Abstract
Introduction:
In observational studies, the association between the Dietary Approaches to Stop Hypertension (DASH) diet and incident heart failure has been inconsistent. It was hypothesized that higher DASH diet concordance has a protective effect on heart failure in a multi-ethnic cohort.
Methods:
The Multi-Ethnic Study of Atherosclerosis cohort includes men and women of multiple ethnicities who were aged 45–84 years and free of clinical cardiovascular disease at baseline. Participants were recruited between 2000 and 2002 from six U.S. communities and followed for incident cardiovascular health events through 2015 for the purpose of this data set. Diet was measured using food-frequency questionnaires. Cox proportional hazards analysis was used to investigate the associations of the DASH diet concordance with incident heart failure in 2017–2018.
Results:
During a median 13 years of follow-up, 179 of 4,478 participants developed heart failure, corresponding to a rate of 3.4 per 1,000 person years. Heart failure incidence rates did not vary significantly by DASH quintile for the population as a whole. In participants younger than 75 years, highest DASH concordance was associated with lower risk of incident heart failure compared with those in the lowest quintile (hazard ratio=0.4, 95% CI=0.2, 0. 9 versus all participants hazard ratio=1.0, 95% CI=0.2, 0. 9) after adjusting for demographics, energy consumption, and known cardiovascular confounders.
Conclusions:
This study supports the hypothesis that DASH is beneficial in heart failure prevention within the individuals aged less than 75 years subgroup, an idea which to date is substantiated only by much smaller studies or in less diverse patient populations.
INTRODUCTION
Heart failure (HF) is a frequent cause of hospitalization in older adults and is associated with substantial healthcare costs. Identifying modifiable risk factors for primary prevention of HF is an important public health goal. The effectiveness of dietary modifications in the prevention and treatment of hypertension (HTN) and cardiovascular diseases (CVDs) is well recognized. The Dietary Approaches to Stop Hypertension (DASH) diet emphasizes intake of fruits, vegetables, whole grains, poultry, fish, nuts, and low-fat dairy products while reducing consumption of red meat, sweets, and sugar-sweetened beverages. The DASH diet has shown evidence-based beneficial effects on HTN,1–4 and is endorsed by the American Heart Association and the American College of Cardiology in their 2013 prevention guidelines.5 HTN has been consistently associated with the incidence of HF.6–9 Individual diet concordance can be ranked and summed to create a DASH diet component score. The use of a component score for estimating concordance to DASH diet, from self-reported dietary intake data, is effective for measuring the risk of cardiovascular events.8,9
Few studies to date have examined the effects of DASH diet on incident HF and they have yielded conflicting results. Using data from the Swedish Mammography Cohort study9 and the Cohort for Swedish Man,10 Levitan and colleagues9,10 reported a 37% lower risk of HF among women (mean age 52.9 years) and 22% in older men (mean age 60.3 years) whose diets were most similar with a DASH dietary pattern. In a prospective cohort study of older white U.S. adults, Del Gobbo et al.11 reported a lack of association between dietary patterns and incident HF. Based on prior protective effects of the DASH diet on left ventricular (LV) parameters found by Nguyen and colleagues12 in the Multi-Ethnic Study of Atherosclerosis (MESA) sample, the same large gender balanced and ethnically diverse U.S. cohort is used to examine the relationship between degree of DASH diet concordance and risk of HF. It is hypothesized that higher DASH diet concordance has a protective effect on HF.
METHODS
Study Sample
Details of the MESA design have been previously described.13 Briefly, 6,814 men and women, aged 45 to 84 years and of four self-reported race/ethnicities (non-Hispanic white, black, Hispanic, and Chinese) and no history of clinical CVD (including valvular disease) were enrolled in six participating centers in the U.S. from 2000 to 2002. The IRB at each of the study sites approved the study protocols. On entry, participants underwent evaluations via questionnaires, physical examination, and laboratory tests. In general, the exclusion criteria followed were described by Nettleton et al.14 A total of 709 participants were excluded from the analysis because of insufficient (n=283 missing food-frequency values, n=5 missing forms) or unreliable dietary data (participants reporting extreme energy intakes >6,000 or <600 kcal/day [n=422]). There were 15 participants who died, four participants had no data on HF, and 1,612 had no heart structure data. Baseline participants with diabetes were not excluded because in MESA substantial dietary pattern differences have not previously been noted relative to participants without diabetes.12 Analyses were conducted in 2017–2018 to examine the effect of DASH diet concordance on the incidence of HF by age, gender, race/ethnicity, adiposity, and diabetes.
Measures
Usual dietary intake was assessed using a 120-item food-frequency questionnaire (FFQ).15 The FFQ was developed in the validated block format and was based on the FFQ used in the Insulin American, and Hispanic individuals and later modified for use in the MESA by including Resistance Atherosclerosis Study.15 It has been validated in non-Hispanic white, African Chinese foods and culinary practices.14,16 Participants recorded serving size (small, medium, or large) and frequency of consumption of specific beverage and food items. Nine frequency options were given, ranging from “rare or never” to a maximum of “2 times per day” for foods and a maximum of “6 times per day” for beverages. Servings per day for each item were calculated as the product of the reported frequency and serving size (small weighted by 0.5, medium by 1.0, and large by 1.50). After quality control measures were taken to minimize underreporting in 19 food items (Appendix List 1), including re-scanning of original questionnaires. In a subset of participants missing original FFQs, missing information on food frequency and serving sizes of 19 FFQ items was imputed using multinomial regression or ordinal regression models. Nutrient intake was estimated for each FFQ item using the Nutrition Data System for Research.
Using these data, DASH diet concordance was assessed with a component score of summed quintile ranks for eight food groups. A DASH score was calculated for each FFQ. For each of the components, participants were classified into quintiles according to their intake ranking.
DASH diet quintile ranges are described in Table 1. Five favorable food groups were positively scored; these were: fruits, vegetables, whole grains, nuts and legumes, and low-fat dairy products. Three unfavorable food groups were reverse scored; these were red and processed meats, sweetened beverages, and sodium. Thus, the range of the final score was 8–40, with higher scores indicating greater DASH diet concordance. Consumption of healthy food components were rated on a scale of 1–5; the higher the score, the more frequent the consumption of that food (i.e., those in Quintile 1 had the lowest consumption and received a score of 1; conversely, those in Quintile 5 had the highest consumption and received a score of 5).
Table 1.
Participant Characteristics by Quintiles (Q) of the DASH Eating Pattern Score (N=4,493)
| Variable | DASH eating pattern score |
|||||
|---|---|---|---|---|---|---|
| Q1 (n=921) 10–19 |
Q2 (n=869) 20–22 |
Q3 (n=944) 23–25 |
Q4 (n=979) 26–29 |
Q5 (n=780) 30–40 |
p-value | |
| Categorical variables, % | ||||||
| Female | 32.8 | 44.5 | 48.6 | 62.9 | 72.1 | <0.001 |
| Race | ||||||
| White | 29.1 | 36.6 | 40.5 | 43.9 | 50.7 | <0.001 |
| Chinese American | 15.6 | 14.3 | 14.6 | 12.5 | 6.3 | <0.001 |
| African American | 33.8 | 25.0 | 21.7 | 23.9 | 20.6 | <0.001 |
| Hispanic American | 21.5 | 24.2 | 23.1 | 21.1 | 21.3 | <0.001 |
| Education | ||||||
| Less than high school | 17.1 | 19.4 | 16.6 | 15.0 | 13.7 | <0.001 |
| High school | 20.9 | 19.5 | 17.4 | 14.6 | 14.6 | <0.001 |
| Some college | 30.5 | 27.7 | 25.7 | 27.1 | 26.8 | <0.001 |
| College graduate or more | 30.5 | 33.5 | 40.3 | 43.3 | 44.8 | <0.001 |
| Smoking status | ||||||
| Never | 43.5 | 51 | 53 | 54.3 | 53.9 | <0.001 |
| Past | 33.9 | 35.1 | 36.3 | 36.8 | 41 | <0.001 |
| Current | 22.6 | 14.3 | 11.4 | 8.5 | 5.5 | <0.001 |
| Diabetes mellitus | 9.2 | 13.0 | 13.3 | 11.3 | 10.3 | 0.029 |
| HTN by JNC | 39.4 | 41.2 | 42.1 | 44.3 | 47.5 | 0.148 |
| HTN drug | 30.18 | 35.4 | 34.0 | 39.1 | 38.7 | <0.001 |
| Lipid lowering drug | 12.6 | 15.0 | 15.6 | 16.6 | 19.7 | 0.002 |
| ACE or ARB | 13.3 | 15.7 | 17.3 | 18.0 | 18.3 | 0.022 |
| Diuretics | 10.3 | 12.0 | 10.4 | 13.6 | 15.4 | 0.005 |
| Alcohol use | ||||||
| Never | 16.2 | 19.2 | 20.9 | 21.5 | 20.9 | 0.100 |
| Former | 23.8 | 25.0 | 22.4 | 21.4 | 22.2 | 0.100 |
| Current | 60.0 | 55.8 | 56.6 | 57.1 | 56.9 | 0.100 |
| Continuous variables, mean (SD) | ||||||
| Age mean, years | 58(10) | 60 (10) | 61(10) | 62(10) | 65(10) | <0.001 |
| BMI (kg/m2) | 28(5.0) | 28(5) | 28 (5) | 27(5) | 26(5) | <0.001 |
| Total daily energy intake (kcal) | 1,934 (901) | 1,799 (849) | 1,560 (780) | 1,560 (675) | 1,381 (545) | <0.001 |
| Intentional exercise (MET minutes/week) | 1,489 (2,676) | 1,435 (2,052) | 1,482 (2,092) | 1,662 (2,399) | 1,979 (2,646) | <0.001 |
| Moderate+vigorous PA | 6,610 (7,280) | 5,805 (5,874) | 5,463 (5,460) | 5,627 (5,712) | 5,641 (5,399) | <0.001 |
| Cholesterol, mg/dl | ||||||
| Total | 193 (35) | 194 (35) | 192 (34) | 195 (36) | 195 (34) | 0.318 |
| LDL | 119 (31) | 119 (32) | 116 (30) | 117 (31) | 114 (29) | 0.003 |
| HDL | 48 (13) | 49 (14) | 50 (14) | 52 (16) | 57 (17) | <0.001 |
| Blood pressure, mmHg | ||||||
| Systolic | 1,265 (20) | 125 (21) | 125 (21) | 126 (21) | 127 (22) | 0.276 |
| Diastolic | 74 (10) | 72 (10) | 72 (10) | 71 (10) | 69 (10) | 0.920 |
| LV ejection fraction (%) | 68 (7) | 68 (8) | 69 (8) | 70 (7) | 70 (6) | <0.001 |
| LV end diastolic volume | 134 (34) | 129 (32) | 127 (32) | 124 (29) | 119 (28) | <0.001 |
| LV mass | 157 (40) | 150 (40) | 145 (39) | 140 (37) | 135 (36) | <0.001 |
| Heart rate (bpm) | 63 (9) | 63 (9) | 63 (10) | 62 (9) | 62 (9) | 0.74 |
| Creatinine (mg/dl) | 1.0 (0.5) | 0.96 (0.2) | 0.95 (0.2) | 0.93 (0.2) | 0.92 (0.29) | <0.001 |
| Estimated GFR | 84.0 (17.8) | 81.6 (17.6) | 81.7 (17.0) | 79.4 (16.1) | 77.7 (16.5) | <0.001 |
| NT-proBNP (pg/ml) # | 92.5 (465.7) | 77.0 (108.4) | 91.7 (174.3) | 106.7 (185.5) | 128.8 (177.0) | <0.001 |
Note: Boldface indicates statistical significance (p<0.05).
DASH, Dietary Approaches to Stop Hypertension; HTN, hypertension; JNC, Joint National Committee; ACE, angiotensin-converting enzyme inhibitor(s); ARB, angiotensin II receptor blocker(s)/antagonist(s); PA, physical activity; LDL, low-density lipoprotein; HDL, high-density lipoprotein; LV, left ventricular; bpm, beats per minute; GFR, glomerular filtration rate; NT-proBNP, N-terminal pro b- type natriuretic peptide.
Cardiac magnetic resonance imaging was performed using 1.5-Telsa magnets at each center; the MESA protocol has been described in detail.17 Briefly, imaging was performed with a four-element, phased-array surface coil placed anteriorly and posteriorly, electrocardiogram gating, and brachial artery blood pressure (BP) monitoring. Cine images of the left ventricle were obtained during short-breath holding (12–15 seconds) at resting lung volume.
Quantitative measurements were performed at one reading center using MASS, version 4.2, analytic software for reader interpretation. LV wall thickness was defined as the average of six mid-ventricle segment thickness measurements. LV end-diastolic volume (EDV) and end systolic volume were calculated by summing the areas on each separate slice multiplied by the sum of the slice thickness. End-diastolic LV mass was determined by the sum of the area between the epicardial and endocardial contours multiplied by the slice thickness; this value was then multiplied by the specific gravity of myocardium (1.05g/ml).
Ejection fraction was calculated as stroke volume divided by EDV. For LV mass and EDV, the inter-reader intra-class correlation coefficients were >0.8. The intra-reader coefficients for these measures ranged from 0.94 to 0.98.
Clinical characteristics and cardiovascular risk factors were obtained as described previously.13 The candidate covariates for this analysis include: age, sex, ethnicity, education, physical activity, total energy intake, smoking status, BP, heart rate, alcohol consumption, HTN medication use, renal function, lipids (total cholesterol, low-density lipoprotein, high-density lipoprotein), lipid lowering medication, and BMI at examination. N-terminal pro b-type natriuretic peptide (NT-proBNP) levels were measured in serum collected at baseline and stored at –70ºC, using the Elecsys 2010 system at a core laboratory.18
HTN was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or taking BP lowering medication. Individuals with diabetes were defined as either having fasting plasma glucose ≥126 mg/dL or receiving drug treatment for diabetes. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Chronic Kidney Disease-Epi equation. Physical activity was assessed via a Typical Week Physical Activity Survey adapted from the Cross-Cultural Activity Participation Study, which was designed to identify the time and frequency spent in various physical activities during a typical week in the past month.19
The following derived variables were considered: moderate to vigorous physical activity (sum of moderate and vigorous MET-hours/week), and intentional exercise variable (sum of walking for exercise, sports/dancing, and conditioning MET-hours/week) which have been used in prior analyses.
MESA participants are regularly queried by phone regarding potential events. Medical records were obtained and outcomes were centrally adjudicated by a committee of physician investigators. Reviewers classified incident hospitalized HF as definite, probable, or absent. Definite or probable HF required HF symptoms, such as shortness of breath or edema; probable HF required HF diagnosed by a physician and patient receiving medical treatment for HF.
Definite HF required one or more other criteria, such as pulmonary edema/congestion by chest x- ray; dilated ventricle or poor LV function by echocardiography or other imaging; or echocardiographic evidence of diastolic dysfunction. Individuals with adjudicated definite or probable HF were used in the analysis as previously described.20
Statistical Analysis
Descriptive statistics were utilized to summarize participant characteristics according to DASH score categories. Data are presented as mean ± SD for continuous variables and number (percentage) for categorical variables. Differences in baseline participant characteristics by quintile of DASH score were tested with Student’s t-test (continuous variables with normal distribution) or chi-square test (categorical variables). Log rank tests were used to compare the incidence of HF by DASH score quintile. Subsequently, Cox proportional hazards models were used to analyze the association of dietary pattern with incident HF. For all regression models, adjustment was made for demographic factors (age, sex, study site, educational level, and race/ethnicity), traditional CVD risk factors (systolic BP and diastolic BP, use of antihypertensive medications, lipids, diabetes mellitus, smoking, alcohol intake, and exercise), BMI, and eGFR. Four sets of models were used: Model 1: gender and age; Model 2: plus race, education, energy, cigarette use, site, exercise, energy and BMI; Model 3 adjusted for Model 2 adjusted for HTN, diabetes, high-density lipoprotein (known disease associations with HF); and Model 4 adjusted for Model 3 plus EF, LV mass (pathophysiology of HF). Analyses were performed using Stata, version 14.2 for Windows.
RESULTS
After exclusions, the analytic sample for these analyses was 4,478 participants. At baseline, 52% of participants were female, the mean age was 62 years, and 40.3% were white, 24.6% black, 22.1% Hispanic, and 12.8% Chinese American. The average DASH score was 24.1 (SD=5.3), range, 10–40, reflecting overall modest levels of concordance with the DASH eating pattern. The specific differences between the components of the diets of participants in Quintile 1 and Quintile 5 are shown in Appendix Table 1; the highest quintile consumed on average more fruits, vegetables, and other components of the DASH eating pattern. Distributions of demographics, lifestyle factors at baseline are shown in Table 1 according to DASH score quintiles. Characteristics associated with being in higher quintiles (indicating healthier diets) included female sex, white race, older age, never smoker, and higher educational attainment. Similarly, lower LV mass and LV EDV, higher level of physical activity, higher NT- Pro BNP, and high-density lipoprotein, as well as lower energy intake, lower eGFR and creatinine were associated with higher quintiles. Total and low-density lipoprotein cholesterol, BP, and heart rate showed no consistent pattern across DASH score quintiles.
During a median of 13 years of follow-up (through 2015), 179 participants developed HF, corresponding to a rate of 3.4 per 1,000 individuals per year. HF incidence rates did not vary significantly by DASH quintile for the population as a whole (Appendix Table 2). Rates of HF were also assessed by DASH quintile according to several parameters known to be associated with HF risk (age, sex, race/ethnicity, adiposity, and diabetes). As expected, rates generally were higher among older participants, men, those with diabetes, and obese participants.
However, there was a suggestion of heterogeneity in the pattern of incident HF by DASH score quintile according to age category (Figure 1). Although in the younger participants the incidence of HF was generally lower in Quintile 5 than Quintile 1, among those aged 75–84 years at baseline, the pattern was reversed but was not statistically significant and the lowest incidence of HF was in Quintile 1 (Appendix Table 2).
Figure 1.
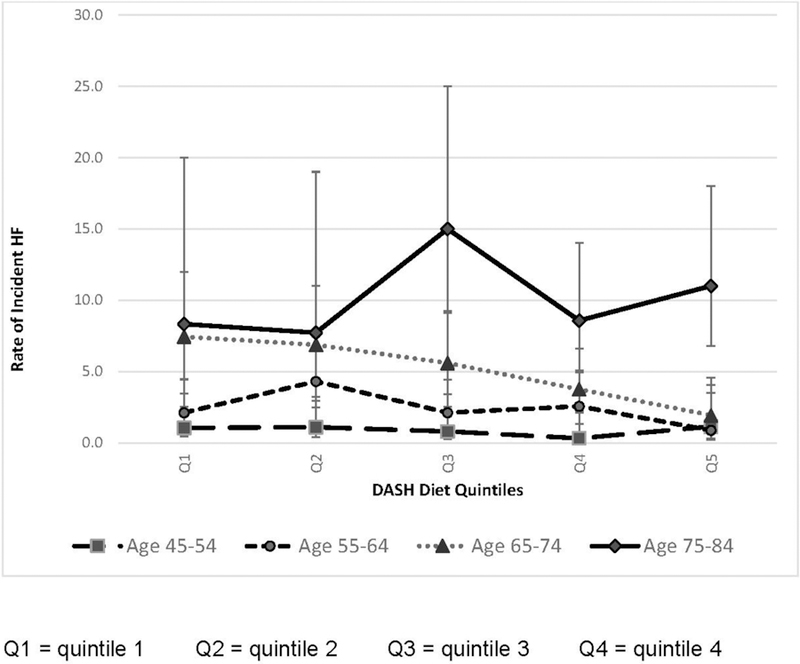
Rate of incident heart failure by DASH diet quintiles (Q1–Q5) and age category, per 1,000 person years (N=4,478).
DASH, Dietary Approaches to Stop Hypertension.
Because of the pattern observed in Figure 1, the analysis continued excluding participants aged ≥75 years. Appendix Table 3 provides incident rates for this subsample; overall among those aged <75 years the highest DASH quintile was associated with a lower rate of incident HF (Table 3). In these participants, highest DASH concordance was associated with lower incidence of HF with a >2-fold greater hazards of HF among those in the lowest quintile (hazard ratio=0.4, 95% CI=0.2, 0. 9). This association was significant after adjustment for demographics, energy intake, behaviors, traditional CVD risk factors, and cardiac structure (Table 3). Analyses of individual food groups showed that highest consumption of low-fat dairy was significantly associated with decreased HF risk in the adjusted model (Model 4): hazard ratio=0.5, 95% CI=0.2, 0.9, p-trend=0.002. Suggestion of a protective effect was also found with most favorable quintile of meat consumption (which was the lowest amount; hazard ratio=0.5, 95% CI=0.2, 1.0, p-trend=0.092; Appendix Table 4).
Table 3.
Hazard Ratios of DASH Eating Pattern Score With Incident HF for Those Aged 45–74 Years at Baseline (N=3,906)
| Models | Q1 | Q2 | Q3 | Q4 | Q5 | p-trend |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI | HR (95% CI) | |||
| Model 1 | ref | 1.3 (0.6, 1.5) | 0.9 (0.5, 1.5) | 0.7 (0.4, 1.3) | 0.4 (0.2, 0.9) | 0.023 |
| Model 2 | ref | 1.3 (0.8, 2.1) | 1.0 (0.6, 1.8) | 0.7 (0.5, 1.2) | 0.6 (0.3, 1.2) | 0.243 |
| Model 3 | ref | 1.2 (0.7, 2.0) | 0.9 (0.5, 1.6) | 0.8 (0.5, 1.6) | 0.5 (0.2, 1.1) | 0.250 |
| Model 4 | ref | 1.2 (0.7, 2.0) | 0.9 (0.5, 1.7) | 0.8 (0.5, 1.5) | 0.4 (0.2, 0.9) | 0.108 |
Notes: Model 1: Adjusted for gender, age; Model 2: Adjusted for Model 1 plus race, education, energy, tobacco, site, exercise, energy, and BMI; Model 3: Adjusted for Model 2 plus HTN, diabetes, HDL; Model 4: Adjusted for Model 3 plus EF, LV mass.
DASH, Dietary Approaches to Stop Hypertension; HF, hear failure; HR, hazard ratio; Q, quintile; HTN, hypertension; HDL, high-density lipoprotein; EF, ejection fraction; LV, left ventricular.
DISCUSSION
Overall the results from the multi-ethnic MESA cohort, evaluating DASH diet concordance and incident HF did not find an association in the entire MESA sample. The findings are consistent with that of a prior study including older adults.11 In the post hoc analysis, this study supports the hypothesis that the DASH diet is beneficial in HF prevention, at least within a younger subgroup, an idea which to date is supported only by much smaller studies or in less diverse patient populations.9,10 All studies used the same FFQ and DASH scoring system created by Fung and colleagues.8 This association was independent of demographics, behavior factors, BP, HTN, and BMI.
Even though DASH is targeted for lowering BP,3 the protective effects against HF go beyond antihypertensive benefits and cannot be explained by HTN alone. The mechanisms by which the DASH diet confers a protective effect on cardiovascular health have been previously studied. Hummel et al.27 found that the DASH diet was associated with reduced BP, oxidative stress, and arterial stiffness as well as improved diastolic function, contractility, arterial elastance, and systolic performance or whether the reduction in effective arterial afterload was responsible. The data in dairy consumption and HF is conflicting. In one previous study, intake of high-fat dairy was related to increased HF risk.21 However, fermented dairy intake has not been related to HF risk in other studies.22,23 Dairy intake appears to be inversely related to the multitude of risk factors for CVD including HTN.24–26 Although associations have been identified between specific food groups and reduced risk for HF, the effects of each group are interrelated and the protective benefits should be considered in the context of the overall DASH diet.
Dietary sodium specifically impacts populations at risk for and mechanisms implicated in the development of HF.27 It also appears that plant-based diets high in antioxidants, micronutrients, dietary nitrate, and fiber but low in saturated/trans fats and sodium are associated with decreased HF incidence/severity. It is likely that these dietary features contribute to decreased oxidative stress, lower homocysteine levels as well as to higher antioxidant defense, nitric oxide bioavailability, and gut microbiome modulation.2
A protective effect of DASH dietary pattern from HF in much older individuals was not seen, despite the fact that their diets were healthier. This may be because of a smaller sample size, but also that pathophysiologic heart changes are irreversible at advanced ages. Another possibility is suggested by the relationship of HTN (and BP drug lowering) to age and DASH quintile. Those in the apparently healthier diet quintiles were more likely to have HTN and be treated. If older participants with HTN had a late adoption of a healthier dietary pattern this may represent a bias similar to confounding by indication. Another reason for the inverse relationship between DASH diet and HF in older ages may be related to people with a perceived higher risk of CVD adopting dietary recommendations. However, MESA participants were free of known clinical CVD and likely unaware at baseline of the extent of subclinical CVD.
A recent meta-analysis, showed that concordance with the DASH diet improves circulating serum inflammatory biomarkers in adults, compared with usual diet.28 Previous studies have shown that interleukin-6, interleukin-2, C-reactive protein, and tumor necrosis factor-α are associated with HF13 and subclinical LV dysfunction. Experimental studies have also shown that interleukin-6 and tumor necrosis factor-α are associated with progressive LV dysfunction, LV remodeling, myocyte hypertrophy, and myocyte apoptosis. Some mechanisms suggested for these associations include immune activation, myocardial biosynthesis of inflammatory markers, under perfusion of systemic tissues, absorption of endotoxins from the edematous intestines, and neurohormonal activation/stabilization. However, it remains unclear whether the association between inflammatory markers and HF is causal or indirectly related to other local or generalized inflammatory state.3 There are few studies focusing specifically on the relationship between C- reactive protein and CVD in older adults.29 Studies including only older participants show that C-reactive protein and fibrinogen may not be as useful as other markers, such as interleukin-6 and tumor necrosis factor-α.30 This may suggest that the pathophysiology of HF may be different in much older adults.
The increasing levels of NT pro-BNT with higher quintiles of DASH diet concordance may be related to the fact that older participants tend to eat healthier. Multiple reports confirm the association of increasing age with higher NT pro-BNP. This relationship appears to reflect the interaction of age with the total burden of comorbidities rather than reflecting an intrinsic characteristic of age per se.4
Limitations
The strengths of this study include the large sample size, long duration of follow-up, adjudicated outcomes, the multi-ethnic cohort, and the fact that unlike other studies, all participants were free of clinical CVD including valvular heart disease at baseline. However, MESA is an observational study; although numerous covariates were considered in the adjusted models, the results may still have been influenced by residual confounding. Another potential weakness is that even though participants with extreme energy intakes were excluded from the analyses, there may be some participants who under- or over-report their food intake. As a result, misclassification may subsequently attenuate the associations.
CONCLUSIONS
High concordance to a DASH diet, as indicated by the quintile score, was associated with a lower risk of developing HF in MESA participants under age 75 years. This research provides a framework for the exploration of the DASH diet, as an effective diet in the primary prevention of HF. As this was found in a post hoc analysis, future prospective studies are needed to further evaluate this hypothesis.
Supplementary Material
Table 2.
Hazard Ratios Between DASH Diet Score Quintile and Incident HF- All Participants (N=4,478)
| Models | Q1 | Q2 | Q3 | Q4 | Q5 | p-trend |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Model 1 | ref | 1.2 (0.8, 2.0) | 1.1 (0.7, 1.7) | 0.8 (0.5, 1.3) | 0.7 (0.4, 1.2) | 0.123 |
| Model 2 | ref | 1.3 (0.8, 2.1) | 1.2 (0.8, 2.0) | 1.0 (0.6, 1.6) | 0.9 (0.5, 1.5) | 0.458 |
| Model 3 | ref | 1.2 (0.7, 1.9) | 1.1 (0.7, 1.8) | 0.9 (0.5, 1.5) | 0.8 (0.5, 1.4) | 0.562 |
| Model 4 | ref | 1.1 (0.7, 1.8) | 1.1 (0.7, 1.8) | 0.9 (0.5, 1.4) | 0.7 (0.4, 1.2) | 0.265 |
Notes: Model 1: Adjusted for gender, age; Model 2: Adjusted for Model 1 plus race, education, energy, tobacco, site, exercise, energy, and BMI; Model 3: Adjusted for Model 2 plus HTN, diabetes, HDL; Model 4: Adjusted for Model 3 plus EF, LV mass.
DASH, Dietary Approaches to Stop Hypertension; HF, heart failure; HR, hazard ratio; Q, quintile; HTN, hypertension; HDL, high- density lipoprotein; EF, ejection fraction; LV, left ventricular.
ACKNOWLEDGMENTS
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR- 001420 from National Center for Advancing Translational Science. The authors thank the other investigators, the staff, and the participants of the Multi-Ethnic Study of Atherosclerosis (MESA) study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org. Dr. Wood is additionally supported by a U.S. Department of Agriculture (USDA) grant (CRIS 309-5-001-058). Dr. Bahrami is supported by NHLBI grant 5K23HL128164A.
This work is a publication of the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine in Houston, Texas, funded in part by the USDA/ARS (Cooperative Agreement 6250-51000). The contents of this publication do not necessarily reflect the view or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement from the U.S. government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.National Heart, Lung, and Blood Institute, NIH. Your Guide to Lowering Your Blood Pressure with DASH Rockville, MD: HHS; 2006. [Google Scholar]
- 2.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure: control main results of the PREMIER clinical trial. JAMA 2003;289(16):2083–2093. 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 3.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. New Engl J Med 1997;336(16):1117–1124. 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. New Engl J Med 2001;344(1):3–10. 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (vol 129, pg S76, 2014). Circulation 2015;131(4):E326. [DOI] [PubMed] [Google Scholar]
- 6.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in U.S. men and women: NHANES I epidemiologic follow- up study. Arch Intern Med 2001;161(7):996–1002. 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KKL. The progression from hypertension to congestive heart failure. JAMA 1996;275(20):1557–1562. 10.1001/jama.1996.03530440037034. [DOI] [PubMed] [Google Scholar]
- 8.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-Style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168(7):713–720. 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 9.Levitan EB, Wolk A, Mittleman MA . Consistency with the DASH diet and incidence of heart failure. Arch Intern Med 2009;169(9):851–857. 10.1001/archinternmed.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitan EB, Wolk A, Mittleman MA. Relation of consistency with the Dietary Approaches to Stop Hypertension diet and incidence of heart failure in men aged 45 to 79 years. Am J Cardiol 2009;104(10):1416–1420. 10.1016/j.amjcard.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Gobbo LC, Kalantarian S, Imamura F, et al. Contribution of major lifestyle risk factors for incident heart failure in older adults: the Cardiovascular Health Study. JACC Heart Fail 2015;3(7):520–528. 10.1016/j.jchf.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen HT, Bertoni AG, Nettleton JA, Bluemke DA, Levitan EB, Burke GL. DASH eating pattern is associated with favorable left ventricular function in the Multi-Ethnic Study of Atherosclerosis. J Am Coll Nutr 2012;31(6):401–407. 10.1080/07315724.2012.10720466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seliger SL, Hong SN, Christenson RH, et al. High-sensitive cardiac troponin t as an early biochemical signature for clinical and subclinical heart failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation 2017;135(16):1494–1505. 10.1161/CIRCULATIONAHA.116.025505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nettleton JA, Steffen LM, Mayer-Davis EJ, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2006;83(6):1369–1379. 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagenknecht LE, Mayer EJ, Rewers M, et al. The Insulin Resistance Atherosclerosis Study (IRAS) objectives, design, and recruitment results. Ann Epidemiol 1995;5(6):464–472. 10.1016/1047-2797(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 16.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a multi-cultural epidemiologic study. Ann Epidemiol 1999;9(5):314–324. 10.1016/S1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 17.Natori S, Lai SH, Finn JP, et al. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006;186(6):S357–S365. 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 18.Chahal H, Bluemke DA, Wu CO, et al. Heart failure risk prediction in the Multi- Ethnic Study of Atherosclerosis. Heart 2015;101(1):58–64. 10.1136/heartjnl-2014-305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertoni AG, Whitt-Glover MC, Chung HJ, The association between physical activity and subclinical atherosclerosis. Am J Epidemiol 2009;169(4):444–454. 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahrarni H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med 2008;168(19):2138–2145. 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nettleton JA, Steffen LM, Loehr LR, Rosamond WD, Folsom AR. Incident heart failure is associated with lower whole-grain intake and greater high-fat dairy and egg intake in the Atherosclerosis Risk in Communities (ARIC) Study. J Am Diet Assoc 2008;108(11):1881–1887. 10.1016/j.jada.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tektonidis TG, Akesson A, Gigante B, Wolk A, Larsson SC. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: a population-based cohort study. Atherosclerosis 2015;243(1):93–98. 10.1016/j.atherosclerosis.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 23.Wirth J, di Giuseppe R, Boeing H, Weikert C. A Mediterranean-style diet, its components and the risk of heart failure: a prospective population-based study in a non-Mediterranean country. Eur J Clin Nutr 2016;70(9):1015–1021. 10.1038/ejcn.2016.140. [DOI] [PubMed] [Google Scholar]
- 24.Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, et al. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta- analysis of prospective cohort studies. Am J Clin Nutr 2011;93(1):158–171. 10.3945/ajcn.2010.29866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drouin-Chartier JP, Gigleux I, Tremblay AJ, Poirier L, Lamarche B, Couture P. Impact of dairy consumption on essential hypertension: a clinical study. Nutr J 2014;13:83 10.1186/1475-2891-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah P, Maxwell K, Shapiro J. Dashing away hypertension: evaluating the efficacy of the dietary approaches to stop hypertension diet in controlling high blood pressure. World J Hypertens 2015;5(4):119–128. 10.5494/wjh.v5.i4.119. [DOI] [Google Scholar]
- 27.Hummel SL, Seymour EM, Brook RD, et al. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail 2013;6(6):1165–1171. 10.1161/CIRCHEARTFAILURE.113.000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soltani S, Chitsazi M, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr 2018;37(2):542–550. 10.1016/j.clnu.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res 2005;66(2):265–275. 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Cesari M, Penninx B, Newman AB, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition Health ABC Study). Am J Cardiol 2003;92(5):522–528. 10.1016/S0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


