Abstract
Early life exposure to a low security setting, charcterized by a scarcity of resources and limited food access, increases the risk for psychiatric illness and metabolic dysfunction. We utilized a translational rat model to mimic a low security environment and determined how this manipulation affected offspring behavior, metabolism, and puberty. Because food insecurity in humans is associated with reduced access to healthy food options the “low security” rat manipulation combined a Western diet with exposure to a limited bedding and nesting manipulation (WD-LB). In this setting, dams were provided with limited nesting materials during the pups’ early life (P2-P10). This manipulation was contrasted with standard rodent caging (SD) and environmental enrichment (EE), to model “medium security” and “high security” environments, respectively. To determine if transitioning from a low to high security environment improved outcomes, some juvenile WD-LB offspring were exposed to EE. Maternal care was impacted by these environments such that EE dams engaged in high quality care when on the nest, but spent less time on the nest than SD dams. Although WD-LB dams excessively chased their tails, they were very attentive to their pups, perhaps to compensate for limited resources. Offspring exposed to WD-LB only displayed subtle changes in behavior. However, WD-LB exposure resulted in significant metabolic dysfunction characterized by increased body weight, precoscious puberty and alterations in the hypothalamic kisspeptin system. These negative effects of WD-LB on puberty and weight regulation were mitigated by EE exposure. Collectively, these studies suggest that both compensatory maternal care and juvenile enrichment can reduce the impact of a low security environment. Moreover, they highlight how utilizing diverse models of resource (in)stability can reveal mechanisms that confer vulnerability and resilience to early life stress.
Keywords: early life stress, limited bedding, western diet, animal models, social economic status, environmental enrichment, precocious puberty, kisspeptin, maternal care
1. Introduction
Both basic and clinical research suggest that early-life stressors (e.g. abuse, neglect) can modify brain development and make an individual prone to mental illness and metabolic dysfunction in later life (Spencer, Korosi, Layé, Shukitt-Hale, & Barrientos, 2017; Walker, et al., 2017; Yam, et al., 2016; Korosi, et al., 2012; Pervanidou & Chrousos, 2012; Avishai-Eliner, Gilles, & Eghbal-Ahma, 2001). For example, exposure to early-life stress increases the risk for mental illnesses, such as mood and depressive disorders, anxiety disorders, and disruptive behavior disorders (Afari, et al., 2013; Green, et al., 2010; Heim, Newport, Mletzko, Miller, & Nemeroff, 2008; Chapman, et al., 2004; Anda, et al., 2002). Moreover, early-life adversity has a positive association with metabolic dysfunctions including metabolic syndrome and the occurrence of precocious puberty (Cowan & Richardson, 2018; Pervanidou & Chrousos, 2012; Björntorp, 2008). To fully understand the relationship between early-life stress and these later life outcomes, it is neccesary to develop ecologically relevant animal models in order to derive their mechanistic underpinnings. This step will be imperative for the design and testing of translational treatments and preventative methods.
The hypothalamic pituitary adrenal (HPA) axis is a fundamental feature of the stress response (Jacobson & Sapolsky, 1991). Normally each component of this axis develops simultaneously, connecting into a functional network. However severely stressful events during the perinatal period can cause them to develop out of sync, resulting in life-long modifications in how the organism responds to stressful experiences (Heim, et al., 2008; Levine, 1994). Even a short period of stressor exposure during the first week of life in rodents can cause behavioral deviations and a vulnerability to physiological disruptions that parallel the clinical symptoms associated with early-life adversity (Walker, et al., 2017; Champagne & Curley, 2007). Moreover, the hypothalamic-pituitary-gonadal (HPG) axis is also susceptible to early life programming, connecting the downstream effects of early adverse experiences on reproductive functioning and stress reponsivity to a larger interactive network (Kentner & Pittman, 2010).
Puberty is the process of hormones orchastrating physiological changes that turns an organism from its sexually immature to its sexually mature state. During early development, kisspeptin in particular plays a role in the secretion of sex steroids (e.g. lutenizing hormone and follicle stimulating hormone) and the onset of puberty (Kauffman, Clifton, & Steiner, 2007; Skorupskaite et al., 2014). Precocious puberty is when an organism experiences puberty significantly ealier than its species predicted time. In humans, the occurrence of precocious puberty is indicated by significant pubertal markers such as the development of secondary sex characteristics (e.g. pubic hair and breast development) before age 8 or menarche before age 9 (Kaplowitz, et al., 2018; Baker & Kappy, 2011; Cesario & Hughes, 2007). Causes of precocious puberty include infection or trauma directed to the parts of the brain that control reproduction (Cowan & Richardson, 2018; Kaplowitz, et al., 2018), in addition to early life stress (Li et al., 2014; Kelly et al., 2017; Virdis et al., 1998). Precocious puberty is associated with short adult stature, emotional distress (i.e. depression), and other central nervous system abnormalaties (Kaplowitz, et al., 2018; Cesario & Hughes, 2007; Chalumeau, et al., 2002; Angold & Worthman, 1993). Both males and females can experience precocious puberty but it effects girls 10 times more frequently (Cesario & Hughes, 2007). Thus, precocious puberty could be considered a sex-dependent characteristic of humans who undergo early-life stress. Precocious puberty due to early life stress has also been reported in animal models of maternal separation and litter isolation (Cowan & Richardson, 2019; Kentner et al., 2018a; Grassi-Oliveira et al., 2016). Notably, sex-dependent precocial puberty is preventable by probiotic treatment (Cowan & Richardson, 2019) and sensory enrichment can also delay reproductive accerated maturation precipitated by early-life adversity (Kentner et al., 2018a).
Parental care has a critical influence on offspring development and negative experiences mediated through the parent-offspring relationship can significantly impair developmental outcomes. In the rodent laboratory, the dynamics of these relationships can be manipulated experimentally by changing the circumstances of the nest environment (Perry, et al., 2018; Walker, et al., 2017; McLaughlin, Verlezz, Gray, Hill, & Walker, 2016; Connors, et al., 2015; Kenny, et al., 2014; Champagne & Curley, 2007; Levine, 2001; Gilles, et al., 1996). One commonly used model of early-life adversity is the limited bedding model (LB) which involves reducing the availability of bedding materials that a dam uses to construct a nest for herself and her pups (Perry, et al., 2018; Walker, et al., 2017; Rice, Sandman, Lenjavi, & Baram, 2008; Gilles, et al., 1996). The lack of materials is a maternal stressor as it decreases the dam’s ability to construct a satisfactory nest (Perry, et al., 2018; Walker, et al., 2017) which influences the way that she interacts with her offspring (Rice, et al., 2008; Champagne & Curley, 2007). Dams housed with limited bedding have lower nest construction quality scores, have poorer nursing habits, and have been reported to step-on and rough handle their pups (Perry, et al., 2018; Heun-Johnson & Levitt, 2016; Heun-Johnson & Levitt, 2016; Sullivan & Holman, 2010; Ivy, Brunson, Sandman, & Baram, 2008). These maternal behavioral patterns show that the LB paradigm can cause care fragmentation and maltreatment (Perry, et al., 2018; Walker, et al., 2017; Gilles, et al., 1996). There is evidence to support that this adversity causes long-term negative consequences on cognitive development, motor skills, and socialization of offspring (Gilles, et al., 1996).
As it stands, the LB protocol can be translated to the experience of a low resource or ‘insecure’ environment for humans. As established earlier, poor access to resources impacts how a dam interacts with her pups and this is also true of human parents. Socioeconomic status can affect parental care quality, for example high economic pressure has been positively correlated to the use of punitive or authoritarian parenting (Leinonen, Solantaus, & Punamaki, 2003). These parenting strategies are associated with conduct disorders and disruptive behavior disorders in children (Stormshak, Bierman, McMahon, & Lengua, 2010). Additionally, lower socioeconomic status is related to low birth weight, asthma, deficient language development, and fewer pre-academic skills (Burchinal, Peisner-Feinberg, Bryant, & Clifford, 2000; Aber, Bennett, Conley, & Li, 1997; ). The rodent LB protocol is most useful for modeling an impoverished environment because, in addition to the stress from lack of physical resources, it can cause behavioral modifications in the mother known to affect a child’s development and adult outcomes.
On the other hand, environmental enrichment (EE) in the animal laboratory can translate to a high resource/high security situation and has been shown to rescue the effects of insecure “stressful” environments (Francis et al., 2002; Bredy et al., 2003; Bredy et al., 2004). Indeed, previous studies have shown that environmental complexity in early life can mitigate the effects of a number of early-life stressors including inflammation, poor maternal care, and neonatal brain injury (Kentner et. al, 2016; Schneider et al., 2006; Bredy et. al., 2004; Bredy et al., 2003; Pedrini Schuch et al., 2016; Durán-Carabali et al., 2018), highlighting its potential to mitigate the effects of LB, which to our knowledge has not been directly explored previously. Quantifying/mapping the therapeutic benefits of this housing condition has translational value in that enrichment protocols have been successfully utilized in clinical settings (Janssen et al., 2014; White et al., 2015; Woo et al., 2013; Woo et al., 2015), underscoring its acceptability and feasibility for use with patients.
Importantly, EE has also been shown to effect the quality of maternal care (Connors et al., 2015; Welberg et al., 2006; Sale et al., 2004; Durán-Carabali et al., 2018), but these effects have been underexplored. While there is some research focusing on defined periods of enrichment exposure (e.g. either pre- or postnatally; Cancedda et al., 2004; Rosenfeld and Weller, 2012), the number of studies evaluating dams in lifelong (or a combined pre- and postnatal) EE exposure are limited (Connors et al., 2015; Welberg et al., 2006; MacRae et al., 2015; Durán-Carabali et al., 2018). Moreover, some using this protocol have utilized co-parenting in the homecage, making the individual contribution of rodent dams difficult to assess. For this reason, we were interested in evaluating maternal care quality across the continuum of resource rich and poor animal laboratory conditions. Given the strength of maternal care quality in shaping offspring outcomes, it is important to understand differences in parent-offspring interactions as a function of environmental complexity. Environmental enrichment is an enhanced laboratory condition promoting the expression of species typical behaviors, when designing animal models for translational research it is imperative to consider environmental complexity and its impact on neurobiological indicies when trying to understand both the underlying etiology of disease and neurotypical development.
One imperative for improving animal models of early-life stressors is to simulate multiple adverse childhood experiences (ACEs). The 2016 National Survey of Children’s Health reported that one in ten children experience three or more ACEs and that poverty is one of the most commonly experienced stressors (Sacks & Murphey, 2018). Experiencing multiple ACEs is associated with increased risk for both metabolic and psychiatric diseases in adulthood (Sacks & Murphey, 2018; Afari, et al., 2013; Pervanidou & Chrousos, 2012; Heim, et al., 2008; Chapman, et al., 2004; Anda, et al., 2002). While there are many early-life stressors that could be incorporated into the LB paradigm, diet is of special interest considering the association of both food insecurity and obesity with a variety of metabolic and psychological outcomes (Spencer, et al., 2017; Yam, et al., 2016). Food insecurity is the condition of ones’ diet being restricted in terms of variety, nutrition, and amount of food (Eicher-Miller & Zhao, 2018). Children who are food insecure are more likely to have poor outcomes including, but not limited to, generally poor health and greater number of hospitalizations, psychosocial and behavioral problems, worse developmental outcomes, and are more likely to suffer from childhood obesity (Gundersen & Kreider, 2009). When examining the relationship between food security and low-income women it was found that food insecurity and diet quality were inversely related; those who were identified as food insecure had lower levels of perceived neighborhood safety, had a higher body mass index, and had lower access to healthy food options (Sanjeevi, Freeland-Graves, & Hersh, 2018). A Western diet (WD) is characterized by high levels of refined sugars and saturated fats, and low fiber content which are all associated with obesity (Francis & Stevenson, 2012). In animal models, high-fat and sugar diets can cause impairments of the hippocampus and dysregulation of the HPA axis (Boitard, et al., 2015; Maniam, Antoniadis, & Morris, 2015). In humans there is a higher occurrence of psychological disturbances (e.g. mood, anxiety, somatoform and eating disorders) among adolescents who are obese in addition to reduced hippocampal volume, which is associated with depression (Kalyan-Masih, et al., 2016; Björntorp, 2008; Campbell, Marriott, Nahmias, & MacQueen, 2004; Britz, et al., 2000). On its own early-life stress causes increased vulnerability to metabolic syndromes which is only exacerbated by food insecurity (Yam, et al., 2016; Tilburg, et al., 2010). For example, early-life stress induced by the LB model paired with a WD in male rats was associated with higher body fat and a positive correlation between white adipose tissue and object recognition scores indicative of cognitive impairments (Yam, et al., 2016).
In the present study, we adapted rodent caging systems to simulate low, medium, and high security environments and evaluated their effects on maternal care and offspring development. The ‘insecure’ housing was modelled by a combination of WD-LB exposure, while standard rodent caging served as a ‘medium security’ environment. In contrast, a high resource environment was modelled by housing a subset of animals in EE. Between these settings we compared differences in maternal care and tested the hypothesis that early life stress caused by a low resource scenario would result in poor maternal care and associated metabolic dysfunction, including precoscious puberty in offspring. Finally, we tested whether weaning into EE could rescue some of the adverse outcomes associated with the early-life stress protocol.
2. Methods
2.1. Animals and housing
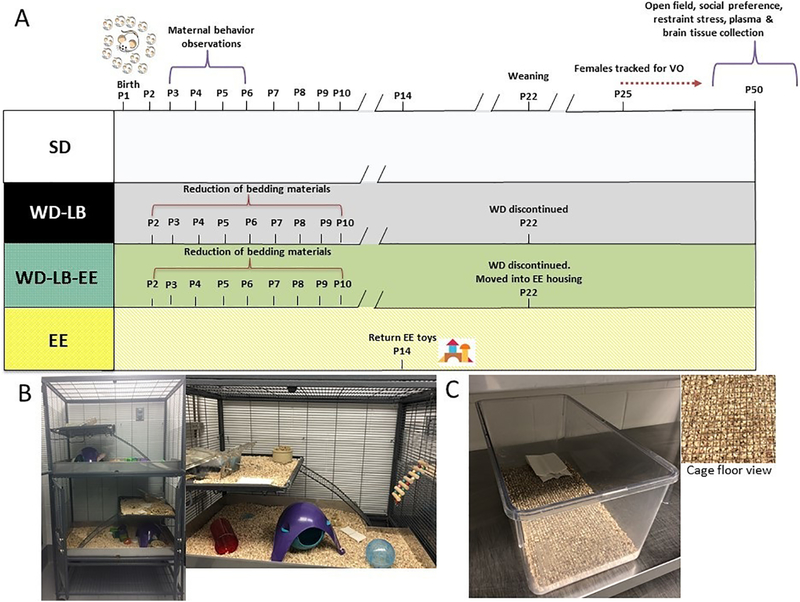
The experiment was approved by the MCPHS Institutional Animal Care and Use Committee and was carried out in compliance with the reccomendations from the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Thirty-six female and twelve male virgin Sprague-Dawley rats were acquired from Charles River (Wilmington, MA) and habituated in same-sex pairs; vivarium kept at 20°C on a twelve-hour light/dark cycle (0700–1900 light) in larger sized cages (51 × 41 × 22 cm). These cages were a one level cage with access to corn bedding, one plastic tube, one chew bone, Nestlets® (Ancare) and ad libitum access to food and water. After a two-week habitation period, a subset of female (n=14) and male (n=4) animals were allocated to a Western diet (WD; LabDiet® 5TJN). These females were assigned to later undergo LB housing (WD-LB; described below) in order to model a low resource or ‘insecure’ housing condition. A separate set of female (n = 22) and male (n = 6) rats were maintained on the standard diet (LabDiet® 5001). All animals were maintained on their respective diet for four weeks, prior to breeding, and remained on their assigned diet until weaning on postnatal day (P)22. One week prior to breeding ten females fed the standard diet were moved into EE (91.5 × 64 × 159 cm; Critter Nation, Muncie IN), maintaining their same-sex dyads. This EE cohort made up our ‘high resource security’ group. The EE housing units were multilevel cages with access to bedding, one tube, one chew bone, ample Nestlets® and toys. The location and type of toys used were changed two times weekly in order to stimulate novelty. The remaining standard chow fed females (n = 12) represented the medium or ‘middle class’ resource control group (standard housed; SD). Animals were weighed once weekly at 12pm. A timeline of the procedures can be found in Figure 1A. In line with initiatives to improve the reporting of experimental methods, we have complete the adapted reporting table from Kentner et al (2018b) and provided it as Supplementary Table 1.
Figure 1.
A) Timeline of study and housing conditions. B) Multilevel environmental enrichment cage. C) Representative picture of the WD-LB cage and bedding floor (inset).
2.2. Breeding and delivery
Breeding consisted of pairing one male with two females until pregnancy was verified by increased weight gain and the observation of visible teats. Pregnant females were kept in pairs until approximately gestational day (G)18, at which point they were housed individually in order to prevent the mixing of pups between litters. With respect to the WD-LB and SD groups, pregnant dams were placed into individual standard sized one level cages (27 × 48 × 20 cm). For the pregnant EE animals, a divider was built into the home cage so that litters could be separated – one litter housed in the top portion and one in the bottom until weaning. Each cage section had two levels (see Figure 1B). Toys were taken away from EE animals on G18 and returned on P14 in order to prevent the risk of pup injury during the early neonatal period. Day of birth was designated as P1; on P2 litters from all housing conditions were culled to n=12 (equal number of males and females where possible). Animals remained undisturbed until the morning of P10 at which point all cages were cleaned. In line with initiatives to reduce the number of animals in research (National Research Council, 2011), on P14, a small subset of male and female animals (n = 2) from the WD-LB litters were quickly administered a single dose of lipopolysaccharide (LPS; Escherichia coli; serotype 026:B6, L-3755; Sigma, St Louis, MO; 100mg/kg) so they could be included as part of a separate study (unpublished). It has previously been shown that this treatment does not disrupt maternal care (Spencer et al., 2006). One male and female animal from each litter was evaluated in the measurements described below. A list of offspring group designations can be found in Table 1.
Table 1:
Offspring Group Designations.
| Group Name | Description |
|---|---|
| SD | Control male and female offspring fed a standard diet and housed in standard laboratory cages. |
| WD-LB | Male and female offspring fed a western diet and housed in standard laboratory cages. Animals were exposed to reduced bedding beginning on the late afternoon of P2 through until the morning of P10. |
| WD-LB- EE | Male and female offspring fed a western diet and housed in standard laboratory cages. Animals were exposed to reduced bedding beginning on the late afternoon of P2 through until the morning of P10.These animals were placed into environmental enrichment at weaning on P22. |
| EE | Male and female offspring fed standard diet housed in enriched multilevel cages; offspring were given novel enrichment toys twice weekly starting on P14. |
2.3. Limited Bedding Model
On the late afternoon of P2 a stainless-steel grate was placed into the cage of WD fed animals (adapted from McLaughlin et al., 2016) creating the WD-LB housing group. In our case, the stainless steel grates sat on top of the bedding, restricting access of the dams to their bedding (see Figure 1C). Additionally, a single piece of paper towel was added in lieu of Nestlets® (Gilles, et al., 1996). Cages were left undisturbed until the morning of P10 at which point the stainless-steel grates were removed and litters placed into fresh clean cages with the same level of access to nesting materials as the SD group.
2.4. Maternal care observations
The first week of life is a critical time for rodents (Champagne & Curley, 2007). Between P3-P6 passive home cage maternal behavior observations were conducted twice daily; once in the light phase (07:45–10:00 h) and once in the dark (19:45–22:00 h) to account for the nocturnality of rodents (Champagne & Curley, 2007). Each observational period consisted of five one-minute observations, with five minutes of no observation occurring between each one-minute bin. Recorded maternal behaviors included the frequency of pup retrievals, dam licking pup, passive/low crouch nursing, active/high crouch nursing, nest building/digging in bedding, dam self-grooms, dam sleeping, dam eating/drinking, dam chasing her tail, and total time on nest (seconds).
2.5. Assessment of puberty onset
Beginning on P25, female rats were evaluated daily for vaginal openings (VO) until all animals were scored as open. Animals were scored by their appearance as being either closed, or having a complete vaginal opening (Cowan & Richardson, 2018; Kentner et al., 2018a; Grassi-Oliveira et al., 2016).
2.6. Open field test
To evaluate the presence of anxiety-like behavior (Crawley, 2007), an open field test was conducted in dams on P22 and offspring on P50. Thirty minutes prior to testing animals were allowed to habituate to the testing room. Dams were placed into a black, square box (72 cm × 72 cm × 36 cm) and video recorded for ten minutes. Juvenile male and female offspring were similarly evaluated in a smaller arena (40 cm × 40 cm ×28 cm) and video recorded for five minutes. Between each recording session all of the equipment was thoroughly cleaned with Quatriside TB. Videos were then scored through an automated behavioral monitoring software program (Cleversys TopScan, Reston, VA) and evaluated for the percent of time (seconds) spent in the center of the arena [((total time in center)/(total time in center + total time in perimeter))*100] and total distance travelled (cm) in the arena.
2.7. Social preference test
Immediately following the open field test, which was used as a habituation period to the test arena, the social preference for a novel conspecific versus an inanimate object (Crawley, 2007) was evaluated. During this assessment, juvenile offspring were placed into the center of the arena and video recorded for five minutes. During this period animals had the choice to visit either a same sex/size/age novel conspecific or an object, each confined within a small wire cup on opposite ends of the arena. Placement of the novel conspecific and the object were counterbalanced between tests and experimental animals groups. Between each recording all of the equipment was thoroughly cleaned with Quatriside TB. Using the software program Stop Watch+ (Atlanta, Georgia), videos were then scored by two blinded observers with an established interrater reliability of >90%. The variables scored were the duration of time (seconds) spent with and frequency of visits to either the same sex/size/age novel conspecific, or object. From these variables, a social preference score was calculated and expressed as a preference ratio arena [((total time with novel rat)/(total time with novel rat + total time with object))*100] where scores >0.5 indicated a preference for the novel rat compared to the object.
2.8. Tissue collection
Two hours after behavioral testing dams and their offspring underwent a 20-minute restraint stress procedure. During this period, animals were individually restrained in a soft open-ended plastic pastry bag (Daymark) and immediately euthanized with a mixture of Ketamine/Xylazine (40–80 mg/kg, i.p. 5–10 mg/kg, i.p.). Cardiac blood was collected in EDTA tubes and plasma collected. Brains were immediately collected and placed on a clean petri dish set on wet ice. Whole hypothalamus was quickly dissected from the freshly collected tissue and frozen on dry ice.All tissues were stored in −70°C for future processing.
2.9. ELISA
Plasma corticosterone and leptin levels were analyzed in duplicate by ELISA using the manufacturer’s instructions. With respect to the plasma corticosterone ELISA we followed the small sample assay protocol provided with the testing kit (ADI-900–097, Enzo Life Sciences, Farmingdale, NY, USA). The minimum detectable concentration was 26.99 pg/ml, and the intra and inter-assay coefficients of variation were 6.6% and 7.8%, respectively. The minimum detectable leptin concentration (abcam ab100773, Abcam) was 30 pg/mL, intra and inter-assay coefficients of variation were <10% and <12%, respectively.
2.10. PCR
Total RNA was extracted from frozen hypothalamus with TRIzol reagent (Thermo Fisher). Isolated RNA was quantified utilizing a NanoDrop 2000 spectrophotometer (Thermo Fisher). cDNA synthesis and qPCR were performed using TaqMan assay probes to evaluate Kiss1, Kiss1R, Gnrh1, Gnrhr and Gapdh (Rn00710914_m1, Rn00576940_m1, Rn00562754_m1, Rn00578981_m1 and Rn01775763_g1, respectively) in a StepOne Plus station (Applied Biosystems) as described previously (Kentner et al., 2018a). Each sample was analyzed in duplicate and relative gene expression levels were evaluated using the ΔΔCt method with Gapdh as the housekeeping gene. Data are presented as mean expression relative to CON-nSaline animals, which have been normalized to 1 for each gene of interest.
2.11. Westerns
Microdissections of the medial prefrontal cortex (mPFC), centered on the infralimbic and prelimbic regions with the ventral anterior cingulate cortex included (A/P = 3.24mm and M/L = 0 mm from Bregma, D/V = −3.2 mm from the surface of the brain) were taken using a trephine from flash froze tissue. Tissue samples were homogenized in RIPA buffer (R0278, Sigma) and HaltTM protease and phosphatase inhibitor cocktail (ThermoFisher, 78440), spun at 40,000 × g for 30 min at 4°C, and the supernatant assayed with the BCA protein Assay (ThermoFisher, 23227). Samples (30ug) were loaded on 4–15% Mini-PROTEAN® TGX™ Precast Protein Gels (BioRad, 456–1084). Gels were run for 35 min, at 200V in 1× Tris/Glycine/SDS buffer (BioRad,1610771). Then gels were transferred to a PVDF membrane (Immobilon-FL, IPFL00010) in 1× Tris/Glycine buffer (BioRad, 1610734) for 1.5 h at 55V. After transfer, membranes were stained with the REVERT Total Protein Stain Kit (BioRad, 926–11016) for total protein normalization according to manufacturer’s instructions and immediately imaged with Odyssey FC Imaging System using Image Studio Life Version 4.0. Then membranes were blocked with Odyssey Blocking Buffer (927–50000) for 1h. Afterwards, membranes were probed with an anti-synaptophysin antibody, clone SY38 (1:1000, Millipore, MAB5258) diluted in 1:1 Odyssey Blocking Buffer:TBS overnight at room temperature. Membranes were washed three times for 5 minutes with TBST and them probed with anti-mouse IRDye 800CW (1:20,000, LiCor) 4 h at room temperature. After three additional 5 min TBST washes, membranes were placed in TBS before imaging with Odyssey FC Imaging System. After scanning, Odyssey Infrared Imaging software quantified the integrated intensity of each band and determined molecular weights based on Biorad Precision Plus Protein Standards (1610374). The ratio of synaptophysin to total protein for the lane was calculated (Kirshner & Gibbs, 2018). For the figures, each channel of the image was adjusted for brightness and contrast individually using the Odyssey Infrared Imaging software and adjustments were applied equally to the entire image.
2.12. Statistical analyses
Statistics were performed using the software package Statistical Software for the Social Sciences (SPSS) version 21.0 (IBM, Armonk, NY) or GraphPad Prism (version 7.0), in the case of the survival curves described below. Alpha levels were set to p< 0.05 for all omnibus tests. A one-or two-way repeated measures ANOVA was used to evaluate maternal (baseline, weeks 1–4, P22) and offspring body weights (P22, P29, P36, P43, P50) respectively. In each case, time was the repeated factor while either housing or housing and sex were the independent variables, as appropriate. The Greenhouse–Geisser correction procedure was applied due to violations to the assumption of sphericity for repeated measure designs. For maternal behavior, Chronbach’s alpha was calculated and interrater reliability between two raters was found to be >80%. Due to the severity of skewness (Shapiro-Wilks) for maternal and offspring behaviors and plasma corticosterone levels, non-parametric Kruskal-Wallis tests (non-parametric equivalent to the one-way ANOVA) were employed. Pairwise comparisons were made using conservative adjusted alpha correction values. Day of VO and female offspring hypothalamic gene expression were evaluated using one way-ANOVAs. With respect to parametric data, LSD post hocs were applied except where there were fewer than three levels, in which case pairwise t-tests and Levene’s (applied in the occurrence of unequal variances on the post hoc assessments) were utilized. The time to VO was also assessed, using the Log-rank (Mantel Cox) test, and plotted as a survival curve. For applicable offspring analyses, if there were no sex differences, then male and female data were collapsed together for analyses and presentation. Data were graphically expressed as mean ±SEM.
3. Results
3.1. Maternal body weights
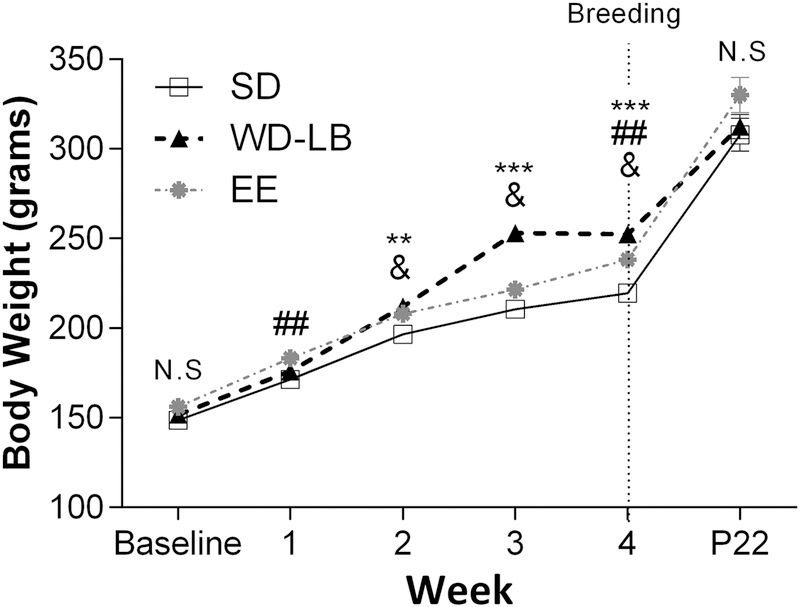
A repeated measures ANOVA revealed a significant interaction between time and housing for maternal body weights (F(3.956, 59.337) = 4.743, p = 0.002; Figure 2). LSD post hocs did not show a difference across housing groups for maternal body weight at either baseline or P22, however significant group differences were observed across the course of the study with WD rats showing significantly greater weight gain compared to SD controls (Week 2: p = 0.001; Weeks 3 and 4: p = 0.0001) and EE animals (Week 3: p = 0.05; Week 4: p = 0.023), validating the western diet treatment. EE females also had significantly more weight gain than SD rats across several points of the study (Week 1: p = 0.008; Week 2: p = 0.023; Week 4: p = 0.006).
Figure 2.
Validation of Western diet on body weight gain (grams) across time. Baseline, weeks 1–4 (prior to breeding), and P22 (weaning). Data expressed as mean ±SEM; n = 8–10 per group. Various symbols are employed (e.g., $, &, *) to delineate differences between groups where *p < 0.05, **p <0.001, ***p<0.001, difference between SD and WD; #indicates differences between SD and EE; &indicates difference between WD and EE.
3.2. Maternal Care
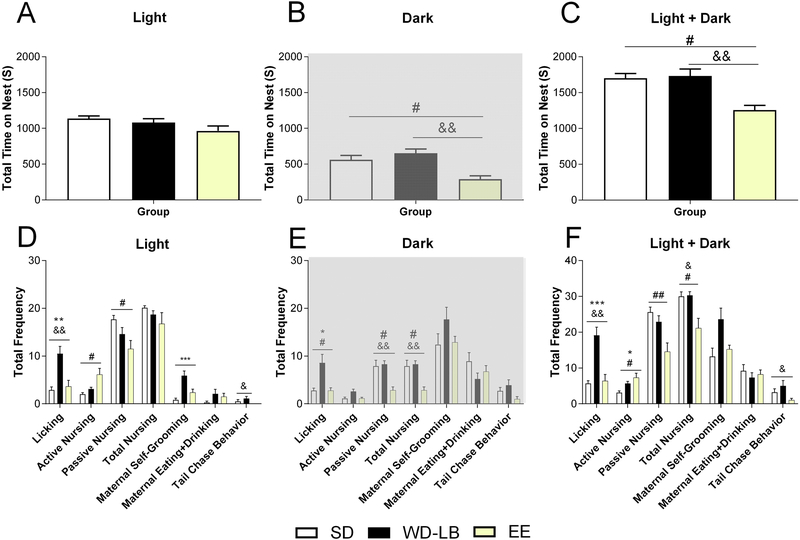
With respect to P3–6 maternal behavior, although there were no treatment differences with respect to time on nest (seconds) during the light phase observation period (X2(2)=4.535, p=0.104; Figure 3A), Kruskal-Wallis tests revealed a significant main effect of housing during the night (X2(2)=12.545, p=0.002; Figure 3B) and for the combined total of the daylight and nightime observations (X2(2)=11.319, p=0.003; Figure 3C) on this measure. Follow-up tests demonstrated that SD (dark: p=0.021; light & dark: p=0.013) and WD-LB (dark: p=0.002; light & dark: p=0.007) dams both spent more time on nest than EE mothers.
Figure 3.
Maternal behavior observations from standard (SD), Western diet-limited bedding (WD-LB), and environmentally enriched (EE) rat dams as a function of postnatal days in the light phase (left panels), dark phase (middle panels) and light and dark phases combined (right panels). Panels A-C display total time on nest (seconds). Panels D-F display the frequency of licking, active and passive nursing, total nursing, maternal self-grooming, maternal eating and drinking and tail chase behaviors. All maternal behavior data are expressed as mean ±SEM; n = 8–10 per group. Various symbols are employed (e.g., $, &, *) to delineate differences between groups where *p<0.05; **p<0.01; ***p<0.0001 indicates differences between SD and WD-LB, #indicates differences between SD and EE. &indicates differences between WD-LB and EE.
During the light phase, there was a significant main effect of housing for licking/grooming (X2(2)=12.763, p=0.002), active nursing (X2(2)=7.539, p=0.023), passive nursing (X2(2)=7.954, p=0.019), maternal self-grooming (X2(2)=14.779, p=0.001), maternal eating and drinking (X2(2)=6.396, p=0.041), and tail chasing (X2(2)=7.495, p=0.024) behaviors (Figure 3D). Post hoc analysis revealed that WD-LB dams licked their pups more frequently than both SD (p=0.004) and EE (p=0.012) mothers. WD-LB dams also groomed themselves more than those reared in SD cages (p=0.0001). EE animals engaged in active nursing significantly more often than SD dams (p=0.019) and SD dams engaged in passive nursing significantly more frequently than EE dams (p=0.016). Finally, WD-LB animals demonstrated more tail chase behaviors than EE dams (p=0.024).
For observations during the dark phase, there were differences in the frequency of licking/grooming (X2(2)=10.478, p=0.005), active nursing (X2(2)=6.491, p=0.039), passive nursing (X2(2)=10.897, p=0.004), and total nursing (X2(2)=13.772, p=0.001) behaviors (Figure 3E). Pairwise comparisons revealed that WD-LB dams licked their pups significantly more than both EE (p=0.014) and SD (p=0.015) mothers. EE animals engaged in fewer bouts of passive nursing postures compared to SD (p=0.014) and WD-LB (p=0.007) animals. The same pattern was also true for the frequency of total nursing behaviors (WD-LB vs SD: p=0.026; WD-LB vs EE: p=0.001).
For the combined day and nighttime observation periods there were differences in licking/grooming pups (X2(2)=16.716, p=0.0001), active nursing (X2(2)=10.235, p=0.006), passive nursing (X2(2)=10.067, p=0.007), total nursing (X2(2)=9.844, p=0.007), maternal self-grooming (X2(2)=6.627, p=0.036), and tail chase (X2(2)=6.337, p=0.042) behaviors (Figure 3F). Post hocs demonstrated that WD-LB dams licked their pups significantly more often than both SD (p=0.001) and EE (p=0.002) mothers. For active nursing, SD dams engaged in significantly fewer bouts than both WD-LB (p=0.036) and EE (p=0.01) animals. SD dams engaged in significantly more passive nursing behaviors than the EE (p=0.006) dams. For total nursing behaviors, EE animals did not nurse as frequently as either the SD (p=0.019) or WD-LB (p=0.013) animals. With regards to maternal self-grooming, there were no significant differences across housing groups. Finally, WD-LB animals chased their tails significantly more than EE (p=0.042) dams.
3.3. Maternal open field test and stress-induced plasma corticosterone
No significant differences were observed across housing groups on measures of P22 maternal anxiety-like behavior and locomotor activity (open field test) or on maternal stress-induced (20 minute restraint stressor) plasma corticosterone (p>0.05, data not shown).
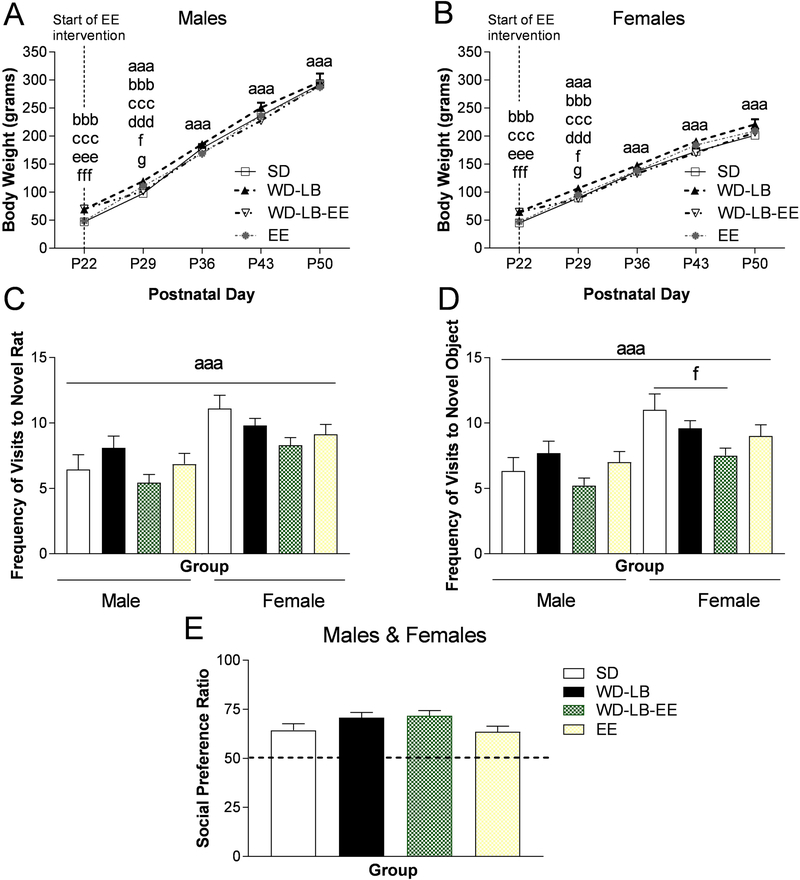
3.4. Offspring body weights
A two way repeated measures design (housing by sex) revealed a sex by time interaction (F(2.199, 142.921) = 79.720, p = 0.0001; n = 8–10; Figure 4AB). Post hocs showed that male offspring were heavier than females (p<0.0001) at P29, P36, P43, and P50. There was also a housing by time interaction (F(6.596, 142.921) = 2.451, p = 0.023; Figure 4AB). WD-LB animals were heavier than SD and EE offspring at weaning on P22 (SD: p =0.0001; EE: p=0.0001) and on P29 (SD: p =0.0001; EE: p=0.0009). WD-LB-EE animals had significantly higher body weights than SD (p=0.0001) and EE (p=0.0001), but were not different than WD-LB offspring on P22. On P29, EE offspring weighed more than SD rat (p=0.017). By P29, WD-LB-EE rats weighed significantly less than WD-LB offspring, suggesting that enrichment was able to quickly rescue the metabolic disruptions induced by a high fat diet. There were no differences in weight across the housing groups by P36, supporting the existence of a resilient weight regulation mechanism that may have turned on after the cessation of the western diet on P22.
Figure 4.
Offspring body weights (grams) for male (A) and female (B) animals. C) Social preference ratios for male and female (collapsed) offspring. Values >0.05 indicate that the experimental rat spent more time visiting with the novel rat compared to the novel object. The total frequency of visits made to the D) novel rat and E) novel object in the social preference test. All data are expressed as mean ±SEM, n = 8–10. Various symbols are employed (e.g., a, b, c) to delineate differences between groups where ap<0.05, aap<0.01, aaap<0.001 indicates differences between males and females; bindicates differences between SD and WD-LB, cindicates differences between WD-LB and EE, dindicates differences between WD-LB and WD-LB-EE, eindicates differences between WD-LB-EE and EE, findicates differences between SD and WD-LB-EE, and gindicates differences between SD and EE.
3.5. Offspring behaviors
Non-parametric Kruskall-Wallis tests did not uncover sex or housing differences for either percent time spent in the center of the open field or for locotomor activity level (n = 8–10; p>0.05, data not shown). Non-parametric analysis revealed significant main effects of sex on the frequency of visits to the novel rat (X2(1) = 16.667, p = 0.0001; Figure 4C) and object (X2(1) = 14.553, p = 0.0001; Figure 4D) in the social preference test, in that females made a higher number of visits overall (p<0.05). There was also a main effect of housing for the total frequency of visits that female offspring made to the novel object (X2(3) = 17.970, p = 0.043; Figure 4D) in that female SD rats visited the novel object more than WD-LB-EE rats. Kruskall-Wallis tests uncovered a main effect of housing on the social preference test (X2(3) = 8.282, p = 0.041; Figure 4E), but follow-up tests did not confirm differences as a function of treatment (p>0.05).
3.6. Precoscious puberty,hypothalamic gene expression, and plasma leptin concentrations
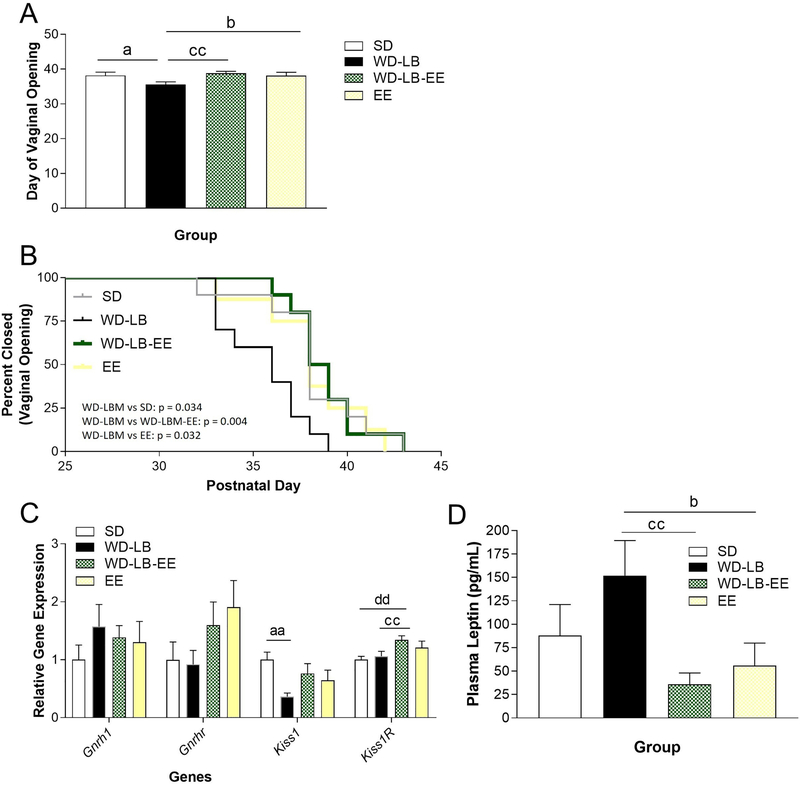
One way ANOVA showed a significant effect of housing on pubertal maturation (F(3, 34) = 3.235, p = 0.034; n = 8–10, Figure 5A). LSD follow up tests revealed that full vaginal opening occurred significantly earlier in WD-LB female offspring compared to SD (p = 0.025), EE (p = 0.040), and WD-LB-EE (p = 0.007) rats. A more in depth evaluation of time to vaginal opening using the Log-rank (Mantel-Cox) test revealed a significant effect of housing (X2(3) = 11.83, p =0.008; Figure 5B). Comparisons of the survival curves revealed significant differences in day of vaginal opening (DVO) between SD (DVO: P38) vs. WD-LB (DVO: P36; X2(1) = 4.685, p =0.0304), WD-LB vs. WD-LB-EE (DVO: P38.5; X2(1) = 8.225, p =0.0041), as well as WD-LB vs. EE (DVO: P38); X2(1) = 4.628, p =0.0315). Animals from all treatment groups displayed full vaginal openings by P43 (Figure 5B).
Figure 5.
A) Day of full vaginal opening for female offspring. B) Survival curves of full vaginal opening for each group expressed across time. C) Relative hypothalamic gene expression of Gnrh1, Gnrhr1, Kiss1, Kiss1R normalized to SD controls. D) Plasma leptin levels (pg/mL) on postnatal day 50. All data are expressed as mean ±SEM. Various symbols are employed (e.g., a, b, c) to delineate differences between groups where ap<0.05, aap<0.01, aaap<0.001 indicates differences between WD-LB and SD; bindicates differences between WD-LB and EE, cindicates differences between WD-LB and WD-LB-EE; dindicates differences between SD and WD-LB-EE.
The kisspeptin/gonadotropin releasing hormone (GnRh) system is an important pathway integral to puberty initiation (Kauffman, Clifton, & Steiner, 2007; Skorupskaite et al., 2014) so we evaluated the P50 expression of several related hypothalamic gene targets (n = 7–10). One way ANOVAs did not indicate differences as a function of housing for Gnrh1 or for its receptor Gnrhr (p>0.05, Figure 5C). However, there were significant main effects of housing for Kiss1 (F(3,30) = 3.089, p = 0.043; Figure 5C) and Kiss1r expression (F(3, 30) = 7.708, p =0.022; Figure 5C). LSD follow-up tests showed that WD-LB female rats had significantly lower levels of Kiss1 compared to SD rats (p = 0.006). Environmental enrichment did not completely rescue this effect (WD-LB vs WD-LB-EE: p =0. 052). However, WD-LB-EE animals were not significantly different from SD rats on this measure (p>0.05), suggesting some modest attenuation following enrichment. Hypothalamic Kiss1r was significantly elevated in WD-LB-EE animals compared to both SD (p=0.005) and WD-LB (p = 0.016) females. This suggests that the elevated levels of Kiss1r following EE may have compensated in part for the depleted Kiss1 levels associated with WD-LB housing.
Given evidence that plasma leptin concentrations are correlated with precocious puberty we elected to measure this hormone in our female animals (Sominsky et al., 2016). A one-way ANOVA revealed a significant housing effect on plasma leptin concentrations (F(3, 25) = 3.172; p = 0.042; Figure 5D). LSD post hoc tests demonstrated that WD-LBM female rats had higher plasma leptin levels compared to WD-EE (p=0.007) and EE (p = 0.033) animals, respectively. There were no significant differences between SD and WD-LBM females (p > 0.05).
3.7. Offspring plasma corticosterone
No significant differences were observed across experimental groups on measures of stress-induced (20 minute restraint stressor) plasma corticosterone for P50 offspring (n = 7–10, p>0.05, data not shown).
3.8. Levels of synaptophysin in the mPFC
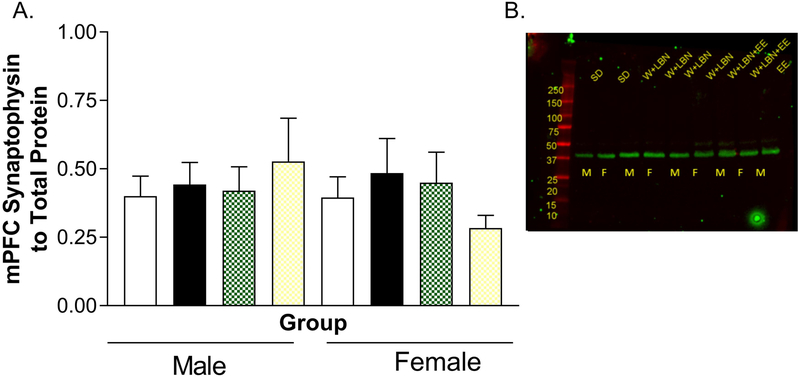
The mPFC continues to develop through puberty, and this development is characterized by alterations in synpases (Drzewiecki et al., 2016). To determine whether sex or housing influenced mPFC maturation we evaluated synaptophysin, a marker of synapses used to assessed cortical synaptic development (Glantz et al., 2007). Values that exceeded 2 standard deviations above or below the group mean were considered outliers and dropped from the analysis. No significant differences were observed in synaptophysin levels in mPFC (n= 8–10, p> 0.05, Figure 6).
Figure 6.
Analysis for synaptophysin in the mPFC. A) Graph shows the mean ratio of the integrated intensity of each band of synaptophysin relative to the total protein signal for the lane of the same sample. There were no significant effects. B) Representative blot showing the levels of synaptophysin. These were normalized to the REVERT total protein stain depicted in Supplementary Figure 1. Please note that the second female EE animal is missing in the representative blot as the membrane only holds nine samples with the molecular weight marker.
Discussion
The present experiments were designed to assess the impact of high, medium, and low security environments on maternal care quality and, in tandem, to evaluate the maturation and neurobehavioral outcomes of male and female offspring. The results show that resource insecurity and environmental complexity differentially affect maternal behavior, but not along a continuum ranging across defined categories from ‘low’ to ‘high’ quality, as one might expect. Instead, circadian timing greatly influenced the temporal pattern of behavioral expression, unveiling potential strengths and weaknesses in maternal care quality across the housing conditions. Offspring born to low resource WD-LB dams were resilient to substantative disruptions in behavior and mPFC plasticity. Only subtle perturbations were observed in the measures evaluated here, and were limited to differences in exploration of the novel object by female WD-LB rats in the social preference test. This did not translate into impairments of species typical preferences for social novelty. Instead, consequences of early life stress were most apparent by metabolic changes such as increased body weight and accelerated puberty, in addition to alterations in the levels of the kisspeptin gene and expression of its receptor. Importantly, placement into EE during the early juvenile period fast-tracked the regulation of body weight after cessation of the Western diet in WD-LB offspring. Moreover, the juvenile enrichment intervention prevented the precocial puberty associated with a history of early life stress. Given that enrichment is a translationally valid intervention for both animals and humans (Woo et al., 2013; Woo et al., 2015; Kentner et al., 2018c), this study offers further insight into additional unexplored uses for this tool. This is in addition to highlighting the utility of developing and incorporating clinically relevant animal models into our basic reserach.
While animal models can be used as a translational approach to evaluate the neurobehavioral effects of early-life stress, or even positive early life experiences, it is important to acknowledge their strengths and shortcomings in terms of test validity. For example, construct, face, and predictive validity satisfy the three-criteria required for an animal disease model: congruent risk factors, symptoms, and efficacy of treatment, respectively (Estes & McAllister, 2016; Belzung & Lemoine, 2011). Although early-life exposure to stressors do not always lead to a disease state, these adverse experiences do increase the risk for mental illnesses (e.g. depression, anxiety) in later life (Kumsta, et al., 2017; Burkholder, Koss, Hostinar, Johnson, & Gunnar, 2016; Bentall, Wickham, Shevlin, & Varese, 2012; Green, et al., 2010; Heim, et al., 2008; Chapman, et al., 2004). Employing animal models of early-life stress such as maternal separation or LB meet construct validity in that they mimic risk factors such as neglect or abuse. Moreover, they demonstrate face validity in that specific symptoms of mental illnesses (e.g. impaired emotional reactivity, disrupted HPA regulation, cognitive disturbances, anxiety-like behavior) can be observed in animals that have experienced these stressors during the perinatal period (Grassi-Oliveira, Honeycutt, Holland, Ganguly, & Brenhouse, 2016; Leussis, Freund, Brenhouse, Thompson, & Andersen, 2012; Raineki, Cortés, Belnoue, & Sillivan, 2012; Chatterjee, et al., 2007; Gonzalez, Lovic, Ward, Wainwright, & Fleming, 2001; Fernandez-Teruel, Escorihuela, Driscoll, Tobena, & Battig, 1991).
Animal models of early-life stress can also be used to predict treatment efficacy. For example, in humans, skin-to-skin contact in the form of Kangaroo Care has been successful in treating the adverse effects of premature birth and improving parent-infant attachment when compared to traditional treatment (Charpak, et al., 2016; Baley, 2015; Tessier, et al., 2003). Similarly, when rat pups are isolated or artificially reared in the laboratory, maternal tactile contact can be simulated by stroking the animal with a paintbrush to emulate licking and grooming behaviors (Chatterjee, et al., 2007). Experimentally this has led to improvements in a number of neural proteins integral to development: higher densities of synaptophysin, neural cell adhesion molecule, growth associated protein 43, brain-derived neurotrophic factor and glucocorticoid receptor expression, when compared to control animals (Chatterjee, et al., 2007; Kentner et al., 2018c). Artificial rearing can have an intergenerational, detrimental behavioral effect. Artificially reared mothers will engage in the same maternal behaviors as control mothers but they will do so for less time, and additionally these dams will show an increase of non-maternal behaviors such as digging, biting at the cage floor, and chasing their own tail (Lomanowska & Melo, 2016; Barrett & Fleming, 2001; Burton, et al., 2007; Gonzalez, et al., 2001). Tactile stimulation during artificial rearing can improve the quality of maternal behaviors that artificially reared mothers provide to their own offspring, compared to artificially reared mothers that did not receive tactile stimulation (Gonzalez et al., 2001). For human infants, similar tactile contact in the form of Kangaroo Care has short-term improvements in birthweight and survival, and long-term improvements in academic success, and higher IQ (Charpak, et al., 2016; Baley, 2015; Tessier, et al., 2003). For human mothers there are improvements in breast milk production, reduced feelings of hopelessness, and better infant attachment (which also extends to fathers; Baley, 2015).
In a similar vein, children who were institutionalized and then moved to foster care showed improvements in cognitive, motor, and behavioral domains (as measured by the Bayley Scale of Infant Development) as well improvements in intellectual functioning in verbal and performance domains (as measured by the Weschler Preschool Primary Scale of Intelligence) when compared to their peers who remained institutionalized (Nelson, et al., 2007). When observing the differences between institutionalized vs never institutionalized children’s brains MRI revealed that those with a history of institutionalization had significantly reduced cortical gray matter volume (Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012). Foster care is superior to institutionalization because of better caregiver contact, better access to resources, and a higher-quality environment all of which are beneficial to cognitive development (Sheridan, et al., 2012). Physical contact from their caregivers to their children is essential to the child’s development. Even in healthy infants, skin-to-skin contact with parents has been shown to stabilize body temperature and blood glucose concentrations, decreases crying, and supports cardiorespiratory stability (Feldman-Winter & Goldsmith, 2016). In animal models, EE has been demonstrated to reverse the negative effects of processive and systemic stressors (Champagne & Curley, 2016; Kentner, Khoury, Queiroz, & MacRae, 2016; Vivinetto, Suárez, & Rivarola, 2013; Schneider, Turczak, & Przewlocki, 2006; Bredy, et al., 2004). Thus, improvements to one’s environment can lead to better outcomes in both humans and rats. While it is important to model the effects of stress in animals, it is also important to model the effects of EE so that efficacy of potential interventions can be established. Indeed, in the present study, we moved juvenile LB animals into EE to measure whether the metabolic consequences of early life stress could be rescued. Overall, each animal model of early-life stress will have its own particular strengths and weaknesses, but the most translational ones will meet several of these tests of validity.
With respect to the current study,the maternal care quality of EE dams was similar to what our laboratory has previously reported (Connors et al., 2015), except here we evaluated individual litters in isolation, as opposed to as part of a co-parenting design. This allowed us to better control for the contributions of individual dams on their litters, which is important given reports that dams may ‘take-turns’ caring for pups in a colony nest setting (Sale et al., 2004). It should be noted that in these co-parenting conditions one dam could not sufficiently meet the needs of both litters on her own, and it is unlikely that pups would receive more maternal attention overall. However, one great strength of the colony nesting design is that group housing may more adequately mimic the living conditions of rats in the wild (see Schultz & Lore, 1993; Hayes, 2000), allowing for a semi-naturalistic assessment of offspring developmental outcomes. That said, evaluating individual litters in the present study revealed a clearer role of circadian rhythms on the temporal patterning of maternal care.
While EE mothers spent less time on their nest as a function of circadian timing, they were perhaps more efficient in their care when in direct contact with pups. Specifically, despite differences in total pup contact time, EE and SD dams did not differ in the quantity of pup licking behavior, which is in alignment with reports of others (Welberg et al., 2006; Connors et al., 2015). However, some investigators have observed higher rates of licking from EE dams (Sale et al., 2004). Additionally, enriched dams spent less time in passive nursing postures across the nycthemeron, compared to standard housed dams, and in turn demonstrated higher frequencies of active nursing, at least in the light phase. This effect is likely not surprising given the longer duration of time that SD dams remained with their litters. In terms of energy expenditure, it would be comparably more difficult to maintain long periods of active nursing postures when spending longer periods nursing on the nest (Sale et al., 2004).
Based on our previous work we had begun to equate SD dams with ‘helicopter parents’ given: a) the increased contact they maintain with their litters and b) observations that SD offspring may not be as ‘adaptable’ as EE animals when exposed to potentially threatening environments (Connors et al., 2015). This is comparable to the maladaptive coping and decision-making skills often assoiciated with children of helicopter parents (Leubbe et al., 2016). Similarly, WD-LB dams also spent longer durations in contact with their litters compared to EE mothers, however there were no indications of maladaptive offspring behaviors, at least among the behavioral tests administered here. Interestingly, WD-LB dams consistently engaged in higher frequencies of pup licking and grooming across both the light and dark periods. High levels of maternal licking and grooming are typically associated with better HPA regulation by offspring in response to stressors, as mediated by upregulation of glucocorticoid receptor expression (Liu et al.,1997). This result raises the intriguing possibility that the the enhanced maternal licking behavior from WD-LB dams counteracted the effects that early life stress typically exerts on offspring behavior. Indeed, similarly to others (McLaughlin et al., 2016) we did not observe any instances of neglectful or abusive maternal behaviors from dams housed with LB, as previously described (Perry, et al., 2018; Heun-Johnson & Levitt, 2016; Sullivan & Holman, 2010; Ivy, Brunson, Sandman, & Baram, 2008). Overall, they appeared to be attentive mothers despite a reduced level of nesting resources. This may have been due in part to the WD as dams maintained on a high fat diet display higher quality maternal care (Purcell et al., 2011; Rincel et al., 2016).
Alongside the increased licking of pups, WD-LB dams also self-groomed significantly more often than EE and SD rats, at least in the light phase. Initially, we speculated that Western fed animals may lick more because of the taste of the diet on themselves and their pups. While this was not assessed directly, informal observations did not suggest that the fur of WD-LB animals was greasy; visually, these animals were no different in appearance from SD or EE dams/offspring. Morever, there were no differences in self-directed maternal grooming behaviors when both the light and dark cycles were considered together as an aggregate measure.
WD-LB mothers did, however, differ in the amount of non-maternal tail-chase behaviors compared to EE dams. Self-directed tail chase behaviors primarily emerge in rats that are individually housed (Hurst, 1997) and may represent a ‘surrogate social response’ in that the tail has spontaneous movements and so the rat responds as it would towards a social conspecific (Baenninger, 1967; Hurst et al., 1997). Higher rates of tail chase behaviors have also been reported in rat dams that underwent social isolation and received limited maternal care in early life (Gonzalez et al., 2001). Either way, tail chasing may suggest that the rat is seeking social interaction (Hurst et al., 1997) or may require additional sensory enrichment. Given the limited resources available to WD-LB dams, it is not surprising that these animals displayed higher levels of tail-chasing. However, we may have to address the question of whether the LB set-up is too impoverished as self-directed tail-chasing is not representative of species typical rat behavior. Additionally, laboratory SD housing as it stands is certainly not resource rich, even by comparison to LB conditions.
Despite a stressful postpartum period, WD-LB rat dams and their offspring demonstrated resiliency against the emergence of long-term behavioral disruptions. This finding is suggestive of compensatory mechanisms that may have made up for a lack of postnatal resources. In our hands, WD-LB dams did not display anxiety-like behaviors at weaning, nor did they or their offspring have exaggerated plasma corticosterone responses following a restraint stressor, though the later could be due to differences in sampling methodology (see Vahl et al., 2005), or a consequence of the high fat diet (see Rincel et al., 2016). While there have been ample investigations into the outcomes of offspring exposed to LB, studies evaluating the concsequences of LB on the dam have been limited. Of the work that has assessed dam behavioral performance after weaning, there has been no evidence of altered anxiety-like or fear-learning behaviors; aberrant behavioral expression appears to be limited to the early life stress period and specific to maternal care quality (Heun-Johnson & Levitt, 2016). In general, the wide variations in maternal behaviors we observed across the low, medium, and high resource settings underscores the need for the scientific community to more thoughtfully consider the utilization of translationally relevant housing conditions in the animal laboratory, particularly given the impact of parental nurturing on offspring outcomes.
Our WD-LB exposed offspring were resilient to behavioral peturbations, as has been similarly reported by others who exposed animals to a maternal high fat diet (Zieba et al., 2018). We only observed minor sex-specific disruptions in the frequency of novel object exploration in the social preference test. In contrast to the negligible behavioral effects reported here, animals exposed to limited resource conditions typically display robust deficits in attachment learning and impaired social behaviors, depressive and anxiety-like behaviors and disrupted maternal care (Moriceau et al., 2009; Raineki et al., 2010; Raineki et al., 2012; Dalle Molle et al., 2012; Brunson et al., 2005; Rincon-Cortes et al., 2016; Roth et al., 2009), although it is appears that whether or not abberant behavior is observed may be task specific (Ivy et al., 2010) or even dependent on age of testing or animal strain (Maniam et al., 2016; Brunson et al., 2005; Dalle Molle et al., 2012).
An important point to consider is that our LB animals were exposed to a WD preconceptionally and maintained on this diet across early development, until weaning, at which point it was replaced with regular chow. A post-weaning high fat diet has been demonstrated to reverse the anxiety-like effects associated with LB (Maniam et al., 2016), suggesting it may have value as an intervention tool (Maniam et al., 2016; Machado et al., 2013). Here, our data indicate that a Western style diet may also have preventative effects against the consequences of LB exposure. Indeed, maternal high-fat diet mitigated a variety of negative outcomes (e.g. anxiety, cognitive and social impairments, nociception and HPA dysregulation) associated with maternal separation including downregulation of Bdnf and Crh mRNA, spine loss and dendritic atrophy (Rincel et al., 2016; Rincel et al., 2018). This could account for the small impact that WD-LB housing had on offspring brain and behavior in the present work. Importantly, maternal high fat diet exposure is associated with increased maternal care (Purcell et al., 2011; Rincel et al., 2016) implicating an indirect pathway by which diet may influence offspring development. While our own WD-LB dams spent relatively less time in active nursing postures, compared to EE mothers, they engaged in higher rates of licking and grooming which conceivably may have counteracted the behavioral effects of early life stress through programming of the HPA-axis (Liu et al., 1997). This is important to note because although EE dams had lower total contact time with their nest, they made-up for this by participating in more active nursing postures. Together, this reminds us that despite the available resources no parent is perfect and for the most part the kids are (behaviorally) alright.
Although offspring of WD-LB animals appeared to be behaviorally resilient to the early life stress, metabolic consequences of this condition were more apparent. Specifically, these animals had higher body weights at weaning and female offspring displayed precocial puberty and downregulated Kiss1 mRNA expression in the hypothalamus. Mutations and deletions of Kiss1 or its receptor are accompanied by impairments in reproductive functioning, including an interruption of pubertal development, associated with reductions in GnRh (Kauffman, 2007). Moreover, prepubertal administration of kisspeptin initiates precoscious puberty, as indicated by LH release and advanced vaginal openings (Navarro et al., 2004). Therefore, we were surprised to measure diminished expression of Kiss1 mRNA in our WD-LB animals. Indeed, we had anticipated that elevated levels of this gene would stimulate GnRh secretion, resulting in the accelerated pubertal onset associated with WD-LB. Since we evaluated whole hypothalamus, it should be considered that we overlooked the tissue specific nature regulating the expression of these genes. For example, sex sterioids inhibit or or stimulate Kiss1 levels in a region specific manner (see Kauffman et al., 2007). We may have observed the predicted gene expression patterns (e.g. higher Kiss1) if we had targeted localized regions of the hypothalamus. It should also be noted that we collected tissue following puberty initation and we likely missed a critical window. Future work will need to include time-dependent sampling methods in order to capture a more accurate developmental picture of how early life stress affects the GnrH-Kisspeptin system and its influence on puberty. In addition to that, potential compensatory mechanisms such as differences in receptor sensitivity and regulatory-feedback systems will need to be evaluated. Finally, one must remember that the proportion of mRNA does not necessarily correlate to protein levels as this relationship is coordinated by several nuanced biological processes (Payne, 2015; Vogel & Marcotte, 2012).
In medicine, the pathophysiology of precocial puberty typically only considers trauma, genetic predisposition, illness or some physical cause for the accelerated maturation (Kaplowitz et al., 2018; Chalumeau et al., 2002; Strauss & Barbieri, 2009). However, early life stress is beginning to garner more attention as being a precipitator of early pubertal onset (Li et al., 2014; Kelly et al., 2017; Cowan & Richardson, 2018; Virdis et al., 1998). Recently, we observed precocious puberty in a rodent model of the neonatal intensive care unit (mimicking reduced parental contact, nosocomial infection, and medical manipulations). However, in this early life stress model we measured reduced GnRh receptor, as opposed to Kiss1 (Kentner et al., 2018a), though this finding is vulnerable to the methodological limitations listed above. Alternatively, these data suggest that different early life stressors may result in similar physiological outcomes (in this case accelerated puberty) that are mediated via a diverse set of mechanistic pathways. This finding is similar to observations that males and females can have similar behavioral outputs mediated by different mechanisms (e.g. Sorge et al., 2015) and the future mapping of this will be interesting to follow. Even though the genes between our early life stress models are differentially affected, they are both suggestive of suppressed gonadotropin levels and possibly a peripheral GnRh-independent mechanism (Barker et al., 2011). The centrally measured changes in GnRh-Kisspeptin genes could consequently be due to disruptions in HPG feedback mechanisms but the underlying programing effects of early life challenges are mostly unclear (Kentner & Pittman, 2010).
We show that environmental enrichment, in the form of a more complex laboratory caging system, prevented several central and metabolic disrputions following early life stress. Indeed, enhanced environmental stimulation accelerated the restoration of body weight to levels of SD regular chow fed rats when access to the WD food ceased. This is a natural metabolic defence mechanism that occurs when regular chow is restored in rodents (Siersbaek et al., 2017; Fischer et al., 2018; Raun et al., 2007), and EE accelerated this process. Moreover, the prepurbertal experience of enhanced laboratory stimulation was associated with elevated levels of hypothalamic Kiss1r in WD-LB-EE females, in addition to the normalization of species typical pubertal onset. Interventions in the form of sensory stimulation replacement and microbiome manipulation have also counteracted the occurance of precocial puberty as a consequency of early life adversity (Kentner et al., 2018a; Cowan & Richardson, 2018). In the low resource stress model used here, the pattern of changes in the kisspeptin system are suggestive of a compensatory mechanism to counteract the reduction in Kiss1 observed in WD-LB exposed rats. While Kiss1r was also lower in SD compared to WD-LB-EE females, SD rats had elevated expression of Kiss1, indicating that hormone levels in these animals may have been sufficient to maintain homeostasis and typical pubertal development. In general, we hypothesize that the enrichment conferred protection in WD-LB-EE females, maintaining an adequate balance between the ligands and receptors of the kisspeptin system, preventing the pubertal timing disturbances associated with early life stress. What needs to be determined is whether this animal model of low resource availability interrupts factors such as sexual motivation and behavior, or even fertility, and if EE might prevent or even restore these systems. As mentioned, future work will also need to address potential changes in the trajectory of kisspeptin and GnRh associated genes expression across development and address the issue of tissue specificity.
In sum, these studies highlight the utility to develop better translational models of low, medium, and high insecurity environments. Our results suggest that the negative consequences of a high insecurity environment can be mitigated by parental care and environmental enrichment in the juvenile period. As these non-pharmacological interventions and their neurobiological effects are underexplored in the basic literature, employing these models can lead to novel insights into mechanisms that promote stress resilience.
Supplementary Material
Highlights.
We compared translationally relevant rodent models of resource (in)stability to compare the effects of low, medium, and high security environments on maternal care and offspring outcomes
Maternal care was differentially impacted by each of these environments
The low security environment (Western diet/ limited bedding manipulation) resulted in metabolic dysfunction such as obesity, precocious puberty and disruptions in the hypothalamic kisspeptin system in offspring
These negative effects of early life stress on puberty and weight regulation were mitigated by environmental enrichment (high security)
These data highlight how utilizing diverse models of resource (in)stability can reveal mechanisms that confer vulnerability and resilience to early life stress.
Acknowlegements
The authors would like to extend their thanks to both Amanda Speno and Isabella Connors for technical ssistance. This project was funded in part by NIMH under Award Number R15MH114035 (to ACK) and by the National Science Foundation (NSF CAREER IOS-1552416 to D.A.B). The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the financial supporters.
Footnotes
Disclosures and Potential Conflict of Interests: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aber JL, Bennett NG, Conley DC, & Li J (1997). The Effects of Poverty on Child Health and Development. Annual Review Public Health, 18, 463–483, doi: 10.1146/annurev.publhealth.18.1.463 [DOI] [PubMed] [Google Scholar]
- Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, & Cuneo JG (2014). Psychological Tramua and Functional Somaic Syndromes: A Systematic Review and Meta-Analysis. Psychosomatic Medicine, 76, 1–20, doi: 10.1097/PSY.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera G (1994). Regulation of Pituitary ACTH Secretion during Chronic Stress. Frontiers in Neuroendocrinology, 15, 321–350, doi: 10.1006/frne.1994.1013 [DOI] [PubMed] [Google Scholar]
- Anda RF, Whitfield CI, Felitti VJ, Chapman D, Edwards VJ, Dube SR, & Williamson DF (2002). Adverse Childhood Experiences, Alcoholic Parents, and Later Risk of Alcoholism and Depression. Psychiatric Services, 1001–1009, doi: 10.1176/appi.ps.53.8.1001 [DOI] [PubMed] [Google Scholar]
- Andre C, Dinel A-L, Ferreria G, Laye S, & Castanon N (2014). Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: Focus on brain indoleamine 2,3-dioxygenase activation. Brain, Behavior, and Immunity, 41, 10–21, doi: 10.1016/j.bbi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Angold A (1993). Puberty onset of gender differences in rates of depression: A developmental, epidemiologic and neuroendocrine perspective. Journal of Affective Disorders, 29(2–3), 145–158. doi: 10.1016/0165-0327(93)90029-j [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Gilles E, & Eghbal-Ahma. (2001). Altered Regulation of Gene and Protein Expression of Hypothalamic-Pituitary-Adrenal Axis Components in an Immature Rat Model of Chronic Stress. Journal of Neuroendocrinology, 13, 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenninger LP (1967). Comparison of behavioural development in socially isolated and grouped rats. Animal Behaviour 15, 312–323. [DOI] [PubMed] [Google Scholar]
- Baley J (2015). Skin-to-Skin Care for Term and Preterm Infants in the Neonatal ICU. Pediatrics, 136, 596–599, doi: 10.1542/peds.2015-2335 [DOI] [PubMed] [Google Scholar]
- Barker JM, & Kappy MS (2011). Precocious puberty in girls. Bermans Pediatric Decision Making, 168–172. doi: 10.1016/b978-0-323-05405-8.00051-6 [DOI] [Google Scholar]
- Barrett J, & Fleming AS (2010). Annual Research Review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry, 52, 368–397, doi: 10.1111/j.1469-7610.2010.02306.x [DOI] [PubMed] [Google Scholar]
- Bauman RW (2014). Microbiology: With Diseases by Taxonomy. Pearson. [Google Scholar]
- Belzung C, & Lemoine M (2011). Criteria of validty for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biology of Mood & Anxiety Disorders, 1–9, doi: 10.1186/2045-5380-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentall RP, Wickham S, Shevlin M, & Varese F (2012). Do Specific Early-Life Adversities Lead to Specific Symptoms of Psychosis? A Study from the 2007 The Adult Psychiatric Morbidity Survey. Schizophrenia Bulletin, 38, 734–740, doi: 10.1093/schbul/sbs049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björntorp P (2008). Do stress reactions cause abdominal obesity and comorbidities? Obesity Reviews, 2, 73–86, doi: 10.1046/j.1467-789x.2001.00027.x [DOI] [PubMed] [Google Scholar]
- Boitard C, Maroun M, Tantot F, Cavaroc A, Sauvant J, Marchand A, . . . Ferreira G (2015). Juvenile Obesity Enhances Emotional Memory and amygdala plasticity through glucocoritcoids. The Journal of Neuroscience, 35, 4092–4103, doi: 10.1523/JNEUROSCI.3122-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C, Parkes SL, Cavaroc A, Tantot F, Castanon N, Laye S, . . . Ferraria G (2016). Switching Adolescent High-Fat Diet to Adult Control Diet Restores Neurocognitive Alterations. Frontiers in Behavoiral Neuroscience. doi: 10.3389/fnbeh.2016.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, & Neumann ID (2008). Brain asporessin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proceedings of the National Avademy of Sciences of the United States of America. doi: 10.1073/pnas.0807412105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower KM, Thorpe RJ, & Rohde C (2014). The Intersection of Neighborhood Racial Segregation, Poverty, and Urbanicity and its Impact on Food Store Availability in the United States. Preventive Medicine, 58, 33–39. doi: 10.1016/j.ypmed.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, & Meaney MJ (2003). Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience, 118, 171–576, doi: 10.1016/S0306-4522(02)00918-1 [DOI] [PubMed] [Google Scholar]
- Bredy TW, Zhang TY, Grant RJ, Diorio J, & Meaney MJ (2004). Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. European Journal of Neuroscience, 20, 1355–1362 doi: 10.1111/j.1460-9568.2004.03599.x [DOI] [PubMed] [Google Scholar]
- Bridges RS (2016). Neuroendocrine Regulation of Maternal Behavior. Frontiers in Neuroendocrinology, 36, 178–196. doi: 10.1016/j.yfrne.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz B, Siegfried W, Ziegler A, Lamertz C, Herpertz-Dahlmann BM, Remschmidt H, . . . Hebebrand J (2000). Rates of psychiatric disorders in a clinical study group of adolescents with extreme obesity and in obese adolescents ascertained via a population based study. International Journal of Obesity, 12, 1707–1714. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. (2005). Mechanisms of late-onset cognitive decline after early-life stress. Journal of Neuroscience, 25, 9328–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari SH, Clark OE, & Williamson LL (2018). Maternal high fructose diet and neonatal immune challenge alter offspring anxiety-like behavior and inflammation across the lifespan. Life Sciences, 197, 114–121, doi: 10.1016/j.lfs.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Burchinal MR, Peisner-Feinberg E, Bryant DM, & Clifford R (2000). Children’s Social and Cognitive development and Child-Care Quality: Testing for Differential Associations Related to Poverty, Gender, or Ethnicity. Applied Developmental Science, 4, 149–165, doi: 10.1207/S1532480XADS0403_4 [DOI] [Google Scholar]
- Burchum J, & Rosenthal L (2016). Lehne’s Pharmacology for Nursing Care. St. Louis: Elsevier. [Google Scholar]
- Burkholder AR, Koss KJ, Hostinar CE, Johnson AE, & Gunnar MR (2016). Early Life Stress: Effects on the Regulation of Anxiety Expression in Children and Adolescents. Social Development, 4, 777–793, doi: 10.1111/sode.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Chatterjee D, Chatterjee-Chakraborty M, Lovic V, Grella SL, Steiner M, & Fleming AS (2007). Prenatal restraint stress and motherless rearing disrupts expression of plasticity markers and stress-induced corticosterone release in adult female Sprague-Dawley rats. Brain Research, 1158, 28–38, doi: 10.1016/j.brainres.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, & MacQueen GM (2004). Lower Hippocampal Volume in Patients Suffering From Depression: A Meta-Analysis. American Journal of Psychiatry, 14, 598–607, doi: 10.1176/appi.ajp.161.4.598 [DOI] [PubMed] [Google Scholar]
- Cancedda L, Putignano E, Sale A, Berardi N, Maffei L, 2004Acceleration of visual system development by environmentalenrichment. J. Neurosci. 24, 4840–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalumeau M, Chemaitilly W, Trivin C, Adan L, Breart G, & Brauner R (2002). Central Precocious Puberty in Girls: An Evidence-Based Diagnosis Tree to Predict Central Nervous System Abnormalities. Pediatrics, 109(1), 61–67. doi: 10.1542/peds.109.1.61 [DOI] [PubMed] [Google Scholar]
- Champagne FA (2008). Epigenetic Mechanisms and the Transgenerational Effects of Maternal Care. Frontiers in Neuroendocrinology, 29, 386–397, doi: 10.1016/j.yfrne.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, & Curley JP (2007). Studying the Epigenetic Influence of Maternal Care in Rodents In Crawley JN, What’s Wrong with My Mouse? Strategies for Rodent Behavior Phenotyping (pp. 71–80). San Diego: Society for Neuroscience. [Google Scholar]
- Champagne FA, & Curley JP (2016). Plasticity of the maternal brain across the lifespan. New Directions for Child and Adolsecent Development, 153, 9–21, doi: 10.1002/cad.20164 [DOI] [PubMed] [Google Scholar]
- Champagne FA, & Meaney MJ (2006). Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biological Psychiatry, 12, 1227–1235, doi: 10.1016/j.biopsych.2005.10.016 [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, & Meaney MJ (2003). Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiology & Behavior, 79, 359–371, doi: 10.1016/S0031-9384(03)00149-5 [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, & Meaney MJ (2001). Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. PNAS, 22, 12736–12741, doi: 10.1073/pnas.221224598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Whitfiled CL, Felitti VJ, Dube SR, Edwards VJ, & Anda RF (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82, 217–225, doi: 10.1016/j.jad.2003.12.013 [DOI] [PubMed] [Google Scholar]
- Charpak N, Tessier R, Ruiz JG, Hernandez JT, Uriza F, Marin J, Maldonado D (2016). Twenty-year Follow-Up of Kangaroo Mother Care Versus Traditional Care. Pediatrics, 139, doi: 10.1542/peds.2016-2063 [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Chatterjee-Chakraborty M, Rees S, Cauchi J, Medeiros CB, & Fleming AS (2007). Maternal isolation alters the expression of neural proteins during development: ‘Stroking’ stimulation reverses these effects. Brain Research, 1158, 11–27, doi: 10.1016/j.brainres.2007.04.069 [DOI] [PubMed] [Google Scholar]
- Cohen S (2006). Social Status and Susceptibility to Respiratory Infections. Annals of the New York Academy of Science. [DOI] [PubMed] [Google Scholar]
- Connors EJ, Migliore MM, Pillsbury SL, Shaik AN, & Kentner AC (2015). Environmental enrichemnet modles a naturalistic form of maternal seperation and shapes the anxiety response patterns of offpsring. Psychoneuroendocrinologu, 52, 153–167, doi: 10.1016/j.psyneuen.2014.10.021 [DOI] [PubMed] [Google Scholar]
- Cooper RA, Richter FR, Bays PM, Plasited-Grant KC, Baron-Cohen S, & Simons JS (2017). Reduced Hippocampal Functional Connectivity During Episodic Memory Retrieval in Autism. Oxford Journals: Cerebral Cortex, 27, 888–902, doi: 10.1093/cercor/bhw417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CS, & Richardson R (2018). Early-life stress leads to sex-dependent changes in pubertal timing in rats that are reversed by a probiotic formulation. Developmental Psychobiology. doi: 10.1002/dev.21765 [DOI] [PubMed] [Google Scholar]
- Crawley JN (2007). Social Behavior Tests for Mice In Crawley JN, What’s Wrong with My Mouse? Strategies for Rodent Behavior Phenotyping (pp. 63–70). San Diego: Society for Neuroscience. [Google Scholar]
- Dalle Molle R, Portella A, Goldani M, Kapczinski F, Leistner-Segala S, Salum G, et al. (2012). Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Translational psychiatry, 2(11), e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, & de Kloet ER (2013). The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology, 38, 1858–1873, doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, Juraska JM (2016). Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats. A role for pubertal onset. Synapse, 70, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán-Carabali LE, Arcego DM, Odorcyk FK, Reichert L, Cordeiro JL, Sanches EF, Freitas LD, Dalmaz C, Pagnussat A, Netto CA (2018). Prenatal and early postnatal environmental enrichment reduce acute cell death and prevent neurodevelopment and memory impairments in rats submitted to neonatal hypoxia ischemia. Mol Neurobiol, 55, 3627–3641. [DOI] [PubMed] [Google Scholar]
- Eicher-Miller HA, & Zhao Y (2018). Evidence for the age-specific relationship of food insecurity and key dietary outcomes among US children and adolescents. Cambridge University Press, 31, 98–113, doi: 10.1017/S0954422417000245 [DOI] [PubMed] [Google Scholar]
- Estes ML, & McAllister KA (2016). Maternal immune activation: implications for neuropsychiatric disorders. Science, 353, 772–777, doi: 10.1126/science.aag3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman-Winter L, & Goldsmith JP (2016). Safe Sleep and Skin-to-Skin Care in the Neonatal Period for Healthy Term Newborns. American Academy of Pediatrics, 138, 1–10, doi: https://doi/org/10.1542/peds.2016-1889 [DOI] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Escorihuela RM, Driscoll P, Tobena A, & Battig K (1991). Infantile (Handling) Stimulation and Behavior in Young Roman High- and Low-Avoidance Rats. Physiology & Behavior, 50, 563–565, doi: 10.1016/0031-9384(91)90546-Z [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, & Cryan JF (2011). High-fat diet selectively protects against the effects of chronic social stress in the mouse. Cognitive, Behavioral, and Systems Neuroscience, 192, 351–360, doi: 10.1016/j.neuroscience.2011.06.072 [DOI] [PubMed] [Google Scholar]
- Finn MV (2006). Evolution and ontogeny of stress response to social challenges in the human child. Developmental Review, 26, 138–174, doi: 10.1016/j.dr.2006.02.003 [DOI] [Google Scholar]
- Fisher IP, Irmler M, Meyer CW, Sachs SJ, Neff F, Hrabê de Angelis M, Beckers J, Tschöp MH, Hofmann SM, Ussar S (2018). A history of obesity leaves and inflammatory fingerprint in liver and adipose tissue. International Journal of Obesity, 42, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TP, Watkins AJ, Velazeuqez MA, Mathers JC, Prentice AM, Stephenson J, . . . Saffery R (2018). Origins of lifetime health around the time of conception: causes and consequences. The Lancet, 391, 1842–1852, doi: 10.1016/S0140-6736(18)30312-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, & Mc Vey Neufeld K-A (2013). Gut-brain axis: How the microbiome influences anxiety and depression. Trends in Neuroscience, 36(5), 305–312. doi: 10.1016/j.tins.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Fram MS, Fongillo EA, Jones SJ, Williams RC, Burke MP, DeLoach KP, & Blake CE (2011). Children Are Aware of Food Insecurity and Take Responsibility for Managing Food Resources. Journal of Nutrition, 141(6), 1114–1119. doi: 10.3945/jn.110.135988 [DOI] [PubMed] [Google Scholar]
- Francis H, & Stevenson R (2012). The longer-term impacts of Western diet on human cognition and the brain. Appetite, 63, 112–128, doi: 10.1016/j.appet.2012.12.018 [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ, 2002. Environmental enrichment reverses the effects of maternal separation on stress reactivity. Journal of Neuroscience, 22(18), 7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes S, Daviu N, Gagliano H, Garrido P, Zelena D, Monasterio N, Nadal R (2014). Sex-dependent effects of an early life treatment in rats that increases maternal care: Vulnerability or resilience? Frontiers in Behavioral Neuroscience, 8, 1–22. doi: 10.3389/fnbeh.2014.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta E, Mairesse J, Deruyter L, Marrocco J, Van Camp G, Bouwalerh H, Maccari S (2018). Reduced maternal behavior caused by gastional stress is predictive of life span changes in risk-taking behavior and gene expression due to altering of stress/ anti-stress balance. NeuroToxicology, 66, 138–149. doi: 10.1016/j.neuro.2018.04.005 [DOI] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, & Baram TZ (1996). Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatric Neurology, 15, 114–119, doi: 10.1016/0887-8994(96)00153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF (2007). Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience, 149, 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Lovic V, Ward GR, Wainwright PE, & Fleming AS (2001). Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Developmental Psychobiology, 38, 11–32, doi: [DOI] [PubMed] [Google Scholar]
- Gowda C, Hadley C, & Aiello AE (2012). The Association Between Food Insecurity and Inflammation in the US Adult Population. American Journal of Public Health, 102, 1579–1586, doi: 10.2105/AJPH.2011.300551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, & Brenhouse HC (2016). Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology, 71, 19–30, doi: 10.1016/j.psyneuen.2016.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JF, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversitites and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) I: Associations with onset of DSM-IV disorders. Archive of General Psychiatry, 67, 1–20, doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønli J, Murison R, Eldbjørg F, Bjorvatn B, Sørensena E, Portas CM, & Ursin R (2005). Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiology & Behavior, 84, 571–577, doi: 10.1016/j.physbeh.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Gundersen C, & Kreider B (2009). Bounding the effects of food insecurity on children’s health outcomes. Journal of Health Economy, 28, 971–983, doi: 10.1016/j.jhealeco.2009.06.012 [DOI] [PubMed] [Google Scholar]
- Hayes LD (2000). To nest communally or not to nest communally: a review of rodent communal nesting and nursing. Animal Behaviour. 59: 677–688. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, & Nemeroff CB (2008). The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology, 33, 693–710, doi: 10.1016/j.psyneuen.2008.03.008 [DOI] [PubMed] [Google Scholar]
- Heun-Johnson H, & Levitt P (2016). Early-Life Stress Paradigm Transiently Alters Maternal Behaviors, Dam-Pup Interactions, and Offspring Vocalizations in Mice. Frontiers in Behavorial Neuroscience, 10, 1–18, doi: 10.3389/fnbeh.2016.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ramsay KM, Marcheva B, & Bass J (2011). Circadian rhythms, sleep, and metabolism. The Journal of Clinical Investigation, 121, 2133–2141, doi: 10.1172/JCI4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JL, Barnard CJ, Nevison CM, West CD (1997). Housing and welfare in laboratory rats: welfare implications of isolation and social contact among caged males. Animal Welfare, 6, 329–347. [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, & Baram TZ (2008). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience, 154, 1132–1142, doi: 10.1016/j.neuroscience.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, et al. (2010). Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. Journal of Neuroscience, 30(39), 13005–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, & Sapolsky R (1991). The Role of the Hippocampus in Feedback Regulation of the Hypothalamic-Pituitary-Adrenocortical Axis. Endocrine Reviews, 12, 118–134, doi: 10.1210/edrv-12-2-118 [DOI] [PubMed] [Google Scholar]
- Janssen H, Ada L, Bernhardt J, McElduff P, Pollack M, Nilsson M, Spratt NJ (2014). An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed-rehabilitation unit: a pilot non-randomized controlled trial. Disability and Rehabilitation, 36, 255–262. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, Alfarez D, Heine VM, Qin Y, & van Riel E (2004). Effects of Chornic Stress on Structure and Cell Function in Rat Hippocampus and Hypothalamus. Stress 7, 221–231, doi: 10.1080/10253890500070005 [DOI] [PubMed] [Google Scholar]
- Kalyan-Masih P, Vega-Torres JD, Miles C, Haddad E, Rainsbury S, Baghchechi M, Figueroa J (2016). Western High-Fat Diet Consumption during Adolescence Increases Susceptibility to Traumatic Stress while Selectively Disrupting Hippocampal and Ventricular Volumes. Cognition and Behavior, 3, 1–24, doi: 10.1523/ENEURO.0125-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz PB, & Hoffman RP (2018, July 05). Precocious Puberty: Background, Pathophysiology, Epidemiology. Retrieved August 10, 2018, from https://emedicine.medscape.com/article/924002-overview#a5
- Kauffman AS, Clifton DK, & Steiner RA (2007). Emerging ideas about kisspeptin–GPR54 signaling in the neuroendocrine regulation of reproduction. Trends in Neurosciences, 30(10), 504–511. doi: 10.1016/j.tins.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Kelly Y, Zilanawala A, Sacker A, Hiatt R, Viner R (2017). Early puberty in 11-year-old girls: Millennium Cohort Study findings. Arch Dis Child, 102, 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny SL, Wright LD, Green A, Mashoodh R, & Perrot TS (2014). Expression of maternal behavior and activation of the bed nucleus of the stria terminalis during predatory threat exposure: modulatory effects of trasport stress. Physiology & Behavior, 123, 148–155, doi: 10.1016/j.physbeh.2013.08.024 [DOI] [PubMed] [Google Scholar]
- Kentner A (2015). Neuroprotection and recovery from early-life adversity: considerations for environemental enrichment. Neural Regeneration Research, 10, 1545–1547, doi: 10.4103/1673-5374.165315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner AC, Khoury A, Queiroz EL, & MacRae M (2016). Environmental enrichement rescues the effects of early life inlammation on markets of synaptic transmission and plasticity. Brain, Behavior, and Immunology, 57, 151–160, doi: 10.1016/j.bbi.2016.03.013 [DOI] [PubMed] [Google Scholar]
- Kentner AC, Scalia S, Shin J, Migliore MM, Rondon-Ortiz AN (2018a). Targeted sensory enrichment interventions protect against behavioral and neuroendocrine consequences of early life stress. Psychoneuroendocrinology, 98, 74–85. [DOI] [PubMed] [Google Scholar]
- Kentner AC, Bilbo AD, Brown AS, Hsiao EY, McAllister AK, Meyer U, Pearce BD, Pletnikov MV, Yolken RH, Bauman MD. (2018b). Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology, 10.1038/s41386-018-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner AC, Cryan JF, Brummelte S (2018c). Resilience Priming: Translational models for understanding resiliency and adaptation to early-life adversity. Developmental Psychobiology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner AC, & Pittman QJ (2010). Minireview: Early-Life Programming by Inflammation of the Neuroendocrine System. Endocrinology, 151(10), 4602–4606. doi: 10.1210/en.2010-0583 [DOI] [PubMed] [Google Scholar]
- King LS, Humphreys KL, Camacho MC, & Gotlib IH (2008). A person-centered approach to the assessment of early life stress: Associations with the volume of stress-sensitive brain regions in early adolescence. Development and Psychopathology, 2, 1–13, doi: 10.1017/S0954579418000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshner ZZ, Gibbs RB (2018). Use of the REVERT® total protein stain as a loading control demonstrates significant benefits over the use of housekeeping proteins when analyzing brain homogenates by Western blot: an analysis of samples representing different gonodal hormone states. Molecular and Cellular Endocrinology, 473, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta R, Schlotz W, Golm D, Moser D, Kennedy M, Knights N, Sonuga-Barke E (2017). HPA axis dysregulation in adult adoptees twenty years after severe institutional deprivation in childhood. Psychoneuroendocrinology, 88, 196–202, doi: 10.1016/j.psyneuen.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Leinonen JA, Solantaus TS, & Punamaki R-L (2003). Social support and the quality of parenting under economic pressure and workload in Finland: The role of family structure and parental gender. Journal of Family Psychology, 17, 409–418, doi: 10.1037/0893-3200.17.3.409 [DOI] [PubMed] [Google Scholar]
- Leng G, & Sabatier N (2016). Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers. Journal of Neuroendocrinology, 28, 1–13, doi: 10.1111/jne.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis MP, Freund N, Brenhouse HC, Thompson BS, & Andersen SL (2012). Depressive-Like Behavior in Adolescents after Maternal Separation: Sex Differences, Controllability, and GABA. Developmental Neuroscience, 34, 210–217, doi: 10.1159/000339162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S (1994). The ontogeny of the hypothalamic-pituitary-adrenal axis: The influence of maternal factors. Annals of the New York Academy of Sciences, 30, 275–288. [DOI] [PubMed] [Google Scholar]
- Levine S (2001). Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in rats. Physiology & Behavior, 73, 255–260, doi: 10.1016/S0031-9384(01)00496-6 [DOI] [PubMed] [Google Scholar]
- Li L, Denholm R, Power C (2014). Child maltreatment and household dysfunction: associations with pubertal development in a British birth cohort. International Journal of Epidemiology, 43(4), 1163–1173. doi: 10.1093/ije/dyu071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Meaney MJ (1997). Maternal Care, Hippocampal Glucocorticoid Receptors, And Hypothalamic-Pituitary-Adrenal Responses to Stress. Science, 227, 1659–1662, doi: 10.1126/science.277.5332.1659 [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, & Melo AI (2016). Deconstructing the function of maternal stimulation in offspring development: Insights from the artificial rearing model in rats. Hormones and Behavior, 77, 224–236, doi: 10.1016/j.yhbeh.2015.05.017 [DOI] [PubMed] [Google Scholar]
- Luebbe AM, Mancini KJ, Kiel EJ, Spangler BR, Semlak JL, Fussner LM (2016). Dimensionality of helicopter parenting and relations to emotional, decision-making, and academic functioning in emerging adults. Assessment, 1–17, 10.1177/1073191116665907 [DOI] [PubMed] [Google Scholar]
- Machado TD, Dalle Molle R, Laureano DP, Portella AK, Werlang ICR., da Silva Benetti C, Noschang C, Silveira PP (2013). Early life stress is associated with anxiety, increased stress responsivity and preference for ‘comfort foods’ in adult female rats. Stress, 16, 549–556. [DOI] [PubMed] [Google Scholar]
- MacRae M, Macrina T, Khoury A, Migliore MM, Kentner AC (2015). Tracing the trajectory of behavioral impairments and oxidative stress in an animal model of neonatal inflammation. Neuroscience, 298, 455–466. [DOI] [PubMed] [Google Scholar]
- Maniam J, Antoniadis CP, & Morris MJ (2015). Sugar Consumption Produces Effects Similar to Early Life Stress Exposure on Hippocampal Markers of Neurogenesis and Stress Response. Frontiers in Molecular Neuroscience, 19, 1–10, doi: 10.3389/fnmol.2015.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniam J, Antoniadis CP, Le V, & Morris MJ (2016). A diet high in fat and sugar reverses anxiety-like behavior induced by limited nesting in male rats: impacts on hippocampal markers. Psychoneuroendocrinology, 68, 202–209. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Verlezz S, Gray JM, Hill MN, & Walker C-D (2016). Inhibition of anandamide hydrolysis dampens the neuroendocrine response to stress in neonatal rats subjected to suboptimal rearing conditions. Journal on the Biology of Stress, 19, 114–124, doi: 10.3109/10253890.2015.1117448 [DOI] [PubMed] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei YT, Regev L, & Baram TZ (2016). Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Translational Anxiety, 6, 1–7, doi: 10.1038/tp.2015.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, & Sullivan RM (2009). Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. Journal of Neuroscience, 29(50), 15745–15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academy of Sciences. [Google Scholar]
- Navarro VM, Fernádez- Fernádez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M (2004). Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol, 561, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA III, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, & Guthrie D (2007). Cognitive Recovery in Socially Deprived Young Children: The Bucharest Early Intervention Project. Science, 318, 1937–1940, doi: 10.1126/science.1143921 [DOI] [PubMed] [Google Scholar]
- Payne SH (2015). The utility of protein and mRNA correlation. Trends Biochem Sci, 40, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Finegood ED, Braren SH, Dejoseph ML, Putrino DF, Wilson DA, Blair C (2018). Developing a neurobehavorial animal model of poverty: Drawing cross-species connections between environments of scarcity-adversity, parenting quality, and infant outcome. Development and Psychopathology, 2, 1–20, doi: 10.1017/S095457941800007X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervanidou P, & Chrousos GP (2012). Metabolic consequences of stress during childhood and adolescence. Metabolism, 61, 611–619, doi: 10.1016/j.metabol.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Young WS, Blanchard DC, & Blanchard RJ (2012). Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors. Hormones and Behavior, 61, 436–444, doi: 10.1016/j.yhbeh.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell RH, Sun B, Pass LL, Power ML, Moran TH, Tamashiro KLK. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav 2011; 104: 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortés MR, Belnoue L, & Sillivan RM (2012). Effects of early life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. Journal of Neuroscience, 32, 7758–7765, doi: 10.1523/JNEUROSCI.5843-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, & Sullivan RM (2010). Developing a Neurobehavioral Animal Model of Infant Attachment to an Abusive Caregiver. Biology of Psychiatry, 67, 1137–1145, doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knedsen LB (2007). Liraglutide, a long-acting glucaogon-like peptide-1 analog reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, Vildagliptin, does not. Diabetes, 56, 8–15. [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, & Baram TZ (2008). A Novel Mouse Model for Acute and Long-Lasting Consequence of Early Life Stress. Endocrinology, 149, 48924900, doi: 10.1210/en.2008-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincel M, Lépinay AL, Delage P, Fioramonti J, Théodorou VS, Layé S, & Darnaudréry M (2016). Maternal high-fat diet prevents developmental programming by early-life stress. Translational Psychiatry, 29, 1–9, doi: 10.1038/tp.2016.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincel M, Lépinay, Janthakhin Y, Soudain, Yvon S, Da Silva S, Joffre C, Aubert A, Séré A, Layé S, Theodorou V, Ferreira G, Darnaudéry A (2018). Maternal high fat diet and early life stress differentially modulate spine density and dendritic morphology in the medial prefrontal cortex of juvenile and adult rats. Brain Struct Funct, 223, 883–895. [DOI] [PubMed] [Google Scholar]
- Rincón-Cortés M, & Sullivan R (2016). Emergence of social behavior deficit, blunted corticolimbic activity and adult depression-like behavior in a rodent model of maternal maltreatment. Translational psychiatry, 6(10), e930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld A, Weller A, 2012. Behavioral effects of environmentalenrichment during gestation in WKY and Wistar rats. Behav. BrainRes. 233, 245–255. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, & Sweatt JD (2009). Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological psychiatry, 65(9), 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RD, Watson PD, Duff MC, & Cohen NJ (2014). The role of the hippocampus in flexible cognition and social behavior. Frontier Human Neuroscience, 8, 1–15, doi: 10.3389/fnhum.2014.00742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks V, & Murphey D (2018). The prevalence of adverse childhood experiences, nationally, by state, and by race or ethnicity. Child Trends. [Google Scholar]
- Sale A, Putignano E, Cancedda L, Landi S, Cirulli F, Berardi N, Maffei L (2004). Enriched environment and acceleration of visual system development. Neuropharmacology, 47, 649–660. [DOI] [PubMed] [Google Scholar]
- Sanjeevi N, Freeland-Graves J, & Hersh M (2018). Food insecurity, diet quality and body mass index of women participating in the Supplemental Nutrition Assistance Program: The role of intrapersonal, home environment, community and social factor. Appetite, 125, 109–117, doi: 10.1016/j.appet.2018.01.036 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, & McEwen BS (1985). Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. Journal of Neuroscience, 5, 1222–1227, doi: 10.1523/JNEUROSCI.05-05-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro S, Geller E, & Eiduson S (1962). Neonatal Adrenal Cortical Response to Stress and Vasopressin. Experimental Biology and Medicine, 109, 937–941. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Levine S, Oitzl MS, van der Mark M, Müller MB, Holsboer F, & de Kloet ER (2005). Glucocorticoid Receptor Blockade Disinhibits Pituitary-Adrenal Activity during the Stress Hyporesponsive Period of the Mouse. Endocrinology, 146, 1458–1464, doi: 10.1210/endo.146.9.9997 [DOI] [PubMed] [Google Scholar]
- Schneider T, Turczak J, & Przewlocki R (2006). Environmental Enrichement Reverses Behavoiral Alterations in Rats Prenatally Exposed to Valproic Acid: Issues for a Therapeutic Approach in Autism. Neuropsychopharmacology, 31, 36–46, doi: 10.1038/sj.npp.1300767 [DOI] [PubMed] [Google Scholar]
- Schultz LA, Lore RK. (1993). Communal reproductive success in rats (Rattus norvegicus): effects of group composition and prior social experience. J Comp Psychol. 107(2):216–22. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler T, Connolly MJ, Prosoki AR, & Pollak SD (2013). Stress-induced elevation of oxytocin in maltreated children: Evolution, neurodevelopment, and social behavior. Child Development, 85, 501–512, doi: 10.1111/cdev.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, & Nelson CA III (2012). Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences of the United States of America, 109, 12927–12932, doi: 10.1073/pnas.1200041109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siersbaek M, Varticovski L, Yang S, Baek S, Nielsen R, Mandrup S, Hager G, Chung JH, Grøntved L (2017). High fat diet-indiced changes of mouse hepatic transcription and enhancer activity can be reversed by subsequent weight loss. Scientific Reports, 7,40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupskaite K, George JT, Anderson RA (2014). The kisspeptin-GnRH pathway in human reproductive health and disease. Human Reproduction Update, 20(4), 485–500. doi: 10.1093/humupd/dmu009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sominsky L, Ziko I, Soch A, Smith JT, Spencer SJ (2016). Neonatal overfeeding induces early decline of the ovarian reserve: implications for the role of leptin. Mol.Cell. Endocrinol. 431, 25–35. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin J-A, Sotocinal SG, Chen D, Yang M, Mogil JS (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neuroscience, 18, 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Korosi A, Layé S, Shukitt-Hale B, & Barrientos R (2017). Food for thought: how nutrition impacts cognition and emotion. NPJ Science of Food, 7, 1–8, doi: 10.1038/s41538-017-0008-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Martin S, Mouihate A, Pittman QJ (2006). Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology, 31, 1910–1918. [DOI] [PubMed] [Google Scholar]
- Stormshak EA, Bierman KL, McMahon RJ, & Lengua LJ (2010). Parenting Practices and Child Disruptive Behavior Problems in Elementary School. Journal of Clinical Child Psychology, 29, 17–29, doi: 10.1207/S15374424jccp2901_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss JF, Barbieri RL Eds. (2009). Yen and Jaffe’s Reproductive Endocrinology: physiology, pathophysiology, and clinical management. 6th edition. Elsevier, China. [Google Scholar]
- Pedrini Schuch C, Diaz R, Deckmann I, Roja J, Deniz B, Pereira L (2016). Early environmental enrichment affects neurobehavioral development and prevents brain damage in rats submitted to neonatal hypoxia-ischemia. Neuroscience Letters, 617, 101–107. doi: 10.1016/j.neulet.2016.02.015 [DOI] [PubMed] [Google Scholar]
- Suchdev PS, Boivin MJ, Forsyth BW, Georgieff MK, Guerrant RL, & Nelson CA III (2017). Assessment of Neurodevelopment, Nutrition, and Inflammation From Fetal Life to Adolescence in Low-Resource Settings. American Academy of Pediatrics, 139, 23–37, doi: 10.1542/peds.2016-2828E [DOI] [PubMed] [Google Scholar]
- Sullivan RM, & Holman PJ (2010). Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neuroscience and Biobehavioral Reviews, 34, 835–844, doi: 10.1016/j.neubiorev.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suschecki D (2018). Maternal regulation of the infants HPA axis stress response: Seymour ‘Gig” Levine’s legacy to Neuroendocrinology. Journal of Neuroendocrinology, 30(7). doi: 10.1111/jne.12610 [DOI] [PubMed] [Google Scholar]
- Tessier R, Cristo MB, Velez S, Giron M, Nadeau L, de Calume F, Charpak N (2003). Kangaroo Mother Care: A method for protecting high-risk low-birth-weight and premature infants against developmental delay. Infant Behavior and Development, 26, 384–397, doi: 10.1016/S0163-6383(03)00037-7 [DOI] [Google Scholar]
- Tilburg MA, Runyan DK, Zoltor AJ, Graham AJ, Dubowtiz JC, & Litrownik AJ (2010). Unexplained Gastrointestinal Symptoms After Abuse in A Prospective Study of Children at Risk for Abuse and Neglect. The Annals of Family Medicine, 8, 134–140, doi: 10.1370/afm.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP (2005). Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab, 289, E823–E828. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli HA, Gandhi CP, & Hill MN (2016A). Acute Psychological Stress Modulates the Expression of Enzymes Involved in the Kynurenine Pathway throughout Corticolimbic Circuits in Adult Male Rats. Neural Plasticity, 2016, 1–12, doi: 10.1155/2016/7215684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarellia HA, Gandhi C, Gray JM, Morena M, Kowther HI, & Hill MN (2016). Divergent responses of inflammatory mediators within the amygdala and medial prefrontal cortex to acute psychological stress. Brain, Behavior, and Immunity, 51, 70–91, doi: 10.1016/j.bbi.2015.07.026 [DOI] [PubMed] [Google Scholar]
- Virdis R, Street ME, Zampolli M, Radetti G, Pezzini B, Benelli M, Ghizzoni L, Volta C (1998). Precocious puberty in girls adopted from developing countries. Arch Dis Child, 78, 152–154. doi: 10.1136/adc.78.2.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivinetto AL, Suárez MM, & Rivarola MA (2013). Neurobiological effects of neonatal maternal separation and post-weaning environmental enrichment. Behavioural Brain Research, 240, 110–118, doi: 10.1016/j.bbr.2012.11.014 [DOI] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM (2012). Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, Baram TZ (2017). Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. The International Journal on the Biology of Stress, 20, 421–448, doi: 10.1080/10253890.2017.1343296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L, Thrivikraman KV, Plotsky PM (2006). Combined pre- and postnatal environmental enrichment programs the HPA axis differentially in male and female rats. Psychoneuroendocrinology, 31(5), 553–564. doi: 10.1016/j.psyneuen.2005.11.011 [DOI] [PubMed] [Google Scholar]
- White JH, Bartley E, Janssen H, Jordan LA, Spratt N (2015). Exploring stroke survivor experience of participation in an enriched environment: a qualitative study. Disability and Rehabilitation, 37, 593–600. doi: 10.3109/09638288.2014.935876 [DOI] [PubMed] [Google Scholar]
- Woo CC, Leon M (2013). Environmental enrichment as an effective treatment for autism: a randomized controlled trial. Behavioral Neuroscience, 127(4), 487–497. doi: 10.1037/a0033010 [DOI] [PubMed] [Google Scholar]
- Woo CC, Donnelly JH, Steinberg-Epstein R, Leon M (2015). Environmental enrichment as a therapy for autism: a clinical trial replication and extension. Behavioral Neuroscience, 129(4), 412–422. doi: 10.1037/bne0000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam KY, Nannick EF, Abbink MR, la Fleur SE, Schipper L, van den Beukel JC,. Korosi A (2016). Exposure to chronic early-life stress lastingly alters the adipose tissue, the leptin system, and changes the vulnerability to western-style diet later in life in mice. Psychoneuroendocrinology, 77, 186–195, doi: 10.1016/j.psyneuen.2016.12.012 [DOI] [PubMed] [Google Scholar]
- Zieba J, Uddin GM, Youngson NA, Karl T, Morris MJ (2018). Long-term behavioural effects of maternal obesity in C57BL/6J mice. Physiology & Behavior, in press 10.1016/j.physbeh.2018.11.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.