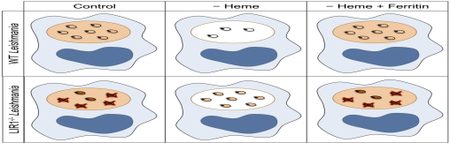
Abstract
The Leishmania plasma membrane transporter Leishmania Iron Regulator 1 (LIR1) facilitates iron export and is required for parasite virulence. By modulating macrophage iron content, we investigated the host site where LIR1 regulates Leishmania amazonensis infectivity. In bone marrow-derived macrophages, LIR1 null mutants demonstrated a paradoxical increase in virulence during infections in heme-depleted media, while wild-type growth was inhibited under the same conditions. Loading the endocytic pathway of macrophages with cationized ferritin prior to infection reversed the effect of heme depletion on both strains. Thus, LIR1 contributes to Leishmania virulence by protecting the parasites from toxicity resulting from iron accumulation inside parasitophorous vacuoles.
Keywords: Heme, Cationic ferritin, Amastigotes, Endocytic pathway, Virulence, Drug target
Graphical Abstract
The protozoan genus Leishmania, the causative agent of the neglected tropical disease leishmaniasis, results in significant death, disability and disfigurement (Desjeux, 2004; Alvar et al., 2012). Therapy remains limited due to the toxicity of drugs, poor implementation and drug resistance (Croft et al., 2006). After transmission to a new mammalian host by a sand fly vector, intracellular replication within macrophages is critical for leishmaniasis pathogenesis (Gossage et al., 2003). This process requires the acquisition of iron and heme from the host, as Leishmania parasites do not have cytosolic iron storage proteins and lack the capacity for heme biosynthesis (Flannery et al., 2013; Kořený et al., 2013; Soares and Hamza, 2016). Furthermore, free iron can be cytotoxic due to unregulated electron exchange and free radical generation ( Kaplan, 2002; Dixon and Stockwell, 2014). Thus, Leishmania must acquire heme and iron for survival, but also must tightly regulate intracellular free iron levels to prevent cellular damage. Macrophages, the principal parasite target cells, contain high levels of iron and heme from recycling senescent red blood cells (Soares and Hamza, 2016). This intracellular environment, rich in iron, further implicates the need for carefully regulated iron metabolism in these parasites. While these parasites must survive in an environment where dynamic iron levels introduce the potential for iron toxicity, Leishmania must also compete with macrophage iron export by ferroportin in order to attain adequate iron for growth (Schaible and Kaufmann, 2004). Previous research has demonstrated that parasites are able to downregulate macrophage ferroportin via increased transcription of hepcidin, a hormone that induces degradation of ferroportin and increases the iron available to parasites over the course of infection (Ben-Othman et al., 2014). In parallel, several proteins involved in iron acquisition and metabolism have been characterized in Leishmania, including Leishmania Iron Transporter 1 (LIT1), Leishmania Heme Response 1 (LHR1), and Leishmania Ferric Reductase 1 (LFR1) (Huynh et al., 2006, 2012; Jacques et al., 2010; Flannery et al., 2011). These proteins are crucial for parasite growth and replication in addition to others, including LMIT1, a mitochondrial iron importer homologous to human mitoferrin-1 (Mittra et al., 2016). Furthermore, superoxide dismutases (SODs), specifically SODA, an iron-dependent SOD found in Leishmania amazonensis, plays a crucial role in both managing oxidative stress in parasites and promoting their differentiation to the infective amastigote form (Mittra et al., 2017). These findings highlight the importance of iron acquisition for Leishmania and the crucial role of iron in promoting parasite differentiation and replication. Our laboratory recently identified and characterized a novel iron-responsive Major Facilitator Superfamily (MFS) protein, Leishmania Iron Regulator 1 (LIR1), a 661 amino acid protein with 14 predicted transmembrane domains, targeted to the parasite plasma membrane. Interestingly, LIR1 showed similarity with nodulin-like proteins that were previously shown to be involved in transmembrane transport of iron in plants (Gollhofer et al., 2011; Denancé et al., 2014). In the promastigote stage, LIR1 is crucial for normal parasite growth; promastigotes deficient in LIR1 display growth delay in response to iron and other transition metals. LIR1 deficiency does not affect iron import, but significantly increases iron retention, implicating LIR1 as the first iron exporter identified in trypanosomatids. Furthermore, LIR1 deficiency significantly decreases replication of parasites in their amastigote stage (Laranjeira-Silva et al., 2018). However, the mechanisms by which LIR1 contributes to virulence in the amastigote stage remain unexplored. As intracellular growth of Leishmania amastigotes is the principal pathogenic form, defining the mechanisms by which LIR1 contributes to this key phase of parasite growth is important. In this report we modulate iron and heme availability in macrophages to define the role of LIR1 in intracellular replication of L. amazonensis. Our findings support the hypothesis that the LIR1 promotes virulence inside the endocytic pathway of host macrophages by optimizing intracellular iron levels to promote growth while controlling toxicity.
Leishmania amazonensis promastigote parasites were cultured at 26°C in medium 199 (M199) (Gibco, USA) at pH 7.2 supplemented with 40 mM HEPES (Gibco), 0.1 mM adenine (Acros Organics, USA), 5 mM L-Glutamine (Gemini Bio-Products, USA), 5% penicillin-streptomycin (Gemini Bio-Products), 0.0001% biotin (J.T. Baker, USA), 0.0005% hemin (25 mg/mL in triethanolamine) (Tokyo Chemical Industry Co. America, USA), and 10% heat-inactivated fetal bovine serum (FBS) (Gemini Bio-Products). Promastigote cultures were passaged every 3-4 days to maintain a mid-log phase growth, and cultures used for in vitro macrophage infections were no older than 15 passages. LIR1-deficient L. amazonensis promastigotes, generated by replacing both LIR1 open reading frames with antibiotic resistance genes via homologous recombination as previously reported, were maintained by the addition of 20 μg/mL of Neomycin (Gibco) and 20 μg/mL of Blasticidin (Gibco) to M199 culture medium (Laranjeira-Silva et al., 2018). In vitro macrophage infections involved the harvest and differentiation of C57BL/6 mouse bone marrow-derived macrophages (BMMs) and infection with purified L. amazonensis metacyclic promastigotes, followed by microscopic quantification of intracellular parasites, as previously described (Sarkar et al., 2018). All infections were conducted at a multiplicity of infection (MOI) of three parasites/macrophage, and infection samples were fixed for quantification at 3, 24, and 48 h following infection. To modulate macrophage iron content, BMM medium was supplemented with heme-depleted FBS instead of regular FBS. Hemedepleted FBS was prepared by treating FBS with 10 mM ascorbic acid for 16 h at room temperature. Heme depletion was verified via optical absorbance at 405 nm, and the heme-depleted FBS was dialyzed three times in cold PBS (Thermo-Fisher, USA), followed by filter sterilization (Zhu et al., 2002). Macrophages for heme-depleted infection samples were plated in BMM medium prepared with heme-depleted FBS 24 h prior to infection. Following 3 h of infection, wells were washed and heme-depleted medium was added. Heme depletion experiments were carried out with macrophages in both heme-depleted medium and heme-depleted medium supplemented with 50 μM desferoxamine (DFO) (Sigma-Aldrich, USA), an iron chelator, to sequester any free iron present in the medium. To supplement macrophages with cationized ferritin, BMMs were plated in heme-depleted medium 24 h prior to infection and 1 h before infection, heme-depleted BMM medium was replaced with the same medium supplemented with 10 μg/mL of cationized ferritin (Sigma-Aldrich). Immediately prior to infection, cells were washed with PBS and heme-depleted BMM medium was added. Following 3 h of infection, cells were washed with PBS and replaced with heme-depleted BMM medium supplemented with 10 μg/mL of cationized ferritin for the remainder of the experiment duration. Images of in vitro macrophage infections were produced using a Leica SPX5 laser-scanning confocal microscope using a 63x N.A. 1.4 oil objective; the excitation wavelength utilized for DAPI was 405 nm. Macrophage viability assays were conducted using alamarBlue® Cell Viability Reagent (Thermo-Fisher). The reagent was added at 10% of the culture volume for each sample. Fluorescence readings (excitation: 544 nm, emission: 590 nm) were collected from 100 μL of culture medium from each sample at 1, 6, and 24 h after addition of the reagent. Fluorescence readings were subtracted by readings taken of corresponding culture media with 10% alamarBlue® reagent and any additional treatments (heme-depletion, addition of DFO).
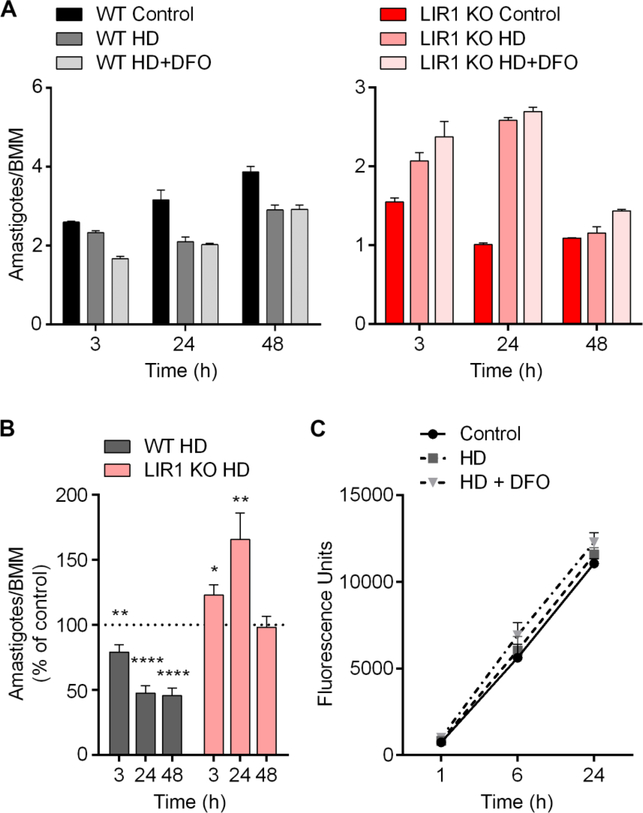
In vitro macrophage infections with wild-type and LIR1 knockout parasites (LIR1 KO) in control medium, heme-depleted medium (HD), and heme-depleted medium supplemented with 50 μM desferoxamine (HD+DFO) reveal that LIR1 KO parasites display an opposite response to the lack of iron compared with wild-type L. amazonensis. Under conditions of heme depletion and heme depletion plus DFO, wild-type parasites exhibited a significant reduction in intracellular replication over the course of 48 h, quantified as the ratio of amastigotes per macrophage (Fig. 1A and B). This reduction is likely due to the crucial role of iron in parasite replication and survival. In contrast, LIR1 KO parasites, which displayed an impaired infectivity under regular conditions, showed an increase in intracellular replication under conditions of heme depletion, which was further supported by the addition of DFO. This increase in virulence appeared to be a transient response, peaking at 24 h of infection (Fig. 1A and B). This recovery supports a role of LIR1 protecting parasites from iron toxicity during the beginning of the infection, as removing heme and iron had a protective effect on LIR1-deficient parasites. The transient nature of this recovery may be due to the parasites’ requirement for iron to support ongoing cell replication and could also indicate additional roles of LIR1, as in the transport of other metals as indicated in previously described results (Laranjeira-Silva et al., 2018). Viability assays performed on macrophages in control medium, heme-depleted medium, and hemedepleted medium plus DFO indicated no significant differences in cell viability based on culture treatments (Fig. 1C), indicating that the observed intracellular parasite load results were not the consequence of changes in macrophage viability.
Fig. 1.
Heme depletion impairs wild-type Leishmania amazonensis infectivity while improving infectivity of Leishmania Iron Regulator 1 (LIR1)-deficient parasites. (A) Microscopic quantification of intracellular parasite load in bone marrow-derived macrophages (BMM) infected with wild-type (WT) and LIR1 double knockout (KO) purified metacyclic L. amazonensis in control medium, heme-depleted medium (HD), and heme depleted medium supplemented with 50 μM desferoxamine (HD+DFO) for 3, 24, and 48 h (multiplicity of infection = 3). Data points represent the mean ± S.E.M. of triplicate determinations in one experiment that is representative of three independent experiments. (B) Pooled data of seven independent experiments showing the intracellular parasite load of WT and KO L. amazonensis in HD medium normalized by the parasite load in control medium at each time point (3, 24, and 48 h). Data points represent the mean ± S.E.M. of triplicate determinations of seven independent experiments. * P = 0.01 (LIR1 KO HD versus control, 3 h); ** P = 0.04 (WT HD versus control, 3 h), P = 0.007 (KO HD versus control, 24 h); **** P < 0.0001 (WT HD versus control, 24, 48 h). (C) AlamarBlue fluorescence viability assay of 106 BMM in control, HD, and HD + DFO media at 1, 6, and 24 h (fluorescence values standardized to readings of sterile culture medium with corresponding treatments). Data points represent the mean ± S.E.M. of three independent experiments.
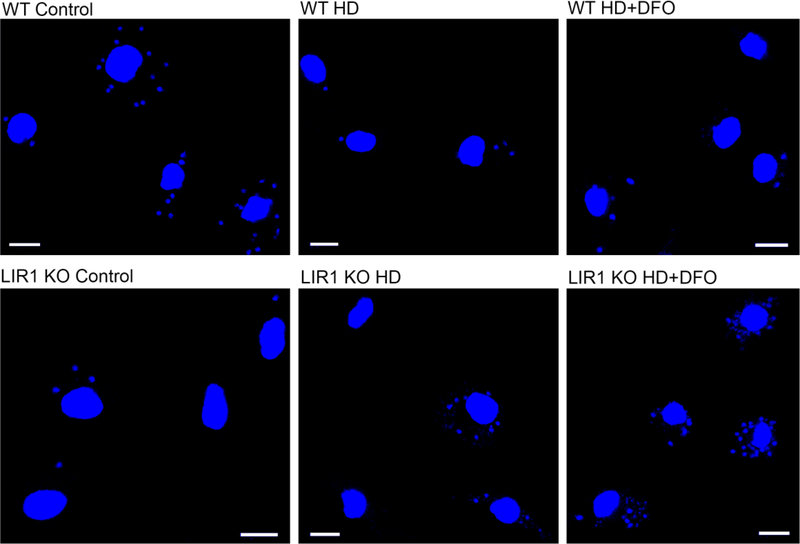
The differences between wild-type and LIR1-deficient parasite virulence under various media conditions (regular, heme-depleted (HD), and HD+DFO) were visibly apparent by altered numbers of DAPI-stained parasite nuclei around each larger macrophage nucleus. Heme depletion and the addition of DFO caused a reduction in the ratio of wild-type parasites to macrophage nuclei, while these treatments caused an increase in this ratio for LIR1-deficient parasites (Fig. 2). Nevertheless, these results do not exclude a possibly different activation of the macrophages by the LIR1-deficient parasites, which could interplay with the ability of these parasites to regulate their iron pool.
Fig. 2.
Changes in wild-type and Leishmania Iron Regulator 1 (LIR1)-deficient parasite infectivity in response to heme depletion and addition of the iron chelator desferoxamine (DFO). Representative images of in vitro macrophage infections at 24 h with wild-type (WT) and LIR1 double knockout (KO) Leishmania amazonensis in regular medium, hemedepleted (HD) medium, and heme-depleted medium + 50 μM DFO (HD+DFO). Blue, DAPI DNA staining. Scale bar = 10 μm.
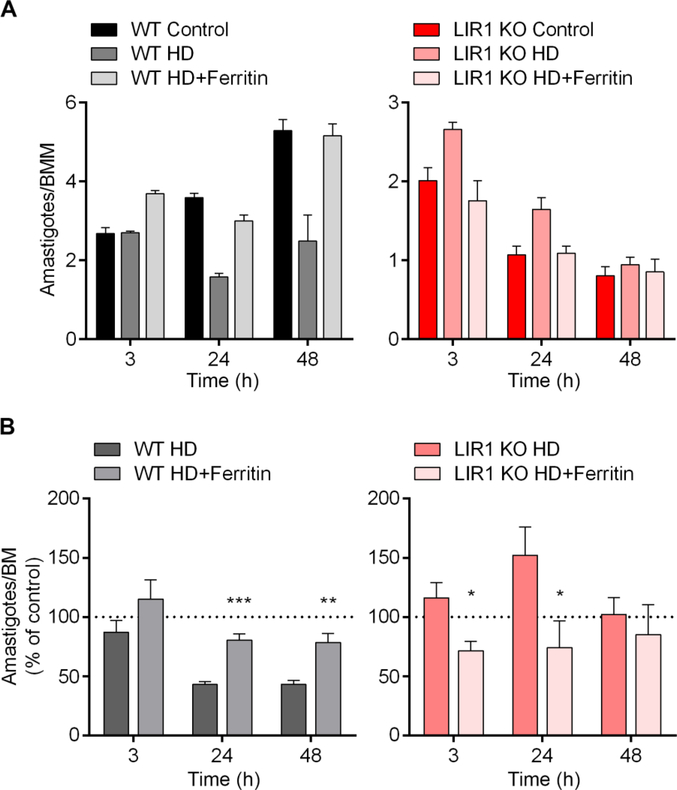
To confirm that these phenotypes were a direct response to iron deficiency, we performed in vitro infections after treating BMM macrophages with cationized ferritin for 1 h followed by infections in heme-depleted media. In this manner, we allowed targeting of iron specifically to the macrophage endocytic pathway, which is known to merge with the L. amazonensis parasitophorous vacuole (Rabinovitch et al., 1985). This iron supplementation significantly reversed the effect of heme depletion on the number of amastigotes per macrophage for both wild-type and LIR1 KO parasites. Wild-type parasites, which displayed a decrease in intracellular replication under heme depletion, were restored to parasite numbers observed in control medium with ferritin supplementation at 24 and 48 h (Fig. 3A and B). In contrast, LIR1 KO parasites, which displayed increased intracellular replication under heme depletion, demonstrated a significant decrease in replication at 3 and 24 h, also restoring the parasite numbers observed in control medium (Fig. 3A and B). These results indicate that ferritin supplementation into the endocytic pathway of macrophages provides wild-type parasites with the iron needed to support intracellular replication under heme-depleted conditions, but is also enough to induce iron toxicity in LIR1 KO parasites even under heme-depleted conditions, causing intracellular parasite death. Altogether, we have provided further evidence for the important role of LIR1 in iron metabolism and regulation during macrophage infections, as the absence of LIR1 had opposite effects in comparison to wildtype on intracellular parasite replication in response to different levels of iron content. Importantly, we directly demonstrated that the early parasitophorous vacuole, where Leishmania parasites reside and replicate after invasion, is the host cell site where LIR1 exerts its important iron regulatory role. These findings highlight iron homeostasis pathways in Leishmania as potential therapeutic targets to control infectivity and disease.
Fig. 3.
Supplementation with cationized ferritin rescues the contrasting heme depletion infectivity phenotypes of wild-type (WT) and Leishmania Iron Regulator 1 (LIR1)-deficient Leishmania amazonensis. (A) Bone marrow-derived macrophages (BMM) were plated for in vitro infection in control medium, heme-depleted medium (HD), or heme-depleted medium supplemented with 10 μg/mL of cationized ferritin for 1 h prior to infection (HD+Ferritin). Infections with WT and LIR1 double knockout (KO) purified metacyclic L. amazonensis under these media conditions were quantified microscopically at 3, 24, and 48 h of infection (multiplicity of infection = 3). Data points represent the mean ± S.E.M. of triplicate determinations in one experiment that is representative of four independent experiments. (B) Pooled data of four independent experiments showing intracellular parasite load of WT and KO L. amazonensis in HD and HD+Ferritin normalized by the parasite load in control media at each time point (3, 24, and 48 h). Data points represent the mean ± S.E.M. of triplicate determinations of four independent experiments. * P = 0.03 (LIR1 KO HD versus LIR1 KO HD+Ferritin, 3 h), P = 0.05 (LIR1 KO HD versus LIR1 KO HD+Ferritin, 24 h); ** P = 0.006 (WT HD versus WT HD+Ferritin, 48 h); *** P = 0.0006 (WT HD versus WT HD+Ferritin, 24 h).
Highlights.
Macrophage heme depletion (HD) impairs Leishmania intracellular replication
Ferritin supplementation of the macrophage endocytic pathway reverts the HD phenotype
The infection phenotype of LIR1−/− mutants is the opposite of wild type under iron modulation
Without LIR1, Leishmania cannot avoid intracellular iron toxicity
The early parasitophorous vacuole is the host cell site where LIR1 exerts its iron regulatory role
Acknowledgements
Our research was supported by the National Institutes of Health, USA (https://www.nih.gov/) grant RO1 AI067979 to NWA. We thank Jason Hauzel for providing excellent technical support and Dr. Fernando Y Maeda for kindly assisting with confocal microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team, 2012. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS One 7, e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Othman R, Flannery AR, Miguel DC, Ward DM, Kaplan J, Andrews NW, 2014. Leishmania-Mediated Inhibition of Iron Export Promotes Parasite Replication in Macrophages. PLoS Pathog. 10, e1003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH, 2006. Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev 19, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denancé N, Szurek B, Noël LD, 2014. Emerging Functions of Nodulin-Like Proteins in Non-Nodulating Plant Species. Plant Cell Physiol. 55, 469–474. [DOI] [PubMed] [Google Scholar]
- Desjeux P, 2004. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis 27, 305–318. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Stockwell BR, 2014. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol 10, 9–17. [DOI] [PubMed] [Google Scholar]
- Flannery AR, Huynh C, Mittra B, Mortara RA, Andrews NW, 2011. LFR1 ferric iron reductase of Leishmania amazonensis is essential for the generation of infective parasite forms. J. Biol. Chem 286, 23266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery AR, Renberg RL, Andrews NW, 2013. Pathways of iron acquisition and utilization in Leishmania. Curr. Opin. Microbiol 16, 716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollhofer J, Schläwicke C, Jungnick N, Schmidt W, Buckhout TJ, 2011. Members of a small family of nodulin-like genes are regulated under iron deficiency in roots of Arabidopsis thaliana. Plant Physiol. Biochem 49, 557–564. [DOI] [PubMed] [Google Scholar]
- Gossage SM, Rogers ME, Bates PA, 2003. Two separate growth phases during the development of Leishmania in sand flies: implications for understanding the life cycle. Int. J. Parasitol 33, 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C, Sacks DL, Andrews NW, 2006. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J Exp Med 203, 2363–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C, Yuan X, Miguel DC, Renberg RL, Protchenko O, Philpott CC, Hamza I, Andrews NW, 2012. Heme Uptake by Leishmania amazonensis Is Mediated by the Transmembrane Protein LHR1. PLoS Pathog. 8, e1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques I, Andrews NW, Huynh C, 2010. Functional characterization of LIT1, the Leishmania amazonensis ferrous iron transporter. Mol. Biochem. Parasitol 170, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J, 2002. Mechanisms of Cellular Iron Acquisition. Cell 111, 603–606. [DOI] [PubMed] [Google Scholar]
- Kořený L, Oborník M, Lukeš J, 2013. Make It, Take It, or Leave It: Heme Metabolism of Parasites. PLoS Pathog. 9, e1003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeira-Silva MF, Wang W, Samuel TK, Maeda FY, Michailowsky V, Hamza I, Liu Z, Andrews NW, 2018. A MFS-like plasma membrane transporter required for Leishmania virulence protects the parasites from iron toxicity. PLoS Pathog. 14, e1007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittra B, Laranjeira-Silva MF, Miguel DC, Perrone Bezerra de Menezes J, Andrews NW, 2017. The iron-dependent mitochondrial superoxide dismutase SODA promotes Leishmania virulence. J. Biol. Chem 292, 12324–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittra B, Laranjeira-Silva MF, Perrone Bezerra de Menezes J, Jensen J, Michailowsky V, Andrews NW, 2016. A Trypanosomatid Iron Transporter that Regulates Mitochondrial Function Is Required for Leishmania amazonensis Virulence. PLoS Pathog. 12, e1005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M, Topper G, Cristello P, Rich A, 1985. Receptor-Mediated Entry of Peroxidases Into the Parasitophorous Vacuoles of Macrophages Infected With Leishmania mexicana amazonensis. J. Leukoc. Biol 37, 247–261. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Khan YA, Laranjeira-Silva MF, Andrews NW, Mittra B, 2018. Quantification of Intracellular Growth Inside Macrophages is a Fast and Reliable Method for Assessing the Virulence of Leishmania Parasites. J. Vis. Exp e57486–e57486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Kaufmann SHE, 2004. Iron and microbial infection. Nat. Rev. Microbiol 2, 946–953. [DOI] [PubMed] [Google Scholar]
- Soares MP, Hamza I, 2016. Macrophages and Iron Metabolism. Immunity 44, 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hon T, Ye W, Zhang L, 2002. Heme deficiency interferes with the Rasmitogen-activated protein kinase signaling pathway and expression of a subset of neuronal genes. Cell Growth Differ. 13, 431–9. [PubMed] [Google Scholar]