Abstract
Although 17β-estradiol (E2) is known to regulate hippocampal function, the specific contributions of hippocampally-synthesized E2 remain unclear. Infusion of the aromatase inhibitor letrozole into the dorsal hippocampus (DH) of ovariectomized mice disrupts object recognition and object placement memory consolidation, suggesting that DH-synthesized E2 is essential for memory. However, the role of DH-synthesized E2 in memory among male rodents is unknown. Here, we examined effects of aromatase inhibition on memory consolidation in male mice. Intact and gonadectomized mice were infused with vehicle or letrozole into the DH immediately post-training in object placement and object recognition tasks. Letrozole blocked memory in both tasks among gonadectomized males only, suggesting that circulating androgens, or a rise in hippocampal androgens due to aromatase inhibition, may support memory consolidation in intact males. To test this hypothesis, intact males were infused with the androgen receptor antagonist flutamide into the DH after object training. A dose-dependent impairment was observed in both tasks, indicating that blocking androgen signaling can impair memory consolidation. To test if hippocampal androgen receptor activation protected intact males from the impairing effects of letrozole, a non-impairing dose of flutamide was co-infused with letrozole. Co-administration of both drugs blocked object placement and object recognition memory consolidation, demonstrating that letrozole impairs memory in intact males only if androgen receptors are blocked. Together, these data suggest that DH-synthesized E2 and androgen receptor activation may work in concert to mediate memory consolidation in intact males, such that androgen receptor activation protects against memory impairments caused by aromatase inhibition.
Keywords: object recognition, spatial memory, memory consolidation, estradiol, neurosteroids, hippocampus, gonadectomy, letrozole, flutamide
Introduction
Although 17β-Estradiol (E2) has long been known to regulate memory formation in both males and females (Daniel, 2013; Duarte-Guterman et al., 2015; Frick et al., 2015; Gibbs and Gabor, 2003; Luine, 2014; Korol and Pisani, 2015; Koss et al., 2018; Packard, 1998), it has typically been assumed that the primary endogenous source of memory-facilitating E2 is the gonads. However, this assumption has recently been challenged by the discovery of E2 synthesis within several brain regions across many species (Azcoitia et al., 2011; Ivanova and Beyer, 2000; Roselli et al., 1985; Roselli and Resko, 1989; Vockel et al., 1990). Specifically, the potential involvement of E2 synthesized within the hippocampus has garnered particular interest, given the importance of this brain region for many types of learning and memory, such as spatial and object recognition memories (Baker and Kim, 2002; Cohen et al., 2013). In female rats, hippocampal levels of E2 fluctuate during the estrous cycle, yet remain detectable after ovariectomy (Kato et al., 2013), and hippocampal E2 levels in males are significantly higher than those in plasma (Hojo et al., 2011), suggesting E2 synthesis in the hippocampus of both sexes. In support, the male and female hippocampus contains all the necessary enzymes for de novo E2 synthesis (Hojo et al., 2004; Hojo et al., 2011; Kawato et al., 2002; Kimoto et al., 2001), including aromatase, the enzyme that converts testosterone to E2, which is present in hippocampal glia and pyramidal cells in humans, birds, and rodents (Azcoitia et al., 2011; Balthazart et al., 1996; Hojo et al., 2004; Peterson et al., 2004; Prange-Kiel et al., 2016; Prange-Kiel et al., 2003; Stoffel-Wagner et al., 1999; Yague et al., 2010). Application of aromatase inhibitors to cultured neonatal hippocampal slices reduces morphological and physiological measures of synaptic plasticity essential for memory formation including CA1 dendritic spine density, synaptic protein levels, and long-term potentiation (Fester et al., 2012; Kretz et al., 2004; Prange-Kiel et al., 2008; Vierk et al., 2012; Zhou et al., 2010). Accordingly, systemic aromatase inhibition impairs hippocampal-dependent spatial memory among gonadally intact female mice (Zameer and Vohora, 2017) and both selectively impairs hippocampal-dependent memory and reduces hippocampal activity in postmenopausal women (Bayer et al., 2015; Shilling et al., 2001). A more direct role for hippocampal E2 was shown in ovariectomized female mice, where dorsal hippocampal infusion of the aromatase inhibitor letrozole blocked a learning-induced increase in hippocampal E2 and prevented memory consolidation in object recognition and object placement tasks (Tuscher et al., 2016). Collectively, these data suggest an important role for hippocampal E2 synthesis in regulating hippocampal-dependent memory in female rodents and humans.
Although memory enhancing effects of E2 have been demonstrated in both gonadectomized and intact males (Koss et al., 2018; Luine and Rodriquez, 1994; Packard et al, 1996), a role for hippocampus-synthesized E2 in memory formation among males has not been well established. In male zebra finches, social interaction with females and exposure to other male’s songs are associated with increased de novo E2 synthesis in the auditory cortex (Remage-Healey et al., 2012; Remage-Healey et al., 2008; Remage-Healey et al., 2011). Moreover, hippocampal aromatase inhibition in male zebra finches impairs spatial memory (Bailey et al., 2013; Bailey et al., 2017), and decreases hippocampal levels of the postsynaptic protein PSD-95, suggesting that aromatase inhibition may block memory in male finches by reducing synapse number (Bailey et al., 2017). Findings in male mammals are considerably more inconsistent. In humans, the only available data on aromatase inhibition in males comes from a study in prepubertal boys with growth disorders who were chronically treated with aromatase inhibitors for 1–2 years. These boys exhibited no significant impairments in verbal or spatial memory compared to their pretreatment baseline measures (Hero et al., 2010), suggesting no effects of long-term aromatase inhibition in juvenile males. Among gonadally-intact adult male rats, two studies report modest improvements in spatial reference memory and non-spatial working memory after intrahippocampal or systemic administration of aromatase inhibitors (Alejandre-Gomez et al., 2007; Moradpour et al., 2006). However, other reports found that short-term systemic aromatase inhibition impairs fear extinction among intact male rats (Graham and Milad, 2014) and passive avoidance in gonadectomized male rats (Nayebi et al., 2014). In one recent study, 4-week systemic administration of letrozole impaired spatial memory in the Morris water maze among intact or gonadectomized male mice (Zhao et al., 2018). This deficit was associated with reduced dendritic spine density, synaptic protein levels, and local protein synthesis, as well as reduced androgen receptor and G-protein-coupled estrogen receptor levels (Zhao et al., 2018), supporting a beneficial role for hippocampal E2 synthesis in mediating spatial memory and hippocampal function in male rats. However, inconsistencies among the few studies in males prevent definitive conclusions about the mnemonic effects of hippocampal E2 synthesis in males at the present time.
Interpreting the effects of aromatase inhibitors in males can be complicated because of high levels of circulating testosterone from the testes. Testosterone can be metabolized by two enzymes: aromatase, which generates estrogens, and 5α-reductase, which produces 5α-dihydrotestosterone (DHT), an androgen that cannot be converted to estrogens (Andriole et al., 2004; Fargo et al., 2009) but can be metabolized into other androgens that bind estrogen receptor β (Pak et al, 2005). Nevertheless, blocking the actions of one of these enzymes provides more substrate for the other, as illustrated by a study in which aromatase inhibition in men increased plasma levels of DHT whereas 5α-reductase inhibition increased levels of E2 (Veldhuis et al., 2009). Because DHT is a potent androgen that binds androgen receptors with a higher affinity than testosterone (Andriole et al., 2004), aromatase inhibition should lead to increased androgen receptor binding. Indeed, testosterone plasma levels and testis volume are increased in prepubertal boys after two years of letrozole treatment (Hero et al., 2010). Testosterone and DHT are present in measurable levels in the hippocampus in rodents of both sexes, but are higher in intact males than GDX males, intact females, or ovariectomized females (Hojo et al., 2011; Hojo and Kawato, 2018). Androgens, like estrogens, can enhance hippocampal memory in both human and rodent males (Aubele et al., 2008; Babanejad et al., 2012; Benice and Raber, 2009; Cherrier et al., 2003; Cherrier et al., 2005; McConnell et al., 2012; Wagner et al., 2018). Thus, high levels of androgens in the hippocampus of males could counteract the memory-impairing effects of aromatase inhibitors observed in females.
The current study explored the role of hippocampally-synthesized E2 in memory consolidation of object recognition and object placement memories in males with (gonadally-intact) and without (gonadectomized) circulating androgens. Bilateral dorsal hippocampal infusion of E2 enhances object recognition and object placement memory consolidation in gonadally-intact male mice (Koss et al., 2018), whereas infusion of the aromatase inhibitor letrozole (0.05 μg/hemisphere) impairs consolidation in ovariectomized mice (Tuscher et al., 2016). Thus, we hypothesized that dorsal hippocampal infusion of letrozole would impair object recognition and object placement memory consolidation in gonadectomized and intact male mice. Because letrozole impaired memory only in gonadectomized, but not intact, male mice, we next hypothesized that circulating androgens protect intact male mice from the memory-impairing effects of letrozole. The resulting data indicate that hippocampal-synthesized E2 is essential for memory consolidation in male mice only when dorsal hippocampal androgen receptor binding is low, and suggest possible cooperative or compensatory actions of these two mechanisms to facilitate memory consolidation in males.
Materials and Methods
Subjects
Male C57BL/6 mice were obtained from Taconic Laboratories (Rensselaer, NY) at 9 weeks of age. Mice were singly-housed in Ancare N10HT high-temp polycarbonate mouse cages with ad libitum access to food (Harlan Teklad 604) and water delivered in Ancare PC9RH8.5 reduced height 9 oz. high-temp polycarbonate bottles. Mice were maintained on a 12:12 light-dark cycle, and all behavioral testing was performed in the light phase of the cycle. Surgeries were performed at 10 weeks of age and mice were approximately 11–13 weeks old at the start of behavioral testing. All procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Milwaukee.
Gonadectomy and Cannula Implantation Surgery
Anesthesia was induced using 5% isoflurane in 100% oxygen and then maintained at 2–3% isoflurane throughout surgery. All mice underwent a two-step surgical procedure in which they were first gonadectomized (GDX) or sham GDX (intact) and then were implanted intracranially with chronic indwelling guide cannulae. During GDX surgeries, a midline incision was made on the scrotal sac, testes were isolated and carefully separated from fat, and then the testes were tied off at the vas deferens and spermatic artery with chromic gut. The incision was closed with monofilament sutures. For intact mice, a midline incision was made and then closed with monofilament sutures without removal of the testes.
Immediately following sham and GDX procedures, all mice were implanted with chronic indwelling guide cannulae as described previously (Boulware et al., 2013; Fortress et al., 2013a; Kim et al., 2016). Mice were secured in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA) and implanted with bilateral guide cannulae (22 gauge; C232G, Plastics One, Roanoke, VA) aimed at each hemisphere of the DH (−1.7 mm AP, ±1.5 mm ML, −2.3 mm DV) or with triple guide cannulae aimed at both hippocampi and the dorsal third ventricle (intracerebroventricular (ICV); −0.9 mm AP, ±0.0 mm ML, −2.3 mm DV). Dummy cannulae (C232DC, Plastics One, Roanoke, VA) were inserted into all guide cannulae to maintain patency. Cannulae were fixed to the skull with dental cement (Darby Dental, Jericho, NY) that served to close the wound.
Drugs and Infusions
Infusions were performed by gently restraining mice, removing the dummy cannulae, and placing an infusion cannula into the guide cannulae (C313I; DH: 28 gauge, extending 0.8 mm beyond the 1.5 mm guide; i.c.v., 28 gauge, extending 1.0 mm beyond the 1.8 mm guide). Infusions were controlled by a microinfusion pump (KDS Legato 180; KD Scientific, Holliston, MA) affixed with a 10 μl Hamilton syringe. Infusions were performed immediately after training at a rate of 0.5 μl/min in the DH or 1 μl/2 min into the dorsal third ventricle as described previously (Boulware et al., 2013; Fortress et al., 2013a; Fortress et al., 2014; Kim et al., 2016; Koss et al., 2018). Infusion cannulae remained in place for 1 min after each infusion to prevent diffusion up the cannula track.
The aromatase inhibitor letrozole (Selleckchem, Houston, TX) was dissolved in 0.9% sterile saline and 2% DMSO saline at a concentration of 0.1 μg/μl. A volume of 0.5 μl was delivered to both hemispheres of the DH, resulting in a dose of 0.05 μg/hemisphere. This dose of letrozole has previously been shown to impair object recognition and object placement memory consolidation in ovariectomized females (Tuscher et al., 2016). Vehicle-treated mice received infusions of 0.9% saline and 2% DMSO of the same volume. The androgen receptor antagonist flutamide (Tocris Biosciences, Minneapolis, MN) was dissolved in 60% DMSO saline. To test the potential memory-impairing effects of flutamide in the object recognition and object placement tasks, 0.5 μl of flutamide was dissolved in 0.9% sterile saline and 60% DMSO was administered to each hemisphere of the DH at concentrations of 0.5, 1.0, or 2.0 μg/μl, resulting in doses of 0.25, 0.5, or 1.0 μg/hemisphere. Vehicle control mice for the flutamide experiment were infused with 60% DMSO saline. Although the concentration of DMSO in this control group is higher than that used for the letrozole control group (necessary due to the higher DMSO concentration needed to fully dissolve flutamide), the greater percentage of DMSO used did not impair memory consolidation in either object task (see Results). Because 0.5 μg/hemisphere flutamide did not impair memory consolidation in either task (see Results), this dose was used in a subsequent experiment testing the combined effects behaviorally-ineffective doses of flutamide and letrozole. In this experiment, mice received bilateral DH infusion of flutamide (0.5 μg/hemisphere) or vehicle (60% DMSO in 0.9% saline) immediately followed by dorsal third ventricle infusion of letrozole (0.05 μg/hemisphere) or vehicle (2% DMSO in 0.9% saline). The triple infusion protocol used in this experiment prevents possible damage to DH tissue from two DH infusions in rapid succession (Boulware et al., 2013; Fan et al., 2010; Fernandez et al., 2008; Fortress et al., 2013a; Koss et al., 2018; Zhao et al., 2012; Zhao et al., 2010).
Object placement and object recognition tasks
Approximately one week after surgery, mice began object placement and object recognition testing to assess spatial and object recognition memory consolidation, respectively. Memory in both tasks depends on intact function of the DH (Baker and Kim, 2002; Cohen et al., 2013). Briefly, both tasks involve a single training and testing trial (Fernandez et al., 2008; Fortress et al., 2013b; Kim et al., 2016; Koss et al., 2018; Tuscher et al., 2016b; Zhao et al., 2010). During training, mice are required to explore two identical objects. All treatments are then administered immediately post-training to pinpoint effects on the consolidation phase of memory formation. After a delay, memory for the training objects is tested by moving one object to a different location (object placement) or replacing one object with a new object (object recognition). Because mice have a natural tendency to explore novelty, they will spend more time than chance exploring the moved or novel object if they remember the identity and location of the training objects. All mice were tested in both the object placement and object recognition tasks, the order of which was counterbalanced within each group. Object placement testing, object recognition testing, and brain tissue collection was separated by two weeks to allow acute effects of each infusion to dissipate prior to the next infusion.
Prior to the start of behavioral testing, mice were handled for 3 days and then were habituated to the testing arena (60 × 60 × 47 cm high) for 5 min/day for 2 days. During habituation, mice moved about freely in the apparatus without objects present. During both handling and habituation, mice were acclimated to objects by placing a Lego Duplo brick (6.3 × 3.1 × 2.3 cm) in their home cage, which was subsequently removed immediately after training. These Duplo bricks do not resemble the objects used in training or testing, but effectively habituate mice to the presence of objects so that they are not anxiogenic during training. This procedure has successfully reduced object-induced neophobia in our previous work (Fortress et al., 2013b; Kim et al., 2016; Koss et al., 2018; Tuscher et al., 2016). Following habituation, mice were trained in either object placement or object recognition. Mice were first briefly reintroduced to the arena by placing them inside without any objects for 2 minutes. They were then returned to their home cage while two identical objects were placed in the northwest or northeast corners of the arena. Mice were then returned to the arena and allowed to explore the objects, which included a plumbing valve, chip clip, mini-stapler, date stamp, master lock, and aquarium figurines. Object exploration was scored only when the mouse’s nose contacted an object. To ensure that all mice received the same amount of exposure to the objects, mice were required to accumulate 30 sec of object exploration within 20 min. If mice did not reach this criterion, it was re-trained 4–7 days later with different objects to account for possible neophobic reactions to the training apparatus and objects. If a mouse did not reach this criterion upon re-training, then it was eliminated from the experiment. All drug infusions were performed immediately after training, after which mice were returned to their home cages.
Testing occurred 4 h later for object placement and 24 h later for object recognition. During object placement testing, one training object was moved to the corner of the arena in which the mouse spent the least amount of time exploring during training to counteract any bias for a preferred corner. During object recognition testing, the training object that was least explored by each mouse during training was replaced with a novel object. In both tasks, mice were required to explore the objects until they accumulated of 30 s of exploration. As in training, if the mouse did not explore the objects for 30 sec within 20 min, the mouse was re-trained and tested with different objects 10–14 days later or eliminated from the experiment. Time spent with the objects was recorded using AnyMaze tracking software (Stoelting, Wood Dale, IL). Mice that remember the identity and location of the training objects should spend more time than chance with the moved and novel objects. Chance is designated as 15 s because this value represents equal exploration of both objects, and hence, no memory of the training objects. The 4 h and 24 h delays were used for object placement and object recognition, respectively, based on previous evidence from ovariectomized females that vehicle-treated mice show intact memory 4 and 24 h after object placement and object recognition training (Boulware et al., 2013; Fortress et al., 2015; Kim et al., 2016). Similarly, gonadally-intact males can remember object identity 24 h after object recognition training (Fortress et al., 2013a; Frick and Gresack, 2003). Thus, the 4 and 24 h delays were used for object placement and object recognition, respectively, to permit potential observation of memory impairments.
Statistical analyses
For all behavioral experiments, two statistical analyses were performed using GraphPad Prism 6 software (La Jolla, CA). To assess learning within each group, one-sample t-tests compared exploration time for each group with chance (set at 50% or 15 s). More time spent with the moved or novel object indicated intact memory for object location and identity, respectively. To assess the effects of surgery and/or treatment among the groups, one- or two-way analyses of variance (ANOVAs) were performed, followed by Fisher’s LSD post-hoc tests. Significance was defined at p < 0.05. Effect sizes were calculated using Cohen’s d for one-sample t-tests and partial eta-squared for ANOVAs.
Results
Dorsal hippocampal infusion of letrozole impairs memory consolidation in GDX but not intact male
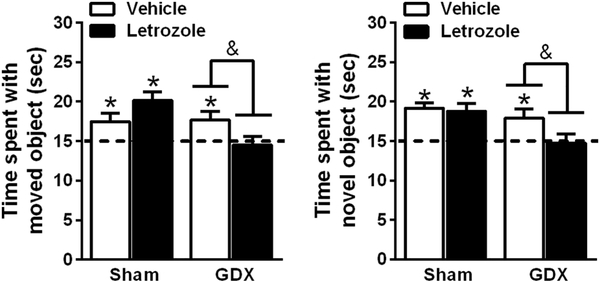
Our laboratory has previously demonstrated that bilateral DH infusion of letrozole (0.05 μg/hemisphere) immediately post-training impairs memory consolidation in ovariectomized mice (Tuscher et al., 2016). Here, we used the same dose of letrozole to investigate the role of hippocampal-synthesized E2 in memory consolidation among intact and GDX male mice. Intact and GDX males were trained in the object placement and object recognition tasks then immediately received a bilateral DH infusion of letrozole (0.05 μg/hemisphere). Memory consolidation was tested 4 h later for object placement and 24 h later for object recognition. Both one-sample t-tests and ANOVAs indicated that letrozole impaired memory in GDX males, but not intact males (Fig. 1). One sample t-tests revealed that both intact and GDX vehicle-treated mice spent significantly more time than chance with the moved object (t(11) = 2.28, p = 0.04, d = 0.66; t(15) = 2.52, p = 0.02, d = 0.63; respectively; Fig. 1a), indicating that control males successfully remembered the original placement of the object. Interestingly, the lack of a deficit in vehicle-treated GDX males suggests that testicular androgens are not required for spatial memory consolidation. In letrozole-treated mice, only intact (t(9) = 4.874, p = 0.002, d = 1.54), not GDX, mice spent significantly more time with the moved object than chance (Fig. 1a). Additionally, a two-way ANOVA revealed a significant surgery x treatment interaction (F(1, 45) = 6.839, p = 0.01, η2 = 0.13) as well as a significant main effect of surgery (F(1, 45) = 6.003, p = 0.02, η2 = 0.12), with post-hoc tests revealing that GDX letrozole-treated mice performed significantly worse than GDX vehicle-treated mice (p = 0.04) or intact letrozole-treated mice (p = 0.002).
Figure 1.
Infusion of letrozole into the dorsal hippocampus impaired memory consolidation in gonadectomized, but not intact, male mice. GDX and intact mice were tested in the object placement (a) and the object recognition (b) tasks immediately after post-training infusion of vehicle or letrozole into the dorsal hippocampus. In the object placement task, intact vehicle-treated (n = 12), intact letrozole-treated (n = 10), and GDX vehicle-treated (n = 16) mice spent significantly more time with the moved object than chance (15 sec; *p < 0.05). In contrast, GDX letrozole-treated mice (n = 11) did not. GDX mice treated with letrozole also spent significantly less time with the moved object than GDX vehicle-treated mice (&p < 0.05). Similarly, in the object recognition task, only the intact vehicle-treated (n = 12), intact letrozole-treated (n = 12), and GDX vehicle-treated (n = 11) mice investigated the novel object significantly more than chance (*p < 0.05).
Consistent with object placement, vehicle-treated intact and GDX males remembered the identity of the training object in object recognition (Fig. 1b), suggesting that testicular hormones are also not required for object recognition memory consolidation. Intact mice spent significantly more than chance with the novel object when treated with vehicle (t(11) = 6.10, p < 0.0001, d = 1.76) or letrozole (t(11) = 3.739, p < 0.003, d = 1.08), suggesting no detrimental effect of letrozole treatment among mice with intact testes. Conversely, GDX mice treated with letrozole did not investigate the novel object more than chance, unlike GDX mice receiving vehicle (t(10) = 2.489, p = 0.03. d = 0.75). A two-way ANOVA revealed a significant main effect of surgery (F(1, 42) = 6.712, p = 0.013, η2 = 0.14), but not treatment. Although a significant interaction was not found (p = 0.18), post hoc tests support a detrimental effect of letrozole in GDX (p = 0.037), but not sham (p = 0.78), males.
Blocking androgen receptors impairs memory consolidation in intact males
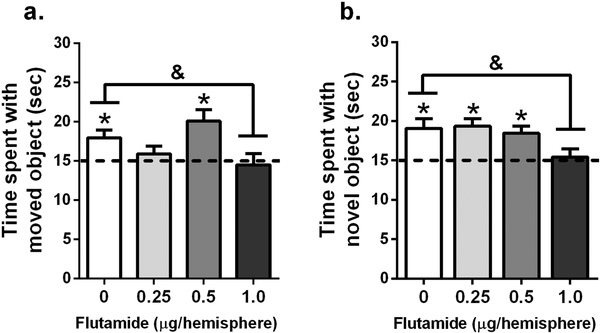
The data illustrated in Fig. 1 indicates that aromatase inhibition impairs memory consolidation only in GDX males, suggesting that androgens of testicular or hippocampal origin may be sufficient to mediate memory consolidation in the absence of hippocampally-synthesized E2. To test this idea, we infused the androgen receptor antagonist flutamide into the dorsal hippocampus. Intact males were used to examine the effects of androgen receptor antagonism because this group was unaffected by aromatase inhibition. Intact males were infused with flutamide directly into the dorsal hippocampus at one of three different doses (0.25, 0.5, or 1.0 μg/hemisphere) immediately after training in the object recognition and object placement tasks (Fig. 2). In the object placement task (Fig. 2a), mice significantly interacted with the moved object more than chance if treated with vehicle (t(11) = 2.897, p = 0.015, d = 0.84) or 0.5 μg (t(7) = 3.444, p = 0.011, d = 1.22), whereas mice receiving 0.25 μg or 1.0 μg flutamide did not investigate the moved object more than chance. Additionally, the main effect of treatment was significant in the one-way ANOVA (F(3, 32) = 2.863, p = 0.05, η2 = 0.19), and post hoc tests revealed that males receiving 1.0 μg flutamide exhibited significantly impaired spatial memory consolidation relative to vehicle-treated males (p = 0.02).
Figure 2.
Infusion of flutamide into the dorsal hippocampus of intact males impaired memory consolidation in both tasks. (a) In the object placement task, mice infused with vehicle (n = 12) or 0.5 μg/hemisphere flutamide (n = 8) spent significantly more time with the moved object than chance (*p < 0.05). However, mice infused with 0.25 μg/hemisphere (n = 11) or 1.0 μg/hemisphere (n = 10) flutamide spent no more time than chance with the moved object, suggesting impairing effects of these doses. Mice infused with 1.0 μg/hemisphere flutamide also spent significantly less time with the moved object than those treated with vehicle (&p < 0.05). (b) In the object recognition task, mice infused with vehicle (n = 10), 0.25 μg/hemisphere (n = 8), or 0.5 μg/hemisphere (n = 9) of flutamide interacted with the novel object significantly more than chance (*p <0.05), whereas the mice treated with 1.0 μg/hemisphere flutamide (n = 9) did not. As in object placement, the time spent with the novel object differed significantly between mice treated with vehicle and 1.0 μg/hemisphere flutamide (&p < 0.05).
In object recognition (Fig. 2b), mice interacted with the novel object more than chance when treated with vehicle (t(9) = 3.316, p = 0.009, d = 1.05), 0.25 μg (t(7) = 4.611, p = 0.0025, d = 1.63), or 0.5 μg (t(8) = 3.697, p = 0.006, d = 1.23) flutamide. One-way ANOVA also revealed a significant treatment effect (F(3, 37) = 3.702, p = 0.02, η2 = 0.26), with post-hoc tests indicating that mice 1.0 μg flutamide exhibited significantly impaired object recognition relative to vehicle-infused controls (p = 0.04). Together, these dose-dependent effects suggest two important conclusions. First, the impairments observed after infusion of 1.0 μg flutamide indicate that androgen signaling in the dorsal hippocampus is important for spatial and object recognition memory consolidation among intact males. Second, the inability of 0.5 μg/hemisphere flutamide to influence memory consolidation reveals this as a behaviorally ineffective non-impairing dose. As such, we then used this subeffective dose in the next experiment to examine the coincident effects of aromatase inhibition and androgen receptor antagonism.
Blocking androgen receptor activation reveals letrozole-induced memory impairments in intact males
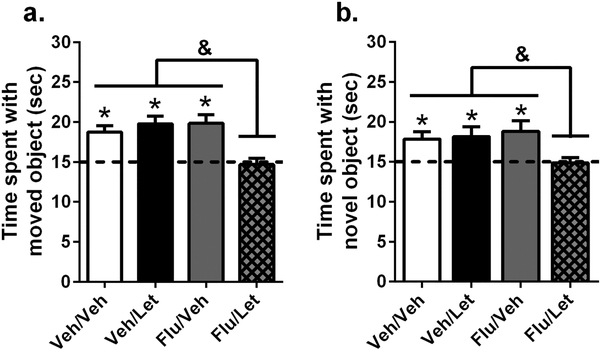
Having established that androgen receptor activation plays a role in memory consolidation among intact males, we next sought to determine if androgen receptor activation might protect intact males from the memory-impairing effects of aromatase inhibition observed in GDX males. To do so, we infused intact mice immediately after object training with behaviorally subeffective doses of flutamide (0.5 μg/hemisphere into the DH as in Fig. 2) and letrozole (0.1 μg ICV, which is the unilateral total of the 0.05 μg/hemisphere dose used in Fig. 1). To prevent tissue damage from multiple infusions, mice first received dorsal hippocampal infusions of vehicle or flutamide immediately followed by infusion of vehicle or letrozole into the adjacent dorsal third ventricle. Our laboratory has successfully used this method in previous studies to determine the necessity of receptor and kinase function to the memory-enhancing effects of E2 (Boulware et al., 2013; Fan et al., 2010; Fernandez et al., 2008; Fortress et al., 2013a; Koss et al., 2018; Zhao et al., 2012; Zhao et al., 2010). As in Fig. 1 and 2, infusion of letrozole alone or flutamide alone did not impair memory; however, memory was impaired significantly when the two drugs were administered together (Fig. 3). A two-way ANOVA for object placement (Fig. 3a) revealed a significant letrozole x flutamide interaction (F(1, 43) = 11.30, p = 0.002, η2 = 0.21) and significant main effects of letrozole treatment (F(1, 43) = 5.083, p = 0.03, η2 = 0.11) and flutamide treatment (F(1, 43) = 4.784, p = 0.03, η2 = 0.10). Post hoc tests revealed that males receiving infusions of both letrozole and flutamide spent significantly less time with the moved object than the vehicle alone (p = 0.02), flutamide alone (p = 0.0003), or letrozole alone (p = 0.0005) groups. Accordingly, one-sample t-tests indicated that only the vehicle alone (t(13) = 4.770, p = 0.0004, d = 1.27), letrozole alone (t(9) = 4.953, p = 0.0008, d = 1.57), and flutamide alone (t(11) = 2.413, p = 0.001, d = 1.27) groups spent significantly more time with moved object than chance. Mice receiving DH infusion of flutamide and ICV infusion of letrozole did not interact with the moved object more than chance, indicating impaired spatial memory consolidation in this group.
Figure 3.
Co-infusion of 0.1 μg letrozole and 0.5 μg/hemisphere flutamide impaired memory consolidation among intact male mice. Intact mice received infusions into the dorsal hippocampus and dorsal third ventricle, respectively, of vehicle+vehicle (Veh/Veh), vehicle+letrozole (Veh/Let), flutamide+vehicle (Flu/Veh), or flutamide+letrozole (Flu/Let). (a) In object placement, the Veh/Veh (n = 14), Veh/Let (n = 10), and Flu/Veh (n = 12) groups spent significantly more time with the moved object than chance; *p < 0.05). In contrast, the Flu/Let group (n = 11) did not spend more time than chance with the moved object, suggesting a detrimental effect of letrozole and flutamide co-administration. This conclusion was supported by post-hoc tests illustrating that the Flu/Let group performed significantly worse than all other groups (&p < 0.05). (b) Similarly, in the object recognition task, mice in the Veh/Veh (n = 13), Veh/Let (n = 11), and Flu/Veh (n = 11) groups investigated the novel object more than chance (*p < 0.05), but mice in the Flu/Let group (n = 11) did not. As with object placement, the Flu/Let group also spent significantly less time with the novel object than all other groups (&p < 0.05).
Parallel results were observed in the object recognition task (Fig. 3b), with vehicle/vehicle (t(12) = 3.008, p = 0.01, d = 0.83), flutamide/vehicle (t(10) = 2.976, p = 0.01, d = 0.9), and vehicle/letrozole (t(10) = 2.566, p = 0.03, d = 0.77) groups all spending more time than chance with the novel object, and mice treated with both flutamide and letrozole investigating the novel object equal to chance. A two-way ANOVA also demonstrated a significant letrozole × flutamide interaction (F(1, 41) = 4.778, p = 0.0346, η2 = 0.10), with post hoc tests revealing that mice receiving letrozole and flutamide exhibited significantly impaired object recognition memory consolidation relative to the vehicle/vehicle (p = 0.047), flutamide/vehicle (p = 0.008), and vehicle/letrozole (p = 0.04) groups. Collectively, the memory impairments observed after co-infusion of doses of letrozole and flutamide that have no detrimental effects on their own suggest that both hippocampal E2 synthesis and androgen receptor activation are involved in memory consolidation in males.
Discussion
The current study was designed to determine whether hippocampally-synthesized E2 mediates memory consolidation in male mice. The aromatase inhibitor letrozole was infused directly into the dorsal hippocampus of gonadally-intact and GDX male mice immediately after training in the object recognition and object placement tasks. Administering an acute infusion of letrozole immediately post-training allowed us to test the effects of aromatase inhibition on memory consolidation independent of possible effects on acquisition or retention. The effects of letrozole differed depending on the presence of the gonads. That is, letrozole impaired memory consolidation in GDX males, but not among intact males. The impairment observed in GDX males is consistent with a previous study from our laboratory in which dorsal hippocampal infusion of the same dose of letrozole used in the current study blocked memory consolidation in both object placement and object recognition tasks in ovariectomized mice (Tuscher et al., 2016). This impairment is also consistent with another recent report that systemic injection of letrozole for 4 weeks impairs spatial memory in the Morris water maze among GDX male mice (Zhao et al., 2018). Although the data thus far are scant, these three studies provide consistent evidence that hippocampally-synthesized E2 is required for memory consolidation in both sexes in the absence of circulating gonadal hormones.
In contrast, the lack of a letrozole-induced impairment among intact males is inconsistent with other studies of intact males reporting that aromatase inhibition impairs spatial learning and memory in zebra finches and fear memory consolidation in rats (Bailey et al., 2013; Bailey et al., 2017; Graham and Milad, 2014; Zhao et al., 2018). These data are also inconsistent with other studies of intact male rats showing that aromatase inhibitors can enhance spatial memory and egocentric working memory (Alejandre-Gomez et al., 2007; Morapour et al., 2006). Discrepancies among these studies could result from myriad differences, including species, route of administration, duration of treatment, and behavioral task used. Importantly, none of the previous studies conducted aromatase inhibition post-training, so effects on non-mnemonic aspects of task performance (e.g., motivation, sensorimotor abilities) may have contributed to the impairments or enhancements observed in other studies. Only one previous study directly compared effects of aromatase inhibition on GDX and intact male mice and found that 4 weeks of daily letrozole injections impaired spatial memory in both groups of males (Zhao et al., 2018). This study also found that treatment in both groups reduced CA1 synaptic density and expression of numerous synaptic proteins and hormone receptors, including estrogen receptors α and β, as well as androgen receptors (Zhao et al., 2018). Thus, chronic letrozole treatment appears to have detrimental effects on multiple elements of hippocampal synaptic plasticity and hormonal responsivity, even in males with normal levels of circulating testicular hormones. It should be noted, however, that other studies of intact male rats report no effects on CA1 spine density of 2 days, 7 days, or 4 weeks of systemic letrozole injections (Fester et al., 2012; Vierk et al., 2012), although neither study assessed memory function. Interestingly, the acute post-training letrozole infusions used here did not impair memory consolidation among intact males, in contrast to its acute memory-impairing effects in GDX males observed here and chronic memory-impairing effects in GDX and intact males observed previously (Zhao et al., 2018). These data may suggest that acute letrozole infusion has few, if any, deleterious effects on hippocampal function in the presence of testicular hormones. Whether a similar situation would be observed among gonadally-intact females is an open question. We previously reported that acute dorsal hippocampal infusion of letrozole blocks memory consolidation in ovariectomized mice (Tuscher et al., 2016), but have never administered letrozole to intact females. Future studies addressing this issue would provide valuable insights into the relative contribution of gonadal vs. hippocampal estrogens in regulating memory.
The absence of letrozole-induced impairment among intact males led us to consider that androgens might protect against the detrimental effects letrozole observed here in GDX males. That is, perhaps memory consolidation in males can be mediated by a combination of hippocampally-synthesized E2 and androgens, such that the loss of both is necessary to impair memory consolidation. Support for this notion comes from the fact that neither GDX alone nor letrozole alone impaired memory consolidation in either task; GDX+vehicle and Sham+letrozole mice exhibited intact memory consolidation in both tasks. The only group that did not display intact memory consolidation was the GDX+letrozole group, suggesting that the loss of both androgens and hippocampal E2 blocks memory formation. Like estrogens, androgens can enhance hippocampal memory in both human and rodent males (Aubele et al., 2008; Babanejad et al., 2012; Benice and Raber, 2009; Cherrier et al., 2003; Cherrier et al., 2005; Locklear and Kritzer, 2014; McConnell et al., 2012; Wagner et al., 2018). To address the possible contribution of androgen receptors to memory enhancement, we infused the androgen receptor antagonist flutamide into the dorsal hippocampus of intact male mice. We selected this method of blocking androgen action over a 5α-reductase inhibitor because such inhibition would also block the metabolism of progesterone to the neurosteroid allopregnanolone. We could have alternatively infused testosterone, DHT, or another androgen, but interpretation of these data would have been difficult because DHT and its neurosteroid metabolites can bind estrogen receptors and GABA-A receptors (Chen et al., 2013; Pak et al., 2005). Here, flutamide dose-dependently impaired memory in both tasks, with the 1.0 μg/hemisphere dose most consistently blocking memory formation. Interestingly, one-sample t-tests indicated that 0.25 μg/hemisphere flutamide impaired object placement, but not object recognition, memory, possibly suggesting a U-shaped dose-response curve for spatial memory only. However, the post-hoc analysis indicated no significant difference between this dose and vehicle for object placement, making it difficult to draw strong conclusions about negative effects of this dose on spatial memory. Nevertheless, the memory-impairing effects of the 1.0 μg/hemisphere dose is consistent with other studies in which flutamide infusion into the hippocampus of intact male rats prior to or immediately after training impaired spatial memory in the Morris water maze and inhibitory avoidance (Edinger and Frye, 2007; Naghdi et al., 2001). Intrahippocampal flutamide also reportedly increases anxiety-like behavior among intact male rats (Edinger and Frye, 2007). Interestingly, systemic flutamide reportedly increases CA1 dendritic spine density (MacLusky et al., 2006; MacLusky et al., 2004), an effect usually associated with enhanced memory. However, the dose-dependence of flutamide’s effects on memory consolidation makes it difficult to directly compare the effects of systemic and dorsal hippocampal administration. Nevertheless, the present and previous behavioral results indicate that blocking androgen receptors impairs various types of memory among intact males, thus, suggesting a key role for these receptors in memory formation.
A second important outcome of our flutamide dose-response study was the identification of a dose that had no effect on memory consolidation, which allowed us to test the hypothesis that androgen receptor activation protects against the memory-impairing effects of letrozole. The use of doses of letrozole and flutamide that had no effect on memory on their own were necessary to ensure that any effects observed after their co-infusion were due to an interaction rather than the result of one compound blocking general memory formation. We found that intact males infused with behaviorally subeffective doses of flutamide and letrozole exhibited significant memory impairments in both object tasks. These data are consistent with other reports that androgens can enhance memory (Aubele et al., 2008; Babanejad et al., 2012; Benice and Raber, 2009; Cherrier et al., 2003; Cherrier et al., 2005; McConnell et al., 2012; Wagner et al., 2018) similar to E2 (Koss et al., 2018; Luine and Rodriquez, 1994; Packard et al, 1996). However, the present findings suggest that hippocampally-synthesized E2 and androgen receptors work in concert to facilitate memory consolidation among intact males. The mechanisms through which this interaction might occur are unknown. Five days of E2 treatment increases expression of androgen receptor mRNA and binding of nuclear proteins to androgen receptors in the cerebral cortex of mice of both sexes (Kumar and Thakur, 2004; Thakur and Kumar, 2007), so a direct interaction that influences classical nuclear transcription is possible, although it is unclear such effects could occur within the 2–3-hour memory consolidation window. Like estrogen receptors, androgen receptors can also influence cellular function via non-classical effects on cell signaling (Fargo et al., 2009), so E2 could have indirect effects on androgen receptor function by influencing activation of various kinases downstream from androgen receptor activation. We think this possibility likely because the effects of E2 on memory consolidation, at least in ovariectomized female mice, depend on rapid non-classical activation of numerous cell signaling cascades (Fernandez et al., 2008; Fortress et al., 2013. However, these possibilities should be addressed in future studies that directly examine how hippocampal E2 influences androgen receptor function.
As the primary source of androgens in males, the testes are the most likely source of the androgens that bind hippocampal androgen receptors. However, testosterone also is made locally in the hippocampus and is present at measurable levels of both sexes (Hojo et al., 2011; Hojo and Kawato, 2018). Moreover, blocking aromatase in this study should have increased levels of hippocampal androgens by making more testosterone substrate available for conversion to androgens such as DHT. An effect such as this has been observed in humans, where systemic administration of aromatase inhibitors increases serum testosterone and DHT levels (Veldhuis et al., 2009). However, hippocampal levels of testosterone in intact male rats are approximately 70% higher than in GDX males and notably, DHT levels are over 90% higher in intact male rats than in GDX males (Hojo et al., 2011; Hojo and Kawato, 2018), so actions of hippocampally-synthesized androgens are likely to pale in comparison to those of androgens in circulation. As suggested by the failure of GDX+letrozole males to display memory consolidation, any increase in hippocampal androgens resulting from aromatase inhibition were not sufficient to preserve memory function in the absence of the gonads. Therefore, the data suggest that the androgens that bind in the hippocampus to influence memory consolidation likely originate in the testes. Interestingly, E2 levels are reduced only by about 10% after GDX in males (Hojo et al., 2011; Hojo and Kawato, 2018), indicating that most hippocampal E2 may not originate from testosterone made from the testes.
Finally, it is notable that effects of letrozole and flutamide were similar for consolidation in both the object recognition and object placement tasks, suggesting generalizability to multiple forms of hippocampal-dependent memory. This influence on both object recognition and spatial memory is consistent with the effects of letrozole on both tasks in ovariectomized female mice (Tuscher et al, 2016) and the beneficial effects of exogenous E2 on both tasks in gonadectomized and intact rodents of both sexes (Boulware et al., 2013; Frye et al., 2007; Inagaki et al., 2010; Ismail and Blaustein, 2013; Kim et al., 2016; Koss et al., 2018; Luine et al., 2003; Phan et al., 2012; Walf et al., 2006). The consistent effects of these hormone manipulations in both object recognition and object placement fit well with data from multiple laboratories indicating that memory in both tasks depends on the intact function of the dorsal hippocampus (Baker and Kim, 2002; Cohen et al., 2013; Haettig et al., 2011). However, the involvement of hippocampal E2 and/or androgen receptors on both tasks in males was not a foregone conclusion at the start of this study because object recognition is complicated by the fact that multiple brain regions are involved this type of memory, including the insular cortex (Bermudez-Rattoni et al., 2005, Balderas et al., 2008), perirhinal cortex (Balderas et al., 2008; Winters and Bussey, 2005), and ventromedial prefrontal cortex (Akirav and Maroun, 2006). Work from our own laboratory using Designer Receptors Exclusively Activated by Designer Drugs (DREADD)-mediated neural inactivation has demonstrated the importance of both the dorsal hippocampus and medial prefrontal cortex for consolidation in both tasks among ovariectomized female mice (Tuscher et al., 2018). The current study found deficits in both object recognition and object placement consolidation after immediate post-training dorsal hippocampal infusion of either an aromatase inhibitor or an androgen receptor blocker, indicating that disruption of E2 synthesis or androgen receptor activation in the dorsal hippocampus is sufficient to impair the consolidation of both object recognition and spatial memory. This result is consistent with those from previous studies demonstrating that pharmacological or chemogenetic inactivation of the dorsal hippocampus impairs memory consolidation in both tasks (Baker and Kim, 2002; Cohen et al., 2013; Haettig et al., 2011; Tuscher et al, 2018). However, the present data does not address the involvement of other brain regions. Given recent findings that dorsal hippocampal cell signaling influences the ability of intracerebroventricularly-infused E2 to increase dendritic spine density in the medial prefrontal cortex, more research is needed to pinpoint the neural circuitry affected by aromatase inhibition and/or androgen receptor antagonism and the resulting effects for memory consolidation as assessed by these object tasks.
In conclusion, the current study demonstrates a key role for both hippocampal E2 synthesis and androgen receptors in memory consolidation among males. Memory consolidation in both the object placement and object recognition tasks was not affected by aromatase inhibition or gonadectomy alone, but was impaired by the combination of both treatments, suggesting that testicular androgens protect against the detrimental effects of hippocampal aromatase inhibition. A beneficial effect of androgen receptor activation on memory consolidation was indicated by the fact that the highest dose of flutamide tested impaired both object recognition and spatial memory among intact males. Also, in intact males, a behaviorally subeffective dose impaired memory when co-infused with letrozole, suggesting that hippocampal androgen receptor activation and E2 synthesis work together to facilitate memory formation. These data add to the growing body of literature demonstrating the importance of hippocampally-synthesized E2 to cognitive function in both males and females. Future work in both sexes should more fully address the relative contributions to memory formation of estrogens and androgens synthesized in the hippocampus and the gonads. Such information would be instrumental in better understanding the effects of aromatase inhibition on memory in humans (e.g., women with breast cancer) and the etiology of sex differences in the incidence of neurodevelopmental, neurodegenerative, and psychiatric diseases.
Highlights.
Hippocampal aromatase inhibition impairs memory in gonadectomized, not intact, males
Hippocampal androgen receptor (AR) antagonism impairs object memory in intact males
Subthreshold inhibition of ARs and aromatase impairs object memory in intact males
Hippocampal estradiol synthesis and androgen receptors regulate memory in male mice
Acknowledgments
We would like to thank Dr. Jennifer Tuscher for helpful advice, and Sarah Philippi, Rachel Gremminger, and Randie Alf for assistance performing the experiments described within this paper. This work was supported by the National Institutes of Health (R01MH107886), a UWM Research Growth Initiative grant (101X334), the UWM College of Letters & Sciences, and the UWM Office of Undergraduate Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akirav I, Maroun M, 2006. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb. Cortex 16, 1759–1765. [DOI] [PubMed] [Google Scholar]
- Alejandre-Gomez M, Garcia-Segura LM, Gonzalez-Burgos I, 2007. Administration of an inhibitor of estrogen biosynthesis facilitates working memory acquisition in male rats. Neurosci. Res 58, 272–277. [DOI] [PubMed] [Google Scholar]
- Andriole G, Bruchovsky N, Chung LW, Matsumoto AM, Rittmaster R, Roehrborn C, Russell D, Tindall D, 2004. Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J. Urol 172, 1399–1403. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer MF, 2008. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm. Behav 54, 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Yague JG, Garcia-Segura LM, 2011. Estradiol synthesis within the human brain. Neuroscience 191, 139–147. [DOI] [PubMed] [Google Scholar]
- Babanejad S, Naghdi N, Haeri Rohani SA, 2012. Microinjection of dihydrotestosterone as a 5alpha-reduced metabolite of testosterone into CA1 region of hippocampus could improve spatial learning in the adult male rats. Iran J Pharm Res 11, 661–669. [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Ma C, Soma KK, Saldanha CJ, 2013. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology 154, 4707–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Makeyeva YV, Paitel ER, Pedersen AL, Hon AT, Gunderson JA, Saldanha CJ, 2017. Hippocampal aromatization modulates spatial memory and characteristics of the synaptic membrane in the male zebra finch. Endocrinology 158, 852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KB, Kim JJ, 2002. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn. Mem 9, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F, 2008. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn. Mem 15, 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball GF, 1996. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J. Neurobiol 31, 129–148. [DOI] [PubMed] [Google Scholar]
- Bayer J, Rune G, Schultz H, Tobia MJ, Mebes I, Katzler O, Sommer T, 2015. The effect of estrogen synthesis inhibition on hippocampal memory. Psychoneuroendocrinology 56, 213–225. [DOI] [PubMed] [Google Scholar]
- Benice TS, Raber J, 2009. Dihydrotestosterone modulates spatial working-memory performance in male mice. J. Neurochem 110, 902–911. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Okuda S, Roozendaal B, McGaugh JL, 2005. Insular cortex is involved in consolidation of object recognition memory. Learn. Mem 12, 447–449. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM, 2013. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J. Neurosci 33, 15184–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang WQ, Lin SX, 2013. Interaction of Androst-5-ene-3beta,17beta-diol and 5alpha-androstane-3beta,17beta-diol with estrogen and androgen receptors: a combined binding and cell study. J. Steroid Biochem. Mol. Biol 137, 316–321. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Craft S, Matsumoto AH, 2003. Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: a preliminary report. J. Androl 24, 568–576. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Ahmed S, Bremner W, Peskind ER, Raskind MA, Johnson M, Craft S, 2005. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology 64, 290–296. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW Jr., 2013. The rodent hippocampus is essential for nonspatial object memory. Curr. Biol 23, 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, 2013. Estrogens, estrogen receptors, and female cognitive aging: The impact of timing. Horm. Behav 63, 231–237. [DOI] [PubMed] [Google Scholar]
- Duarte‐Guterman P, Yagi S, Chow C, Galea LA, 2015. Hippocampal learning, memory, and neurogenesis: effects of sex and estrogens across the lifespan in adults. Horm. Behav 74, 37–52. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA, 2007. Androgens’ performance-enhancing effects in the inhibitory avoidance and water maze tasks may involve actions at intracellular androgen receptors in the dorsal hippocampus. Neurobiol. Learn. Mem 87, 201–208. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM, 2010. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J. Neurosci 30, 4390–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargo KN, Pak TR, Foecking EM, Jones KJ, 2009. Molecular biology of androgen action, in: Etgen AM, Pfaff DW (Eds.), Molecular Mechanisms of Hormone Actions on Behavior. Elsevier Inc., San Diego, CA, pp. 127–152. [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM, 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J. Neurosci 28, 8660–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H, Schumacher M, Rune GM, 2012. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J. Steroid Biochem. Mol. Biol 131, 24–29. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM, 2013a. Estradiol-induced object recognition memory consolidation is dependent on activation on mTOR signaling in the dorsal hippocampus. Learn. Mem 20, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Heisler JD, Frick KM, 2015. The mTOR and canonical Wnt signaling pathways mediate the mnemonic effects of progesterone in the dorsal hippocampus. Hippocampus 25, 616–629. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM, 2014. 17β-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn. Mem 21, 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Schram SL, Tuscher JJ, Frick KM, 2013b. Canonical Wnt signaling is necessary for object recognition memory consolidation. J. Neurosci 33, 12619–12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE, 2003. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav. Neurosci 117, 1283–1291. [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM, 2015. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn. Mem 22, 472–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA, 2007. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol. Learn. Mem 88, 208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, 2003. Estrogen and cognition: Applying preclinical findings to clinical perspectives. J. Neurosci. Res 74, 637–643. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR, 2014. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn. Mem 21, 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA, 2011. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn. Mem 18, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hero M, Maury S, Luotoniemi E, Service E, Dunkel L, 2010. Cognitive effects of aromatase inhibitor therapy in peripubertal boys. Eur. J. Endocrinol 163, 149–155. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S, 2004. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. U. S. A 101, 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Kawato S, Hatanaka Y, Ooishi Y, Murakami G, Ishii H, Komatsuzaki Y, Ogiue-Ikeda M, Mukai H, Kimoto T, 2011. Hippocampal synthesis of sex steroids and corticosteroids: essential for modulation of synaptic plasticity. Front Endocrinol (Lausanne) 2, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Kawato S, 2018. Neurosteroids in Adult Hippocampus of Male and Female Rodents: Biosynthesis and Actions of Sex Steroids. Front Endocrinol (Lausanne) 9, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine VN, 2010. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm. Behav 58, 415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Blaustein JD, 2013. Pubertal immune challenge blocks the ability ofestradiol to enhance performance on cognitive tasks in adult female mice. Psychoneuroendocrinology 38, 1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova T, Beyer C, 2000. Ontogenetic expression and sex differences of aromatase and estrogen receptor-alpha/beta mRNA in the mouse hippocampus. Cell Tissue Res. 300, 231–237. [DOI] [PubMed] [Google Scholar]
- Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S, 2013. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits 7, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato S, Hojo Y, Kimoto T, 2002. Histological and metabolism analysis of P450 expression in the brain. Methods Enzymol. 357, 241–249. [DOI] [PubMed] [Google Scholar]
- Kim J, Szinte JS, Boulware MI, Frick KM, 2016. 17β-estradiol and agonism of G-protein Coupled Estrogen Receptor (GPER) enhance hippocampal memory via different cell-signaling mechanisms. J. Neurosci 36, 3309–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N, Kawato S, 2001. Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology 142, 3578–3589. [DOI] [PubMed] [Google Scholar]
- Korol DL, Pisani SL, 2015. Estrogens and cognition: friends or foes? An evaluation of the opposing effects of estrogens on learning and memory. Horm. Behav 74, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Haertel JM, Philippi SM, Frick KM, 2018. Sex differences in the rapid cell signaling mechanisms underlying the memory-enhancing effects of 17β-Estradiol. eNeuro. 5(5). pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM, 2004. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci 24, 5913–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RC, Thakur MK, 2004. Sex steroids reduce DNaseI accessibility of androgen receptor promoter in adult male mice brain. Mol. Brain Res 134, 1–7. [DOI] [PubMed] [Google Scholar]
- Locklear MN, Kritzer MF, 2014. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm. Behav 66, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Rodriguez M, 1994. Effects of estradiol on radial arm maze performance of young and aged rats. Behav. Neural. Biol 62, 230–236. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ, 2003. Rapid enhancement of visual and placememory by estrogens in rats. Endocrinology 144, 2836–44. [DOI] [PubMed] [Google Scholar]
- Luine VN, 2014. Estradiol and cognitive function: past, present and future. Horm. Behav 66, 602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C, 2006. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology 147, 2392–2398. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C, 2004. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology 145, 4154–4161. [DOI] [PubMed] [Google Scholar]
- McConnell SE, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE, 2012. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Horm. Behav 61, 479–486. [DOI] [PubMed] [Google Scholar]
- Moradpour F, Naghdi N, Fathollahi Y, 2006. Anastrozole improved testosterone-induced impairment acquisition of spatial learning and memory in the hippocampal CA1 region in adult male rats. Behav. Brain Res. 175, 223–232. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H, 2002. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem 9, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghdi N, Nafisy N, Majlessi N, 2001. The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain Res. 897, 44–51. [DOI] [PubMed] [Google Scholar]
- Nayebi AM, Pourrabi S, Hossini S, 2014. Testosterone ameliorates streptozotocin-induced memory impairment in male rats. Acta Pharmacol Sin 35, 752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, 1998. Posttraining estrogen and memory modulation. Horm. Behav 34, 126–139. [DOI] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM, 1996. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic systems. Behav. Neurosci 110, 626–632. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ, 2005. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology 146, 147–155. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Lee DW, Fernando G, Schlinger BA, 2004. Radial glia express aromatase in the injured zebra finch brain. J. Comp. Neurol 475, 261–269. [DOI] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, et al. , 2012. Low doses of 17beta-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology 37, 2299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J, Dudzinski DA, Prols F, Glatzel M, Matschke J, Rune GM, 2016. Aromatase expression in the hippocampus of AD patients and 5xFAD mice. Neural Plast 2016, 9802086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, Rune GM, 2008. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J. Cell Biol 180, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM, 2003. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus 13, 226–234. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA, 2012. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J. Neurophysiol 107, 1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA, 2008. Forebrain steroid levels fluctuate rapidly during social interactions. Nat. Neurosci 11, 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Saldanha CJ, Schlinger BA, 2011. Estradiol synthesis and action at the synapse: evidence for “synaptocrine” signaling. Front Endocrinol (Lausanne) 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA, 1985. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology 117, 2471–2477. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA, 1989. Testosterone regulates aromatase activity in discrete brain areas of male rhesus macaques. Biol. Reprod 40, 929–934. [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Fallowfield L, Howell A, 2001. The effects of oestrogens and anti-oestrogens on cognition. Breast 10, 484–491. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmuller D, 1999. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J. Steroid Biochem. Mol. Biol 70, 237–241. [DOI] [PubMed] [Google Scholar]
- Thakur MK, Kumar RC, 2007. 17Beta-estradiol modulates age-dependent binding of 40 kDa nuclear protein to androgen receptor promoter in mouse cerebral cortex. Biogerontology. 8(5), 575–82. [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM, 2016. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm. Behav 83, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Taxier LR, Fortress AM, Frick KM, 2018. Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol. Learn. Mem 156, 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Mielke KL, Cosma M, Soares-Welch C, Paulo R, Miles JM, Bowers CY, 2009. Aromatase and 5alpha-reductase inhibition during an exogenous testosterone clamp unveils selective sex steroid modulation of somatostatin and growth hormone secretagogue actions in healthy older men. J. Clin. Endocrinol. Metab 94, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, Rune GM, 2012. Aromatase inhibition abolishes LTP generation in female but not in male mice. J. Neurosci 32, 8116–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vockel A, Prove E, Balthazart J, 1990. Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res. 511, 291–302. [DOI] [PubMed] [Google Scholar]
- Wagner BA, Braddick VC, Batson CG, Cullen BH, Miller LE, Spritzer MD, 2018. Effects of testosterone dose on spatial memory among castrated adult male rats. Psychoneuroendocrinology 89, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA, 2006. Ovarian steroids enhance object recognition innaturally cycling and ovariectomized, hormone-primed rats. Neurobiol. Learn. Mem 86, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ, 2005. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J. Neurosci 25, 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yague JG, Azcoitia I, DeFelipe J, Garcia-Segura LM, Munoz A, 2010. Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 1315, 41–52. [DOI] [PubMed] [Google Scholar]
- Zameer S, Vohora D, 2017. Effect of aromatase inhibitors on learning and memory and modulation of hippocampal dickkopf-1 and sclerostin in female mice. Pharmacol Rep 69, 1300–1307. [DOI] [PubMed] [Google Scholar]
- Zhao J, Bian C, Liu M, Zhao Y, Sun T, Xing F, Zhang J, 2018. Orchiectomy and letrozole differentially regulate synaptic plasticity and spatial memory in a manner that is mediated by SRC-1 in the hippocampus of male mice. J. Steroid Biochem. Mol. Biol 178, 354–368. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM, 2012. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J. Neurosci 32, 2344–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM, 2010. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc. Natl. Acad. Sci. U. S. A 107, 5605–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM, 2010. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology 151, 1153–1160. [DOI] [PubMed] [Google Scholar]