Abstract
Little is known about the temporal trends and outcomes for extra-corporeal membrane oxygenation (ECMO) in patients with high-risk pulmonary embolism (PE) in the United States. We queried the National Inpatient Sample (NIS) database from 2005 to 2013 to identify patients admitted with high-risk PE. Our objective was to determine trends for ECMO use in patients with high-risk PE. We also assessed in-hospital outcomes among patients with high-risk PE receiving ECMO. We evaluated 77,809 hospitalizations for high-risk PE. There was an upward trend in the utilization of ECMO from 0.07% in 2005 to 1.1% in 2013 (p = 0.015). ECMO was utilized more in urban teaching hospitals and large hospitals. ECMO use was associated with lower mortality in patients with massive PE (p < 0.001). In-hospital mortality for patients receiving ECMO was 61.6%, with no change over the observational period (p = 0.68). Our investigation revealed several independent predictors of increased mortality in patients with high-risk PE using ECMO as hemodynamic support, including: age, female sex, obesity, congestive heart failure, and chronic pulmonary disease. ECMO, therefore, as a rescue strategy or bridge to definitive treatment, may be effective in the management of high-risk PE when selecting patients with favorable clinical characteristics.
Keywords: extra-corporeal membrane oxygenation (ECMO), high-risk pulmonary embolism (PE), respiratory failure, shock
Introduction
High-risk or ‘massive’ pulmonary embolism (PE) manifests as acute right ventricular dysfunction and is defined by sustained hypotension (systolic blood pressure < 90 mmHg) for at least 15 minutes assuming there is no secondary cause for the hypotension.1,2 High-risk PE has a 30-day mortality ranging from 25% to 65%.3 Despite advances in the therapeutic armamentarium, including systemic thrombolytic agents, catheter-directed therapies (CDT), and surgical embolectomy, many patients remain refractory to treatment.4 Venous-arterial extra-corporeal membrane oxygenation (VA-ECMO) is a promising treatment modality to unload the acutely failing right ventricle (RV).5 The use of ECMO for high-risk PE has been evaluated only in case series or small retrospective analyses.6 Little is known about recent temporal trends regarding the use of ECMO in the context of high-risk PE. Our aim was to use the National Inpatient Sample (NIS) database as a platform to evaluate clinical features associated with ECMO use for high-risk PE in the United States, then determine if certain clinical variables predicted morbidity and mortality.
Methods
Clinical information was obtained from the NIS administrative database. The NIS is the largest all-payer inpatient database, which includes more than 100 data elements from over 7 million unweighted hospital stays each year, representing ~ 20% of hospital admissions in the US. Data from the NIS database is de-identified and publicly available and so this study was exempt from institutional review board evaluation. We queried the NIS from 2005 to 2013. We identified hospitalizations for PE with the International Classification of Diseases, Ninth Revision (ICD-9) (415.1, 415.13, and 415.19). We included hospitalizations with a primary diagnosis of PE and those with a secondary diagnosis of PE if the primary diagnosis was either respiratory failure (518.81, 518.82, 518.84, and 799.1) or deep vein thrombosis (453.4, 453.40, 453.41, 453.42, 453.8, 453.82, 453.83, and 453.9). Patients with high-risk PE were identified by mechanical ventilation (procedure codes 96.70, 96.71, and 96.72), vasopressors (procedure code 00.17), and non-septic shock (diagnosis codes 785.50, 785.51, and 785.59). Among those encounters with high-risk PE, we identified patients who received ECMO (procedural code 36.95). This search methodology for NIS was previously described.7–10 We identified individuals receiving pulmonary arteriography (procedure codes 88.43, 88.62), suggesting the possibility of catheter-directed therapy (CDT). The use of systemic thrombolytic therapy (procedure code 99.10), surgical pulmonary embolectomy (procedure code 380.5), and inferior vena cava (IVC) filter (procedure code 387) was also reported.
We report temporal trends in the utilization of ECMO in cases of high-risk PE as well as the trends for in-hospital mortality during the observational period. Baseline characteristics for patients who received ECMO were described. We reported in-hospital clinical outcomes among patients who received ECMO, including acute renal failure, length of hospital stay, a mechanical ventilation requirement, a blood transfusions requirement, hematomas, and retroperitoneal hematomas.
A multivariate regression analysis was conducted among patients with high-risk PE for in-hospital mortality, using binary regression analysis. The model included the following independent variables: age, sex, ethnicity, diabetes, hyper-tension, chronic pulmonary disease, chronic kidney disease, peripheral vascular disease, obesity, congestive heart failure, and the use of ECMO. A second model was constructed for patients with high-risk PE who received ECMO to identify predictors of mortality. This model included: age, sex, ethnicity, diabetes, hypertension, chronic pulmonary disease, chronic kidney disease, peripheral vascular disease, obesity, congestive heart failure, and the use of CDT.
All analyses were conducted by weighting samples for national estimates in conjunction with the Healthcare Cost and Utilization Project (HCUP) regulations for using the NIS database. We analyzed categorical variables using the chi-squared test and represented the numbers as percentages. Continuous variables were reported as mean ± SD of the mean, or median and IQR depending on the normality of distribution. Effect sizes of outcomes were expressed using log-transformed odds ratios (OR) with 95% CIs. Associations were considered significant if the p-value was < 0.05. SPSS software (Version 24.0, 2016; IBM Corp., Armonk, NY, USA) was used for all analyses and R software (R Foundation for Statistical Computing, Vienna, Austria) for all statistical analysis.
Results
From 2005 to 2013, 77,809 hospitalizations for high-risk PE were identified. After excluding cases with missing data for in-hospital mortality (n = 60), 77,749 unique hospitalization encounters were examined, of which 219 (0.3%) indicated the use of ECMO for high-risk PE. Table 1 describes the baseline clinical characteristics for each encounter in which ECMO was initiated for massive PE. The majority of patients receiving ECMO were male (67.6%), with a mean age of 42 ± 17.9 years. ECMO was performed 1.9 ± 4.1 days from the index admission date. The median hospital stay for encounters receiving ECMO for high-risk PE was 10 days (IQR = 19.7). ECMO utilization was more frequent in urban teaching hospitals compared to non-teaching hospitals (95.6% vs 4.4%, p < 0.001).
Table 1.
Baseline characteristics of the study population in the National Inpatient Sample with massive PE receiving ECMO therapy, 2005–2013 (n = 219 hospitalizations).
| Baseline characteristics | n = 219 |
| Age ± SD | 42 years (± 17.9) |
| Male sex | 148 (67.6%) |
| Ethnicity | |
| White | 113 (51.7%) |
| Black | 40 (18.4%) |
| Hispanic | 20 (9.1%) |
| Other | 16 (6.9%) |
| Diabetes | 65 (29.6%) |
| Hypertension | 89 (40.9%) |
| Peripheral vascular disease | 15 (6.9%) |
| Chronic kidney disease | NR |
| Congestive heart failure | 93 (42.5%) |
| Coagulopathy | 95 (43.4%) |
| Chronic pulmonary disease | 20 (9.1%) |
| Urban non-teaching hospital | NR |
| Urban teaching hospital | 209 (95.6%) |
ECMO, extra-corporeal membrane oxygenation; NR, not reportable (events less than 11 should not be reported per Healthcare Cost and Utilization Project regulations).
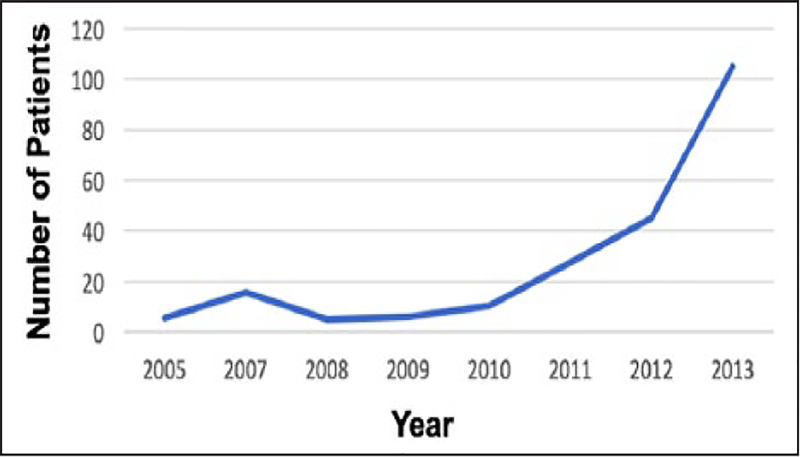
There was an upward trend in the utilization of ECMO in the population examined from 0.07% in 2005 to 1.1% in 2013 (p = 0.015) (Figure 1). Similarly, we observed an upward trend in the use of systemic thrombolytic agents in patients with high-risk PE from 7.9% in 2005 to 17.2% in 2013 (p < 0.001). In-hospital mortality was 61.6% for patients who received ECMO for high-risk PE. Between 2005 and 2013 there was no significant change in the trend of in-hospital mortality in patients receiving ECMO (p = 0.68). Among patients with high-risk PE receiving ECMO, 144 patients (66%) developed acute renal failure, 194 patients (88.8%) required mechanical ventilation, 59 patients (27%) required blood transfusion, 4.6% had hematomas, and 2.3% had retroperitoneal hematomas. The use of advanced therapies in conjunction with ECMO was noted as follows: 45 cases (20.4%) with surgical embolectomy, 54 cases (24.9%) with thrombolysis, and 69 cases (31.7%) with IVC filter placement.
Figure 1.
Interrogation of the National Inpatient Sample over the 8-year observation period revealed 77,749 hospital encounters in which massive PE was identified. In 219 of these encounters (0.28%), ECMO was utilized, with an upward trend between 2005 and 2013 (p = 0.015).
ECMO, extra-corporeal membrane oxygenation; PE, pulmonary embolism.
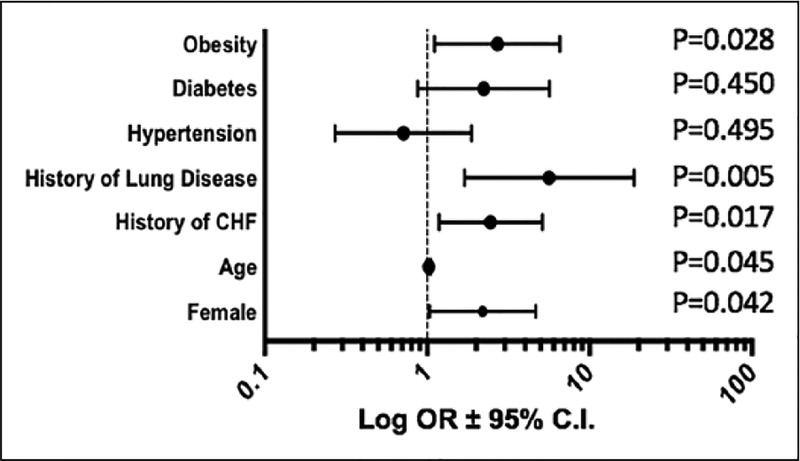
Multivariate regression analysis for patients with high-risk PE overall showed lower in-hospital mortality with ECMO use (OR = 0.34; 95% CI = 0.25 to 0.45, p < 0.001). The second multivariate regression model for patients with high-risk PE on ECMO identified the following predictors of in-hospital mortality (Figure 2): older age (OR = 1.03; 95% CI = 1.01 to 1.05, p = 0.045), female sex (OR = 2.19; 95% CI = 1.03 to 4.67, p = 0.042), congestive heart failure (OR = 2.45; 95% CI = 1.18 to 5.13, p = 0.017), chronic pulmonary disease (OR = 5.65; 95% CI = 1.70 to 18.79, p = 0.005), and obesity (OR = 2.70; 95% CI = 1.11 to 6.57, p = 0.028). Catheter-directed pulmonary procedures did not improve in-hospital mortality (OR = 1.04; 95% CI = 0.37 to 2.93, p = 0.941).
Figure 2.
Multivariate regression analysis revealed that obesity, advanced age, female sex, and a history of heart failure or chronic lung disease were independent predictors of mortality when ECMO was utilized for massive PE.
Data are shown as a forest plot with log10-transformed ORs with 95% CIs. Statistical significance is noted beside each variable.
CHF, congestive heart failure; ECMO, extra-corporeal membrane oxygenation; OR, odds ratio.
Discussion
In this observational analysis of 77,749 hospitalizations, we sought to explore the trends and outcomes for ECMO use in high-risk PE in the US. ECMO use, which increased between 2005 and 2013, was independently associated with reduced in-hospital mortality among patients with high-risk PE. The in-hospital mortality for patients receiving ECMO for high-risk PE remained high at 61.6%, which did not change during the observational period, reflecting that many patients with refractory shock from massive PE are in extremis, with few options for treatment. ECMO use for high-risk PE was utilized more frequently in urban teaching hospitals, and was associated with significant co-morbidities including acute renal failure, mechanical ventilation, and prolonged hospital stay. Among those receiving ECMO for high-risk PE, advanced age, female sex, a history of heart failure or chronic pulmonary disease, and obesity were all independently associated with higher in-hospital mortality.
The use of cardiopulmonary bypass – which ECMO is based upon – for treating high-risk PE was first described by Cooley in 1961, and may be under-utilized in patients with high-risk PE in whom circulation and/or oxygenation are inadequate.6,11 ECMO decompresses the right ventricle, bypassing the obstructed pulmonary arterial circulation ex vivo, and returns oxygenated blood to the arterial system.6 ECMO can be used as stand-alone therapy, as a bridge to surgical embolectomy, or catheter-based interventions.12 A prospective, randomized, clinical trial to evaluate ECMO in managing cardiogenic shock secondary to high-risk PE, such as massive PE, has never been conducted. The largest published study evaluating ECMO use was a systematic review in 78 patients and showed no improvement in mortality with CDT use.13 Similarly, George et al. documented that CDT in 32 patients with massive PE was not associated with improvement in adverse clinical events compared to patients managed without CDT.4 Ain et al. conducted a single-center study and reported survival rates of 58.6% for high-risk PE following the initiation of ECMO, which supports our present data.14 Meneveau et al. conducted a multicenter retrospective analysis to evaluate the outcomes for ECMO use among 52 patients with high-risk PE,15 reporting a 30-day mortality of 61.5% in patients who underwent ECMO, which was further decreased when surgical embolectomy was employed.15 In our present investigation, there was an uptrend in the use of ECMO in the US for high-risk PE associated with lower in-hospital mortality. The increase in the use of systemic thrombolytic agents during this observational period might have impacted hospital mortality also. Institutional multidisciplinary pulmonary embolism response teams (PERTs) began around 2012 in the US, and may play a role in delivering more advanced therapies, including ECMO, for patients with high-risk PE.16 One center reported that after 394 PERT activations, only 2% of patients with high-risk PE received ECMO, implying institutional capabilities and practice patterns remain important contributors to patient outcomes.17 Critical illness, derangement in the coagulation cascade, and vascular access complications are common in ECMO recipients. These may also impact in-hospital mortality.18,19 Our query of the NIS revealed that 27% of patients requiring ECMO for high-risk PE had an inpatient blood transfusion requirement.
The SAVE-score is a validated scoring system used to predict mortality for general ECMO use in refractory cardiogenic shock and is based on 13 pre-ECMO variables.5 Our data for ECMO use in high-risk PE further validate the SAVE-score, identifying advanced age, obesity, and congestive heart failure as predictors of poor survival among patients with cardiogenic shock.5 Female sex was identified by us as being associated with worse outcomes for ECMO use in the setting of high-risk PE. This observation may be attributed to the higher incidence of bleeding and vascular complications in females.
Limitations
Our study has limitations. Although the use of the NIS database provides the opportunity to study outcomes in a relatively large number of patients with high-risk PE managed by ECMO, some important prognostic data and clinical variables to account for possible confounders are not available with this analysis strategy. In addition, NIS provides only in-hospital outcomes without longitudinal data to assess long-term outcomes. Follow-up data including detailed information on RV function, pulmonary artery pressures, and incidence of chronic thromboembolic pulmonary hypertension were also lacking. We were not able to determine if this dataset represented ECMO use as stand-alone treatment or as a bridge to other definitive treatments. Certain interventions such as CDT may not be accurately coded using an administrative database such as NIS, and the available ICD-9 codes might not accurately distinguish thrombolysis delivered systemically versus catheter delivered thrombolysis. This may impact conclusions drawn about the effectiveness of CDT. Also, the observational nature of this study and lack of randomization carries the inherent risk of selection bias. Lastly, and most importantly, PERTs were initiated toward the end of the observational period used in this study, and so advanced treatment modalities and an increase in our understanding of how to manage high-risk PE will ultimately affect the incidence of ECMO use and appropriate patient selection. Despite these limitations, this analysis helps in addressing the current gap in the literature regarding the utility of ECMO in patients with high-risk PE.
Conclusion
Real-world data using the largest national database showed an increase in the utilization of ECMO in patients with high-risk PE. ECMO use was associated with lower mortality among patients with high-risk PE. Overall, ECMO as a rescue strategy in the setting of hemodynamic compromise, may be more effective in the management of high-risk PE if selecting patients with favorable clinical characteristics such as those we identify.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: K08HL128856 and NIH HL12020 to SJC.
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011; 123: 1788–1830. [DOI] [PubMed] [Google Scholar]
- 2.Konstantinides SV, Torbicki A, Agnelli G; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 3033–3069. [DOI] [PubMed] [Google Scholar]
- 3.Corsi F, Lebreton G, Bréchot N, et al. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care 2017; 21: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George B, Wallace EL, Charnigo R, et al. A retrospective analysis of catheter-based thrombolytic therapy for acute submassive and massive pulmonary embolism. Vasc Med 2015; 20: 122–130. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015; 36: 2246–2256. [DOI] [PubMed] [Google Scholar]
- 6.George B, Parazino M, Omar HR, et al. A retrospective comparison of survivors and non-survivors of massive pulmonary embolism receiving veno-arterial extracorporeal membrane oxygenation support. Resuscitation 2018; 122: 1–5. [DOI] [PubMed] [Google Scholar]
- 7.Smith SB, Geske JB, Kathuria P, et al. Analysis of national trends in admissions for pulmonary embolism. Chest 2016; 150: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai H, Natt B, Bime C, et al. Pulmonary embolism with right ventricular dysfunction: Who should receive thrombolytic agents? Am J Med 2017; 130: 93.e29–93.e32. [DOI] [PubMed] [Google Scholar]
- 9.Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: Saves lives but underused. Am J Med 2012; 125: 465–470. [DOI] [PubMed] [Google Scholar]
- 10.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf 2012; 21: 154–162. [DOI] [PubMed] [Google Scholar]
- 11.Cooley DA, Beall AC, Alexander JK. Acute massive pulmonary embolism: Successful surgical treatment using temporary cardiopulmonary bypass. JAMA 1961; 177: 283–286. [DOI] [PubMed] [Google Scholar]
- 12.Friedman O, Horowitz JM, Ramzy D. Advanced cardiopulmonary support for pulmonary embolism. Tech Vasc Interv Radiol 2017; 20: 179–184. [DOI] [PubMed] [Google Scholar]
- 13.Yusuff H, Zochios V, Vuylsteke A. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: A systematic review. Perfusion 2015; 30: 611–616. [DOI] [PubMed] [Google Scholar]
- 14.Ain DL, Albaghdadi M, Giri J, et al. Extra-corporeal membrane oxygenation and outcomes in massive pulmonary embolism: Two eras at an urban tertiary care hospital. Vasc Med 2018; 23: 60–64. [DOI] [PubMed] [Google Scholar]
- 15.Meneveau N, Guillon B, Planquette B, et al. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: A multicentre series of 52 cases. Eur Heart J 2018; 39: 4196–4204. [DOI] [PubMed] [Google Scholar]
- 16.Elbadawi A, Wright C, Patel D, et al. The impact of a multi-specialty team for high risk pulmonary embolism on resident and fellow education. Vasc Med 2018; 23: 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: Initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest 2016; 150: 384–393. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka D, Hirose H, Cavarocchi N, et al. The impact of vascular complications on survival of patients on venoarterial extracorporeal membrane oxygenation. Ann Thorac Surg 2016; 101: 1729–1734. [DOI] [PubMed] [Google Scholar]
- 19.Thomas J, Kostousov V, Teruya J. Bleeding and thrombotic complications in the use of extracorporeal membrane oxygenation. Semin Thromb Hemost 2018; 44: 20–29. [DOI] [PubMed] [Google Scholar]