Abstract
The development of infection resistant materials is of substantial importance as seen with an increase in antibiotic resistance. In this project, the nitric oxide (NO)-releasing polymer has an added topcoat of zinc oxide nanoparticle (ZnO-NP) to improve NO-release and match the endogenous NO flux (0.5 – 4 × 10−10 mol cm−2 min−1). The ZnO-NP is incorporated to act as a catalyst and provide the additional benefit of acting synergistically with NO as an antimicrobial agent. The ZnO-NP topcoat is applied on a polycarbonate-based polyurethane (CarboSil) that contains blended NO donor, S-nitroso-N-acetylpenicillamine (SNAP). This sample, SNAP-ZnO, continuously sustained NO release above 0.5 × 10−10 mol cm−2 min−1 for 14 days while samples containing only SNAP dropped below physiological levels within 24 hours. The ZnO-NP topcoat improved NO release and reduced the amount of SNAP leached by 55% over a 7-day period. ICP-MS data observed negligible Zn ion release into the environment, suggesting longevity of the catalyst within the material. Compared to samples with no NO-release, the SNAP-ZnO films had a 99.03% killing efficacy against Staphylococcus aureus and 87.62% killing efficacy against Pseudomonas aeruginosa. A cell cytotoxicity study using mouse fibroblast 3T3 cells also noted no significant difference in viability between the controls and the SNAP-ZnO material, indicating no toxicity towards mammalian cells. The studies indicate that the synergy of combining a metal ion catalyst with a NO-releasing polymer significantly improved NO-release kinetics and antimicrobial activity for device coating applications.
Keywords: Nitric Oxide, Antimicrobial, Zinc Oxide Nanoparticle, Catalysis, Medical Device Coating
1. INTRODUCTION
One of the most common problems with implanted medical devices is the increased susceptibility of the patients to infections.1 Infections attributed to medical devices, otherwise known as healthcare associated infections (HAIs), have led to various complications like increased healthcare costs, medical device failure, and unnecessary deterioration in a patient’s health.2 While the Centers for Disease Control and Prevention estimates that 1 out of every 25 hospitalized patients is affected, HAIs are increasingly linked to mortality and morbidity.3 Some of the most common types of HAIs include catheter associated urinary tract infections, surgical site infections, and bloodstream infections. The need to prevent and control HAIs is evident; such infections can be transmitted between different healthcare facilities and their prevention can result up to $31.5 billion in medical cost savings.3
While infection can be managed using several strategies, prevention of infections by anti-fouling and antimicrobial materials for medical devices has been studied extensively.4 Although some of the most successful strategies include both active and passive agents, active agents are most widely studied due to their higher rate of success in preventing infections in the long term as passive surfaces can be fouled over time. Passive materials such as polyethylene glycol, zwitterionic polymers and other hydrophilic polymers are unable to kill the pathogens themselves and can also be fouled over time through settling of other biomacromolecules, which in turn can attract microbes. Therefore, agents such as silver nanoparticles (Ag-NPs), antibiotics, chlorhexidine, triclosan, quaternary ammonium ions, antimicrobial peptides, and nitric oxide (NO) have been studied widely.5
The often miraculous roles of NO in several biological applications, ranging from nerve signals to gut functions, have been studied aggressively since 1992 when it was awarded the “Molecule of the Year” by The American Association for the Advancement of Science. Since NO’s half-life is very short in physiological conditions, NO is transported in the form of endogenous S-nitrosothiols (RSNOs, e.g. S-nitrosoglutathione (GSNO), S-nitrosoalbumin, S-nitrosocysteine).6 S-nitrosothiols degrade to release NO and form a disulfide.7 However, over the last two decades, NO release from both endogenous and synthetic donors has also been studied for the purpose of antimicrobial medical device coatings and wound healing applications.5,8–14 While NO donors like N-diazeniumdiolates have been researched extensively, their disadvantages include low NO release, cytotoxicity towards mammalian cells, and by-products that are not approved by the FDA.15–17 Similar to GSNO, S-nitroso-N-acetylpenicillamine (SNAP) is another RSNO, but is synthetic and has a longer shelf-life with increased NO donor capacity in polymers.18,19 SNAP has been studied extensively in different polymers and thus is a well-characterized NO donor with the least cytotoxicity towards mammalian cells since the release of NO leads to FDA approved by-products.20
Zinc oxide is another antimicrobial but it is already commercially used and is known to have less cytotoxicity towards mammalian cells while having similar antibacterial effects at the same concentration when compared to other commonly used antimicrobial metal ions such as copper and silver.21 It inhibits the growth of dental caries-related bacteria, Streptococcus mutans, Actinomyces viscosus, Lactobacillus casei, Staphylococcus aureus (S. aureus), and Candida albicans.22 Zinc oxide nanoparticles (ZnO-NPs) have been found to inhibit growth and cause loss of cell viability in Escherichia coli (E. coli) and S. aureus at concentrations ranging from as low as 1 mM up to 3.4 mM. The same concentrations also had minimal effects on primary human T cell viability.23 Metal ions with high affinity for sulfur like Zn ions tend to inhibit glycolysis within microorganisms by oxidizing thiols groups in essential glycolytic enzymes. Coupled with low toxicity towards mammalian cells, ZnO-NPs are a good example of metal ion nanoparticles that are required in low concentrations for higher antimicrobial effects.24
As of yet, no studies have been conducted to demonstrate the increased antimicrobial activity of biomaterials that contain both NO releasing properties and ZnO-NP coated surfaces. The hybrid material fabricated in this study containing both ZnO-NP and NO donor capacity will serve two purposes: 1) provide a synergistic effect of antimicrobial properties by combining different mechanisms of bactericidal properties exhibited by NO and ZnO -NPs, and 2) the catalytic release of NO in the presence of a ZnO-NP topcoat.
While the enhanced biological effects of NO releasing materials have been studied with metal ions like iron and copper,25–27 and polyurethane/metal organic framework composite materials,28 the catalytic effects of a much more mammalian cell friendly metal ion, ZnO-NPs, has not been studied until now. In the past, the effect of Zn2+ on its ability to generate NO from SNAP has been studied using a Zn wire and a solution for in vivo biodegradable bare stent and has been found to elevate NO release.29 However, the enhanced biological effects including increased antimicrobial activity and lower cytotoxic effects of ZnO-NPs on NO releasing polymers have not been studied.
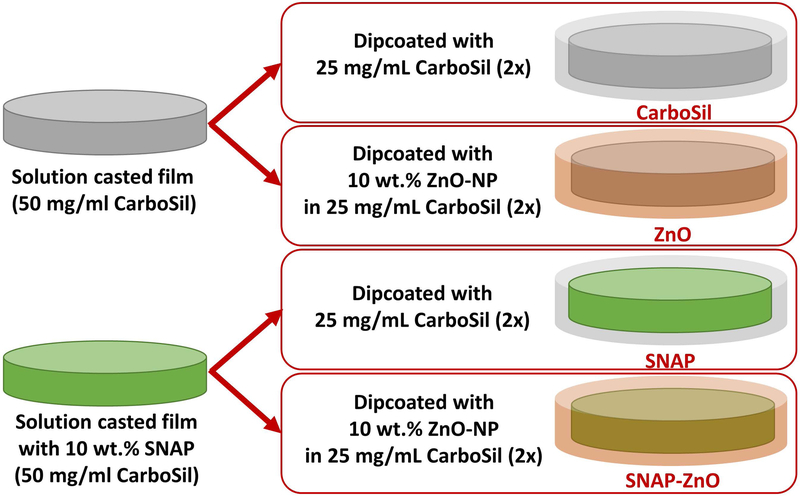
As discussed herein, we have attempted to fabricate, study, and demonstrate the catalytic and antimicrobial properties of a hybrid material SNAP-ZnO (Figure 1). The base polymer used for the fabrication was CarboSil, a thermoplastic silicone-polycarbonate-urethane (TSPCU, DSM Biomedical). It is a biocompatible and biostable polymer that is thromboresistant in nature and can be processed using different techniques. ZnO-NPs were topcoated on the NO-releasing polymer to enhance infection resistant properties of potential medical coatings. Different concentrations of ZnO-NPs were dispersed in previously established concentrations of NO-releasing polymer topcoats and studied for leaching properties of SNAP. Once the lowest leaching (highest SNAP storage) combination is determined, the hybrid sample is then used to investigate synergistic properties of NO and ZnO-NP in antimicrobial and cytotoxicity studies. Studies for up to 14 days of elevated NO release and 24-hour antimicrobial effects have been presented. Along with proof of antimicrobial efficacy of the material, cytotoxic studies are performed to ensure mammalian cell friendly nature of the final product.
Figure 1.
Fabrication process of four main tested samples in antimicrobial and cytotoxicity tests. CarboSil, ZnO, SNAP, SNAP-ZnO.
2. Materials and Methods
2.1. Materials
CarboSil® 2080A UR STPU (referred to as CarboSil hereon) was acquired from DSM Biomedical Inc. (Berkeley, CA). Anhydrous tetrahydrofuran (THF), N-acetyl-D-penicillamine (NAP), sodium nitrite (NaNO2), concentrated sulfuric acid (H2SO4), phosphate buffered saline (PBS), ZnO-NPs, cell counting kit-8 and ethylenediamine tetraacetic acid (EDTA) were obtained from Sigma Aldrich (St. Louis, MO). Concentrated hydrochloric acid (conc. HCl), and methanol were bought from Fisher-Scientific (Hampton, NH). CarboSil™ 2080A (CarboSil) was obtained from DSM Biomedical Inc. (Berkeley, CA). Gram-positive Staphylococcus aureus (ATCC 6538) and Gram-negative Pseudomonas aeruginosa (ATCC 27853, P. aeruginosa) were originally obtained from American Type Culture Collection (Manassas, VA). Milli-Q filter was used to obtain deionized (DI) water for all the aqueous solution preparations. Nitrogen and oxygen gas cylinders were purchased from Airgas (Kennesaw, GA). LB Agar (LA), Miller and Luria broth (LB), Lennox were purchased from Fischer BioReagents (Fair Lawn, NJ).
2.2. Synthesis of S-nitroso-N-acetyl-penicillamine (SNAP)
The protocol for the synthesis of SNAP was followed from a previously reported method with slight modifications.30 Briefly, 2M HCl and 2M H2SO4 were added to a beaker containing a 1:1 mixture by volume of methanol and water, followed by an equimolar ratio of NAP and NaNO2. The solution was stirred for 30 minutes then moved to an ice bath to facilitate the precipitation of the SNAP crystals. After 6 hours, the crystals were collected via vacuum filtration and dried for 24 hours. The entire process and crystals obtained were shielded from light throughout the entire duration of the experiment.
2.3. Fabrication of ZnO-NP loaded-NO Releasing Films
The bulk of the films were made using solvent casting method and top coats were added using dip coating (both techniques have been previously described and published).30,31 Briefly, SNAP films were prepared by dissolving CarboSil in THF for a final concentration of 50 mg ml-1. CarboSil is a polycarbonate-based polyurethane that contains a silicone segment and is marketed by DSM. It has been used previously by our and other groups and has been found to have stable NO-releasing properties when incorporated with SNAP.30–32 (Exact properties are not public knowledge but all details are available on the company’s website.) When CarboSil was fully dissolved, 10 wt.% SNAP was added to the solution. The solution was then poured into a Teflon™ mold and left to dry in the dark, overnight. Dried films were cut into circular disks with diameters of 8 mm. Then, various solutions of 25 mg ml−1 of CarboSil were prepared separately (containing 0, 1, 5, and 10 wt.% ZnO-NP). The circular films were top coated twice with the prepared solution containing 0, 1,5 or 10 wt.% ZnO-NP by dipping the films in the solution and allowing 10 minutes of drying between coats. The ZnO-NP used was purchased from Sigma-Aldrich. The size of the ZnO-NP was specified as <50nm in size and >97% purity. All films were allowed 24 hours to fully dry before being used for experiments.
Following is a table (Table 1) for all the compositions used along with the sample names.
Table 1.
Composition for each sample.
| Sample Fabrication | ||
|---|---|---|
| SAMPLE NAME | BASE FILM | TOPCOAT |
| CarboSil | 50 mg/ml CarboSil | 2 dips of 25 mg/ml CarboSil solution |
| ZnO-1 | 50 mg/ml CarboSil | 2 dips of 25 mg/ml CarboSil solution containing 1 wt.% ZnO-NP |
| ZnO-5 | 50 mg/ml CarboSil | 2 dips of 25 mg/ml CarboSil solution containing 5 wt.% ZnO-NP |
| ZnO | 50 mg/ml CarboSil | 2 dips of 25 mg/ml CarboSil solution containing 10 wt.% ZnO-NP |
| SNAP | 50 mg/ml CarboSil with 10 wt.% SNAP | 2 dips of 25 mg/ml CarboSil solution |
| SNAP-ZnO-1 | 50 mg/ml CarboSil with 10 wt.% SNAP | 2 dips of 25 mg/ml CarboSil solution containing 1 wt.% ZnO-NP |
| SNAP-ZnO-5 | 50 mg/ml CarboSil with 10 wt.% SNAP | 2 dips of 25 mg/ml CarboSil solution containing 5 wt.% ZnO-NP |
| SNAP-ZnO | 50 mg/ml CarboSil with 10 wt.% SNAP | 2 dips of 25 mg/ml CarboSil® solution containing 10 wt% ZnO-NP |
2.4. SNAP Leaching Analysis
Prepared circular films were tested for leaching of SNAP using UV-Vis spectrophotometry. The SNAP leached into the PBS used to soak the films was measured by detecting the absorbance at 340 nm wavelength (maximum absorbance for S-nitroso bond in SNAP) at various time points over 7 days. Samples were weighed before applying the topcoats to determine the amount of SNAP initially present. After application of topcoats, films were soaked in PBS (with EDTA) at 37°C for the duration of the study. Measurements were compared to a calibration curve.
2.5. Energy-dispersive X-ray spectroscopy
Scanning electron microscopy (SEM, FEI Teneo, FEI Co.) fitted with a large detector Energy dispersive X-ray spectroscopy (EDS, Oxford Instruments) system was employed at an accelerating voltage of 10.00 kV to examine the presence and elemental mapping of SNAP and ZnO-NP particles throughout the surfaces fabricated.
2.6. Nitric Oxide Release Measurements
Chemiluminescence of NO release from SNAP-ZnO films versus the SNAP films was measured using a Siever’s Nitric Oxide Analyzer (NOA) (Boulder, CO). Films containing SNAP (both with and without ZnO coatings) were measured for their NO release. Each sample was placed in an amber reaction cell containing PBS buffer at 37°C. EDTA was added to this PBS buffer as a chelating agent and prevent any catalytic release of NO by free metal-ions in the solution. Nitrogen gas was bubbled through the solution to purge NO from the solution. Sweep gas carried the purged NO to the detection chamber, where it was measured in PPB. Samples were measured from the NOA on days 0, 1, 3, 5, and 7. Between measurements samples were kept in PBS buffer in a 37°C incubator.
2.7. Inductively Coupled Plasma – Mass Spectroscopy (ICP-MS)
To measure the amount of metal-ion nanoparticles (ZnO in this case) in the sample leachates for the duration of the study, an ICP-MS study was conducted using a VG ICP-MS Plasma Quad 3 instrument.25 In this study, the samples containing ZnO topcoats were soaked in DMEM for 2 weeks and kept in 37°C. At the end of 2 weeks, the films were removed from the media and the media was analyzed for presence of 64Zn and 66Zn isotopes using a previously published method.33
2.8. Bacterial Adhesion Study
Bacterial adhesion study for the fabricated materials was carried out using a previously established ASTM E2180 protocol.31 This protocol was performed with very minor modifications. The samples used for the bacterial adhesion study were CarboSil, ZnO, SNAP and SNAP-ZnO. The bacteria used for antimicrobial efficacy analyses were S. aureus and P. aeruginosa. The bacteria were grown to a mid-log phase of ~106-108 CFU ml−1 in LB broth at 37°C. Following this, the bacteria were then resuspended in PBS to incubate the samples. 2 ml of bacterial solution was used for each sample and kept in a shaker incubator (37°C, 200 rpm) for 24 h. After 24 h of incubation with the bacteria, samples were rinsed with DI water to remove any unattached bacteria. The samples were then homogenized for 1 min each to remove any adhered bacteria into buffer solutions. The buffer solutions, now containing bacteria from the materials, were serially diluted (up to 10−5), plated on LB agar, and kept in the incubator (37°C). The colonies of bacteria were counted after 18 h of incubation of the plates. The average number of CFUs were normalized for the surface area of each sample exposed to the bacteria according to the following formula:
2.9. Cytotoxicity Analysis
The cytotoxicity test was performed on mouse fibroblast cells (3T3) using a recommended and previously published cell cytotoxicity assay.25 The mouse fibroblast cells were cultured from a cryopreserved vial Dulbecco’s Modified Eagle’s Media (DMEM) containing 5% glucose, 10% Fetal bovine serum and 1% antibiotics (Penn-Strep). The culture media was changed every second day until the cell confluency was 80–90%. After this step, 100 µl of 5000 cells ml−1 were seeded per well of a cell culture grade 96-well plate (n=5) and kept in a humidified incubator with 5% CO2 maintained at 37°C.
Meanwhile, leachates from the CarboSil films, SNAP-CarboSil, ZnO-CarboSil and ZnO-SNAP-CarboSil films were collected by adding 10 mg of each type of sample in 10 ml of DMEM media (n=5 for each sample type). The resulting mixture was covered in an amber vial in the incubator at 37°C for 24 hours to allow the films to leach in the DMEM medium.
After 24 hours, 10 µL of the leachates were added to each of the well-containing cells followed by incubation for 24 hours in the incubator. 10 µL of the WST-8 solution (CCK-8 kit, Sigma Aldrich) was added to the resulting mixture and incubated for 4 hours according to the manufacturer’s recommendation. The NADH released by only viable fibroblast cells converted WST-8 to formazan, an orange color product that was quantified at 430 nm using a photo plate reader. The relative viability of the cells was measured with respect to CarboSil (cells exposed to CarboSil leachates) using the formula below.
2.10. Statistical Analysis
All data are stated as mean ± standard deviation. The number of replicates for every experiment have been mentioned under methods used.
3. Results
3.1. Film Fabrication, Surface Characterization and NO Release Kinetics
Films of varying ZnO-NPs were made to test for SNAP leaching on consistent SNAP content films as mentioned on Table 1. This was done to establish the wt.% of ZnO-NP required for a longer NO-release with ideal SNAP storage. The amount of SNAP, 10 wt.%, required for sustained NO-release has already been established in previously published results.31 To determine if ZnO-NP topcoated films helped to retain SNAP within the film, a 7-day study on films stored in PBS (pH of 7.4, 37°C) was conducted. It is ideal to have high SNAP retention within the polymer in order to ensure prolonged NO release from the material, as well as avoid adverse cytotoxic effects, if any exist. After 7 days of soaking, all film types showed a high amount of SNAP retention (Figure 2, Table 2). Minimal leaching was demonstrated in the SNAP-ZnO films with only 7.75 ± 0.51 wt.% SNAP leached after 7 days, while films containing only SNAP had the most SNAP leached (13.86 ± 3.62 wt.%).
Figure 2.
SNAP leaching profile for SNAP, SNAP-ZnO-1, SNAP-ZnO-5, and SNAP-ZnO-10 films. SNAP leaching was tested over 7 days/168 hours. (n=3).
Table 2.
Complementary table for Figure 2: Weight percentage of SNAP leached.
| Wt. % of SNAP Leached | |||||||
|---|---|---|---|---|---|---|---|
| 1 hour | 4 hours | 24 hours | 48 hours | 72 hours | 96 hours | 168 hours | |
| SNAP | 1.70 ±1.55 | 3.73 ±1.94 | 5.34 ±2.11 | 7.81 ±1.98 | 9.81 ±1.98 | 11.53±2.51 | 13.86 ±2.96 |
| SNAP-ZnO-1 | 0.45 ±0.40 | 2.06 ±0.50 | 3.94 ±0.59 | 6.22 ±0.83 | 8.10 ±0.96 | 10.89 ±2.35 | 12.70 ±2.61 |
| SNAP-ZnO-5 | 0.00 ±0.00 | 1.06 ±0.32 | 2.37 ±0.41 | 4.39 ±0.51 | 5.39 ±0.26 | 6.86 ±0.34 | 8.82 ±0.26 |
| SNAP-ZnO | 0.21 ±0.30 | 1.38 ±0.34 | 2.52 ±0.30 | 4.47 ±0.66 | 4.99 ±0.39 | 6.08 ±0.40 | 7.75 ±0.41 |
(Note: The loading efficiency of SNAP in all the films with SNAP is estimated to be ~100% since the SNAP crystals are allowed to dissolve and blend into the polymer/THF solution and casted into the molds.)
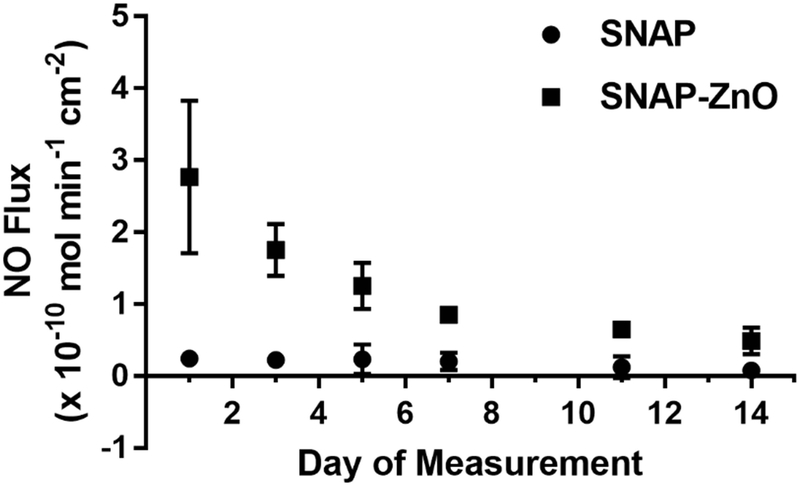
The low water uptake property of CarboSil allows for SNAP crystal formation within the polymer matrix. Exceeding the SNAP solubility threshold allows for crystallization of the molecule, thus stabilizing the NO donor to increase longevity of NO release.34,35 To reach the optimum crystallization of SNAP without sacrificing mechanical properties of the polymer, 10 wt.% SNAP was used in all films, as determined from previous work.35 It was observed that the NO flux for the SNAP-ZnO films remained in the physiological range released from the endothelium of 0.5 to 4.0 (x10−10 mol min−1 cm−2) for over 14 days (Figure 3, Table 3). While the SNAP samples had an initial burst (Day 0 not included in figure) in NO flux of 3.57 ± 0.814 (x10−10 mol min−1 cm−2), within 24 hours the flux was 0.24 ± 0.045 (x10−10 mol min−1 cm−2) and below 0.10 (x10−10 mol min−1 cm−2) by day 14. Although the average NO Flux for the SNAP-ZnO films on day 14 was 0.487 ± 0.075 (x10−10 mol min−1 cm−2), just below physiological levels, recent work has shown these levels still exhibit an antimicrobial effect.36
Figure 3.
NO release profile for SNAP vs. SNAP-ZnO films for 14 days. (n=3)
Table 3.
Complementary table for Release of NO (x10−10 mol cm−2 min−1) from SNAP films vs. SNAP-ZnO films for 14 days.
| Day 1 | Day 3 | Day 5 | Day 7 | Day 11 | Day 14 | |
|---|---|---|---|---|---|---|
| SNAP | 0.241 ±0.045 | 0.222 ±0.023 | 0.235 ±0.084 | 0.203 ±0.048 | 0.123 ±0.061 | 0.079 ±0.043 |
| SNAP-ZnO | 2.766 ±0.427 | 1.752 ±0.145 | 1.253 ±0.129 | 0.851 ±0.019 | 0.649 ±0.026 | 0.487 ±0.075 |
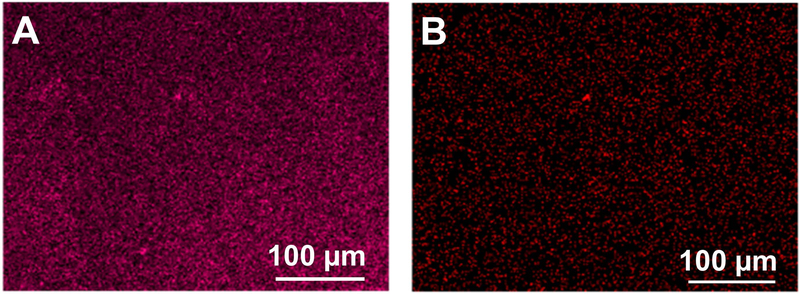
To confirm that SNAP crystals were blended and ZnO-NPs were present and evenly distributed on the surface of the samples, fabricated films (SNAP-ZnO) were mapped by EDS and analyzed for the uniform presence of zinc and sulfur. The blending of SNAP and ZnO-NPs were found to be uniform as shown in Figure 4 A and B indicating that the fabrication method had no adverse effect on the stability of SNAP or ZnO-NPs.
Figure 4.
Energy dispersive X-ray Spectroscopy images of the elements present in different coatings. A) Sulfur element map for SNAP-ZnO films and B) Zinc element map for SNAP-ZnO films.
3.2. Analysis of ZnO-NP Leaching
While Zn has many beneficial effects, and is vital for numerous physiological pathways, it is important that a majority of the ZnO-NP stays within the tested polymer films to help facilitate the catalytic NO-release from the blended SNAP. To detect for any ZnO-NP diffusion, ICP-MS was performed on 1 cm2 measured samples. Only the highest weight percent (10%) of ZnO-NPs was used for all ICP-MS studies to observe any potential leaching into the surrounding environment. After 14 days of soaking in DMEM at 37°C, ZnO films demonstrated only 1.08% of the total Zn leached into solution while SNAP-ZnO films also had a negligible 3.17% leached.
3.3. Enhanced Antimicrobial Efficacy and Low Cytotoxicity of NO-Releasing Materials Topcoated with ZnO-NP
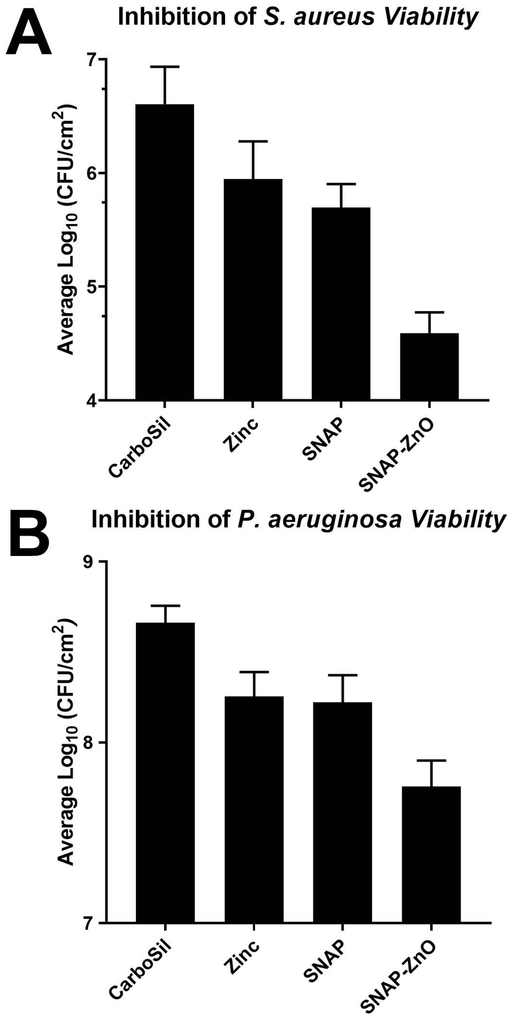
Due to the antibacterial properties of NO, active release of NO from the donor molecule incorporated in the hydrophobic polymeric films can reduce the chances of biomedical device related infections or HAIs. The adhesion of bacteria to the NO-releasing material can further be reduced increasing the NO-release or having an initial burst release followed by the synergistic bactericidal activity of a metal ion. As seen from Figure 5A, in case of S. aureus, there is a 78.02 ±25.03% reduction (~0.5 log) when only ZnO-NPs are applied as a topcoat on CarboSil samples. This is due to the bactericidal properties of ZnO-NPs as mentioned in the introduction. NO-releasing CarboSil (SNAP films) in comparison have a higher killing efficiency at 87.72 ±7.53% (~1 log) reduction due to even better bactericidal properties of diffusion based bacterial cytotoxicity of NO. However, the synergistic effects are clearly seen and very prominent as there is a 99.03 ±0.50% (~2 log) reduction in case of SNAP-ZnO films. This reduction is seen to increase when ZnO-NPs are applied as topcoat to SNAP containing polymer and hence it can be concluded that ZnO-NPs and NO have synergistic bactericidal effects against S. aureus. It is also important to note here that in addition to the higher reduction with SNAP-ZnO materials, there was also a high reduction between ZnO vs SNAP-ZnO (95.59 ±2.29%) and SNAP vs SNAP-ZnO (92.11 ±4.10%) materials.
Figure 5.
Inhibition of viable bacteria adhesion over 24-hour exposure in physiological conditions. A) Comparison in adhesion of S. aureus between CarboSil, ZnO, SNAP and SNAP-ZnO films. B) Comparison in adhesion of P. aeruginosa between CarboSil, ZnO, SNAP and SNAP-ZnO films. (n=4 for S. aureus; n=3 for P. aeruginosa).
Similar results were observed in case of P. aeruginosa but with a smaller log reduction in all the bactericidal agent containing films (Figure 5B). This may be attributed to the extra cell membrane that Gram negative bacteria like P. aeruginosa have. A 60.98 ±14.18% (~0.5 log) reduction was seen in ZnO, and a 63.76 ±14.88% reduction for SNAP materials was seen when compared to CarboSil. Although when both the bactericidal agents were combined, SNAP-ZnO materials yielded an 87.63 ±4.86% (~1 log) reduction when compared to CarboSil samples. All of these reductions were significant with a p value<0.05. This higher reduction is seen as a synergistic effect of ZnO-NPs and NO’s antimicrobial activity. In addition to these reductions, there was also a high reduction between ZnO vs SNAP-ZnO (65.86 ±13.42%) and SNAP vs SNAP-ZnO (68.29 ±12.46) materials. Thus, from the antimicrobial assays used to test the synergistic combination of ZnO nanoparticles with NO donor, the hybrid materials were able to demonstrate superior infection-resistant properties that can be applied to medical device coatings (Table 4).
Table 4.
Reduction of bacteria/cm2 seen on test samples compared to CarboSil
| Reduction in S. aureus (%) | Reduction in P. aeruginosa (%) | |
|---|---|---|
| CarboSil vs. ZnO | 78.02 ±25.03 | 60.98 ±14.18 |
| CarboSil vs. SNAP | 87.72 ±7.53 | 63.76 ±14.88 |
| CarboSil vs. SNAP-ZnO | 99.03 ±0.50 | 87.63 ±4.86 |
| ZnO vs. SNAP-ZnO | 95.59 ±2.29 | 65.86 ±13.42 |
| SNAP vs. SNAP-ZnO | 92.11 ±4.10 | 68.29 ±12.46 |
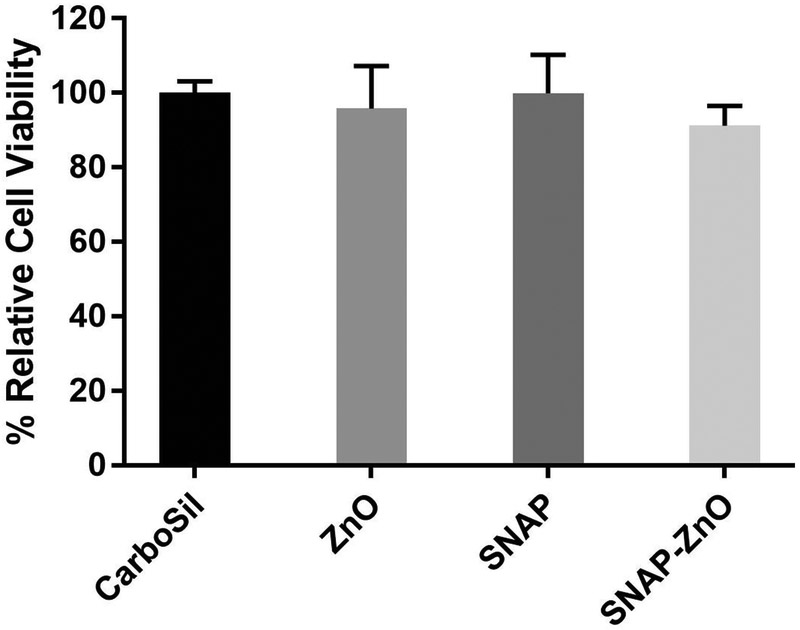
The WST-8 dye-based test showed that there was no significant difference in the viability when the CarboSil was compared with cells exposed to leachates from SNAP, ZnO or SNAP-ZnO materials (Figure 6). This means that the material does not possess any cytotoxicity toward mouse fibroblast cells. This negligible cytotoxicity was expected based from the leaching test results of Zn ions (ICP-MS results) and SNAP and serves as a proof-of-concept for the potential biocompatibility of the material.
Figure 6.
Percentage relative cell viability of mouse fibroblast cells after 24-hour exposure to leachates from CarboSil, ZnO, SNAP and SNAP-ZnO films. (n=3).
4. Discussion
Previous work has shown SNAP-blended polymers to release NO on the low end of the physiological range.20 This is in part due to the need for hydrophobic polymers requiring a thin top coat as a support for SNAP to prevent rapid leaching of the molecule. The hydrophobicity of these polymers delays or prevents moisture from reaching the SNAP molecule to elicit NO release. We hypothesized that the addition of a metal ion, specifically Zn, would catalytically increase NO release from SNAP within the hydrophobic polymers without unnecessary SNAP leaching. For this study we used a biocompatible, medical grade thermoplastic urethane copolymer, CarboSil, as a support for SNAP.
This higher retention of SNAP within SNAP-ZnO may be contributed to the ZnO-NP content in the topcoat as all other variables are left consistent among the tested samples. The metal ion-blended topcoat may slow SNAP’s ability to pass through the polymer layer. The catalytic effect of the ZnO-NP within the topcoat could also be facilitating a quicker degradation of the SNAP as it diffuses, resulting in the leaching of NAP instead which would be undetectable at the measured SNAP UV wavelength (340 nm). However, since NAP has been demonstrated in the past to be non-cytotoxic and is even used in heavy metal chelation therapy, the possible leaching of this would not be problematic.37 Due to the low leaching results of SNAP from the 10wt.% of ZnO-NPs, subsequent studies (NO release measurement, EDS mapping, antibacterial efficacy, cytotoxicity) were performed with SNAP-ZnO as the proposed hybrid material demonstrated reduced loss of SNAP from the material despite having the exact same coatings as the other materials and differing only in the ZnO-NP content (10 wt.% compared to 1% and 5%).
From the low leaching characteristics of SNAP-ZnO samples, it was predicted that the samples would also have an extended NO release. As expected, the improved NO release from SNAP-ZnO films showed a promising outlook for long-term indwelling medical device applications to reduce device-associated infections. The constantly higher release of NO from the SNAP-ZnO samples confirmed them to be ideal for testing antimicrobial efficacy and cytocompatibility.
Following NO storage and release characterization, the materials were tested for distribution of the SNAP and metal ion elements. The uniform distribution was a good indication that the coating method is reliable to be used for microbial adhesion prevention on the medical device’s surface.
The decomposition of all RSNOs into radical NO and disulfides is facilitated through the homolytic cleavage of the sulfur-nitroso bond which can be accelerated by the administration of certain transition metal ions. The reduction of Cu2+ metal ions to Cu+ has been thoroughly investigated in its catalytic properties with RSNOs.38 While the exact mechanism is still being investigated, Zn has previously been reported to display a similar catalytic effect.29 The Zn2+ mediated reactivity of RSNOs has a more unique proposed mechanism when compared to Cu+ as it keeps its ionic state consistent throughout the catalytic process. After RSNO degradation to RS- + NO, residual RS- molecules end up forming disulfide RSSR compounds when in an aqueous environment. Zn is able to form a complex with these residual RS- ions to prevent disulfide bond formation, allowing for possible regeneration of RSH molecules after complete NO-release and subsequent renitrosation into its original RSNO form.39 The presence of these thiols would also assist in the increased NO release of Zn incorporated SNAP films as RSH molecules have been demonstrated to have destabilizing effects to RSNOs.40
The minimal leaching of Zn ions from the SNAP-ZnO samples (3.17%) shows how well encapsulated the nanoparticles were within the topcoats of the synthesized CarboSil polymer films and demonstrates the potential longevity of their catalytic activity. As NO is emitted from the polymer into an aqueous environment, trace amounts of nitric acid are formed at the material interface which can increase the potential Zn solubility and account for the slightly higher leachate result. The increase in Zn leaching from the SNAP-ZnO films could also assist in an increased antimicrobial activity.
Bacterial adhesion is a common challenge faced by medical implants. It is triggered in response to medical devices coming in contact with the fluidic biological milieu provided by the human physiology and the surgical wound provided during the insertion of the medical device. This bacterial adhesion in the first few hours of implantation can lead to infection of the site and further cause medical device failure or even death. Furthermore, S. aureus and P. aeruginosa are two of the most commonly found nosocomial pathogens. Due to the stated reasons, a bacterial adhesion study was carried out for the fabricated materials for 24 hours using S. aureus and P. aeruginosa. Another important point to note here is that both bacteria studies were done after an initial 24 hours soaking of the materials in PBS. This step allowed for initial metal-ion and SNAP leachates to be removed from the study and hence prevent any false results due to higher percentage of leachates during bacterial incubation. For both the bacteria tested, the SNAP-ZnO samples showed a greater amount of reduction in viable bacteria adhesion than any other samples. This increased antimicrobial activity was a consequence of improved NO release as well as the combined antimicrobial activity of ZnO-NPs with NO. As seen from the results, even though NO and ZnO-NPs do exhibit antimicrobial activities by themselves, they definitely work additively when combined in the SNAP-ZnO material.
While the material demonstrated controlled NO release and antibacterial efficacy, it was important to validate that the material is not toxic to the mammalian cells. In a real-life scenario, this would mean protecting the host tissue from toxic side effects during the time of application.
While the same concentration of SNAP by itself for NO-release has shown negligible cytotoxicity in the past,41 copper nanoparticles have also assisted NO-release from SNAP and demonstrated no toxicity towards mammalian cells.25 However, this was the first time that similar results have been recorded with the SNAP-ZnO combination. Another advantage of such NO based strategies is that it is highly compatible with other bacteriostatic or bactericidal approaches such as zwitterions, quaternary ammonium ions, silicone oil, and diatomaceous earth particle.31,42–44 The non-cytotoxic nature combined with the antibacterial properties offers a potential alternative therapeutic option instead of silver nanoparticles or antibiotics which have the issue of cytotoxicity or bacterial resistance.
5. Conclusion
This work demonstrates the potential beneficial effects of ZnO-NPs on the catalytic release and antimicrobial effects of NO under physiological conditions. The hybrid material, SNAP-ZnO, was composed of a base film (50 mg ml−1 CarboSil with 10 wt.% SNAP) and a topcoat (2 dips of 25 mg ml−1 CarboSil solution containing 10 wt.% ZnO-NP). The 10 wt.% of ZnO-NPs in SNAP-ZnO samples was able to retain more SNAP molecules within it (7.75 wt.% loss of SNAP) when compared to samples with no (13.85 wt.%), 1 (12.697 wt.%) or 5 (8.821 wt.%) wt.% ZnO-NPs in them. EDS-mapping showed that the hybrid material, SNAP-ZnO, had a uniform distribution of ZnO-NPs and SNAP molecules in it. NO release measurements also demonstrated SNAP-ZnO’s ability to maintain physiological levels of NO release for up to 14 days. Antibacterial efficacy at 99.03 ±0.50% and 87.63 ±4.86% reduction for S. aureus and P. aeruginosa, respectively, were promising results. Finally, low cytotoxicity results demonstrated by SNAP-ZnO samples establish the need to fabricate antibacterial materials that are mammalian cell friendly.
These studies represent preliminary data that can be used to design a long-term antimicrobial and biocompatible device coating that has high potential for use in different implantable materials. In the future, more similar mammalian cell friendly metal-ions, such as iron or magnesium, that can be combined with nitric oxide donors to tune NO release for biomedical purposes can be designed for longer term use that can be tested with in vivo conditions.45
Acknowledgments
Funding Sources
The authors acknowledge the financial support of the National Institutes of Health (K25HL111213 and R01HL134899).
Footnotes
Conflict of Interest
The authors declare no conflict of interest. No benefit of any kind will be received either directly or indirectly by all the authors in this manuscript.
References
- 1.von Eiff C, Jansen B, Kohnen W, Becker K. Infections associated with medical devices: pathogenesis, management and prophylaxis. Drugs 2005;65(2):179–214. [DOI] [PubMed] [Google Scholar]
- 2.Harding JL, Reynolds MM. Combating medical device fouling. Trends Biotechnol 2014;32(3):140–6. [DOI] [PubMed] [Google Scholar]
- 3.Prevention CfDCa. Healthcare-Associated Infections. [Google Scholar]
- 4.Donlan RM. Biofilms and device-associated infections. Emerging Infectious Diseases 2001;7(2):277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singha P, Locklin J, Handa H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomaterialia 2017;50:20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Sa’doni H, Ferro A. S-Nitrosothiols: a class of nitric oxide-donor drugs. Clin Sci (Lond) 2000;98(5):507–20. [PubMed] [Google Scholar]
- 7.Al-Sa’doni HH, Khan IY, Poston L, Fisher I, Ferro A. A Novel Family of S-Nitrosothiols: Chemical Synthesis and Biological Actions. Nitric Oxide 2000;4(6):550–560. [DOI] [PubMed] [Google Scholar]
- 8.Nablo BJ, Chen T-Y, Schoenfisch MH. Sol−Gel Derived Nitric-Oxide Releasing Materials that Reduce Bacterial Adhesion. Journal of the American Chemical Society 2001;123(39):9712–9713. [DOI] [PubMed] [Google Scholar]
- 9.Hetrick EM, Schoenfisch MH. Antibacterial nitric oxide-releasing xerogels: Cell viability and parallel plate flow cell adhesion studies. Biomaterials 2007;28(11):1948–1956. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter AW, Schoenfisch MH. Nitric oxide release: part II. Therapeutic applications. Chem Soc Rev 2012;41(10):3742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Wang X, Suchyta DJ, Schoenfisch MH. Antibacterial Activity of Nitric Oxide-Releasing Hyperbranched Polyamidoamines. Bioconjugate Chemistry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Annich GM, Miskulin J, Stankiewicz K, Osterholzer K, Merz SI, Bartlett RH, Meyerhoff ME. Nitric Oxide-Releasing Fumed Silica Particles: Synthesis, Characterization, and Biomedical Application. Journal of the American Chemical Society 2003;125(17):5015–5024. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds MM, Frost MC, Meyerhoff ME. Nitric Oxide-Releasing Hydrophobic Polymers: Preparation, Characterization, And Potential Biomedical Applications. Free Radical Biology & Medicine 2004;37(7):926–936. [DOI] [PubMed] [Google Scholar]
- 14.Brisbois EJ, Handa H, Major TC, Bartlett RH, Meyerhoff ME. Long-term nitric oxide release and elevated temperature stability with S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As polymer. Biomaterials 2013;34(28):6957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backlund CJ, Worley BV, Schoenfisch MH. Anti-biofilm action of nitric oxide-releasing alkyl-modified poly (amidoamine) dendrimers against Streptococcus mutans. Acta biomaterialia 2016;29:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai W, Wu J, Xi C, Meyerhoff ME. Diazeniumdiolate-doped poly(lactic-co-glycolic acid)-based nitric oxide releasing films as antibiofilm coatings. Biomaterials 2012;33(32):7933–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handa H, Brisbois EJ, Major TC, Annich GM, Meyerhoff ME, Bartlett RH. Prevention of thrombosis and infection using diazeniumdiolate-based nitric oxide releasing coatings in short-term and long-term animal models. 2013. AMER CHEMICAL SOC 1155 16TH ST, NW, WASHINGTON, DC 20036 USA. [Google Scholar]
- 18.Hogg N THE BIOCHEMISTRY AND PHYSIOLOGY OF S-NITROSOTHIOLS. Annual Review of Pharmacology and Toxicology 2002;42(1):585–600. [DOI] [PubMed] [Google Scholar]
- 19.Goudie MJ, Singha P, Hopkins SP, Brisbois EJ, Handa H. Active release of an antimicrobial and antiplatelet agent from a non-fouling surface modification. ACS Applied Materials & Interfaces 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goudie MJ, Brisbois EJ, Pant J, Thompson A, Potkay JA, Handa H. Characterization of an S-nitroso-N-acetylpenicillamine–based nitric oxide releasing polymer from a translational perspective. International Journal of Polymeric Materials and Polymeric Biomaterials 2016;65(15):769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning C, Wang X, Li L, Zhu Y, Li M, Yu P, Zhou L, Zhou Z, Chen J, Tan G and others. Concentration Ranges of Antibacterial Cations for Showing the Highest Antibacterial Efficacy but the Least Cytotoxicity against Mammalian Cells: Implications for a New Antibacterial Mechanism. Chemical research in toxicology 2015;28(9):1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yakoob J, Abbas Z, Usman MW, Awan S, Naz S, Jafri F, Hamid S, Jafri W. Comparison of Antimicrobial Activity of Zinc Chloride and Bismuth Subsalicylate Against Clinical Isolates of Helicobacter pylori. Microbial Drug Resistance 2013;20(4):305–309. [DOI] [PubMed] [Google Scholar]
- 23.Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Applied Physics Letters 2007;90(21):213902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi E-K, Lee H-H, Kang M-S, Kim B-G, Lim H-S, Kim S-M, Kang I-C. Potentiation of bacterial killing activity of zinc chloride by pyrrolidine dithiocarbamate. The Journal of Microbiology 2010;48(1):40–43. [DOI] [PubMed] [Google Scholar]
- 25.Pant J, Goudie MJ, Hopkins SP, Brisbois EJ, Handa H. Tunable Nitric Oxide Release from S-Nitroso-N-acetylpenicillamine via Catalytic Copper Nanoparticles for Biomedical Applications. ACS Applied Materials & Interfaces 2017;9(18):15254–15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wonoputri V, Gunawan C, Liu S, Barraud N, Yee LH, Lim M, Amal R. Copper Complex in Poly(vinyl chloride) as a Nitric Oxide-Generating Catalyst for the Control of Nitrifying Bacterial Biofilms. ACS Applied Materials & Interfaces 2015;7(40):22148–22156. [DOI] [PubMed] [Google Scholar]
- 27.Wonoputri V, Gunawan C, Liu S, Barraud N, Yee LH, Lim M, Amal R. Iron Complex Facilitated Copper Redox Cycling for Nitric Oxide Generation as Nontoxic Nitrifying Biofilm Inhibitor. ACS Applied Materials & Interfaces 2016;8(44):30502–30510. [DOI] [PubMed] [Google Scholar]
- 28.Harding JL, Reynolds MM. Composite materials with embedded metal organic framework catalysts for nitric oxide release from bioavailable S-nitrosothiols. Journal of Materials Chemistry B 2014;2(17):2530–2536. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy CW, Guillory RJ, Goldman J, Frost MC. Transition-Metal-Mediated Release of Nitric Oxide (No) from S-Nitroso-N-Acetyl-D-Penicillamine (SNAP): Potential Applications for Endogenous Release of No at the Surface of Stents Via Corrosion Products. ACS applied materials & interfaces 2016;8(16):10128–10135. [DOI] [PubMed] [Google Scholar]
- 30.Singha P, Pant J, Goudie MJ, Workman CD, Handa H. Enhanced antibacterial efficacy of nitric oxide releasing thermoplastic polyurethanes with antifouling hydrophilic topcoats. Biomaterials Science 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q, Singha P, Handa H, Locklin J. Covalent Grafting of Antifouling Phosphorylcholine-Based Copolymers with Antimicrobial Nitric Oxide Releasing Polymers to Enhance Infection-Resistant Properties of Medical Device Coatings. Langmuir 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha KH, Meyerhoff ME. Compatibility of Nitric Oxide Release with Implantable Enzymatic Glucose Sensors Based on Osmium (III/II) Mediated Electrochemistry. ACS Sensors 2017;2(9):1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanhoe H, Vandecasteele C, Versieck J, Dams R. Determination of iron, cobalt, copper, zinc, rubidium, molybdenum, and cesium in human serum by inductively coupled plasma mass spectrometry. Analytical Chemistry 1989;61(17):1851–1857. [DOI] [PubMed] [Google Scholar]
- 34.Wo Y, Li Z, Colletta A, Wu J, Xi C, Matzger AJ, Brisbois EJ, Bartlett RH, Meyerhoff ME. Study of crystal formation and nitric oxide (NO) release mechanism from S-nitroso-N-acetylpenicillamine (SNAP)-doped CarboSil polymer composites for potential antimicrobial applications. Composites Part B: Engineering 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wo Y, Li Z, Brisbois EJ, Colletta A, Wu J, Major TC, Xi C, Bartlett RH, Matzger AJ, Meyerhoff ME. Origin of long-term storage stability and nitric oxide release behavior of carbosil polymer doped with S-nitroso-N-acetyl-D-penicillamine. ACS applied materials & interfaces 2015;7(40):22218–22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundaram J, Pant J, Goudie MJ, Mani S, Handa H. Antimicrobial and physicochemical characterization of biodegradable, nitric oxide-releasing nanocellulose–chitosan packaging membranes. Journal of agricultural and food chemistry 2016;64(25):5260–5266. [DOI] [PubMed] [Google Scholar]
- 37.Kark RP, Poskanzer DC, Bullock JD, Boylen G. Mercury poisoning and its treatment with N-acetyl-D, L-penicillamine. New England Journal of Medicine 1971;285(1):10–16. [DOI] [PubMed] [Google Scholar]
- 38.Williams DLH. The chemistry of S-nitrosothiols. Accounts of chemical research 1999;32(10):869–876. [Google Scholar]
- 39.Kozhukh J, Lippard SJ. Zinc Thiolate Reactivity toward Nitrogen Oxides: Insights into the Interaction of Zn2+ with S-Nitrosothiols and Implications for Nitric Oxide Synthase. Inorganic chemistry 2012;51(13):7346–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of Nitric Oxide Release from S-Nitrosothiols. Journal of Biological Chemistry 1996;271(31):18596–18603. [DOI] [PubMed] [Google Scholar]
- 41.Pant J, Goudie MJ, Chaji SM, Johnson BW, Handa H. Nitric oxide releasing vascular catheters for eradicating bacterial infection. Journal of Biomedical Materials Research Part B: Applied Biomaterials;0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pant J, Gao J, Goudie MJ, Hopkins SP, Locklin J, Handa H. A multi-defense strategy: Enhancing bactericidal activity of a medical grade polymer with a nitric oxide donor and surface-immobilized quaternary ammonium compound. Acta Biomaterialia 2017;58(Supplement C):421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goudie MJ, Pant J, Handa H. Liquid-infused nitric oxide-releasing (LINORel) silicone for decreased fouling, thrombosis, and infection of medical devices. Scientific Reports 2017;7(1):13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grommersch B, Pant J, Hopkins SP, Goudie MJ, Handa H. Bio-Templated Synthesis and Characterization of Mesoporous Nitric Oxide-Releasing Diatomaceous Earth Silica Particles. ACS Applied Materials & Interfaces 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T, Janczuk AJ. Nitric Oxide Donors: Chemical Activities and Biological Applications. Chemical Reviews 2002;102(4):1091–1134. [DOI] [PubMed] [Google Scholar]