Abstract
Stroke risk and poor stroke outcomes in postmenopausal women have usually beeen attributed to decreased levels of estrogen. However, two lines of evidence suggest that this hormone may not be solely responsible for elevated stroke risk in this population. First, the increased risk for CVD and stroke occurs much earlier than menopause at a time when estrogen levels are not yet reduced. Second, estrogen therapy has not successfully reduced stroke risk in all studies. Other sex hormones may therefore also contribute to stroke risk. Prior to menopause, levels of the gonadotrophin Follicle Stimulating Hormone (FSH) are elevated while levels of the gonadal peptide inhibin are lowered, indicating an overall decrease in ovarian reserve. Similarly, reduced estrogen levels at menopause significantly increase the ratio of androgens to estrogens. In view of the evidence that androgens may be unfavorable for CVD and stroke, this elevated ratio of testosterone to estrogen may also contribute to the postmenopause-associated stroke risk. This review synthesizes evidence from different clinical populations including natural menopause, surgical menopause, women on chemotherapy, and preclinical stroke models to dissect the role of ovarian hormones and stroke risk and outcomes.
Keywords: menopause, ischemic stroke, FSH, testosterone
Menopause, estrogen deficiency and stroke outcomes:
The importance of ovarian hormones as a risk factor for stroke is evident in comparisons of the incidence of female strokes before and after menopause. Premenopausal women have a much lower incidence of stroke as compared to young males, however at the menopause transition (ages 45–54), the incidence of stroke is double that of men1,2. In tandem with increased risk, stroke outcomes are also worse (reviewed in3). Women account for 60% of stroke-related deaths4, even after normalization for age. A Canadian stroke registry study reported that 10% of women stroke patients were discharged to long term care as compared to 5% men5, despite the fact that stroke size tends not to be different in males and females6. Moreover, 5-yr stroke recurrence is disproportionately higher in females (20%) as compared to males (10%) in the 45–64 age range7.
Increased stroke risk and severity among older women led to the hypothesis that the loss of ovarian hormones, principally estrogens, at menopause may be a contributory factor. However, analysis of hormone use and stroke incidence in pre and postmenopausal women does not support this conclusion entirely. For example, a multicenter case-controlled study showed that increased lifetime exposure to estrogen was associated with a lower risk of stroke, supporting the idea that estrogens are benefical8. In contrast, a case-control study in Northern California Kaiser Permanente facilities reported no benefit for stroke risk in postmenopausal women who took hormone therapy relative to those not taking hormones9. More ominously, the Women’s Health Initiative (WHI) study, which had a signifcant impact on menopause medicine, concluded that hormone use actually increased stroke risk. This randomized, double blind, placebo-controlled multicenter trial compared the risk of myocardial infarction, stroke and dementia in women who consumed daily conjugated equine estrogens (CEE)10, CEE+progestins11 or placebo. Hormone therapy groups showed an increased risk for stroke; however, subgroup analyses indicated that most of this risk was seen in the older age groups. In the CEE trial, stroke risk was significantly elevated in the 60–69 year old group but not the 50–59 year old group10. In an observational analysis of postmenopausal women in the Nurse’s Health Study, estrogen and estrogen+progestin use increased the risk of stroke irrespective of the age of the user or time since menopause12. However, the observational arm of the WHI study showed no increased risk for stroke in the CEE or CEE+progestin arm13,14. A possible factor in the discrepancy between the WHI trial and the WHI observational study was that the initiation of hormones was much earlier in the latter study. However other health characteristics among this group can also impact stroke risk in conjunction with hormone therapy (HT). In the observational trial (SHOW study) HT users were more likely to be normotensive and lean as compared to non-users in this study15 which was not the case in the WHI study, where hypertension incidence was similar in CEE users and non-users10. A similar interaction between HT and hypertension was seen in the Danish Nurses study, where normotensive women who used hormone therapy were not different from controls, while the risk for stroke was elevated among hypertensive women who used hormone therapy16.
In addition to comorbid conditions, hormone treatment effects are also modified by the timing of treatment. Data from a prospective study of Swedish women showed that stroke risk was significantly decreased in women who initiated hormone treatment prior to menopause17. In a population-based nested case-control study of 50–69 year old women, HT did not significantly elevate ischemic stroke risk18, further supporting the idea that HT at ages closer to the menopause may be harmless for stroke. Coronary artery calcification, a surrogate marker of cardiac disease, was reduced by estrogen in the youngest cohort of the WHI study (50–59 years)19, also signifying that estrogen’s effects can be modulated by the age of the user. Finally, a study of non-users of HT found that stroke-related mortality in women 65 and older was higher in women with higher levels of endogenous estrogen20, implying that elevated levels of hormones in late life, whether exogenous or endogenous, may exert a deleterious effect on stroke. The issue of timing of treatment was directly tested in the Kronos Early Estrogen Replacement study (KEEPS) 21 and the Early versus Late Intervention Trial with Estradiol (ELITE) study22. The KEEP study was a prospective, randomized, controlled trial study where the primary outcome measure was cardiovascular risk measured by carotid intima thickness, coronary artery calcium, as well as other ancillary measures. Participants were women who were within 3 years of menopause and received either oral CEE or transdermal 17b-estradiol21. The study found that there was no difference in carotid intima thickness (CIMT) in the oral CEE, transdermal 17b-estradiol or placebo-treated groups23. Moreover, although hormone treatment did not affect cognitive function24, it improved sleep quality and vasomotor symptoms25. These findings suggest that hormone therapy for healthy, early postmenopausal women does not increase cardiac disease indicators. The ELITE trial tested the effect of oral 17b-estradiol treatment (with or without progesterone by vaginal gel) on early (<6 years) and late (>10 years) post-menopausal women. This study showed that progression of CIMT was influenced was influenced by the timing of estradiol treatment. Thus estradiol treatment to the early menopausal group had a lower rate of progression of CIMT as compared to placebo controls while CIMT measures in estradiol treated late post-menopausal group were no different from the placebo group22. The ELITE study therefore suggested a protective role for 17b estradiol for early menopausal females. Neither study examined stroke as an endpoint, but extrapolating from the CVD marker, these studies suggest that estrogens are not deleterious when given to women at the early stage of menopausal. In summary, the evidence linking estrogen therapy and stroke risk, is modified by several intervening variables. The modification by ‘age’ (early or late menopause) suggest that other sex hormones may also influence this association.
1. If not estrogen, then what?
The aging ovary: The normal reproductive ovary secretes hormones under the regulation of two pituitary gonadotropins, follicle-stimulating hormones (FSH) and luteinizing hormone (LH) in a tightly regulated cycle26 (Figure 1). In this section, three types of ovarian secretions, steroids, gonadal peptides, and growth factors, will be reviewed.
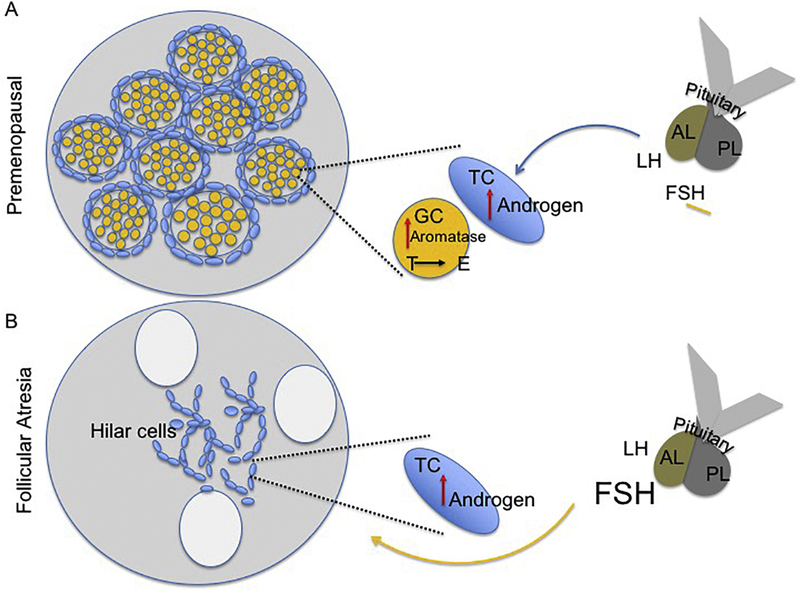
Figure 1:
Schematic representation of the pituitary-ovary axis at (A) pre-menopause and (B) menopause. (A) Pituitary gonadotrophin release acts on theca cells to stimulate androgen synthesis, while FSH increases aromatase in granulosa cells, which facilitates conversion of testosterone to estrogen. Estrogen subsequently suppresses FSH. Menopause: Age-related follicular atresia is associated with loss of granulosa cells and theca cells that accumulate as hilar cells. Pituitary secretions elevate androgens in theca cells, which is not converted to estrogens due to granulosa cell loss, thus increasing T:E ratio and FSH due to loss of negative feedback. LH: Luteinizing hormone, FSH: Follicle stimulating hormone, TC: theca cells, GC: granulosa cells, AL: anterior lobe of the pituitary, PL: posterior lobe of the pituitary.
Ovarian steroids are essential for preparing the uterine endometrium for pregnancy27. Specifically, in response to LH stimulation, ovarian theca cells produce the androgens, DHEA, testosterone, and androstenedione, which diffuse into the granulosa cells28,29. The granulosa cells, in turn, upregulate aromatase in response to FSH to produce 17β-estradiol (E2)30 (Figure 1). After ovulation, the corpus luteum forms, and its theca and granulosa cells increase its production of the other major sex steroid, progesterone31.
The ovarian peptide hormones regulate ovarian function and health. Inhibin, activin, and follistatin modulate pituitary gonadotropin release32,33,34,35. Insulin-like growth factors (IGF) −1 & −2 are produced by ovarian granulosa cells and are involved in follicle development36,37,38,39,26. FSH also regulates IGF binding proteins and stimulates ovarian IGF-1 synthesis40. Antimullerian hormone (AMH) is produced by pre-antral and early antral follicles and reflect the approximate size of the primordial follicle pool and maybe the best biochemical marker of ovarian function41. AMH levels gradually decline with age and loss of the primordial follicle pool. At menopause, AMH is undetectable42.
After menopause, the ovary becomes unresponsive to pituitary gonadotropins, and ovarian hormone production declines43. Estrone (E1), which is produced mostly by the aromatization of androstenedione in fat, replaces 17β-estradiol (E2) as the dominant circulating estrogen44,45,46. However, estrone levels are at lower quantities than premenopausal E247,48. Ovarian testosterone production is preserved and regulated by the gonadotropin LH49,50. The growth factors and gonadal peptides are not produced in appreciable amounts in postmenstrual women51,49. In non-human primates, the data is contradictory with studies showing ovarian hormone decline with age, and other showing that menstruation occurs in late life52. Rodents, like humans, also cease cyclic hormones expression during reproductive senescence. Rats and mice both have 4–5 day estrus cycles, and both species will display age-related changes in cycle length and pattern, although these changes may occur at different chronological ages depending on the species and strain. Thus, Fisher rats do not become acyclic until 18+ months of age53, while Sprague Dawley rats are acyclic at 10–12 months of age54,55. Among mice strain differences have not been well studied, however, C57/B6 mice, one the most commonly used transgenic and wild type model, are acyclic at 13–16 months of age56. With age, rodents display an acyclic pattern of persistent estrus where estradiol levels are still measurable, and FSH levels are low57. This is followed by persistent diestrus, where estradiol levels are undetectable and FSH levels are elevated58. In rodents, the role of estrogen has been thoroughly studied, however, the actions of other ovarian and pituitary hormones is poorly understood (see Table 1 for summary).
Table 1.
| Sex differences in stroke outcomes | Potential mechanism |
|---|---|
| Young female rats have smaller infarcts than male rats and this sex difference is eradicated by bilateral ovariectomy161, 186 | Estrogen deficiency worsens stroke outcomes |
| Estrogen treatment to ovariectomized young females is neuroprotective164, 167, 168, 170 |
| Age differences in stroke outcomes | Potential mechanism | Future direction |
|---|---|---|
| Worse stroke outcomes in middle-aged/aged females as compared to young females or to age-matched males | Toxicity due to low estrogen levels | Use estrogen levels that suppress FSH or improve T:E ratio, instead of matching pre-senescent levels of estrogen |
| -Estrogen treatment to ovariectomized middle-aged or aged females is toxic187, 188 | ||
| Toxicity due to elevated levels of FSH | Test stroke outcomes in animals with FSH over-abundance or FSH receptor k/o. | |
|
FSH receptor knockout in mice:
Promote bone health75–77 | ||
| Estrogen + FSH treatment: No atheroprotection68 | ||
| Toxicity due to increased T:E ratio | Decreasing free testosterone during postmenopausal transition could improve stroke outcome | |
| Testosterone exacerbates stroke infarction Castrated males have better stroke outcomes than gonadally-intact males183–185 |
FSH as a risk factor for CVD and stroke:
Follicle Stimulating Hormone (FSH) stimulates maturation of follicles and estrogen synthesis, and free estrogen, in a negative feedback loop, inhibits FSH secretion from the pituitary. However, serum FSH starts rising above the normal level before menopause, when the level of estrogen is still normal59. Unlike estrogen, FSH has an unfavorable effect on lipid profiles. FSH promotes lipogenesis and fat storage, which is reduced in the FSH receptor knockout animals60,61. Pre-menopausal women with serum FSH >7 IU/l have significant elevation of serum total cholesterol compared to pre-menopausal women with serum FSH < 7 IU/l62. Adipose tissue, which is the main repository for cholesterol, secretes pro-inflammatory molecules such as tumor necrosis factor-alpha (TNF-a) and Interleukin-6 (IL-6), which are associated with development of atherosclerotic plaques and insulin resistance63,64. Not surprisingly, in postmenopausal women, FSH levels also correlate well with vascular inflammation65,66. In the SWAN study, low FSH was associated with low intima media thickness (a measure of cardiac health), as compared to medium or high FSH levels67. Higher levels of FSH and Vascular Cell Adhesion Molecule (VCAM)1, an adhesion molecule associated with atherosclerotic lesions, are found in blood samples from postmenopausal women as compared to premenopausal women, with a positive correlation between these molecules68. In vitro, FSH is shown to elevate VCAM1 in endothelial cells and increase adhesion of human monocytes to endothelial cells68. Interestingly, in a 22-site population-based study of postmenopausal women in East China, high levels of FSH were associated with a low risk of atherosclerotic cardiovascular disease, and low obesity69. In fact two recent studies report that FSH levels are better predictor of metabolic disease in postmenopausal women than CRP (C Reactive Protein), adiponectin and leptin70,71. Thus, the effect of FSH effect is non-linear, and may affect risk factors for cardiovascular disease differently from vascular inflammation.
In addition to the cardiovascular health, FSH also negatively impacts bone health. Elevated level of circulating FSH in the premenopausal period (when estrogen levels are normal) is associated with post-menopausal bone turnover; especially bone resorption72. When estrogen level is low, such as cases of amenorrhea, a high level of FSH is strongly correlated with decreasing bone density73. Hormone therapy is generally shown to prevent bone loss in estrogen-deficient women, but a subgroup of postmenopausal women still lose bone mass despite HT. A retrospective study showed that serum FSH level is a good predictor of bone mineral density in patients with hormonal replacement therapy74. These finding are supported by animal studies which showed that mice with FSH receptors knock out or simple hypophysectomy can preserve bone density despite severe hypogonadism75–77.
1.2. Androgens:
The ratio of estrogen and androgen is also a critical factor for physiologic homeostasis. Estradiol, the most active estrogen, is primarily produced by ovary, whereas testosterone is produced by the ovary (25%), the adrenal gland (25%) and by peripheral conversion of androstenedione and dehydroepiandrosterone (produced by ovarian stroma and adrenal gland) (50%)78. After menopause, the source of circulating testosterone is: 50% by ovaries, 10% by adrenal gland and 40% by peripheral conversion of androstenedione and dehydroepiandrosterone79. During the postmenopause period, the ovary increases secretion of testosterone possibly via stimulation by elevated LH, and this secretion sometimes span up to 10 years after menopause80–82. As a consequence, estrogen levels decline steeply after menopause79,83 whereas testosterone level remain more or less unchanged, leading to a state of relative androgen excess84–86. Like FSH, excess androgen also has unfavorable effects on lipid profiles. It decreases HDL level and increases triglyceride, LDL and total cholesterol87–89. Testosterone is also positively correlated with insulin resistance and type-2 diabetes in elderly population90. Thus one possibility is that estrogen therapy may be more effective if the imbalance between androgen and estrogen is normalized, especially during the transition during the pre- and post- menopause stage.
1.3. Sex hormone binding globulin (SHBG):
Studies have stressed that along with androgen and estrogen, sex hormone binding globulin (SHBG) be included in evaluating risk factors for cardiovascular diseases and mortality in postmenopausal women. SHBG levels tend to decrease across the menopause transition91,92. Low SHBG significantly correlates with the incidence of non-insulin-dependent diabetes mellitus (NIDDM), and stroke93–95, and high level of SHBG in postmenopaual women is strongly associated with decreased risk of type-2 diabetes96. Similar to high level of FSH and androgen, low SHBG is associated with increased triglyceride97, decreased HDL-C98 and, in elderly men, it is associated with coronary heart disease (CHD) mortality99. A high level of free testosterone and low level of SHBG is also associated with elevated triglyceride and low level of HDL100. FAI (Free Androgen Index: Free Testosterone level / SHBG) increase during the menopausal transition is also strong risk factor for metabolic syndromes occurring around the menopausal phase86,101. Thus, in addition to low estrogen levels SHBG may also modify cardiovascular pathologies.
Early menopause:
Two populations can inform the debate on early menopause and stroke risk:
2.1. Chemotherapy for breast cancer:
More than 250,000 women will be diagnosed with breast cancer every year, and women with ER+ tumors102 will be prescribed adjuvant therapy involving endocrine agents. Tamoxifen (TAM), a selective estrogen receptor modulator (SERM) is the most prescribed therapy for premenopausal breast cancer patients. Alternately, women may be prescribed aromatase inhibitors (AI) which block estrogen synthesis. The third-generation AIs such as anastrozole, exemestane and letrozole have largely replaced tamoxifen as the preferred treatment for HR+ breast cancer in postmenopausal women103 and are more effective in women with ER+/PR-tumors. In premenopausal females, AIs are only used if TAM treatment fails104, and then usually in conjunction with ovarian suppression drugs such as GnRH agonists104. Despite their overall effectiveness, however, long term usage of these treatments reveal moderate to severe side effects.
Both drugs are shown to increase disease-free survival, and to reduce cancer-related mortality. At the same time, cardiovascular disease has emerged as the single greatest non-cancer cause of death, accounting for approximately 35% of non-breast cancer mortality for survivors 50 years of age and older. The accelerated menopause phenotype is well recognized in this population, including changes in bone, uterine and cardiovascular health. Neurologic changes due to breast cancer therapies are only recently recognized and only infrequently included in the overall assessment of breast cancer survivors. A recent paper105 clearly outlines this gap in our knowledge of estrogen signaling on neurologic disease such as AD. A similar case may be made for cerebrovascular stroke, since TAM and LTZ causes menopause-like phenotype and effectively places women into a stroke-prone demographic, where outcomes are very poor.
Tamoxifen:
Initially a failed contraceptive compound, tamoxifen (TAM) or ICI146,474 was successfully repurposed as adjuvant breast cancer therapy due to its antagonist actions on estrogen receptors (ER) in this tissue106. TAM is metabolized to 4-hydoxytamoxifen and can exert both antagonist and agonist actions at the estrogen receptor, depending on the cis/trans conformation of its metabolites107, and/or the type of steroid receptor coactivators present in the cell108. Thus, while its action on breast tumor cells is antagonistic, TAM is an agonist on bone tissue and the uterus. Studies have repeatedly shown that TAM use increases the risk for endometrial cancer109,110,111, pulmonary emboli (ATLAS study)110, and increased mortality due to ischemic heart disease and stroke (NSABP B-14) although patient events were low in the latter111. Neural effects of these endocrine therapies are also noted, specifically in cognitive function. In a Dutch study, TAM treatment resulted in significantly lower scores on cognitive performance, especially verbal, memory and executive functioning112. Patients treated for 5–6 months with either TAM or anestrozole (a related AI) showed significant cognitive decline from baseline scores113, although most studies show that the two classes of drugs themselves do not differ in their cognitive effects (reviewed in114). In addition to tissue-specific effects, TAM is also anti-angiogenic, preventing angiogenesis and endothelial tube formation in vitro115, reducing VEGF-induced angiogenesis in vivo in a matrigel preparation116 as well as in the uterus117, indicating the potential for widespread inhibition of angiogenic signaling in multiple organs.
Letrozole:
Letrozole, a 3rd generation aromatase inhibitor, effectively inhibits estrogen synthesis throughout the body, and is a highly effective adjuvant therapy for post-menopausal patients. LTZ is a potent non-steroidal aromatase inhibitor, both in vivo and in vitro118. However, LTZ results in significant side effects such arthralgia and higher grade cardiac events119. Unlike, TAM which has tissue-specific effects, LZT inhibits estrogen synthesis in all tissues. As a result, it has significant side effects that resemble menopausal symptoms including vaginal dryness, hot flashes, loss of libido, musculoskeletal pain and loss of bone mineral density. Joint pain or arthralgia which is associated with increased levels of inflammatory cytokines, occurs in 50% of women prescribed AI, leading to 20% non-compliance with an otherwise effective drug120. Women on LTZ have elevated levels of inflammatory mediators including CRP, eotaxin, MCP-1 (Monocyte Chemoattractant Protein-1)121 and in rodents, a three week course of LTZ causes ovarian cysts with elevation of CRP and oxidative stress122. Similar to TAM, LTZ exerts suppressive effects on angiogenesis in ovarian tissue123. By inhibiting estrogen synthesis and signaling, both LTZ and TAM have the potential to impair stroke recovery.
Among breast cancer survivors, cardiovascular disease (CVD) accounts for approximately 35% of non-breast cancer mortality for survivors 50 years of age and older, making this the single largest non-cancerous cause of death. Cancer is commonly seen in stroke patients and a recent study reported that the rates of hospitalization for stroke among cancer patients (urogenital, breast, prostate being the most frequent) were significantly higher than non-cancer patients in 1997, and remained high in 2006, while hospitalization rates actually fell among non-cancer patients for stroke during the same time frame124. Meta-analyses of breast cancer treatment trials showed that tamoxifen is associated with an increased risk of stroke125–127. Compared to AI, thromoboembolic events and transient ischemic attacks (TIAs) are more common with TAM treatment128.
Unlike TAM, LTZ is more likely to lead to elevated levels of FSH129. Interestingly, this group is also more likely to suffer bone fractures compared to TAM130, although estrogens are equally reduced in both these treatments131. Indirectly, these data support the idea that elevated FSH levels, occuring as a result of estrogen depletion, is likely to be deletrious to traditional targets of estrogen. Bone loss is reported to occur 2–3 years prior to the final menstrual period (FMP)132, a time at which estrogens are not yet low but FSH levels are significantly elevated133.
Far less, however, is known about the effects of breast cancer hormone therapy on stroke recovery. A retrospective analysis of stroke among cancer patients (urogenital, breast and gastrointestinal, being the most frequent) and controls found that cancer patients had a poorer neurological condition at discharge and a trend towards a longer stay in the stroke unit134, both indicative of worse stroke outcomes. The Bergen NORSTROKE study found that ischemic stroke was more prevalent in cancer patients, and the median NIHSS score (an indicator of stroke severity) was significantly higher in cancer patients than non-cancer patients135. Given the mixed cancer population in these study, and the lack of information on endocrine therapy use in this group, the recovery from stroke in patients with TAM or LTZ treatment remains an important gap in the health literature. A report from the Swedish National Hospital Discharge Registry combined with the Swedish Cause of Death Registry showed that stroke incidence was increased during the active phase of TAM treatment and reduced after the active period of treatment. Moreover, mortality from stroke also increased during the active drug period and fell during the post-treatment period136, indicating that both the risk and severity of stroke is affected by TAM treatment. Currently no studies are available on the effects of LTZ on stroke recovery. Long term TAM or LTZ therapy is understudied in the context of stroke and is poorly studied in the preclinial literature. This group of patients show premature menopause, where both FSH levels are likely to be elevated (due to estrogen suppression) as well as an increased ratio of testosterone to estrogen.
2.2. Premature menopause resulting from Bilateral Salpingo-Oophorectomy (BSO):
Bilateral salpingo oophorectomy is a prophylactic surgery for women positive for BRCA1 and BRCA2 mutations to prevent ovarian or fallopian cancer, after their childbearing age. However the majority of cases involve benign disease. BSO is also usually performed along with hysterectomy in order to avoid ovarian pathologies or adnexal pain from postsurgical adhesions. In 1984, 1 out of 8 US women were reported to have undergone oophorectomy prior to natural menopause137, however oophorectomy surgeries have decreased overall nationwide since that time. A recent study shows that the rate of elective bilateral salpingo-oophorectomy was 7.8 per 10,000 in 1998, which increased to 9.0 per 10,000 in 2001 and then fell to 7.4 per 10,000 in 2006138. Due to the increased prevalence of outpatient BSO surgeries, these numbers may be an underestimate since most databases reflect inpatient surgeries139.
Oophorectomy reduces estrogens and testosterone levels and causes a corresponding rise in FSH levels140. In a community-dwellers study (Ranch Bernardo), testosterone levels were significantly reduced in women with BSO when compared to age-matched women with no surgery and women who undergo hysterectomy with preservation of ovaries141. In contrast, FSH levels are significantly elevated in this population. Typically, FSH levels of 30 IU/L with one year without menses is indicative of menopause. Using a surgically intact referent group of women, women over 40years of age with hysterectomy and unilateral oophorectomy were 2.49× more likely to exceed a 40 IU/L criteria and 19.17X more likely to exceed this criteria in women under 40 years of age142.
While BSO reduces ovarian cancer-related mortality in women inherited with BRCA1 and BRCA2 mutations143, it may do more harm than good for women144, including increased non-cancer mortality145, 146. Among the earliest studies on this population were short term (3–6 months) assessments of cognition. Oophorectomy decreased scores on tests of cognition and recall and the MMSE, while estrogen therapy maintained scores at presurgical levels147–149. Perhaps the most definitive studies of health risks for BSO come from longitudinal studies on a well characterized cohort in Olmstead County, MN, called the Mayo Clinic Cohort Study of Oophorectomy and Aging. Women in this cohort showed significant neurological and cardiovascular health risks145. In this cohort, bilateral oophorectomy performed at a younger age, in the absence of estrogen therapy, leads to higher risk for neurological diseases such as Parkinson (Hazard Ratio (HR): 1.8), Dementia (HR=1.7), and depression (HR=1.54)144,150–152. Similarly, an increased risk of cognitive impairment was also found after unilateral or bilateral oophorectomy before menopause153. Estrogen therapy to women with BSO after 49 years of age showed no increased risk for dementia144.
In addition to neurological disorders, bilateral oophorectomy also led to cardiovascular disorders in women. Compared to pre-menopausal women, oophorectomy in the absence of estrogen therapy in age-matched women increased the risk for MI by more than 2-fold144,154. Similarly, an observational study on 29,380 women participating in The Nurses’ Health Study (NHS) with age range of 30–55 also found that compared to women with ovarian conservation, oophorectomy without estrogen therapy increased the risk for MI155. BSO after the age of 50 increases the risk for stroke, which is attenuated by estrogen therapy156.
Increase in serum lipids, reduced carotid artery blood flow, and increased atherosclerosis due to the reduced circulating level of estrogen in the oophorectomized women could be the reason for these cardiovascular conditions. BSO before 45 years, with no estrogen therapy, increased all-cause mortality143,146,155,157. In many of these studies, HT reduced the risk for stroke. While this may indicate that estrogen is a mediator of stroke effects158, it does not preclude the involvement of FSH, since estrogen therapy is known to suppress FSH159.
3. Preclinical models:
Lessons learnt from Preclinical Studies
While it is not practical to study stroke risk in preclinical models, a significant literature is available on the role of sex hormones in stroke recovery. Ischemic stroke is usually caused by mechanical or biochemical occlusion of the lumen of a major brain vessel, typically the middle cerebral artery (MCA) which results in a corticostriatal infarct, or bilateral carotid artery occlusion that results in hippocampal cell death, among others160. Stroke-induced neural damage is usually assessed by a variety of measures such as infarct volume (extent of dead tissue), changes in vascularity (microvessel density, tortuosity, length), changes in blood brain barrier permeability, central and peripheral inflammation, and stroke impairment and recovery is measured by short term and long-term deficits in sensory motor function, cognition, depressive-like behaviors, among others.
In rodents, ischemic stroke results in a smaller infarct and better cerebral blood flow in young females compared to age-matched, normoglycemic161 or diabetic162 males. This sex difference is eradicated when females are bilaterally ovariectomized, supporting the idea that gonadal hormones may underlie these sex differences. This idea received further support from studies showing that the extent of ischemic damage was inversely related to circulating levels of estrogen163. Over the last 20 years, studies have overwhelmingly shown that estrogen treatment to ovariectomized female rats or mice is neuroprotective, an umbrella term typically referring to reduced infarct volume, reduced inflammation and improved motor (behavioral) performance164–167. Thus, replacement with 17β-estradiol or its inactive stereoisomer 17α estradiol168 as well as the conjugate equine estrogen preparations169, all reduced infarct volume in female animals. Exogenous estrogen replacement was shown to be neuroprotective when given prior170 or subsequent to the injury171,172.
However, the effects of estrogens on stroke are not always neuroprotective. In some cases, this has been attributed to the age of the animal or the type of ischemic injury.
Type of injury: Most studies use a transient focal ischemia, where blood flow to the middle cerebral artery (MCAo) is disrupted and then reinstated. In this model, estradiol is typically shown to improve stroke infarction. However, in permanent ischemia models, several studies have shown that estrogen increased infarct volume173–175.
Hormone effects are more complicated in the context of aging. In general, studies agree that older female rats/mice have worse outcomes as compared to young females. In fact, older females have larger infarct volumes than age matched males176, indicating a virtual reversal of the sex difference seen in young animals. This loss of ‘female advantage’ appears to be related to ovarian function, such that acyclic middle-aged female rats also display significantly larger infarct volumes as compared to young females167. These studies used reproductive senescent rats, defined as animals with multiple previous successful pregnancies, current reproductive failure, and vaginal cytology indicative of constant diestrus (a low estrogen state). In this group, serum levels of estradiol are low and FSH levels are elevated, consistent with a ‘menopausal’ pattern58. Thus, the large infarcts seen in this group compared to young normally cycling females is consistent with the hypothesis that ovarian aging impairs stroke recovery. Moreover estradiol treatment for 2–4 weeks, which decreased infarct volumes167,177 and reduced sensory motor impairment in young females, paradoxically, increased infarct volume in reproductive senescent females167.
The anomalous effects of estrogens on stroke outcomes in animal models is reminiscent of the paradoxical effects of this hormone in clinical studies. We proposed (above) that some of these paradoxical effects of estrogen may be related to the altered endocrine environment in aging such as testosterone levels or other sex hormones (FSH). Preclinical studies could be informative on the role of these hormones, especially in the context of aging, however, they have not been exploited. Most studies use a 2-group approach, comparing ovariectomized (OVX) vehicle-treated animals with OVX+estrogen-treated animals, or a 3-group approach comparing gonadally intact, OVX and OVX+estradiol treated animals. Few studies have incorporated an estrogen receptor antagonist, to define the locus of estrogen action. One study that used the pan-estrogen receptor antagonist, ICI182,780, noted that this drug increased striatal (but not cortical) infarction178 presumably by blocking endogenous estrogen signaling. However, no differences were reported in infarct volume between the wild type and ER-alpha knock out (ERKO) mouse, suggesting that estrogen may act via receptor independent process179. Conversely, exogenous estradiol is reported to improve infarct volume in the OVX wild type but not in the OVX ERKO mouse180 or in neuron-specific ER knock out mouse181. These studies did not report whether infarct volume in the untreated OVX mouse is similar to WT, thus it is not clear if the baseline in these models is altered. A third strategy involves the use of the aromatase knock-out or ARKO, where all endogenous estrogen synthesis is inhibited. In this model, MCAo results in a larger infarct volume in the ARKO mouse as compared WT or the ovariectomized WT mouse, and infarct volume is reduced by estradiol treatment182. Moreover, WT animals pretreated with the aromatase inhibitor fadrozole for 1 week also showed worse infarct volumes after MCAo182
The OVX model, which shares similarities with the BSO population, may also result in elevated FSH. Thus, an alternate explanation for the effects of estrogen on stroke outcomes in the OVX model is that this treatment improves stroke outcomes by suppressing FSH. Few studies have tested if estrogen treatment to OVX rats (or mice) reduces FSH levels and whether co-treatment with FSH negates the effects of estradiol. In a study of aortic atherosclerotic lesions in the ApoE−/−, OVX mice showed increased lesions which were reduced by estradiol. However, co-treatment of estradiol and FSH abolished the protective effect of estradiol68, suggesting that the atheroprotective effect of estradiol was driven by its negative regulation of FSH. No such studies have been performed for stroke studies.
In the case of ovary-intact populations, there is some concordance of the human and preclinical data, such that stroke risk is elevated in older postmenopausal women and stroke outcomes are worse in reproductive senescent rats. In these groups, toxicity of elevated FSH levels may be a concern, as also the increased testosterone to estrogen ratio may be a probable cause for worse stroke outcomes. In rodent models, testosterone has been shown to exacerbate infarction. Thus, castrate males display lower infarct volumes as compared to gonadal-intact males and testosterone replaced castrate males183,184,185. Thus, larger infarct sizes in reproductive senescent females may also result from elevated testosterone levels. A corollary to this hypothesis is that estrogen treatment to this group is toxic or ineffective because it fails to suppress elevated FSH levels or fails to restore homeostatic levels of testosterone and estrogen. It also raises the intriguing idea that ovariectomy to this group may actually improve stroke outcomes as compared to ovary intact females. These alternate strategies have not been tested thus far.
Conclusions:
Overall, the evidence linking estrogen loss to increased stroke risk is fairly strong, however, the data on estrogen therapy and stroke risk is more ambiguous. In reviewing other cohorts of early menopause (surgical or through chemotherapy) suggests that other sex hormones may also be modify this risk. In the preclinical literature, the variability in stroke outcomes based on age, dose, type of injury may be better explained by considering a global change in ovarian hormones and gonadotrophins.
Highlights.
Loss of ovarian hormones increases the risk for ischemic stroke in women, and increases stroke severity in animal models
Estrogen treatment, however, does not consistently reduce stroke risk in women, or stroke outcomes in animal studies.
The increase in gonadotropins and altered ratio of androgens to estrogens after menopause may be important modifiers of stroke risk and stroke outcomes
Acknowledgements
Supported by AG042189 and NS074895 to FS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reeves MJ, et al. , Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol, 2008. 7(10): p. 915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Towfighi A, et al. , A midlife stroke surge among women in the United States. Neurology, 2007. 69(20): p. 1898–904. [DOI] [PubMed] [Google Scholar]
- 3.Sohrabji F, Chapter 9 - Cerebrovascular Stroke: Sex Differences and the Impact of Estrogens A2 - Duncan, Kelli A, in Estrogen Effects on Traumatic Brain Injury. 2015, Academic Press: San Diego: p. 125–141. [Google Scholar]
- 4.Lloyd-Jones D, et al. , Heart disease and stroke statistics−−2010 update: a report from the American Heart Association. Circulation, 2010. 121(7): p. e46–e215. [DOI] [PubMed] [Google Scholar]
- 5.Kapral MK, et al. , Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke, 2005. 36(4): p. 809–14. [DOI] [PubMed] [Google Scholar]
- 6.Silva GS, et al. , Gender differences in outcomes after ischemic stroke: role of ischemic lesion volume and intracranial large-artery occlusion. Cerebrovasc Dis, 2010. 30(5): p. 470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roger VL, et al. , Heart disease and stroke statistics−−2011 update: a report from the American Heart Association. Circulation, 2011. 123(4): p. e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso de Lecinana M, et al. , Risk of ischemic stroke and lifetime estrogen exposure. Neurology, 2007. 68(1): p. 33–8. [DOI] [PubMed] [Google Scholar]
- 9.Petitti DB, et al. , Ischemic stroke and use of estrogen and estrogen/progestogen as hormone replacement therapy. Stroke, 1998. 29(1): p. 23–8. [DOI] [PubMed] [Google Scholar]
- 10.Hendrix SL, et al. , Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation, 2006. 113(20): p. 2425–34. [DOI] [PubMed] [Google Scholar]
- 11.Wassertheil-Smoller S, et al. , Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. Jama, 2003. 289(20): p. 2673–84. [DOI] [PubMed] [Google Scholar]
- 12.Grodstein F, et al. , Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med, 2008. 168(8): p. 861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prentice RL, et al. , Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol, 2008. 167(12): p. 1407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prentice RL, et al. , Combined analysis of Women’s Health Initiative observational and clinical trial data on postmenopausal hormone treatment and cardiovascular disease. Am J Epidemiol, 2006. 163(7): p. 589–99. [DOI] [PubMed] [Google Scholar]
- 15.Bushnell C, Stroke Hormones and Outcomes in Women (SHOW) study: is the ‘healthy-user effect’ valid for women after stroke? Womens Health (Lond), 2009. 5(5): p. 485–96. [DOI] [PubMed] [Google Scholar]
- 16.Lokkegaard E, et al. , Increased risk of stroke in hypertensive women using hormone therapy: analyses based on the Danish Nurse Study. Arch Neurol, 2003. 60(10): p. 1379–84. [DOI] [PubMed] [Google Scholar]
- 17.Li C, et al. , Risk of stroke and hormone replacement therapy. A prospective cohort study. Maturitas, 2006. 54(1): p. 11–8. [DOI] [PubMed] [Google Scholar]
- 18.Arana A, et al. , Hormone therapy and cerebrovascular events: a population-based nested case-control study. Menopause, 2006. 13(5): p. 730–6. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, et al. , Estrogen therapy and coronary-artery calcification. N Engl J Med, 2007. 356(25): p. 2591–602. [DOI] [PubMed] [Google Scholar]
- 20.Maggio M, et al. , Relationship between higher estradiol levels and 9-year mortality in older women: the Invecchiare in Chianti study. J Am Geriatr Soc, 2009. 57(10): p. 1810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller VM, et al. , Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). J Cardiovasc Transl Res, 2009. 2(3): p. 228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodis HN, et al. , Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. New England Journal of Medicine, 2016. 374(13): p. 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harman SM, et al. , Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med, 2014. 161(4): p. 249–60. [DOI] [PubMed] [Google Scholar]
- 24.Gleason CE, et al. , Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS–Cognitive and Affective Study. PLoS Medicine, 2015. 12(6): p. e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cintron D, et al. , Effects of oral versus transdermal menopausal hormone treatments on self-reported sleep domains and their association with vasomotor symptoms in recently menopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). Menopause (New York, N.y.), 2018. 25(2): p. 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman BL, et al. , Reproductive Endocrinology, in Williams Gynecology, 3e 2016, McGraw-Hill Education: New York, NY. [Google Scholar]
- 27.Alford C and Nurudeen S, Chapter 4 Physiology of Reproduction in Women, in CURRENT Diagnosis & Treatment: Obstetrics & Gynecology, 11e, DeCherney AH, et al., Editors. 2013, The McGraw-Hill Companies: New York, NY. [Google Scholar]
- 28.Jeppesen JV, et al. , LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab, 2012. 97(8): p. E1524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Channing CP, et al. , Ovarian follicular and luteal physiology. Int Rev Physiol, 1980. 22: p. 117–201. [PubMed] [Google Scholar]
- 30.Richards JS and Kersey KA, Changes in theca and granulosa cell function in antral follicles developing during pregnancy in the rat: gonadotropin receptors, cyclic AMP and estradiol-17 β. Biology of Reproduction, 1979. 21(5): p. 1185–1201. [DOI] [PubMed] [Google Scholar]
- 31.Niswender GD, et al. , Mechanisms controlling the function and life span of the corpus luteum. Physiological reviews, 2000. 80(1): p. 1–29. [DOI] [PubMed] [Google Scholar]
- 32.Sharpe R, Cellular aspects of the inhibitory actions of LH-RH on the ovary and testis. Journal of reproduction and fertility, 1982. 64(2): p. 517–527. [DOI] [PubMed] [Google Scholar]
- 33.Welt CK, et al. , Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab, 1999. 84(1): p. 105–11. [DOI] [PubMed] [Google Scholar]
- 34.Vale W, et al. , The inhibin/activin family of hormones and growth factors, in Peptide growth factors and their receptors II. 1990, Springer; p. 211–248. [Google Scholar]
- 35.Carroll RS, et al. , Inhibin, activin, and follistatin: regulation of follicle-stimulating hormone messenger ribonucleic acid levels. Molecular Endocrinology, 1989. 3(12): p. 1969–1976. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez E, et al. , Rat ovarian insulin-like growth factor I (IGF-I) gene expression is granulosa cell-selective: 5′-untranslated mRNA variant representation and hormonal regulation. Endocrinology, 1989. 125(1): p. 572–574. [DOI] [PubMed] [Google Scholar]
- 37.Mason H, et al. , Insulin-like growth factor-I (IGF-I) inhibits production of IGF-binding protein-1 while stimulating estradiol secretion in granulosa cells from normal and polycystic human ovaries. The Journal of Clinical Endocrinology & Metabolism, 1993. 76(5): p. 1275–1279. [DOI] [PubMed] [Google Scholar]
- 38.OLIVER JE, et al. , Insulin-like growth factor I gene expression in the rat ovary is confined to the granulosa cells of developing follicles. Endocrinology, 1989. 124(6): p. 2671–2679. [DOI] [PubMed] [Google Scholar]
- 39.Parikh G, et al. , Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Hormone and metabolic research, 2010. 42(10): p. 754–757. [DOI] [PubMed] [Google Scholar]
- 40.Adachi T, et al. , Regulation of IGF binding proteins by FSH in human luteinizing granulosa cells. J Assist Reprod Genet, 1995. 12(9): p. 639–43. [DOI] [PubMed] [Google Scholar]
- 41.Peluso C, et al. , AMH: An ovarian reserve biomarker in assisted reproduction. Clin Chim Acta, 2014. 437: p. 175–82. [DOI] [PubMed] [Google Scholar]
- 42.La Marca A and Volpe A, Anti-Mullerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf), 2006. 64(6): p. 603–10. [DOI] [PubMed] [Google Scholar]
- 43.Seifer DB, et al. , Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertility and sterility, 1999. 72(1): p. 63–65. [DOI] [PubMed] [Google Scholar]
- 44.Szymczak J, et al. , Concentration of sex steroids in adipose tissue after menopause. Steroids, 1998. 63(5–6): p. 319–321. [DOI] [PubMed] [Google Scholar]
- 45.Pasqualini J, et al. , Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre-and postmenopausal breast cancer patients. The Journal of Clinical Endocrinology & Metabolism, 1996. 81(4): p. 1460–1464. [DOI] [PubMed] [Google Scholar]
- 46.Batrinos ML, Premenopause: the endocrinology of reproductive decline. Hormones (Athens), 2013. 12(3): p. 334–49. [DOI] [PubMed] [Google Scholar]
- 47.Grodin J, Siiteri P, and MacDonald P, Source of estrogen production in postmenopausal women. The Journal of Clinical Endocrinology & Metabolism, 1973. 36(2): p. 207–214. [DOI] [PubMed] [Google Scholar]
- 48.Cauley JA, et al. , The epidemiology of serum sex hormones in postmenopausal women. American journal of epidemiology, 1989. 129(6): p. 1120–1131. [DOI] [PubMed] [Google Scholar]
- 49.Longcope C, Endocrine function of the postmenopausal ovary. Journal of the Society for Gynecologic Investigation, 2001. 8(1_suppl): p. S67–S68. [DOI] [PubMed] [Google Scholar]
- 50.Ushiroyama T and Sugimoto O, Endocrine Function of the Peri–and Postmenopausal Ovary. Hormone Research in Paediatrics, 1995. 44(2): p. 64–68. [DOI] [PubMed] [Google Scholar]
- 51.Rinaudo P and Strauss JF, Endocrine function of the postmenopausal ovary. Endocrinology and Metabolism Clinics, 2004. 33(4): p. 661–674. [DOI] [PubMed] [Google Scholar]
- 52.Walker ML and Herndon JG, Menopause in nonhuman primates? Biology of reproduction, 2008. 79(3): p. 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeynalov E, et al. , Reproductive Senescence Blunts Response of Estrogen Receptor-α Expression to Estrogen Treatment in Rat Post-Ischemic Cerebral Microvessels. PLOS ONE, 2014. 9(7): p. e102194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LeFevre J and McClintock MK, Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod, 1988. 38(4): p. 780–9. [DOI] [PubMed] [Google Scholar]
- 55.Jezierski MK and Sohrabji F, Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging, 2001. 22(2): p. 309–19. [DOI] [PubMed] [Google Scholar]
- 56.Nelson JF, et al. , A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod, 1982. 27(2): p. 327–39. [DOI] [PubMed] [Google Scholar]
- 57.Koebele SV and Bimonte-Nelson HA, Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas, 2016. 87: p. 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sohrabji F, Bake S, and Lewis DK, Age-related changes in brain support cells: Implications for stroke severity. Neurochem Int, 2013. 63(4): p. 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klein NA and Soules MR, Endocrine changes of the perimenopause. Clin Obstet Gynecol, 1998. 41(4): p. 912–20. [DOI] [PubMed] [Google Scholar]
- 60.Liu XM, et al. , FSH regulates fat accumulation and redistribution in aging through the Gαi/Ca2+/CREB pathway. Aging cell, 2015. 14(3): p. 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zareba P, et al. , Androgen deprivation therapy and cardiovascular disease: what is the linking mechanism? Therapeutic advances in urology, 2016. 8(2): p. 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chu MC, et al. , Elevated basal FSH in normal cycling women is associated with unfavourable lipid levels and increased cardiovascular risk. Human Reproduction, 2003. 18(8): p. 1570–1573. [DOI] [PubMed] [Google Scholar]
- 63.Choi SH, Hong ES, and Lim S, Clinical implications of adipocytokines and newly emerging metabolic factors with relation to insulin resistance and cardiovascular health. Front Endocrinol (Lausanne), 2013. 4: p. 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crawford ED, et al. , The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol Oncol, 2017. 35(5): p. 183–191. [DOI] [PubMed] [Google Scholar]
- 65.Figueroa-Vega N, Moreno-Frias C, and Malacara JM, Alterations in adhesion molecules, pro-inflammatory cytokines and cell-derived microparticles contribute to intima-media thickness and symptoms in postmenopausal women. PLoS One, 2015. 10(5): p. e0120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poljak Z, et al. , Are GnRH and FSH potentially damaging factors in the cardiovascular system? Pharmazie, 2018. 73(4): p. 187–190. [DOI] [PubMed] [Google Scholar]
- 67.El Khoudary SR, et al. , Trajectories of estradiol and follicle-stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. European Journal of Preventive Cardiology, 2016. 23(7): p. 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, et al. , Follicular Stimulating Hormone Accelerates Atherogenesis by Increasing Endothelial VCAM-1 Expression. Theranostics, 2017. 7(19): p. 4671–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang N, et al. , Follicle‐Stimulating Hormone, Its Association with Cardiometabolic Risk Factors, and 10‐Year Risk of Cardiovascular Disease in Postmenopausal Women. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease, 2017. 6(9): p. e005918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stefanska A, et al. , Association of FSH with metabolic syndrome in postmenopausal women: a comparison with CRP, adiponectin and leptin. Biomark Med, 2014. 8(7): p. 921–30. [DOI] [PubMed] [Google Scholar]
- 71.Stefanska A, et al. , Association of follicle-stimulating hormone and sex hormone binding globulin with the metabolic syndrome in postmenopausal women. Clin Biochem, 2012. 45(9): p. 703–6. [DOI] [PubMed] [Google Scholar]
- 72.Sowers MR, et al. , Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int, 2003. 14(3): p. 191–7. [DOI] [PubMed] [Google Scholar]
- 73.Devleta B, Adem B, and Senada S, Hypergonadotropic amenorrhea and bone density: new approach to an old problem. J Bone Miner Metab, 2004. 22(4): p. 360–4. [DOI] [PubMed] [Google Scholar]
- 74.Kawai H, Furuhashi M, and Suganuma N, Serum follicle-stimulating hormone level is a predictor of bone mineral density in patients with hormone replacement therapy. Archives of gynecology and obstetrics, 2004. 269(3): p. 192–195. [DOI] [PubMed] [Google Scholar]
- 75.Yeh J, Chen M-M, and Aloia J, Ovariectomy-induced high turnover in cortical bone is dependent on pituitary hormone in rats. Bone, 1996. 18(5): p. 443–450. [DOI] [PubMed] [Google Scholar]
- 76.Yeh J, Chen M, and Aloia J, Effects of 17β-estradiol administration on cortical and cancellous bone of ovariectomized rats with and without hypophysectomy. Bone, 1997. 20(5): p. 413–420. [DOI] [PubMed] [Google Scholar]
- 77.Sun L, et al. , FSH directly regulates bone mass. Cell, 2006. 125(2): p. 247–60. [DOI] [PubMed] [Google Scholar]
- 78.Burger HG, Androgen production in women. Fertil Steril, 2002. 77 Suppl 4: p. S3–5. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y, et al. , Relative androgen excess and increased cardiovascular risk after menopause: a hypothesized relation. Am J Epidemiol, 2001. 154(6): p. 489–94. [DOI] [PubMed] [Google Scholar]
- 80.Ala-Fossi SL, et al. , Ovarian testosterone secretion during perimenopause. Maturitas, 1998. 29(3): p. 239–45. [DOI] [PubMed] [Google Scholar]
- 81.Sluijmer AV, et al. , Endocrine activity of the postmenopausal ovary: the effects of pituitary down-regulation and oophorectomy. J Clin Endocrinol Metab, 1995. 80(7): p. 2163–7. [DOI] [PubMed] [Google Scholar]
- 82.Fogle RH, et al. , Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab, 2007. 92(8): p. 3040–3. [DOI] [PubMed] [Google Scholar]
- 83.Burger HG, The menopausal transition. Baillieres Clin Obstet Gynaecol, 1996. 10(3): p. 347–59. [DOI] [PubMed] [Google Scholar]
- 84.Longcope C, et al. , Steroid and gonadotropin levels in women during the perimenopausal years. Maturitas, 1986. 8(3): p. 189–96. [DOI] [PubMed] [Google Scholar]
- 85.Rannevik G, et al. , A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas, 1995. 21(2): p. 103–13. [DOI] [PubMed] [Google Scholar]
- 86.Torréns JI, et al. , Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in mid-life women: SWAN. Menopause (New York, NY), 2009. 16(2): p. 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarrel PM, Cardiovascular aspects of androgens in women. Semin Reprod Endocrinol, 1998. 16(2): p. 121–8. [DOI] [PubMed] [Google Scholar]
- 88.Reiner Z, [The effects of androgens and other sex hormones on serum lipoproteins]. Lijec Vjesn, 1996. 118 Suppl 1: p. 33–7. [PubMed] [Google Scholar]
- 89.Kaunitz AM, The role of androgens in menopausal hormonal replacement. Endocrinol Metab Clin North Am, 1997. 26(2): p. 391–7. [DOI] [PubMed] [Google Scholar]
- 90.Oh JY, et al. , Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care, 2002. 25(1): p. 55–60. [DOI] [PubMed] [Google Scholar]
- 91.Burger HG, et al. , A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. The Journal of Clinical Endocrinology & Metabolism, 2000. 85(8): p. 2832–2838. [DOI] [PubMed] [Google Scholar]
- 92.Gambera A, et al. , Androgens, insulin-like growth factor-I (IGF-I), and carrier proteins (SHBG, IGFBP-3) in postmenopause. Menopause, 2004. 11(2): p. 159–66. [DOI] [PubMed] [Google Scholar]
- 93.Lindstedt G, et al. , Low sex-hormone-binding globulin concentration as independent risk factor for development of NIDDM: 12-yr follow-up of population study of women in Gothenburg, Sweden. Diabetes, 1991. 40(1): p. 123–128. [DOI] [PubMed] [Google Scholar]
- 94.Lapidus L, et al. , Concentrations of sex-hormone binding globulin and corticosteroid binding globulin in serum in relation to cardiovascular risk factors and to 12-year incidence of cardiovascular disease and overall mortality in postmenopausal women. Clinical chemistry, 1986. 32(1): p. 146–152. [PubMed] [Google Scholar]
- 95.Laaksonen DE, et al. , Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care, 2004. 27(5): p. 1036–41. [DOI] [PubMed] [Google Scholar]
- 96.Ding EL, et al. , Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med, 2009. 361(12): p. 1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Masarei JR, et al. , HDL-cholesterol and sex-hormone status. Lancet, 1980. 1(8161): p. 208. [DOI] [PubMed] [Google Scholar]
- 98.Haffner SM, et al. , Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. The Journal of Clinical Endocrinology & Metabolism, 1993. 77(1): p. 56–60. [DOI] [PubMed] [Google Scholar]
- 99.Kalme T, et al. , Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. The Journal of Clinical Endocrinology & Metabolism, 2005. 90(3): p. 1550–1556. [DOI] [PubMed] [Google Scholar]
- 100.Haffner SM and Valdez RA, Endogenous sex hormones: impact on lipids, lipoproteins, and insulin. Am J Med, 1995. 98(1A): p. 40S–47S. [DOI] [PubMed] [Google Scholar]
- 101.Shelley JM, et al. , Relationship of endogenous sex hormones to lipids and blood pressure in mid-aged women. Ann Epidemiol, 1998. 8(1): p. 39–45. [DOI] [PubMed] [Google Scholar]
- 102.Hammond ME, et al. , American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract, 2010. 6(4): p. 195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fabian CJ, The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. International Journal of Clinical Practice, 2007. 61(12): p. 2051–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Forward DP, et al. , Clinical and endocrine data for goserelin plus anastrozole as second-line endocrine therapy for premenopausal advanced breast cancer. British Journal of Cancer, 2004. 90(3): p. 590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fiske ST and Blaustein JD, Treatments for Breast Cancer That Affect Cognitive Function in Postmenopausal Women. Policy Insights from the Behavioral and Brain Sciences, 2017. 4(2): p. 170–177. [Google Scholar]
- 106.Jordan VC, Tamoxifen: the herald of a new era of preventive therapeutics. J Natl Cancer Inst, 1997. 89(11): p. 747–9. [DOI] [PubMed] [Google Scholar]
- 107.Robertson DW, et al. , Tamoxifen antiestrogens. A comparison of the activity, pharmacokinetics, and metabolic activation of the cis and trans isomers of tamoxifen. J Steroid Biochem, 1982. 16(1): p. 1–13. [DOI] [PubMed] [Google Scholar]
- 108.Gallo MA and Kaufman D, Antagonistic and agonistic effects of tamoxifen: significance in human cancer. Semin Oncol, 1997. 24(1 Suppl 1): p. S1–71–s1–80. [PubMed] [Google Scholar]
- 109.Chen J-Y, et al. , Endometrial Cancer Incidence in Breast Cancer Patients Correlating with Age and Duration of Tamoxifen Use: a Population Based Study. Journal of Cancer, 2014. 5(2): p. 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davies C, et al. , Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet, 2013. 381(9869): p. 805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fisher B, Highlights from recent National Surgical Adjuvant Breast and Bowel Project studies in the treatment and prevention of breast cancer. CA Cancer J Clin, 1999. 49(3): p. 159–77. [DOI] [PubMed] [Google Scholar]
- 112.Schilder CM, et al. , Effects of Tamoxifen and Exemestane on Cognitive Functioning of Postmenopausal Patients With Breast Cancer: Results From the Neuropsychological Side Study of the Tamoxifen and Exemestane Adjuvant Multinational Trial. Journal of Clinical Oncology, 2010. 28(8): p. 1294–1300. [DOI] [PubMed] [Google Scholar]
- 113.Collins B, et al. , Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psychooncology, 2009. 18(8): p. 811–21. [DOI] [PubMed] [Google Scholar]
- 114.Phillips KA, Ribi K, and Fisher R, Do aromatase inhibitors have adverse effects on cognitive function? Breast Cancer Research : BCR, 2011. 13(1): p. 203–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnson KE, et al. , Tamoxifen Directly Inhibits Platelet Angiogenic Potential and Platelet-Mediated Metastasis. Arterioscler Thromb Vasc Biol, 2017. 37(4): p. 664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McNamara DA, et al. , Tamoxifen inhibits endothelial cell proliferation and attenuates VEGF-mediated angiogenesis and migration in vivo. Eur J Surg Oncol, 2001. 27(8): p. 714–8. [DOI] [PubMed] [Google Scholar]
- 117.Helmestam M, et al. , Tamoxifen modulates cell migration and expression of angiogenesis-related genes in human endometrial endothelial cells. Am J Pathol, 2012. 180(6): p. 2527–35. [DOI] [PubMed] [Google Scholar]
- 118.Bhatnagar AS, The discovery and mechanism of action of letrozole. Breast Cancer Research and Treatment, 2007. 105(Suppl 1): p. 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Regan MM, et al. , Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1–98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol, 2011. 12(12): p. 1101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Niravath P, Aromatase inhibitor-induced arthralgia: a review. Annals of Oncology, 2013. 24(6): p. 1443–1449. [DOI] [PubMed] [Google Scholar]
- 121.Bauml J, et al. , Arthralgia among women taking aromatase inhibitors: is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Research, 2015. 17(1): p. 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kafali H, et al. , Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res, 2004. 35(2): p. 103–8. [DOI] [PubMed] [Google Scholar]
- 123.Hirakawa H, et al. , Inhibitory effects of aromatase inhibitor on estrogen receptor-alpha positive ovarian cancer in mice. J Ovarian Res, 2014. 7: p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanossian N, et al. , Trends in Cancer Diagnoses among Inpatients Hospitalized with Stroke. Journal of Stroke and Cerebrovascular Diseases, 2013. 22(7): p. 1146–1150. [DOI] [PubMed] [Google Scholar]
- 125.Bushnell C, Depression and the Risk of Stroke in Women: An Identification and Treatment Paradox. Stroke, 2011. 42(10): p. 2718–2719. [DOI] [PubMed] [Google Scholar]
- 126.Sohrabji F, Park MJ, and Mahnke AH, Sex differences in stroke therapies. J Neurosci Res, 2017. 95(1–2): p. 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Braithwaite RS, et al. , Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med, 2003. 18(11): p. 937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coates AS, et al. , Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol, 2007. 25(5): p. 486–92. [DOI] [PubMed] [Google Scholar]
- 129.Bajetta E, et al. , Double-blind, randomised, multicentre endocrine trial comparing two letrozole doses, in postmenopausal breast cancer patients. Eur J Cancer, 1999. 35(2): p. 208–13. [DOI] [PubMed] [Google Scholar]
- 130.Rabaglio M, et al. , Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1–98 trial. Ann Oncol, 2009. 20(9): p. 1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rossi E, et al. , Endocrine Effects of Adjuvant Letrozole Compared With Tamoxifen in Hormone-Responsive Postmenopausal Patients With Early Breast Cancer: The HOBOE Trial. Journal of Clinical Oncology, 2009. 27(19): p. 3192–3197. [DOI] [PubMed] [Google Scholar]
- 132.Sowers MR, et al. , Amount of Bone Loss in Relation to Time around the Final Menstrual Period and Follicle-Stimulating Hormone Staging of the Transmenopause. The Journal of Clinical Endocrinology & Metabolism, 2010. 95(5): p. 2155–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Randolph JJF, et al. , Change in Follicle-Stimulating Hormone and Estradiol Across the Menopausal Transition: Effect of Age at the Final Menstrual Period. The Journal of Clinical Endocrinology & Metabolism, 2011. 96(3): p. 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brumm AJ, et al. , Astrocytes Can Adopt Endothelial Cell Fates in a p53-Dependent Manner. Mol Neurobiol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Selvik HA, et al. , Prior Cancer in Patients with Ischemic Stroke: The Bergen NORSTROKE Study. Journal of Stroke and Cerebrovascular Diseases, 2014. 23(5): p. 919–925. [DOI] [PubMed] [Google Scholar]
- 136.Rosell J, et al. , Time dependent effects of adjuvant tamoxifen therapy on cerebrovascular disease: results from a randomised trial. Br J Cancer, 2011. 104(6): p. 899–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Howe HL, Age-specific hysterectomy and oophorectomy prevalence rates and the risks for cancer of the reproductive system. Am J Public Health, 1984. 74(6): p. 560–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Asante A, et al. , Elective oophorectomy in the United States: trends and in-hospital complications, 1998–2006. Obstet Gynecol, 2010. 116(5): p. 1088–95. [DOI] [PubMed] [Google Scholar]
- 139.Wright JD, et al. , Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol, 2013. 122(2 Pt 1): p. 233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cooper GS and Thorp JM Jr., FSH levels in relation to hysterectomy and to unilateral oophorectomy. Obstet Gynecol, 1999. 94(6): p. 969–72. [DOI] [PubMed] [Google Scholar]
- 141.Laughlin GA, et al. , Hysterectomy, Oophorectomy, and Endogenous Sex Hormone Levels in Older Women: The Rancho Bernardo Study1. The Journal of Clinical Endocrinology & Metabolism, 2000. 85(2): p. 645–651. [DOI] [PubMed] [Google Scholar]
- 142.Moorman PG, et al. , Effect of Hysterectomy With Ovarian Preservation on Ovarian Function. Obstetrics and gynecology, 2011. 118(6): p. 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Domchek SM, et al. , Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol, 2006. 7(3): p. 223–9. [DOI] [PubMed] [Google Scholar]
- 144.Parker WH, et al. , Effect of bilateral oophorectomy on women’s long-term health. Womens Health (Lond), 2009. 5(5): p. 565–76. [DOI] [PubMed] [Google Scholar]
- 145.Rocca WA, et al. , Accelerated Accumulation of Multimorbidity After Bilateral Oophorectomy: A Population-Based Cohort Study. Mayo Clin Proc, 2016. 91(11): p. 1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Evans EC, et al. , Salpingo-oophorectomy at the time of benign hysterectomy: a systematic review. Obstetrics & Gynecology, 2016. 128(3): p. 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Phillips SM and Sherwin BB, Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology, 1992. 17(5): p. 485–95. [DOI] [PubMed] [Google Scholar]
- 148.Sherwin BB, Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology, 1988. 13(4): p. 345–57. [DOI] [PubMed] [Google Scholar]
- 149.Farrag AK, et al. , Effect of surgical menopause on cognitive functions. Dement Geriatr Cogn Disord, 2002. 13(3): p. 193–8. [DOI] [PubMed] [Google Scholar]
- 150.Rocca W, et al. , Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology, 2008. 70(3): p. 200–209. [DOI] [PubMed] [Google Scholar]
- 151.Rocca WA, et al. , Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause, 2008. 15(6): p. 1050–9. [DOI] [PubMed] [Google Scholar]
- 152.Rocca WA, et al. , Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology, 2007. 69(11): p. 1074–83. [DOI] [PubMed] [Google Scholar]
- 153.Rocca W, et al. , Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology, 2007. 69(11): p. 1074–1083. [DOI] [PubMed] [Google Scholar]
- 154.Garcia M, et al. , Cardiovascular disease in women: clinical perspectives. Circulation research, 2016. 118(8): p. 1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Parker WH, et al. , Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstetrics and gynecology, 2009. 113(5): p. 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lai JC-Y, et al. , The risk of stroke after bilateral salpingo-oophorectomy at hysterectomy for benign diseases: A nationwide cohort study. Maturitas, 2018. 114: p. 27–33. [DOI] [PubMed] [Google Scholar]
- 157.Rocca WA, et al. , Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol, 2006. 7(10): p. 821–8. [DOI] [PubMed] [Google Scholar]
- 158.Rocca WA, et al. , Premature menopause or early menopause and risk of ischemic stroke. Menopause (New York, N.y.), 2012. 19(3): p. 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lambrinoudaki I, et al. , Sex hormones in postmenopausal women receiving low-dose hormone therapy: the effect of BMI. Obesity (Silver Spring), 2011. 19(5): p. 988–93. [DOI] [PubMed] [Google Scholar]
- 160.Macrae IM, Preclinical stroke research--advantages and disadvantages of the most common rodent models of focal ischaemia. Br J Pharmacol, 2011. 164(4): p. 1062–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alkayed NJ, et al. , Gender-linked brain injury in experimental stroke. Stroke, 1998. 29(1): p. 159–65; discussion 166. [DOI] [PubMed] [Google Scholar]
- 162.Toung TK, et al. , Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke, 2000. 31(11): p. 2701–6. [DOI] [PubMed] [Google Scholar]
- 163.Liao S, et al. , Association of serum estrogen level and ischemic neuroprotection in female rats. Neurosci. Lett, 2001. 297(3): p. 159–62. [DOI] [PubMed] [Google Scholar]
- 164.Rusa R, et al. , 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke, 1999. 30(8): p. 1665–70. [DOI] [PubMed] [Google Scholar]
- 165.Dubal DB, et al. , Estradiol protects against ischemic injury. J Cereb Blood Flow Metab, 1998. 18(11): p. 1253–8. [DOI] [PubMed] [Google Scholar]
- 166.Simpkins JW, et al. , Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg, 1997. 87(5): p. 724–30. [DOI] [PubMed] [Google Scholar]
- 167.Selvamani A and Sohrabji F, Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging, 2010. 31(9): p. 1618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Simpkins J, et al. , Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J. Neurosurg, 1997. 87: p. 724–730. [DOI] [PubMed] [Google Scholar]
- 169.McCullough LD, et al. , Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke, 2001. 32(3): p. 796–802. [DOI] [PubMed] [Google Scholar]
- 170.Dubal D, et al. , Estradiol protects against ischemic injury. J. Cereb. Blood Flow Metab, 1998. 18: p. 1253–1258. [DOI] [PubMed] [Google Scholar]
- 171.Liu R, et al. , 17beta-Estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res, 2005. 1060(1–2): p. 55–61. [DOI] [PubMed] [Google Scholar]
- 172.Yang SH, et al. , The use of estrogens and related compounds in the treatment of damage from cerebral ischemia. Ann. N. Y. Acad. Sci, 2003. 1007: p. 101–7. [DOI] [PubMed] [Google Scholar]
- 173.Bingham D, Macrae IM, and Carswell HV, Detrimental Effects of 17β-Oestradiol after Permanent Middle Cerebral Artery Occlusion. Journal of Cerebral Blood Flow & Metabolism, 2005. 25(3): p. 414–420. [DOI] [PubMed] [Google Scholar]
- 174.Carswell HV, et al. , Differential Effects of 17β-Estradiol upon Stroke Damage in Stroke Prone and Normotensive Rats. Journal of Cerebral Blood Flow & Metabolism, 2004. 24(3): p. 298–304. [DOI] [PubMed] [Google Scholar]
- 175.Gordon KB, Macrae IM, and Carswell HVO, Effects of 17β-oestradiol on cerebral ischaemic damage and lipid peroxidation. Brain Research, 2005. 1036(1): p. 155–162. [DOI] [PubMed] [Google Scholar]
- 176.Manwani B, et al. , Functional recovery in aging mice after experimental stroke. Brain Behav Immun, 2011. 25(8): p. 1689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Glendenning ML, Lovekamp-Swan T, and Schreihofer DA, Protective effect of estrogen in endothelin-induced middle cerebral artery occlusion in female rats. Neurosci Lett, 2008. 445(2): p. 188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sawada M, et al. , Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab, 2000. 20(1): p. 112–8. [DOI] [PubMed] [Google Scholar]
- 179.Sampei K, et al. , Stroke in estrogen receptor-alpha-deficient mice. Stroke, 2000. 31(3): p. 738–43; discussion 744. [DOI] [PubMed] [Google Scholar]
- 180.Dubal DB, et al. , Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A, 2001. 98(4): p. 1952–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Elzer JG, et al. , Neuronal estrogen receptor-alpha mediates neuroprotection by 17beta-estradiol. J Cereb Blood Flow Metab, 2010. 30(5): p. 935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McCullough LD, et al. , Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci, 2003. 23(25): p. 8701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cheng J, et al. , Age-dependent effects of testosterone in experimental stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 2009. 29(3): p. 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang SH, et al. , Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J Appl Physiol (1985), 2002. 92(1): p. 195–201. [DOI] [PubMed] [Google Scholar]
- 185.Hawk T, et al. , Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res, 1998. 796(1–2): p. 296–8. [DOI] [PubMed] [Google Scholar]
- 186.Park EM, et al. , Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab, 2006. 26(3): p. 392–401. [DOI] [PubMed] [Google Scholar]
- 187.Leon RL, et al. , Worsened outcome from middle cerebral artery occlusion in aged rats receiving 17beta-estradiol. Endocrinology, 2012. 153(7): p. 3386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Selvamani A and Sohrabji F, The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of IGF-1. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2010. 30(20): p. 6852–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]