Abstract
Active surveillance (AS) is increasingly used to monitor patients with low-risk prostate cancer; however, approximately 50% of AS patients experience disease reclassification requiring definitive treatment and little is known about patient characteristics that modify the risk of reclassification. Obesity may be one of the major contributing factors.
The Prostate Cancer Active Lifestyle Study (PALS) is a clinical trial evaluating the impact of weight loss among overweight/obese (Body Mass Index (BMI) > 25 kg/m2) men with clinically localized prostate cancer on AS. Two hundred participants will be randomized to either the PALS intervention, a 6-month structured diet and exercise program adapted from the Diabetes Prevention Program followed by 6 months of maintenance, or control (general diet and physical activity guidelines delivered in a single session). The PALS intervention involves one-on-one instruction with a registered dietitian and exercise physiologist to achieve the study goal of loss of 7% of baseline weight. Participation is coordinated so that the 6-month time point coincides with the participants’ standard-of-care AS prostate biopsy. Primary outcomes will evaluate the intervention effects on circulating and tissue markers of glucose and insulin regulation, health-related quality of life and pathologic upgrading on follow-up prostate biopsies. Additional analyses will determine whether changes in weight and glucose regulation can be sustained for 6 months after the end of instruction. Findings from this trial may have wide reaching implications for men diagnosed with clinically-localized prostate cancer by providing an active lifestyle-based approach to improve prostate cancer patient outcomes.
Keywords: Diet, Nutrition, Physical Activity, Exercise, Prostate cancer, Active Surveillance, glucose regulation, insulin regulation, Outcomes, Randomized Trial
INTRODUCTION
Prostate cancer (PCa) is the most common cancer diagnosis among men, with over 164,000 men diagnosed in the United States in 2017.(1) Due to widespread prostate-specific antigen (PSA) screening, approximately 50% of men diagnosed with PCa present with clinically low-risk disease.(1) Increasingly, men with low-risk PCa are managed with active surveillance (AS), where patients are monitored via PSA blood tests, physical exams and surveillance biopsies with definitive treatment undertaken if evidence of disease reclassification (i.e. increase in PCa volume or aggressiveness).(2) Considering the significant quality of life and functional side effects associated with PCa interventions such as surgery and radiation, the goal of AS is to minimize treatment- related side effects in those least likely to experience PCa-related morbidity and mortality. Despite their initial lower risk status, roughly 50% of men on AS will experience disease reclassification requiring active treatment(3).
It is well established that being overweight/obese and weight gain are risk factors for poor PCa outcomes.(4) Epidemiologic studies have consistently associated obesity with an increased risk of PCa progression and PCa-specific mortality(5–12) and an increased risk of recurrence after definitive treatment(13). Increasing evidence also identifies being overweight/obese as a predictor of poorer prognosis for men on AS. Several studies have suggested that overweight/obese men with low-risk PCa who are otherwise good candidates for AS but chose instead to undergo immediate radical prostatectomy were more likely to experience upstaging and upgrading than normal weight men.(10, 14–16) Furthermore, among men on AS, obese men have a greater than 2-fold increased risk of pathologic progression compared with normal weight men(17, 18), suggesting that obesity may be a modifiable risk factor for PCa progression.
The causal mechanisms underlying the obesity-PCa progression relationship are unclear; though obesity-induced metabolic changes, including impaired glucose regulation, may be responsible.(19–21) Several lines of evidence suggest the importance of glucose regulation in PCa progression, including animal models of hyperinsulinemia and high glucose feeding(22, 23), observational human studies showing hyperglycemia and hyperinsulinemia are associated with worse PCa outcomes(19, 20, 24, 25), and tissue level evidence of overexpression of the insulin receptor on PCa cells(26, 27). These data support the notion that improved glucose regulation in men with PCa may reduce the risk of PCa progression and its associated morbidity and mortality.
To test the potential impact of weight loss and improved glucose regulation on men with low-risk, localized PCa, we developed the Prostate Cancer Active Lifestyle Study (PALS). PALS is a phase III, randomized clinical trial evaluating the effects of a diet and exercise program to promote weight loss among overweight/obese men (body mass index (BMI) ≥25 kg/m2) on AS. This novel trial will enhance our understanding of the biologic mechanisms linking obesity and PCa outcomes. The overall goal is to test the effect of a weight loss intervention on serum and tissue markers associated with PCa mortality. Findings from this study may have wide-reaching implications for improving both the overall and disease-specific outcomes in men diagnosed with clinically localized PCa, and provide an alternative, non-invasive approach for men with low-risk PCa on AS.
2. Research Design and Methods
Overview
PALS is a randomized, phase III clinical trial of a diet and exercise lifestyle intervention vs general healthy lifestyle recommendations among men with low-risk PCa on AS. The trial is funded by the National Cancer Institute and is registered at clinicaltrials.gov (NCT02454517). As a clinical trial, a data safety monitoring board (DSMB) consisting of clinical, statistical and epidemiologic experts, has been selected to oversee trial progress. The DSMB meets annually to assess the safety and efficacy of the intervention. The study was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board and the Veteran’s Administration Puget Sound Health Care System Institutional Review Board and all participants are asked to provide written informed consent for participation.
Eligibility and exclusion criteria
Eligible men have histologically-confirmed, clinically-localized low or low-intermediate risk adenocarcinoma of the prostate (defined as stage T1c/T2a, Gleason grade group 1 (3+3) or 2 (3+4), and PSA < 20 ng/ml) and have elected AS as primary treatment. Additionally, eligible patients must be overweight or obese (defined as BMI ≥ 25 kg/m2), able to make the required dietary changes, and physically able to undertake an exercise program.
Exclusion criteria include enrollment in a structured weight loss program (e.g. Weight Watchers® or Jenny Craig®) within the previous 12 months, planned weight loss surgery, use of appetite suppressants, use of androgen deprivation therapy within the previous 12 months, insulin dependent diabetes mellitus, use of metformin, and alcohol or narcotics abuse. Additional exclusion criteria limit participation to men without significant cardiovascular disease that would preclude participation in an exercise program (defined as a history of myocardial infarction or stroke within the previous 6 months, pulmonary edema, myocarditis, pericarditis, unstable angina, pulmonary embolism or deep vein thrombosis, uncontrolled hypertension (systolic and diastolic blood pressure above 200 and 110, respectively), uncontrolled arrhythmia, or heart failure).
Recruitment
Potential participants are identified in two different ways. (1) At collaborating University of Washington clinical sites (University of Washington Medical Center (UWMC), Valley Medical Center and the Seattle Puget Sound Veterans Affairs Heath Care System), clinic staff pre-screen urology clinic patients to determine medical eligibility. Eligible patients are approached during their clinic visit to introduce the study. Interested men are contacted by study staff to finalize recruitment into the study. (2) The Cancer Surveillance System (CSS) of Western Washington Surveillance Epidemiology and End Results (SEER) program, which collects population-based data on cancer incidence and survival in 13 counties in western Washington State, is also used to identify potentially eligible men who have newly-diagnosed PCa and meet the clinical eligibility criteria (grade group 1 or 2, clinical T-stage≤T2a, PSA<20 ng/ml). Men identified via CSS are sent a prior notification mailing that introduces PALS, states the mission and purpose of the CSS, to informs men how to “opt out” of being contacted by study staff. Men who have not opted out within 10 days are then called by study staff and approached about participating in PALS.
PALS intervention
The PALS intervention is based on the Diabetes Prevention Program (DPP), an evidence-based lifestyle intervention that combines structured curriculum on nutrition and physical activity with goal-based behavior instruction to achieve weight loss(28), with modifications to condense the delivery of the intervention and tailor the materials to older men. The overall PALS lifestyle intervention goal is a 7% reduction in baseline. Weight loss of 7% has been shown to significantly decrease fasting glucose levels(29), reduce the risk of incident diabetes mellitus(29)(28), was sustainable over 10 years(30), and reduces the risk of cardiovascular disease(31). Weight loss is to be met via a reduction in caloric intake and an increase in physical activity. Participants are provided an individualized calorie goal, representing a reduction in total energy of 500 to 1000 calories/day, depending on the participants’ starting body weight. The physical activity goal for all participants is 150 minutes per week of moderate to vigorous exercise. To help participants achieve moderate to vigorous intensity during exercise, they are provided an individualized target heart rate range, which is equivalent to 70 to 85% of their peak heart rate reached during the baseline submaximal exercise test.
The PALS intervention is delivered via a registered dietitian, who has received formal DPP training, and an exercise physiologist. The instructional materials are covered in a total of 11 sessions (Table 1). During the first 6-months of the trial participants meet one-on-one with the dietitian weekly for the first 4 weeks, then once every other week for 8 weeks, then once a month for the remaining 3 months. Concurrent with the first two dietitian sessions, participants also complete one-on-one instruction with the exercise physiologist. Thereafter, participants are invited to complete weekly supervised exercise sessions (overseen by the exercise physiologist, but no one-on-one instruction is provided). Nutrition sessions last between 30 and 60 minutes and one-on-one exercise instruction sessions lasts 60 minutes, both of which follow a prespecified curriculum (Table 1). At the beginning of each session, participants are weighed, and their progress reviewed by the dietitian and exercise physiologist. During each session the PALS intervention materials are reviewed, and individualized information is provided to help participants achieve study goals and stay motivated. Participants are asked to record their daily dietary intake and physical activity daily using a “Keeping Track™” booklet.
Table 1.
PALS Lifestyle Intervention Curriculum
| Week | Topic | Objectives |
|---|---|---|
| 0 | Introduction to PALS |
|
| 1 | ||
| Move Those Muscles |
|
|
| 2 | ||
| Jumpstart and Strengthen your Physical Activity |
|
|
| 3 | Healthy Eating |
|
| 4 | Four Keys to Healthy Eating Out |
|
| 6 | Tip the Balance |
|
| 8 | Take Charge of What’s Around You |
|
| 10 | Problem Solving |
|
| 13 | The Slippery Slope of Lifestyle |
|
| 16 | Make Social Cues Work for You |
|
| 20 | You Can Manage Stress |
|
| 24 | Ways to Stay Motivated |
|
To support their progress in the intervention, participants receive a PALS binder containing the diet and exercise curriculum. Men are provided a ‘punch card’ to promote attendance of weekly supervised exercise sessions (months 1 – 6) at the Fred Hutchinson Cancer Research Center Prevention Clinic’s exercise facility. Participants also receive several tools to assist in achieving the PALS weight loss and physical activity goals, including a heart rate monitor (Polar Ft1®), Calorie King® book to tally the calorie and fat content of foods they eat, and measuring cups and spoons. In addition to the standardized curriculum, a “toolbox” approach is used to tailor the intervention to optimize behavior change for individual participants.(32) Examples of some toolbox topics include exercising in inclement weather, wearing appropriate footwear, healthy snack choices, making healthier food choices, and choosing healthier fats.
General healthy lifestyle (control)
Men assigned to the control arm receive general instruction on a healthy lifestyle during the baseline visit. This instruction reviews the United States Dietary and Physical Activity Guidelines for Americans(33, 34) and includes discussion of the health benefits of weight loss. At the end of their participation in the 12-month study, men assigned to the control arm will receive the PALS intervention materials as a notebook but they will not receive a full delayed intervention with specific, individual instructional sessions.
Data Collection
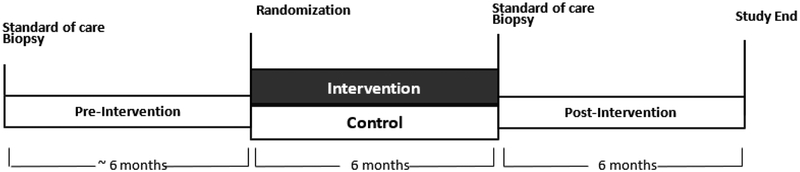
Both intervention and control participants complete 4 clinic visits over the 12- month study, which are scheduled at baseline, 3, 6 and 12 months. Participation in the study is coordinated so that the 6-month PALS clinic visit coincides with the participants’ planned AS prostate biopsy (Figure 1). Pre-intervention biopsy tissue will be acquired from stored pathologic specimens from the most recent pre-study biopsy. Data and specimens collected at all clinic visits include anthropometric measures, fasting blood, and self-administered questionnaires on quality of life (Table 2). A 3-day food record and sub-maximal exercise tests are completed at baseline and 6 months, and a dual X- ray absorptiometry scan (DXA) is completed at baseline and 12 months. Participants who have abnormal results on the sub-maximal exercise test are required to receive clearance to participate from their physician. All intervention instruction and clinic visits are conducted in the Prevention Center at the Fred Hutchinson Cancer Research Center in Seattle, WA.
Figure 1.
PALS study Schema
Table 2.
Data and specimen collection in PALS
| Pre-study | Baseline | 3 months | 6 months | 12months | |
|---|---|---|---|---|---|
| Fasting Blood Draw | X | X | X | X | |
| Anthropometrics | X | X | X | X | |
| QOL | X | X | X | X | |
| Lifestyle Questionnaire | X | ||||
| 3-day Food Record | X | X | |||
| Physical Activity Questionnaire | X | X | X | ||
| Medication use | X | X | X | ||
| DXA scan | X | X | |||
| Exercise test | X | X | |||
| Standard of care Biopsy | X | X |
Outcomes
The primary outcomes of PALS include changes in glucose regulation from baseline (fasting glucose, C-peptide, insulin, Insulin like Growth Factor (IGF-1), IGF binding protein 3 and adiponectin) and changes in expression of insulin receptor, IGF-1 receptor and phosphorylated-AKT in PCa epithelial cells at 6-months. Selected blood- based biomarkers of glucose regulation have been found to be associated with PCa aggressiveness and outcomes. (19–21, 24, 25, 35)) The tissue-based markers of glucose regulation selected have been shown to be overexpressed in prostate cancer(26), found to be responsive to dietary restriction among animals with PCa(22, 36–39), or are a downstream signaling molecule for the IGF-1R and IR(40). An additional primary outcome is to evaluate whether patients randomized to the intervention arm are able to sustain the beneficial changes in weight loss and glucose regulation 6 months after the active intervention.
Secondary outcomes include several aspects of health-related quality of life (QOL), specifically PCa-related anxiety (measured by the Memorial Anxiety Scale for Prostate Cancer (MAX-PC)(41), urinary and sexual function and bother (measured by the Expanded Prostate Cancer Index (EPIC) short form-26)(42), and general quality of life (measured by the EQ-5D-5L)(43). Additional secondary outcomes include adverse pathology (defined as Gleason upgrading, increase in number of positive cores, greater than 50% positive cores) on follow-up biopsy.
Data Analysis
Adaptive randomization is used to assign participants by baseline age (<65 and ≥65 years) and BMI (<30 vs. ≥ 30 kg/m2) to either the PALS lifestyle intervention or control arm. This design randomizes participants using an algorithm that minimizes treatment imbalances within each baseline age and BMI subgroup.(44) The total expected sample size is 210 men, which includes an anticipated 5% drop out in each arm.
Primary analyses will compare changes in circulating and tissue markers of glucose and insulin regulation between the intervention and control arms. Global assessment of the intervention effects will be evaluated using a two-sided t-test (or Wilcoxon rank sum test if normality of the measurement is questionable). Further analysis will quantify effects of patient age, BMI and other body composition measures on change blood and tissue measures. Differential effects between intervention and control arms will be quantified using interaction terms. Effects of missing data will be investigated using extreme case analysis. All analyses will be based on intention-to-treat principles.
To test whether PCa patients randomized to the intervention arm can sustain the beneficial changes in weight and glucose regulation an additional 6 months after the active intervention, sustained weight loss will be characterized as maintenance of a 7% reduction in baseline weight(30), and sustained glucose regulation as maintenance within 5% of 6-month levels. Because not all participants will achieve 7% weight loss at 6 months, analyses will look in the subset of participants who did and did not achieve this goal both separately and combined. A one-sample test of proportions will be used to determine whether the proportion of participants that are able to sustain lifestyle changes differs from zero.
Secondary analyses will test whether changes in PCa-specific QOL differs between the intervention and control arms. Intervention effects will be evaluated using a two-sided t-test, and models will be adjusted for age, socioeconomic status, marital status, and baseline BMI. An additional secondary analysis will evaluate whether the proportion of participants with adverse pathology (Gleason upgrading, increase in number of positive cores, increase in the number of cores with >50% positive involvement) on follow-up surveillance biopsy differs between intervention and control arms using a two-sample test of proportions.
Allowing for a drop-out rate of 5% and assuming correlations 0.70 between baseline and follow-up measures, the final sample size of 100 men in each arm provides 80% power to detect differences in changes in serum glucose regulation biomarkers between arms ranging from 5.3% for glucose to 33.1% for insulin with a 2-sided alpha of 0.05 (Table 3). Similarly, for changes in tissue markers of glucose regulation, the minimum detectable differences in expression of markers range from 26.5% for AKT to 46.3% for IGF-1 receptor (Table 3).
Table 3.
Minimal detectable differences (MDD) in serum and tissue biomarkers at β=80%, α=0.05 given a sample size of 200 (100 each in intervention and control arms)
| Biomarker | Baseline Mean (SD) or n (%) | MDD | |
|---|---|---|---|
| Mean | % of mean | ||
| Primary Aim 1 - Serum | |||
| Fasting glucose, mg/dL | 106.3 (12.6) | 5.6 | 5.3% |
| Primary Aim 2 - Serum | |||
| C-peptide, ng/mL | 1.7 (1.2) | 0.54 | 31.6% |
| Insulin, pmol/L | 138.0 (102.0) | 45.7 | 33.1% |
| IGF-1, ng/mL | 232.7 (75.4) | 33.8 | 14.5% |
| IGF-BP3, ng/mL | 4317.6 (975.2) | 436.5 | 10.1% |
| Adiponectin, ug/mL | 6.1(3.9) | 1.8 | 29.0% |
| Primary Aim 3 - Tissue | |||
| Insulin receptor | 30 (34) | 13.9 | 29.2% |
| IGF-1 receptor | 112 (80) | 32.7 | 46.3% |
| AKT | 128 (83) | 33.9 | 26.5% |
DISCUSSION
Obesity is a well-established risk factor for the incidence of aggressive PCa and for PCa mortality.(5–12) More recently, obesity has also been identified as an independent risk factor of poor outcomes among men on AS. In two separate cohorts of men with low-risk PCa on AS, obesity was associated with significant increases in risk of pathologic progression.(17, 18) In addition, one of these studies also reported that obese men were also more likely than normal weight men to initiate active treatment for their PCa (HR=1.8; 95%CI, 1.0–1.9; p=0.08).(17) Furthermore, patient BMI is used an independent clinical variable in calculators designed to estimate risk of progression on subsequent biopsy among men using AS to manage PCa (https://canarypass.org/pass-risk-calculator, manuscript in preparation). Despite this observational evidence, clinical trial data are lacking as to whether weight loss after diagnosis will improve outcome.
Lifestyle interventions in patients who choose AS is an emerging area of research with potential to yield insights on biological pathways underlying disease progression, though few studies have been conducted to date. The Prostate Cancer Lifestyle Trial (PCLT), a 1-year trial evaluating an intensive lifestyle intervention (vegan diet supplemented with soy, fish oil, vitamin E, selenium and vitamin C, 3 hours weekly moderate exercise, and 1 hour daily stress management) among 90 men with low-risk PCa on AS, reported LNCaP cell growth declined an average of 70% in the lifestyle intervention arm, compared to a 9% decline in the control arm (p<0.001).(45) In a study of 30 men undergoing a similar 3-month intensive lifestyle intervention, which achieved a mean 2.6 unit decline in BMI, changes in gene expression from pre- and post- intervention prostate biopsies included down-regulation of IGF-1R genes.(46) In addition, the Men’s Eating and Living (MEAL) study, a 6-month randomized trial, evaluated a behavior telephone-based intervention to increased vegetable (particularly cruciferous and tomato) intake, compared to control, among nearly 500 men on AS.(47) Preliminary analyses from this study have reported significant increases in the daily number of servings of vegetables consumed and in plasma carotenoid levels concentrations, although no significant differences in the rate of PCa progression between intervention and control groups.(48) No trials to date have specifically targeted weight loss and underlying glucose regulation mechanisms through diet and exercise among men on AS. Evaluating the effects of a lifestyle intervention among PCa survivors on AS also provides a novel approach to studying the biological pathways by which obesity may delay or prevent disease progression. Men with PCa on AS have untreated cancer and undergo routine surveillance biopsies, which allows the use of repeat biopsies to investigate the intervention’s effects on tumor tissue and cancer biomarkers.
Men with PCa on AS represent an important group in which to test a lifestyle intervention. The growing concern of over-diagnosis and over-treatment of more indolent PCa(49) has led to a substantial increase in men choosing AS. However, patient acceptance of AS for primary treatment remains low and approximately 50% of men on AS will experience disease progression requiring treatment.(3) Of concern for patients is the anxiety about doing “nothing” for their PCa. The PALS intervention has the potential to provide both patients and providers with an active yet non-invasive therapy to improve PCa patient outcomes.
The use of the DPP as the basis of the PALS intervention has several strengths. First, the DPP has a proven track record of weight loss and improved glucose regulation among a pre-diabetic population(29), and long-term follow-up has demonstrated sustained weight loss at 10 years(30). This is an important consideration as other studies of dietary interventions in men with PCa have used diets that are difficult to maintain over the long-term (e.g. vegan(45), low-glycemic(50) and prepared meals(51). Although the DPP has not previously been studied in cancer survivors, there are data to suggest that older men may be an ideal population for the DPP lifestyle intervention. (52) In the primary trial, older participants (≥60 yrs) compared to younger groups (25–44 yrs and 45–59 yrs) had the greatest weight loss (mean −6.4 kg vs. −4.1 and −5.0 kg respectively, p<0.001) and more commonly met the exercise goal (48% vs. 34% and 38% respectively, p<0.001).(52) In addition, compared to women, men lost more weight (p<0.01) and performed higher levels of physical activity (p<0.05) in the lifestyle intervention.(53) Lastly, if successful, expanding the delivery of the PALS program to PCa patients within the general community would be extremely feasibly as many community-based organizations, including hospitals, medical centers and YMCA’s across the nation already deliver the DPP lifestyle intervention program.
Conclusion
With the growing recognition that many men with lower-risk PCa features do not require definitive treatment and can instead be monitored through AS protocols, it becomes imperative to identify modifiable risk factors for reducing the rate of disease progression in these men. A lifestyle modification that could delay or prevent disease progression would represent a significant savings of health care expenditures from treatments such as surgery and radiation, as well as lowering morbidity and mortality in the patients requiring further treatment. Findings from this study may have wide reaching implications in improving both the overall and disease-specific outcomes in men diagnosed with clinically-localized PCa, and provide an alternative approach of an active lifestyle for AS.
Acknowledgements
We would like to thank the clinical site coordinators Branda Levchak and Alexandria Lahydia as well as urologists Atreya Dash, Bill Ellis, George Schade, Bruce Dalkin, Dan Simon, Franklin Lee, Scott Van Appledorn, Jim Downey and Timothy Roddy for helping recruit patients to participate in the PALS study.
Funding Support: R01 CA184075–01, R50 CA221836, P30 CA015704
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Ganz PA, Barry JM, Burke W, Col NF, Corso PS, Dodson E, Hammond ME, Kogan BA, Lynch CF, Newcomer L, Seifter EJ, Tooze JA, Viswanath K, Wessells H. National Institutes of Health State-of-the- Science Conference: role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med. 2012;156(8):591–5. doi: 10.7326/0003-4819-156-8-201204170-00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomsen FB, Brasso K, Klotz LH, Roder MA, Berg KD, Iversen P. Active surveillance for clinically localized prostate cancer--a systematic review. J Surg Oncol. 2014;109(8):830–5. doi: 10.1002/jso.23584. [DOI] [PubMed] [Google Scholar]
- 4.Patlak M, Nass SJ. The role of obesity in cancer survival and recurrence: Workshop summary: National Academies Press; 2012. [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109(4):675–84. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 7.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109(6):1192–202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 8.Andersson SO, Wolk A, Bergstrom R, Adami HO, Engholm G, Englund A, Nyren O. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89(5):385–9. [DOI] [PubMed] [Google Scholar]
- 9.Strom SS, Wang X, Pettaway CA, Logothetis CJ, Yamamura Y, Do KA, Babaian RJ, Troncoso P. Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clin Cancer Res. 2005;11(19 Pt 1):6889–94. doi: 10.1158/1078-0432.CCR-04-1977. [DOI] [PubMed] [Google Scholar]
- 10.Kane CJ, Im R, Amling CL, Presti JC Jr., Aronson WJ, Terris MK, Freedland SJ, Group SDS. Outcomes after radical prostatectomy among men who are candidates for active surveillance: results from the SEARCH database. Urology. 2010;76(3):695–700. doi: 10.1016/j.urology.2009.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayachandran J, Banez LL, Aronson WJ, Terris MK, Presti JC Jr., Amling CL, Kane CJ, Freedland SJ, Group SDS. Obesity as a predictor of adverse outcome across black and white race: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2009;115(22):5263–71. doi: 10.1002/cncr.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banez LL, Sun L, Trock BJ, Han M, Partin AW, Aronson WJ, Terris MK, Presti JC Jr., Kane CJ, Amling CL, Moul JW, Freedland SJ. Body mass index and prostate specific antigen as predictors of adverse pathology and biochemical recurrence after prostatectomy. J Urol. 2009;182(2):491–6; discussion 6–8. doi: 10.1016/j.juro.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800–9. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ploussard G, de la Taille A, Bayoud Y, Durand X, Terry S, Xylinas E, Allory Y, Vacherot F, Abbou CC, Salomon L. The risk of upstaged disease increases with body mass index in low-risk prostate cancer patients eligible for active surveillance. Eur Urol. 2012;61(2):356–62. doi: 10.1016/j.eururo.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 15.Truong M, Slezak JA, Lin CP, Iremashvili V, Sado M, Razmaria AA, Leverson G, Soloway MS, Eggener SE, Abel EJ, Downs TM, Jarrard DF. Development and multi-institutional validation of an upgrading risk tool for Gleason 6 prostate cancer. Cancer. 2013;119(22):3992–4002. doi: 10.1002/cncr.28303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Cobelli O, Terracciano D, Tagliabue E, Raimondi S, Galasso G, Cioffi A, Cordima G, Musi G, Damiano R, Cantiello F, Detti S, Victor Matei D, Bottero D, Renne G, Ferro M. Body mass index was associated with upstaging and upgrading in patients with low-risk prostate cancer who met the inclusion criteria for active surveillance. Urol Oncol. 2015;33(5):201 e1–8. doi: 10.1016/j.urolonc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Bhindi B, Kulkarni GS, Finelli A, Alibhai SM, Hamilton RJ, Toi A, van der Kwast TH, Evans A, Hersey K, Jewett MA, Zlotta AR, Trachtenberg J, Fleshner NE. Obesity is associated with risk of progression for low-risk prostate cancers managed expectantly. Eur Urol. 2014;66(5):841–8. doi: 10.1016/j.eururo.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Macleod LC, Ellis WJ, Newcomb LF, Zheng Y, Brooks JD, Carroll PR, Gleave ME, Lance RS, Nelson PS, Thompson IM Jr., Wagner AA, Wei JT, Lin DW. Timing of Adverse Prostate Cancer Reclassification on First Surveillance Biopsy: Results from the Canary Prostate Cancer Active Surveillance Study. J Urol. 2017;197(4):1026–33. doi: 10.1016/j.juro.2016.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammarsten J, Hogstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005;41(18):2887–95. doi: 10.1016/j.ejca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, Gaziano JM, Pollak M, Stampfer MJ. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9(11):1039–47. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuhouser ML, Till C, Kristal A, Goodman P, Hoque A, Platz EA, Hsing AW, Albanes D, Parnes HL, Pollak M. Finasteride modifies the relation between serum C-peptide and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer Prev Res (Phila). 2010;3(3):279–89. doi: 10.1158/1940-6207.CAPR-09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkateswaran V, Haddad AQ, Fleshner NE, Fan R, Sugar LM, Nam R, Klotz LH, Pollak M. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99(23):1793–800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 23.Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ, Cohen P, Hwang D, Peterson B, Fields T, Pizzo SV, Isaacs WB. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68(1):11–9. doi: 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright JL, Plymate SR, Porter MP, Gore JL, Lin DW, Hu E, Zeliadt SB. Hyperglycemia and prostate cancer recurrence in men treated for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013;16(2):204–8. doi: 10.1038/pcan.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HS, Presti JC Jr., Aronson WJ, Terris MK, Kane CJ, Amling CL, Freedland SJ. Glycemic control and prostate cancer progression: results from the SEARCH database. Prostate. 2010;70(14):1540–6. doi: 10.1002/pros.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox ME, Gleave ME, Zakikhani M, Bell RH, Piura E, Vickers E, Cunningham M, Larsson O, Fazli L, Pollak M. Insulin receptor expression by human prostate cancers. Prostate. 2009;69(1):33–40. doi: 10.1002/pros.20852. [DOI] [PubMed] [Google Scholar]
- 27.Winters B, Plymate S, Zeliadt SB, Holt S, Zhang X, Hu E, Lin DW, Morrissey C, Wooldridge B, Gore JL, Porter MP, Wright JL. Metformin effects on biochemical recurrence and metabolic signaling in the prostate. Prostate. 2015;75(15):1694–703. doi: 10.1002/pros.23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Association AD. The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes care. 1999;22(4):623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diabetes Prevention Program Research G, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond). 2005;29(10):1153–67. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- 32.Group DPPR. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes care. 2002;25(12):2165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Health UDo, Services H. Dietary guidelines for Americans 2015–2020: Skyhorse Publishing Inc.; 2017. [Google Scholar]
- 34.Alliance NPAP. U.S. National Physical Activity Plan. Retrieved from: http://physicalactivityplanorg/docs/2016NPAP_Finalforwebsitepdf. 2016.
- 35.Hong SK, Lee ST, Kim SS, Min KE, Byun SS, Cho SY, Choe G, Lee SE. Significance of preoperative HbA1c level in patients with diabetes mellitus and clinically localized prostate cancer. Prostate. 2009;69(8):820–6. doi: 10.1002/pros.20932. [DOI] [PubMed] [Google Scholar]
- 36.Narita S, Tsuchiya N, Saito M, Inoue T, Kumazawa T, Yuasa T, Nakamura A, Habuchi T. Candidate genes involved in enhanced growth of human prostate cancer under high fat feeding identified by microarray analysis. Prostate. 2008;68(3):321–35. doi: 10.1002/pros.20681. [DOI] [PubMed] [Google Scholar]
- 37.Powolny AA, Wang S, Carlton PS, Hoot DR, Clinton SK. Interrelationships between dietary restriction, the IGF-I axis, and expression of vascular endothelial growth factor by prostate adenocarcinoma in rats. Mol Carcinog. 2008;47(6):458–65. doi: 10.1002/mc.20403. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi N, Barnard RJ, Said J, Hong-Gonzalez J, Corman DM, Ku M, Doan NB, Gui D, Elashoff D, Cohen P, Aronson WJ. Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-Myc transgenic mouse model. Cancer Res. 2008;68(8):3066–73. doi: 10.1158/0008-5472.CAN-07-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mavropoulos JC, Buschemeyer WC 3rd, Tewari AK, Rokhfeld D, Pollak M, Zhao Y, Febbo PG, Cohen P, Hwang D, Devi G, Demark-Wahnefried W, Westman EC, Peterson BL, Pizzo SV, Freedland SJ. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res (Phila). 2009;2(6):557–65. doi: 10.1158/1940-6207.CAPR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 41.Roth A, Nelson CJ, Rosenfeld B, Warshowski A, O’Shea N, Scher H, Holland JC, Slovin S, Curley- Smart T, Reynolds T, Breitbart W. Assessing anxiety in men with prostate cancer: further data on the reliability and validity of the Memorial Anxiety Scale for Prostate Cancer (MAX-PC). Psychosomatics. 2006;47(4):340–7. doi: 10.1176/appi.psy.47.4.340. [DOI] [PubMed] [Google Scholar]
- 42.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. [DOI] [PubMed] [Google Scholar]
- 43.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frane JW. A Method of Biased Coin Randomization, Its Implementation, and Its Validation. Drug Information Journal. 1998;32:423–32. [Google Scholar]
- 45.Ornish D, Weidner G, Fair WR, Marlin R, Pettengill EB, Raisin CJ, Dunn-Emke S, Crutchfield L, Jacobs FN, Barnard RJ, Aronson WJ, McCormac P, McKnight DJ, Fein JD, Dnistrian AM, Weinstein J, Ngo TH, Mendell NR, Carroll PR. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174(3):1065–9; discussion 9–70. doi: 10.1097/01.ju.0000169487.49018.73. [DOI] [PubMed] [Google Scholar]
- 46.Ornish D, Magbanua MJ, Weidner G, Weinberg V, Kemp C, Green C, Mattie MD, Marlin R, Simko J, Shinohara K, Haqq CM, Carroll PR. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci U S A. 2008;105(24):8369–74. doi: 10.1073/pnas.0803080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parsons JK, Pierce JP, Mohler J, Paskett E, Jung SH, Morris MJ, Small E, Hahn O, Humphrey P, Taylor J. Men’s Eating and Living (MEAL) study (CALGB 70807 [Alliance]): recruitment feasibility and baseline demographics of a randomized trial of diet in men on active surveillance for prostate cancer. BJU international. 2018;121(4):534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parsons JK, Zarieh D, Pierce JP, Mohler J, Paskett E, Hansel D, AKibel A, Hahn O, J T, Grubb R, S S, E S, Van Veldhuizen P, Morris MJ, Marshall J. The Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]): A Randomized Clinical Trial of a Diet Intervention in Men on Active Surveillance for Prostate Cancer. The Journal of Urology. 2018;199(4S). [Google Scholar]
- 49.Dall’Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, Freedland SJ, Klotz LH, Parker C, Soloway MS. Active surveillance for prostate cancer: a systematic review of the literature. European urology. 2012;62(6):976–83. [DOI] [PubMed] [Google Scholar]
- 50.Lin DW, Neuhouser ML, Schenk JM, Coleman IM, Hawley S, Gifford D, Hung H, Knudsen BS, Nelson PS, Kristal AR. Low-fat, low-glycemic load diet and gene expression in human prostate epithelium: a feasibility study of using cDNA microarrays to assess the response to dietary intervention in target tissues. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2150–4. doi: 10.1158/1055-9965.EPI-07-0154. [DOI] [PubMed] [Google Scholar]
- 51.Aronson WJ, Kobayashi N, Barnard RJ, Henning S, Huang M, Jardack PM, Liu B, Gray A, Wan J, Konijeti R, Freedland SJ, Castor B, Heber D, Elashoff D, Said J, Cohen P, Galet C. Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer Prev Res (Phila). 2011;4(12):2062–71. doi: 10.1158/1940-6207.CAPR-11-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diabetes Prevention Program Research G, Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett- Connor E, Fowler S, Dagogo-Jack S, Andres R. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61(10):1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perreault L, Ma Y, Dagogo-Jack S, Horton E, Marrero D, Crandall J, Barrett-Connor E, Diabetes Prevention P. Sex differences in diabetes risk and the effect of intensive lifestyle modification in the Diabetes Prevention Program. Diabetes Care. 2008;31(7):1416–21. doi: 10.2337/dc07-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]