Abstract
Fetal placental macrophages and microglia (resident brain macrophages) have a common origin in the fetal yolk sac. Yolk-sac-derived macrophages comprise the permanent pool of brain microglia throughout an individual’s lifetime. Inappropriate fetal microglial priming may therefore have lifelong neurodevelopmental consequences, but direct evaluation of microglial function in a living fetus or neonate is impossible. We sought to test the hypothesis that maternal obesity would prime both placental macrophages and fetal brain microglia to overrespond to an immune challenge, thus providing a window into microglial function using placental cells. Obesity was induced in C57BL/6J mice using a 60% high-fat diet. On embryonic day 17.5, fetal brain microglia and corresponding CD11b+ placental cells were isolated from fresh tissue. Cells were treated with media or lipopolysaccharide (LPS). Tumor necrosis factor-alpha (TNF-α) production by stimulated and unstimulated cells was quantified via ELISA. We demonstrate for the first time that the proinflammatory cytokine production of CD11b+ placental cells is strongly correlated with that of brain microglia (Spearman’s ρ= 0.73, p= 0.002) in the setting of maternal obesity. Maternal obesity-exposed CD11b+ cells had an exaggerated response to LPS compared to controls, with a 5.1-fold increase in TNF-α production in placentas (p=0.003) and 3.8-fold increase in TNF-α production in brains (p=0.002). In sex-stratified analyses, only male obesity-exposed brains and placentas had significant increase in TNF-α production in response to LPS. Taken together, these data suggest that maternal obesity primes both placental macrophages and fetal brain microglia to overproduce a proinflammatory cytokine in response to immune challenge. Male brain and placental immune response is more marked than female in this setting. Given that fetal microglial priming may impact neuroimmune function throughout the lifespan, these data could provide insight into the male predominance of certain neurodevelopmental morbidities linked to maternal obesity, including cognitive dysfunction, autism spectrum disorder, and ADHD. Placental CD11b+ macrophages may have the potential to serve as an accessible biomarker of aberrant fetal brain immune activation in maternal obesity. This finding may have broader implications for assaying the impact of other maternal exposures on fetal brain development.
Keywords: maternal obesity, fetal brain, microglia, placenta, Hofbauer cells, inflammation
Graphical abstract
1. Introduction
One in three women in the United States now starts pregnancy obese (Deputy, Dub, and Sharma 2018), with slightly lower but comparable rates of pre-pregnancy obesity in Europe and around the world in developed settings (WHO 2014). Human epidemiologic studies have identified long-term neurodevelopmental morbidities in offspring born to obese mothers, including an increased risk of autism spectrum disorder, cognitive deficits, attention deficit hyperactivity disorder (ADHD), anxiety and depression, and disordered eating (Edlow 2017a). Obesity is a state of chronic low-level immune activation (Gregor and Hotamisligil 2011). Pregnancy may augment this low-grade metabolically-induced inflammation, as normal pregnancy is associated with increased levels of certain pro-inflammatory cytokines (Christian and Porter 2014). In addition, levels of circulating pro-inflammatory cytokines such as IL-6 and CRP increase in pregnancy with increasing maternal BMI (Aye et al. 2014; Friis et al. 2013; Christian and Porter 2014). In normal pregnancy, the placenta is relatively protected from maternal peripheral inflammation, with both maternal decidual and placental macrophages typically maintained in an anti-inflammatory state to enable immune tolerance of the fetus (Bolton and Bilbo 2014). This placental immune quiescence is likely deranged in the setting of maternal obesity, with activation of pro-inflammatory pathways in the placenta and histopathological evidence of placental inflammation described in the literature (Aye et al. 2014; Bar et al. 2012; Challier et al. 2008). Animal model studies have also demonstrated brain inflammation in the offspring of obese females, with developmental neuroinflammation identified as an important candidate mechanism underlying postnatal cognitive and behavioral morbidities in offspring of obese dams (Hanamsagar and Bilbo 2016; Bilbo and Tsang 2010; Edlow et al. 2016; Kang et al. 2014; White et al. 2009). However, relatively little is known about how placental inflammation might correlate with fetal brain inflammation in the setting of maternal obesity.
In considering the potential impact of maternal obesity on fetal brain inflammation, the logical starting point is to assess the functional impact on microglia, the resident brain immune cells. Microglia function not only as immune sentinels, they also play a key role in normal brain development and brain homeostasis (Bilbo et al. 2018; Koyama and Ikegaya 2015; Bilimoria and Stevens 2015; Paolicelli et al. 2011; Rakic and Zecevic 2000; Streit 2001). Aberrant microglial activation and subsequent pro-inflammatory cytokine production in utero may result in abnormal development, with the developing brain particularly vulnerable to inflammatory disruption during critical windows (Bilbo and Schwarz 2009, 2012; Deverman and Patterson 2009;Urakubo et al. 2001; Yu et al. 2004; Rodier 1980).
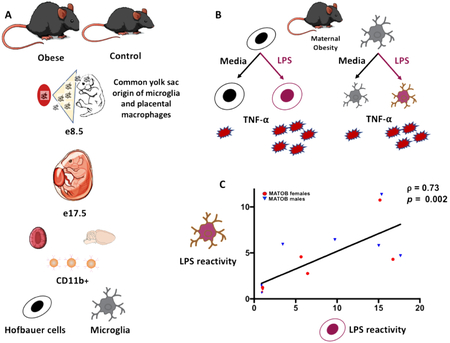
Microglia are of particular interest in evaluating correlations between brain and placental inflammation, given that subpopulations of placental macrophages and brain microglia have a common origin in the fetal yolk sac (Ginhoux et al. 2010; Reyes, Wolfe, and Golos 2017; Takahashi et al. 1991). Fate-mapping studies have demonstrated that these primitive yolk-sac-derived macrophages colonize the developing brain as early as e 9.5 in rodents, entering the parenchyma via the blood stream and ventricles (Ginhoux et al. 2010). Microglia continue to proliferate throughout the first postnatal weeks in humans and rodents, and form a self-renewing pool that lasts throughout the lifespan, without contribution from peripheral hematopoietic cells in the periphery under normal conditions (Ajami et al. 2007; Ginhoux et al. 2010).
Given the embryonic origins of adult microglia, fetal exposure to inflammation–as may occur in the setting of maternal obesity—can have enduring consequences for microglial function across the lifespan. Aberrant microglial activation or priming has been implicated in a number of later-onset neurodevelopmental morbidities, including autism spectrum disorder, schizophrenia, obsessive compulsive disorder, and neurodegenerative conditions such as Alzheimer’s or Parkinson’s Disease (Bilbo et al. 2018; Bilimoria and Stevens 2015; Koyama and Ikegaya 2015; Perry and Teeling 2013). Despite the knowledge that maternal immune activation or inflammation in the setting of maternal obesity may be programming microglia to overrespond to immune challenge, there is no reliable way to directly assay the impact of maternal obesity on the developing brain. Unlike microglia, however, the placenta is accessible in pregnancy (via chorionic villus sampling) and immediately after delivery, so if a subpopulation of placental cells could give information about microglial function in the setting of a maternal exposure such as obesity, this could inform evaluations of offspring risk for later development of neurodevelopmental morbidities. We therefore sought to determine whether placental macrophages could provide information about the function of brain microglia, and whether maternal obesity primed both placental macrophages and fetal brain microglia to overrespond to an immune challenge.
2. Materials and Methods
2.1. Animals and diet
Female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were placed on either a lard-based, high-fat diet containing 60% calories from fat (Research Diets D12492, New Brunswick, NJ), or a matched control diet containing 10% calories from fat (Research Diets D12450J), starting at 4-5 weeks of age. Diets were matched for protein, fiber, sucrose, and micronutrients (content information available at https://researchdiets.com). Dams were continued on their allocated diet for 12-14 weeks pre-breeding, and throughout pregnancy. Male C57BL/6J mice were fed the control diet. For females, obesity was defined as at least a 30% increase in weight compared to age-matched controls (Gallou-Kabani et al. 2007; Edlow et al. 2016). Mice were housed in same-sex groups of 4 throughout the pre-breeding feeding period, with ad libitum access to food and water. The colony was maintained at 22°C on a 12:12 hour light-dark cycle. Obese and lean females were paired with control males for breeding. Female breeders were examined daily during breeding, and the presence of a vaginal plug was defined as embryonic day 0.5 (e0.5). Males were separated upon observation of a vaginal plug. Females were weighed at pregnancy days 0 (P0), P10 and P15. All procedures were approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital.
2.2. Tissue collection
On embryonic day e17.5, pregnant mice were euthanized with isoflurane followed by decapitation. E17.5 was selected as the timepoint for examination because the male testes begin to secrete testosterone at e18, while the ovaries remain quiescent in females. We thus selected a timepoint that would permit assessment of placental and brain immune function prior to sex hormone exposure, which would be imbalanced in male and female fetuses. Embryos were rapidly dissected from the uterine horns and placed in ice-cold phosphate buffered saline (PBS 1x). Theiler staging of embryos was performed to confirm embryonic day 17.5 at the time of sacrifice (Theiler 1989). Crown-rump lengths, fetal, and placental weights were recorded. Forebrains were rapidly dissected from skulls and corresponding/matched placentas were dissected from each amniotic sac. Sex genotyping was subsequently performed on tail snip DNA using real time PCR with specific probes for the Sry gene (Transnetyx, Cordova, TN). Only one to two fetuses per sex per litter were used for experiments.
2.3. Microglial and placental macrophage isolation
Isolated fresh forebrains and placentas were minced into small pieces using a sterile razor and homogenized in 5 mLs of enzyme digestion mix comprised of Hanks Balanced Salt Solution without magnesium and calcium (HBSS, Gibco/ThermoFisher Scientific, Waltham, MA), 5% fetal bovine serum (FBS, ThermoFisher Scientific), Hepes 10M (Gibco/ThermoFisher Scientific), collagenase A (2mg/mL, Roche, Indianapolis, IN), and DNaseI (Invitrogen, Carlsbad, CA). Tissue was incubated for 45 minutes in a 37°C water bath, and every 15 minutes samples were removed from the bath and passed through successively smaller glass Pasteur pipettes to ensure complete dissociation. Samples were then passed through a nylon filter and centrifuged at 1,200 rpm for 10 min at 4°C. Cell pellets were resuspended in 30% Percoll (GE Healthcare, Uppsala, Sweden) in 1X sterile phosphate buffered saline (PBS) prepared from isotonic Percoll (90% Percoll and 10% 10X PBS, Thermo Fisher Scientific, NY, USA). The 30% resuspension was then carefully underlayed with 70% Percoll in 1X PBS. Microglia and other mononuclear cells are known to accumulate at the interface of a 30%/70% Percoll gradient (Jin and Kim 2015). Samples were centrifuged at 1,400 rpm for 15 minutes at 23°C. Mononuclear cells were pipetted from the interface of the Percoll gradient, and centrifuged with a post-Percoll solution containing HBSS with calcium and magnesium, FBS, and Hepes 1M at 1200 rpm for 10 minutes at 4°C. Cells were then resuspended in MACS buffer and incubated with CD11b antibody-conjugated magnetic beads (MACS Miltenyi Biotec, San Diego CA) for 15 min at 4°C.
CD11b is a subunit of the complement receptor 3 complex (CD11b/CD18), also known as macrophage-1 antigen or Mac1. As an integrin family member, CD11b regulates leukocyte adhesion and migration, and may play a role in cell-mediated cytotoxicity, chemotaxis, and phagocytosis. CD11b is one of the most widely used microglial markers, and microglia are the only intra-parenchymal CNS cell type that express CD11b under normal conditions (Brandon 1995; Salter and Stevens 2017; Greter, Lelios, and Croxford 2015; Jeong et al. 2013). In the placenta, CD11b+ cells will be highly enriched for fetal macrophages, including both Hofbauer cells (fetal macrophages of yolk sac origin), and possibly fetal peripheral monocytes (Schliefsteiner et al. 2017; Sisino et al. 2013; Challier et al. 2008). After washing, cells were again centrifuged at 1200 rpm for 10 minutes at 4°C, resuspended in MACS buffer and then passed through nylon filters onto magnetic bead columns (LS columns with the MidiMACS separator, Miltenyi Biotec, San Diego CA) and CD11b positive (CD11b+) versus negative (CD11b−) populations were separated and placed on ice. Samples were centrifuged at 1200 rpm for 10 minutes at 4°C to isolate the final purified CD11b+ and CD11b− cells.
2.4. Ex vivo LPS stimulation
Purified CD11b+ and CD11b− cells were resuspended in neuro media, containing high glucose DMEM, L-glutamine, Pen Strep, N2 media supplement, sodium pyruvate, and 5μg/mL Forskolin. An aliquot of the cell suspension for each sample was treated with Trypan Blue in a 1:2 or 1:10 dilution, depending on cell density, and 10 μl of the treated cell suspension was applied to a standard hemacytometer with Neubauer rulings. Live cells were hand-counted in 3 large corner squares. Cell counts in the 3 quadrants were averaged for the final density determination. Total number of cells was calculated using the following formula: (average cell count over 3 quadrants) × (Trypan Blue dilution factor) × 104. Cells were plated in 96-well round-bottom plates and incubated with LPS (1250 ng/ml) or media alone for 4 hours at 37°C, 5% CO2. The LPS dose was based on pilot dosing experiments, coupled with evaluation of the literature regarding response of rodent microglia to varying doses of LPS ex vivo (Frank et al. 2010; Frank et al. 2006; Njie et al. 2012; Turano, Lawrence, and Schwarz 2017; Williamson et al. 2011). We selected a dose at the highest end of the reported range for rodent microglia because studies reporting a dose-response for ex vivo treatment of microglia with LPS found that increasing the dose of LPS up to the range of 1000 ng/mL was associated with increased production of pro-inflammatory cytokines (Frank et al. 2010; Frank et al. 2006; Lee et al. 1993; Njie et al. 2012). In addition, there are limited data regarding response of fetal microglia to LPS (Cao et al. 2015; Lee et al. 1993; Schaafsma et al. 2017), but these data suggest that fetal microglia might have less robust production of pro-inflammatory cytokines in response to LPS (Cao et al. 2015) compared to neonatal or adult microglia (Frank et al. 2010; Frank et al. 2006; Njie et al. 2012; Turano, Lawrence, and Schwarz 2017; Williamson et al. 2011). The potential for decreased fetal microglial responsiveness to LPS provided additional rationale for selecting a high dose. For each condition, samples were run in duplicate. At the end of incubation, the plate was centrifuged at 2400 rpm for 5 minutes at 4°C to pellet cells. Supernatants were removed and frozen at −80°C until quantification of TNF-α production.
2.5. Quantification of TNF-α production by CD11b+ and CD11b− fetal brain and placental cells
Tumor necrosis factor alpha or TNF-α is a pro-inflammatory cytokine primarily secreted by activated macrophages. TNF-α has been demonstrated to be increased in maternal plasma and the fetal brain in the setting of maternal immune activation with Poly (I:C), and in the placenta, fetal plasma, and offspring brain in rodent models of maternal high-fat diet (Desai et al. 2013; Garay et al. 2013; Winther et al. 2018). TNF-α has been implicated in microglial regulation of synaptic maturation and plasticity (Salter and Stevens 2017), and increased serum and brain levels of TNF-α correlate with neurobehavioral morbidity in offspring (Xie et al. 2017; Winther et al. 2018).
TNF-α levels were quantified in the supernatant from each sample using a commercially available ELISA kit (Mouse TNF-alpha Quantikine ELISA kit, R&D Systems, Minneapolis, MN). TNF-α level for each sample was determined by averaging the replicates. Cytokine expression data were adjusted for sample cell counts using two different methods. For each sample, an “LPS reactivity ratio” was generated by dividing the mean TNF-α pg/mL signal in the LPS treatment wells by the mean TNF-α pg/mL signal in the media-treated wells. This ratio represents a fold-change increase in TNF-α after LPS treatment, and contains a built-in correction for the cellularity of the sample (for a given sample, the media-treated and the LPS-treated wells contained the same number of cells). For analyses evaluating the baseline production of TNF-α in the unstimulated/media condition, the raw TNF-α signal was adjusted to cell count per well for each sample, and data are expressed as pg/mL TNF-α produced per 100,000 cells.
2.6. Statistical analyses
Differences between obesity-exposed and control animals were determined using Student’s t-test (normally-distributed data) and Mann-Whitney testing (non-normally distributed data), with significance defined as p<0.05. To help control for litter effects, only 1-2 fetuses per sex per litter were used for these experiments. In addition, we built a linear mixed effects model controlling for the random effect of dam, to evaluate the effect of maternal obesity on TNF-α production while accounting for any clustering by litter. Main effects and significant interactions between maternal obesity status and fetal sex on LPS reactivity of brain and placental cells were evaluated using 2-way ANOVA. Correlation between brain and placental cell reactivity to LPS was determined using Spearman’s rank correlation. Given our own prior data demonstrating sex differences in embryo size and embryonic brain gene expression signatures in this model of maternal diet-induced obesity (Edlow et al. 2016; Edlow et al. 2017b), as well as the work of others demonstrating significant impact of fetal sex on placental structure, gene expression and function in the setting of obesity (Evans and Myatt 2017; Kim et al. 2014; Muralimanoharan et al. 2015), and impact of fetal sex on microglia in the developing brain (Hanamsagar et al. 2017; Schwarz, Sholar, and Bilbo 2012), we planned to evaluate for sex differences. A pre-specified sex-stratified analysis was therefore performed to evaluate for significant differences in LPS reactivity between obesity-exposed and control cells within the males and female study populations. Analyses were performed using GraphPad Prism (Prism 7, GraphPad Software, San Diego, CA) and StataIC (v.14, StataCorp LLC, College Station, TX). Data are expressed as mean ± SEM unless otherwise indicated.
3. Results
3.1. Diet-induced obesity model and study population characteristics
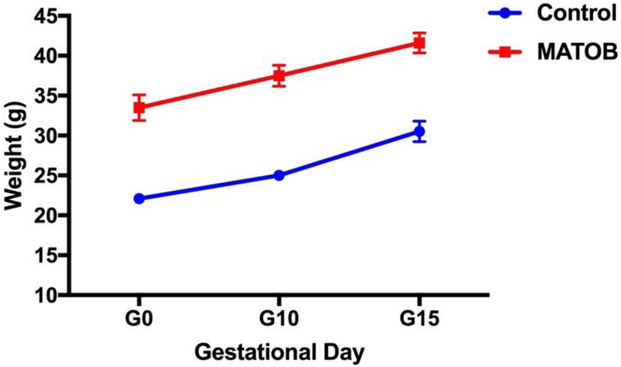
CD11b+ cells from brains and corresponding placentas were isolated from 18 control fetuses (9 females, 9 males) and 16 obesity-exposed fetuses (8 males, 8 females), reflecting 9 litters (N=4 obese, N=5 control, cells isolated from 1-2 fetuses/sex/litter). Dam pregnancy weight trajectories are depicted in Figure 1. Dam and fetus characteristics are depicted in Table 1. Obese dams were significantly heavier at breeding and at time of sacrifice on e17.5 than their lean counterparts. There were no statistically significant differences in litter size, fetal weight, or placental weight between study groups. Significantly more CD11b+ cells were isolated from brains of obesity-exposed fetuses compared to controls, per hemocytometer cell counts. There were no significant differences between groups in CD11b+ cells isolated from the placenta.
Figure 1: Pregnancy weight trajectory in obese and lean dams.
G0: gestational day 0 (day of mating); G10: gestational day 10; G15: gestational day 15; MATOB: Maternal obesity
Table 1:
Study population characteristics
| Characteristic | Control | Maternal Obesity | p-value |
|---|---|---|---|
| Dam weight at breeding (g)* | 20.60 ± 0.58 | 31.83 ± 0.94 | 0.02 |
| Dam weight at sacrifice (g) | 36.38 ± 1.13 | 42.97 ± 1.80 | 0.03 |
| Litter size | 8.8 ± 1.2 | 6.25 ± 1.49 | 0.24 |
| Fetal weight (g) | |||
| Male | 0.96 ± 0.03 | 0.97 ± 0.03 | 0.93 |
| Female | 0.86 ± 0.04 | 0.91 ± 0.02 | 0.67 |
| Both sexes combined | 0.90 ± 0. 03 | 0.93 ± 0.02 | 0.57 |
| Placental weight (g) | |||
| Male | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.59 |
| Female | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.19 |
| Both sexes combined | 0.12 ± 0. 01 | 0. 10 ± 0. 01 | 0.13 |
| CD11b+ cells isolated from brain | |||
| Male | 3.85 × 105± 0.63 | 7.42 × 105 ± 1.14 | 0.02 |
| Female | 4.65 × 105 ± 0.50 | 7.75 × l05 ± 0.81 | 0.003 |
| Both sexes combined | 4.25 × 105 ± 0.40 | 7.60 × 105 ± 0.66 | <0.001 |
| CD11b+ cells isolated from placenta | |||
| Male | 7.02 × 105 ± 1.54 | 8.32 × 105 ± 1.37 | 0.47 |
| Female | 7.21 × 105 ± 1.86 | 6.23 × 105 ± 0.76 | 0.99 |
| Both sexes combined | 7.171× 105 ± 1.16 | 7.27 × 105 ± 0.80 | 0.59 |
| CD11b− cells isolated from brain | |||
| Male | 9.13 × 106 ± 2.12 | 10.33 × 106 ± 1.30 | 0.35 |
| Female | 10.03 × 106 ± 1.56 | 14.52 × 106 ± 2.89 | 0.24 |
| Both sexes combined | 9.57 × 106 ± 1.28 | 12.56 × 106 ± 1.69 | 0.16 |
| CD11b− cells isolated from placenta | |||
| Male | 4.42 × 106 ± 0.77 | 4.22 × 106 ± 0.36 | 0.91 |
| Female | 3.12 × 106 ± 0.60 | 4.90 × 106 ± 0.28 | 0.03 |
| Combined | 3.77 × 106 ± 0.50 | 4.58 × 106 ± 0.24 | 0.09 |
Data are expressed as mean ± SEM unless otherwise indicated
3.2. Responsiveness of microglia and placental CD11b+ cells to immune challenge in the setting of maternal obesity
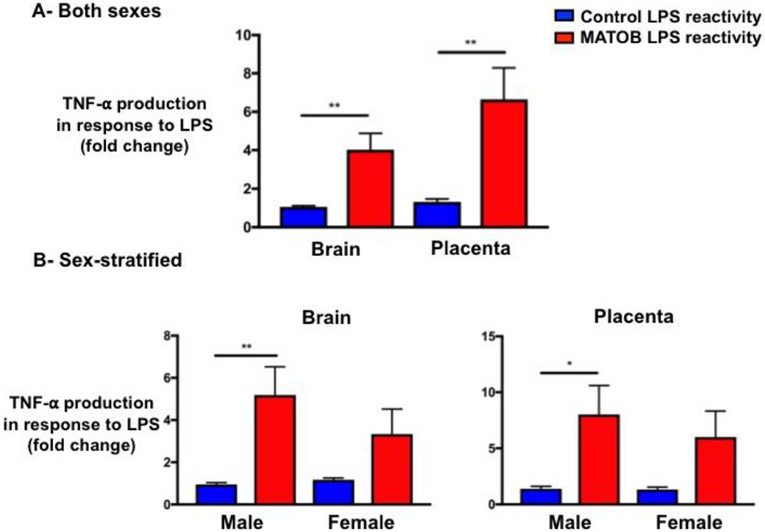
Maternal obesity status did not significantly impact baseline TNF-α production by CD11b+ cells isolated from brains and corresponding placentas (Supplemental Figure 1). However, the pro-inflammatory response to LPS was significantly increased by maternal obesity, with maternal obesity priming CD11b+ cells in the brain and placenta to overrespond to LPS treatment compared to controls (Figure 2 A/B). In analyses combining males and females, maternal obesity-exposed CD11b+ cells had an exaggerated response to LPS compared to controls, with a 3.8-fold increase in TNF-α production in brains (p=0.002), and a 5.1-fold increase in TNF-α production in placentas (p=0.003), Figure 2A.
Figure 2: Responsiveness of microglia and placental CD11b+ cells to immune challenge in the setting of maternal obesity.
Maternal obesity is associated with significantly increased pro-inflammatory response to LPS by both brain microglia and placental CD11b+ cells. Sex-stratified analyses demonstrate that only obesity-exposed males have a statistically significant increase in TNF-α production in response to LPS.
LPS: lipopolysaccharide; MATOB: Maternal obesity; **p<0.01; *p<0.05
The effect of maternal obesity on TNF-α production by CD11b+ cells in brain and placenta remained significant, even after controlling for dam. For all analyses, TNF-α release was adjusted for cell count or to each subject’s own untreated media baseline, so the increased responsiveness of maternal obesity-exposed CD11b+ cells to LPS was not attributable simply to increased cell numbers. In addition, the same priming effect was seen in the placenta as in the fetal brain, but the number of CD11b+ cells did not differ significantly between obese and lean groups in the placenta.
3.2.1. Sex differences in responsiveness of microglia and placental CD11b+ cells to immune challenge in the setting of maternal obesity
In the pre-specified sex-stratified analysis, only male obesity-exposed brain and placental cells had statistically-significant increases in LPS reactivity (5.5-fold increase in TNF-α production over controls in brain, p=0.008, 5.8-fold increase in TNF-α production over controls in placenta, p= 0.02, Figure 3B). Female cells displayed a similar trend but didn’t achieve statistical significance (2.8-fold increase in TNF-α production over controls in brain, p=0.19, 4.6-fold increase in TNF-α production over controls in placenta, p=0.06, Figure 3B).
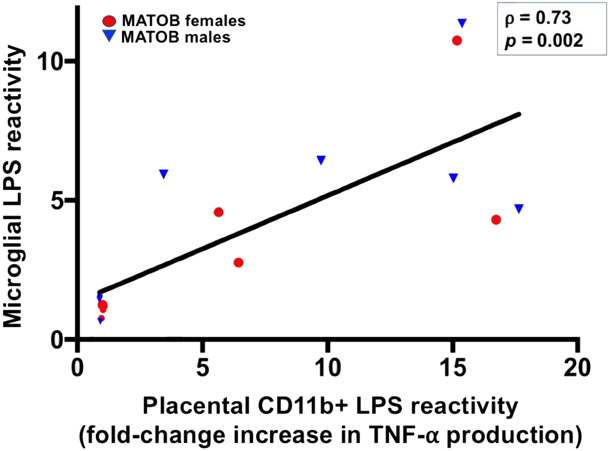
Figure 3: Response of fetal brain microglia and placental CD11b+ cells to immune challenge is highly correlated in the setting of maternal obesity.
TNF-α production in response to LPS challenge by CD11b+ cells in the placenta and fetal brain is highly correlated. Axis values reflect fold-change increase in TNF-α production after LPS administration.
MATOB: maternal obesity, red circles (female) and blue triangles (male) represent matched fetal brains and placentas of obese dams.
LPS: lipopolysaccharide
2-way ANOVA analyses (maternal obesity status × fetal sex) demonstrated a main effect of maternal obesity status on LPS reactivity of brain and placental CD11b+ cells (F(1,25) = 12.01, p=0.002 for fetal brain, F(1,28)= 10.65, p=0.003 for placenta). For both brains and placentas, there was no significant main effect of fetal sex on LPS reactivity (F(1,25)= 0.8, p=0.38 brain; F(1,28)= 0.36, p=0.55 placenta), and the interaction terms between maternal obesity and fetal sex on LPS reactivity for both brain and placenta were nonsignificant (F(1,25)= 1.25, p=0.27 brain, F(1,28)= 0.32 p=0.58 placenta).
3.3. Placental CD11b+ cell reactivity is highly correlated with reactivity of brain microglia
To determine how correlated placental CD11b+ cell reactivity and microglial reactivity were in the setting of immune challenge, Spearman’s correlation analysis was performed for brain and placenta values for each fetus. Only obesity-exposed fetal brains and placentas were included in the correlation, given the lack of significant response to LPS in control samples. Placental CD11b+ cell reactivity was strongly correlated with brain microglial reactivity in the setting of maternal obesity (Spearman’s ρ= 0.73, p=0.002, Figure 3). This analysis suggests that the behavior of placental CD11b+ cells in response to immune challenge may serve as a reliable proxy for the behavior of brain microglia in the setting of maternal obesity.
3.4. Responsiveness of CD11b− brain and placental cells to immune challenge in the setting of maternal obesity
Production of TNF-α by the negatively-selected cell population in fetal brain and placental tissue (all CD11b− cells isolated from these tissues) was also evaluated by ELISA. For the majority of samples, TNF-α production by CD11b− brain and placental cells fell below the detectable range of the assay (<7.21 pg/mL). Analyses performed on the subset of fetal brain and placenta samples with TNF-α values above the limit of detection for the assay demonstrated no significant response to LPS in obesity-exposed or control CD11b− cells.
4. Discussion
Here we demonstrate for the first time that a subset of placental immune cells may provide insight into the behavior of microglia in the fetal brain. Specifically, we report that maternal obesity primes CD11b+ cells in both the placenta and fetal brain to overrespond to immune challenge with LPS, compared to controls. This effect was most pronounced in male fetal brains and placentas. There was a strong correlation between production of pro-inflammatory cytokine TNF-α by placental CD11b+ cells and fetal brain microglia. Given that the pool of microglia throughout an individual’s lifespan is fetal in origin, maternal obesity-associated priming of microglia to overrespond to immune challenge has potential implications for understanding the increased risk for autism spectrum disorder, cognitive dysfunction, and other neurodevelopmental morbidities noted in offspring of obese women. It is difficult to identify which offspring may be most at risk for aberrant microglial activation in the setting of maternal obesity (and other disorders of maternal chronic immune activation), due to the inability to directly assay the developing brain in an ongoing pregnancy or after birth. These data represent an initial step toward the use of placental macrophages as a biomarker, accessible during pregnancy and at the time of birth, of fetal neuroimmune activation. If these placental macrophages and corresponding brain microglia can be further characterized in a variety of maternal exposure states, these data may have broader implications for assaying the impact not only of maternal obesity but other maternal exposures on fetal brain development.
A strength of this study is the novel use of placental macrophages to provide information about the behavior of fetal brain immune cells, and the demonstration of a strong correlation between the behavior of these two cell types in response to immune challenge. The common yolk-sac origin of fetal placental macrophages and microglia (Ginhoux et al. 2010; Ginhoux and Prinz 2015) inspired our examination of whether both cell types would behave similarly after in vivo exposure to maternal obesity, followed by a second ex vivo immune challenge (LPS administration). This “two-hit” model, in which maternal obesity may not in isolation change the behavior of fetal microglia and placental macrophages (as evidenced by the lack of baseline differences in TNF-a production between obesity-exposed and lean-exposed cells), but primes them to overrespond to a second immune challenge, may help explain why maternal obesity is a risk factor for neurodevelopmental morbidity, but not all offspring are affected.
Our finding that maternal obesity alone did not alter baseline levels of TNF-α production by fetal brain microglia or placental macrophages is consistent with the findings of two rodent models of maternal immune activation via polyinosinic:polycytidylic acid (Poly(I:C)), which did not find alterations in microglial TNF-α mRNA or protein expression at e16.5 (Pratt et al. 2013), and did not identify an effect on microglia at e17.5 even in the setting of repeated Poly(I:C) administration (Smolders et al. 2015). The absence of maternal obesity-induced baseline increase in TNF-α production by either microglia or placental macrophages in our study is also consistent with the absence of detectable fetal inflammation and limited placental inflammation observed in a rat model of maternal obesity induced by a cafeteria diet (Crew, Waddell, and Mark 2016). In contrast to these two models of maternal immune activation/inflammation, maternal LPS administration, whether one-time (O'Loughlin et al. 2017), or repeated (Schaafsma et al. 2017), was sufficient to increase TNF-α mRNA expression in embryonic brain at e17.5 and e18. It may be that maternal obesity is a more comparable exposure to Poly(I:C) than it is to LPS, and a second hit may be required to induce overexpression of pro-inflammatory cytokines by microglia in this setting. The two-hit model is well described in models of maternal immune activation, and has been implicated in neuropsychiatric outcomes ranging from anxiety to cognitive dysfunction and schizophrenia, among others (Estes and McAllister 2016; Debost et al. 2017; Bolton et al. 2013; Giovanoli et al. 2013; Williamson et al. 2011).
The absence of a significant response of control CD11b+ fetal brain or placental cells to LPS merits further discussion. In rodent models, ex vivo LPS challenge has been demonstrated to produce a pro-inflammatory response in control/wild-type adult microglia (Frank et al. 2010; Frank et al. 2006; Njie et al. 2012) and in postnatal day 4 microglia (Turano, Lawrence, and Schwarz 2017). However, the limited available data suggest that normal fetal microglia may have a less consistent pro-inflammatory response to LPS administration (Cao et al. 2015; Schaafsma et al. 2017; Lee et al. 1993). The few studies that have examined fetal microglial response to LPS report conflicting results: some report no detectable increase in TNF-α (Cao et al. 2015) or IL-1β (Lee et al. 1993) in control supernatants after LPS administration measured by ELISA, but increased TNF-α, IL-6, and IL-1β RNA after LPS administration has been detected via quantitative PCR (RTqPCR) or Northern blot (Lee et al. 1993; Schaafsma et al. 2017). These differing results may be partly attributable to different sensitivities of ELISA versus RTqPCR for low levels of cytokine expression (Amsen, de Visser, and Town 2009; Peinnequin et al. 2004). Examination of cytokine production via ELISA is a strength of this study. Many studies examining the impact of maternal immune activation on fetal and offspring microglial function have focused primarily on mRNA levels, and in the limited number of studies that have examined both message levels and protein, discrepancies have been noted between pro-inflammatory cytokine expression by mRNA versus protein.(Schaafsma et al. 2017; Pratt et al. 2013; Lee et al. 1993) Characterization of cytokine expression by ELISA is closer to phenotype.
The joint focus on fetal brain microglia and placental macrophages in the setting of maternal obesity is a unique contribution of our study. As the interface between the maternal and fetal environments and the source of fetal nutrition, the placenta has been implicated in developmental programming (Jansson and Powell 2007), including of the developing fetal brain (Hsiao and Patterson 2012; Bale 2016; Bronson and Bale 2016). While the placenta is involved in mediating immune protection of the fetus, these protective mechanisms can become deranged in the setting of inflammation or maternal immune activation, contributing to long-term programming of the fetal brain and offspring behavior in later life (Hsiao and Patterson 2012; Bronson and Bale 2014). In normal pregnancy, both maternal decidual and placental macrophages are maintained in an anti-inflammatory state to enable immune tolerance of the fetus (Bolton and Bilbo 2014). Human studies have demonstrated that maternal obesity is associated with accumulation of placental macrophages (Hofbauer cells) in the placenta, and accompanying evidence of increased inflammation (Aye et al. 2014; Bar et al. 2012; Challier et al. 2008; Roberts et al. 2011). Placental inflammation may in turn have direct effects on the developing fetal brain (Bronson and Bale 2014; Goeden et al. 2016). A proposed underlying mechanism is increased placental serotonin production in the setting of inflammation, which inhibits neurogenesis and axon outgrowth in the developing forebrain (Bonnin et al. 2011; Goeden et al. 2016). Increased placental conversion of maternal l-tryptophan to serotonin may be mediated in part through IL-6 (Howell and Powell 2017), which has been demonstrated to be increased in the setting of maternal obesity (Christian and Porter 2014; Friis et al. 2013; Stewart et al. 2007). The relative contributions of maternal obesity compared to high-fat diet on placental and brain serotonin signaling may be difficult to disentangle: in a non-human primate model of maternal high-fat diet, fetal central serotonergic signaling was perturbed and offspring demonstrated increased anxiety-like behavior, independent of maternal obesity status (Sullivan et al. 2010).
The neurodevelopmental morbidities observed with increased frequency in offspring of obese mothers include not only mood disorders (anxiety and depression), but also autism spectrum disorder, developmental delay and/or disorders of cognitive function, ADHD, disordered eating, cerebral palsy, and schizophrenia in some studies (Brion et al. 2011; Crisham Janik et al. 2013; Edlow 2017a; Heikura et al. 2008; Hinkle et al. 2012; Huang et al. 2014; Krakowiak et al. 2012; Rising and Lifshitz 2005; Rodriguez et al. 2008; Tanda et al. 2013). Microglial number, activation state, and function has been implicated as potentially contributing to the majority of these pathologies (Koyama and Ikegaya 2015; Lenz and McCarthy 2015; Matcovitch-Natan et al. 2016; Sekar et al. 2016). The fetal origin of the permanent pool of microglia in the brain suggests that pregnancy and the perinatal period are key windows during which brain microglial function may be permanently altered.
While prior work has demonstrated that maternal high-fat diet is associated with chronic priming of microglia in offspring, and increased pro-inflammatory cytokine expression in the adult offspring brain in response to a bacterial challenge (Bilbo and Tsang 2010), this the first study to report microglial and placental macrophage priming by maternal obesity detectable even in fetal life. It is biologically plausible that maternal obesity could program macrophages to overrespond to immune challenge, given that maternal obesity has been shown to be associated with placental inflammation, fetal and neonatal peripheral inflammation, and offspring brain inflammation (Aye et al. 2014; Challier et al. 2008; Desai et al. 2013; Zhu et al. 2010; Bilbo and Tsang 2010; Kang et al. 2014).
The examination of both male and female fetal brains and placentas allowed us to investigate sex differences in response to immune challenge in the setting of maternal obesity. The finding that male brain and placental macrophages have a significantly increased response to LPS in maternal obesity is consistent with other studies that have demonstrated increased male brain vulnerability to early-life immune challenge (Schwarz, Sholar, and Bilbo 2012; Iwasa et al. 2010; Llorente et al. 2009; Rebuli et al. 2016; Ruggiero et al. 2018; Hilton, Nunez, and McCarthy 2003; Nunez, Alt, and McCarthy 2003; Walker, Nakamura, and Hodgson 2010), and may provide insight into the male predominance of certain neurodevelopmental morbidities linked to maternal obesity (autism spectrum disorders, developmental/cognitive delay, ADHD, and in some studies schizophrenia) (Bilbo, Smith, and Schwarz 2012; Lenz and McCarthy 2015; Edlow 2017a; Koyama and Ikegaya 2015). Recent studies have demonstrated that the placenta may transmit sex-specific signals to the developing fetal brain in the setting of maternal environmental perturbations, and that such signaling may be mediated in part via X-linked genedosage effects and sex differences in placental inflammation (Bale 2016; Howerton et al. 2013; Bronson and Bale 2014). Differences in the male placenta may therefore render the developing male brain more vulnerable to insults such as maternal stress (Bronson and Bale 2014; Bale 2016). The finding of a more pronounced priming effect of maternal obesity on male brain and placental macrophages should interpreted with caution, however, given that significant differences between males and females were noted in the sex-stratified analyses only, while the interaction term on 2-way ANOVA did not achieve statistical significance. The absence of a statistically-significant response of female macrophages to LPS in the setting of maternal obesity may be because only males have a significant response, or may be because more female fetuses than 8-9/diet group are needed to see a statistically significant effect.
Our study was limited in its ability to fully characterize the CD11b+ placental macrophage population. While placental CD11b+ macrophages are fetal in origin, and therefore enriched for Hofbauer cells (resident fetal macrophages of yolk sac origin within the placental villus core) (Reyes, Wolfe, and Golos 2017), further characterization of the CD11b+ placental macrophages is an important area for future study. There are at least three possibilities for the identity of the CD11b+ cells isolated from the placenta: (1) Hofbauer cells (2) circulating blood monocytes of fetal origin, or (3) circulating blood monocytes of maternal origin (less likely but some contamination of placentas with maternal blood despite PBS rinsing cannot be ruled out). Distinguishing between these three possibilities via flow cytometry/fluorescence-activated cell sorting may be challenging, due to the inherent complexity of the mononuclear phagocyte system and significant phenotypic variability of placental macrophages which has been elucidated in recent years (Hume 2008a; Reyes, Wolfe, and Golos 2017). There has been increasing recognition of many shared markers between what are typically considered “resident” versus “inflammatory” macrophages, and recognition of more than 20 macrophage markers expressed by Hofbauer cells, most of which are gestational age-specific (Gordon and Taylor 2005; Hume 2008a, 2008b; Reyes, Wolfe, and Golos 2017). In addition, there may be some overlap between Hofbauer cells and peripheral fetal macrophages as pregnancy progresses. Hofbauer cells are exclusively of fetal yolk sac origin in early pregnancy, but during later stages of pregnancy, may originate from fetal monocytes recruited to the placenta (Kim et al. 2008; Kim et al. 2009; Takahashi et al. 1991; Seval, Korgun, and Demir 2007).
Similarly, while the brain CD11b+ macrophage population is highly enriched for microglia, we cannot rule out the possibility of some infiltrating immune cells from the periphery. Data are limited regarding the potential for infiltration of peripheral monocytes into the brain, as this is a more recent consideration. The original fetal pool of yolk-sac derived macrophages is the lifelong source of myeloid cells in the CNS; this microglial pool is self-renewing and its maintenance does not depend on circulating monocytes under normal conditions (Salter and Stevens 2017; Greter, Lelios, and Croxford 2015; Jeong et al. 2013; Bruttger et al. 2015; Hashimoto et al. 2013; Sheng, Ruedl, and Karjalainen 2015). There is controversy, however, regarding whether peripheral CD11b+ monocytes could contribute to the brain population under inflammatory conditions (Greter, Lelios, and Croxford 2015; Bruttger et al. 2015; Tay et al. 2017; Sevenich 2018; Wohleb et al. 2014), and whether the increased permeability of the blood brain barrier in fetal life might permit some influx of peripheral immune cells into the developing brain (Ginhoux and Prinz 2015; Saunders, Liddelow, and Dziegielewska 2012; Garay et al. 2013). Novel markers highly specific to microglia that can distinguish between microglia and other monocytes or macrophages present in the brain have been recently described (transcriptional repressor Spalt Like Transcription Factor 1 or Sall 1, and Transmembrane protein 119 or Tmem119).(Bennett et al. 2016; Buttgereit et al. 2016). However, the accuracy of these markers to distinguish microglia from other immune cells in the embryonic brain is unknown at this time (Smolders et al. 2018). Future experiments will therefore focus on additional characterization of CD11b+ cells in the developing brain and placenta via flow cytometry and/or transcriptional profiling, to better understand whether some of these cells could be infiltrating peripheral macrophages.
5. Conclusions
These experiments provide two novel insights into fetal immune function in the setting of maternal obesity. First, we demonstrate for the first time the potential for placental macrophages to provide information about the behavior of brain microglia, true for both obese and lean pregnancy. While further characterization of the CD11b+ cells in the placenta is needed, the fact that these cells behave similarly to brain microglia in response to immune challenge may have broader implications for assaying fetal brain development in a variety of maternal exposures. Second, we demonstrate that maternal obesity primes both placental macrophages and fetal brain microglia to overrespond to an ex vivo immune challenge. Sex-stratified analyses suggest that male placental macrophages and microglia have a stronger pro-inflammatory response in the setting of this priming. This observation may provide insight into the male predominance of neurodevelopmental morbidities observed with increased frequency in the setting of maternal obesity, such as autism spectrum disorder, cognitive dysfunction, and ADHD. All of these morbidities have been linked to microglial function, although the role of placental inflammation in these conditions has been less well-described. These experiments will form the foundation for an experimental program to investigate placental-brain crosstalk in maternal chronic inflammatory states, such as obesity.
Supplementary Material
Highlights.
Reactivity of CD11b+ placental macrophages is highly correlated with that of fetal brain microglia
Maternal obesity primes CD11b+ placental and brain macrophages to overrespond to immune challenge
Male fetuses are more vulnerable to this priming effect of maternal obesity than females
Placental CD11b+ cells may be an accessible biomarker of fetal brain immune activation in maternal obesity
Acknowledgments
Funding sources: This work was supported by the National Institutes of Health: 2K12HD000849-28 (PI: Edlow, Program PI: Schust)
R01ES025549 (PI: Bilbo)
American Board of Obstetrics and Gynecology (Edlow)
March of Dimes (Edlow)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of the funding sources: the funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication.
Declarations of interest: None
Contributor Information
Andrea G Edlow, Email: aedlow@mgh.harvard.edu.
Ruthy M Glass, Email: ruthymsher@gmail.com.
Caroline J Smith, Email: csmith97@mgh.harvard.edu.
Phuong Kim Tran, Email: sisiktran@gmail.com.
Kaitlyn James, Email: kjames5@mgh.harvard.edu.
Staci Bilbo, Email: sbilbo@mgh.harvard.edu.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, and Rossi FM. 2007. 'Local self-renewal can sustain CNS microglia maintenance and function throughout adult life', Nat Neurosci, 10: 1538–43. [DOI] [PubMed] [Google Scholar]
- Amsen D, de Visser KE, and Town T. 2009. 'Approaches to determine expression of inflammatory cytokines', Methods Mol Biol, 511: 107–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aye IL, Lager S, Ramirez VI, Gaccioli F, Dudley DJ, Jansson T, and Powell TL. 2014. 'Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways', Biol Reprod, 90: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL 2016. 'The placenta and neurodevelopment: sex differences in prenatal vulnerability', Dialogues Clin Neurosci, 18: 459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar J, Schreiber L, Saruhanov E, Ben-Haroush A, Golan A, and Kovo M. 2012. 'Placental histopathological findings in obese and nonobese women with complicated and uncomplicated pregnancies', Arch Gynecol Obstet, 286: 1343–7. [DOI] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, and Barres BA. 2016. 'New tools for studying microglia in the mouse and human CNS', Proc Natl Acad Sci U S A, 113: E1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Block CL, Bolton JL, Hanamsagar R, and Tran PK. 2018. 'Beyond infection - Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders', Exp Neurol, 299: 241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, and Schwarz JM. 2009. 'Early-life programming of later-life brain and behavior: a critical role for the immune system', Front Behav Neurosci, 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2012. 'The immune system and developmental programming of brain and behavior', Front Neuroendocrinol, 33: 267–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Smith SH, and Schwarz JM. 2012. 'A lifespan approach to neuroinflammatory and cognitive disorders: a critical role for glia', J Neuroimmune Pharmacol, 7: 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, and Tsang V. 2010. 'Enduring consequences of maternal obesity for brain inflammation and behavior of offspring', FASEB J, 24: 2104–15. [DOI] [PubMed] [Google Scholar]
- Bilimoria PM, and Stevens B. 2015. 'Microglia function during brain development: New insights from animal models', Brain Res, 1617: 7–17. [DOI] [PubMed] [Google Scholar]
- Bolton JL, and Bilbo SD. 2014. 'Developmental programming of brain and behavior by perinatal diet: focus on inflammatory mechanisms', Dialogues Clin Neurosci, 16: 307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Huff NC, Smith SH, Mason SN, Foster WM, Auten RL, and Bilbo SD. 2013. 'Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice', Environ Health Perspect, 121:1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, and Levitt P. 2011. 'A transient placental source of serotonin for the fetal forebrain', Nature, 472: 347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon JM 1995. 'Macrophage distribution in decidual tissue from early implantation to the periparturient period in mice as defined by the macrophage differentiation antigens F4/80, macrosialin and the type 3 complement receptor', J Reprod Fertil, 103: 9–16. [DOI] [PubMed] [Google Scholar]
- Brion MJ, Zeegers M, Jaddoe V, Verhulst F, Tiemeier H, Lawlor DA, and Smith GD. 2011. 'Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts', Pediatrics, 127: e202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, and Bale TL. 2014. 'Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment', Endocrinology, 155: 2635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2016. 'The Placenta as a Mediator of Stress Effects on Neurodevelopmental Reprogramming', Neuropsychopharmacology, 41: 207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, Mack M, Pinteaux E, Muller W, Zipp F, Binder H, Bopp T, Prinz M, Jung S, and Waisman A. 2015. 'Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System', Immunity, 43: 92–106. [DOI] [PubMed] [Google Scholar]
- Buttgereit A, Lelios I, Yu X, Vrohlings M, Krakoski NR, Gautier EL, Nishinakamura R, Becher B, and Greter M. 2016. 'Sall1 is a transcriptional regulator defining microglia identity and function', Nat Immunol, 17:1397–406. [DOI] [PubMed] [Google Scholar]
- Cao M, Cortes M, Moore CS, Leong SY, Durosier LD, Burns P, Fecteau G, Desrochers A, Auer RN, Barreiro LB, Antel JP, and Frasch MG. 2015. 'Fetal microglial phenotype in vitro carries memory of prior in vivo exposure to inflammation', Front Cell Neurosci, 9: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, and Hauguel-de Mouzon S. 2008. 'Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta', Placenta, 29: 274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, and Porter K. 2014. 'Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index', Cytokine, 70: 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew RC, Waddell BJ, and Mark PJ. 2016. 'Maternal obesity induced by a 'cafeteria' diet in the rat does not increase inflammation in maternal, placental or fetal tissues in late gestation', Placenta, 39: 33–40. [DOI] [PubMed] [Google Scholar]
- Crisham Janik MD, Newman TB, Cheng YW, Xing G, Gilbert WM, and Wu YW. 2013. 'Maternal diagnosis of obesity and risk of cerebral palsy in the child', J Pediatr, 163: 1307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debost JP, Larsen JT, Munk-Olsen T, Mortensen PB, Meyer U, and Petersen L. 2017. 'Joint Effects of Exposure to Prenatal Infection and Peripubertal Psychological Trauma in Schizophrenia', Schizophr Bull, 43: 171–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deputy NP, Dub B, and Sharma AJ. 2018. 'Prevalence and Trends in Prepregnancy Normal Weight - 48 States, New York City, and District of Columbia, 2011-2015', MMWR Morb Mortal Wkly Rep, 66: 1402–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N, Roman A, Rochelson B, Gupta M, Xue X, Chatterjee PK, Tam Tam H, and Metz CN. 2013. 'Maternal metformin treatment decreases fetal inflammation in a rat model of obesity and metabolic syndrome', Am J Obstet Gynecol, 209: 136 e1-9. [DOI] [PubMed] [Google Scholar]
- Deverman BE, and Patterson PH. 2009. 'Cytokines and CNS development', Neuron, 64: 61–78. [DOI] [PubMed] [Google Scholar]
- Edlow AG 2017a. 'Maternal obesity and neurodevelopmental and psychiatric disorders in offspring', Prenat Diagn, 37: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow AG, Guedj F, Pennings JL, Sverdlov D, Neri C, and Bianchi DW. 2016. 'Males are from Mars, and females are from Venus: sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity', Am J Obstet Gynecol, 214: 623 e1-23 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow AG, Mattei LH, Khattaby IO, Williamson CA, Daruvala S, Guedj F, Bianchi DW 2017b. 'Sex differences in offspring memory and anxiety in a mouse model of maternal diet-induced obesity', Am J Obstet Gynecol, 216 (1): S15–16. [Google Scholar]
- Estes ML, and McAllister AK. 2016. 'Maternal immune activation: Implications for neuropsychiatric disorders', Science, 353: 772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L, and Myatt L. 2017. 'Sexual dimorphism in the effect of maternal obesity on antioxidant defense mechanisms in the human placenta', Placenta, 51: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, and Maier SF. 2010. 'Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo', J Neuroimmunol, 226: 181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, and Maier SF. 2006. 'Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics', J Neurosci Methods, 151: 121–30. [DOI] [PubMed] [Google Scholar]
- Friis CM, Paasche Roland MC, Godang K, Ueland T, Tanbo T, Bollerslev J, and Henriksen T. 2013. 'Adiposity-related inflammation: effects of pregnancy', Obesity (Silver Spring), 21: E124–30. [DOI] [PubMed] [Google Scholar]
- Gallou-Kabani C, Vige A, Gross MS, Rabes JP, Boileau C, Larue-Achagiotis C, Tome D, Jais JP, and Junien C. 2007. 'C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome', Obesity (Silver Spring), 15: 1996–2005. [DOI] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, and McAllister AK. 2013. 'Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development', Brain Behav Immun, 31: 54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, and Merad M. 2010. 'Fate mapping analysis reveals that adult microglia derive from primitive macrophages', Science, 330: 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, and Prinz M. 2015. 'Origin of microglia: current concepts and past controversies', Cold Spring Harb Perspect Biol, 7: a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, Schedlowski M, and Meyer U. 2013. 'Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice', Science, 339: 1095–9. [DOI] [PubMed] [Google Scholar]
- Goeden N, Velasquez J, Arnold KA, Chan Y, Lund BT, Anderson GM, and Bonnin A. 2016. 'Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain', J Neurosci, 36: 6041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, and Taylor PR. 2005. 'Monocyte and macrophage heterogeneity', Nat Rev Immunol, 5: 953–64. [DOI] [PubMed] [Google Scholar]
- Gregor MF, and Hotamisligil GS. 2011. 'Inflammatory mechanisms in obesity', Annu Rev Immunol, 29: 415–45. [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, and Croxford AL. 2015. 'Microglia Versus Myeloid Cell Nomenclature during Brain Inflammation', Front Immunol, 6: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, and Bilbo SD. 2017. 'Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity', Glia, 65: 1504–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, and Bilbo SD. 2016. 'Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development', J Steroid Biochem Mol Biol, 160: 127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, and Merad M. 2013. 'Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes', Immunity, 38: 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikura U, Taanila A, Hartikainen AL, Olsen P, Linna SL, von Wendt L, and Jarvelin MR. 2008. 'Variations in prenatal sociodemographic factors associated with intellectual disability: a study of the 20-year interval between two birth cohorts in northern Finland', Am J Epidemiol, 167: 169–77. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, and McCarthy MM. 2003. 'Sex differences in response to kainic acid and estradiol in the hippocampus of newborn rats', Neuroscience, 116: 383–91. [DOI] [PubMed] [Google Scholar]
- Hinkle SN, Schieve LA, Stein AD, Swan DW, Ramakrishnan U, and Sharma AJ. 2012. 'Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age', Int J Obes (Lond), 36: 1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KR, and Powell TL. 2017. 'Effects of maternal obesity on placental function and fetal development', Reproduction, 153: R97–R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton CL, Morgan CP, Fischer DB, and Bale TL. 2013. 'O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development', Proc Natl Acad Sci U S A, 110: 5169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, and Patterson PH. 2012. 'Placental regulation of maternal-fetal interactions and brain development', Dev Neurobiol, 72: 1317–26. [DOI] [PubMed] [Google Scholar]
- Huang L, Yu X, Keim S, Li L, Zhang L, and Zhang J. 2014. 'Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project', Int J Epidemiol, 43: 783–92. [DOI] [PubMed] [Google Scholar]
- Hume DA 2008a. 'Differentiation and heterogeneity in the mononuclear phagocyte system', Mucosal Immunol, 1: 432–41. [DOI] [PubMed] [Google Scholar]
- ———. 2008b. 'Macrophages as APC and the dendritic cell myth', J Immunol, 181: 5829–35. [DOI] [PubMed] [Google Scholar]
- Iwasa T, Matsuzaki T, Kinouchi R, Fujisawa S, Murakami M, Kiyokawa M, Kuwahara A, Yasui T, and Irahara M. 2010. 'Neonatal LPS injection alters the body weight regulation systems of rats under non-stress and immune stress conditions', Int J Dev Neurosci, 28: 119–24. [DOI] [PubMed] [Google Scholar]
- Jansson T, and Powell TL. 2007. 'Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches', Clin Sci (Lond), 113: 1–13. [DOI] [PubMed] [Google Scholar]
- Jeong HK, Ji K, Min K, and Joe EH. 2013. 'Brain inflammation and microglia: facts and misconceptions', Exp Neurobiol, 22: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, and Kim BS. 2015. 'Isolation of CNS-infiltrating and Resident Microglial Cells', Bio Protoc, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Kurti A, Fair DA, and Fryer JD. 2014. 'Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring', J Neuroinflammation, 11: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Young SL, Grattan DR, and Jasoni CL. 2014. 'Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse', Biol Reprod, 90: 130. [DOI] [PubMed] [Google Scholar]
- Kim JS, Romero R, Kim MR, Kim YM, Friel L, Espinoza J, and Kim CJ. 2008. 'Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology', Histopathology, 52:457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, and Kim JS. 2009. 'Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease', J Immunol, 182: 3919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama R, and Ikegaya Y. 2015. 'Microglia in the pathogenesis of autism spectrum disorders', Neurosci Res, 100: 1–5. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, and Hertz-Picciotto I. 2012. 'Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders', Pediatrics, 129: e1121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, and Berman JW. 1993. 'Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta', J Immunol, 150: 2659–67. [PubMed] [Google Scholar]
- Lenz KM, and McCarthy MM. 2015. 'A starring role for microglia in brain sex differences', Neuroscientist, 21: 306–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente R, Gallardo ML, Berzal AL, Prada C, Garcia-Segura LM, and Viveros MP. 2009. 'Early maternal deprivation in rats induces gender-dependent effects on developing hippocampal and cerebellar cells', Int J Dev Neurosci, 27: 233–41. [DOI] [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada Gonzalez F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, Itzkovitz S, Elinav E, Sieweke MH, Schwartz M, and Amit I. 2016. 'Microglia development follows a stepwise program to regulate brain homeostasis', Science, 353: aad8670. [DOI] [PubMed] [Google Scholar]
- Muralimanoharan S, Guo C, Myatt L, and Maloyan A. 2015. 'Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity', Int J Obes (Lond), 39: 1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, and Streit WJ. 2012. 'Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function', Neurobiol Aging, 33: 195 e1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Alt JJ, and McCarthy MM. 2003. 'A novel model for prenatal brain damage. II. Long-term deficits in hippocampal cell number and hippocampal-dependent behavior following neonatal GABAA receptor activation', Exp Neurol, 181: 270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Loughlin E, Pakan JMP, Yilmazer-Hanke D, and McDermott KW. 2017. 'Acute in utero exposure to lipopolysaccharide induces inflammation in the pre- and postnatal brain and alters the glial cytoarchitecture in the developing amygdala', J Neuroinflammation, 14: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, and Gross CT. 2011. 'Synaptic pruning by microglia is necessary for normal brain development', Science, 333: 1456–8. [DOI] [PubMed] [Google Scholar]
- Peinnequin A, Mouret C, Birot O, Alonso A, Mathieu J, Clarencon D, Agay D, Chancerelle Y, and Multon E. 2004. 'Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green', BMC Immunol, 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, and Teeling J. 2013. 'Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration', Semin Immunopathol, 35: 601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L, Ni L, Ponzio NM, and Jonakait GM. 2013. 'Maternal inflammation promotes fetal microglial activation and increased cholinergic expression in the fetal basal forebrain: role of interleukin-6', Pediatr Res, 74: 393–401. [DOI] [PubMed] [Google Scholar]
- Rakic S, and Zecevic N. 2000. 'Programmed cell death in the developing human telencephalon', Eur J Neurosci, 12: 2721–34. [DOI] [PubMed] [Google Scholar]
- Rebuli ME, Gibson P, Rhodes CL, Cushing BS, and Patisaul HB. 2016. 'Sex differences in microglial colonization and vulnerabilities to endocrine disruption in the social brain', Gen Comp Endocrinol, 238: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes L, Wolfe B, and Golos T. 2017. 'Hofbauer Cells: Placental Macrophages of Fetal Origin', Results Probl Cell Differ, 62: 45–60. [DOI] [PubMed] [Google Scholar]
- Rising R, and Lifshitz F. 2005. 'Relationship between maternal obesity and infant feeding-interactions', Nutr J, 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, Hor K, Jabbour HN, Norman JE, and Denison FC. 2011. 'Placental structure and inflammation in pregnancies associated with obesity', Placenta, 32: 247–54. [DOI] [PubMed] [Google Scholar]
- Rodier PM 1980. 'Chronology of neuron development: animal studies and their clinical implications', Dev Med Child Neurol, 22: 525–45. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Miettunen J, Henriksen TB, Olsen J, Obel C, Taanila A, Ebeling H, Linnet KM, Moilanen I, and Jarvelin MR. 2008. 'Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts', Int J Obes (Lond), 32: 550–7. [DOI] [PubMed] [Google Scholar]
- Ruggiero MJ, Boschen KE, Roth TL, and Klintsova AY. 2018. 'Sex Differences in Early Postnatal Microglial Colonization of the Developing Rat Hippocampus Following a Single Day Alcohol Exposure', J Neuroimmune Pharmacol, 13: 189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, and Stevens B. 2017. 'Microglia emerge as central players in brain disease', Nat Med, 23: 1018–27. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Liddelow SA, and Dziegielewska KM. 2012. 'Barrier mechanisms in the developing brain', Front Pharmacol, 3: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma W, Basterra LB, Jacobs S, Brouwer N, Meerlo P, Schaafsma A, Boddeke Ewgm, and Eggen BJL. 2017. 'Maternal inflammation induces immune activation of fetal microglia and leads to disrupted microglia immune responses, behavior, and learning performance in adulthood', Neurobiol Dis, 106: 291–300. [DOI] [PubMed] [Google Scholar]
- Schliefsteiner C, Peinhaupt M, Kopp S, Logl J, Lang-Olip I, Hiden U, Heinemann A, Desoye G, and Wadsack C. 2017. 'Human Placental Hofbauer Cells Maintain an Antiinflammatory M2 Phenotype despite the Presence of Gestational Diabetes Mellitus', Front Immunol, 8: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, and Bilbo SD. 2012. 'Sex differences in microglial colonization of the developing rat brain', J Neurochem, 120: 948–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Consortium Schizophrenia Working Group of the Psychiatric Genomics, Daly MJ, Carroll MC, Stevens B, and McCarroll SA. 2016. 'Schizophrenia risk from complex variation of complement component 4', Nature, 530: 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seval Y, Korgun ET, and Demir R. 2007. 'Hofbauer cells in early human placenta: possible implications in vasculogenesis and angiogenesis', Placenta, 28: 841–5. [DOI] [PubMed] [Google Scholar]
- Sevenich L 2018. 'Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer', Front Immunol, 9: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J, Ruedl C, and Karjalainen K. 2015. 'Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells', Immunity, 43: 382–93. [DOI] [PubMed] [Google Scholar]
- Sisino G, Bouckenooghe T, Aurientis S, Fontaine P, Storme L, and Vambergue A. 2013. 'Diabetes during pregnancy influences Hofbauer cells, a subtype of placental macrophages, to acquire a pro-inflammatory phenotype', Biochim Biophys Acta, 1832: 1959–68. [DOI] [PubMed] [Google Scholar]
- Smolders S, Notter T, Smolders SMT, Rigo JM, and Brone B. 2018. 'Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders', Brain Behav Immun. [DOI] [PubMed] [Google Scholar]
- Smolders S, Smolders SM, Swinnen N, Gartner A, Rigo JM, Legendre P, and Brone B. 2015. 'Maternal immune activation evoked by polyinosinic:polycytidylic acid does not evoke microglial cell activation in the embryo', Front Cell Neurosci, 9: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, and Ferrell WR. 2007. 'Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers', J Clin Endocrinol Metab, 92: 969–75. [DOI] [PubMed] [Google Scholar]
- Streit WJ 2001. 'Microglia and macrophages in the developing CNS', Neurotoxicology, 22: 619–24. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Smith MS, Coleman K, and Grove KL. 2010. 'Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring', J Neurosci, 30: 3826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Naito M, Katabuchi H, and Higashi K. 1991. 'Development, differentiation, and maturation of macrophages in the chorionic villi of mouse placenta with special reference to the origin of Hofbauer cells', J Leukoc Biol, 50: 57–68. [DOI] [PubMed] [Google Scholar]
- Tanda R, Salsberry PJ, Reagan PB, and Fang MZ. 2013. 'The impact of prepregnancy obesity on children's cognitive test scores', Matern Child Health J, 17: 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grun D, Ronneberger O, and Prinz M. 2017. 'A new fate mapping system reveals context-dependent random or clonal expansion of microglia', Nat Neurosci, 20: 793–803. [DOI] [PubMed] [Google Scholar]
- Theiler K 1989. The House Mouse- Atlas of Embryonic Development. (Springer-Verlag: Edinburgh, UK: ). [Google Scholar]
- Turano A, Lawrence JH, and Schwarz JM. 2017. 'Activation of neonatal microglia can be influenced by other neural cells', Neurosci Lett, 657: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakubo A, Jarskog LF, Lieberman JA, and Gilmore JH. 2001. 'Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain', Schizophr Res, 47: 27–36. [DOI] [PubMed] [Google Scholar]
- Walker AK, Nakamura T, and Hodgson DM. 2010. 'Neonatal lipopolysaccharide exposure alters central cytokine responses to stress in adulthood in Wistar rats', Stress, 13: 506–15. [DOI] [PubMed] [Google Scholar]
- White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, and Bruce-Keller AJ. 2009. 'Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet', Neurobiol Dis, 35: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2014. "Global Status Report on Non-Communicable Diseases." In.: World Health Organization. [Google Scholar]
- Williamson LL, Sholar PW, Mistry RS, Smith SH, and Bilbo SD. 2011. 'Microglia and memory: modulation by early-life infection', J Neurosci, 31: 15511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther G, Elfving B, Muller HK, Lund S, and Wegener G. 2018. 'Maternal High-fat Diet Programs Offspring Emotional Behavior in Adulthood', Neuroscience, 388: 87–101. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Sheridan JF, and Godbout JP. 2014. 'Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior', Front Neurosci, 8: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Huang L, Li X, Li H, Zhou Y, Zhu H, Pan T, Kendrick KM, and Xu W. 2017. 'Immunological cytokine profiling identifies TNF-alpha as a key molecule dysregulated in autistic children', Oncotarget, 8: 82390–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Yuan TM, Gu WZ, and Li JP. 2004. 'Expression of glial fibrillary acidic protein in developing rat brain after intrauterine infection', Neuropathology, 24: 136–43. [DOI] [PubMed] [Google Scholar]
- Zhu MJ, Du M, Nathanielsz PW, and Ford SP. 2010. 'Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta', Placenta, 31: 387–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.