Abstract
Surgical sutures are vulnerable to bacterial infections and biofilm formation. At the suture site, pain and undesirable, excess inflammation are additionally detrimental to wound healing. The development of a polymerized cyclodextrin (pCD) coated surgical suture introduces the capability to locally deliver both anti-inflammatory and anti-microbial drugs throughout the phases of acute and chronic healing. Local delivery allows for the improvement of wound healing while reducing related systemic side effects and drug resistance. Through testing, it has been shown that the fabrication of our pCD coating minimally affects the suture’s mechanical properties. In-vitro studies show measurable and consistent drug delivery for nearly five weeks. The therapeutic level of this delivery is sufficient to show inhibition of bacterial growth for four weeks, and free-radical scavenging (an in-vitro anti-inflammatory activity approximation) for two weeks. With this pCD coating technique, we maintain clinical performance standards while also introducing a long-term dual delivery system relevant to the wound healing timeframe.
Keywords: Drug Delivery, Anti-Microbial, Anti-Inflammatory, Suture, Wound Healing
1. Introduction:
Surgical sutures are used to hold tissue together after injury or surgery, and represent a market estimated to be around $1.3 billion annually.(1) Sutures come in a wide variety of sizes and materials, and their usage primarily depends on the location of the suture site. Absorbable sutures do not necessitate removal like their traditional non-absorbable counterparts, as they fully degrade after 4 to 8 weeks. This makes them effective for internal or inaccessible suture sites.(2),(3) Recently, there have been successes in the creation of sutures which have the capability to improve wound healing. These include antimicrobial sutures(4)-(8) and bio-active sutures such as drug-eluting(9)-(11) and stem cell(12)-(14) or growth factor coated(15)-(18) sutures. These newly designed sutures represent a response to a critical market, as there has been extensive research done which indicates increased potential for bacterial growth and undesirable inflammatory response when traditional surgical sutures are used.(19)-(21)
The first FDA approved antibacterial suture was Vicryl Plus in 2002.(22),(23) Made of a polyglactin suture coated with triclosan, it successfully reduced the risk of surgical site infections(24),(25), and opened the door for the development of a variety of anti-microbial suture types. The growing resistance to triclosan(26), and a limit to the approved and effective usages of Vicryl Plus has caused sutures alternatives with improved properties to be desired. With the inclusion of anti-microbial effects, sutures sites would be less likely to develop infections, and sutures themselves are less likely to build biofilms, a challenging complication of long-term bacterial colonization.(27) Addressing infections and biofilm prevention will result in overall easier healing for the patient, and lower medical costs.
While a limited variety of antimicrobial sutures exist, infection prevention is still a major concern for any sutured wound. As mentioned previously, surgical sutures are vulnerable to bacterial biofilm formation. While biofilm incidence is low (with an estimated 5% occurrence rate)(28),(29), cost of infection is high, as it requires high drug dosages and can even necessitate repeat surgery. Additionally, once biofilms have formed, the difficulty of eradicating infection is drastically increased, and typical antibiotic regimens can be ineffective. Preventative anti-microbial drugs would eradicate this issue, but as with the administration of NSAIDs, long-term systemic exposure is problematic.
Pain from the wound site is an inevitable outcome for patients requiring stitches, whether it be post-operative or simply due to injury. To treat this pain, drugs such as non-steroidal anti-inflammatories (NSAIDs) are often administered systemically. However, these drugs are not given throughout the entire healing process, to reduce systemic exposure to the drug. As a result, they do not exhibit effects throughout the entire healing window. There has been recent research into the production of anti-inflammatory sutures, which would allow for localized pain relief and better wound healing. Common designs are coatings on clinically used sutures(30),(31), or entirely new suture fabrications.(32)-(35)
The rationale behind using sutures for drug delivery of both drug types is the simultaneous addressing of both issues with localized drug release, avoiding the need for excessive systemic levels. Surgical sutures are used in almost all surgical procedures. Therefore, the introduction of drug delivery to them allows additional therapy without the need to introduce extra material or an implant, which can have detrimental effects on healing. By introducing anti-microbial and anti-inflammatory drugs locally via new drug delivery sutures, therapeutic concentrations can be achieved for sustained periods of time without risk of systemic toxicity or resistance development.
While existing smart suture designs have been moderately successful, there are only limited suture systems which incorporate multiple therapies.(36) This project aims to produce a dual-delivery suture system, which shows both anti-microbial and anti-inflammatory effects. In doing so, we hope to have the best possible wound healing outcome for patients.
Furthermore, existing smart sutures often only release drug for a short period of time, and the majority of research reports delivery time frames of 1–2 weeks.(37) Wound healing can be a slow process, with high cell infiltration and neutrophil activity giving way to macrophage/monocyte activity and tissue remodeling after 4 long weeks. In addition, the percutaneous nature of many sutures leaves them open to infection risk over the entire time they are in place, not just the first 1–2 weeks. To best impact wound healing and patient quality of life, therapeutic drugs should be used throughout this entire healing window. Even absorbable sutures can take up to 2 months to be fully broken down and removed from the body, further highlighting the chronic time scale of many suture applications. Based on these time scales, four weeks was chosen as a benchmark goal for drug delivery at therapeutic levels. While the majority of non-absorbable sutures are removed before this point, it is a good goal to meet if there is any applying pCD to absorbable sutures as well, which can take 4 to 8 weeks to fully degrade.
Additionally, many existing methods for fabrication of drug-eluting sutures significantly affect the mechanical properties of the suture itself, rendering it less useful clinically.(35) Currently used non-absorbable nylon sutures (4–0 monofilament) handle tensile loads up to 14N, while comparable polyester and polypropylene sutures can take about 11N of tensile force.(38) Tensile strength is the most important strength property for surgical sutures, therefore it is vital that drug-eluting sutures have strength equal to at least that of polyester and polypropylene sutures.
Cyclodextrin (CD) is a good material for generating a drug delivery coating due to its ability to be polymerized during in situ on a device, and due to its long-term drug release capabilities.(39),(40) The capacity for CD to improve drug delivery rates is due to its molecular structure. The monomer form of CD is a cyclic oligosaccharide, and it is this ring-shaped structure which allows the molecule to act as a ‘pocket’ for holding small molecule drugs. The inner space of this pocket is hydrophobic, and drug molecules have an affinity to localize to this area. When CD is insolubilized by forming a high molecular weight polymer, we can take advantage of this molecular affinity to alter the rate of release beyond that capable of diffusion alone. Past work from our lab and others has shown that this affinity reduces the initial drug burst effect, retaining more drug to be delivered at later time points.(41) Additionally, loading CD polymers is very straightforward, and can be done post-polymerization, allowing drug incorporation for dual drug loading and release.
By introducing a novel pCD coating technique to non-absorbable surgical sutures, it is our aim to maintain current clinical performance standards while also introducing a long-term dual delivery system relevant to the wound healing timeframe.
2. Materials and Methods:
2.1. Materials.
A lightly epichlorohydrin-crosslinked β-cyclodextrin pre-polymer (βCD, 2–15 kDa, average 10 CDs per chain) was purchased from CycloLab Ltd, (Hungary). Braided Nylon sutures in size 0 (Surgilon) were purchased from eSutures.
1,6-diisocyanatohexane (HDI) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) from Sigma Aldrich (St. Louis, MO), N,N-dimethylformamide (DMF, extra dry) from Applied Biosystems (Foster City, CA), rifampicin (RMP) from Research Products International (Mt. Prospect, IL), and trans-resveratrol (RSV) from PureBulk (Roseburg, OR) were used as received. All other reagents, solvent, and chemicals were purchased from Fisher Scientific in the highest grade available.
Green fluorescent protein (GFP)-labeled Staphylococcus aureus culture was kindly provided by Dr. Ed Greenfield (Case Western Reserve University).
2.2. Methods.
Fabrication of polymerized cyclodextrin (pCD) coated sutures
Polymerization of CD onto existing non-absorbable nylon sutures
Cyclodextrin (CD) was polymerized according to previously published protocol.(42) Briefly, 250 mg of β-CD prepolymer is dissolved into 750 mL of dimethylformamide (DMF) and polymerized using a hexamethylene diisocyanate (HDI) crosslinker in a molar ratio of 1:0.32 (glucose residue:HDI) at room temperature. With two reactive groups on either end, HDI is able to react to two different CD hydroxyls, allowing CD to form an insoluble network polymer that is a hydrogel. The hydrogel structure remains well hydrated, resulting in good transport properties, and consistent drug delivery rates. To produce polymerized cyclodextrin (pCD) coated sutures for testing, braided nylon (Surgilon) size 0 sutures were cut into 1 inch segments and hung. These segments were dipped into the CD mixture at hours 2 and 7 in the polymerization process, allowing the CD hydrogel to form onto the suture surface as the reaction progresses. In between these time points, the polymerizing CD mixture was kept at room temperature, and sealed to reduce solvent evaporation. After the 7 hour dip coating, sutures were left to dry overnight. This simple and safe reaction has no excessive heat or other toxic byproducts, which allows it to proceed and penetrate the microstructure of the suture material.
Characterization of surface coating using Scanning Electron Microscopy
Sutures both with and without pCD coating were characterized by Scanning Electron Microscopy (SEM)(JSM-6510 Series, JEOL) to determine surface morphology. To prepare samples for SEM, suture samples were coated with 5nm of palladium in vacuum by a sputter coater. Images were taken at an excitation voltage of 25 kV.
Evaluation of tensile properties – Pull Test
Suture samples of approximately 2.5” were used for tensile strength tests. Gauge length for each sample was 1”. Samples were kept in ambient environment before testing, and testing was carried out at room temperature as well. An Instron Model 1130 Universal Testing machine (CWRU, Advanced Manufacturing and Mechanical Reliability Center (AMMRC)) was used to obtain measurements. The samples were pulled at 10 mm/min until complete fracture occurred. The force was measured with a 100 N load cell, and data acquisition occurred at a rate of 100 Hz. Stiffness was taken as the slope of the best-fit line acquired by performing linear regression on all data points in the elastic region of force-displacement curves between 45% to 55% of the maximum load (minimum 40 data points per specimen, all r2 values exceeded 0.999). Replicas of 5 samples were used to ensure adequate statistical power as several properties were analyzed: max load, work to failure, stiffness, and strain to failure.
Loading of anti-microbial drug Rifampicin and anti-inflammatory drug Resveratrol into pCD suture coating
The usage of rifampicin (RMP) and resveratrol (RSV) is due to both their known interaction kinetics with CD(39),(42), and their capability to be easily read in the same solution due to non-overlapping absorbance wavelengths and the fluorescent properties of RSV.
To load coated sutures equally with both rifampicin (RMP) and resveratrol (RSV), each 1 inch segment was immersed in 1 mL of highly concentrated, mixed loading solution of both drugs and left on a shaker at low speed at 23ºC for 72 hours. The optimal loading solution was determined through condition testing on pCD disks, which is illustrated in Supplementary Figure 1. Conditions tested include equal and non-equal drug amounts (1:1 and 2:1 v/v of RMP and RSV respectively), alternate drug concentrations (20 and 50 mg/mL w/v), and varying loading solvents (100 and 90% DMF v/v).
For the pCD-coated sutures, an equal mixture (1:1) of RMP and RSV was chosen as the drug-loading solution. Both drugs were dissolved in a 90% DMF/10% v/v water mixture at 5 wt% concentration. These conditions were found to show both high and comparable loading of RMP and RSV simultaneously.
Once the dual-loading solution was determined, drug loading of pCD was carried out as mentioned previously, and according to previously determined protocol.(43) After 72 hours of loading, sutures were blotted on KimWipes and briefly rinsed with water to remove surface DMF and any free drug which is not loaded. Rinsed sutures were left to dry at 23ºC overnight.
Evaluation of loading efficiency of rifampicin and resveratrol and total drug loading capacity of pCD suture coating using a solvent extraction
To determine the total drug in the pCD coating which is available for release, an organic solvent extraction was carried out to measure the total weight percent of loaded/available drug. Dual-loaded pCD coated sutures were incubated in 1 mL of DMF. Every 12±4 hours, the DMF was removed and replaced with fresh to create infinite sink conditions. All DMF samples were added together in a single test tube, and the concentration of drug in this solution was determined spectrophotometrically, giving a quantitative measurement of the total released drug. For spectroscopy, extraction samples were diluted in PBS until DMF background was no longer significant.
Extraction samples were analyzed using UV/Vis at 321 nm with a standard curve of RMP in PBS to determine total RMP loading. The same samples were also analyzed using fluorescence, with an excitation wavelength of 318 nm and emission wavelength of 385 nm,(44) using a standard curve of RSV in PBS to determine total RSV loading.
Quantify in-vitro release of both anti-inflammatory and anti-microbial drugs from pCD coated sutures
RMP and RSV release – Infinite sink model
Dual-loaded pCD coated sutures were incubated in 1 mL of phosphate buffer saline (PBS) pH 7.4 at 37ºC with mild shaking. Every 24±4 hours, the PBS release media was removed and replaced with fresh to create infinite sink conditions. The concentration of both RMP and RSV in each time point sample was determined spectrophotometrically using the same wavelengths and standards as in the leach experiment. The release was continued until concentration of drug was no longer accurately measurable (<1 ug/mL RMP and <0.5 ug/mL RSV). Measureable amounts are determined by the lowest point on the standard curve.
Bacterial clearance – in-vitro zone of inhibition study
A zone of inhibition assay was used to determine the efficacy of the anti-microbial properties of the loaded sutures in-vitro. Dual-loaded pCD coated sutures were evaluated against Staphylococcus aureus (S. aureus) according to previously published protocol.(45) S. aureus was cultured overnight and 70mL was then spread on a trypticase soy broth (BD BBL™) agar plate. A dual-loaded pCD coated suture was placed on this fresh S. aureus lawn and incubated at 37ºC overnight. After 24 hours, the zone of inhibition around each suture was measured with calipers at three points and recorded. Each suture was then transferred onto a new S. aureus plate and the process repeated daily until the zone is no longer visible (<0.5mm).
Anti-inflammatory activity – DPPH Scavenging
In order to determine the in-vitro efficacy of the anti-inflammatory properties of the loaded sutures, a 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging assay was used. DPPH is a free radical which changes color when scavenged. RSV’s anti-inflammatory activity comes from its antioxidant properties, scavenging free radicals involved in inflammation to help prevent chronic inflammation from occurring. A 100μM solution of DPPH in ethanol was created, according to published protocol.(46) This DPPH solution was incubated with dual-loaded pCD coated suture release samples in a 1:1 ratio for 30 minutes, after which scavenging can be determined by reading color change spectrophotometrically at 517 nm. A positive control of 1mg/mL RSV and a negative control of PBS were used to determine % DPPH scavenging.
Statistical Analysis
If not otherwise stated, experiments were carried out in triplicate for statistical analysis (n=3). Data displayed is the mean of each condition, and error bars represent the standard deviation of the triplicate set. Student’s t test and ANOVA were done using Prism to determine reported statistical significance.
3. Results:
Fabrication of polymerized cyclodextrin (pCD) coated sutures
Polymerization of CD onto existing non-absorbable nylon sutures
CD was successfully polymerized onto the surface of the braided nylon suture. This pCD coating can be seen by eye as a translucent surface. This surface can also be seen in digital camera photos, as demonstrated in Image 1. The coating holds up to moderate handling and submersion in solutions such as water and PBS. However, extreme bending of pCD coated sutures seemed to cause microscale cracking of the coating at stress points. This would indicate that the dried coating is more brittle than the nylon suture core. Nevertheless, once broken at a stress point, the coating remains adhered to the nylon suture core, indicating that cracking does not render the coated suture more susceptible to further breakdown or delamination.
Characterization of surface coating using Scanning Electron Microscopy
From the SEM images in Figure 1, it is clear that polymerized cyclodextrin has successfully coated the non-absorbable braided nylon sutures. In the non-coated images (Figure 1.a and 1.b), the individual nylon threads can be clearly seen. In the coated images (Figure 1.c and 1.d), individual threads are no longer readily apparent, due to the pCD coating. The coating appears fairly uniform, and the braids can still be determined. However, the pCD coating shows noticeable cracking at this microscale.
Figure 1.
Scanning Electron Microscopy (SEM) images of non-absorbable nylon sutures before (A, B) and after (C, D) polymerized cyclodextrin (pCD) coating. Scale bar represents 100μm. pCD coated sutures show smoother surface in comparison to individual braided threads which can be seen in the uncoated image, but there is significant micro cracking throughout the coating itself.
The preparation process caused the pCD hydrogel to transition from an aqueous to dry state, due to the vacuum conditions required for SEM imaging. In the aqueous state, pCD is highly hydrated. Therefore, it is likely that the cracks shown in these images occurred are solely due to the stresses experienced during drying, and that hydrated coatings would not have these same cracks.
Interestingly, the majority of cracks appear to follow the lines of the braided nylon filament. The cracks do not appear to induce delamination while the suture is under tensile stress, but the cracks have the potential to propagate across the suture-coating interface if alternate stresses or strains are applied.
Evaluation of tensile properties – Pull Test
Force-displacement curves from suture tensile tests were analyzed to determine several properties, namely max load, work to failure, stiffness, and strain to failure. The overall shape of the force-displacements graphs for both pCD coated and uncoated sutures are very similar (Supplementary Figure 1). However, the maximum displacement is reduced in the case of the coated suture samples, significantly affecting the values for max load (p < 0.001), work-to-failure (p < 0.005), and strain-to-failure (p < 0.001) for the coated sutures.
In pCD coated samples, the determined average values for max load, work-to-failure, and strain-to-failure of the coated sutures were 94.1%, 74.8%, and 84.1% respectively of the average uncoated suture sample values. There is no significant difference between determined stiffness values (Figure 2).
Figure 2.

Tensile testing of polymerized cyclodextrin (pCD) coated and uncoated nylon sutures shows significant difference between Max Load, Work to Failure, and Strain to Failure of the two sample conditions. (*p < 0.005 and **p < 0.001) There is no significant difference between Stiffness values.
Polyester and polypropylene sutures are used for many of the same procedures as nylon. However, they have been reported to exhibit values for max load which are 79% those for nylon sutures.(38) With this in mind, the significant differences in mechanical properties should prove acceptable in vivo, since the reduction is less than that which is clinically accepted in the case of polyester and polypropylene.
Evaluation of loading efficiency of rifampicin and resveratrol and total drug loading capacity of pCD suture coating using a solvent extraction
From the extraction experiment, it was found that a 1 inch segment of pCD coated nylon suture loaded 71.06±6.03 and 68.7±12.94 μg of RMP and RSV respectively. These raw amounts can also be expressed in terms of weight percent of the coating (Figure 3): 11.84±1.01 and 11.45±2.16 wt% of RMP and RSV respectively. Both RMP and RSV load similarly under the 50mg/mL, 90% DMF loading solution.
Figure 3.

Leach study of polymerized cyclodextrin (pCD) coated sutures, dual-loaded with rifampicin (RMP) and resveratrol (RSV), averaged (n = 3) with error bars representing standard deviation. Statistically similar loading of each drug is seen under optimal loading conditions.
However, in the preliminary loading studies (Supplementary Figure 2), which used pCD disks to determine optimal loading conditions, weight % loading was significantly reduced (4.23±0.13 and 5.33±0.74 wt% of RMP and RSV respectively). This is likely due to the different surface to area ratio that is seen in the pCD disks used in the optimization studies when compared to the coated suture fibers. Disks are much thicker, and have overall more mass than the suture coating. Increased weight % loading is ideal, as it allows for more drug to be originally introduced to the same length of suture material. This, in turn, allows for a longer-term release profile.
In many clinical settings, larger wounds will require more stitches and therefore a greater length of suture material. This greater length will increase the raw weight of drug loaded and available for delivery. With increased suture material, the release profile should maintain the same shape, but with increased values, as there is more polymer and drug undergoing release.
Quantify in-vitro release of both anti-inflammatory and anti-microbial drugs from pCD coated sutures
RMP and RSV Release - Infinite sink model
A profile of in-vitro drug release over time can be seen in Figure 4. There is a small burst of drug release at the beginning of release, which can be seen as increased values in the daily release curve (Figure 4.a) or as an increased slope in the cumulative release curve (Figure 4.b). Specifically, 5.16±0.82 μg of RMP and 1.59±0.50 μg of RSV are released within the first hour, and 10.65±1.07 μg of RMP and 9.55±1.55 μg of RSV are released within the first day. This small initial burst represents about 15 and 14% of total RMP and RSV release respectively overall. The rest of the curve appears linear, due to the consistent daily release of both drugs. A linear regression of the data shows that the RMP release profile after initial burst release can be fit to the line y = 1.545x + 7.715 with an R2 value of 0.9916, and the RSV release can be fit to the line y = 1.356x + 7.855 with an R2 value of 0.9596. This matches the average daily release of 1.56 μg and 1.37 μg of rifampicin and resveratrol respectively from the pCD coated suture segments, which has been recorded for nearly five weeks.
Figure 4.
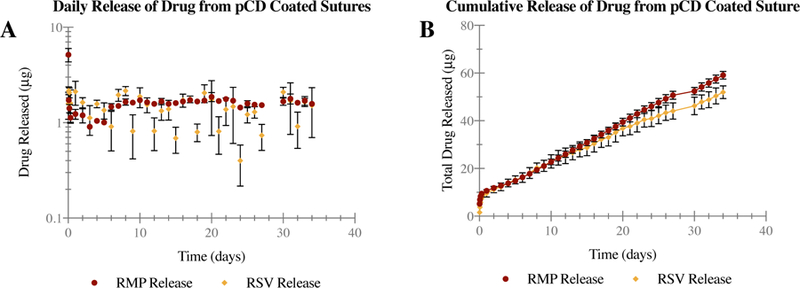
Infinite sink release study of polymerized cyclodextrin (pCD) coated sutures, dual-loaded with rifampicin (RMP) and resveratrol (RSV). Release points for each drug over time (t = 34 days) are averaged (n = 3) with error bars representing standard deviation, and plotted to visualize both daily (A) and cumulative (B) release. pCD coated sutures demonstrate consistent daily release for nearly 5 weeks, resulting in fairly linear release over time.
Spectroscopy confirms continued release of both RMP and RSV at fairly consistent levels throughout a five-week period. RMP has been shown to have a minimum inhibitory concentration of <0.06 μg/mL against S. aureus.(47) With the completed release showing an average daily release of 1.56 μg/mL (Figure 4.a) from pCD coated sutures, there should be more than enough RMP to prevent infection.
In published literature, 5–25 μM (1.14–5.7 μg/mL) has been cited as an effective RSV dosage to modulate inflammation in neural tissue. (46) The completed release shows an average daily release of 1.37 μg/mL (Figure 4.a) from the sutures, which is appropriate for therapeutic effectiveness.
Bacterial clearance – in-vitro zone of inhibition study
To evaluate anti-microbial activity, dual-loaded pCD coated sutures were used in a zone of inhibition assay against S. aureus. Figure 5 shows that the fabricated dual-loading sutures are capable of eradicating S. aureus for at least 24 days. The zone of inhibition measured shows a slow decrease in size, starting at 10.09±2.02 mm, but seems to hold steady at an average of 1.41 mm in the last week of testing. Linear regression of the data shows that the first phase of Figure 5 (days 1–18) can be fit to the line y = 0.426x + 10.76 with an R2 value of 0.7882, while the second phase (days 19–24) does not fit to a significantly non-zero slope. ANOVA was also used to compare the day-to-day change in the zone of inhibition, but no significant statistical differences were found between adjacent time points. When all time points are compared against day 1, the first statistically significant difference is seen at day 7 (p < 0.01). In this study, both RMP and RSV are loaded and released. In the zone of inhibition study, dual drug delivering sutures are challenged against a fresh host of S. aureus bacteria every day, showing the efficacy of pCD coated sutures in a worst-case scenario, where there is continual introduction of healthy bacterial cells. The results of this study (Figure 5) confirm that the fabricated pCD coated sutures are capable of releasing a therapeutic dose of active antibiotic which is sufficient to inhibit the growth of bacteria for approximately four weeks.
Figure 5.

Zone of inhibition study of polymerized cyclodextrin (pCD) coated sutures, dual-loaded with rifampicin (RMP) and resveratrol (RSV), against S. Aureus (t = 24 days) averaged (n = 3) with error bars representing standard deviation. pCD coated sutures demonstrate antibacterial activity against S. Aureus for at least 24 days. ANOVA shows no statistically significant difference between adjacent time points.
Anti-inflammatory activity – DPPH Scavenging
To evaluate anti-inflammatory activity, release samples from dual-loaded pCD coated sutures were used in a DPPH scavenging assay. Figure 6 shows that the fabricated dual-loading sutures exhibit anti-inflammatory activity for approximately 14 days, with an average scavenging activity of 9.25±4.83%. Overall, the results of this study confirm that the fabricated pCD coated sutures are capable of releasing a measurable dose of an active anti-inflammatory for a two-week period. Analysis of the data shows that while Figure 6 has a clear trend, a linear fit does not represent the data well, with an R2 value of only 0.6384. ANOVA was used to compare the day-to-day change in scavenging, but no significant statistical differences were found between adjacent time points.
Figure 6.
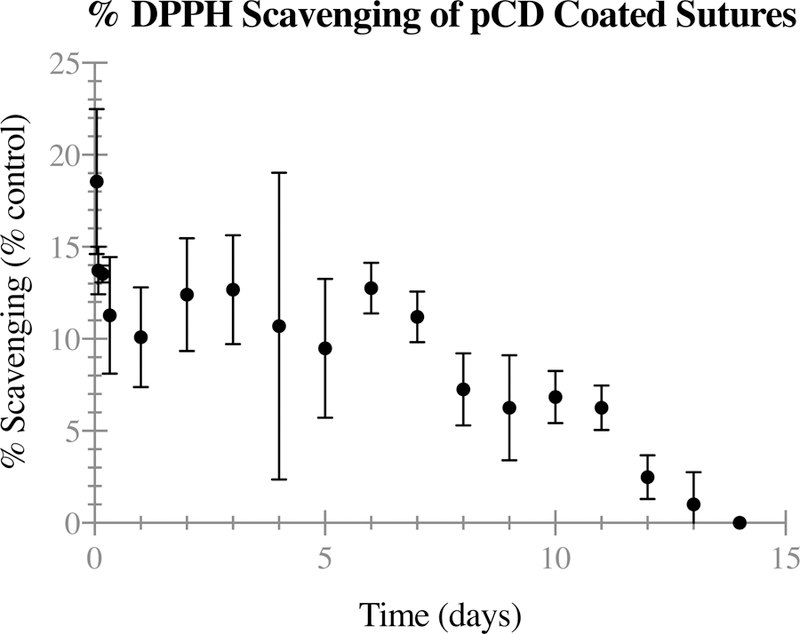
DPPH scavenging study of polymerized cyclodextrin (pCD) coated sutures, dual-loaded with rifampicin (RMP) and resveratrol (RSV) (t = 14 days) averaged (n = 3) with error bars representing standard deviation. pCD coated sutures demonstrate anti-inflammatory activity through two weeks. ANOVA shows no statistically significant difference between adjacent time points.
Based on the release study and an experimentally determined standard for the anti-inflammatory activity of RSV (Supplementary Figure 3), DPPH % scavenging was as expected. The average daily release of 1.37 μg (Figure 4.a) corresponds to a scavenging of ~12% on the standard curve. With an initial 18.56±3.94% scavenging within the first hour, and an average of 9.25% scavenging throughout the two-week period, the anti-inflammatory activity recorded is only slightly below expected values.
The timing of suture removal varies with wound location and patient, but in the majority of cases, non-absorbable suture removal occurs after 10 to 14 days. With this in mind, the two-week time frame of anti-inflammatory activity is acceptable for pain reduction and improved wound healing.
4. Discussion
The dual loading of a pre-formed polymer is a fairly novel concept, both for our lab and the general scientific space. Most drug releasing polymers must be loaded with drug as they are formed, not after the polymerization/coating process. Our pCD coatings can be loaded after polymerization is complete, and can be loaded with drug cocktails when desired.
This manuscript shows a successful potential application where pCD coatings are necessary and advantageous. The polymer coating is required to successfully integrate with surgical sutures, possibly reducing the barrier to clinical entry by simplifying manufacture. Mechanical changes were found to be significant, and there is evidence that polymer coatings are vulnerable to cracking. Even though polymer cracking does not induce delamination, there is evidence based on previous work(43), that fragments of polymer coating (i.e. particles) remain equally good delivery vehicles as compared to coated materials.
This system meets all desired properties as originally designed, with therapeutic time frames of 2 and 4 weeks for anti-inflammatory and anti-microbial drugs respectively – the times for removal and tissue remodeling/suture breakdown. If delamination or breakage of coating is found to still be a concern, our next steps would be a different way to further integrate pCD into the suture (e.g. chemical conjugation). For absorbable situations, a degradable version of the polymer would be an ideal way to adapt the system to a greater clinical space. Now that general loading and release kinetics have been quantified, different drugs could be used due to the simple nature of the loading process. An anti-inflammatory with higher activity (simvastatin) or an antibiotic with additional anti-inflammatory activity (minocycline) could be used just as easily. The dual-loading process could even be altered to allow for the loading of three drugs, to take advantage of known synergistic antibiotic interactions (rifampicin/minocycline) in more extreme or at-risk suture sites.
Supplementary Material
Supplementary Figure 1. Force-Displacement curves produced during tensile testing of uncoated (A) and polymerized cyclodextrin (pCD) coated (B) nylon sutures. pCD coated sutures show decreased maximum displacement before failure, and less consistent mechanical behavior.
Supplementary Figure 2. Weight percent loading of rifampicin (RMP) and resveratrol (RSV) into polymerized cyclodextrin (pCD) gel disks under various loading conditions (20mg/mL vs 50mg/mL concentration, 100% DMF vs 90% DMF, and at varying ratios of drug). Optimal condition of 50:50, 50mg/mL, 90% DMF was used for studies in this manuscript due to high and fairly similar loading of both drugs.
Supplementary Figure 3. DPPH scavenging standard curve of resveratrol (RSV). Relationship between RSV concentration and in-vitro anti-inflammatory activity is neither linear nor truly logarithmic. Even at fairly high concentrations (100μg/mL) the activity of RSV is low, indicating that it is a weak anti-inflammatory.
5. Acknowledgements
The authors would like to thank Kathleen Young for assistance with SEM imaging and Dr. Evon Ereifej for assistance with the anti-inflammatory DPPH scavenging assay. We would also like to thank the NSF Undergraduate Summer Research Experience. Research reported in this publication was supported by the National Institute of General Medical Sciences of the Natural Institute of Health under award number R01 GM121477. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Declaration of Interest: HvR is a co-founder of Affinity Therapeutics, but does not receive salary.
Image 1. Digital camera images of non-absorbable nylon sutures with (bottom) and without (top) polymerized cyclodextrin (pCD) coating. Scale bar represents 0.2 in.
6. References
- 1.Champeau M, Thomassin J-M, Tassaing T, Jérôme C. Current manufacturing processes of drug-eluting sutures. Expert Opin Drug Deliv 2017;14:1293–303. [DOI] [PubMed] [Google Scholar]
- 2.Sajid MS, McFall MR, Whitehouse PA, Sains PS. Systematic review of absorbable vs non-absorbable sutures used for the closure of surgical incisions. World J Gastrointest Surg Baishideng Publishing Group Inc; 2014;6:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gys T, Hubens A. A prospective comparative clinical study between monofilament absorbable and non-absorbable sutures for abdominal wall closure. Acta Chir Belg 1989;89:265–70. [PubMed] [Google Scholar]
- 4.Leaper D, McBain AJ, Kramer A, Assadian O, Sanchez JLA, Lumio J, Kiernan M. Healthcare associated infection: novel strategies and antimicrobial implants to prevent surgical site infection. The Annals of The Royal College of Surgeons of England. The Royal College of Surgeons of England; 2010;92:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallo AL, Paladini F, Romano A, Verri T, Quattrini A, Sannino A, Pollini M. Efficacy of silver coated surgical sutures on bacterial contamination, cellular response and wound healing. Materials Science and Engineering: C 2016;69:884–93. [DOI] [PubMed] [Google Scholar]
- 6.Serrano C, García-Fernández L, Fernández-Blázquez JP, Barbeck M, Ghanaati S, Unger R, Kirkpatrick J, Arzt E, Funk L, Turón P, del Campo A. Nanostructured medical sutures with antibacterial properties. Biomaterials 2015;52:291–300. [DOI] [PubMed] [Google Scholar]
- 7.López-Saucedo F, Flores-Rojas GG, López-Saucedo J, Magariños B, Alvarez-Lorenzo C, Concheiro A, Bucio E. Antimicrobial silver-loaded polypropylene sutures modified by radiation-grafting. European Polymer Journal 2018;100:290–7. [Google Scholar]
- 8.Dubas ST, Wacharanad S, Potiyaraj P. Tunning of the antimicrobial activity of surgical sutures coated with silver nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2011;380:25–8. [Google Scholar]
- 9.Choudhury AJ, Gogoi D, Chutia J, Kandimalla R, Kalita S, Kotoky J, Chaudhari YB, Khan MR, Kalita K. Controlled antibiotic-releasing Antheraea assama silk fibroin suture for infection prevention and fast wound healing. Surgery 2016;159:539–47. [DOI] [PubMed] [Google Scholar]
- 10.Weldon CB, Tsui JH, Shankarappa SA, Nguyen VT, Ma M, Anderson DG, Kohane DS. Electrospun drug-eluting sutures for local anesthesia. J Control Release 2012;161:903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Ge L, Mueller A, Carlson MA, Teusink MJ, Shuler FD, Xie J. Twisting electrospun nanofiber fine strips into functional sutures for sustained co-delivery of gentamicin and silver. Nanomedicine: Nanotechnology, Biology and Medicine 2017;13:1435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen KJ, Favreau JT, Guyette JP, Tao Z-W, Coffin ST, Cunha-Gavidia A, D’Amore B, Perreault LR, Fitzpatrick JP, DeMartino A, Gaudette GR. Functional Effects of Delivering Human Mesenchymal Stem Cell-Seeded Biological Sutures to an Infarcted Heart Biores Open Access. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA; 2016;5:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao Z-W, Favreau JT, Guyette JP, Hansen KJ, Lessard J, Burford E, Pins GD, Gaudette GR. Delivering stem cells to the healthy heart on biological sutures: effects on regional mechanical function. J Tissue Eng Regen Med 2017;11:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao J, Woon CY-L, Behn A, Korotkova T, Park D-Y, Gajendran V, Smith RL. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am 2012;37:1639–45. [DOI] [PubMed] [Google Scholar]
- 15.Cummings SH, Grande DA, Hee CK, Kestler HK, Roden CM, Shah NV, Razzano P, Dines DM, Chahine NO, Dines JS. Effect of recombinant human platelet-derived growth factor-BB-coated sutures on Achilles tendon healing in a rat model: A histological and biomechanical study J Tissue Eng. SAGE PublicationsSage UK: London, England; 2012;3:2041731412453577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs TF, Surke C, Stange R, Quandte S, Wildemann B, Raschke MJ, Schmidmaier G. Local delivery of growth factors using coated suture material. ScientificWorldJournal Hindawi; 2012;2012:109216–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uggen C, Dines J, McGarry M, Grande D, Lee T, Limpisvasti O. The Effect of Recombinant Human Platelet-Derived Growth Factor BB–Coated Sutures on Rotator Cuff Healing in a Sheep Model. Arthroscopy: The Journal of Arthroscopic & Related Surgery 2010;26:1456–62. [DOI] [PubMed] [Google Scholar]
- 18.Rijcken E, Fuchs T, Sachs L, Kersting CM, Bruewer M, Krieglstein CF. Insulin-like growth factor 1-coated sutures improve anastomotic healing in an experimental model of colitis. British Journal of Surgery Wiley-Blackwell; 2010;97:258–65. [DOI] [PubMed] [Google Scholar]
- 19.Matalon S, Kozlovsky A, Kfir A, Levartovsky S, Mazor Y, Slutzky H. The effect of commonly used sutures on inflammation inducing pathogens – An in vitro study. Journal of Cranio-Maxillofacial Surgery 2013;41:593–7. [DOI] [PubMed] [Google Scholar]
- 20.Akhter MSJ, Verma R, Madhukar KP, Vaishampayan AR, Unadkat PC. Incidence of surgical site infection in postoperative patients at a tertiary care centre in India. J Wound Care MA Healthcare London; 2016;25:210–2–214–7. [DOI] [PubMed] [Google Scholar]
- 21.Hess DJ, Henry-Stanley MJ, Wells CL. Interplay of antibiotics and bacterial inoculum on suture-associated biofilms. Journal of Surgical Research 2012;177:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gartti-Jardim EC, de Souza AP, Carvalho ACG de S, Pereira CCS, Okamoto R, Magro Filho O. Comparative study of the healing process when using Vicryl®, Vicryl Rapid®, Vicryl Plus®, and Monocryl® sutures in the rat dermal tissue. Oral Maxillofac Surg Springer Berlin Heidelberg; 2013;17:293–8. [DOI] [PubMed] [Google Scholar]
- 23.Dennis C, Sethu S, Nayak S, Mohan L, Morsi YY, Manivasagam G. Suture materials - Current and emerging trends. J Biomed Mater Res A 3rd ed. Wiley-Blackwell; 2016;104:1544–59. [DOI] [PubMed] [Google Scholar]
- 24.Renko M, Paalanne N, Tapiainen T, Hinkkainen M, Pokka T, Kinnula S, Sinikumpu J-J, Uhari M, Serlo W. Triclosan-containing sutures versus ordinary sutures for reducing surgical site infections in children: a double-blind, randomised controlled trial. The Lancet Infectious Diseases 2017;17:50–7. [DOI] [PubMed] [Google Scholar]
- 25.Defazio A, Datta M, Nezhat C. Does the use of Vicryl™ Plus Antibacterial Suture Decrease the Incidence of Umbilical Infection When Compared to Vicryl™ Suture? Fertility and Sterility 2005;84:S161. [Google Scholar]
- 26.Joseph B, George A, Gopi S, Kalarikkal N, Thomas S. Polymer sutures for simultaneous wound healing and drug delivery – A review. International Journal of Pharmaceutics 2017;524:454–66. [DOI] [PubMed] [Google Scholar]
- 27.Leonardo J, Rozzelle CJ. Antimicrobial Suture Use Associated with a Decreased Incidence of Cerebrospinal Fluid Shunt Infections. Neurosurgery 2006;59:478. [Google Scholar]
- 28.Hood CT, Lee BJ, Jeng BH. Incidence, Occurrence Rate, and Characteristics of Suture-Related Corneal Infections After Penetrating Keratoplasty. Cornea 2011;30:624–8. [DOI] [PubMed] [Google Scholar]
- 29.Kathju S, Nistico L, Hall-Stoodley L, Post JC, Ehrlich GD, Stoodley P. Chronic surgical site infection due to suture-associated polymicrobial biofilm. Surgical Infections. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA; 2009;10:457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melendez Ortiz HI, Díaz Rodríguez P, Alvarez-Lorenzo C, Concheiro A, Bucio E. Binary Graft Modification of Polypropylene for Anti‐Inflammatory Drug–Device Combo Products. Journal of Pharmaceutical Sciences 2014;103:1269–77. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Chen D, Sun J. Layer-by-Layer Deposition of Polymeric Microgel Films on Surgical Sutures for Loading and Release of Ibuprofen. Langmuir. American Chemical Society; 2009;25:7990–4. [DOI] [PubMed] [Google Scholar]
- 32.Huh BK, Kim BH, Kim S-N, Park CG, Lee SH, Kim KR, Heo CY, Choy YB. Surgical suture braided with a diclofenac-loaded strand of poly(lactic- co -glycolic acid) for local, sustained pain mitigation. Materials Science and Engineering: C 2017;79:209–15. [DOI] [PubMed] [Google Scholar]
- 33.Catanzano O, Acierno S, Russo P, Cervasio M, Del Basso De Caro M, Bolognese A, Sammartino G, Califano L, Marenzi G, Calignano A, Acierno D, Quaglia F. Melt-spun bioactive sutures containing nanohybrids for local delivery of anti-inflammatory drugs. Materials Science and Engineering: C 2014;43:300–9. [DOI] [PubMed] [Google Scholar]
- 34.Padmakumar S, Joseph J, Neppalli MH, Mathew SE, Nair SV, Shankarappa SA, Menon D. Electrospun Polymeric Core–sheath Yarns as Drug Eluting Surgical Sutures. ACS Applied Materials & Interfaces. American Chemical Society; 2016;8:6925–34. [DOI] [PubMed] [Google Scholar]
- 35.Zurita R, Puiggalí J, Rodríguez-Galán A. Loading and Release of Ibuprofen in Multi- and Monofilament Surgical Sutures. Macromolecular Bioscience WILEY‐VCH Verlag; 2006;6:767–75. [DOI] [PubMed] [Google Scholar]
- 36.Shukla A, Fuller RC, Hammond PT. Design of multi-drug release coatings targeting infection and inflammation. J Control Release 2011;155:159–66. [DOI] [PubMed] [Google Scholar]
- 37.Haley RM, Recum von HA. Localized and targeted delivery of NSAIDs for treatment of inflammation: A review. Exp Biol Med (Maywood) 2018;6:1535370218787770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J-C, Lee Y-K, Lim B-S, Rhee S-H, Yang H-C. Comparison of tensile and knot security properties of surgical sutures. Journal of Materials Science: Materials in Medicine Springer US; 2007;18:2363–9. [DOI] [PubMed] [Google Scholar]
- 39.Thatiparti TR, Shoffstall AJ, Recum von HA. Cyclodextrin-based device coatings for affinity-based release of antibiotics. Biomaterials 2010;31:2335–47. [DOI] [PubMed] [Google Scholar]
- 40.Shelley H, Babu RJ. Role of Cyclodextrins in Nanoparticle Based Drug Delivery Systems. Journal of Pharmaceutical Sciences 2018. [DOI] [PubMed]
- 41.Cyphert EL, Learn GD, Hurley SK, Lu C-Y, Recum von HA. An Additive to PMMA Bone Cement Enables Postimplantation Drug Refilling, Broadens Range of Compatible Antibiotics, and Prolongs Antimicrobial Therapy. Adv Healthc Mater Wiley-Blackwell; 2018;10:e1800812. [DOI] [PubMed] [Google Scholar]
- 42.Thatiparti TR, Recum von HA. Cyclodextrin complexation for affinity-based antibiotic delivery. Macromolecular Bioscience Wiley-Blackwell; 2010;10:82–90. [DOI] [PubMed] [Google Scholar]
- 43.Grafmiller KT, Zuckerman ST, Petro C, Liu L, Recum von HA, Rosen MJ, Korley JN. Antibiotic-releasing microspheres prevent mesh infection in vivo. Journal of Surgical Research 2016;206:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Díaz TG, Merás ID, Rodríguez DA. Determination of resveratrol in wine by photochemically induced second-derivative fluorescence coupled with liquid–liquid extraction. Anal Bioanal Chem. Springer-Verlag; 2007;387:1999–2007. [DOI] [PubMed] [Google Scholar]
- 45.Cyphert EL, Zuckerman ST, Korley JN, Recum von HA. Affinity interactions drive post-implantation drug filling, even in the presence of bacterial biofilm. Acta Biomater 2017;57:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen JK, Jorfi M, Buchanan KL, Park DJ, Foster EJ, Tyler DJ, Rowan SJ, Weder C, Capadona JR. Influence of resveratrol release on the tissue response to mechanically adaptive cortical implants. Acta Biomater 2016;29:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bahl D, Miller DA, Leviton I, Gialanella P, Wolin MJ, Liu W, Perkins R, Miller MH. In vitro activities of ciprofloxacin and rifampin alone and in combination against growing and nongrowing strains of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. American Society for Microbiology Journals; 1997;41:1293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Force-Displacement curves produced during tensile testing of uncoated (A) and polymerized cyclodextrin (pCD) coated (B) nylon sutures. pCD coated sutures show decreased maximum displacement before failure, and less consistent mechanical behavior.
Supplementary Figure 2. Weight percent loading of rifampicin (RMP) and resveratrol (RSV) into polymerized cyclodextrin (pCD) gel disks under various loading conditions (20mg/mL vs 50mg/mL concentration, 100% DMF vs 90% DMF, and at varying ratios of drug). Optimal condition of 50:50, 50mg/mL, 90% DMF was used for studies in this manuscript due to high and fairly similar loading of both drugs.
Supplementary Figure 3. DPPH scavenging standard curve of resveratrol (RSV). Relationship between RSV concentration and in-vitro anti-inflammatory activity is neither linear nor truly logarithmic. Even at fairly high concentrations (100μg/mL) the activity of RSV is low, indicating that it is a weak anti-inflammatory.



