ABSTRACT
Enterococcus cecorum is a commensal bacteria and opportunistic pathogen that can cause outbreaks of Enterococcal spondylitis (“kinky back”) in poultry, with a growing concern worldwide. Numerous Bacillus-based probiotic strains are commercially available with proven effects in supporting gut health and growth performance, but efficacy against pathogenic E. cecorum is unknown. This study compared the in vitro inhibitory potential of cell-free supernatants (CFSs) of 18 Bacillus strains (14 commercial probiotic strains, 1 internal negative control and 3 type strains) on the growth of 9 clinical E. cecorum isolates. Standardized biomass cultures of live Bacillus were harvested and filtered to obtain CFSs. Inhibitory potential against E. cecorum isolates was assessed via a microdilution assay in which the final pathogen concentration was ∼ 104 CFU/mL. Absorbance (OD) was measured every 15 min for 15 h and used to calculate percentage growth inhibition at an OD equivalent to 0.4 in the positive control (PC) (pathogen but no CFS), and growth delay vs. PC. Growth kinetic responses of pathogen isolate-Bacillus strain combinations ranged from total pathogen inhibition to partial inhibition, lag in growth, no effect, or increased growth vs. PC. Percentage inhibition of individual isolates varied markedly among Bacillus strains, from 100% to −100% (growth promotion as recorded for the type strain) (B. amyloliquefaciens DSM7T). Five B. amyloliquefaciens CFSs produced higher average inhibition rates (>75%) than 2 out of 3 Bacillus licheniformis CFSs (−2.5, and −8.39% vs. PC, respectively) and 1 out of 2 Bacillus subtilis CFSs (7.3% vs. PC) (P < 0.05). Commercial strain 3AP4 exhibited the highest average percentage inhibition vs. PC (85.0% ± 7.9) and the most consistent inhibitory effect across pathogen isolates. The findings indicate that some commercially available poultry probiotic Bacillus strains are effective at inhibiting pathogenic E. cecorum in vitro, but effects are highly strain and pathogen isolate-dependent. Further work is required to confirm effects in vivo and isolate the inhibitory substances.
Keywords: Enterococcus cecorum, Bacillus, probiotic, pathogen inhibition
INTRODUCTION
Enterococcus cecorum is a commensal, Gram-positive bacteria that has been identified in the intestinal tract of a diverse range of mammals and birds, including poultry (Devriese et al., 1983; Devriese et al., 1991a; Devriese et al., 1992). Non-pathogenic strains are present in the intestines of chickens from approximately 3 wk of age and apparently dominate the gut microbiota of healthy birds by 3 mo (Devries et al., 1991b). However, pathogenic strains also occur and can result in Enterococcal spondylitis (ES), also known as “kinky back”, a serious disease of commercial poultry production in which the bacteria translocate from the intestine to the free thoracic vertebrae and adjacent notarium or synsacrum, causing lameness, hind-limb paresis and, in 5 to 15% of cases, mortality (de Herdt et al., 2008; Martin et al., 2011; Jung and Rautenschlein, 2014). The clinical significance of E. cecorum infections in broilers was first described in 2002 (Devriese et al., 2002; Wood et al., 2002). Recent evidence from a variety of articles and case reports has suggested that pathogenic E. cecorum is emerging (or re-emerging) as a significant challenge in poultry production worldwide, causing significant losses to commercial flocks when outbreaks occur, especially in the US (Harada et al., 2012; Aitchison et al., 2014; Jung and Rautenschlein, 2014; Dolka et al., 2016; Dolka et al., 2017). The reasons for this rise are currently unclear. Proposed explanations include a general reduction in the use of antibiotic growth promoters that may create more favorable conditions for the re-emergence of pathogens and/or the emergence of clonal isolates of E. cecorum; recent studies have revealed evolutionary divergent genomic features and increased virulence of pathogenic strains compared with commensal strains of E. cecorum (Borst et al., 2015). The existence of certain predisposing factors in the bird, such as osteochondrosis dissecans lesions in the free thoracic vertebra, may also increase the pathogenicity of E. cecorum and likely development of ES (Borst et al., 2016). Altered prevalence of concurrent infections, changing nutritional requirements of birds or genetic selection pressures could also be at play (de Herdt et al., 2008). Against this background antibiotic alternatives to preventing and combatting pathogenic E. cecorum infections in poultry production are highly desirable.
Probiotics, also known as direct-fed microbials, have been produced commercially from a range of source microorganisms (bacteria, yeasts, and fungi), and have shown considerable success in poultry production in supporting gut health and improving growth performance (FAO, 2016). Many of the currently available commercial probiotics for poultry incorporate strains of Bacillus sp. (typically Bacillus subtilis, Bacillus amyloliquefaciens, and/or Bacillus licheniformis), favored because of their spore-forming capacity and associated ability to survive harsh processing conditions as well as digestion in the stomach (Cutting, 2011). Individual species and strains vary in the precise nature of their effects and their beneficial modes of action (Lee et al., 2010), but most have been selected based on their ability to reduce gut colonization by a wide range of major pathogenic bacteria including Escherichia coli (Wu et al., 2011; Ahmed et al., 2014; Lei et al., 2015), Salmonella spp. (Jeong and Kim, 2014; Park and Kim, 2014), Clostridium perfringens (Gebert et al., 2007; Jeong and Kim, 2014), and Campylobacter spp. (Fritts et al., 2000). It is biologically plausible that strains of Bacillus may also be effective at inhibiting pathogenic strains of E. cecorum, but this has not previously been investigated in any systematic way. Pathogenic E. cecorum isolated from extra-intestinal sites of diseased birds are known to exhibit significant genetic heterogeneity, differ in their pathogenesis and do not always harbor known virulence genes (Borst et al., 2015; Dolka et al., 2016; Dolka et al., 2017). Therefore, it is likely that different clinically isolated strains of E. cecorum may differ in their pathogenicity and potentially also in their susceptibility to the inhibitory effects of probiotic Bacillus spp.
This study aimed to systematically evaluate the capacity of a range of commercially produced strains of probiotic Bacillus spp. to inhibit or delay the growth of E. cecorum isolates, in vitro via the synthesis of antimicrobial compounds. The E. cecorum strains were sourced from broilers showing clinical signs of ES and were collected from avian production sites located in two geographical markets (US and EU) across several years, in order to maximize the genetic diversity of the pathogen represented in the study.
MATERIALS AND METHODS
Enterococcus cecorum Isolates and Culture Conditions
Isolates of 9 different clinical strains of pathogenic E. cecorum were obtained from the internal collections of DuPont Industrial Biosciences, or purchased from Poulpharm (Izegem, Belgium). The identification of each pathogenic isolate was confirmed by PCR or MALDI-TOF mass spectrometry. All strains had originally been isolated from extra-intestinal lesions and confirmed ES outbreaks in poultry production (broilers or breeders) allowing confidence that the tested strains were virulent and capable of causing disease. Details of the isolates and their origin are given in Table 1.
Table 1.
Origin and details of the pathogenic E. cecorum isolates (n = 9).
| Geographic origin of isolate | Year of isolation | Strain designation | Identification confirmation | Biological origin | Supplier |
|---|---|---|---|---|---|
| North America (NC) Carolina) | 2013 | 12147-1 | PCR + 16S | Broiler, spinal abscess | DuPont Internal Collection |
| North America (NC) Carolina) | 2013 | 12696M-1 | PCR + 16S | Broiler, spinal abscess | DuPont Internal Collection |
| North America (NC) Carolina) | 2013 | 11957-3 | PCR + 16S | Broiler, spinal abscess | DuPont Internal Collection |
| North America (NC) Carolina) | 2013 | 11951-1 | PCR + 16S | Broiler, spinal abscess | DuPont Internal Collection |
| European Union (BE) | 2014 | E.59.56 | MaldiTof | Broiler, femoral head | Poulpharm, Izegem, Belguim |
| European Union (BE) | 2015 | F.68.19 | MaldiTof | Broiler, joint | Poulpharm, Izegem, Belgium |
| European Union (BE) | 2013 | C.34.19 | MaldiTof | Broiler, bone marrow | Poulpharm, Izegem, Belgium |
| European Union (BE) | 2013 | D.42.11 | MaldiTof | Broiler, joint | Poulpharm, Izegem, Belgium |
| European Union (BE) | 2015 | G.75.17 | MaldiTof | Broiler, articulation | Poulpharm, Izegem, Belgium |
NC: North Carolina
BE: Belgium
Isolates were supplied in frozen vials in culture media and glycerol and were subsequently cultured in Brain Heart Infusion broth (BHI, Conda, Madrid, Spain) upon arrival in the laboratory to check for viability and purity. Aliquots were frozen in vials and stored at −80°C prior to further use.
Bacillus Strains and Preparation of Cell Free Supernatants (CFS)
The inhibitory potential of 14 different commercial strains of probiotic Bacillus, 1 internal negative control and 3 Bacillus-type strains were tested. These included both DuPont proprietary probiotic strains and Bacillus strains isolated from other poultry probiotic products available on the market in 2015. The origin and identity of the Bacillus strains used in the study are given in Table 2. The DuPont proprietary Bacillus strains were supplied in-house. All other strains were purchased and isolated in triplicate from 3 separate production batches. All strains were identified by Illumina sequencing to ensure that the strains recovered matched those declared on the product label.
Table 2.
Origin and identity of the probiotic Bacillus strains used in this study.
| Probiotic Product | Manufacturer | Bacillus species | Internal strain designation | Commercial strain designation |
|---|---|---|---|---|
| Enviva®Pro | Danisco Animal Nutrition, DuPont Industries, US | B. amyloliquefaciens | 15AP4 | PTA-6507 |
| Enviva®Pro | Danisco Animal Nutrition, DuPont | B. amyloliquefaciens | BS8 | NRRL B-50104 |
| Enviva®Pro | Danisco Animal Nutrition, DuPont | B. amyloliquefaciens | 2084 | NRRL B-50013 |
| Calsporin | Calpis Co. Ltd, Japan | B. amyloliquefaciens | #4–1 | C-3102/DSM 15544 |
| Clostat | Kemin Industries Inc., US | B. amyloliquefaciens | #1–1 | unknown |
| Sporulin | Novus International, Inc., US | B. amyloliquefaciens | #10/4 | unknown |
| Sporulin | Novus International, Inc., US | B. amyloliquefaciens | #10B/1 | unknown |
| Sporulin | Novus International, Inc., US | B. amyloliquefaciens | #10B/4 | unknown |
| GalliPro | CHR Hansen, Denmark | B. subtilis | #11/1 | DSM 17299 |
| Galliprotect | CHR Hansen, Denmark | B. licheniformis | #12/1 | DSM 17236 |
| CSI | DuPont | B. amyloliquefaciens | 22CP1 | n/a |
| CSI | DuPont | B. amyloliquefaciens | 3AP4 | n/a |
| CSI | DuPont | B. amyloliquefaciens | BS18 | n/a |
| CSI | DuPont | B. amyloliquefaciens | ABP278 | n/a |
| Internal negative control | DuPont | B. amyloliquefaciens | BS27 | BS27 |
| n/a | DSMZ, Germany | B. amyloliquefaciens | DSM7T | Type Strain |
| n/a | DSMZ, Germany | B. licheniformis | DSM13T | Type Strain |
| n/a | DSMZ, Germany | B. subtilis | DSM10T | Type Strain |
n/a: not applicable
Cell-free supernatants (CFS) were prepared from Bacillus strains according to the following procedure: a small amount of frozen Bacillus from the stock was removed with a sterile inoculating loop and streaked onto tryptic soy agar (TSA, Biokar Diagnostics, Beauvais, France) plates for overnight culture at 32°C. The next day, a small amount of the respective colony was added to 10 mL tryptic soy broth (TSB, Biokar Diagnostics, Beauvais, France) in a 50 mL conical tube and shaken at 100 rpm at 32°C for 6 to 8 h. Cultures were then streaked onto TSA plates and incubated at 32°C for 24 h to check for purity. A 10 μL aliquot of the pure culture was then transferred to a 250 mL Erlenmeyer flask containing 50 mL TSB. Flasks were shaken at 100 rpm at 32°C for 16 h, re-checked for purity, and then the culture was diluted 1:10 in Luria-Bertani broth (containing tryptones 10 g/L, NaCl 10 g/L and yeast extract 5 g/L) before measuring absorbance at an optical density (OD) of 600 nm, using a microplate reader (CLARIOstar, BMG labtech). The aim was to compare CFSs obtained from bacterial growth with comparable OD fixed at 4 ± 0.5 (equivalent to 1.109 CFU/mL). A blank sample of the TSB culture medium was used to calibrate the spectrophotometer. Once cultures had reached the required absorbance range, the Bacillus growth was re-checked for purity and transferred to a sterile 250 mL bottle and centrifuged at 10,000 rpm for 10 min. The supernatant was then transferred into a 50 mL tube, centrifuged again at 8,000 g for 5 min, and the supernatant filtered through a 0.2 μm Nalgene filter (ThermoFisher Scientific) to obtain CFS. The CFSs were stored at −20°C until further use.
Enterococcus cecorum Inhibition Assay
Enterococcus cecorum isolates were inoculated from deep frozen stock cultures in a BHI broth and a BHI agar plate (to check purity) and incubated overnight at 37°C. All strains were subcultured at least twice before inclusion in the assay to ensure adaptation to the growth medium. The E. cecorum cultures were then diluted in Tryptone Salt Broth (Casein enzymic hydrolysate 1 g/L; sodium chloride 8.5 g/L) and ino-culated in BHI medium (pre-heated for 1 h at 37°C to avoid thermic shock of E. cecorum cells) in 96-well UV-treated microtiter plates with flat-bottomed wells, at a final concentration of 104 CFU/mL. The Frozen CFS samples were thawed at room temperature for 30 min, and then administered to the 96 well-plate into the treated wells (10% v/v) containing BHI and E. cecorum isolates (1% v/v). Non-treated wells contained BHI and E. cecorum isolate (1% v/v) only. This produced the following treatments: positive control (BHI medium plus 1% (v/v) E. cecorum culture) (PC); negative control (BHI medium) (NC); CFS only (BHI medium plus 10% (v/v) CFS); E. cecorum plus CFS (BHI medium plus 1% (v/v) E. cecorum culture plus 10% (v/v) CFS). All microtiter plates were covered and incubated at 37°C for 15 h in a FlexStation® multi-mode microplate reader coupled with Soft Max pro software (Molecular Devices LLC, US), for the measurements of optical densities (following homogenization) at 595 nm every 15 min.
The OD data (averaged across two biological replicates with SD < 0.05) were used to calculate the percentage of pathogen growth inhibition, where inhibition is defined as the percentage reduction in growth in the experimental sample compared with that in the respective PC sample (containing pathogen in BHI medium but no Bacillus CFS), with growth being measured as biomass (absorbance OD), and being determined at the time-point equivalent to that producing an OD of 0.4 in the PC (equivalent to 1.107 CFU/mL). This OD had been selected during validation of the experimental procedure as being indicative of the middle of the exponential growth phase of the pathogen. The following equation, where “t” is time, was used to calculate the percentage of inhibition:
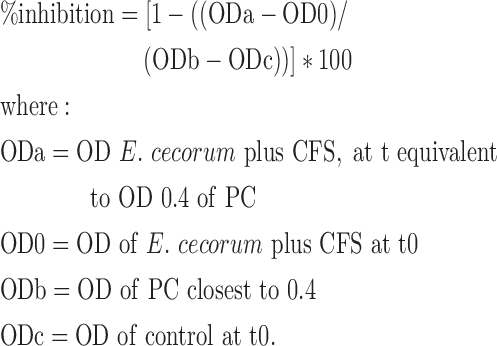 |
The kinetics of E. cecorum growth in the presence/absence of the Bacillus CFSs were also studied. The delay in pathogen growth in the presence of Bacillus CFS was calculated as the difference in time (minutes) to reach an OD of 0.4 between the PC and CFS plus E. cecorum supplemented wells.
Statistical Analysis
The results of the inhibition assays were averaged across duplicates and then analyzed by analysis of variance (ANOVA) to investigate the differences among Bacillus strains in their inhibitory effects. Post-hoc means separation was achieved using Tukey's Honest Standard Difference test. Statistical analysis was performed using the Fit Model platform of JMP 11.0 (SAS Institute Inc., Car, NC, 1989–2013). Differences were considered significant at P < 0.05.
RESULTS
A variety of different growth kinetic responses were exhibited by the E. cecorum isolates when exposed in vitro to the Bacillus CFSs. Graphical representations of these are provided in Supplementary material S1. For some Bacillus CFS-E. cecorum isolate combinations, total or almost total pathogen inhibition was evident throughout the exposure period (15 h or 900 min). For others, pathogen growth tracked that of the PC during the initial lag phase but was partially/totally inhibited during the exponential growth phase (compared with the PC). A further response was seen in which pathogen growth tracked that of the PC but was delayed by 1 to 2 h and modified by an extended lag phase, leading to a reduced final microbial population. Conversely, in a few cases the CFS-promoted pathogen growth leading to a reduced lag phase and increased final microbial population, and in yet other cases there was no effect of the CFS on pathogen growth.
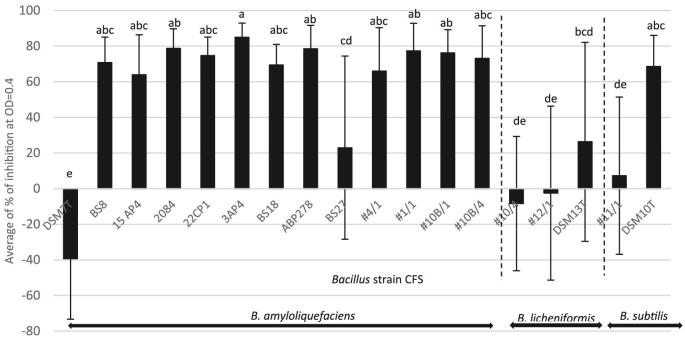
Figure 1 shows the mean percentage growth inhibition (biomass reduction as measured by sample absorbance) of the 9 pathogenic E. cecorum isolates by the 18 Bacillus CFSs, determined at a time-point equivalent to an OD in the PC of 0.4 (results for each pathogen isolate individually are displayed in Supplementary material S2). Except for the B. amyloliquefaciens type strain (DSM7T), all of the Bacillus CFSs were capable of inhibiting the growth of one or more of the pathogenic E. cecorum isolates, but effects varied markedly both between and within Bacillus species and strains (Figure 1). Percentage inhibition (vs. PC) across individual Bacillus strain-pathogen isolate combinations ranged from as low as −108%, indicating markedly increased pathogen growth in the presence of the CFS (as in B. amyloliquefaciens type strain DSM7T against E. cecorum isolate 11951-1), to 100%, indicating total inhibition of pathogen growth (as in B. amyloliquefaciens CFSs BS8, 15AP4, 2084 and #10B/4 against E. cecorum isolate 11957-3, and B. amyloliquefaciens CFS #1/1 against E. cecorum isolate 12696M-1). Si-gnificant variation in response across E. cecorum isolates was also evident, to the extent that some of the Bacillus CFSs inhibited the growth of certain isolates of E. cecorum (vs. PC) whilst promoting the growth of others (Supplementary material S2). This was particularly evident in the case of CFS BS27–a DuPont internal control strain known not to exhibit strong and consistent antimicrobial potential against Gram-positive and Gram-negative bacteria (data not shown)–which showed very wide variation in inhibitory effects across pathogen strains.
Figure 1.
Mean percentage growth inhibition 1 of pathogenic E. cecorum isolates (based on 2 biological replicates; n = 9) by 18 Bacillus cell-free supernatants, measured at a time-point equivalent to that which produced an optical density of 0.4 in the positive control.1 Defined as the percentage reduction in pathogen isolate growth in the experimental sample compared with that in the respective PC sample (containing pathogen in BHI medium but no Bacillus CFS), with growth being measured as biomass (absorbance (OD)), not as CFU, and being determined at the time-point equivalent to that producing an OD of 0.4 in the PC. Thus, 90% inhibition would mean a 90% reduction in growth (biomass). a, b, c, d, e Bars with no common letters are significantly different (P < 0.01).
The majority of the CFSs obtained from the existing proprietary Bacillus probiotic strains that were available on the market in 2015 belonged to B. amyloliquefaciens. With a few exceptions (notably the type strain DSM7T CFS and BS27 CFS), all of the CFSs from this species showed some degree of inhibitory effect against all isolates of pathogenic E. cecorum tested. There was a greater predominance of positive inhibition results (vs. PC) among the B. amyloliquefaciens CFSs than was evident among the other Bacillus sp. CFSs, and the error bars accompanying the mean inhibition values produced by the B. amyloliquefaciens CFSs were markedly smaller (Figure 1). Comparison of the mean pathogen inhibition rates (%) by the Bacillus CFSs using ANOVA and Tukey's HSD confirmed that there were differences among Bacillus strains in their abi-lity to inhibit E. cecorum (P < 0.05) (Figure 1). A total of 8 CFSs, all of which were from commercial pro-biotic strains of B. amyloliquefaciens, exhibited average pathogen inhibition rates of >70% and small standard errors compared to the other strains tested, indicating a more consistent effect across pathogen strains. Five of these (CFSs 3AP4, 2084, ABP278, #1/1, and #10B/1) produced higher average inhibition rates (>75%) than 2 out of 3 B. licheniformis CFSs (#12/1 and #10/4, −2.5 and −8.39% vs. PC, respectively) and 1 out of 2 B. subtilis CFSs (#11/1, 7.3% vs. PC) (P < 0.05) (Figure 1). Commercial strain 3AP4 exhibited the highest mean percentage inhibition vs. PC and the lowest degree of variation across pathogen strains (85.0% ± 7.9). The lowest inhibition was produced by the CFS from the B. amyloliquefaciens type strain (DSM7T) which appeared to actively support growth of the pathogen (vs. PC) (mean % inhibition −39.3 ± 34.1).
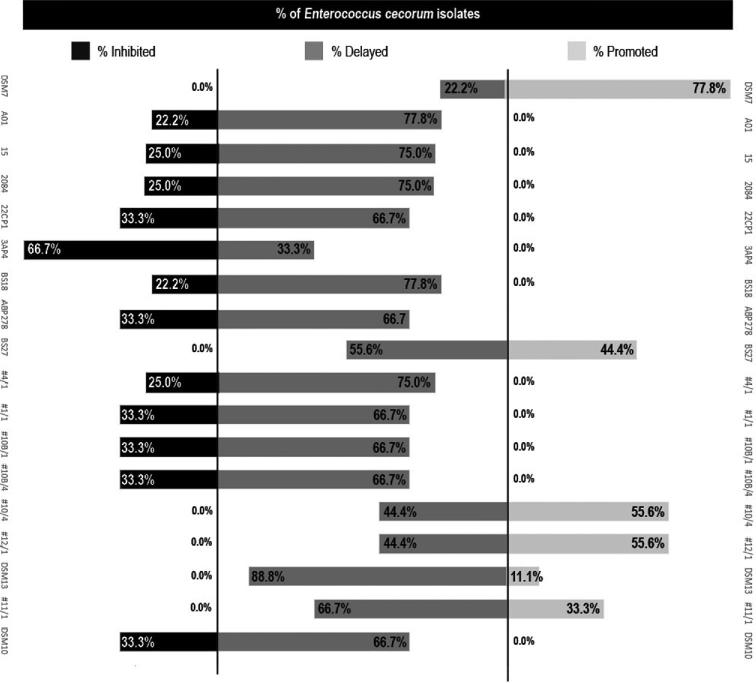
The wider comparative ability of the different Bacillus CFSs to inhibit, delay or promote the growth of all 9 pathogenic E. cecorum isolates is shown in Figure 2. The most consistent inhibitory effect across E. cecorum isolates was produced by the CFS from Bacillus strain 3AP4, which inhibited the growth of 6 of the pathogenic strains for the entire 15 h experimental period, and delayed growth in the remaining 3 isolates by an average of 6.53 h ± 2.1. This means that, in the least efficacious scenario, CFS 3AP4 delayed the growth of the pathogen by at least 4 h. In contrast, B. amyloliquefaciens CFSs DSM7T, BS27, #10/4, #12/1, B. licheniformis CFSs DSM13 and #11/1 delayed the growth of 22.2 to 88.8% of the pathogenic isolates but did not fully inhibit any of them. Meanwhile, certain Bacillus CFSs promoted the growth of one or more of the E. cecorum isolates (CFSs obtained from the type strain DSM7T and DSM13T; the internal negative control BS27, and 3 commercial probiotic strains #10/4, #12/1, and #11/1) (Figure 2).
Figure 2.
Percentage of pathogenic E. cecorum isolates inhibited, delayed, or promoted by cell-free supernatants of probiotic Bacillus spp.
DISCUSSION
Competitive exclusion is a probiotic mode of action that can occur via several different mechanisms, one of which is the production of secondary metabolites or other antimicrobial substances that inhibit the growth of pathogens. Growth inhibitory and/or bacteriocidal effects against certain poultry pathogens in vitro have previously been described for a number of probiotic bacteria, including strains of Bacillus spp. (Svetoch et al., 2005; Teo and Tan, 2005; Latorre et al., 2016; Poormontaseri et al., 2017). The present study sought to extend the current knowledge by testing whether substances within the CFSs of commercially available probiotic and type strains of Bacillus spp. could inhibit the activity of clinical isolates of E. cecorum - a commensal bacteria present in healthy birds which is emerging as an opportunistic pathogen capable of causing significant clinical disease outbreaks.
The results revealed that CFSs from some of the Bacillus strains tested were effective at inhibiting the growth of clinical E. cecorum isolates in vitro, but this was not the case for all strains. Effects were highly Bacillus-strain dependent, both as to whether inhibitory activity was evident as well as the degree of effect observed. This varied from total (100%) inhibition for the duration of the experimental period (15 h), to delayed growth of the pathogen and partial inhibition, to an apparent promotion of pathogen growth of up to 100% compared with the PC (in which no Bacillus CFS was present). It is worth noting that three of the tested commercial strains that were highly effective at inhibiting pathogenic E. cecorum (CFSs 15AP4, BS8, and 2084) are used in combination in the commercial product DuPont™ Enviva® PRO and as a combination should be more consistent than other single strain-based commercial products. The results here demonstrated that CFSs 15AP4, BS8, and 2084 have a diverse and a complementary coverage of inhibition.
The study's findings have highlighted significant strain specificity among probiotic Bacillus spp. in their in vitro effects on pathogenic microorganisms, namely pathogenic isolates of E. cecorum. This has not been reported previously and contrasts with recent concepts put forward by Sanders et al. (2018) that human clinical benefits of probiotics derived from Bifidobacterium and Lactobacillus genera are likely to derive from shared mechanisms at a sub-species, species, or genus level, rather than at strain level. Our findings suggest that, for Bacillus-based probiotics applied to poultry production, antimicrobial effects cannot be attributed at the species level. Nevertheless, the results also suggest that some of the existing proprietary Bacillus strains that are effective against major poultry pathogens may also be effective at controlling pathogenic E. cecorum growth and preventing gut colonization in vivo, whilst other strains may actively enhance growth of the pathogen. Thus, there may be potential for widening the application of the effective proprietary strains in poultry production. Under the in vitro conditions used here, CFSs from B. amyloliquefaciens proprietary strains 3AP4, 2084, ABP278, #1/1, and #10B/1 exhibited the most potent and consistent inhibitory effects against the E. cecorum isolates (>75% inhibition over the 15 h experimental period, on average, vs. the PC), whilst those from B. licheniformis strains #10/4 and #12/1, and B. subtilis strain #11/1 exhibited the lowest inhibitory effect (<10% inhibition, on average, vs. PC).
The Bacillus genus is known to be an extremely diverse taxonomic group, exhibiting huge genetic, phenotypic and functional variation, and differences between species and strains in relation to a range of functional properties and modes of probiotic action have been reported previously in the literature. Nevertheless, the apparent growth promotion effect of the CFSs from certain strains (BS27, #10/4, #12/1, #11/1) on the growth of some, but not all, of the E. cecorum isolates was unanticipated, and suggests that the antimicrobial activity of substances present in the CFSs was specific for particular Bacillus strain—E. cecorum isolate combinations. The CFS from the B. amyloliquefaciens type strain DSM7T, appeared to promote the growth of all the tested E. cecorum isolates under the study conditions (above the level seen in the PC), suggesting that substances present in the secretions of that particular strain were beneficial to E. cecorum growth. It is worth noting that type strains are not established based on any specific probiotic or pathogen-inhibitory activity per se, but rather as the definitive points of reference for a species (Lapage et al., 1990). Thus, it may be the case that the type strain DSM7T exhibits quite different functional properties towards pathogens such as E. cecorum compared with strains that have been selected based on their probiotic properties. In fact, E. cecorum pathogen inhibitory activity varied markedly across the 3 type strains (DSM7T, DSM13T, and DSM10T) and was generally inconsistent with the observed activity of the proprietary probiotic strains. This would seem to indicate that the Bacillus type strains for B. amyloliquefaciens, B. licheniformis, and B. subtilis do not serve as reliable reference points for establishing the E. cecorum inhibitory activities of probiotic strains of these species.
The study design did not allow the identification of the inhibitory substances present in the CFSs tested, or their potency; the observed growth inhibitory effects could have been bacteriocidal or bacteriostatic in nature, or a combination of both. Five of the Bacillus strains (BS8, 15AP4, 2084, #1/1, and #10B/4) were effective at totally inhibiting growth of individual E. cecorum isolates for the duration of the experimental period (15 h), which would seem to suggest a bacteriocidal effect. Whilst for other Bacillus strain-pathogen isolate combinations growth was more moderately suppressed and, whilst delayed, appeared to otherwise track that seen in the control treatment (for example Bacillus strain CFS 22CP1 and CFS 15AP4 against E. cecorum isolate D4211). This is more suggestive of a bacteriostatic effect, in which reproduction is somewhat inhibited but the bacteria are not necessarily killed. Effects of some of the tested Bacillus strains were also more consistent (across E. cecorum isolates) than others, which may be a reflection of the concentration of antimicrobial substances released in the supernatant and/or to the variety of substances present. The pHs of all the CFSs were found to be broadly similar (between 6.4 and 6.8, data not shown), which may be reflective of their pH optima, this range being notably similar to the pH range found in the poultry small intestine (pH range 5.5 to 6.6; Shafey and McDonal, 1991), where effects would be manifested in vivo. Further studies are needed to isolate the active compound(s) and determine their precise mode(s) of action.
The inhabiting microbial community, physiological, and environmental conditions of the broiler small intestine are much more complex and variable than was represented by the controlled in vitro conditions of the study. Whether and to what degree the observed effects would be replicated in vivo, and what impact this might have on the ability of pathogenic E. cecorum to colonize the gut lining and cause clinical ES disease, remains to be determined. However, it seems likely that those Bacillus strains whose CFSs did not exhibit antimicrobial activity under study conditions (that excluded any competition effects) might be even less likely to do so in the more challenging environment of the gastrointestinal tract. The different pathogen growth kinetic responses to CFS indicated that where there was a delay, this frequently exceeded or approached the upper end of the 4 to 8 h average total tract retention time of the chicken (Svihus, 2010). This suggests that, if replicated in vivo, inhibitory substances contained in the extracellular secretions of the implicated strains may be effective in reducing opportunities for E. cecorum to colonize the gut lining of broilers. The potential transfer of ES disease from poultry to humans is a further factor to consider because E. cecorum is a zoonotic organism and in rare cases can cause human infections, presumably through animal–human transfer (Stubljar and Skvarc, 2015; Delaunay et al., 2015).
There is some evidence from existing studies of ES pathogenesis and of probiotic effects in poultry that suggest a second plausible mechanism of effect in vivo. The leakage of bacteria across the intestinal epithelial barrier and into the blood circulation is thought to be a key step in the pathogenesis of ES and is the route through which the bacteria gain access to bone sites for infection (Wideman, 2016). The integrity of this barrier is maintained by the activities of tight junction (TJ) complexes sited between adjacent epithelial cells, and a range of stress factors have been shown to be capable of compromising TJ activity, leading to a “leaky gut” in which there is increased opportunity for pathogens to cross the gut-epithelial barrier (Saunders et al., 1994; Quinteiro-Filho et al., 2010; Ulluwishewa et al., 2011). The ability of the commercial probiotic product Enviva® PRO (which is a 1:1:1 combination of tested strains 15AP4, BS8, and 2084) to strengthen the gut barrier of the ileum and caecum in broilers and laying hens challenged with coccidia, Campylobacter, or E. coli, has already been demonstrated in vivo (Murugesan, 2013). Increased microbial challenge is one of the factors that can reduce TJ integrity, leading to the translocation of bacteria across the epithelium of the small intestine (Murugesan et al., 2014), and evidence suggests that certain Bacillus-based probiotics can enhance intestinal barrier integrity, prevent bacterial translocation in vitro and in vivo (Ulluwishewa et al., 2011; Pastorelli et al., 2013; Murugesan et al., 2014; Gadde et al., 2017) and support the immune system, as has been demonstrated in broilers for strains 15AP4, BS8, and 2084 (Amerah et al., 2013). No information about such effects in the presence of challenge with pathogenic strains of E. cecorum is currently available, but a dual-benefit hypothesis might be envisaged whereby strains of probiotic Bacillus could both enhance intestinal barrier activity at the same time as having a direct bacteriocidal or bacteriostatic effect on E. cecorum in the poultry small intestine.
In conclusion, the present study represents the first report of inhibitory activities of proprietary poultry Bacillus strains against pathogenic isolates of E. cecorum in vitro, but effects are highly strain dependent and vary significantly among different pathogenic isolates. This warrants the interest of a multi-strains probiotic product, especially because it is not E. cecorum specific and the variation in coverage is also true for other poultry pathogens (E. coli). Further work is required to establish whether these effects are also evident in vivo in broiler production conditions, as well as to isolate and characterize the specific inhibitory substances responsible for the observed effects.
SUPPLEMENTARY DATA
Supplementary material S1. Examples of E. cecorum growth kinetics seen during exposure to different probiotic Bacillus strain cell-free supernatants.
Supplementary material S2. Percentage growth inhibition1 of pathogenic E. cecorum isolates by 18 Bacillus cell-free supernatants, measured at a time-point equivalent to that which produced an optical density of 0.4 in the positive control (obtained from duplicate).1 Defined as the percentage reduction in pathogen isolate growth in the experimental sample compared with that in the respective PC sample (containing pathogen in BHI medium but no Bacillus CFS), with growth being measured as biomass (absorbance OD), not as CFU, and being determined at the time-point equivalent to that producing an OD of 0.4 in the PC. Thus, 90% inhibition would mean a 90% reduction in growth (biomass). Note: values of greater than 100% occurred where the measured OD (nm) at timex is less than the OD at time0. This may happen, for example, where lysis occurs in the pathogen+CFS suspension.
ACKNOWLEDGEMENTS
Thanks to Joelle Buck (Reading, UK) for her assistance with the writing of this manuscript. Thanks also to Michael Perry (DuPont IB, Wilmington, US) for producing the CFSs, Fiona Bucquet (ABTE, Unicaen, FR) for optimization of the method, Laurence Rohmer (DuPont Pioneer, Johnston, US) and Mark Kolkman (DuPont IB, Leiden, NL) for the genome sequencing, assembling, and annotation.
REFERENCES
- Ahmed A. T., Islam Md. M., Mun H.-S., Sim H.-J., Kim Y.-J.. 2014. Effects of Bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult. Sci. 93:1963–1971. [DOI] [PubMed] [Google Scholar]
- Amerah A. M., Quiles A., Medel P., Sanchez J., Lehtinen M. J., Gracia M. I.. 2013. Effect of pelleting temperature and probiotic supplementation on growth performance and immune function of broilers fed maize/soy-based diets. Anim. Feed Sci. Technol. 180:55–63. [Google Scholar]
- Aitchison H., Poolman P., Coetzer M., Griffiths C., Jacobs J., Meyer M., Bisschop S.. 2014. Enterococcal-related vertebral osteoarthritis in South African broiler breeders: A case report. J. S. Afr. Vet. Assoc. 85:1077–1082. [DOI] [PubMed] [Google Scholar]
- Borst L. B., Suyemoto M. M., Scholl E. H., Fuller F. J., Barnes H. J.. 2015. Comparative genomic analysis identifies divergent genomic features of pathogenic Enterococcus cecorum including a type IC CRISPR-Cas system, a capsule locus, an epa-like locus, and putative host tissue binding proteins. PLoS One. 10:e0121294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst L. B., Suyemoto M. M., Sarsour A. H., Harris M. C., Martin M. P., Strickand J. D., Oviedo E. O., Barnes H. J.. 2017. Pathogenesis of Enterococcal Spondylitis caused by Enterococcus cecorum in broiler chickens. Vet. Pathol. 54:61–73. [DOI] [PubMed] [Google Scholar]
- Cutting S. M. 2011. Bacillus probiotics. Food Microbiol. 28:214–220. [DOI] [PubMed] [Google Scholar]
- DeHerdt P., Defoort J., van Steelan H., Swam L., Tanghe S., Van Goethem M., Vanrobaeys M.. 2008. Enterococcus cecorum osteomyelitis and arthritis in broiler chickens. Vlaams Diergeneeskundig Tijdschrift. 78:44–48. [Google Scholar]
- Delaunay E., Abat C., Rolain J.-M.. 2015. Enterococcus cecorum human infection, France. New Microbes New Infect 7:50–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devriese L. A., Dutta G. N., Farrow J. A. E., Vandekerckhove A., Philips B. A., 1983. Streptococcus cecorum, a new species isolated from chickens. Int. J. Syst. Bacteriol. 33:772–776. [Google Scholar]
- Devriese L. A., Ceyssens K., Haesebrouck F.. 1991. Characteristics of Enterococcus cecorum strains from the intestines of different animal species. Lett. Appl. Microbiol. 12:137–139. [Google Scholar]
- Devriese L. A., Hommez J., Wijfels R., Haesebrouck F.. 1991. Composition of the enterococcal and streptococcal intestinal flora of poultry. J. Appl. Microbiol. 71:46–50. [PubMed] [Google Scholar]
- Devriese L. A., Colque J. I. C., Deherdt P., Haesebrouck F.. 1992. Identification and composition of the tonsillar and anal enterococcal and streptococcal flora of dogs and cats. J. Appl. Bacteriol. 73:421–425. [DOI] [PubMed] [Google Scholar]
- Devriese L. A. K. Cauwerts, Hermans K., Wood A. M.. 2002. Enterococcus cecorum septicemia as a cause of bone and joint lesions resulting in lameness in broiler chickens. Vlaams Diergeneeskundig Tijdschrift. 71:219–221. [Google Scholar]
- Dolka B., Chrobak-Chmiel D., Makrai L., Szeleszczuk P.. 2016. Phenotypic and genotypic characterization of Enterococcus cecorum strains associated with infections in poultry. BMC Vet. Res. 12:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolka B., Chrobak-Chmiel D., Czopowicz M., Szeleszczuk P.. 2017. Characterization of pathogenic Enterococcus cecorum from different poultry groups: Broiler chickens, layers, turkeys, and waterfowl. PLoS One. 12:e0185199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO 2016. Probiotics in animal nutrition – Production, impact and regulation by Y. S. Bajagai, A. V. Klieve, P. J. Dart and Wayne L. Bryden. Makkar Harinder P. S., ed. FAO Animal Production and Health Paper No. 179, Rome. [Google Scholar]
- Fritts C. A., Kersey J. H., Motl M. A., Kroger E. C., Yan F., Si. Q. Jiang J., Campos M. M., Waldroup A. L., Waldroup P. W.. 2000. Bacillus subtilis C-3102 (Calsporin) improves live performance and microbiological status of broiler chickens1. J. Appl. Poult. Res. 9:149–155. [Google Scholar]
- Gadde U., Oh S. T., S. Y. S. Lee, Davis E., Zimmerman N., Rehberger T.. 2017. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics & Antimicro. Prot. 9:397–405. [DOI] [PubMed] [Google Scholar]
- Gebert S., Kromm C., Rehberger T.. 2007. Effect of a Bacillus-based direct-fed microbial on turkey poultry performance and changes within the gastrointestinal microflora. Poult. Sci. 86(Suppl. 1):249.17234837 [Google Scholar]
- Harada T., Kawahara R., Kanki M., Taguchi M., Kumeda Y.. 2012. Isolation and characterization of vanA genotype vancomycin-resistant Enterococcus cecorum from retail poultry in Japan. Int. J. Food Microbiol. 153:372–377. [DOI] [PubMed] [Google Scholar]
- Jeong J. S., Kim I. H.. 2014. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult. Sci. 93:3097–3103. [DOI] [PubMed] [Google Scholar]
- Jung A., Rautenshlein S.. 2014. Comprehensive report of an Enterococcus cecorum infection in a broiler flock in Northern Germany. BMC Vet. Res. 10:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapage S. P., Sneath P H. A., Lessel E. F., Skerman V. B. D., Seeliger H. P. R., Clark W. A., (eds.). 1990 Revision International Code of Nomenclature of Bacteria, ASM Press, Washington, DC. [PubMed] [Google Scholar]
- Latorre J. D., Hernandez-Velasco X., Wolfenden R. E., Vicente J. L., Wolfenden A. D., Menconi A., Bielke L. R., Hargis B. M., Tellez G.. 2016. Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Vet. Sci. 3:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Lillehoj H. S., Siragusa G. R.. 2010. Direct-fed microbials and their impact on the intestinal microflora and immune system of chickens. J. Poult. Sci. 47:106–114. [Google Scholar]
- Lei X., Piao X., Ru Y., Zhang H., Peron A., Zhang H.. 2015. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian Australas. J. Anim. Sci. 28:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. T., Martin M. P., Barnes H. J.. 2011. Experimental reproduction of enterococcal spondylitis in male broiler breeder chickens. Avian Dis. 55:273–278. [DOI] [PubMed] [Google Scholar]
- Murugesan G. R. 2013. Characterization of the effects of intestinal physiology modified by exogenous enzymes and direct-fed microbials on intestinal integrity, energy metabolism, body composition and performance of laying hen and broiler chicks. PhD Thesis No. 13175 Iowa State University, Ames, Iowa. [Google Scholar]
- Murugesan G. R., Gabler N. K., Persia M. E.. 2014. Effects of direct-fed microbial supplementation on broiler performance, intestinal nutrient transport and integrity under experimental conditions with increased microbial challenge. Br. Poult. Sci. 55:89–97. [DOI] [PubMed] [Google Scholar]
- Pastorelli L., De Salvo C., Mercado J. R., Vecchi M., Pizarro T. T.. 2013. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Frontiers in Science (Front. Immunol.) 4:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poormontaseri M., Hosseinzadeh S., Shekarforoush S. S., Kalantari T.. 2017. The effects of probiotic Bacillus subtilis on the cytotoxicity of Clostridium perfringens type a in Caco-2 cell culture. BMC Microbiol. 17:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteiro-Filho W. M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M. L., Sakai M., Sá L. R., Ferrieira J. P., Palermo-Neto J.. 2010. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 89:1905–1914. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Benson A., Lebeer S., Merenstein D. J., Klaenhammer T. R.. 2018. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 49:207–216. [DOI] [PubMed] [Google Scholar]
- Saunders P. R. U., Kosecka U., McKay D. M., Perdue M. H.. 1994. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am. J. Physiol. 267:G794–G799. [DOI] [PubMed] [Google Scholar]
- Shafey T. M., McDonal M. W.. 1991. The effects of dietary calcium, phosphorus, and protein on the performance and nutrient utilization of broiler chickens. Poult. Sci. 70:548–553. [DOI] [PubMed] [Google Scholar]
- Stubljar D., Skvarc M.. 2015. Enterococcus cecorum infection in two critically ill children and in two adult septic patients. Slov. Vet. Res. 52:39–44. [Google Scholar]
- Svetoch E. A., Stern N. J., Eruslanov B. V., Kovalev Y. N., Volodina L. I., Perelygin V. V., Mitsevich E. V., Mitsevich I. P., Pokhilenko V. D., Borzenkov V. N., Levchuk V. P., Svetoch O. E., Kudriavtseva T. Y.. 2005. Isolation of Bacillus circulans and Paenibacillus polymyxa strains inhibitory to Campylobacter jejuni and characterization of associated bacteriocins. J. Food Prot. 68:11–17. [DOI] [PubMed] [Google Scholar]
- Svihus B. 2010. Effect of digestive tract conditions, feed processing and ingredients on response to NSP enzymes. Pages 129–159 in Enzymes in Farm Animal Nutrition Bedford M. R., Partridge G. G., eds. CABI; Wallingford, UK. [Google Scholar]
- Teo A. Y., Tan H. M.. 2005. Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Appl. Environ. Microbiol. 71:4185–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R. C., Mcnabb W. C., Moughan P. J., Wells J. M., Roy N. C.. 2011. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 141:769–776. [DOI] [PubMed] [Google Scholar]
- Wideman R. F. 2016. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: A review. Poult. Sci. 95:325–344. [DOI] [PubMed] [Google Scholar]
- Wood A. M., MacKenzie N. C., Brown L., Devriese L. A., Baele M.. 2002. Isolation of Enterococcus cecorum from bone lesions in broiler chickens. Vet. Rec. 150:27. [PubMed] [Google Scholar]
- Wu B. Q., Zhang T., Guo L. Q., Lin J. F.. 2011. Effects of Bacillus subtilis KD1 on broiler intestinal flora. Poult. Sci. 90:2493–2499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.