Abstract
While psychosocial risk factors for peripartum depression are well-researched, studies on neural risk factors are scarce. Previous studies suggest a blunted neural response to reward may be a biomarker of depression and risk. In a sample of 86 pregnant women, the present study examined whether a reduced Reward Positivity (RewP), an event-related potential (ERP) elicited to feedback indicating monetary reward, relates to greater antenatal depressive symptoms. We also examined whether the RewP explains unique variance in antenatal depressive symptoms relative to other indices of risk, including annual income, history of a major depressive episodes, and score on a validated psychosocial risk measure, the Pregnancy Risk Questionnaire (PRQ). Zero-order correlations revealed that lower annual income, greater PRQ scores, and a blunted RewP were associated with greater antenatal depressive symptoms. The RewP and PRQ scores were identified as the best predictors of antenatal depressive symptoms in a stepwise regression, and together predicted 48 percent of the variance in antenatal depressive symptoms. PRQ scores accounted for 44% of the variance in antenatal depressive symptoms while the RewP accounted for 4% of additional incremental variance. This is the first study to combine self-report and neural activity to examine depressive symptoms in pregnant women. Future directions for research on perinatal depression are discussed.
Introduction
The American Psychological Association reports that up to one in seven women develop postpartum depression (PPD) within several months after delivering a baby. PPD is characterized by the symptoms of major depressive disorder and typically leads to impairment in functioning, severe distress, and at times, thoughts of harming oneself or the baby (APA, 2018). In addition to its impacts on women, PPD has deleterious effects on offspring. Offspring of mothers with PPD show high incidences of insecure attachment, depressed affect, behavioral disturbances, and cognitive impairment (Clark et al., 2003; Murray et al., 2011; Verbeek et al., 2012). Chronicity of maternal depression in infanthood has been shown to lead to delays in verbal abilities, behavioral problems, and lower school readiness skills (Clark et al., 2003). Additionally, PPD has been linked to depression and other mental health problems in adolescent offspring (Murray et al., 2011; Verbeek et al., 2012). Importantly, depressive symptoms during pregnancy (i.e., the antenatal period) have also been shown to have adverse effects on offspring, including irregular fetal heart rate, increased cortisol and norepinephrine levels, and internalizing and externalizing psychopathology (Gentile, 2017). Thus, the impact on mothers and offspring underscore the importance of identifying reliable predictors of peripartum depression (i.e. depression occurring around the time of birth) and developing effective systems of risk detection.
Antenatal depression itself has been shown to be one of the best predictors of PPD. In a meta-analysis, Beck (2001) found 13 significant predictors of PPD. In order of most to least predictive, they are: antenatal depression, self-esteem, childcare stress, prenatal anxiety, life stress, social support, marital relationship satisfaction, history of depression, infant temperament, postpartum blues, marital status, socioeconomic status, and unplanned pregnancy (Beck, 2001). Other reviews have similarly shown antepartum depression to be one of the most significant risk factors of PPD, in addition to cognitive attributional style, delivery stress, and stressful life events (O’Hara et al., 1982). More recent reviews and meta-analyses have replicated these findings and have placed emphasis on three categories of risk factors—past psychopathology, life stress, and poor social support—and report similar risk factors for depression in the antenatal period (O’Hara & Wisner, 2014). In addition to examining psychosocial and psychiatric risk factors, recent reviews have summarized biological predictors of peripartum depression, such as endocrine, immune, and genetic influences (Yim et al., 2015; Serati et al., 2016); however, few studies have examined neural correlates of peripartum depression (Moses-Kolko et al., 2011; Moses-Kolko et al., 2014; Silverman et al., 2007). One functional magnetic resonance imaging (fMRI) study examining postpartum depression found rapid attenuation of ventral striatal response to the receipt of rewards in comparison to healthy controls (Moses-Kolko et al., 2011). Similarly, other studies found attenuated striatal activation to positive words (Silverman et al., 2007) and to infants’ cries (Laurent & Ablow, 2012), in those with greater PPD symptoms.
Relatedly, etiological models of major depression have focused on neural abnormalities in reward function. Behavioral responses to reward have been found to be decreased in depressed individuals as compared to healthy controls (Pizzagalli et al., 2005). FMRI studies examining depressed adults have shown reduced brain activity in regions central to reward processing, such as the ventral striatum and caudate (Pizzagalli et al., 2009; Steele et al., 2007). While fMRI studies have been instrumental in examining reward-related deficits in postpartum and major depression, fMRI is inappropriate for studying reward processing in antenatal depression due to unknown risks for pregnant women and the fetus.
Event-related potentials (ERPs) are a tool for examining neural activity in response to rewards that pose minimal risk to pregnant women and their offspring. Approximately 300 milliseconds (ms) after feedback indicating monetary reward, the ERP at frontocentral recording sites is characterized by a relative positivity; an apparent negativity is observed following feedback indicating monetary loss (Holroyd et al., 2006; Hajcak et al., 2007). The ERP response to reward is referred to as the Reward Positivity (RewP; Holroyd et al., 2008; Baker & Holroyd, 2011; Proudfit, 2015). In many studies using ERPs, a smaller RewP has been consistently associated with depression (Belden et al., 2016; Bress et al., 2013; Liu et al., 2014) and greater depressive symptoms (Bress et al., 2012; Foti & Hajcak, 2009). Thus, the RewP is an ideal measure for studying neural reward responsivity in pregnant women given its well-documented associations with depressive symptoms and safety for use in pregnant women.
The present study is the first study to utilize the RewP to examine reward function in pregnant women in relation to antenatal depressive symptoms. To this end, the present study examined cross-sectional associations between RewP amplitude, self-reported risk factors (i.e., annual income, past major depressive episodes (MDE), and scores on a psychosocial risk factor questionnaire) and depressive symptoms in the antenatal period. Since we are employing a cross-sectional design, we are not able to address causality. Therefore, this study could lay the groundwork for future work testing the utility of the RewP as a predictor of risk for depression in prospective studies, as has been done previously with adolescent depression (Nelson et al., 2016). In the present study, we hypothesized that the RewP would explain variance in current depressive symptoms that is independent from variance explained by other socioeconomic, psychological, and psychosocial risk factors.
Methods
Participants
Eighty-six pregnant women recruited from an OB/GYN clinic in Tallahassee, FL participated in the study and were provided with monetary compensation for their participation. Front desk staff at the OB/GYN clinic provided study flyers to all pregnant patients and asked if they were interested in participating. If interested, patients provided contact information for research staff who contacted interested patients with more information and to schedule study visits. Demographic information for the sample can be found in Table 1. Average gestational weeks at the time of testing was 26.73 [SD = 9.75]. Informed consent was obtained prior to participation and the research protocol was approved by the Institutional Review Board at Florida State University.
Table 1. Demographics (top), depression symptoms, and occurrence of past depressive episodes (bottom).
| M | SD | |
|---|---|---|
| Demographics | ||
| Age (years) | 29.45 | 5.52 |
| Gestation (weeks) | 19.95 | 9.46 |
| Income (dollars) | 73,002.19 | 50,676.45 |
| Race | ||
| Asian | 3.4% | |
| Black | 19.8% | |
| Caucasian | 69.8% | |
| Latino | 7% |
| M | SD | |
|---|---|---|
| PHQ-9 Depression | 4.60 | 4.04 |
| PRQ Score | 30.93 | 11.77 |
| PHQ-9 Above Clinical Cutoff | 8.1% | |
| Past MDE | 18.6% |
Note. PHQ-9 = Patient Health Questionnaire; PRQ = Pregnancy Risk Questionnaire; M = mean; MDE = Major Depressive Episode; SD = standard deviation; PHQ-9 clinical cutoff ≤ 10.
Measures
Patient Health Questionnaire Depression Scale (PHQ-9):
The PHQ-9 is a 9-item self-report questionnaire that scores each of the nine DSM-IV criteria for depressive disorders over the past two weeks as “0” (not at all) to “3” (nearly every day). The PHQ-9 is a widely used measure of depression severity that is both reliable and valid (Kroenke et al., 2001), including during the peripartum period (Flynn et al., 2011; Yawn et al., 2009).
Mini-International Neuropsychiatric Interview (MINI):
The MINI is a short and structured diagnostic interview for DSM-IV and ICD-10 psychiatric disorders which is designed to be conducted in less time than other diagnostic interviews. The MINI has been found to exhibit excellent inter-rater and test-retest reliability and good concordance with the Structured Interview for DSM-III-R (SCIP-P; Sheehan et al., 1998; Sheehan et al., 1997).
Pregnancy Risk Questionnaire (PRQ):
The PRQ is an 18-item self-report questionnaire that assesses presence of multiple psychosocial risk factors of postpartum depression, such as the woman’s attitude toward her pregnancy, her experience of parenting in childhood, history of depression, history of physical and sexual abuse, presence of social support, etc. (Austin et al., 2005). The PRQ is shown to have greater sensitivity and specificity than previously reported tools developed for the antenatal prediction of postpartum depression (Austin et al., 2005).
Procedure
Subjects participated in one data collection session during pregnancy at the North Florida Women’s Care center immediately before or after one of their regularly scheduled OB/GYN appointments. These data are part of a larger, longitudinal, and ongoing study on neural and psychosocial predictors of perinatal depression. All participants first provided written informed consent, and then completed self-report questionnaires. After completion of the questionnaires and EEG setup, participants completed the doors task (described below) while EEG was recorded. Following the doors task, two other brief tasks were collected, but are not reported here. Finally, the MINI was administered and audio recorded.
The doors task was administered using Presentation software (Neurobehavioral Systems, Inc., Albany, CA, USA) and was similar to the version used in previous studies (Proudfit, 2015). The task consisted of two blocks of 20 trials. Each trial began with the presentation of two identical doors. Participants were instructed to select the left or right door by clicking the left or right mouse button, respectively. Participants were told that they could either win $0.50 or lose $0.25 on each trial. These values were chosen to equalize the subjective value of gains and losses (Tversky & Kahneman, 1981; Tversky & Kahneman, 1992). Participants were explicitly informed that they would keep their earnings and that the goal of the task was to earn as much money as possible. The image of the doors was presented until the participant made a selection. After stimulus offset, a fixation cross (+) was presented for 1,000 ms, and feedback was then presented on the screen for 2,000 ms. A gain was indicated by a green arrow pointing upward (↑), and a loss was indicated by a red arrow pointing downward (↓). The feedback stimulus was followed by a fixation cross (+) presented for 1,500 ms, immediately followed by the message “Click for next round.” This prompt remained on the screen until the participant responded with a button press to initiate the next trial. There were an equal number of gain and loss trials (20 each), such that participants had an equal likelihood of receiving gain and loss feedback over the course of the task.
EEG Recording and Processing
Continuous EEG was recorded using an elastic cap with 34 electrode sites placed according to the 10/20 system. Electrooculogram (EOG) was recorded using four additional facial electrodes: two placed approximately 1 cm outside of the right and left eyes, and two placed approximately 1 cm above and below the right eye. All electrodes were sintered Ag/AgCl electrodes. Data were recorded using the Active Two BioSemi system (BioSemi, Amsterdam, Netherlands). The EEG was digitized with a sampling rate of 1024 Hz using a low-pass fifth order sinc filter with a half-power cutoff of 204.8 Hz. A common mode sense active electrode producing a monopolar (i.e., nondifferential) channel was used as recording reference. EEG data were analyzed using Brain Vision Analyzer (Brain Products, Gilching, Germany). Data were referenced offline to the average of left and right mastoids, and band-pass filtered (0.01 to 30 Hz with a 24 dB/oct roll-off).
Feedback-locked epochs were extracted with a duration of 1,200 ms, including a 200 ms pre-stimulus and 1000 ms post-stimulus interval; these segments were then corrected for eye movement artifacts using a regression-based approach (Gratton, Coles, & Donchin, 1983). Epochs containing a voltage greater than 50 μV between sample points, a voltage difference of 175 μV within a segment, or a maximum voltage difference of less than 0.50 μV within 100 ms intervals were automatically rejected. Additional artifacts were identified and removed based on visual inspection. The 200 ms pre-stimulus interval was used as the baseline.
Feedback-locked ERPs were averaged separately for gains and losses. The number of trials per condition that remained after artifact rejection at the FCz electrode site were as follows: Gain (M = 19.93, SD = .30), Loss (M = 19.85, SD = .74). The average ERP response to gains and losses between 250 and 350 ms were exported. The RewP was analyzed by entering both the averaged ERP response to gains (i.e., the RewP) and losses (i.e., the feedback negativity, or the FN) into the regression. This approach essentially produces residualized difference scores (e.g., the RewP controlling for the FN; Meyer et al., 2017).
Data Analysis
Pearson and point-biserial (i.e., for the dichotomous history of MDE variable) correlations were utilized to examine relationships between antenatal depressive symptoms, the RewP, and other risk factors (i.e., annual household income, history of major depressive episodes, and scores on a psychosocial risk factor questionnaire, the Pregnancy Risk Questionnaire, PRQ). Additionally, a stepwise regression was conducted to examine whether these factors predict unique variance in antenatal depressive symptoms. Finally, the RewP and PRQ variables were mean-centered and multiplied together to compute an interaction term, and a linear regression predicting PHQ-9 depressive symptoms with RewP, PRQ scores, and their interaction term entered as predictors was conducted to examine whether the RewP and PRQ interact to predict antenatal depressive symptoms.
Results
Greater antenatal depressive symptoms were associated with lower annual household income (r(86)= −.30, p < .01), a blunted RewP (r(86)= −.21, p < .05), and a greater score on the PRQ (r(86)= .66, p < .001). Antenatal depressive symptoms were unrelated to history of MDE (r(86)= .13, p = .24).
Next, a stepwise regression was conducted to predict antenatal depressive symptoms based on history of MDE, annual household income, PRQ score, and the RewP. In the first step (F(1, 85) = 66.07, p < .001), PRQ score was the best single predictor of antenatal symptoms (β = .23, t(85) = 8.13, p < .001), accounting for 44% of the variance in symptoms. In the second step (F(2, 85) = 37.50, p < .001), PRQ score (β = .23, t(85) = 8.26, p < .001) and the RewP (β = −.10, t(85) = −2.33, p = .02) were significant independent predictors of antenatal depressive symptoms, with the addition of the RewP accounting for an additional 4% of the variance in depressive symptoms. Both other variables (i.e., annual income and history of a depressive episode) failed to account for a significant increment in current depressive symptoms at the .05 level. Thus, the stepwise regression analysis suggested that PRQ score and the RewP were the best independent predictors of antenatal depressive symptoms—and together accounted for 48% of the variance in current antenatal depressive symptoms.1
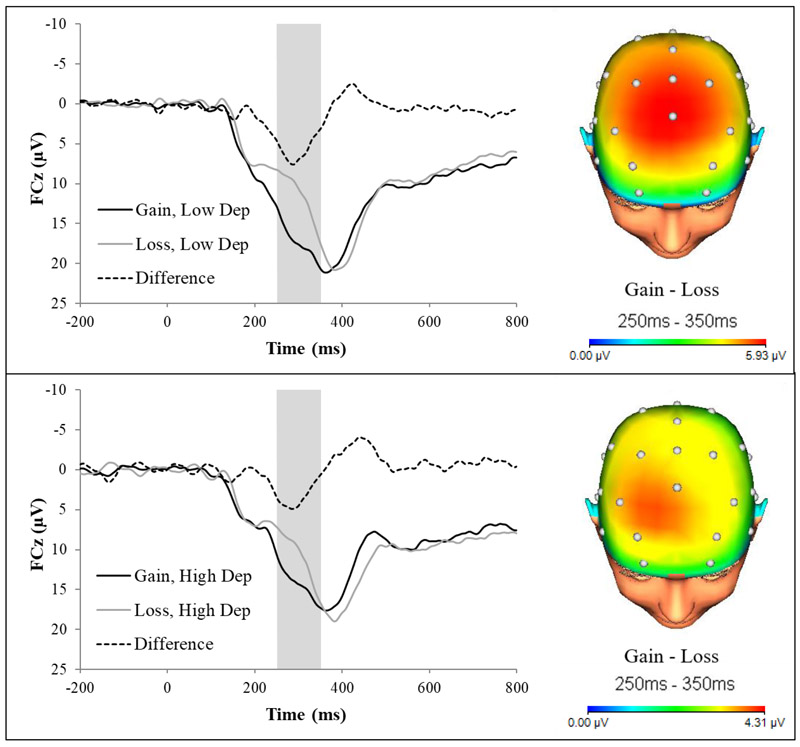
To visualize the association between reduced RewP and greater antenatal depressive symptoms revealed in zero-order correlations and the stepwise regression, symptoms were dichotomized using a median split (median = 4.00), and the ERP response to gains and losses were graphed for those with low versus high depression symptoms. As evident from Fig. 1, individuals with higher depressive symptoms showed a reduced RewP (i.e., the difference in activity between gain and loss trials) as compared to individuals with lower depressive symptoms.
Figure 1.
Feedback-locked ERPs (left) for gains and losses, and topographic maps for the gain-loss difference (right) in individuals low (top) and high (bottom) in depressive symptoms in pregnancy. Individuals with high depressive symptoms showed a reduced RewP (i.e., the difference in amplitude between gain and loss conditions) as compared to individuals with low depressive symptoms.
Finally, we examined whether PRQ scores and the RewP might interact to predict antenatal depressive symptoms. PRQ score and RewP variables were mean-centered and an interaction term was computed by multiplying the two variables. Next, a linear regression predicting PHQ-9 scores was conducted with mean-centered RewP, PRQ score, and their interaction term entered as independent variables. The regression model was significant (F(3, 85) = 26.11, p < .001) and the RewP (β = .20, t(85) = 2.59, p = .01) and PRQ scores (β = −.66, t(85) = −8.34, p < .001) were independent significant predictors of depressive symptoms. However, the interaction term was non-significant (β = −.12, t(85) = −1.49, p = .14). Thus, while the RewP and PRQ independently predict antenatal depressive symptoms, they do not interact to predict symptoms.
Discussion
The present study examined the cross-sectional associations between depressive symptoms in the antenatal period and both neural response to reward and self-reported risk factors (i.e., annual income, past depressive episodes, and scores on the Pregnancy Risk Questionnaire). Zero-order correlations revealed that greater depressive symptoms in pregnancy were related to lower annual income, past depressive episodes, and an elevated score on the PRQ. These findings align with previous reviews that identify socioeconomic status and past major depressive episodes as strong correlates of perinatal depressive symptoms (Beck, 2001).
Moreover, greater depressive symptoms were also related to a reduced RewP. Indeed, when all measures were entered into a stepwise regression, PRQ score and RewP amplitude emerged as the best independent predictors of antenatal depressions symptoms, which together accounted for 48% of the variance in current antenatal depressive symptoms. Specifically, PRQ score accounted for 44% of the variance in current antenatal depressive symptoms, while the RewP accounted for another 4% of incremental variance. While the RewP predicts a relatively small incremental amount of variance in depressive symptoms, the RewP shares no method variance with the PHQ-9, unlike the PRQ. Finally, the RewP × PRQ interaction term was not a significant predictor of antenatal depressive symptoms, suggesting that RewP impacted depressive symptoms equally across all levels of PRQ.
This study replicates previous work linking greater depressive symptoms to a reduced RewP (Foti & Hajcak, 2009; Bress et al., 2012), and extends this research to antenatal depressive symptoms. Our findings also align with previous research implicating reduced-reward related neural activation in striatal regions in postpartum depression (Moses-Kolko et al., 2011; Silverman et al., 2007; Laurent & Ablow, 2012), as previous research has suggested that the RewP reflects reward-circuit activation, including the striatum (Foti et al., 2011; Carlson et al., 2011).
Furthermore, our study provides novel evidence that the RewP indexes distinct variability in antenatal depressive symptoms from other well-researched psychosocial risk factors. Our results lend further support to the notion that the RewP is a distinct biomarker of risk for major depression (Bress et al., 2013; Nelson et al., 2016; Proudfit, 2015), and suggest that the RewP might be investigated in relation to risk for later increases in depressive symptoms (e.g., as a predictor of postpartum depression). In a large sample of never-depressed adolescent girls, a reduced RewP prospectively predicted first-onset depressive disorder and greater depressive symptoms 18 months later, even when controlling for depressive symptoms at initial testing and parental lifetime psychiatric history (Nelson et al., 2016). Employing a similar approach in future studies, we can examine whether the RewP predicts new-onset cases of depression and increases in depressive symptoms in the postpartum period. Such findings would suggest that reward insensitivity is a trait that confers risk for depression in the peripartum period, and as such, could be a target for novel interventions and prevention efforts. In particular, interventions that address maternal pleasure / reward associated with thoughts and behaviors about their infants are urgently needed.
Thus, pending future studies replicating the current findings in independent samples, RewP and the PRQ could potentially be utilized in improving precision of risk screening for antenatal and postpartum depression. In our previous work, we demonstrated that the RewP could significantly enhance the positive predictive value of first-onset depressive disorders when applied in series with self-report measures (Nelson et al., 2016). In the context of perinatal depression, using the RewP and the PRQ in tandem has the potential to increase the sensitivity and specificity of screenings. Further research on the sensitivity and specificity of these measures, used together in series or in parallel, in prospectively detecting cases of peripartum depression is needed.
The present study has multiple limitations that warrant consideration. First, the present study was a preliminary study that aimed to examine whether cross-sectional associations between antenatal depressive symptoms and the RewP were present and whether they are independent from associations between depressive symptoms and other well-research psychosocial predictors. Given the cross-sectional design of the study, causality cannot be inferred from our results. Thus, the present results do not imply that a reduced RewP or heightened scores on the PRQ cause antenatal depressive symptoms, but rather that these factors are associated with heightened depressive symptoms. Thus, future studies should utilize longitudinal designs to determine whether a reduced RewP and heightened psychosocial risk factors prospectively predict heightened depressive symptoms in the antenatal period. Second, given that the present study is the first study to report on associations between the RewP and antenatal depressive symptoms, the present findings should be replicated in larger samples.
In conclusion, the current study examined the relationship between antenatal depressive symptoms, neural correlates of reward sensitivity, and psychosocial predictors of risk for antenatal depressive symptoms. While antenatal depressive symptoms were found to be associated with annual income, the RewP, and Pregnancy Risk Questionnaire scores in zero-order correlations, the RewP and PRQ scores were identified in a stepwise regression as the best predictors of antenatal depressive symptoms, and together predicted 48 percent of the variance in current depressive symptoms. This is the first study to identify the RewP as a correlate of depressive symptoms in women in the antenatal period and sets the stage for examining whether the RewP could aid in the early detection of risk for perinatal increases in depression and to target interventions for the most disabling symptoms.
Highlights:
Lower annual income was associated with greater antenatal depressive symptoms.
Greater Pregnancy Risk Questionnaire (PRQ) scores were related to increased symptoms.
A reduced neural response to rewards was related to increased depressive symptoms.
Reward response and PRQ score independently predicted antenatal depressive symptoms.
Acknowledgments
We would like to thank Bill Hambsh, David O’Bryan, Erin Ryals, and the rest of the staff at North Florida Women’s Care for their support with subject recruitment and data collection.
This work was supported by the following grant: NIMH T32 MH 093311.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Upon adding two additional variables as predictors to the stepwise regression—gestational weeks and a dichotomous variable indicating whether subjects participated before or after their doctor’s appointment—results of the stepwise regression remained consistent such that only PRQ score and the RewP emerged as the best predictors of antenatal depressive symptoms. In the first step (F(1, 79) = 59.60, p < .001), PRQ score was the best single predictor of antenatal symptoms (β = .23, t(79) = 7.72, p < .001), accounting for 43% of the variance in symptoms. In the second step (F(2, 79) = 34.60, p < .001), PRQ score (β = .22, t(79) = 7.59, p < .001) and the RewP (β = −.12, t(79) = −2.43, p = .02) were significant independent predictors of antenatal depressive symptoms, with the addition of the RewP accounting for an additional 4% of the variance in depressive symptoms.
References
- American Psychological Association. (2018). Postpartum Depression. Retrieved February 22, 2018, from http://www.apa.org/pi/women/resources/reports/postpartum-depression.aspx [Google Scholar]
- Austin MP, Hadzi- Pavlovic D, Saint K, & Parker G (2005). Antenatal screening for the prediction of postnatal depression: validation of a psychosocial Pregnancy Risk Questionnaire. Acta Psychiatrica Scandinavica, 112(4), 310–317. [DOI] [PubMed] [Google Scholar]
- Baker TE, & Holroyd CB (2011). Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biological psychology, 87(1), 25–34. [DOI] [PubMed] [Google Scholar]
- Beck CT (2001). Predictors of postpartum depression: an update. Nursing research, 50(5), 275–285. [DOI] [PubMed] [Google Scholar]
- Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, … & Barch DM (2016). Neural correlates of reward processing in depressed and healthy preschool-age children. Journal of the American Academy of Child & Adolescent Psychiatry, 55(12), 1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, & Hajcak G (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology, 50(1), 74–81. [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, & Hajcak G (2012). Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological psychology, 89(1), 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, & Hajcak G (2011). Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage, 57(4), 1608–1616. [DOI] [PubMed] [Google Scholar]
- Clark R, Tluczek A, & Wenzel A (2003). Psychotherapy for postpartum depression: a preliminary report. American Journal of Orthopsychiatry, 73(4), 441. [DOI] [PubMed] [Google Scholar]
- Flynn HA, Sexton M, Ratliff S, Porter K, Zivin K (2011). Comparative performance of the Edinburgh Postnatal Depression Scale and the Patient Health Questionnaire-9 in pregnant and postpartum women seeking psychiatric services. Psychiatry Res.187(1-2): 130–4 [DOI] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2009). Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology, 81(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, & Hajcak G (2011). Event- related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human brain mapping, 32(12), 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile S (2017). Untreated depression during pregnancy: Short-and long-term effects in offspring. A systematic review. Neuroscience, 342, 154–166. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, & Simons RF (2007). It's worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology, 44(6), 905–912. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, & Larsen JT (2006). The good, the bad and the neutral: Electrophysiological responses to feedback stimuli. Brain Research, 1105(1), 93–101. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, & Krigolson OE (2008). The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology, 45(5), 688–697. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The phq- 9. Journal of general internal medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, & Ablow JC (2011). A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience, 7(2), 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, & Chan RC (2014). The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia, 53, 213–220. [DOI] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, & Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114–122. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, & Phillips ML (2011). Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biological psychiatry, 70(4), 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses- Kolko EL, Horner MS, Phillips ML, Hipwell AE, & Swain JE (2014). In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. Journal of neuroendocrinology, 26(10), 665–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Arteche A, Fearon P, Halligan S, Goodyer I, & Cooper P (2011). Maternal postnatal depression and the development of depression in offspring up to 16 years of age. Journal of the American Academy of Child & Adolescent Psychiatry, 50(5), 460–470. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, & Hajcak G (2016). Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. American Journal of Psychiatry, 173(12), 1223–1230. [DOI] [PubMed] [Google Scholar]
- O'hara MW, Rehm LP, & Campbell SB (1982). Predicting depressive symptomatology: cognitive-behavioral models and postpartum depression. Journal of Abnormal Psychology, 91(6), 457. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, & Wisner KL (2014). Perinatal mental illness: definition, description and aetiology. Best Practice & Research Clinical Obstetrics & Gynaecology, 28(1), 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, … & Fava M (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry, 166(6), 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, & O’Shea JP (2005). Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological psychiatry, 57(4), 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52(4), 449–459. [DOI] [PubMed] [Google Scholar]
- Serati M, Redaelli M, Buoli M, & Altamura AC (2016). Perinatal major depression biomarkers: a systematic review. Journal of affective disorders, 193, 391–404. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Sheehan K, Amorim P & Janavs J (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59, 22–33. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, … & Dunbar GC (1997). The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry, 12(5), 232–241. [Google Scholar]
- Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, & Goldstein M (2007). Neural dysfunction in postpartum depression: an fMRI pilot study. CNS spectrums, 12(11), 853–862. [DOI] [PubMed] [Google Scholar]
- Steele JD, Kumar P, & Ebmeier KP (2007). Blunted response to feedback information in depressive illness. Brain, 130(9), 2367–2374. [DOI] [PubMed] [Google Scholar]
- Tversky A & Kahneman D (1981). The framing of decisions and the psychology of choice. Science, 211(4481), 453–458. [DOI] [PubMed] [Google Scholar]
- Tversky A & Kahneman D (1992). Advances in prospect theory: Cumulative representation of uncertainty. Journal of Risk and Uncertainty, 5(4), 297–323. [Google Scholar]
- Verbeek T, Bockting CL, van Pampus MG, Ormel J, Meijer JL, Hartman CA, & Burger H (2012). Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. Journal of affective disorders, 136(3), 948–954. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Pace W, Wollan PC, Bertram S, Kurland M, Graham D, Dietrich A (2009). Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J Am Board Fam Med;22(5):483–91 [DOI] [PubMed] [Google Scholar]
- Yim IS, Stapleton LRT, Guardino CM, Hahn-Holbrook J, & Schetter CD (2015). Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annual review of clinical psychology, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]