Abstract
Endocrine-disrupting chemicals (EDCs) are pervasive in the environment. They are found in plastics and plasticizers (bisphenol A (BPA) and phthalates), in industrial chemicals such as polychlorinated biphenyls (PCBs), and include some pesticides and fungicides such as vinclozolin. These chemicals act on hormone receptors and their downstream signaling pathways, and can interfere with hormone synthesis, metabolism, and actions. Because the developing brain is particularly sensitive to endogenous hormones, disruptions by EDCs can change neural circuits that form during periods of brain organization. Here, we review the evidence that EDCs affect developing hypothalamic neuroendocrine systems, and change behavioral outcomes in juvenile, adolescent, and adult life in exposed individuals, and even in their descendants. Our focus is on social, communicative and sociosexual behaviors, as how an individual behaves with a same- or opposite-sex conspecific determines that individual’s ability to exist in a community, be selected as a mate, and reproduce successfully.
Keywords: Endocrine-disrupting chemicals (EDCs), Polychlorinated biphenyls (PCBs), Aroclor 1221, BPA, Phthalate, Vinclozolin, Ultrasonic vocalization, Social behavior, Sociosexual behavior, Sex difference, Gene expression, Hypothalamus, Transgenerational
1. Endocrine-disrupting Chemicals (EDCs)
An endocrine-disrupting chemical is “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action” (Zoeller et al., 2012). The World Health Organization (WHO), U.S. Environmental Protection Agency (EPA), United Nations Environment Programme (UNEP), European Food and Safety Agency (EFSA), scientific/ medical societies such as the Endocrine Society, and many others, recognize EDCs as a public health threat (Bergman et al., 2013; Di Renzo et al., 2015; Zoeller et al., 2012). Exposure of humans and wildlife to EDCs comes from a variety of sources including plastics, flame retardants, agrichemicals, personal care products, household chemicals, and many others.
EDCs perturb hormonal systems. However, the molecular mechanism of action of an EDC in a target cell is complex; this is attributable in part to the complexity of endocrine physiology, but it is also due to chemical properties of EDCs that make them problematic. Most EDCs were never intended for consumption, and with the exception of pesticides, were usually not meant to have biological activity. Despite that, most EDCs are lipophilic (Fernández et al., 2004), can cross cell membranes, and exert actions within cells including via binding of intracellular receptors (Balaguer et al., 2017). A single EDC may bind to one, or more than one, hormone receptor, and act as an agonist, antagonist, or have mixed effects (Gore et al., 2015). EDCs may also travel through the circulatory system as free chemicals, rather than in association with binding proteins as is the case for many endogenous hormones, meaning that EDCs have relatively higher bioavailability. Some EDCs can affect hormone metabolism or degradation (Aluru and Vijayan, 2006; Kester et al., 2000; Kester et al., 2002), thereby changing the ability of a hormone to function in the organism.
To date, EDCs are best studied for their actions via estrogen receptor signaling (Alonso-Magdalena et al., 2006; Dickerson and Gore, 2007). However, EDCs can also be anti-estrogenic, androgenic, anti-androgenic, thyroid-disrupting, and act on membrane hormone receptors (Alonso-Magdalena et al., 2005; Gray et al., 2001; Kelce et al., 1994; Zoeller, 2005). Thus, in considering actions of EDCs, it is important to keep in mind these pleiotropic effects on endocrine systems.
Events during the 20th century influenced our current understanding of how the environment affects reproductive and endocrine systems. In the 1940s, sheep grazing on clover became infertile (Bennetts et al., 1946); this was later attributable to plant phytoestrogens that interfered with reproductive physiology (Adams, 1995). In her groundbreaking book Silent Spring (Carson, 1962), Rachel Carson made the case that environmental chemicals, especially the pesticide DDT, were contributing to serious reproductive problems in wildlife. Subsequent work on wildlife, comparing animals in areas of known contamination to those considered relatively pristine, revealed malformations of reproductive tissues (Orlando and Guillette, 2007). The relevance of environmental chemicals to humans, especially xenoestrogens, came from the connection between the use of diethylstilbestrol (DES), a powerful pharmaceutical estrogen, during pregnancy, and the increased incidence of a rare clear-cell adenocarcinoma of the vagina in girls exposed in utero (Herbst et al., 1971). In this case, a pharmaceutical chemical acted as an EDC by exposing the developing fetus. The widespread detection of estrogenic oral contraceptives and menopausal treatments in the watershed means that aquatic wildlife are exposed to environmental pharmaceutical estrogens (Milnes et al., 2006). As more observations in wildlife, experimental laboratory studies, and human epidemiological work have converged, it is clear that all humans and wildlife are exposed to EDCs, that we have numerous chemicals in our bodies, and that endocrine systems are vulnerable to actions of these environmental chemicals (Gore et al., 2015).
In this review, we will discuss the evidence for effects of EDCs on neuroendocrine systems and the neurobiology of social behavior in mammals. We begin by focusing on how direct EDC exposure during key developmental windows, especially to the fetus and infant, change the developing brain and subsequently, adult behavior of the exposed individuals. We will also provide examples of EDCs influencing behavior several generations later.
There are about 1000 chemicals identified to date as EDCs. For this review, we selected 4 from different classes as examples (Table 1), selected based on knowledge for their direct and/or transgenerational effects on neuroendocrine function and behavior: polychlorinated biphenyls (PCBs), widespread and persistent industrial contaminants; the fungicide vinclozolin; bisphenol A (BPA), commonly found in plastics; and phthalates, used as plasticizers.
Table 1.
EDC families associated with neuroendocrine and neurological effects
| Family | Example EDCs | Structure | Best-known mechanisms of action |
|---|---|---|---|
| Bisphenols | Bisphenol A (BPA) and other bisphenols (BPS, BPN, BPF, etc.) | 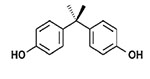 |
Actions on estrogen receptors, androgen receptors, aromatase |
| Phthalates | Di(2-ethylhexyl)phthalate (DEHP); Dibuty1 phthalate (DBP) | 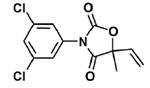 |
Anti-androgenic, andi-estrogenic |
| Fungicides | Vinclozolin | 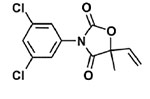 |
Anti-androgenic |
| Polychlorinated biphenyls (PCBs) | Aroclor 1221 | 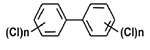 |
Depending upon the degree of chlorination, can be estrogenic, anti-androgenic, bind to thyroid hormone receptors, and bind to aryl hydrocarbon receptors |
2. EDCs and the development of neuroendocrine systems
As the interface between the nervous and endocrine systems, neuroendocrine systems integrate information about the external and internal environment and orchestrate appropriate responses. In response to an environmental challenge, be it predator stress, a social contextual change, climate, or chemical contaminants, the organism must be able to attain and maintain a physiological state that allows survival, adaptation, and ultimately a return to homeostasis when the challenge has passed. Neuroendocrine systems coordinate physiology with behavior, and their actions enable an individual to attain reproductive competence, exhibit sex-appropriate behaviors, choose a mate, reproduce, and care for offspring. In order for this to happen, the brain circuits that regulate or modulate these behaviors must develop under the influence of the correct temporal hormonal and social influences that, if disrupted, may change development, behavior, and impair social interactions and reproductive success.
2.1. The Developmental Origins of Adult Disease (DOHaD) and Neuroendocrine Systems
The DOHaD hypothesis postulates that environmental influences in early development shape the propensity for health or disease states later in life. While initially applied to the concept of prenatal nutritional deprivation in humans (Barker, 2003), DOHaD is fundamental to understanding environmental EDC actions in the developing organism (Heindel et al., 2015a). DOHaD is perfectly illustrated by the concept of brain sexual differentiation. During critical pre- and postnatal developmental stages, gonadal steroids organize the brain, thereby establishing the developmental trajectory of the individual (Arnold and Gorski, 1984). In male mammals, the developing fetal testes produce testosterone, which masculinizes reproductive and non-reproductive tissues. In the brain, fetal testosterone acts upon androgen receptors; it is also converted to estradiol via the aromatase enzyme. Estradiol binds to estrogen receptors in the male brain and activates signaling cascades responsible for both masculinization and defeminization. In female mammals, the ovary produces estradiol, albeit at low concentrations, but in non-human mammals, circulating estradiol is bound by alpha-fetoprotein, preventing it from crossing the blood-brain barrier (Bakker and Baum, 2008; Bakker et al., 2006). This relative lack of exposure to gonadal hormones results in brain feminization and demasculinization. Organizational effects of hormones on the brain are necessary for the development of neural circuitry and the expression of sexually dimorphic physiology and behavior later in life (Nugent et al., 2011). Not only are reproductive behaviors sexually dimorphic – much of the entire social behavior repertoire is dimorphic.
Published work on neuroendocrine disruption is based on experimental and laboratory work mainly conducted in rats and mice. This is the focus of our review, but when available, we include evidence from other rodent species. Humans are exposed to the same chemicals as wildlife, perhaps more due to household chemicals. However, experimental studies on hypothalamic development, hormone and EDC actions, and latent effects on brain and behavior are not possible or ethical in humans. Thus, epidemiological work fills a key gap in knowledge about EDC exposures and their consequences on the propensity to develop disease. Such studies show that humans with higher concentrations of EDC s in their bodies are more likely to be those with endocrine and neurological/ neurobehavioral problems [reviewed in (Gore et al., 2015)]. However, the direct cause-and-effect is not proven (Lee, 2018). Some interesting recent research has also related proxies of early life exposure – chemicals detected in amniotic fluid, urine/serum from pregnant women, and umbilical cord blood – and adverse outcomes (Braun, 2017; Buck Louis et al., 2018). By connecting results of this human work to the experimental mammalian work, confidence in (and concern about) neuroendocrine effects of EDCs emerges. There is an excellent literature on neuroendocrine effects of EDCs in bird, amphibian, reptile and fish species, but we do not have the space to review this field. Several reviews on the subject are here: (Le Page et al., 2011; León-Olea et al., 2014; Ottinger et al., 2013; Rosenfeld et al., 2017; Wingfield and Mukai, 2009).
Hormones exert effects at exquisitely low doses, particularly during sensitive life phases. This concept extrapolates to EDCs, for which extensive research shows biological relevance of dosages of certain EDCs below regulatory “safe” levels (Vandenberg et al., 2012). Neuroendocrine systems are among the most hormonally-sensitive in the body due to high abundance of receptors for steroid hormones, and expression of neuropeptides and their receptors involved in hypothalamic-pituitary regulation, the central control of energy balance, and social/reproductive behaviors, among others. Early in life, the consequences of EDCs on the organization of the brain’s neuroendocrine systems can be both latent and profound. Before we discuss neuroendocrine effects of EDCs, to follow is an introduction to the four classes of EDCs that are the focus of this review .
2.2. Properties of Different EDC Classes
2.2.1. Bisphenol A (BPA)
BPA (Table 1), used in plastics and thermal receipts, became a “poster child” of EDCs, when it was discovered that chemical leaching from improperly washed mouse cages resulted in aneuploidy in mouse oocytes (Hunt et al., 2003). Laboratory work has gone on to show that BPA exposure causes a variety of hormone-sensitive cancers, affects thyroid hormones, is linked to diabetes and obesity, and leads to many other reproductive problems beyond aneuploidy (Chapin et al., 2008; Vandenberg et al., 2009). Since then, this chemical has received a great deal of public attention, leading to consumer pressure to remove it from the marketplace. The label “BPA-free” now appears on a large number of products, although less commonly known is that other members of the bisphenol family such as BPS and BPF have replaced BPA and are on the market. Although beyond the scope of this review, research indicates that these bisphenols may be just as problematic as BPA in acting upon hormone receptor-mediated and other (e.g. aromatase) pathways [(Kinch et al., 2015; Viñas and Watson, 2013); reviewed in (Rosenfeld, 2017)]. While most work on BPA has focused on its estrogenic mechanisms, BPA also acts upon androgenic, thyroid, progesterone, insulin and other endocrine signaling systems (Aldad et al., 2011; Alonso-Magdalena et al., 2010; Brannick et al., 2012), and it influences the control of energy balance (Chamorro-García et al., 2018; Ohlstein et al., 2014; Roepke et al., 2016). The half-life of BPA is short, on the order of hours (Gerona el al., 2013). However, its pervasiveness means that it is constantly replenished, with over 90% of people tested having detectable levels of BPA in their bodies (Braun et al., 2011; Calafat et al., 2008).
2.2.2. Phthalates
Phthalates (Table 1) are used as plasticizers in the production of commercial plastics and plastic coatings, medical tubing, vinyl flooring materials, children’s toys, and in some cosmetics. Phthalates are found in human urine and serum, as well as in breast milk and umbilical cord blood (Fromme et al., 2011; Hines et al., 2009). Food contamination with phthalates is common and likely the main route of exposure. The use of phthalates in intravenous tubing also introduces the chemicals directly into the body, as does application of personal care products to the skin, where dermal absorption can occur. Phthalates have a short half-life in the body, estimated at about 12 hours (Gore et al., 2015). They are best studied as anti-androgens, although anti-estrogenic effects have been shown (Czemych et al., 2017).
2.2.3. Polychlorinated biphenyls (PCBs)
PCBs are a family of about 200 stable and persistent chemicals that were widely used in industry from the 1930s until their ban in the late 1970s. The number and position of chlorines around the double phenolic ring (Table 1) determines structural and functional properties of each specific PCB congener (Giesy and Kannan, 1998), as well as its half-life in the environment or the body. PCBs typically are found in mixtures, including the commercial Aroclor mixtures used in industry. Those PCBs that are dioxin-like exert their toxic effects primarily through the aryl hydrocarbon receptor (AhR) system (Zhang et al., 2012). Lightly chlorinated PCBs (1-3 chlorines per biphenyl), including Aroclor 1221, the mixture used in the neuroendocrine studies discussed below, are weakly estrogenic (Frame, 1997). More heavily chlorinated PCBs are the most persistent, with half-lives in the environment of 20 years or more (Seegal et al., 2010). Thus, despite their ban in most countries today, PCBs are detectable in body tissues, umbilical cord blood, and serum and urine of virtually all humans and wildlife (Calafat et al., 2008; Centers for Disease Control and Prevention, 2005; Haines and Murray, 2012). With climate change and the melting of polar ice caps, more PCBs are liberated into the environment such that the global burden is actually increasing in parts of the world (Colgan et al., 2016; Jepson et al., 2016; Jepson and Law, 2016). Epidemiological evidence consistently shows that PCB exposures impair the quality of life and increase the risk of chronic disease and reproductive dysfunctions [reviewed in (Gore et al., 2015)].
2.2.4. Vinclozolin
Vinclozolin (Table 1), a commercially available fungicide that is used on turf, vineyards and other crops (Cabras and Angioni, 2000), is anti-androgenic, as are its metabolites, M1 and M2 (Kelce et al., 1994). In the environment, although some of its metabolites degrade via soil metabolism and hydrolysis, its terminal metabolite (3,5-DCA) is resistant to the degradation process. Vinclozolin itself dissipates in the environment with half-lives of 90 days, but with its metabolites, the estimated half-life is approximately three years (EPA, 2000). In the body, vinclozolin has a half-life of about 1 day.
3. EDC effects on the hypothalamus
In order for an individual to survive and reproduce, he/she must exhibit social communicative skills, reproductive behaviors, parental care, anxiety responses, and affective states that are appropriate to their sex, life stage, and other factors. Sex differences in these behaviors are due in large part to differences in hormone exposures during organizational and activational periods of life. The control of social behavior involves a network of brain regions involved in mediating and integrating the sensory, motor, affective, and motivational aspects of how conspecifics interact and make choices about social and sexual interactions. We refer readers to papers by Newman (Newman, 1999) and O’Connell and Hofmann (O’Connell and Hofmann, 2012) on this subject. In brief, conserved neural circuits that include the basal ganglia and limbic system enable the evaluation of a conspecific and the subsequent decision-making process involved in interacting with that individual, be it in a social or sexual context.
In the following sections, we will present the relevant literature on effects of pre- and/or perinatal exposure to BPA, phthalates, and PCBs on hypothalamic development. To our knowledge, there are no studies that specifically determined effects of vinclozolin on hypothalamic development or differentiation.
3.1. Hypothalamic Structure and Neurochemistry
3.1.1. BPA
Developmental EDC exposures on hypothalamic morphology and neurochemistry, particularly in rodents, are well-established [reviewed in (Gore et al., 2015)]. This review will focus specifically on hypothalamic regions involved in the control of social and sociosexual behavior and reproductive physiology. The majority of this research has been conducted for BPA, with results varying depending upon experimental models (e.g. species, strain, dose and route of BPA administration, age at administration, age at phenotyping, etc.) and endpoint; experimental details of BPA studies are in Table 2. The most consistent literature is for effects of BPA on the anteroventral periventricular nucleus (AVPV), a sexually dimorphic brain region that in rats is larger in females than males, and is abundant in dopaminergic neurons and estrogen receptors (Chakraborty et al., 2005; Davis et al., 1996; Herbison, 2008; Orikasa et al., 2002; Simerly and Swanson, 1987). In an early study, numbers of tyrosine hydroxylase labeled neurons in the AVPV, indicative of dopaminergic neurons, were increased in male but not female rats (Patisaul et al., 2006). In that same study, whereas ERα-immunoreactive cells were unchanged in either sex, the number (females) and percentage (females and males) of tyrosine hydroxylase cells that co-expressed ERα was decreased. In mice, Rubin et al. also reported that the sex differences in the size of the AVPV, and expression of tyrosine hydroxylase, were diminished by BPA (Rubin et al., 2006).
Table 2.
Developmental BPA and Phthalate Effects on Hypothalamic Morphology, Neurochemistry, and Gene Expression
| EDC | Exposure | Region | EDC Effect | Reference |
|---|---|---|---|---|
| BPA | BPA injected into newborn rats (250 μg twice a day on P1 and P2) | AVPV | ↑ # TH-ir neurons in ♂ but not ♀ rats. No change # ERα neurons in either sex. ↓ # and % of TH cells that co-expressed ERα. |
(Patisaul et al., 2006) |
| BPA (25 or 250 ng/kg) administered s.c. by osmotic pump to pregnant mouse (G8 to P16) | AVPV | The sex difference in AVPV size and TH expression (♀ > ♂) was diminished by BPA. | (Rubin et al., 2006) | |
| BPA (10 to 10,000 μg/kg/day) fed through pregnancy to rats | SDN-POA | ↓ SDN-POA volume in male rats at all dosages. ↓ # calbindin neurons in male rats at the two lowest dosages. No effect in ♀. |
(McCaffrey et al., 2013) | |
| AVPV | ↓ # TH neurons in the AVPV of both sexes, dependent upon dosage. | |||
| BPA (10, 20, or 40 μg/kg/day) fed to pregnant mice from G11 to P8 | Medial POA | ↑ # neuronal nitric oxide synthase-ir cells in ♀s. | (Martini et al., 2010) | |
| BPA (2.5, 25, 2500 μg/kg/day) given by gavage to rats through gestation and postnatally (CLARITY-BPA) | AVPV | ↑ AVPV volume in both sexes. No change in SDN-POA volume. |
(Arambula et al., 2017) | |
| BPA (0.05 or 5 mg/kg/day) from G15 until weaning to mice | SDN-POA | No change in SDN-POA volume (assessed by calbindin-D28-ir neuron #). | (Naule et al., 2014) | |
| Rostral PEN | ↑ # kisspeptin-ir neurons in ♀ rats; this was selective to rostral PEN and not seen in caudal PEN or AVPV. No change in # GnRH neurons, GnRH-kisspeptin appositions, or ERα cell numbers in medial POA, VMN, ARC. |
|||
| BPA (50 μg or 5 mg/kg/day) given orally to mice from G15 to P21 | Medial POA | No change in # calbindin-ir neurons in ♂. ↑ #ERα and AR cells in ♂. |
(Picot et al., 2014) | |
| AVPV | No change in # kisspeptin neurons in ♂. | |||
| BPA (12, 25, 50 mg/kg/day) by gavage, from G1 to P20 in mice | Whole hypothalamus | ↑ Gnrh, Kiss1 by higher-dose BPA in both sexes; no change in Gper. | (Xi et al., 2011) | |
| BPA (50 or 50,000 μg/kg/day) from G7 through P7 in rats | ARC | ↓ Adipor1, Chrm3, Bdnf, Cck2r, Igf1, Htr2c in ♀. | (Roepke et al., 2016) | |
| BPA (2.5 or 25 μg/kg/day) by gavage on G6-21 in rats | AVPV, medial POA | No change Esr1, Esr2 in AVPV, medial POA. | (Cao et al., 2013) | |
| ARC, VMN | The sex difference in Esr1 (♀ > ♂) in the rostral ARC was diminished by BPA. ↑ Esr1 in caudal ARC and VMN in both sexes. ↑ Esr2 in rostral and caudal VMN. |
|||
| BPA (2.5, 250, 25,000 μg/kg/day) given by gavage to rats through gestation (CLARITY-BPA) in rats | Whole hypothalamus | ↑ Esr1, Esr2 in ♀ at P1. | (Arambula et al., 2016) | |
| Phthalates | DBP (0.5, 5, or 50 mg/kg/day, s.c.) from P1-5 in rats | AVPV | ↑ Kiss1, Gper, Esr2 in a dose-dependent manner in adult ♀. ↑ kisspeptin immunoreactivity (0.5 mg/kg). |
(Hu et al., 2013) |
| ARC | ↑ Kiss1 (50 mg/kg), ↓ Gper (all doses), ↓ Esr1 (50 mg/kg), ↓ Esr2 (5 mg/kg). ↑ kisspeptin immunoreactivity (5 mg/kg). |
|||
| DEHP (3 or 30 mg/kg/day via drinking water) from gestation through P15 in rats | POA-medial basal hypothalamus | ↑ aspartate, ↓ GABA in ♂; no change in glutamate. | (Carbone et al., 2012) | |
| Phthalate mixture (200 or 1000 μg/kg in food) from G2 to P10 in rats | Whole hypothalamus | No effect on Avp, Avpr1a, Avpr1b, Cd38, Oxt, Oxtr at P10 or P90 in either sex. | (Kougias et al., 2018) | |
| DEHP (2, 10 or 50 mg/kg/day) given by gavage to rats from G14 to G19 | Whole hypothalamus | ↓ Esr2, Cyp19a1, Grin2a, Avpr1a, Kiss1r, Tac3r, Arntl, Clock, Dbp, Mtnr1a, Per2 in P1 ♂s, especially at the 10 mg/kg dose. | (Gao et al., 2018) | |
| DEHP (2, 10 or 50 mg/kg/day) given by gavage to rats from G14 to G19 | AVPV | ↑ Crhr1, Drd2 (2 mg/kg), ↓ Avp, Tac3r (10 mg/kg) in P70 ♂s. | ||
| ARC | ↓ Npy, Pomc, ↑ Trh (10 mg/kg); ↑ Avp, Trh, ↓ Esr1, Esr2, Ghrh, Kiss1, Npy, Pomc, Tac2 (50 mg/kg) in P70 ♂s. | |||
| MPN | ↓ Avp, ↑ Hctr2 (50 mg/kg) in P70 ♂s. | |||
| ARC, AVPV, MPN | ↓ ERα protein in ARC; no effect in AVPV or MPN. |
↑, increase; ↓, decrease; ♂, male; ♀, female. Abbreviations: ARC, arcuate nucleus; AVPV, anteroventral periventricular nucleus; DBP, dibutyl phthalate; DEHP, di(2-ethylhexyl)phthalate); G, gestational day of age; ir: immunoreactive; MPN, medial preoptic nucleus; P, postnatal day of age; PEN, periventricular nucleus; POA, preoptic nucleus; SDN-POA, sexually dimorphic nucleus of the preoptic area; TH, tyrosine hydroxylase (marker of dopaminergic neurons); VMN, ventromedial nucleus.
Other work has measured size and neurochemistry of the sexually dimorphic nucleus (SDN) of the preoptic area (POA), which is larger in male than female rats (Gorski et al., 1980). A study feeding BPA to pregnant rats showed that male but not female offspring had decreased SDN-POA volume and fewer calbindin-immunoreactive neurons in a dose-dependent manner (McCaffrey et al., 2013). Numbers of tyrosine-hydroxylase immunoreactive cells in the AVPV were decreased in both sexes; again this was dependent upon dose (McCaffrey et al., 2013). In mice exposed perinatally to BPA, numbers of neuronal nitric oxide synthase (nNOS)-expressing neurons, the enzyme involved in the synthesis of nitric oxide in the brain, was increased in females (Martini et al., 2010). Other brain regions were largely unaffected in that latter study. Data from one arm of the CLARITY-BPA study (Heindel et al., 2015b), in which daily gavage of BPA was done throughout gestation and postnatally until weaning, BPA consistently increased the volume of the AVPV in both sexes; the SDN-POA was unaffected (Arambula et al., 2017). Naule et al. (Naule et al., 2014) reported that BPA did not affect morphology of the SDN-POA. By contrast, numbers of kisspeptin-immunoreactive cells were significantly and selectively increased in the rostral periventricular nucleus (PEN), although not the AVPV or caudal PEN (Naule et al., 2014). No effects of BPA were found on numbers of GnRH neurons, appositions between GnRH neurons and kisspeptin fibers, or ERα cell numbers in the medial POA, ventromedial nucleus (VMN), or arcuate nucleus (ARC).
Gene expression in the hypothalamus has also been measured following BPA exposure. In whole hypothalamus of mice, Kiss1 and GnRH1 were increased by BPA in both sexes; Gper (the membrane G-protein coupled ER) was unaffected (Xi et al., 2011). Another group assayed gene expression in the ARC using a 48-gene qPCR platform (Roepke et al., 2016). Of these, 6 genes were suppressed by BPA (high dose, low dose, or both): adiponectin receptor 1, cholinergic muscarinic receptor 3, brain-derived neurotrophic factor, cholecystokinin receptor 2, serotonin 2C receptor, and insulin-like growth factor 1. Prenatal BPA was tested for effects on ERα and β genes, Esr1 and Esr2 (Cao et al., 2013). The AVPV and medial POA were unaffected; however, in the rostral ARC, BPA abolished the sex difference in Esr1 (female > male). In caudal ARC, as well as the VMN, Esr1 was increased by BPA in male and female rats. Esr2 was up-regulated by BPA in both rostral and caudal VMN (Cao et al., 2013). Finally, again from the CLARITY-BPA study, BPA increased Esr1 and Esr2 in whole female hypothalamus at P1; no effect was found in males (Arambula et al., 2016).
3.1.2. Phthalates
Research on phthalates shows effects of developmental exposure on hypothalamic genes and proteins; experimental details of these studies are in Table 2. In female Sprague-Dawley rats exposed neonatally, gene expression in adulthood of Kiss1, Gper and Esr2 in the AVPV were increased in a dose-dependent manner by dibutyl phthalate (Hu et al., 2013). In the ARC, neonatal DBP increased Kiss1, and decreased Gpr54, Esr1, and Esr2 (see Table 2 for dosages). Consistent with the Kiss1 results, kisspeptin immunoreactivity was increased in the AVPV and ARC (Hu et al., 2013). In another study, DEHP (di(2-ethylhexyl)phthalate) changed expression of the monoaminergic neurotransmitters aspartate and GABA, but not glutamate, in a sex-specific manner in the hypothalamus of P15 rats (Carbone et al., 2012). When a phthalate mixture was fed to rat dams from gestational day (G)2 to postnatal day (P)10, gene expression of Avp, Avpr1a, Avpr1b, Cd38, Oxt, Oxtr was unaffected in whole hypothalamus at P10 or P90 in male and female rats (Kougias et al., 2018). Effects of prenatal DEHP were assessed in whole hypothalamus of rats at PI (Gao et al., 2018). Eleven genes were down-regulated by DEHP (Esr2, Cyp19a1, Grin2a, Avpr1a, Kiss1r, Tac3r, Arntl, Clock, Dbp, Mtnr1a, Per2). In adult male rats at P70, DEHP affected a large number of genes in a region- (AVPV, ARC, medial preoptic nucleus (MPN)) and dose-dependent manner (summarized in Table 2). ERα protein expression was decreased in the ARC at the highest DEHP dose, and was unaffected by treatment in the AVPV and MPN (Gao et al., 2018).
3.1.3. PCBs
The third class of EDCs, PCBs, has effects on hypothalamic structure and function in developmentally-exposed individuals. Work from our lab has utilized a weakly estrogenic PCB mix, Aroclor 1221 (A1221), for which we have reported sex- and age-dependent outcomes of prenatal exposure. For the majority of these studies, Sprague-Dawley rats were exposed prenatally on G16 and G18, and male and female F1 offspring were characterized phenotypically. A summary of endpoints changed in the hypothalamus by A1221 is presented in Table 3A. Among genes and proteins affected were ERα and ERβ, kisspeptin and its receptor, androgen receptor, and other neurotransmitter and receptor genes. As a whole, we found more effects of A1221 on hypothalamic gene expression and morphology in females than males.
Table 3.
Summary of Neuroendocrine and Behavioral Effects of Prenatal PCBs (Aroclor 1221)
| A. Hypothalamic effects of A1221 | ||||
| Age Evaluated | Endpoint | Females | Males | Reference |
| P1 | Apoptosis (AVPV) | ↑ A1221 | No effect | (Dickerson et al., 2011a) |
| Apoptosis (MPN) | No effect | No effect | ||
| Gene expression (POA) | ↑ A1221: Arnt, Gabbr1, Kiss1r, Grin2b, Vdr | ↓ A1221: Bdnf, Kiss1r; ↑ A1221: Igf1 | ||
| P42 | ERβ cell number (AVPV; ♀ only) | ↓ A1221 (given G16, P1, P4) | (Not measured) | (Salama et al., 2003) |
| ERβ cell number (SON; ♀ only) | No effect | (Not measured) | ||
| P60 | Gene expression (POA) | ↓ A1221: Ar, Igr1, Grin2b, Tgfb1 | No effect | (Dickerson et al., 2011b) |
| AVPV volume | ↓ A1221 | No effect | ||
| ERα cell number (AVPV) | ↓ A1221 | No effect | ||
| Kisspeptin fiber density (AVPV) | ↓ A1221 | No effect | ||
| GnRH-Fos co-expression in POA on proestrus (♀ only) | ↓ A1221 | (Not measured) | ||
| P15-P90 | Sexual dimorphisms in gene expression induced by A1221 (AVPV) | Kiss1 | Kiss1 | (Walker et al., 2014) |
| Genes masculinized in the ♀ (AVPV) | Ar, Thra, Gper, Gal, Kiss1r, Dnmt1, Arnt1, Per2 | (Not applicable) | ||
| P90 | Gene expression (MPN) | ↑ A1221: Ar, Esr1, Esr2, Drd3, Kissl, Oprml | ↑ A1221: Per2 | (Topper et al., 2018) |
| Gene expression (VMN) | No effect | No effect | ||
| P93-P108 | Gene expression (MPN, PVN, VMN) | No effect A1221 (given G16, 18, 20) in MPN (Ar, Esr1, Oxtr, Oprm1), PVN (Crh, Esr1, Avp, Oxt), VMN (Esr1, Oprm1). | ↓ A1221: Ar, Esr1, Oprm1, Oxtr in MPN. | (Bell et al., 2016a) |
| 9 months | Gene expression (ARC) | ↓ A1221 in acyclic ♀, : Dnmt3a, Ar, Pdyn, Lepr, Mc3r, Oxt, Grin2b, Grin2d | No effect | (Walker et al., 2013) |
| Gene expression (ME) | ↓ A1221 in cyclic ♀: Esr1 | No effect | ||
| Gene expression (AVPV) | No effect | No effect | ||
| B. Behavioral effects of A1221 | ||||
| Age | Endpoint | Females | Males | Reference |
| P30-P39 | Adolescent behaviors: Affiliative behaviors, sociability, anxiety-like behaviors (EPM, LD box) | No effect of prenatal A1221, although a second set of A1221 “hits” at P24, 26, 28 ↓ frequency-modulated USVs, ↑ latency to hop to reach the social stimulus in a sociability test. In EPM, ↑ A1221: time in open arm, # entries into open arm. | No effect | (Bell et al., 2016b) |
| P50 | Paced mating (♀ only) | ↑ A1221: Mount return latency and post-ejaculatory return latency | (Not measured) | (Steinberg et al., 2007) |
| ↑ A1221: # trials to successfully mate | (Not measured) | |||
| P60-P90 | Sociability (choice between same-sex rat vs. empty cage) | No effect | No effect | (Reilly et al., 2015) |
| Social novelty (choice between a familiar and a novel same-sex rat) | The greater time spent in proximity to, and nose touching with, a novel over a familiar rat, was lost in A1221 ♀ rats. | ↓ A1221 (0.5 mg/kg): overall time spent nose touching, and the preference for a novel over familiar rat for this behavior was lost in these ♂ rats. | ||
| P56-P90 | Four-choice test (“FourPlex”) in which a rat is given a choice between 2 same-sex gonadectomized rats (one given hormone, one no hormone) and 2 opposite-sex gonadectomized rats (one given hormone, one no hormone) | All females showed a preference for the opposite-sex, testosterone-treated male. Treatment effects were small: A1221 females spent more time with the gonadectomized no-hormone female compared to vehicle, and made more arm entries towards this stimulus. | All males showed a preference for the opposite-sex, estradiol-treated female. Treatment effects were small: A1221 males spent more time than vehicle males exploring the no-hormone ♀s. | (Reilly et al., 2018) |
| P60-P90 | Anxiety-like behaviors (LD box, EPM) | No treatment effect in the EPM or LD box. | ↑ A1221: In the LD box, time spent in the light box, # line crossings, speed, and distance traveled. ↓ A1221: time spent immobile. | (Gillette et al., 2017) |
| P60-P90 | Sociosexual choice (3-chambered apparatus, with a rat given a choice between 2 gonadectomized rats. One was hormone treated, the other untreated). | No effect | ↓ A1221: Time spent nose-touching with the ♀ stimulus rats, irrespective of their hormone treatment. | (Topper et al., 2018) |
| P90-P110 | Adult sociosexual USVs, sociosexual choice (3-chambered apparatus), anxiety-like behaviors (EPM, LD box). | No effect | ↓ A1221: time close to non-hormone stimulus rat in sociability test. | (Bell et al., 2016b) |
Unless otherwise noted, PCBs (A1221) or vehicle were given i.p. on G16 and G18 at 1 mg/kg. No effect indicates no significant difference between A1221 and vehicle groups. ↑, increase; ↓, decrease; ♂, male; ♀, female. Abbreviations: ER, estrogen receptor; G, gestational day of age; P, postnatal day of age; AVPV, anteroventral periventricular nucleus; ARC, arcuate nucleus; ME, median eminence; MPN, medial preoptic nucleus; PVN, paraventricular nucleus; VMN, ventromedial nucleus; USV: ultrasonic vocalization; LD box: light:dark box; EPM: elevated plus maze.
Other laboratories have utilized another Aroclor mixture, A1254, and showed that treatment of pregnant Sprague-Dawley rats (G15 to G19 via gavage, 25 mg/kg) increased expression of hypothalamic aryl hydrocarbon receptor RNA when measured on P20 in males (the increase in females was not significant) (Pravettoni et al., 2005). In another study, A1254 (10 mg/kg/day, s.c.) was administered to pregnant rats from G10 to G18, and exposed offspring were euthanized at 4 months of age (Faass et al., 2013). In VMN, the normal sex difference in progesterone receptor mRNA (Pgr; female > male) was lost in the A1254 rats due to decreased Pgr in females to the male level. There was no A1254 treatment effect on Esr1 or Esr2, but expression of preproenkephalin mRNA decreased in the VMN of PCB-exposed rats of both sexes. In the medial POA, A1254 increased Esr2 and decreased preproenkephalin mRNA in both sexes. Esr1 and Pgr were unaffected in the medial POA (Faass et al., 2013).
3.2. Hypothalamic vasopressin and oxytocin systems
The key roles vasopressin and oxytocin play in the control of social behavior make them an obvious target for research on EDCs. These nonapeptides are synthesized in neurons in the supraoptic nucleus (SON) and paraventricular nucleus (PVN), and act via receptors in regions such as the medial amygdala and POA to regulate social recognition, affiliative behaviors, and maternal behaviors, among others (Bosch and Neumann, 2010; Carter et al., 2008; Choleris et al., 2007; Donaldson and Young, 2008). Vasopressin and oxytocin neurons are highly sensitive to the steroid hormone milieu (Alves et al., 1998; Choleris et al., 2003; Choleris et al., 2006; Pierman et al., 2008; Scordalakes and Rissman, 2004). However, there is a surprising dearth of research on EDC effects, summarized in Table 4. We also refer readers to recent reviews on this subject (Patisaul, 2017; Rosenfeld, 2015). We are unaware of research on phthalates and vinclozolin in this domain, other than a study by Kougias et al. that found no effect of a phthalate mix on Oxt, Oxtr, Avp and Avpr1a (genes for oxytocin, oxytocin receptor, vasopressin, and vasopressin 1a receptor, respectively) in the hypothalamus (Kougias et al., 2018). Work on BPA (Table 4) shows that depending upon the route, dosage, and age of exposure, effects on central oxytocin and vasopressin neurons are observed. For PCBs, limited work from our lab shows relatively few effects of prenatal A1221 (Table 4), but research in this area is in early days, with future research needed.
Table 4.
Developmental EDC effects on vasopressin and oxytocin neurons and their receptors
| Animal | Exposure | EDC effect on vasopressin, oxytocin | Reference |
|---|---|---|---|
| BPA | |||
| Prairie vole | 5 μg, 50 μg, or 50 mg/kg/day, given orally to pups from P8-P14. | BPA (50 mg/kg/day) ↑# vasopressin neurons in ♀ anterior PVN, ↑ oxytocin neurons in ↓ posterior PVN. No effect in ♂ | (Sullivan et al., 2014) |
| Rat | 50 μg, or 50 mg/kg/day, given s.c. daily to pups from P0 to P3. | BPA ↑# oxytocin neurons in ♀ anterior PVN. Double-labeling of oxytocin and Fos was unaffected. Males were not studied. | (Adewale et al., 2011) |
| Rat | 1 mg/L in drinking water, throughout gestation and postnatally until puberty. | No effect on Oxt, Oxtr, Avpr1a in the amygdala. | (Patisaul et al., 2012) |
| Rat | 2.5, 25, 250, 2500, 25,000 μg/kg/day by gavage throughout gestation. | In whole hypothalamus of P1 pups, BPA (25, 250) ↑ Oxt in ♀ and BPA (25) ↑ Oxt in ♂ | (Arambula et al., 2016) |
| Mouse | 5 μg BPA daily in chow, during gestation through G18.5. | BPA ↓ Oxtr in whole embryonic ♂ brain. No change in ♀ | (Wolstenholme et al., 2012) |
| PCBS | |||
| Rat | A1221 (i.p. on G16, 18). | A1221 ↓ Oxt in acyclic ♀ ARC. No effect in ♂ | (Walker et al., 2013) |
| Rat | A1221 (i.p. on G16, 18, 20). | A1221 ↓ Oxtr in ♂ MPN. No effect in ♀. Oxt (PVN), Avp (PVN) were unaffected in both sexes. | (Bell et al., 2016a) |
| Rat | A1221 (i.p. on G16, 18). | No effect on # of vasopressin or oxytocin immunoreactive neurons in the SON or PVN of ♂s or ♀s. | Reilly and Gore, unpublished |
| Phthalates | |||
| Rat | Phthalate mixture (200 or 1000 μg/kg in food) from G2 to P10 in rats | No effect on Avp, Avpr1a, Oxt, Oxtr in whole hypothalamus at P10 or 90 in either sex. | (Kougias et al., 2018) |
↑, increase; ↓, decrease; ♂, male; ♀, female. Abbreviations: G, gestational day of age; P, postnatal day of age; MPN, medial preoptic nucleus; PVN, paraventricular nucleus; SON, supraoptic nucleus. Modified from: (Gore et al., 2015)
4. Social and Sociosexual Behaviors: Effects of EDCs
4.1. Social and sexual behaviors
A growing body of literature shows that developmental perturbations caused by EDCs play out as deficits in the acquisition and manifestation of appropriate social behaviors. Rodents such as mice and rats are social species that live in groups and engage in a variety of social behaviors throughout their lives. This begins with the litter, in which infants communicate with each other and with their mother (and father, in biparental species); the mother (parents) must exhibit behaviors such as licking and grooming, nursing, and pup retrieval, that are necessary for the maturation and survival of the offspring (Champagne et al., 2003; Crews et al., 2004; de Medeiros et al., 2010). As pups mature, they display affiliative behaviors as juveniles (Auger and Olesen, 2009). These social behaviors evolve into adult behaviors that include affiliation, establishment and maintenance of the social hierarchy, and territorial and aggressive behaviors (Engelmann et al., 1995; Williams et al., 2013). Most of these behaviors are qualitatively and/or quantitatively sexually dimorphic, presumably due to organizational/activational effects of hormones and their actions on the neural network involved in social decision-making (Stack et al., 2010).
Work on EDCs has taken advantage of most rodents’ preference for novel over familiar conspecifics, for opposite-sex over same-sex animals, for intact/hormone-treated gonadectomized individuals over gonadectomized individuals without hormone, and in the case of males, for estrous over non-estrous females (Bakker, 2003; Henley et al., 2011; Reilly et al., 2018; Reilly et al., 2015; Xiao et al., 2004). There is also a small EDC literature on maternal behavior of F0 mothers towards F1 offspring that, for the most part, shows little or no effect; we will not discuss these articles in the prose, but some are included in Table 5 for reference.
Table 5.
EDC Effects on Social and Sociosexual Behavior
| Exposure | Social Behavior(s) Tested | EDC Effect | Reference |
|---|---|---|---|
| BPA | |||
| BPA 5 μg, 50 μg, or 50 mg/kg/day, given orally to prairie vole pups from P8-P14. | Novel social test at P30 (interactions with tethered, unrelated same-sex and -age stimulus vole) | Sex difference in control voles (♂ > ♀ in time spent investigating the stimulus vole) was reversed in BPA (50 mg) group | (Sullivan et al., 2014) |
| Partner preference test at P60-75 (opposite sex conspecific) | Control ♀ formed a partner preference; BPA females did not. No ♂s formed a partner preference. | ||
| BPA 50 mg/kg/day in feed (exposure dose estimated at 0.15 mg/day) beginning 2 weeks prior to mating, and through pregnancy and lactation in California mice | Territorial marking (urination pattern) by ♂ when placed as dyads across a barrier | ↓ territorial marking on day 7 of testing; no difference on days 0 or 1 | (Williams et al., 2013) |
| BPA 50 mg/kg in feed prior to mating and through pregnancy and lactation in mice | Social novelty at P27-30 in a 3-chambered apparatus, one side with an empty cage, one with a novel untreated adult male mouse | ↓ time spent interacting with the male in mice raised by a foster but not biological dam | (Cox et al., 2010) |
| BPA 1.25 mg/kg in diet (exposure dose estimated at 5 μg BPA daily) in mice | Juvenile social interactions on P21 (interaction with same age, sex, and treatment mouse) | ↑ side-by-side interactions, ↓ time spent self-grooming in ♀ | (Wolstenholme et al., 2012) |
| Social preference on P24 in a 3-chambered apparatus, one side with an empty cage, one with a novel untreated adult male mouse | No effect | ||
| BPA 2, 20 or 200 μg/kg/day given orally through gestation in mice | Maternal behavior on P1 to P6 toward F1 offspring | ↑ pup licking and arched-back nursing at the highest BPA dose | (Kundakovic and Champagne, 2011) |
| Home cage social behavior from P30-P40 (huddling, sniffing, grooming, play) | Reduction in the sexual dimorphism in play behaviors | ||
| BPA 10 μg or 10 mg/kg/day in diet beginning 2 weeks prior to mating through weaning at P21 in mice | Maternal behavior | No effect | (Xin et al., 2018) |
| Social choice in a 3-chambered apparatus (non-social vs. social cue) | No effect | ||
| BPA 40 μg/kg/day to rat dams through gestation and lactation | Social investigation and play from P35-55 in ♀ toward ♂ | ↑ ♀ social exploration at P35 and P45 | (Porrini et al., 2005) |
| ↓ ♀ play with ♂ at P45 | |||
| ↓ ♀ social grooming at P45 | |||
| BPA 40 μg/kg/day to rat dams either through gestation or lactation | Intruder test (same-sex and size unfamiliar intruder) | No effect in ♀; ↓ proportion of ♂ showing defensive behaviors, and ↑ ratio of defensive to agonistic behavior | (Farabollini et al., 2002) |
| Sexual preference between a sexually experienced ♂ and a receptive ♀ | No effect in ♀ or ♂ | ||
| ♂ sexual behavior | ↑ # intromissions (postnatal BPA); ↑ intromission latency, genital sniffing (prenatal BPA) | ||
| ♀ sexual behavior | ↓ exit latency, ↑ lordosis frequency | ||
| BPA 5, 50, 500 or 5000 μg/kg/day given orally from G7 through P14 in rats | ♂ sexual behavior | Non-linear dose responses, with lower doses impairing behavior and the highest dose having no impairment | (Jones et al., 2011) |
| ♀ sexual behavior | No effect on proceptive or receptive behaviors | ||
| BPA 0.05 or 20 mg/kg injected to rat pups s.c. every other day from P1 to P7 | ♀ sexual behavior (Ovariectomy, hormone-primed in adulthood) | ↓ hops and darts; no effect on ear wiggling, lordosis quotient or lordosis rating | (Monje et al., 2009) |
| BPA 50 μg or 5 mg/kg/day given orally to mice from G15 to P21 | ♂ sexual behavior | No effect | (Picot et al., 2014) |
| Olfactory preference between soiled bedding of male and estrous female, or between anesthetized mice | No effect | ||
| BPA (0.05 or 5 mg/kg/day from G15 until weaning at P21) to mice | ♀ sexual behavior (Ovariectomy, hormone-primed in adulthood) | ↑ lordosis quotient (BPA 0.05) | (Naule et al., 2014) |
| Olfactory preference between soiled bedding of male and estrous female, or between anesthetized mice | No effect | ||
| BPA (2.5, 25 μg/kg/day) by gavage from G6-21 in rats | Play behavior at P34 | No effect | (Ferguson et al., 2014) |
| Manual lordosis | No effect | ||
| ♀ sexual behavior | No effect | ||
| BPA (2, 20, 200 μg/kg/day) by gavage from G7 to P18 | Lordosis behavior | No effect | (Ryan et al., 2010) |
| Phthalates | |||
| DEHP (0.5, 40, 400 μg/kg/day) orally to mice throughout gestation to P10 | Maternal behavior on P2, 4, 6 | No effect | (Quinnies et al., 2017) |
| Social interaction (same-sex, age and treatment) from P28-32 | ↓ time sitting side-by-side (♂ 40, 400), time sitting alone (both sexes, 40, 400); ↑ time sniffing (both sexes, 40) and time exploring (both sexes, 400) | ||
| Phthalate mixture (200 or 1000 μg/kg in food) from G2 to P10 in rats | Maternal behavior daily from P3 to 10 | No effect | (Kougias et al., 2018) |
| Social play behavior for 4 consecutive days between P32-40 | ↓ social play, ↑ passive contact in ♂ (200); ↑ time spent alone in ♀ (200) | ||
| DBP (20, 200, 2000, 10,000 mg/kg), DINP (40, 400, 4000, 20,000 mg/kg), DEHA (480, 2400, 12,000 mg/kg) in food from G15 to P21 in rats | ♂ sexual behavior | ↓ # mounts and intromissions (DINP 40, DEHA 480); ↓ # ejaculations (DBP 200, 2000, DINP 40, DEHA 48, 12,000) | (Lee et al., 2006) |
| ♀ sexual behavior | ↓ lordosis quotient at all doses and chemicals | ||
| DEHP (30 mg/kg orally) 4 weeks prior to mating, and through pregnancy and lactation in mice | Social interaction with a strange or familiar rat placed into a box in the testing chamber | ↓ time spent near a stranger; no change in time near a familiar mouse | (Lee et al., 2016) |
| PCBs* | |||
| PCB47 and PCB77 (12.5, 25 mg/kg) fed in chow from G1 through weaning at P20 in rats | Juvenile (P21) ♂ social interactions and recognition: choice between an adult ♂ conspecific or an empty box, followed by a social port test of preference after social isolation | ↑ time in social compartment in PCB (25) ♂s; no effect on entries into the social or nonsocial chamber. Few effects in the social port test, although PCB (25) animals lost the social preference when socially isolated | (Jolous-Jamshidi et al., 2010) |
| PCB47 and PCB77 (12.5, 25 mg/kg) fed in chow from G1 through weaning in rats | Maternal odor conditioning in P12 to P14 pups | Aspects of odor preference were changed in the PCB rats (both sexes) | (Cromwell et al., 2007) |
| PCB47 (2, 4 mg/kg) G6-18 in rats | Maternal behavior | ↑ time spent on the nest, licking and grooming (4) | (Simmons et al., 2005) |
| PCB77 (100 μg/kg) or PCB126 (10 μg/kg) on G15 in rats | ♂ sexual behavior | ↑ # intromissions (PCB126); no change in impregnantion or litter outcomes | (Faqi et al., 1998) |
| PCB47 (1, 20 mg/kg) or PCB77 (0.25, 1 mg/kg) injected from G7-18 in rats | ♂ sexual behavior | No effect | (Wang et al., 2002) |
| ♀ sexual behavior - paced mating | ↓ lordosis quotient (PCB47 (20), PCB77 (0.25, 1); ↓ approach latency in a paced mating paradigm (PCB77 (0.25, 1) | ||
| A1221, A1254 (14 or 42 mg/kg) injected on G14, P1, P10 in rats | ♀ sexual behavior - paced mating | ↑ intromission return latency, % intromission leave, ↓ lordosis quotient (A1221 5); ↑ %mount leave and intromission leave (A1254 5, 15) | (Chung and Clemens, 1999) |
| A1221, A1254 (2.5, 5 mg) on P1 to 7 in rats | ♀ sexual behavior - paced mating | ↓ lordosis quotient, intromission return latency (A1254 2.5, 5); no effect A1221 | (Chung et al., 2001) |
| PCB77 (2 mg/kg) injected s.c. from G6-18 in rats (prenatal); pups were cross-fostered to result in individuals with prenatal only, postnatal only (via lactation) or both exposures to vehicle or PCBs | Partner preference in ♀ (3-chambered apparatus, choice between a sexually experienced ♂ and a receptive ♀) | ↓ preference for ♂ over ♀ stimulus rat in PCB (pre + postnatal, postnatal only) ♀s | (Cummings et al., 2008) |
| ♀ sexual behavior - paced mating | No effect | ||
| PCB mix of PCBs 28, 52, 101, 138, 153, 180 (10 or 1000 ng/kg) fed to mice from G6 until P21 | Same-sex social behavior in 3-chambered apparatus, first between a stranger mouse and an empty cage; then a test of social novelty between the familiar and a novel mouse. Mice were tested at young adult (P50) and middle-aged (P330) ages | In both tests of sociability (stranger vs. empty cage) and social novelty (familiar vs. novel mouse), there were various sex- and age-dependent changes in the PCB animals | (Karkaba et al., 2017) |
| Vinclozolin | |||
| Vinclozolin (200 mg/kg) injected to ♂ rat pups on P2 and 3 | Play behavior on P35-36 | ↓ # of chases and total amount of play behavior; no effect on sniffing or dorsal contacts | (Hotchkiss et al., 2003) |
| Vinclozolin (3, 6, 12 mg/kg) from G14 to P3 given orally in rats | Social play on P22 and P34 with a same-sex littermate | ↑ play on P34, but no effect on P22 | (Colbert et al., 2005) |
See Table 3B for a summary of effects of A1221 on behaviors in work from the Gore laboratory
↑, increase; ↓, decrease; ♂, male; ♀, female. Abbreviations: A1221, Aroclor 1221 (PCB mix); A1254, Aroclor 1254 (PCB mix); DEHP, di(2-ethylhexyl)phthalate); G, gestational day of age; P, postnatal day of age. Behavioral studies were conducted on adult animals unless otherwise indicated. In column C, numbers refer to dosages specified in column A, with units omitted in column C.
4.1.1. BPA
A substantial literature demonstrates effects of pre- or perinatal exposures to BPA on the social and sexual behavioral phenotypes (Table 5). We have selected a subset of studies that illustrate juvenile behaviors, as well as adult same-sex social and opposite-sex sociosexual behaviors. As was the case for neurobiological effects, BPA’s effects on behavior vary depending upon animal mode, dose, timing of exposure, route, age at phenotyping, and endpoints.
Juvenile behaviors were evaluated in prairie voles, for which early life BPA exposure reversed the normal sex difference (males > females) in time spent by a juvenile (P30) investigating a stimulus animal (Sullivan et al., 2014). In laboratory mice, BPA-exposed females increased time engaged in side-by-side interactions with a same-sex conspecific, and decreased time spent self-grooming (Wolstenholme et al., 2012). Males were unaffected by treatment. In that same study, social choice between a conspecific or an empty cage was unaffected in juvenile male and female mice. Another study of BPA effects on juvenile/adolescent behaviors in mice reported a loss in the sexual dimorphism of play behaviors (Kundakovic and Champagne, 2011). In rats, social exploration and play behavior of female towards male rats was assessed from P35-P55; BPA increased exploration but decreased play and social grooming in the females (Porrini et al., 2005). Finally, Ferguson et al. (Ferguson et al., 2014) did not observe any effects of prenatal BPA on play behavior at P34 in rats. More research is needed to resolve these differences; it should also be noted that treatment details varied among the studies that could account for differential results (Table 5).
Effects of developmental BPA on adult social and sociosexual behaviors have been reported in several species. California mice (Peromyscus californicus) exposed to BPA showed reduced territorial marking in adult males (Williams et al., 2013). In laboratory mice, BPA decreased time spent with a novel male in a 3-chambered apparatus, an effect that was seen in animals cross-fostered but not raised by a biological dam (Cox et al., 2010). In a resident intruder test given to rats at P100, males but not females changed defensive and agonistic behaviors (Farabollini et al., 2002). In that same study, although there were no effects of BPA in a choice between a sexually receptive female and a sexually experienced male rat, aspects of sexual behaviors in both sexes were affected by BPA. Another study found non-monotonic dose-response effects of perinatal BPA on sexual behavior in male rats (impairments at low doses; no effect at the highest dose), and no change in sexual behavior in females (Jones et al., 2011). For female rats given BPA in early postnatal life and tested for sexual behavior after ovariectomy plus steroid priming, only the proceptive behavior of hops and darts was affected (decreased by BPA), as lordosis behavior was unchanged (Monje et al., 2009). Two additional studies reported no effects of prenatal BPA on lordosis behavior and/or sex behavior in female rats (Ferguson et al., 2014; Ryan et al., 2010). This contrasts with work in mice, in which perinatal BPA increased the lordosis quotient in females (Naule et al., 2014). This latter lab also tested male mice for sexual behavior and found no developmental treatment effect (Picot et al., 2014).
4.1.2. Phthalates
Most research on phthalates has focused on their effects on reproductive development and physiology, especially in males, because of their anti-androgenic mechanisms of action. Therefore, only a few studies have evaluated how perinatal phthalate exposure affected social and sociosexual behaviors (see Table 5 for experimental details). Exposure of rats to a phthalate mixture caused small but significant effects on juvenile play behavior in rats. Males exposed to phthalates spent less time in juvenile social play and increased time in passive contact, whereas females spent more time alone (Kougias et al., 2018). A study on juvenile mice showed that phthalate exposure decreased social interactions (Quinnies et al., 2017). High dose phthalates decreased male sexual behavior and diminished female lordosis behavior in rats (Lee et al., 2006). In a test of social interaction with a novel or a familiar mouse, DEHP exposed mice decreased time spent near the stranger but exhibited no difference in time near a familiar mouse (Lee et al., 2016).
4.1.3. PCBs
Details of behavioral experiments conducted on animals developmentally exposed to PCBs are in Tables 3 and 5. Rats exposed to PCB77 and tested as pups showed a decreased preference for maternal-associated cues, but no overall impact on novel (odor) preference (Cromwell et al., 2007). Male rats exposed to PCBs 47 and 77 perinatally showed subtle effects in juvenile social behavior (Jolous-Jamshidi et al., 2010). Our lab also evaluated effects of A1221 on juvenile social behavior in rats (Table 3B). Whereas prenatal A1221 did not have any effects, females receiving a second hit of A1221 postnatally, in juvenile life, decreased ultrasonic vocalization calling and increased their latency to hop in a sociability test (Bell et al., 2016b). That study did not find any effects in juvenile males.
Tests of adult social preference have also revealed effects of PCBs. Using a mix of PCBs (see Table 5), Karkaba et al. (Karkaba et al., 2017) found complex effects on social choice (novel mouse vs. an empty cage) and social novelty (novel vs. familiar) that were dependent upon the sex and age of the experimental mouse. In our lab, rats given prenatal A1221 showed few effects in a test of sociability (novel rat vs. empty cage), but in a test of social novelty, A1221-exposed males spent less time nose touching with the stimulus rats compared to the vehicle rats (Reilly et al., 2015). Females were also affected, with the greater time spent in proximity to a novel over a familiar rat being lost in A1221 exposed rats (Table 3). We also tested social behavior in a more complex testing arena that we refer to as a FourPlex: it is an X-shaped apparatus with four arms, giving rats a choice among same- and opposite-sex gonadectomized rats that were hormone or non-hormone treated (Reilly et al., 2018). Male rats showed the expected preference for opposite-sex, hormone-primed females, but the effects of PCB treatment were small, with A1221 rats spending more time exploring no-hormone females.
Sexual behavior studies in male rats showed that developmental PCB77 or PCB126 increased numbers of intromissions but did not affect pregnancy outcomes (Faqi et al., 1998). Another report in male rats showed no effect of PCB47 or PCB77 (Wang et al., 2002). By contrast, measures of sexual behavior in females have revealed effects of developmental PCB exposures. In a paced mating paradigm, prenatal PCB47 or PCB77 decreased lordosis quotient and approach latency (Wang et al., 2002), although another study from this same lab using a similar model of PCB77 exposure did not see any effects on female paced mating behavior (Cummings et al., 2008). Cummings et al. (Cummings et al., 2008) found no effect of PCB77 (prenatal, postnatal, or both) on paced mating in female rats, but in a test of preference between an adult male and a sexually receptive female, the natural preference for the male over the female rat was diminished in the PCB-exposed females, especially due to postnatal exposure. Treatment of rats with A1221 or A1254 (industrial PCB mixtures) on G14, P1 and P10 changed the timing of events as measured in a paced mating test, with increased time the female spent away from the male, and decreased lordosis quotient in the PCB females compared to their vehicle counterparts (Chung and Clemens, 1999). A slightly different treatment regime (Aroclors given from P1–7) revealed effects of A1254 but not A1221 on paced mating behavior (Chung et al., 2001), underscoring the importance of the timing of exposure on behavioral outcomes. Work from our lab, in which A1221 was administered on G16 and G18, showed that paced mating behavior in rats was impaired, with time away from the male rat increased, and the number of trials needed to mate successfully also increased in the A1221 females (Steinberg et al., 2007). As a whole, the literature on PCBs suggests greater sensitivity of female than male rodents, and disruption of reproductive behaviors.
4.1.4. Vinclozolin
We are aware of only two published studies of direct perinatal exposure to vinclozolin on the social behavioral phenotype. High-dose vinclozolin administered to male rats in early postnatal life decreased play behavior (Hotchkiss et al., 2003). By contrast, lower-dose vinclozolin enhanced play behavior in males (Colbert et al., 2005), with the opposite effect presumably attributable to the differences in dose and/or the exposure period (Colbert et al. exposed from G14 to P3; Hotchkiss et al. on P2-3).
4.2. Social communication: Ultrasonic vocalizations (USVs)
Communication in rodents is accomplished by ultrasonic calls in a range (20 kHz and above) that is beyond human hearing (20 Hz to ~20 kHz). In adult rats, the species most commonly studied for USVs, there are two types of ultrasonic vocalizations that are associated with the affective state of the individual. Low frequency calls, in the range of 20 to 40 kHz, are emitted in aversive situations such as pain, aggression, or drug or alcohol withdrawal, or as alarm calls about intruders or predators (Brudzynski, 2007; Brudzynski and Chiu, 1995; Mittal et al., 2017). High frequency calls, in the range of 50 to 90 kHz, are emitted in general communicative or in hedonic situations. They are induced by the removal of a partner or mate from the home cage, or when placed into a new cage, and are therefore thought to maintain, establish or reestablish social contact (Garcia et al., 2017a; Garcia et al., 2017b; Harding and McGinnis, 2003; Wöhr et al., 2008). High frequency calls are also emitted in rewarding and positive affective situations such as juvenile play (Knutson et al., 1998), mating behavior (Burgdorf et al., 2008; Willadsen et al, 2014) and other social situations (Brudzynski, 2013). Features of USVs such as call numbers, modulation of a call’s frequency and amplitude, and other properties, probably provide other nuances to an animal’s affective state (Burgdorf et al., 2011; Wöhr et al., 2008).
Minimal research has documented the effects of prenatal EDCs on communicative behavior in rodents but we consider this an important gap in knowledge considering the wide variety of information conveyed by USV calls. Our lab published a study showing that prenatal exposure to A1221 altered certain aspects of ultrasonic calling, particularly in female rats (Bell et al., 2016b). Adult exposure to phthalates (DEHP) reduced the emission of ultrasonic vocalizations and altered the types of syllables emitted in mice (Dombret et al., 2017). Perinatal BPA exposure also increased the duration and median frequency distribution of offspring USV calls (Harris et al., 2018). Collectively, these results indicate that vocalizations are indeed susceptible to prenatal as well as adult EDC disruption, and suggest that this endpoint should be included as part of the diagnostic of social behavioral disruption by EDCs.
5. Multi- and transgenerational effects of EDCs on the social behavioral phenotype
5.1. EDC effects can be manifested across generations
In utero exposure to EDCs impacts not only the offspring (F1 generation), but subsequent generations as well. In a seminal study, Anway, Skinner et al. (Anway et al., 2005) demonstrated that high dose vinclozolin exposure to pregnant rat dams resulted in a complex disease phenotype in the male offspring, an effect that persisted at least three generations later. Although the mechanism for transmission is still not fully understand, epigenetic molecular reprogramming such as heritable DNA methylation “imprints,” histone retention, and non-coding RNAs are implicated in contributing to these outcomes (Ben Maamar et al., 2018). This field is not without controversy and others have found different outcomes with similar high-dose vinclozolin treatments (Iqbal et al., 2015; Schneider et al., 2008; Stouder and Paoloni-Giacobino, 2010), so more research is needed [see review article for some perspective: (Heindel and Blumberg, 2018)]. Nevertheless since the time of the original observation of transgenerational effects of vinclozolin, others have published clear evidence that BPA, phthalates, and other EDCs result in an inter- or transgenerational disease phenotype (Bansal et al., 2017; Brehm et al., 2018; Chamorro-Garcia et al., 2013; Chamorro-García et al., 2018; Crews et al., 2007; Drobná et al., 2018; Krishnan et al., 2018; Manikkam et al., 2013; Quinnies et al., 2017; Rattan et al., 2018; Salian et al., 2009; Susiarjo et al., 2015).
When an F0 pregnant dam is exposed to EDCs (the most common experimental laboratory model) both the dam and her fetal offspring (F1 generation) have direct exposure of their bodies to EDCs. The F2 generation individuals, present as developing germ cells in the F2 fetus, are also directly exposed to the chemical (Skinner, 2008; Xin et al., 2015). The bulk of research cited above has evaluated effects of EDCs in the F3 generation – the first to be entirely unexposed to any personal contact with EDCs – as evidence for transgenerational effects.
Of research on multi- or transgenerational EDC effects, little has considered the behavioral phenotype or neuroendocrine endpoints. An exception is the body of work showing that ancestral, high-dose vinclozolin affected the F3 descendants’ stress responsiveness in their own lifetimes (Crews et al., 2012; Gillette et al., 2014; Skinner et al., 2008). These studies did not evaluate social or neuroendocrine phenotypes, but they provide an important precedent for how an individual’s transgenerational history of EDC exposures changes the life trajectory.
5.2. Inter- and transgenerational EDC effects on social behaviors and neuroendocrine systems
Because transgenerational high-dose vinclozolin increases the predisposition to develop a disease phenotype (Anway et al., 2005), Crews, Gore et al. (Crews et al., 2007) used male and female F3 rats, provided by the Skinner laboratory, to determine consequences on attractiveness in a mate-preference test. Female rats were given a choice between an F3-vinclozolin and an F3-vehicle male in a 3-chambered apparatus; the females unambiguously preferred the vehicle over the vinclozolin descendant. When male rats were given a similar choice (F3-vinclozolin female, F3-vehicle female) they showed no preference. These results are consistent with sexual selection theory in which the sex with the greater investment is choosier (in rats, a polygamous species, females bear all of the energetic costs of pregnancy, lactation, and pup care) (Gore et al., 2018). The mechanism by which female rats distinguished the F3-vinclozolin and -vehicle males is unknown: a test of discrimination of male odors did not reveal any clear preference (Crews et al., 2007). Despite that, odorants, pheromones, behavioral cues, USVs, and others, likely account for the mate preference results for vehicle over vinclozolin descendants.
Since then, intergenerational (F2) or transgenerational (F3 and beyond) exposures to EDCs have been evaluated in a few more reports. Our lab recently published a study showing that F2 males of patrilineal descent from vinclozolin-treated rats had decreased numbers of USV calls; F2 PCB (A1221) descendants had changes in certain qualities of the USV calls [power, frequency; (Krishnan et al., 2018)]. Sexual behavior in both the A1221 and vinclozolin F2 patrilineal descendants was also changed (Krishnan et al., 2018). This was one of few studies to look at descent via the male vs. the female lineage, and it is interesting that the effects were in the patrilineal but not matrilineal line, suggestive of different mechanisms of transmission of a trait across generations. In our females, F2 matrilineal and patrilineal descendants each had unique effects of A1221 or vinclozolin on sexual behavior (Krishnan et al., 2018).
The Rissman laboratory has conducted transgenerational social behavioral work for BPA and phthalate exposures in mice. Wolstenholme et al. (Wolstenholme et al., 2012) evaluated effects of BPA in F1 mice exposed prenatally, and in the F2 and F4 descendants. It is informative to discuss results of the 3 generations together, to illustrate differences in social-behavioral results. In the FI generation, juvenile mice exposed to BPA spent more time sitting side-by-side, but spent less time interacting with one another. The BPA mice also had fewer anogenital investigations but made more solicitations to play. Juvenile BPA F1 males also had fewer social interactions with an adult male whereas female BPA mice increased their social behaviors (Wolstenholme et al., 2012). In the F2 and F4 generations, in general, social behaviors were increased – the reverse of what was seen in the F1 generation. In another study, this laboratory conducted tests of social recognition in the F1 and F3 generations of mice exposed to BPA (Wolstenholme et al., 2013). When juvenile mice (both sexes) were repeatedly exposed to a control mouse (ovariectomized female), BPA increased time investigating the stimulus mouse. When a novel female was introduced, there were no differences between BPA and vehicle mice (Wolstenholme et al., 2013). In the F3 generation, male and female BPA descendants again showed greater investigation of the repeatedly-introduced stimulus mouse compared to vehicle descendants. In the test of social novelty, F3 vehicle but not BPA lineage mice increased investigation time; the authors speculated that social memory may be impaired in F3 but not F1 mice (Wolstenholme et al., 2013).
Transgenerational effects of high-dose phthalates (DEHP) on juvenile social behavior were tested in F3 male descendants (Quinnies et al., 2015). While DEHP F3 males spent more time engaged in the non-social behaviors of digging and less time self-grooming, they did not differ from controls in social behaviors such as side-by-side sitting, approaching, following, crawling, sniffing, or social grooming. A second study evaluated lower DEHP exposures on social behaviors in F1 and F3 mice (Quinnies et al., 2017). Overall, results in the F1 generation were opposite of those in the F3 generation. In a juvenile social interaction test with same-sex and -treatment conspecifics, F1 mice in the higher dose DEHP exposure groups had fewer interactions than lower dose DEHP or vehicle mice. In the F3 generation, higher dose DEHP exposure groups were generally more interactive (Quinnies et al., 2017). Thus, direct exposure to EDCs can affect the social behavioral phenotype, but this does not predict transgenerational effects. This is not surprising considering that the F1 generation is undergoing neural development during the EDC exposure, when the chemicals can directly act upon cells and receptors in the developing brain. The F3 generation is unexposed, and any EDC effect presumably is due to an epigenetic modification to the germline that would be completely independent of somatic EDC effects on the F1 brain (Walker and Gore, 2017).
6. Summary and Conclusions
The evidence that EDCs affect the developing brain in a manner that alters social behavior has grown considerably in the last decade. The maturing hypothalamus is highly sensitive to endogenous hormones from the gonads and adrenals of the fetus, as well as placental and maternal hormones (Gore et al., 2014), making it vulnerable to EDC effects. Although the current review of the literature shows that the structural and behavioral outcomes of EDCs differ in the details, several commonalities emerge. First, males and females have differential sensitivity and responses to EDCs. In fact, some of the EDC effects appear to be a reduction in sexual dimorphisms in brain and behavior. Second, the social behavior literature is consistent with work on EDCs in other domains showing that there are both low-dose effects, as well as non-monotonic dose-response curves to EDCs (Andrade et al., 2006; Vandenberg et al, 2012; Vandenberg et al., 2007). Third, effects of EDCs can be relatively subtle, in the sense that the entire social behavioral repertoire is not eradicated; rather, the EDCs discussed here, when used at dosages that are comparable to environmental exposures, disrupt specific aspects of neurobiological functions.
It is informative to consider the human epidemiological literature on behavioral effects of EDCs [see review,(Braun, 2017)]. There is no “smoking gun” directly linking EDCs to human social impairments. Studies of proxies of EDC exposures to fetuses/infants, through measurements of EDCs in urine or serum of pregnant women, umbilical cord blood, or breast milk, have shown some associations with autism-spectrum disorders [reviewed in (Gore et al., 2014)]. More subtle impairments in social behavior are difficult to diagnose in humans (especially in the first few years of life) but associations between certain pesticides and poorer cognitive development (Horton et al., 2012), and between BPA and anxiety/hyperactivity in children (Harley et al., 2013) suggests that humans, like their other mammalian relatives, are sensitive to developmental EDCs.
Figure 1.
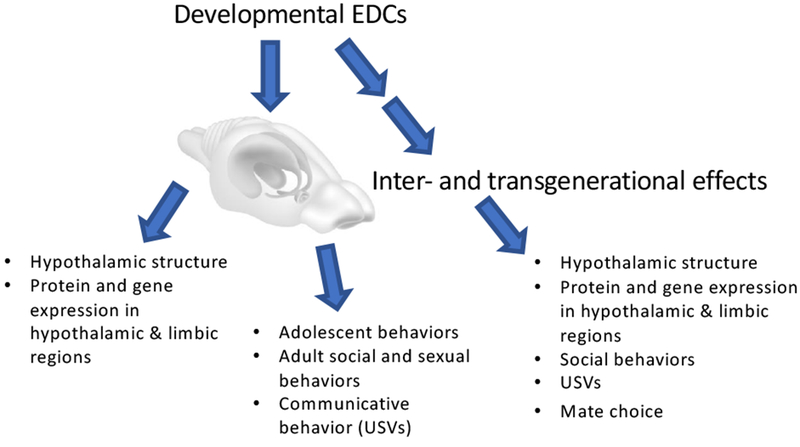
Schematic overview of effects of developmental EDC exposures on social and sociosexual behaviors, and on brain circuits involved in these processes. Along with direct EDC exposure effects on the F1 generation, we show the potential for inter- and transgenerational effects to be propagated across generations.
Highlights:
Bisphenol A, PCBs, phthalates and vinclozolin are endocrine-disrupting chemicals (EDCs)
EDC exposures change hypothalamic development and organization.
Social and sociosexual behaviors are perturbed by developmental EDC exposures
There are transgenerational effects of EDCs on social behavior
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: Funding was provided by the NIEHS (RO1 ES020662, R56 ES020662) to A.C.G. The authors have nothing to disclose.
Competing interests: The authors have no competing interests.
References
- Adams NR, 1995. Detection of the effects of phytoestrogens on sheep and cattle. J Anim Sci 73, 1509–1515. [DOI] [PubMed] [Google Scholar]
- Adewale HB, Todd KL, Mickens JA, Patisaul HB, 2011. The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology 32, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldad TS, Rahmani N, Leranth C, Taylor HS, 2011. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertility and Sterility 96, 175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, Soria B, Nadal A, 2005. Low Doses of Bisphenol A and Diethylstilbestrol Impair Ca2+ Signals in Pancreatic α-Cells through a Nonclassical Membrane Estrogen Receptor within Intact Islets of Langerhans. Environ Health Perspec 113, 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A, 2006. The Estrogenic Effect of Bisphenol -A Disrupts Pancreatic β-Cell Function In Vivo and Induces Insulin Resistance. Environ Health Perspec 114, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A, 2010. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect 118, 1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluru N, Vijayan MM, 2006. Aryl hydrocarbon receptor activation impairs cortisol response to stress in rainbow trout by disrupting the rate-limiting steps in steroidogenesis. Endocrinology 147, 1895–903. [DOI] [PubMed] [Google Scholar]
- Alves SE, Lopez V, McEwen BS, Weiland NG, 1998. Differential colocalization of estrogen receptor b (ERb) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: An immunocytochemical study. Proc Natl Acad Sci USA 95, 3281–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade AJ, Grande SW, Talsness CE, Grote K, Chahoud I, 2006. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology 227, 185–92. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK, 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arambula SE, Belcher SM, Planchart A, Turner SD, Patisaul HB, 2016. Impact of Low Dose Oral Exposure to Bisphenol A (BPA) on the Neonatal Rat Hypothalamic and Hippocampal Transcriptome: A CLARITY-BPA Consortium Study. Endocrinology 157, 3856–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arambula SE, Fuchs J, Cao J, Patisaul HB, 2017. Effects of perinatal bisphenol A exposure on the volume of sexually-dimorphic nuclei of juvenile rats: A CLARITY-BPA consortium study. NeuroToxicology 63, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA, 1984. Gonadal steroid induction of structural sex differences in the central nervous system. Ann Rev Neurosci 7, 413–442. [DOI] [PubMed] [Google Scholar]
- Auger AP, Olesen KM, 2009. Brain sex differences and the organisation of juvenile social play behaviour. Journal of Neuroendocrinology 21, 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, 2003. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J Neuroendocrinol 15, 615–621. [DOI] [PubMed] [Google Scholar]
- Bakker J, Baum MJ, 2008. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol 29, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, DeMees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C, 2006. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9, 220–226. [DOI] [PubMed] [Google Scholar]
- Balaguer P, Delfosse V, Grimaldi M, Bourguet W, 2017. Structural and functional evidences for the interactions between nuclear hormone receptors and endocrine disruptors at low doses. Comptes Rendus Biologies 340, 414–420. [DOI] [PubMed] [Google Scholar]
- Bansal A, Rashid C, Xin F, Li C, Polyak E, Duemler A, van der Meer T, Stefaniak W, Wajid S, Doliba N, Bartolomei MS, Simmons RA, 2017. Sex- and dose-specific effects of maternal bisphenol A exposure on pancreatic islets of first- and second-generation adult mice offspring. Environmental Health Persp 125, 097022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, 2003. The developmental origins of adult disease. Eur J Epidemiol 18, 733–736. [DOI] [PubMed] [Google Scholar]
- Bell MR, Hart BG, Gore AC, 2016a. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 2. Sex-specific neuromolecular effects in the brain. Mol Cell Endocrinol 420, 125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Thompson LM, Rodriguez K, Gore AC, 2016b. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Horm Behav 78, 168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Maamar M, Sadler-Riggleman I, Beck D, McBirney M, Nilsson E, Klukovich R, Xie Y, Tang C, Yan W, Skinner MK, 2018. Alterations in sperm DNA methylation, non-coding RNA expression, and histone retention mediate vinclozolin-induced epigenetic transgenerational inheritance of disease. Environ Epigenetics 4, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetts HW, Underwood EJ, Shier FL, 1946. Specific breeding problem of sheep on subterranean clover pastures in Western Australia. Australian veterinary journal 22, 2–12. [DOI] [PubMed] [Google Scholar]
- Bergman A, Heindel JJ, Jobling S, Kidd KA, Zoeller RT, (Eds.), State of the science of endocrine disrupting chemicals, United Nations Environment Programme and the World Health Organization, Geneva, Switzerland, 2013. [Google Scholar]
- Bosch OJ, Neumann ID, 2010. Vasopressin released within the central amygdala promotes maternal aggression. The European journal of neuroscience 31, 883–91. [DOI] [PubMed] [Google Scholar]
- Brannick KE, Craig ZR, Himes AD, Peretz JR, Wang W, Flaws JA, Raetzman LT, 2012. Prenatal exposure to low doses of bisphenol A increases pituitary proliferation and gonadotroph number in female mice offspring at birth. Biol Reprod 87, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, 2017. Early Life Exposure to Endocrine Disrupting Chemicals and Childhood Obesity and Neurodevelopment. Nature reviews. Endocrinology 13, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP, 2011. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 128, 873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm E, Rattan S, Gao L, Flaws JA, 2018. Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice. Endocrinology 159, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM, 2007. Ultrasonic calls of rats as indicator variables of negative or positive states: Acetylcholine–dopamine interaction and acoustic coding. Behavioural Brain Research 182, 261–273. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, 2013. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol 23, 310–317. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Chiu EMC, 1995. Behavioural responses of laboratory rats to playback of 22 kHz ultrasonic calls. Physiol Behav 57, 1039–1044. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Zhai S, Smarr MM, Grewal J, Zhang C, Grantz KL, Hinkle SN, Sundaram R, Lee S, Honda M, Oh J, Kannan K, 2018. Endocrine disruptors and neonatal anthropometry, NICHD Fetal Growth Studies - Singletons. Environ Int 119, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J, 2008. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol 122, 357–67. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Moskal JR, 2011. Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neurosci Biobehav Rev 35, 1831–6. [DOI] [PubMed] [Google Scholar]
- Cabras P, Angioni A, 2000. Pesticide residues in grapes, wine, and their processing products. J Agric Food Chem 48, 967–973. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect 116, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, Patisaul HB, 2013. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci 133, 157–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone S, Samaniego YA, Cutrera R, Reynoso R, Cardoso N, Scacchi P, Moguilevsky JA, Ponzo OJ, 2012. Different effects by sex on hypothalamic–pituitary axis of prepubertal offspring rats produced by in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP). NeuroToxicology 33, 78–84. [DOI] [PubMed] [Google Scholar]
- Carson R, 1962. Silent Spring. Houghton Mifflin, Boston. [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW, 2008. Oxytocin, vasopressin and sociality. Prog Brain Res 170, 331–6. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, D.H.H.S., 2005. Third National Report on Human Exposure to Environmental Chemicals, NCEH Pub. No. 05–0570, National Center for Environmental Health D. o. L. S., Atlanta, GA, pp. 174–248. [Google Scholar]
- Chakraborty TR, Rajendren G, Gore AC, 2005. Expression of estrogen receptor α in the anteroventral periventricular nucleus of hypogonadal mice. Exp Biol Med (Maywood) 230, 49–56. [DOI] [PubMed] [Google Scholar]
- Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B, 2013. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect 121, 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro-García R, Shoucri BM, Willner S, Kach H, Janesick A, Blumberg B, 2018. Effects of Perinatal Exposure to Dibutyltin Chloride on Fat and Glucose Metabolism in Mice, and Molecular Mechanisms, in Vitro. Environ Health Perspect 126, 057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ, 2003. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 79, 359–71. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LEJ, Hayward SW, Lees PSJ, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR, National Toxicology Program Center for the Evaluation of Risks to Human Reproduction monograph on the ppotential human reproductive and developmental effects of bisphenol A, Research Triangle Park, 2008. [Google Scholar]
- Choleris E, Gustafsson J-A, Korach KS, Muglia LJ, Pfaff DW, Ogawa S, 2003. An estrogen-dependent four-gene micronet regulating social recognition: A study with oxytocin and estrogen receptor- a and - b knockout mice. Proc Natl Acad Sci USA 100, 6192–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW, 2007. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci USA 104, 4670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, 2006. Involvement of estrogen receptor α, β and oxytocin in social discrimination: a detailed behavioral analysis with knockout female mice. Genes, Brain and Behavior 5, 528–539. [DOI] [PubMed] [Google Scholar]
- Chung Y-W, Clemens LG, 1999. Effects of perinatal exposure to polychlorinated biphenyls on development of female sexual behavior. Bull Environ Contam Toxicol 62, 664–670. [DOI] [PubMed] [Google Scholar]
- Chung YW, Nunez AA, Clemens LG, 2001. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol Behav 74, 363–370. [DOI] [PubMed] [Google Scholar]
- Colbert NK, Pelletier NC, Cote JM, Concannon JB, Jurdak NA, Minott SB, Markowski VP, 2005. Perinatal exposure to low levels of the environmental antiandrogen vinclozolin alters sex-differentiated social play and sexual behaviors in the rat. Environ Health Perspec 113, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan W, Machguth H, MacFerrin M, Colgan JD, van As D, MacGregor JA, 2016. The abandoned ice sheet base at Camp Century, Greenland, in a warming climate. Geophys Res Lett 43. [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF, 2010. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav 58, 754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Fuller T, Mirasol EG, Pfaff DW, Ogawa S, 2004. Postnatal environment affects behavior of adult transgenic mice. Exp Biol Med 229, 935–939. [DOI] [PubMed] [Google Scholar]
- Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK, 2012. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci USA 109, 9143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK, 2007. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA 104, 5942–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Johnson A, McKnight L, Horinek M, Asbrock C, Burt S, Jolous-Jamshidi B, Meserve LA, 2007. Effect of polychlorinated biphenyls on maternal odor conditioning in rat pups. Physiol Behav 91, 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Clemens LG, Nunez AA, 2008. Exposure to PCB 77 affects partner preference but not sexual behavior in the female rat. Physiol Behav 95, 471–5. [DOI] [PubMed] [Google Scholar]
- Czemych R, Chraniuk M, Zagozdzon P, Wolska L, 2017. Characterization of estrogenic and androgenic activity of phthalates by the XenoScreen YES/YAS in vitro assay. Environ Toxicol Pharmacol 53, 95–104. [DOI] [PubMed] [Google Scholar]
- Davis EC, Shryne JE, Gorski RA, 1996. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology 63, 142–148. [DOI] [PubMed] [Google Scholar]
- de Medeiros CB, Rees SL, Llinas M, Fleming AS, Crews D, 2010. Deconstructing early life experiences: distinguishing the contributions of prenatal and postnatal factors to adult male sexual behavior in the rat. Psychol Sci 21, 1494–501. [DOI] [PubMed] [Google Scholar]
- Di Renzo GC, Conry JA, Blake J, DeFrancesco MS, DeNicola N, Martin JN Jr., McCue KA, Richmond D, Shah A, Sutton P, Woodruff TJ, van der Poel SZ, Giudice LC, 2015. International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. International journal of gynaecology and obstetrics 131, 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Gore AC, 2011a. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicol Appl Pharmacol 252, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC, 2011b. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology 152, 581–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Gore AC, 2007. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord 8, 143–59. [DOI] [PubMed] [Google Scholar]
- Dombret C, Capela D, Poissenot K, Parmentier C, Bergsten E, Pionneau C, Chardonnet S, Hardin-Pouzet H, Grange-Messent V, Keller M, Franceschini I, Mhaouty-Kodja S, 2017. Neural Mechanisms Underlying the Disruption of Male Courtship Behavior by Adult Exposure to Di(2-ethylhexyl) Phthalate in Mice. Environmental Health Perspectives 125, 097001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ, 2008. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–4. [DOI] [PubMed] [Google Scholar]
- Drobná Z, Henriksen AD, Wolstenholme JT, Montiel C, Lambeth PS, Shang S, Harris EP, Zhou C, Flaws JA, Adli M, Rissman EF, 2018. Transgenerational Effects of Bisphenol A on Gene Expression and DNA Methylation of Imprinted Genes in Brain. Endocrinology 159, 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R, 1995. Social discrimination procedure: An alternative method to investigate juvenile recognition abilities in rats. Physiology & Behavior 58, 315–321. [DOI] [PubMed] [Google Scholar]
- EPA, 2000. R.E.D. FACTS.
- Faass O, Ceccatelli R, Schlumpf M, Lichtensteiger W, 2013. Developmental effects of perinatal exposure to PBDE and PCB on gene expression in sexually dimorphic rat brain regions and female sexual behavior. Gen Comp Endocrinol 188, 232–41. [DOI] [PubMed] [Google Scholar]
- Faqi AS, Dalsenter PR, Merker HJ, Chahoud I, 1998. Effects on developmental landmarks and reproductive capability of 3,3’,4,4’-tetrachlorobiphenyl and 3,3’4,4’,5-pentachlorobiphenyl in offspring of rats exposed during pregnancy. Human & Experimental Toxicology 17, 365–372. [DOI] [PubMed] [Google Scholar]
- Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessì-Fulgheri F, 2002. Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environmental Health Perspectives 110, 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA, Law CD, Kiss1ing GE, 2014. Developmental treatment with ethinyl estradiol, but not bisphenol A, causes alterations in sexually dimorphic behaviors in male and female Sprague Dawley rats. Toxicol Sci 140, 374–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández MF, Rivas A, Olea-Serrano F, Cerrillo I, Molina-Molina JM, Araque P, Martínez-Vidal JL, Olea N, 2004. Assessment of total effective xenoestrogen burden in adipose tissue and identification of chemicals responsible for the combined estrogenic effect. Analytical and Bioanalytical Chemistry 379, 163–170. [DOI] [PubMed] [Google Scholar]
- Frame G, 1997. A collaborative study of 209 PCB congeners and 6 Aroclors on 20 different HRGC columns, 2. Semi-quantitative Aroclor congener distributions. Fresenius J Anal Chem 357, 714–722. [Google Scholar]
- Fromme H, Gruber L, Seckin E, Raab U, Zimmermann S, Kiranoglu M, Schlummer M, Schwegler U, Smolic S, Voelkel W, 2011. Phthalates and their metabolites in breast milk--results from the Bavarian Monitoring of Breast Milk (BAMBI). Environ Int 37, 715–722. [DOI] [PubMed] [Google Scholar]
- Gao N, Hu R, Huang Y, Dao L, Zhang C, Liu Y, Wu L, Wang X, Yin W, Gore AC, Sun Z, 2018. Specific effects of prenatal DEHP exposure on neuroendocrine gene expression in the developing hypothalamus of male rats. Arch Toxicol 92, 501–512. [DOI] [PubMed] [Google Scholar]
- Garcia AN, Bezner K, Depena C, Yin W, Gore AC, 2017a. The effects of long-term estradiol treatment on social behavior and gene expression in adult female rats. Horm Behav 87, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AN, Depena C, Bezner K, Yin W, Gore AC, 2017b. The timing and duration of estradiol treatment in a rat model of the perimenopause: Influences on social behavior and the neuromolecular phenotype. Horm Behav 97, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, Friesen MW, Fujimoto VY, Hunt PA, 2013. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environmental science & technology 47, 12477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesy JP, Kannan K, 1998. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment. Crit Rev Toxicol 28, 511–69. [DOI] [PubMed] [Google Scholar]
- Gillette R, Miller-Crews I, Nilsson EE, Skinner MK, Gore AC, Crews D, 2014. Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. Endocrinology 155, 3853–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, Reilly MP, Topper VY, Thompson LM, Crews D, Gore AC, 2017. Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Horm Behav 87, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, 2015. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36, E1–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Holley AM, Crews D, 2018. Mate choice, sexual selection, and endocrine-disrupting chemicals. Horm Behav 101, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Martien KM, Gagnidze K, Pfaff D, 2014. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev 35, 961–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM, 1980. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol 193, 529–539. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L, 2001. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update 7, 248–64. [DOI] [PubMed] [Google Scholar]
- Haines DA, Murray J, 2012. Human biomonitoring of environmental chemicals--early results of the 2007–2009 Canadian Health Measures Survey for males and females. Int J Hyg Environ Health 215, 133–7. [DOI] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY, 2003. Effects of testosterone in the VMN on copulation, partner preference, and vocalizations in male rats. Horm Behav 43, 327–335. [DOI] [PubMed] [Google Scholar]
- Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, Eskenazi B, 2013. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environmental research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EP, Allardice HA, Schenk AK, Rissman EF, 2018. Effects of maternal or paternal bisphenol A exposure on offspring behavior. Hormones and Behavior 101, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, Landrigan PJ, Sly PD, Suk W, Cory Slechta D, Thompson C, Hanson M, 2015a. Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology 156, 3416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, 2018. Environmental Obesogens: Mechanisms and Controversies. Annual Review of Pharmacology and Toxicology. DOI 10.1146/annurev-pharmtox-010818-021304, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Newbold RR, Bucher JR, Camacho L, Delclos KB, Lewis SM, Vanlandingham M, Churchwell MI, Twaddle NC, McLellen M, Chidambaram M, Bryant M, Woodling K, Gamboa da Costa G, Ferguson SA, Flaws J, Howard PC, Walker NJ, Zoeller RT, Fostel J, Favaro C, Schug TT, 2015b. NIEHS/FDA CLARITY-BPA research program update. Reproductive Toxicol 58, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley CL, Nunez AA, Clemens LG, 2011. Hormones of choice: the neuroendocrinology of partner preference in animals. Front Neuroendocrinol 32, 146–54. [DOI] [PubMed] [Google Scholar]
- Herbison AC, 2008. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, Poskanzer DC, 1971. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 284, 878–881. [DOI] [PubMed] [Google Scholar]
- Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE, 2009. Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ Health Perspec 117, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Kahn LG, Perera F, Barr DB, Rauh V, 2012. Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicol Teratol 34, 534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AK, Ostby JS, Vandenbergh JG, Gray LE, 2003. An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiology & Behavior 79, 151–156. [DOI] [PubMed] [Google Scholar]
- Hu J, Du G, Zhang W, Huang H, Chen D, Wu D, Wang X, 2013. Short-term neonatal/prepubertal exposure of dibutyl phthalate (DBP) advanced pubertal timing and affected hypothalamic kisspeptin/GPR54 expression differently in female rats. Toxicology 314, 65–75. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ, 2003. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol 13, 546–553. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Tran DA, Li AX, Warden C, Bai AY, Singh P, Wu X, Pfeifer GP, Szabó PE, 2015. Deleterious effects of endocrine disruptors are corrected in the mammalian germline by epigenome reprogramming. Genome Biology 16, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson PD, Deaville R, Barber JL, Aguilar A, Borrell A, Murphy S, Barry J, Brownlow A, Barnett J, Berrow S, Cunningham AA, Davison NJ, Ten Doeschate M, Esteban R, Ferreira M, Foote AD, Genov T, Gimenez J, Loveridge J, Llavona A, Martin V, Maxwell DL, Papachlimitzou A, Penrose R, Perkins MW, Smith B, de Stephanis R, Tregenza N, Verborgh P, Fernandez A, Law RJ, 2016. PCB pollution continues to impact populations of orcas and other dolphins in European waters. Scientific reports 6, 18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson PD, Law RJ, 2016. Persistent pollutants, persistent threats. Science 352, 1388–1389. [DOI] [PubMed] [Google Scholar]
- Jolous-Jamshidi B, Cromwell HC, McFarland AM, Meserve LA, 2010. Perinatal exposure to polychlorinated biphenyls alters social behaviors in rats. Toxicol Lett 199, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BA, Shimell JJ, Watson NV, 2011. Pre- and postnatal bisphenol A treatment results in persistent deficits in the sexual behavior of male rats, but not female rats, in adulthood. Horm Behav 59, 246–51. [DOI] [PubMed] [Google Scholar]
- Karkaba A, Soualeh N, Soulimani R, Bouayed J, 2017. Perinatal effects of exposure to PCBs on social preferences in young adult and middle-aged offspring mice. Horm Behav 96, 137–146. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE Jr., 1994. Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol 126, 276–85. [DOI] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ, 2000. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology 141, 1897–900. [DOI] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, van Toor H, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Schuur AG, Brouwer A, Visser TJ, 2002. Potent inhibition of estrogen sulfotransferase by hydroxylated metabolites of polyhalogenated aromatic hydrocarbons reveals alternative mechanism for estrogenic activity of endocrine disrupters. J Clin Endocrinol Metab 87, 1142–50. [DOI] [PubMed] [Google Scholar]
- Kinch CD, Ibhazehiebo K, Jeong JH, Habibi HR, Kurrasch DM, 2015. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci U S A 112, 1475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J, 1998. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol 112, 65–73. [DOI] [PubMed] [Google Scholar]
- Kougias DG, Cortes LR, Moody L, Rhoads S, Pan Y-X, Juraska JM, 2018. Effects of Perinatal Exposure to Phthalates and a High-Fat Diet on Maternal Behavior and Pup Development and Social Play. Endocrinology 159, 1088–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Mittal N, Thompson Lindsay M, Rodriguez-Santiago M, Duvauchelle Christine L, Crews D, Gore Andrea C, 2018. Effects of the Endocrine-Disrupting Chemicals, Vinclozolin and Polychlorinated Biphenyls, on Physiological and Sociosexual Phenotypes in F2 Generation Sprague-Dawley Rats. Environmental Health Perspectives 126, 097005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA, 2011. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun 25, 1084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page Y, Vosges M, Servili A, Brion F, Kah O, 2011. Neuroendocrine effects of endocrine disruptors in teleost fish. J Toxicol Environmental Health. Part B, Critical reviews 14, 370–86. [DOI] [PubMed] [Google Scholar]
- Lee D-H, 2018. Evidence of the Possible Harm of Endocrine-Disrupting Chemicals in Humans: Ongoing Debates and Key Issues. Endocrinol Metab 33, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-C, Yamanouchi K, Nishihara M, 2006. Effects of Perinatal Exposure to Phthalate/Adipate Esters on Hypothalamic Gene Expression and Sexual Behavior in Rats. J Reproduction Development 52, 343–352. [DOI] [PubMed] [Google Scholar]
- Lee K-I, Chiang C-W, Lin H-C, Zhao J-F, Li C-T, Shyue S-K, Lee T-S, 2016. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch Toxicol 90, 1211–1224. [DOI] [PubMed] [Google Scholar]
- León-Olea M, Martyniuk CJ, Orlando EF, Ottinger MA, Rosenfeld C, Wolstenholme J, Trudeau VL, 2014. Current Concepts in Neuroendocrine Disruption. Gen Comp Endocrinol 203, 158–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK, 2013. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PloS One 8, e55387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini M, Miceli D, Gotti S, Viglietti-Panzica C, Fissore E, Palanza P, Panzica G, 2010. Effects of perinatal administration of Bisphenol A on the neuronal nitric oxide synthase expressing system in the hypothalamus and limbic system of CD1 mice. Journal of Neuroendocrinology 22, 1004–12. [DOI] [PubMed] [Google Scholar]
- McCaffrey KA, Jones B, Mabrey N, Weiss B, Swan SH, Patisaul HB, 2013. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. Neurotoxicology 36, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnes MR, Bermudez DS, Bryan TA, Edwards TM, Gunderson MP, Larkin IL, Moore BC, Guillette LJ, 2006. Contaminant-induced feminization and demasculinization of nonmammalian vertebrate males in aquatic environments. Environ Res 100, 3–17. [DOI] [PubMed] [Google Scholar]
- Mittal N, Thakore N, Bell RL, Maddox WT, Schallert T, Duvauchelle CL, 2017. Sex-specific ultrasonic vocalization patterns and alcohol consumption in high alcohol-drinking (HAD-1) rats. Physiology & Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje L, Varayoud J, Munoz-de-Toro M, Luque EH, Ramos JG, 2009. Neonatal exposure to bisphenol A alters estrogen-dependent mechanisms governing sexual behavior in the adult female rat. Reproductive Toxicology 28, 435–42. [DOI] [PubMed] [Google Scholar]
- Naule L, Picot M, Martini M, Parmentier C, Hardin-Pouzet H, Keller M, Franceschini I, Mhaouty-Kodja S, 2014. Neuroendocrine and behavioral effects of maternal exposure to oral bisphenol A in female mice. J Endocrinology 220, 375–88. [DOI] [PubMed] [Google Scholar]
- Newman SW, 1999. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann N Y Acad Sci 877, 242–257. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Schwarz JM, McCarthy MM, 2011. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: implications for sexual differentiation. Horm Behav 59, 338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA, 2012. Evolution of a vertebrate social decision-making network. Science 336, 1154–7. [DOI] [PubMed] [Google Scholar]
- Ohlstein JF, Strong AL, McLachlan JA, Gimble JM, Burow ME, Bunnell BA, 2014. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J Mol Endocrinol 53, 345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y, 2002. Sexually dimorphic expression of estrogen receptor b in the anteroventral periventricular nucleus of the rat preoptic area: Implication in luteinizing hormone surge. Proc Natl Acad Sci USA 99, 3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando EF, Guillette LJ, 2007. Sexual dimorphic responses in wildlife exposed to endocrine disrupting chemicals. Environ Res 104, 163–173. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Carro T, Bohannon M, Baltos L, Marcell AM, McKernan M, Dean KM, Lavoie E, Abdelnabi M, 2013. Assessing effects of environmental chemicals on neuroendocrine systems: Potential mechanisms and functional outcomes. Gen Comp Endocrinol 190, 194–202. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, 2017. Endocrine Disruption of Vasopressin Systems and Related Behaviors. Front Endocrinol (Lausanne) 8, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK, 2006. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol 28, 111–118. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, Coughlin JL, Buckley B, Gore AC, 2012. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PloS One 7, e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M, Naule L, Marie-Luce C, Martini M, Raskin K, Grange-Messent V, Franceschini I, Keller M, Mhaouty-Kodja S, 2014. Vulnerability of the neural circuitry underlying sexual behavior to chronic adult exposure to oral bisphenol A in male mice. Endocrinology 155, 502–12. [DOI] [PubMed] [Google Scholar]
- Pierman S, Sica M, Allieri F, Viglietti-Panzica C, Panzica GC, Bakker J, 2008. Activational effects of estradiol and dihydrotestosterone on social recognition and the arginine-vasopressin immunoreactive system in male mice lacking a functional aromatase gene. Horm Behav 54, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrini S, Belloni V, Seta DD, Farabollini F, Giannelli G, Dessì-Fulgheri F, 2005. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res Bull 65, 261–266. [DOI] [PubMed] [Google Scholar]
- Pravettoni A, Colciago A, Negri-Cesi P, Villa S, Celotti F, 2005. Ontogenetic development, sexual differentiation, and effects of Aroclor 1254 exposure on expression of the arylhydrocarbon receptor and of the arylhydrocarbon receptor nuclear translocator in the rat hypothalamus. Reprod Toxicol 20, 521–30. [DOI] [PubMed] [Google Scholar]
- Quinnies KM, Doyle TJ, Kim KH, Rissman EF, 2015. Transgenerational Effects of Di-(2-Ethylhexyl) Phthalate (DEHP) on Stress Hormones and Behavior. Endocrinology 156, 3077–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnies KM, Harris EP, Snyder RW, Sumner SS, Rissman EF, 2017. Direct and transgenerational effects of low doses of perinatal di-(2-ethylhexyl) phthalate (DEHP) on social behaviors in mice. PLOS ONE 12, e0171977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Brehm E, Gao L, Flaws JA, 2018. Di(2-Ethylhexyl) Phthalate Exposure During Prenatal Development Causes Adverse Transgenerational Effects on Female Fertility in Mice. Toxicol Sci 163, 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly MP, Weeks CD, Crews D, Gore AC, 2018. Application of a Novel Social Choice Paradigm to Assess Effects of Prenatal Endocrine-Disrupting Chemical Exposure in Rats (Rattus norvegicus). J Comp Psychol 132, 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly MP, Weeks CD, Topper VY, Thompson LM, Crews D, Gore AC, 2015. The effects of prenatal PCBs on adult social behavior in rats. Horm Behav 73, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Yang JA, Yasrebi A, Mamounis KJ, Oruc E, Zama AM, Uzumcu M, 2016. Regulation of Arcuate Genes by Developmental Exposures to Endocrine-Disrupting Compounds in Female Rats. Reproductive Toxicology (Elmsford, N.Y.) 62, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, 2015. Bisphenol A and phthalate endocrine disruption of parental and social behaviors. Front Neurosci 9, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, 2017. Neuroendocrine disruption in animal models due to exposure to bisphenol A analogues. Front Neuroendocrinol 47, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Denslow ND, Orlando EF, Gutierrez-Villagomex JM, Trudeau VL, 2017. Neuroendocrine disruption of organizational and activational hormone programming in poikilothermic vertebrates. J Toxicol Environ Health B Crit Rev. 20, 276–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM, 2006. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology 147, 3681–3691. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Hotchkiss AK, Crofton KM, Gray LEJ, 2010. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol Sci 114, 133–148. [DOI] [PubMed] [Google Scholar]
- Salama J, Chakraborty TR, Ng L, Gore AC, 2003. Effects of polychlorinated biphenyls (PCBs) on female reproductive development and estrogen receptor b expression. Environ Health Perspect 111, 1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G, 2009. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A. Life Sciences 85, 11–8. [DOI] [PubMed] [Google Scholar]
- Schneider S, Kaufmann W, Buesen R, van Ravenzwaay B, 2008. Vinclozolin--the lack of a transgenerational effect after oral maternal exposure during organogenesis. Reprod Toxicol 25, 352–60. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF, 2004. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor á. Genes, Brain and Behavior 3, 20–26. [DOI] [PubMed] [Google Scholar]
- Seegal R, Fitzgerald EF, Hills EA, Wolff MS, Haase RF, Todd AC, Parsons P, Molho ES, Higgins DS, Factor SA, Marek KL, Seibyl JP, Jennings DL, McCaffrey RJ, 2010. Estimating the half-lives of PCB congeners in former capacitor workers measured over a 28 year interval. J Expos Sci Environ Epidemiol 21, 234–246. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, 1987. The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: Implications for the control of gonadotropin secretion in the rat. Brain Res 400, 11–34. [DOI] [PubMed] [Google Scholar]
- Simmons SL, Cummings JA, Clemens LG, Nunez AA, 2005. Exposure to PCB 77 affects the maternal behavior of rats. Physiol Behav 84, 81–86. [DOI] [PubMed] [Google Scholar]
- Skinner MK, 2008. What is an epigenetic transgenerational phenotype? Reprod Toxicol 25, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D, 2008. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE 3 (e3745), e3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M, 2010. Sex Differences in Social Interaction in Rats: Role of the Immediate-Early Gene zif268. Neuropsychopharmacology 35, 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, Gore AC, 2007. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav 51, 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouder C, Paoloni-Giacobino A, 2010. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction 139, 373–9. [DOI] [PubMed] [Google Scholar]
- Sullivan AW, Beach EC, Stetzik LA, Perry A, D’Addezio AS, Cushing BS, Patisaul HB, 2014. A novel model for neuroendocrine toxicology: Neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster). Endocrinology 155, 3867–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susiarjo M, Xin F, Bansal A, Stefaniak M, Li C, Simmons RA, Bartolomei MS, 2015. Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 156, 2049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper VY, Reilly MP, Wagner LM, Thompson LM, Gillette R, Crews D, Gore AC, 2018. Social and neuromolecular phenotypes are programmed by prenatal exposures to endocrine-disrupting chemicals. Mol Cell Endocrinol, DOI 10.1016/j.mce.2018.09.010, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP, 2012. Hormones and endocrine disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocrine Rev 33, 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM, 2009. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30, 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM, 2007. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology 148, 116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñas R, Watson CS, 2013. Bisphenol S Disrupts Estradiol-Induced Nongenomic Signaling in a Rat Pituitary Cell Line: Effects on Cell Functions. Environ Health Perspec 121, 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Goetz BM, Gore AC, 2014. Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Molecular Endocrinology 28, 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Gore AC, 2017. Epigenetic impacts of endocrine disruptors in the brain. Front Neuroendocrinol 44, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Kermath BA, Woller MJ, Gore AC, 2013. Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinology 154, 2129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Fang J, Nunez AA, Clemens LG, 2002. Developmental exposure to polychlorinated biphenyls affects sexual behavior of rats. Physiol Behav 75, 689–696. [DOI] [PubMed] [Google Scholar]
- Willadsen M, Seffer D, Schwarting RKW, Wöhr M, 2014. Rodent ultrasonic communication: Male prosocial 50-kHz ultrasonic vocalizations elicit social approach behavior in female rats (Rattus norvegicus). J Comp Psychol 128, 56–64. [DOI] [PubMed] [Google Scholar]
- Williams SA, Jasarevic E, Vandas GM, Warzak DA, Geary DC, Ellersieck MR, Roberts RM, Rosenfeld CS, 2013. Effects of developmental bisphenol A exposure on reproductive-related behaviors in California mice (Peromyscus californicus): a monogamous animal model. PloS one 8, e55698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield JC, Mukai M, 2009. Endocrine disruption in the context of life cycles: Perception and transduction of environmental cues. Gen Comp Endocrinol 163, 92–96. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Houx B, Schwarting RKW, Spruijt B, 2008. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav 93, 766–776. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ, 2012. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology 153, 3828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Goldsby JA, Rissman EF, 2013. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav 64, 833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Lee CK, Yeung WS, Giesy JP, Wong MH, Zhang X, Hecker M, Wong CK, 2011. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reproductive Toxicology 31, 409–17. [DOI] [PubMed] [Google Scholar]
- Xiao K, Kondo Y, Sakuma Y, 2004. Sex-specific effects of gonadal steroids on conspecific odor preference in the rat. Horm Behav 46, 356–361. [DOI] [PubMed] [Google Scholar]
- Xin F, Fischer E, Krapp C, Krizman EN, Lan Y, Mesaros C, Snyder NW, Bansal A, Robinson MB, Simmons RA, Bartolomei MS, 2018. Mice exposed to bisphenol A exhibit depressive-like behavior with neurotransmitter and neuroactive steroid dysfunction. Horm Behav 102, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin F, Susiarjo M, Bartolomei MS, 2015. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Seminars in cell & developmental biology 43, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sargis RM, Volden PA, Carmean CM, Sun XJ, Brady MJ, 2012. PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. PLoS ONE 7, e37103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, 2005. Environmental chemicals as thyroid hormone analogues: New studies indicate that thyroid hormone receptors are targetes of industrial chemicals? Mol Cell Endocrinol 242, 10–15. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS, 2012. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from The Endocrine Society. Endocrinology 153, 4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]


