ABSTRACT
The objective of the current research was to evaluate transgenerational effects of maternal dietary energy and protein on growth, efficiency, and yield of broiler offspring. A factorial arrangement of treatments consisting of high and low ME and CP levels fed during the rearing and laying phases was used. The study was a final 2 × 2 × 2 × 2 factorial arrangement of treatments, including broiler sex. Ross 708 broiler breeder pullets (n = 933) were fed diets containing 2,736 (HEREAR) or 2,528 kcal/kg ME (LEREAR) with either 15.3% (HPREAR) or 13.7% CP (LPREAR). From 25 wk, dams were fed a 15% CP laying diet containing 2,900 (HELAY) or 2,800 kcal/kg ME (LELAY). Following artificial insemination of the dams at 35 wk, eggs were collected for 1 wk, incubated, and pedigree hatched to preserve maternal identity. Broiler offspring were placed sex-separately into 32 pens, according to laying phase maternal treatments, with rearing maternal treatments nested within pens. Individual BW and pen level feed intake were recorded weekly. Broilers were processed at 40 d of age to evaluate yield. Maternal diet effects on offspring BW were sex dependent and transient. Female LPREAR × LELAY broilers had lower pectoralis major and carcass yield than HPREAR × LELAY females. Male HPREAR × HELAY broilers had increased breast yield (19.8%) compared with 18.4% in HPREAR × LELAY broilers. Carcass yield was lower in LEREAR × HPREAR broilers (63.7%) compared with HEREAR × HPREAR broilers (64.9%). LEREAR × HPREAR dams had the lowest ME to CP ratio (E: P) diets and highest rearing phase CP intake. Maternal diet did not influence offspring FCR. The most consistent contributor to increased BW was higher maternal dietary CP and ME during rearing. Low ME maternal laying phase diets increased BW of male offspring more consistently than of female offspring. Maternal nutrition also influenced broiler yield, and is thus economically important. Energy and protein dilution in broiler breeder pullet diets may have detrimental effects on offspring performance.
Keywords: intergenerational effects, nutrition, progeny, caloric restriction, epigenetics
INTRODUCTION
Awareness about the importance of maternal diet on offspring health is increasing. Maternal nutrition is being studied in livestock species as a way of improving offspring growth and production (Rehfeldt et al., 2011; Long et al., 2012; van Emous et al., 2015b). The poultry industry is constantly working to increase carcass yield and obtain a fast growing broiler, and manipulation of maternal diet can be one way of increasing broiler performance (Calini and Sirri, 2007).
Peebles et al. (2002) fed broiler breeder hens diets with different ME levels (2,709, 2,826, or 2,940 kcal/kg) and observed that broiler BW at 43 d was higher for offspring of hens fed a low-energy diet compared to BW of offspring of hens fed a high-energy diet. Spratt and Leeson (1987) reported that higher energy in the diet of broiler breeder hens increased BW in male but not in female broilers. Proudfoot and Hulan (1986) fed different combinations of CP and ME treatments to broiler breeders during rearing (12.9% CP, 2,902 kcal/kg; or 15.8% CP, 2,699 kcal/kg) and laying phases (15.3% CP, 2,746 kcal/kg; 17.6% CP, 2,746 kcal/kg; and 17.8% CP, 2,651 kcal/kg), but found no maternal dietary effects on offspring BW and FCR at processing. More recently, van Emous et al. (2015b) found no effect on broiler offspring BW and FCR when broiler breeders were fed different levels of protein during rearing.
There are few recent papers evaluating the effects of modern broiler breeder dietary energy and protein on offspring performance. It is possible that the increasing relative degree of feed restriction in modern broiler breeders might influence mechanisms governing offspring performance. Therefore, the objective of this research was to evaluate the effect of different dietary energy and protein levels in broiler breeder female rearing diets and different dietary energy levels during lay on offspring growth, carcass yield, and FCR.
MATERIALS AND METHODS
Experimental Design
The protocol for the current study was approved by the University of Alberta Animal Care and Use Committee for Livestock and followed principles established by the Canadian Council on Animal Care Guidelines and Policies (CCAC, 1993).
The effect of maternal nutrition on broiler performance was studied using a 2 × 2 × 2 × 2 factorial arrangement of treatments with 2 maternal dietary ME levels during rearing (HEREAR: 2,736 kcal/kg and LEREAR: 2,528 kcal/kg); 2 maternal dietary CP levels during rearing (HPREAR: 15.3% and LPREAR: 13.7%); 2 maternal dietary ME levels during lay (HELAY: 2,900 kcal/kg and LELAY: 2,800 kcal/kg), both with 15% CP and 2 broiler sexes.
Maternal Stocks and Management
A detailed description of the maternal broiler breeder treatments and management was reported by Moraes et al. (2014). Briefly, broiler breeder hens were artificially inseminated at 35 wk of age with 0.5 mL of pooled fresh undiluted semen. Eggs (n = 1,250) were collected during the following week. All eggs were weighed individually, identified by hen and date laid, and incubated (37.4 ± 0.2°C, 84.2 ± 2% RH). Eggs were transferred to pedigree hatch baskets at 18 d of incubation. Each egg was isolated from the others in individual cells in the hatcher tray, thus retaining maternal treatment information of each chick upon hatching.
Broiler Stocks and Management
Standard broiler management procedures were used, and were the same as those reported previously (Moraes et al., 2014). Broiler chicks (n = 933) were individually identified and placed, 25 to 32 per pen, depending on the numbers hatched in each treatment, into 32 pens. Broiler starter diet (0 to 14 D) contained 23% CP and 3,067 kcal/kg ME; the grower diet (15 to 28 D) contained 20% CP and 3,152 kcal/kg ME; the finisher I diet (29 to 39 D) contained 19% CP and 3,196 kcal/kg ME; and the finisher II diet (40 to 54 D) contained 17.7% CP and 3,262 kcal/kg ME. Broilers were weighed weekly (Weltech BW-1050, Weltech International Ltd; St Ives, Cambridgeshire, UK), and pen level feed intake was recorded weekly by weighting back unused feed. Mortality was 4.3% overall, and did not differ between treatments. A total of 201 birds were processed at 40 D. Carcass, breast muscle, legs and wings weight were reported as percentage of live BW. Carcass weight did not include viscera, neck, feet, or abdominal fat pad.
Statistical Analysis
BW and dissection data were analyzed as a 4-way ANOVA using the MIXED procedure of SAS (Version 9.2. SAS Institute Inc., Cary, NC) with rearing dietary ME, rearing dietary CP, laying dietary ME, and broiler sex as main effects. Hen nested in pen was included as a random effect in the statistical model. FCR data was analyzed as a 2-way ANOVA with dietary energy during lay and broiler sex as main effects. Pearson correlation coefficients were calculated using the CORR procedure of SAS to evaluate relationships among breeder BW and intake and broiler variables. Pairwise differences between means were reported, within sex, where P ≤ 0.05.
RESULTS AND DISCUSSION
Body Weight
Significance of all effects and interactions on offspring BW was reported systematically in Table 1. There was a correlation between maternal 35 wk BW and broiler 39 d BW (r = 0.10; P = 0.03). At 8 and 15 d of age, female broilers had greater BW than males; however, by 39 d of age, males were 127 g heavier than females (Table 2). At hatch, male LEREAR × HPREAR chicks had greater BW compared with HEREAR × HPREAR chicks (Table 2). At 15 d of age, female HEREAR × HPREAR broilers were heavier than female LEREAR × HPREAR broilers, whereas male LEREAR × HPREAR broilers were heavier than male LEREAR × LPREAR broilers (Table 2). The first column (HPREAR × HEREAR) and the last row (LELAY) in the heat map in Table 3 show the most consistent positive effects of maternal nutritional treatments on overall broiler BW. The most consistent contributor to increased BW was the highest combination of both maternal dietary CP and ME during rearing. Hens on the LEREAR × HPREAR maternal diet had the highest daily maternal protein intake (8.5 g protein/kg BW0.75). As a consequence, upon switching to laying phase diets, any increase in the protein intake for this rearing treatment would have been minimal. During lay, higher protein intake, which occurs as a result of lower maternal dietary ME level, may provide some advantage in terms of greater offspring BW, as relative BW was 1% higher across the LELAY treatment, compared with the HELAY treatment (100.5 vs. 99.5, respectively; Table 3). The data suggest that a low maternal dietary protein signal at the time of transition to the laying diet may reduce female offspring growth rates. Little is known about the underlying mechanisms driving incorporation of protein into tissues, and it is interesting that there are sex-specific broiler responses to broiler dietary protein levels (Eits et al., 2003). We hypothesize that the mechanisms that dictate that male broilers are more responsive to dietary amino acids may somehow also be related to this observation.
Table 1.
Significance of the effects of broiler age and sex, and maternal dietary treatments applied during rearing and lay on broiler BW.
| Effect | F Value | Prob > F |
|---|---|---|
| Age | 27,791 | <0.001 |
| Sex | 10.91 | 0.001 |
| MEREAR (ER) | 2.79 | 0.095 |
| CPREAR (PR) | 7.93 | 0.005 |
| MELAY (EL) | 2.04 | 0.15 |
| Age × sex | 10.9 | <0.001 |
| Age × ER | 0.82 | 0.53 |
| Age × PR | 2.08 | 0.065 |
| Age × EL | 1.82 | 0.11 |
| Sex × ER | 1.23 | 0.27 |
| Sex × PR | 0.39 | 0.53 |
| Sex × EL | 4.33 | 0.038 |
| ER × PR | 2.14 | 0.14 |
| ER × EL | 0.02 | 0.88 |
| PR × EL | 0.03 | 0.87 |
| Age × sex × ER | 0.91 | 0.47 |
| Age × sex × PR | 0.27 | 0.93 |
| Age × sex × EL | 1.77 | 0.12 |
| Age × ER × PR | 0.63 | 0.68 |
| Age × ER × EL | 0.07 | 1.00 |
| Age × PR × EL | 0.26 | 0.94 |
| Sex × ER × PR | 5.53 | 0.019 |
| Sex × ER × EL | 0.55 | 0.46 |
| Sex × PR × EL | 0.11 | 0.74 |
| Age × sex × ER × PR | 3.14 | 0.008 |
| Age × sex × PR × EL | 0.66 | 0.65 |
| Age × sex × ER × EL | 0.43 | 0.82 |
| Age × ER × PR × EL | 0.51 | 0.77 |
| Sex × ER × PR × EL | 4.58 | 0.032 |
| ER × PR × EL | 1.43 | 0.23 |
| Age × sex × ER × PR × EL | 1.28 | 0.27 |
MEREAR = maternal dietary ME treatment during rearing; CPREAR = maternal dietary CP treatment during rearing; MELAY = maternal dietary ME treatment during lay.
Table 2.
Effects of sex and maternal dietary ME and CP and broiler sex on broiler BW.
| Age (D) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | MELAY | MEREAR | CPREAR | E:P1 | 0 | 8 | 15 | 22 | 29 | 39 |
| kcal/g | ––––––––––––––––––––––– BW (g) ––––––––––––––––––––––– | |||||||||
| Main effects | ||||||||||
| Female | 42.0 | 188a | 469a | 839 | 1,365 | 2,309b | ||||
| Male | 42.0 | 180b | 455b | 834 | 1,377 | 2,436a | ||||
| HELAY | 19.4 | 42.1 | 184 | 458 | 829 | 1,356 | 2,375 | |||
| LELAY | 18.5 | 41.9 | 184 | 466 | 844 | 1,386 | 2,370 | |||
| HEREAR | 19.0 | 41.8 | 185 | 465 | 841 | 1,377 | 2,388 | |||
| LEREAR | 17.5 | 42.1 | 184 | 460 | 832 | 1,365 | 2,358 | |||
| HPREAR | 17.2 | 42.0 | 185 | 466 | 847 | 1,383 | 2,394 | |||
| LPREAR | 19.2 | 41.9 | 183 | 459 | 826 | 1,360 | 2,352 | |||
| SEM | 0.15 | 1.3 | 3.6 | 14.2 | 9.0 | 16.6 | ||||
| Sex × MEREAR × CPREAR × age interaction | ||||||||||
| Female | HEREAR | HPREAR | 17.9 | 42.2 | 193a | 485a | 865 | 1,402a | 2,375 | |
| LPREAR | 20.0 | 41.7 | 187a,b | 463b | 823 | 1,356a,b | 2,287 | |||
| LEREAR | HPREAR | 16.5 | 42.1 | 185b | 459b | 828 | 1,339b | 2,286 | ||
| LPREAR | 18.5 | 42.0 | 188a,b | 470a,b | 841 | 1,364a,b | 2,289 | |||
| Male | HEREAR | HPREAR | 17.9 | 41.4b | 179 | 452a,b | 844 | 1,388 | 2,473 | |
| LPREAR | 20.0 | 42.0a,b | 180 | 457a,b | 834 | 1,362 | 2,416 | |||
| LEREAR | HPREAR | 16.5 | 42.4a | 184 | 468a | 852 | 1,401 | 2,442 | ||
| LPREAR | 18.5 | 41.9a,b | 179 | 444b | 807 | 1,357 | 2,414 | |||
| SEM | 0.30 | 2.6 | 7.2 | 28.3 | 17.9 | 33.3 | ||||
a–bMeans within effect, age, and sex with no common superscript differ significantly (P < 0.05).
MEREAR = maternal dietary ME treatment during rearing; CPREAR = maternal dietary CP treatment during rearing; MELAY = maternal dietary ME treatment during lay; HEREAR = high maternal dietary ME (2,736 kcal/kg) during rearing; LEREAR = low maternal dietary ME (2,528 kcal/kg) during rearing; HPREAR = high maternal dietary CP (15.3%) during rearing; LPREAR = low maternal dietary CP (13.7% CP) during rearing; HELAY = high maternal dietary ME (2,900 kcal/kg) during lay; LELAY = low maternal dietary ME (2,800 kcal/kg) during lay.
1E:P = dietary ME:CP ratio.
Table 3.
Heat map showing overall relative effect (P = 0.032) of maternal dietary treatments on male and female broiler BW.
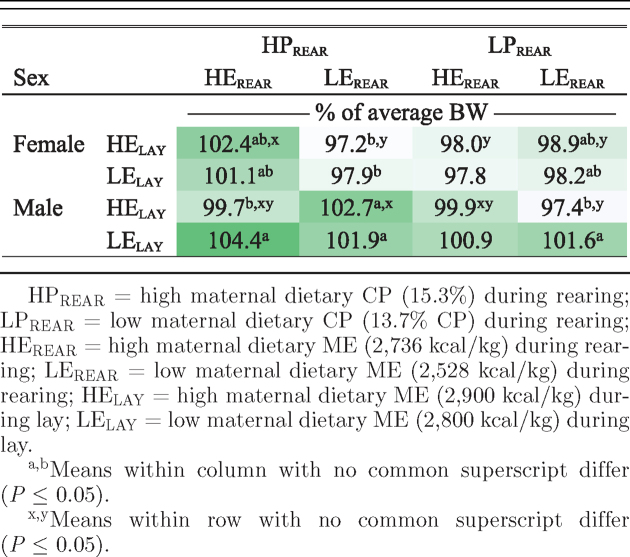
|
HPREAR = high maternal dietary CP (15.3%) during rearing; LPREAR = low maternal dietary CP (13.7% CP) during rearing; HEREAR = high maternal dietary ME (2,736 kcal/kg) during rearing; LEREAR = low maternal dietary ME (2,528 kcal/kg) during rearing; HELAY = high maternal dietary ME (2,900 kcal/kg) during lay; LELAY = low maternal dietary ME (2,800 kcal/kg) during lay.
a,bMeans within column with no common superscript differ (P ≤ 0.05).
x,yMeans within row with no common superscript differ (P ≤ 0.05).
At the end of lay, LEREAR × HPREAR hens had lower liver weights compared with HEREAR × HPREAR hens (59.4 vs. 67.1 ± 2.3 g, respectively; P < 0.05). In rats, Muaku et al. (1995) observed that lower liver weight in protein-restricted mothers can result in reduced offspring BW. Proudfoot and Hulan (1986) did not find a maternal dietary nutrient effect (during rearing) on offspring performance; however, broiler genetics have changed a lot in the 30 yr between that time and the current study (Zuidhof et al., 2014). The current result is similar to the findings of Zhu et al. (2012), who observed that maternal diets had a transient effect on BW until 21 d of age, and disappeared by processing age in Chinese Yellow broilers. Although in the current study BW effects did not remain significant to processing age, the interaction effect of all of the maternal treatments was significant overall BW (Tables 1 and 3). Koppenol et al. (2015) reported that subtle maternal effects may not prevail through the entire broiler growout because numerous environmental and nutritional factors could overshadow maternal effects. Between the time that the hen consumes her feed and the broiler offspring are processed, many factors such as egg storage, incubation and hatching conditions, transportation, rearing conditions, feed quality, and pathogen exposure may additively or synergistically mask the effect of maternal nutrition on the broiler. In contrast with the current study, van Emous et al. (2015b) reported no effect of maternal dietary protein during rearing on offspring BW and BW gain. The current result is partially consistent with results from an earlier flock of offspring from the same maternal experiment, where female HEREAR × LPREAR offspring had lower BW from 22 to 36 d compared with offspring from hens that consumed more CP as pullets (Moraes et al., 2014). The results from the current study confirm that maternal nutrition during the broiler breeder pullet stage can impose a lasting influence on offspring BW, which is greater in magnitude than those resulting from laying phase nutrition. This may be the result of more intense feed restriction during the pullet phase, and it suggests epigenetic mechanisms may be in play.
Carcass Yield
Significance of all treatment effects and interactions on offspring yield was reported systematically in Table 4. As a percentage of BW, female broilers had higher pectoralis major, pectoralis minor, total breast, and overall carcass yield compared with male broilers (Table 5). Broilers from the HEREAR × HPREAR treatment had the highest overall carcass yield (Table 6). This was the same maternal treatment that had the most consistent increases in offspring BW (Table 3).
Table 4.
Significance of the effects of sex and maternal dietary treatments on broiler carcass parts yield (% of live BW).
| Pectoralis major | Pectoralis minor | Breast | Legs | Wings | Carcass | |
|---|---|---|---|---|---|---|
| Source | –––––––––––––––––––––––––– Prob > F –––––––––––––––––––––––––––––––––––– | |||||
| Sex | 0.002 | < 0.001 | < 0.001 | < 0.001 | 0.81 | 0.005 |
| MEREAR (ER) | 0.93 | 0.46 | 0.96 | 0.17 | 0.21 | 0.089 |
| CPREAR (PR) | 0.45 | 0.43 | 0.64 | 0.13 | 0.046 | 0.95 |
| MELAY (EL) | 0.29 | 0.23 | 0.24 | 0.48 | 0.52 | 0.56 |
| Sex × ER | 0.63 | 0.58 | 0.59 | 0.71 | 0.24 | 0.35 |
| Sex × PR | 0.31 | 0.79 | 0.34 | 0.079 | 0.051 | 0.13 |
| Sex × EL | 0.25 | 0.56 | 0.27 | 0.54 | 0.96 | 0.45 |
| ER × PR | 0.88 | 0.39 | 0.78 | 0.33 | 0.55 | 0.036 |
| ER × EL | 0.71 | 0.25 | 0.93 | 0.36 | 0.97 | 0.55 |
| PR × EL | 0.87 | 0.31 | 0.97 | 0.55 | 0.29 | 0.59 |
| Sex × ER × PR | 0.37 | 0.75 | 0.47 | 0.24 | 0.86 | 0.44 |
| Sex × ER × EL | 0.18 | 0.088 | 0.15 | 0.46 | 0.53 | 0.48 |
| Sex × PR × EL | 0.008 | 0.061 | 0.009 | 0.35 | 0.74 | 0.038 |
| ER × PR × EL | 0.80 | 0.82 | 0.82 | 0.92 | 0.72 | 0.18 |
| Sex × ER × PR × EL | 0.89 | 0.95 | 0.87 | 0.27 | 0.72 | 0.17 |
MEREAR = maternal dietary ME treatment during rearing; CPREAR = maternal dietary CP treatment during rearing; MELAY = maternal dietary ME treatment during lay.
Table 5.
Effects of sex and maternal dietary treatments on carcass yield.
| Sex | MELAY | MEREAR | CPREAR | E:PREAR1 | E:PLAY1 | Pectoralis major | Pectoralis minor | Breast | Carcass |
|---|---|---|---|---|---|---|---|---|---|
| ––––––––––––– % of live BW ––––––––––––– | |||||||||
| Main effects | |||||||||
| Female | 16.5a | 3.71a | 20.0a | 64.7a | |||||
| Male | 15.7b | 3.35b | 19.1b | 63.9b | |||||
| HELAY | 19.4 | 16.2 | 3.56 | 19.8 | 64.4 | ||||
| LELAY | 18.5 | 16.0 | 3.50 | 19.5 | 64.2 | ||||
| HEREAR | 19.0 | 16.1 | 3.55 | 19.6 | 64.6 | ||||
| LEREAR | 17.5 | 16.1 | 3.51 | 19.6 | 64.0 | ||||
| HPREAR | 17.2 | 16.2 | 3.51 | 19.7 | 64.3 | ||||
| LPREAR | 19.2 | 16.0 | 3.55 | 19.6 | 64.3 | ||||
| SEM | 0.17 | 0.04 | 0.20 | 0.21 | |||||
| MEREAR × CPREAR interaction | |||||||||
| HEREAR | HPREAR | 17.9 | 16.2 | 3.50 | 19.7 | 64.9a | |||
| LPREAR | 20.0 | 16.0 | 3.60 | 19.6 | 64.2a,b | ||||
| LEREAR | HPREAR | 16.5 | 16.2 | 3.51 | 19.7 | 63.7b | |||
| LPREAR | 18.5 | 16.0 | 3.51 | 19.5 | 64.4a,b | ||||
| SEM | 0.25 | 0.06 | 0.29 | 0.30 | |||||
| Sex x MELAY × CPREAR interaction | |||||||||
| Female | HELAY | HPREAR | 17.2 | 19.4 | 16.3a,b | 3.70 | 20.0 | 64.8a,b | |
| LPREAR | 19.2 | 19.4 | 16.6a,b | 3.77 | 20.3 | 65.1a | |||
| LELAY | HPREAR | 17.2 | 18.5 | 17.0a | 3.70 | 20.7 | 65.1a | ||
| LPREAR | 19.2 | 18.5 | 15.9b | 3.69 | 19.7 | 63.9b | |||
| Male | HELAY | HPREAR | 17.2 | 19.4 | 16.3a | 3.44a | 19.8a | 63.9 | |
| LPREAR | 19.2 | 19.4 | 15.7a,b | 3.35a,b | 19.0a,b | 63.9 | |||
| LELAY | HPREAR | 17.2 | 18.5 | 15.2b | 3.19b | 18.4b | 63.5 | ||
| LPREAR | 19.2 | 18.5 | 15.8a,b | 3.41a,b | 19.2a,b | 64.3 | |||
| SEM | 0.33 | 0.08 | 0.39 | 0.42 | |||||
a,bMeans within column, sex, and effect with no common superscript differ significantly (P < 0.05).
MEREAR = maternal dietary ME treatment during rearing; CPREAR = maternal dietary CP treatment during rearing; MELAY = maternal dietary ME treatment during lay; HEREAR = high maternal dietary ME (2,736 kcal/kg) during rearing; LEREAR = low maternal dietary ME (2,528 kcal/kg) during rearing; HPREAR = high maternal dietary CP (15.3%) during rearing; LPREAR = low maternal dietary CP (13.7% CP) during rearing; HELAY = high maternal dietary ME (2,900 kcal/kg) during lay; LELAY = low maternal dietary ME (2,800 kcal/kg) during lay.
1E:PREAR = energy to protein ratio for rearing diets (kcal/g); E:PLAY = energy to protein ratio for lay diets (kcal/g).
Table 6.
Heat map showing relative effect of maternal rearing dietary treatments on male and female broiler breast and carcass yield.
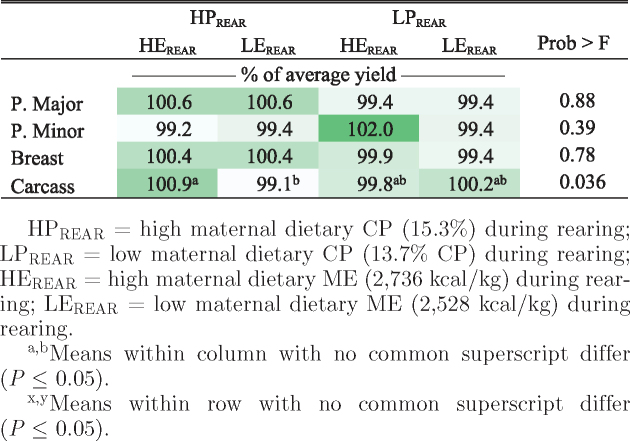
|
HPREAR = high maternal dietary CP (15.3%) during rearing; LPREAR = low maternal dietary CP (13.7% CP) during rearing; HEREAR = high maternal dietary ME (2,736 kcal/kg) during rearing; LEREAR = low maternal dietary ME (2,528 kcal/kg) during rearing.
a,bMeans within column with no common superscript differ (P ≤ 0.05).
x,yMeans within row with no common superscript differ (P ≤ 0.05).
Female LPREAR × LELAY broilers had lower pectoralis major and carcass yield than female broilers from hens fed HPREAR × LELAY. In female broilers, the maternal HPREAR diet increased pectoralis major yield, but only when hens were fed the LELAY diet. In males, the opposite was true; an increase in pectoralis major yield was seen in the maternal HPREAR diet, but only when their mothers were fed the HELAY diet (Table 5). The HPREAR had a lower energy to protein (E: P) ratio (17.2 kcal/g), compared with 19.2 kcal/g in the LPREAR treatment. A decrease in E: P ratio for LPREAR × LELAY upon switching from rearing to laying phase diets (LELAY = 18.5 kcal/g) may play some role in the observed decrease in offspring breast yield, possibly related to overfeeding of protein during the laying phase. van Emous et al. (2015b) reported no effect of maternal pullet phase dietary CP on female offspring breast yield; however, a low maternal CP diet (12.5% CP) increased breast yield in males compared with a high maternal CP diet during rearing (14.1% CP). Henry and Burke (1998) described sexual dimorphism in muscle development in broiler embryos such that male broilers usually develop greater myofiber numbers (more myofibers per 30,000 μm2), while female broilers develop larger myofibers. Furthermore, myofiber number and size can be differently influenced by protein levels in maternal diet (Rehfeldt et al., 2012). Changes in E: P ratios require a metabolic adjustment by the animal (Wagle et al., 1962), which we hypothesize may have triggered an epigenetic effect and influenced gene expression relating to growth and breast muscle development in the offspring. However, epigenetic mechanisms were not evaluated in the current study.
Carcass yield was lower (63.8%) in LEREAR × HPREAR offspring compared with that of HEREAR × HPREAR broilers (64.9%, Table 5). Hens that received the LEREAR × HPREAR diet consumed 67.5 g/d of feed, consisting of 10.3 g/d of protein and 170.7 kcal/d. Hens fed the HEREAR × HPREAR diet consumed 61.4 g/d of feed (9.3 g of protein and 168 kcal).
HPREAR hens receiving the lower ME level had lower liver weights compared to hens on the HEREAR × HPREAR (data not shown). The liver is responsible for important metabolic functions such as lipogenesis (Taouis et al., 2001), and lipogenesis is positivity correlated with E: P ratio (Donaldson, 1985). In a study conducted with rats, protein-restricted dams had a lower liver weight, resulting in lower liver weight and lower concentration of liver IGF-I in the offspring (Muaku et al., 1995). A decrease in IGF-I in the offspring could result in a decreased carcass yield because IGF-I is a regulator of muscle development (Duclos, 2005).
An increase in carcass or breast yield generated by changes in dietary energy and protein level in broiler breeder diets can be economically advantageous for the poultry industry. The current study confirms that manipulation of dietary ME and CP in broiler breeders can influence broiler yield. The current study points to a positive effect from higher CP levels in pullet phase diets. This adds complexity to broiler breeder nutrition decisions since there is some consensus around the hypothesis that lower CP diets may help to fatten the breeder pullet and better prepare for egg production. For example, van Emous (2015b) fed broiler breeders low or high levels of protein during rearing and found that birds fed the low protein diet had lower breast muscle and higher abdominal fat pad at 22 wk. Birds fed low protein during rearing also had higher hatchability and increased egg production when compared to the birds fed high protein at rearing. This is not the first broiler breeder paradox ever identified.
Feed Conversion Ratio
Broiler FCR up to 39 d of age ranged from 1.60 (HELAY) to 1.62 (LELAY) but was not significantly influenced by maternal dietary energy during lay (P = 0.65), broiler sex (0.86), or the interaction of maternal dietary energy during lay and broiler sex (P = 0.59). This result concurs with previous work that did not find any influence of maternal diet on FCR of the offspring (Moraes et al., 2014). Proudfoot and Hulan (1986) fed different maternal CP and ME levels during rearing (12.9%, 2,902 kcal/kg; 15.8%, 2,699 kcal/kg) and laying periods (15.3%, 2,746 kcal/kg; 17.6%, 2,746 kcal/kg; and 17.8%, 2,651 kcal/kg) to 3 strains of broiler breeders and found no effect on offspring FCR. van Emous et al. (2015b) also found no effect of maternal dietary treatments varying in CP during rearing (12.5, 13.3, and 14.1%) on offspring FCR.
In conclusion, effects of maternal rearing diet on the BW of their broiler offspring were sex dependent and transient, but significant. Broiler breast and carcass yield effects were also sex dependent. High (15.3%) maternal dietary protein during rearing showed the most consistent overall greatest BW and economically important yields. The low maternal ME level during lay (2,800 kcal/kg) had consistently higher BW, but may have negative effects on offspring yield, particularly in males. Maternal diet did not influence offspring FCR. Maternal nutrition influenced broiler yield, and thus may be more economically important than previously thought. Confirming previous work (Moraes et al., 2014), there was a significant effect related to pullet phase nutrition. A systematic and careful investigation is needed to complete our understanding of these interacting and time-confounded effects and their underlying mechanisms.
ACKNOWLEDGEMENTS
Financial support for this study was provided by Alberta Livestock and Meat Agency (Edmonton, Alberta, Canada), Alberta Chicken Producers (Edmonton, Alberta, Canada), Aviagen (Huntsville, Alabama), Poultry Industry Council (Guelph, Ontario, Canada), and Sofina Foods (Edmonton, Alberta, Canada). The authors gratefully acknowledge the technical assistance provided by the staff and students of the University of Alberta Poultry Research Centre, Edmonton, Alberta.
REFERENCES
- Calini F., Sirri F.. 2007. Breeder nutrition and offspring performance. Rev. Bras. Cienc. Avic. 9:77–83. [Google Scholar]
- CCAC 1993. Guide to the Care and Use of Experimental Animals, 2nd ed Vol. 1 Canadian Council on Animal Care, Ottawa, Ontario, Canada. [Google Scholar]
- Donaldson W. E. 1985. Lipogenesis and body fat in chicks: effects of calorie-protein ratio and dietary fat. Poult. Sci. 64:1199–1204. [DOI] [PubMed] [Google Scholar]
- Duclos M. J. 2005. Insulin-like growth factor-I (IGF-1) mRNA levels and chicken muscle growth. J. Physiol. Pharmacol. 56:25–35. [PubMed] [Google Scholar]
- Eits R. M., Kwakkel R. P., Verstegen M. W. A., Emmans G. C.. 2003. Responses of broiler chickens to dietary protein: effects of early life protein nutrition on later responses. Br. Poult. Sci. 44:398–409. [DOI] [PubMed] [Google Scholar]
- Henry M. H., Burke W. H.. 1998. Sexual dimorphism in broiler chick embryos and embryonic muscle development in late incubation. Poult. Sci. 77:728–736. [DOI] [PubMed] [Google Scholar]
- Koppenol A., Delezie E., Wang Y., Franssens L., Willems E., Ampe B., Buyse J., Everaert N.. 2015. Effects of maternal dietary EPA and DHA supplementation and breeder age on embryonic and post-hatch performance of broiler offspring: age and n-3 pufa affect embryonic and post-hatch performance. J. Anim. Physiol. Anim. Nutr. 99:36–47. [DOI] [PubMed] [Google Scholar]
- Long N. M., Tousley C. B., Underwood K. R., Paisley S. I., Means W. J., Hess B. W., Du M., Ford S. P.. 2012. Effects of early- to mid-gestational undernutrition with or without protein supplementation on offspring growth, carcass characteristics, and adipocyte size in beef cattle. J. Anim. Sci. 90:197–206. [DOI] [PubMed] [Google Scholar]
- Moraes T. G. V., Pishnamazi A., Mba E. T., Wenger I. I., Renema R. A., Zuidhof M. J.. 2014. Effect of maternal dietary energy and protein on live performance and yield dynamics of broiler progeny from young breeders. Poult. Sci. 93:2818–2826. [DOI] [PubMed] [Google Scholar]
- Muaku S. M., Beauloye V., Thissen J. P., Underwood L. E., Ketelslegers J. M., Maiter D.. 1995. Effects of maternal protein malnutrition on fetal growth, plasma insulin-like growth factors, insulin-like growth factor binding proteins, and liver insulin-like growth factor gene expression in the rat. Pediatr. Res. 37:334–342. [DOI] [PubMed] [Google Scholar]
- Peebles E. D., Zumwalt C. D., Gerard P. D., Latour M. A., Smith T. W.. 2002. Market age live weight, carcass yield, and liver characteristics of broiler offspring from breeder hens fed diets differing in fat and energy contents,. Poult. Sci. 81:23–29. [DOI] [PubMed] [Google Scholar]
- Proudfoot F. G., Hulan H. W.. 1986. The performance of one normal and two dwarf meat maternal genotypes and their progeny as affected by rearing and adult dietary treatments. Can. J. Anim. Sci. 66:245–256. [Google Scholar]
- Rehfeldt C., Lang I. S., Görs S., Hennig U., Kalbe C., Stabenow B., Brüssow K. P., Pfuhl R., Bellmann O., Nürnberg G., Otten W., Metges C. C.. 2011. Limited and excess dietary protein during gestation affects growth and compositional traits in gilts and impairs offspring fetal growth. J. Anim. Sci. 89:329–341. [DOI] [PubMed] [Google Scholar]
- Rehfeldt C., Stabenow B., Pfuhl R., Block J., Nürnberg G., Otten W., Metges C. C., Kalbe C.. 2012. Effects of limited and excess protein intakes of pregnant gilts on carcass quality and cellular properties of skeletal muscle and subcutaneous adipose tissue in fattening pigs. J. Anim. Sci. 90:184–196. [DOI] [PubMed] [Google Scholar]
- Spratt R. S., Leeson S.. 1987. Effect of protein and energy intake of broiler breeder hens on performance of broiler chicken offspring. Poult. Sci. 66:1489–1494. [DOI] [PubMed] [Google Scholar]
- Taouis M., Dridi S., Cassy S., Benomar Y., Raver N., Rideau N., Picard M., Williams J., Gertler A.. 2001. Chicken leptin: properties and actions. Domest. Anim. Endocrinol. 21:319–327. [DOI] [PubMed] [Google Scholar]
- van Emous R. A., Kwakkel R. P., van Krimpen M. M., Hendriks W. H.. 2015a. Effects of dietary protein levels during rearing and dietary energy levels during lay on body composition and reproduction in broiler breeder females. Poult. Sci. 94:1030–1042. [DOI] [PubMed] [Google Scholar]
- van Emous R. A., Kwakkel R. P., van Krimpen M. M., van den Brand H., Hendriks W. H.. 2015b. Effects of growth patterns and dietary protein levels during rearing of broiler breeders on fertility, hatchability, embryonic mortality, and offspring performance. Poult. Sci. 94:681–691. [DOI] [PubMed] [Google Scholar]
- Wagle D. S., Marfatia U., Sreenivasan A.. 1962. The effect of variation in the calorie: protein ratio of the diet on nitrogen retention and body composition in the rat. Br. J. Nutr. 16:369–377. [DOI] [PubMed] [Google Scholar]
- Zhu C., Jiang Z. Y., Jiang S. Q., Zhou G. L., Lin Y. C., Chen F., Hong P.. 2012. Maternal energy and protein affect subsequent growth performance, carcass yield, and meat color in Chinese Yellow broilers. Poult. Sci. 91:1869–1878. [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J., Schneider B. L., Carney V. L., Korver D. R., Robinson F. E.. 2014. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 93:2970–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]


