affects multiple pathways in the cell, further work will be required to identify the exact contribution of autophagy induction to the positive effects of metformin therapy.
Direct inhibition of mTOR via administration of rapamycin has shown encouraging results in the fight against aging. Accumulating reports suggest rapamycin can improve function in aging hearts as well as increase lifespan in general. Again, the contribution of autophagy to these effects remains to be elucidated.
Finally, in a recent study by Eisenberg et al., cardioprotection and extension of lifespan were observed in aged mice given oral supplementation of the polyamine spermidine.101 Mechanistically, spermidine feeding engendered a multitude of positive effects in treated animals including enhanced cardiac autophagy and increased mitochondrial clearance and respiration, in addition to a reduction in systemic blood pressure. Interestingly, the cardioprotective effects of spermidine feeding were lost in mice with cardiac specific deletion of Atg5, clearly demonstrating the importance of autophagy to the efficacy of spermidine treatment. Furthermore, analysis of epidemiological data identified an inverse correlation between dietary spermidine consumption and cardiovascular disease in humans. These results are particularly promising, since treatment may only require minor modification or supplementation of diet.
Conclusion
A decline in cardiomyocyte autophagy is likely a direct contributor to aging in the heart, with negative consequences for cardiac structure and function. However, it is still unclear why autophagy declines with age and whether specific proteins or pathways involved in regulating autophagy are altered with age. Thus, there is a need to further our understanding of the mechanistic underpinnings of autophagy to aid in the design of novel therapeutics to combat aging. To date, most studies on autophagy and mitophagy have been done using immortalized cell lines combined with overexpression of regulators and/or treatments with various toxins or drugs. However, although these systems are valuable to dissect the signaling mechanism, they do not mimic conditions in vivo and have limited physiological relevance to aging. Future studies on autophagy in aging need to prioritize the use of primary cell lines and animal models. Autophagy is a complex process regulated by a number of different pathways; yet, while this makes the study of autophagy more challenging, it also provides a greater potential number of opportunities and targets for therapeutic intervention.
All pharmaceutical therapies currently under investigation as potential autophagy activators also affect other pathways besides that of autophagy; it’s therefore difficult to assert what contribution autophagy makes to the positive outcomes arising from these treatments. One goal of future research efforts should be the development of more selective autophagy activators. This will require a deepening of our knowledge of the mechanism of autophagy beyond our current level of understanding. However, many of the off-target effects of activating AMPK, for example, are also of potential benefit for the aging heart and may have a synergistic effect in combination with autophagy activation. It may be the case then, that targeting autophagy in isolation will not be as an effective treatment. Again, further study and greater understanding will be required in order to answer these questions.
References
- 1.Lakatta EG, Levy D: Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation, 107: 346–354, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D: Arterial and cardiac aging: major shareholders in cardiovasculardisease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation, 107: 139–146, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Larson ED, St Clair JR, Sumner WA, Bannister RA, Proenza C: Depressed pacemakeractivity of sinoatrial node myocytes contributes to the age-dependent decline in maximum heart rate. Proc Natl Acad Sci U S A, 110: 18011–18016, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J: Evidence for cardiomyocyte renewal in humans. Science, 324: 98–102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K,Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS: Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation, 119: 2789–2797, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, Stehouwer CD: Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol, 63: 1739–1747, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Hilfiker-Kleiner D, Landmesser U, Drexler H: Molecular Mechanisms in Heart Failure. Journal of the American College of Cardiology, 48: A56–A66, 2006. [Google Scholar]
- 8.Kolwicz SC Jr., Purohit S, Tian R: Cardiac metabolism and its interactions withcontraction, growth, and survival of cardiomyocytes. Circ Res, 113: 603–616, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaper J, Meiser E, Stammler G: Ultrastructural morphometric analysis of myocardiumfrom dogs, rats, hamsters, mice, and from human hearts. Circ Res, 56: 377–391, 1985. [DOI] [PubMed] [Google Scholar]
- 10.Sack MN, Fyhrquist FY, Saijonmaa OJ, Fuster V, Kovacic JC: Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J Am Coll Cardiol, 70: 196–211, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamed SA, Hanke T, Erasmi AW, Bechtel MJ, Scharfschwerdt M, Meissner C, Sievers HH, Gosslau A: Mitochondrial DNA deletions and the aging heart. Exp Gerontol, 41: 508–517, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE,Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS: Extension of murine life span by overexpression of catalase targeted to mitochondria. Science, 308: 1909–1911, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE,Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG:Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature, 429: 417–423, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY,Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA: Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science, 309: 481–484, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA,Rabinovitch PS: Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell, 9: 536–544, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li WW, Li J, Bao JK: Microautophagy: lesser-known self-eating. Cell Mol Life Sci, 69:1125–1136, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawabata T, Yoshimori T: Beyond starvation: An update on the autophagic machineryand its functions. J Mol Cell Cardiol, 95: 2–10, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Xilouri M, Stefanis L: Chaperone mediated autophagy in aging: Starve to prosper. Ageingresearch reviews, 32: 13–21, 2016. [DOI] [PubMed] [Google Scholar]
- 19.Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, Menzies FM,Rubinsztein DC: Mammalian Autophagy: How Does It Work? Annu Rev Biochem, 85: 685–713, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J: Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell, 141: 656–667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge L, Zhang M, Kenny SJ, Liu D, Maeda M, Saito K, Mathur A, Xu K, Schekman R:Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis. EMBO reports, 18: 1586–1603, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morozova K, Sridhar S, Zolla V, Clement CC, Scharf B, Verzani Z, Diaz A, Larocca JN,Hajjar KA, Cuervo AM, Santambrogio L: Annexin A2 promotes phagophore assembly by enhancing Atg16L(+) vesicle biogenesis and homotypic fusion. Nat Commun, 6: 5856, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Kundu M, Viollet B, Guan KL: AMPK and mTOR regulate autophagy through directphosphorylation of Ulk1. Nat Cell Biol, 13: 132–141, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T,Ohsumi Y, Yoshimori T: Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci, 116: 1679–1688, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Xie Z, Nair U, Klionsky DJ: Atg8 controls phagophore expansion during autophagosomeformation. Mol Biol Cell, 19: 3290–3298, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G,Johansen T: p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem, 282: 24131–24145, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Webb JL, Ravikumar B, Rubinsztein DC: Microtubule disruption inhibitsautophagosome-lysosome fusion: implications for studying the roles of aggresomes in polyglutamine diseases. IntJ Biochem Cell Biol, 36: 2541–2550, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW,Jimenez-Sanchez M, Korolchuk VI, Lichtenbergr M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC: Regulation of mammalian autophagy in physiology andpathophysiology. Physiol Rev, 90: 1383–1435, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ: Mitochondrial membranepotential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol, 191: 933–942, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ:PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS biology, 8: e1000298, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, Harper JW:Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature, 496: 372–376, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI,Youle RJ: The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature, 524: 309–314, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inuzuka Y, Okuda J, Kawashima T, Kato T, Niizuma S, Tamaki Y, Iwanaga Y, Yoshida Y, Kosugi R, Watanabe-Maeda K, Machida Y, Tsuji S, Aburatani H, Izumi T, Kita T, Shioi T: Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation, 120: 1695–1703, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Takuji Shirasawa TS, Mizushima N, Otsu K: Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy, 6: 600–606, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Ren X, Chen L, Xie J, Zhang Z, Dong G, Liang J, Liu L, Zhou H, Luo P: Resveratrol Ameliorates Mitochondrial Elongation via Drp1/Parkin/PINK1 Signaling in Senescent-Like Cardiomyocytes. Oxidative medicine and cellular longevity, 2017: 4175353, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, Chong SY, Lim A, Singh BK, Sinha RA, Salmon AB, Yen PM: Changes inmacroautophagy, chaperone-mediated autophagy, and mitochondrial metabolism in murine skeletal and cardiac muscle during aging. Aging, 9: 583–599, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi W, Watanabe E, Fujimura L, Watanabe-Takano H, Yoshidome H, Swanson PE, Tokuhisa T, Oda S, Hatano M: Kinetics and protective role of autophagy in a mouse cecal ligation and puncture-induced sepsis. Crit Care, 17: R160, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagakannan P, Iqbal MA, Yeung A, Thliveris JA, Rastegar M, Ghavami S, Eftekharpour E: Perturbation of redox balance after thioredoxin reductase deficiency interrupts autophagy-lysosomal degradation pathway and enhances cell death in nutritionally stressed SH-SY5Y cells. Free Radic Biol Med, 101: 53–70, 2016. [DOI] [PubMed] [Google Scholar]
- 39.Frudd K, Burgoyne T, Burgoyne JR: Oxidation of Atg3 and Atg7 mediates inhibition ofautophagy. Nat Commun, 9: 95, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li-Harms X, Milasta S, Lynch J, Wright C, Joshi A, Iyengar R, Neale G, Wang X, Wang YD, Prolla TA, Thompson JE, Opferman JT, Green DR, Schuetz J, Kundu M: Mito-protective autophagy is impaired in erythroid cells of aged mtDNA-mutator mice. Blood, 125:162–174, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren J: Akt2 ablation prolongs life span and improves myocardial contractile functionwith adaptive cardiac remodeling: role of Sirt1 - mediated autophagy regulation. 16: 976–987, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME: Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell, 96: 857–868,1999. [DOI] [PubMed] [Google Scholar]
- 43.Moeinifard M, Hassan ZM, Fallahian F, Hamzeloo-Moghadam M, Taghikhani M:Britannin induces apoptosis through AKT-FOXO1 pathway in human pancreatic cancer cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 94: 1101–1110, 2017. [DOI] [PubMed] [Google Scholar]
- 44.Chaker Z, Aid S, Berry H, Holzenberger M: Suppression of IGF-I signals in neural stemcells enhances neurogenesis and olfactory function during aging. Aging Cell, 14: 847–856, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bharill P, Ayyadevara S, Alla R, Shmookler Reis RJ: Extreme Depletion of PIP3 Accompanies the Increased Life Span and Stress Tolerance of PI3K-null C. elegans Mutants. Frontiers in genetics, 4: 34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J, Gontier G, Chaker Z, Lacube P, Dupont J, Holzenberger M: Longevity effect of IGF-1R(+/−) mutation depends on genetic background-specific receptor activation. Aging Cell, 13: 19–28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubli DA, Quinsay MN, Gustafsson AB: Parkin deficiency results in accumulation ofabnormal mitochondria in aging myocytes. Commun Integr Biol, 6: e24511, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, Jimenez R, Petrosyan S,Murphy AN, Gustafsson AB: Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem, 288: 915–926, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sachs HG, Colgan JA, Lazarus ML: Ultrastructure of the aging myocardium: amorphometric approach. Am J Anat, 150: 63–71, 1977. [DOI] [PubMed] [Google Scholar]
- 50.Hiruta Y, Chin K, Shitomi K, Ichihara T, Mochizuki M, Adachi K, Obayashi T, Tanaka M,Ozawa T: Mitochondrial encephalomyopathy with A to G transition of mitochondrial transfer RNA(Leu(UUR)) 3,243 presenting hypertrophic cardiomyopathy. Intern Med,34: 670–673, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Kamogashira T, Hayashi K, Fujimoto C, Iwasaki S, Yamasoba T: Functionally andmorphologically damaged mitochondria observed in auditory cells under senescence-inducing stress. NPJAging Mech Dis, 3: 2, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K,Egashira K, Ohishi M, Abdellatif M, Sadoshima J: Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res, 116: 264–278, 2015. [DOI] [PubMed] [Google Scholar]
- 53.Gomes LC, Di Benedetto G, Scorrano L: During autophagy mitochondria elongate, arespared from degradation and sustain cell viability. Nat Cell Biol, 13: 589–598, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunk UT, Terman A: The mitochondrial-lysosomal axis theory of aging: accumulationof damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem, 269: 1996–2002, 2002. [DOI] [PubMed] [Google Scholar]
- 55.de Grey AD: A proposed refinement of the mitochondrial free radical theory of aging.Bioessays, 19: 161–166, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Jung T, Bader N, Grune T: Lipofuscin: formation, distribution, and metabolicconsequences. Ann N YAcad Sci, 1119: 97–111, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Hohn A, Jung T, Grimm S, Catalgol B, Weber D, Grune T: Lipofuscin inhibits theproteasome by binding to surface motifs. Free Radic Biol Med, 50: 585–591, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Terman A, Dalen H, Brunk UT: Ceroid/lipofuscin-loaded human fibroblasts showdecreased survival time and diminished autophagocytosis during amino acid starvation. Exp Gerontol, 34: 943–957,1999. [DOI] [PubMed] [Google Scholar]
- 59.Martinez-Cisuelo V, Gomez J, Garcia-Junceda I, Naudi A, Cabre R, Mota-Martorell N,Lopez-Torres M, Gonzalez-Sanchez M, Pamplona R, Barja G: Rapamycin reverses age-related increases in mitochondrial ROS production at complex I, oxidative stress, accumulation of mtDNA fragments inside nuclear DNA, and lipofuscin level, and increases autophagy, in the liver of middle-aged mice. Exp Gerontol, 83: 130–138, 2016. [DOI] [PubMed] [Google Scholar]
- 60.Liu A, Guo E, Yang J, Yang Y, Liu S, Jiang X, Hu Q, Dirsch O, Dahmen U, Zhang C,Gewirtz DA, Fang H: Young plasma reverses age-dependent alterations in hepatic function through the restoration of autophagy. Aging Cell, 17, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guarente L: Calorie restriction and sirtuins revisited. Genes Dev, 27: 2072–2085, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S,Bénit P, Rustin P, Criollo A, Kepp O, Galluzzi L, Shen S, Malik SA, Maiuri MC, Horio Y, López-Otín C, Andersen JS, Tavernarakis N, Madeo F, Kroemer G: Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. The Journal of Cell Biology, 192: 615–629, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T: A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A, 105: 3374–3379, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF,Sadoshima J: Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res, 100: 1512–1521, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J: Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res, 107: 1470–1482, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J: Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation, 122: 2170–2182, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA: Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science, 305: 390–392, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Haigis MC, Sinclair DA: Mammalian sirtuins: biological insights and disease relevance. Annual review of pathology, 5: 253–295, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A,Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G: Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis, 1: e10, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verdin E: NAD(+) in aging, metabolism, and neurodegeneration. Science, 350: 1208–1213, 2015. [DOI] [PubMed] [Google Scholar]
- 71.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J: Nicotinamidephosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res, 105: 481–491, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baixauli F, Acin-Perez R, Villarroya-Beltri C, Mazzeo C, Nunez-Andrade N, Gabande-Rodriguez E, Ledesma MD, Blazquez A, Martin MA, Falcon-Perez JM, Redondo JM, Enriquez JA, Mittelbrunn M: Mitochondrial Respiration Controls Lysosomal Function during Inflammatory T Cell Responses. Cell Metab, 22: 485–498, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J: Nicotinamidemononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PloS one, 9: e98972, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardie DG: AMP-activated protein kinase: an energy sensor that regulates all aspects ofcell function. Genes Dev, 25: 1895–1908, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mukhopadhyay S, Saqcena M, Chatterjee A, Garcia A, Frias MA, Foster DA: Reciprocalregulation of AMP-activated protein kinase and phospholipase D. J Biol Chem, 290: 6986–6993, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS,Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ: Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science, 331: 456–461, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ,Puigserver P, Auwerx J: AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature, 458: 1056–1060, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R: Metformin improves healthspan and lifespan in mice. Nat Commun, 4: 2192, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fryer LG, Parbu-Patel A, Carling D: The Anti-diabetic drugs rosiglitazone and metforminstimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem, 277: 25226–25232, 2002. [DOI] [PubMed] [Google Scholar]
- 80.Barnes BR, Long YC, Steiler TL, Leng Y, Galuska D, Wojtaszewski JF, Andersson L,Zierath JR: Changes in exercise-induced gene expression in 5’-AMP-activated protein kinase gamma3-null and gamma3 R225Q transgenic mice. Diabetes, 54: 3484–3489, 2005. [DOI] [PubMed] [Google Scholar]
- 81.Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO,Kirkland KT, Fields S, Kennedy BK: Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science, 310: 1193–1196, 2005. [DOI] [PubMed] [Google Scholar]
- 82.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S: Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol, 14: 885–890, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F: Genetics: influence ofTOR kinase on lifespan in C. elegans. Nature, 426: 620, 2003. [DOI] [PubMed] [Google Scholar]
- 84.Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, Chen T, Gu H,Djukovic D, Raftery D, Beyer RP, MacCoss MJ, Rabinovitch PS: Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell, 13: 529–539, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA: Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460: 392–395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schröder S, Adler T, Afonso LC,Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Hölter SM, Moreth K, Prehn C, Puk O, Rácz I, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski J, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Höfler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Ehninger D: Rapamycin extends murine lifespan but has limited effects on aging. The Journal of Clinical Investigation,123: 3272–3291, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Wang C, Zhou J, Sun A, Hueckstaedt LK, Ge J, Ren J: Complex inhibition ofautophagy by mitochondrial aldehyde dehydrogenase shortens lifespan and exacerbates cardiac aging. Biochimica et biophysica acta, 1863: 1919–1932, 2017. [DOI] [PubMed] [Google Scholar]
- 88.Alvers AL, Wood MS, Hu D, Kaywell AC, Dunn WA Jr., Aris JP: Autophagy is requiredfor extension of yeast chronological life span by rapamycin. Autophagy, 5: 847–849,2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R: A C. elegans mutant that lives twiceas long as wild type. Nature, 366: 461–464, 1993. [DOI] [PubMed] [Google Scholar]
- 90.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E: Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS medicine, 4: e76, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL,Williamson DA, Smith SR, Ravussin E: Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis, 203: 206–213,2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Someya S, Kujoth GC, Kim MJ, Hacker TA, Vermulst M, Weindruch R, Prolla TA: Effectsof calorie restriction on the lifespan and healthspan of POLG mitochondrial mutator mice. PloS one, 12: e0171159, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B: Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature, 481: 511–515, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, Sadoshima J, Robbins J: Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest,123: 5284–5297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crawford D, Jeffery RW, French SA: Can anyone successfully control their weight?Findings of a three year community-based study of men and women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity, 24: 1107–1110, 2000. [DOI] [PubMed] [Google Scholar]
- 96.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D,Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA: A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PloS one, 3: e2264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM,Yellon DM: Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol, 103: 274–284, 2008. [DOI] [PubMed] [Google Scholar]
- 98.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ: Acutemetformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes, 57: 696–705, 2008. [DOI] [PubMed] [Google Scholar]
- 99.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M: Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation, 119: 2568–2577, 2009. [DOI] [PubMed] [Google Scholar]
- 100.Sun D, Yang F: Metformin improves cardiac function in mice with heart failure aftermyocardial infarction by regulating mitochondrial energy metabolism. Biochemical and biophysical research communications, 486: 329–335, 2017. [DOI] [PubMed] [Google Scholar]
- 101.Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A,Schipke J, Zimmermann A, Schmidt A, Tong M, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K, Carmona-Gutierrez D, Buttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, Horsch M, Moreth K, Beckers J, Fuchs H, Gailus-Durner V, Neff F, Janik D, Rathkolb B, Rozman J, de Angelis MH, Moustafa T, Haemmerle G, Mayr M, Willeit P, von Frieling-Salewsky M, Pieske B, Scorrano L, Pieber T, Pechlaner R, Willeit J, Sigrist SJ, Linke WA, Muhlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, Madeo F: Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med, 22: 1428–1438, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]