Abstract
Pneumonia results in significant morbidity and mortality worldwide. However, chest radiography may not be accessible in primary care setting. We aimed to evaluate clinical features and its diagnostic value to identify pneumonia among adults in primary care settings. Three academic databases were searched and included studies that assessed clinical predictors of pneumonia, adults without serious illness, have CXR and have conducted in primary care settings. We calculated sensitivity, specificity, positive and negative likelihood ratios, diagnostic odds ratio of each index test and the pool estimates for index tests. We identified 2,397 articles, of which 13 articles were included. In our meta-analysis, clinical features with the best pooled positive likelihood ratios were respiratory rate ≥20 min−1 (3.47; 1.46–7.23), temperature ≥38 °C (3.21; 2.36–4.23), pulse rate >100 min−1 (2.79; 1.71–4.33), and crackles (2.42; 1.19–4.69). Laboratory testing showed highest pooled positive likelihood ratios with PCT >0.25 ng/ml (7.61; 3.28–15.1) and CRP > 20 mg/l (3.76; 2.3–5.91). Cough, pyrexia, tachycardia, tachypnea, and crackles are limited as a single predictor for diagnosis of radiographic pneumonia among adults. Development of clinical decision rule that combine these clinical features together with molecular biomarkers may further increase overall accuracy for diagnosis of radiographic pneumonia among adults in primary care setting.
Subject terms: Respiratory signs and symptoms, Respiratory distress syndrome
Introduction
Pneumonia is an infection of the lungs caused by bacteria, virus or fungi. It is a leading cause of morbidity and mortality worldwide, especially in elder patients and patients with comorbidities. Globally, 3.2 million of the 56.4 million deaths in 2015 were cauesd by lower respiratiry tract infection1. The annual incidence of pneumonia was estimated at 1.07–1.2 cases per 1,000 persons per year in Europe and 16.9 cases per 1,000 persons per year in Asia2. Diagnosis of pneumonia in adults presenting with signs of lower respiratory tract infection is important because it requires specific treatment and follow up. Pneumonia is usually diagnosed by a combination of clinical history, physical examination and/or laboratory tests. According to most clinical guidelines globally, the supposed gold standard tool for diagnosing pneumonia is a chest X-ray (CXR) which can distinguish pneumonia from other respiratory tract infections3,4. Other diagnostic tests such as laboratory tests (white blood cell count (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin), blood culture, serology, and computed tomography scan (CT scan) have been reported with different rates of accuracy5,6. However, chest radiography and other diagnostic procedures, such as sputum and blood cultures, may not be accessible or not routinely measured in primary care setting for economic and logistic reasons. The superior gold standard, CT scan, is very far from available in primary care patients. Therefore, primary care physicians usually rely on patient’s medical history and physical examinations to diagnose or exclude pneumonia. Similarly, performing CXR to all suspected pneumonia cases is also challenging in the community and thereby will not always be performed for all patients. This then necessitate the need for decision aids for ordering CXR for pneumonia in the community to assess the risk more appropriately.
Several prediction rules have been identified to improve detection of pneumonia in outpatient settings7–13. Only one study of systematic review and meta-analysis to assess the diagnostic value of clinical features to identify pneumonia in children was conducted14. A systematic review and meta-analysis of the clinical features is lacking in adults. Therefore, the objective is to assess the predictive performance of clinical features associated with CXR-confirmed pneumonia compared to non-pneumonia patients in primary care settings among adults aged ≥18 years without serious illness and pre-existing immune suppression.
Materials and Methods
Search strategy and selection criteria
The study was performed in accordance with the recommendations of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA, Appendix S1)15. The meta-review does not involve human participants or experiments on live vertebrates and/or higher invertebrates, only published aggregate data from the selected studies is used in the meta-analysis. The study protocol is available as Supplementary material (Appendix S2). The search strategy consists of two phases. The first phase was an extensive search using the identified index terms and keywords in three databases: PubMed, EMBASE and the Cochrane Library. The second phase was an additional search of the references of retrieved articles to find any articles that did not appear in the databases search. The keywords that were used in the search are ‘pneumonia’ or ‘community acquired pneumonia’ or ‘community-acquired pneumonia’ or ‘respiratory tract infection’ or ‘respiratory tract infections’ and ‘predictive value of tests’ or ‘sensitivity and specificity’ or ‘diagnostic test’ or ‘diagnostic tests’ or ‘medical history taking’ or ‘medical history’ or ‘physical examination’ or ‘physical examinations’ or ‘clinical laboratory techniques’ or ‘laboratory diagnoses’ or ‘laboratory examinations’ or ‘laboratory testing’ and ‘ambulatory care’ or ‘primary care’ or ‘outpatient care’ or ‘general practitioner’ or ‘emergency clinic’. The bibliographical software package, EndNote version X7 (Thomas Reuters, New York, NY, USA), was used to import references and to remove duplicates references. The remaining studies were checked against the inclusion and exclusion criteria. Two reviewers (HTP and CHL) independently screened eligibility based on title, abstracts and assessed full reports, resolving discrepancies by consensus.
Studies were selected if they were published studies that assessed clinical predictors of community-acquired pneumonia without date restrictions to maximize the search. The first search was employed on Dec 4, 2017, with an update on Mar 5, 2018. Narrative review, letters to editors, case reports and case series were excluded. Studies were included if participants aged ≥18 years without serious illness (e.g. mechanical ventilation) and pre-existing immune suppression (HIV, malnutrition, and immunosuppressant medication). To be eligible, studies had to have reference standard of CXR for diagnosing pneumonia, and have conducted in ambulatory care or primary care settings. Index tests assessed were patient’s socio-demographic, clinical signs and symptoms and laboratory tests.
Quality assessment
After identifying studies that fulfilled the selection criteria and verifying their eligibility by reading the full articles, the quality assessment of the studies were done by using QUADAS-216 as recommended by the Cochrane collaboration. Studies were assessed for selection of patient, index test, reference standard, and flow and timing. Signalling questions were made to facilitate the rating of risk of bias into low, unclear or high.
Data extraction
The following variables were extracted from each study using pre-designed forms: study characteristics (study design, year of publication, country and setting), study population (age, number of participants recruited, prevalence of pneumonia, inclusion and exclusion criteria), reference standard (number of readers, masking and interpretation criteria), and index tests. Index tests were classified as related to socio-demographic, clinical symptoms or signs, and laboratory tests to diagnose pneumonia. Outcome data were extracted and compiled in a table by one author (HTP). After which, all extracted data were cross-checked by another author (SY) by comparing them to the original data from the selected articles.
Data analysis
Data analysis was based on a published methodological review – a systematic review of evaluations of diagnostic and screening tests17. We constructed 2 × 2 tables for each study included in the review to calculate sensitivity, specificity, positive and negative likelihood ratios and diagnostic odds ratio with 95% confidence intervals (CI). The likelihood ratio indicates the value of the test for increasing certainty about a positive diagnosis. The positive likelihood ratio (LR+) is the probability of a positive result in patients with the disease, compared to the probability in patients not having the disease; while a negative likelihood ratio (LR−) is the probability of obtaining a negative test result in patients without the disease, compared to the probability in patients with the disease18. Clinical signs and symptoms and laboratory tests with an LR+ greater than 2.0 and an LR− less than 0.5 are clinically useful for diagnosis of pneumonia19. Diagnostic odds ratio is defined as the ratio of odds of the test being positive for a patient with the disease in relation to odds of the test being positive for a patient without the disease. Considering the correlation between sensitivity and specificity within and across studies, we performed bivariate model to calculate the pooled estimates of sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and diagnostic odds ratio with 95% CIs. To avoid the large variances between studies, we conducted random effect meta-analysis20 approach as the final model. The final bivariate model was computed using the mada package in R version 3 3 421. Finally, we performed the pool estimates of meta-analyses for index tests with at least four or more studies. Pool estimates for less than four studies have limited validity and hence, was excluded22. The index tests assessed at different thresholds were pooled together and analysed. The Summary Receiver Operating Characteristics (SROC) curve for index tests (at least four included studies) were computed using the Reitsma SROC model to obtain the summary point estimates of sensitivity and specificity as well as 95% predicting region and 95% confidence region for the summary operating point23.
Results
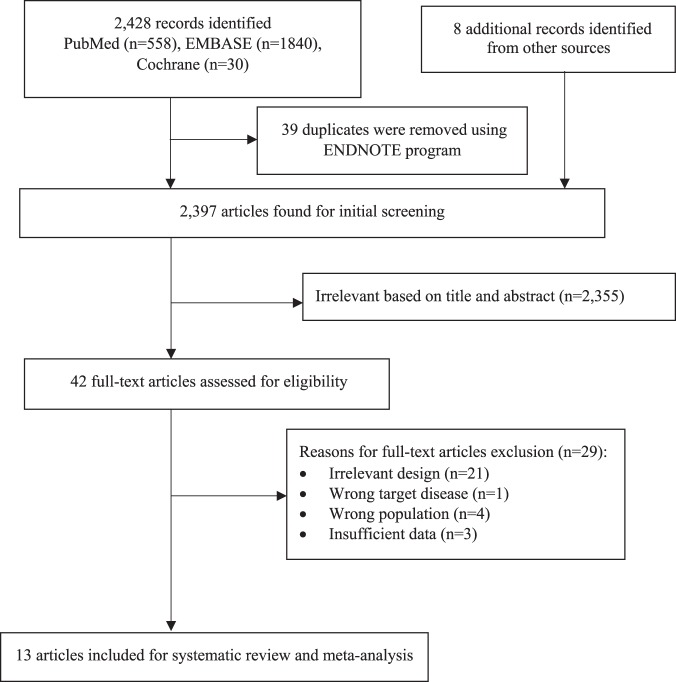
The selection process (PRISMA flow-diagram) is showed in Fig. 1. A total of 2,428 records were identified from the initial database search and additional records from other sources. The articles were curated using EndNote and 39 duplicates were removed. Following this, 2,397 were included for initial screening and 2,355 articles were excluded based on relevance of titles and abstracts. 42 full text articles were retrieved, reviewed and selected based on relevance and quality for eligibility. A further 29 articles were excluded because of irrelevant design (i.e. irrelevant content, unmet inclusion criteria), wrong target disease (i.e. diseases other than pneumonia e.g. influenza), wrong populations (i.e. performed in age group less than 18 years) and insufficient data. This brings the total number of included articles for this review to 1313,24–35.
Figure 1.
Study selection.
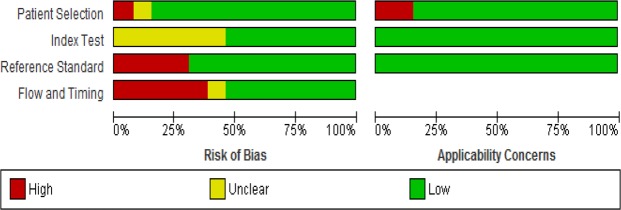
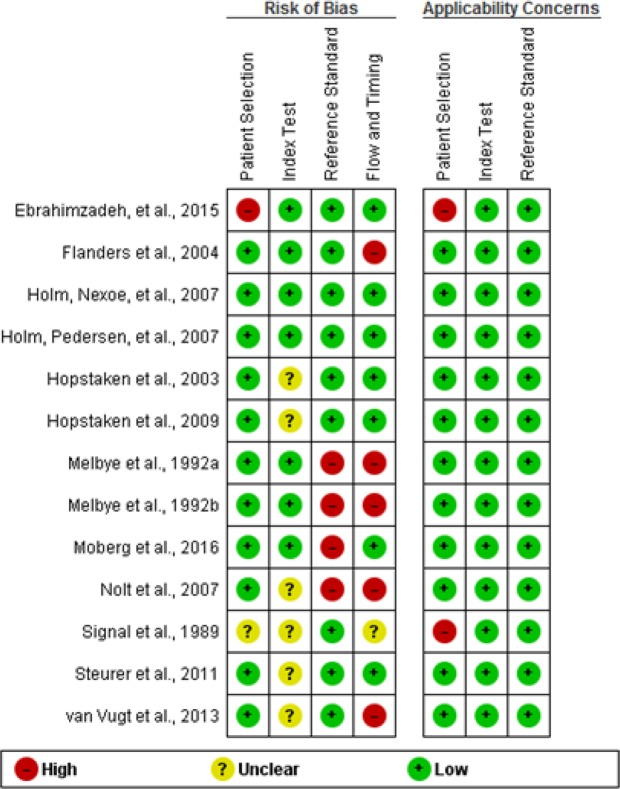
The methodological quality of included studies are summarised in Figs 2 and 3. In risk of bias, eleven studies had low risk in patient selection and one study had high risk for enrolling participants with confirmed diagnosis and control group without the condition. Seven studies had low risk in index test and six studies were unclear for pre-specifying of threshold of index test. Four studies had high risk in reference standard due to lack of blinding in interpretation of radiograph and extraction of data from medical records. Seven studies had low risk in flow and timing. Five studies had high risk bias due to attrition of some participants and selectively receiving the reference standards. In applicability concerns, eleven studies had low risk concerns in patient selection, whereas two studies had high risk concerns. All studies addressed low risk concerns for index test and reference standard.
Figure 2.
Graphical illustration of risk of bias and applicability concerns.
Figure 3.
Summary of risk of bias and applicability concerns.
The summary characteristics of the 13 included studies are shown in Table 1. A total of 11,144 participants were obtained from the studies and they are from varying locations: Iran (n = 1), USA (n = 3), Denmark (n = 2), Netherlands (n = 2), Norway (n = 2), Sweden (n = 1), Switzerland (n = 1) and Europe (n = 1). The studies were done in outpatient clinics (n = 6), emergency clinics (n = 6), primary care centres (n = 2) and GP clinic (n = 1). All the participants were adults aged ≥18 years and the sample size varied from 95 to 4,464. The study designs were prospective cohort (n = 8), case-control (n = 1), cross sectional (n = 3) and retrospective chart review (n = 1). The studies included consecutive patients with history of respiratory tract infection. In these studies, the inclusion criterion was cough alone (n = 3) and clinically suspected pneumonia (n = 10). The proportion of radiographic pneumonia in the studied populations varied from 5% to 50%.
Table 1.
Characteristics of included studies.
| Author, Year | Setting | Age, sample size | Study design | Prevalence of radiographic pneumonia | Inclusion criteria | Exclusion criteria | CXR | Index test | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Readers | Blinding | Interpretation | ||||||||
| Ebrahimzadeh, et al., 2015 |
Iran; Outpatient clinics and emergency clinics |
≥18 years; 840 |
Case control study | 50% | Acute respiratory symptoms with positive CXR | Acute respiratory symptoms with insignificant findings on CXR | A board certified radiologist | Yes | New consolidation without an air bronchogram, pleural effusion, abscess or empyema |
Socio-demographic: Age, gender Symptoms: Cough, sputum, dyspnea, chest pain Signs: Temperature ≥38 °C, pulse rate ≥100 min−1 respiratory rate ≥20 min−1 Laboratory tests: WBC, CRP |
| Flanders et al., 2004 |
USA; Outpatient clinics and emergency clinics |
≥18 years; 150 |
Prospective cohort | 13.3% | Acute cough (within past 3 weeks) | Pregnancy, systematic inflammatory disorders, coexistence infections, traumas, burns, myocardial infarct or unstable angina, cancer, HIV or immunosuppressive disorders | Radiologist | Yes | Infiltrate or consolidation on chest radiograph |
Socio-demographic: Age, gender, smoking Symptoms: Fever, muscle pain, fatigue, runny nose, sore throat, cough, yellow phlegm, blood in sputum, wheezing, dyspnea, chest pain Signs: Temperature ≥37.8 °C, pulse rate ≥100 min−1, respiratory rate ≥24 min−1, O2 saturationv ≤93%, decreased breath sounds, rales, wheezes Laboratory tests: CRP |
| Holm, Nexoe, et al., 2007 | Denmark; Outpatient clinics |
≥18 years; 364 |
Prospective cohort | 13% | Clinical diagnosis of LRTI | Pregnancy, hospitalization within preceding 7 days, severe illness requiring hospitalization, former participation in the study | Experienced specialist in infectious lung disease | Yes | Transient, non-malignant infiltrate on chest film |
Signs: Temperature ≥38 °C, pulse rate ≥100 min−1, respiratory rate ≥22 min−1, O2 saturation < 95% Laboratory tests: WBC and CRP |
| Holm, Pedersen, et al., 2007 | Denmark; Outpatient clinics |
≥18 years; 364 |
Prospective cohort | 13% | Clinical diagnosis of LRTI | Pregnancy, hospitalization within preceding 7 days, severe illness requiring hospitalization, former participation in the study | Experienced specialist in infectious lung disease | Yes | Transient, non-malignant infiltrate on chest film | Laboratory tests: PCT |
| Hopstaken et al., 2003 |
Netherlands; Outpatient clinics |
≥18 years; 243 |
Cross- sectional | 13% | New or increasing cough, combined with other clinical characteristics | Pregnancy and lactation, allergy to penicillin, concomitant treatment with ergot alkaloids and/or terfenadine, severe clinical disease, antibiotics treatment within 14 days, hospital stay for previous 4 weeks | 2 radiologists independently and 1 senior radiologist in case of disagreement | Yes | Infiltrates on chest radiograph |
Socio-demographic: Age Symptoms: Dry cough, purulent sputum, dyspnea, chest pain, fever, chills, diarrhea Signs: Temperature ≥38 °C, respiratory rate > 20 min−1, dullness on percussion, bronchial breathing, crackles Laboratory tests: ESR, CRP |
| Hopstaken et al., 2009 |
Netherlands; Outpatient clinics |
≥18 years; 95 |
Cross- sectional | 11.7% | New or increasing cough, combined with other clinical characteristics | Pregnancy and lactation, allergy to penicillin, concomitant treatment with ergot alkaloids and/or terfenadine, severe clinical disease, antibiotics treatment within 14 days, hospital stay for previous 4 weeks | 2 radiologists independently and 1 senior radiologist in case of disagreement | Yes | Infiltrates on chest radiograph |
Signs: Temperature ≥38 °C Laboratory tests: CRP, LBP, fibrinogen |
| Melbye et al., 1992 | Norway; Municipal emergency clinic |
≥18 years; 402 |
Prospective cohort |
41% (21 out of 51 CXR patients) |
Symptoms of respiratory tract or throat infection | Pregnancy, severe dyspnea patients | 2 radiologists and 1 senior chest physician independently | NR | A density on chest film |
Typical symptoms: Dry cough, purulent sputum, dyspnea, chest pain, fever, chills Atypical symptoms: Fatigue, myalgia/arthralgia, coryza, sore throat Signs: Wheezes, crackles, decreased breath sounds, dullness to percussion |
| Melbye et al., 1992 | Norway; Municipal emergency clinic |
≥18 years; 402 |
Prospective cohort |
41% (21 out of 51 CXR patients) |
Symptoms of respiratory tract or throat infection | Pregnancy, severe dyspnea patients | 2 radiologists and 1 senior chest physician independently | NR | A density on chest film | Laboratory tests: ESR, WBC and CRP |
| Moberg et al., 2016 | Sweden; Primary care centres |
≥18 years; 103 |
Prospective cohort | 45% | Respiratory tract infection symptoms for 24 hour | Pregnancy, COPD, received antibiotics less than 2 weeks, patients living in nursing home | Radiologists on duty and a board certified radiologist | No | Infiltrates on chest radiograph |
Socio-demographic: Gender, smoking Symptoms: Chest pain Signs: Temperature > 38 °C, pulse rate > 100 min−1, respiratory rate > 20 min−1, O2 saturation < 95% crackles, rales, decreased breath sounds, dullness on percussion Laboratory tests: WBC, CRP |
| Nolt et al., 2007 | USA; Emergency clinics |
≥18 years; 4464 |
Retrospective charts review | 12% | Acute cough illness | Any visits without a chief complaint of cough | Radiography notes were abstracted by research coordinators | NR | Haziness, density, consolidation, inflammation, infiltration or acute pulmonary abnormality in radiology report |
Socio-demographic: Age, smoking Signs: Temperature ≥100.4 °F, pulse rate >100 min−1, respiratory rate ≥20 min−1, O2 saturation <95% |
| Signal et al., 1989 |
USA; Emergency clinics |
≥18 years; 255 |
Prospective cohort | 15.6% | Patients who perform chest radiography | Critically ill patients | A board certified radiologist and final typed report was reviewed by the investigators | NR | Infiltrates on chest radiograph |
Socio-demographic: Age, gender Symptom: Cough, chest pain and dyspnea Signs: Crackles, wheezes, tachycardia, tachypnea |
| Steurer et al., 2011 | Switzerland; GP clinics |
≥18 years; 642 |
Prospective cohort | 20.5% | New or worsening cough for 24 hour, with increased body temperature | Pregnancy, chronic lung diseases, HIV patients taking oral steroid, on chemotherapy, organ transplantation, mental disorder | Radiologists | Yes | Shadow on radiograph |
Socio-demographic: Age, gender, smoking Symptoms: Cough, fever, dyspnea, wheezing, chest pain, muco-purulent sputum, bloody sputum Signs: Decreased breath sound, bronchial breath sound, dullness on percussion Laboratory tests: CRP |
| van Vugt et al., 2013 | Europe; Primary care centres |
≥18 years; 2820 |
Cross sectional | 5% | Acute cough | No chest radiograph performed or insufficient quality of radiograph | Radiologists | Yes | Diagnosis by selecting one of the following fixed option responses such as normal chest radiograph, acute bronchitis, pneumonia, or other diagnosis |
Socio-demographic: Age, gender, smoking Symptoms: Cough, phlegm, dyspnea, runny nose, fever, chest pain, diarrhea Signs: Diminished vesicular breath sound, crackles, temperature > 37.8 °C, pulse rate > 100 min−1, respiratory rate > 24 min−1 Laboratory tests: PCT and CRP |
COPD = chronic obstructive pulmonary disease. CRP = C-reactive protein. CXR = chest X-ray. ESR = erythrocyte sedimentation rate. HIV = human immunodeficiency virus. LBP = lipopolysaccharide binding protein.
LRTI = lower respiratory tract infection. NR = not reported. PCT = procalcitonin. WBC = white blood cell count.
A total of 25 different clinical history and features studied for their accuracy in diagnosis of radiographic pneumonia: related to socio-demographic (n = 3), symptoms (n = 13), signs (n = 9). The 13 included papers comprised 40 clinical index tests. Of the 40 index tests, the most frequently assessed index tests were: history of fever (n = 5), cough (n = 7), sputum (n = 6), dyspnea (n = 7), chest pain (n = 8), crackles (n = 7), elevated temperature (n = 9), increased pulse rate (n = 6), respiratory rate (n = 7), and decreased breath sounds (n = 5). Different thresholds were used to measure age, temperature, pulse rate, respiratory rate, and O2 saturation. Six different laboratory tests (white blood cell count (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin (PCT), lipopolysaccharide binding protein (LBP) and fibrinogen were used to examine the diagnostic value of radiographic pneumonia. CRP was the most frequently assessed index test (n = 10), however, each with a different standard.
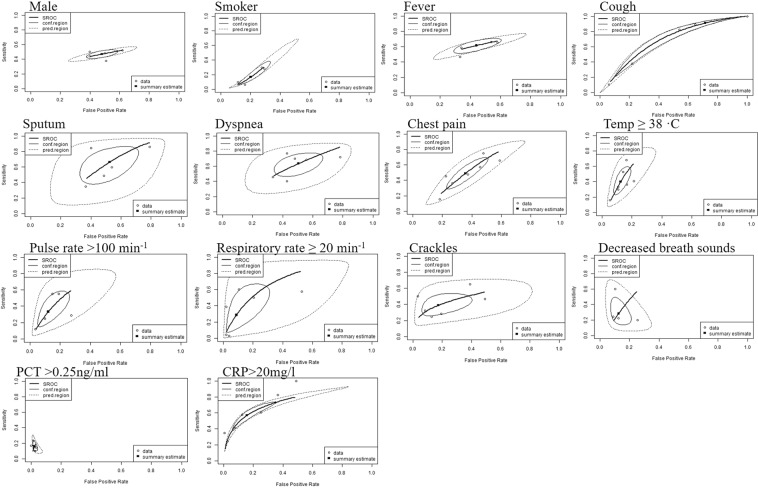
The diagnostic performance measures (sensitivity, specificity, positive and negative likelihood ratios, and diagnostic odds ratio) of each index test was prescribed detail in Supplementary material (Appendix S3). Pooled estimates for gender (male), smoker, fever (≥37.5 °C), cough, sputum, dyspnoea, chest pain, temperature, pulse rate, respiratory rate, crackles, decreased breath sounds, PCT, and CRP were obtained by meta-analysis. The summary estimates of each index test’s diagnostic performance measures (sensitivity, specificity, positive, negative likelihood ratio and diagnostic odds ratio) are shown in Table 2. Among the index tests, cough had high sensitivity 0.91 (0.36–0.99) but had low specificity 0.28 (0.03–0.83). We would estimate that 91% of patients with radiographic pneumonia would have symptoms of cough. Some index tests had specificities higher than 0.80, such as temperature ≥ 38 °C 0.88 (0.82–0.91), pulse rate >100 min−1 0.88 (0.77–0.94), respiratory rate ≥20 min−1 0.91 (0.75–0.97), crackles 0.83 (0.65–0.92), decreased breath sounds 0.87 (0.81–0.92), PCT >0.25 ng/ml 0.98 (0.96–0.99) and CRP >20 mg/l 0.84 (0.7–0.93). The clinical features with pooled estimates of significantly high positive likelihood ratios as defined by LR+ >2.0 were temperature ≥ 38 °C, pulse rate >100 min−1, respiratory rate ≥20 min−1, crackles, PCT >0.25 ng/ml and CRP > 20 mg/l. The clinical features with pooled estimates of significantly high negative likelihood ratio (LR− <0.5) was cough. The highest positive likelihood ratio observed was PCT (7.61) followed by CRP (3.76), respiratory rate ≥20 min−1 (3.47), temp ≥ 38 °C (3.21), pulse rate >100 min−1 (2.79), and crackles (2.42). Overall, based on diagnostic odds ratio, cough, crackles, respiratory rate ≥20 min−1, fever with temperature ≥ 38 °C, pulse rate >100 min−1, decreased breath sounds, CRP and PCT were potential useful diagnostic indicators of pneumonia. The SROC plot of summary point estimates of sensitivity and specificity with 95% confidence region and 95% prediction region are shown in Fig. 4.
Table 2.
Summary estimates of diagnostic performance measures of each index test assesses in four studies or more.
| Factor | Number of studies | Total population | Sensitivity (95% CI) |
Specificity (95% CI) |
Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | Diagnostic odds ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| Socio-demographic | |||||||
| Male | 5 | 4,549 | 0.47 (0.42–0.52) | 0.52 (0.43–0.6) | 0.98 (0.86–1.14) | 1.03 (0.91–1.18) | 0.96 (0.73–1.25) |
| Smoker | 4 | 3,707 | 0.17 (0.08–0.33) | 0.80 (0.70–0.87) | 0.84 (0.56–1.15) | 1.03 (0.94, 1.09) | 0.82 (0.52–1.22) |
| Symptoms | |||||||
| Fever | 4 | 3,849 | 0.61 (0.53–0.69) | 0.56 (0.43–0.68) | 1.41 (1.15–1.78) | 0.70 (0.59–0.82) | 2.06 (1.4–2.91) |
| Cough* | 6 | 4,945 | 0.91 (0.36–0.99) | 0.28 (0.03–0.83) | 1.36 (1.03–2.10) | 0.36 (0.15–0.78) | 4.23 (2.44–6.83) |
| Sputum** | 5 | 4,690 | 0.66 (0.44–0.83) | 0.48 (0.32–0.64) | 1.27 (0.90–1.72) | 0.72 (0.39–1.13) | 1.95 (0.79–4.04) |
| Dyspnea | 6 | 4,946 | 0.63 (0.50–0.75) | 0.49 (0.36–0.63) | 1.27 (0.99–1.63) | 0.75 (0.53–1.01) | 1.77 (0.98–2.97) |
| Chest pain | 7 | 5,044 | 0.49 (0.32–0.66) | 0.64 (0.52–0.75) | 1.37 (1.14–1.60) | 0.79 (0.62–0.93) | 1.76 (1.23–2.44) |
| Signs | |||||||
| Temp ≥ 38 °C† | 7 | 4,593 | 0.40 (0.26–0.56) | 0.88 (0.82–0.91) | 3.21 (2.36–4.23) | 0.68 (0.53–0.82) | 4.80 (2.96–7.38) |
| Pulse rate > 100 min−1‡ | 5 | 4,256 | 0.33 (0.18–0.53) | 0.88 (0.77–0.94) | 2.79 (1.71–4.33) | 0.76 (0.57–0.90) | 3.78 (1.99–6.57) |
| Respiratory rate ≥ 20 min−1¥ | 6 | 4,468 | 0.29 (0.10–0.59) | 0.91 (0.75–0.97) | 3.47 (1.46–7.23) | 0.77 (0.50–0.95) | 4.74 (1.6–11.00) |
| Crackles¶ | 6 | 3,671 | 0.39 (0.28–0.51) | 0.83 (0.65–0.92) | 2.42 (1.19–4.69) | 0.75 (0.61–0.91) | 3.34 (1.13–7.06) |
| Decreased breath sounds | 4 | 3,394 | 0.28 (0.16–0.45) | 0.87 (0.81–0.92) | 2.43 (0.98–4.87) | 0.82 (0.61–1.00) | 3.17 (0.97–7.78) |
| Lab investigations | |||||||
| PCT > 0.25 ng/ml$ | 4 | 6,042 | 0.16 (0.11–0.22) | 0.98 (0.96–0.99) | 7.61 (3.28–15.1) | 0.86 (0.79–0.92) | 8.98 (3.59–18.8) |
| CRP > 20 mg/l§ | 9 | 9,476 | 0.57 (0.42–0.70) | 0.84 (0.70–0.93) | 3.76 (2.30–5.91) | 0.52 (0.42–0.63) | 7.21 (5.08–9.94) |
*Dry cough in one study is included. **Yellowish purulent sputum in three studies are included. †Temperature ≥37.8 °C in two studies are included. ‡Pulse rate ≥100 min−1 in one study is included. ¥Respiratory rate ≥22 min−1 in one study, respiratory rate ≥24 min−1 in two studies are included. ¶Rales in two studies are included. $PCT >0.50 ng/ml in two studies are included. §CRP >50 mg/l in two studies and CRP > 100 mg/l in three studies are included.
Figure 4.
Summary ROC plot for socio-demographic, symptoms, signs and laboratory tests.
Discussion
Clinicians have traditionally used certain clinical signs and symptoms to diagnose pneumonia in the community. We aimed to assess the clinical predictors for diagnosis of pneumonia in adults to complement the clinical judgement for the need of CXR in a primary care setting, where CXR may not be readily available. The results of the pooled diagnostic odds ratio for clinical signs and laboratory tests were promising in our findings. However, the pooled diagnostic odds ratio for socio-demographic and symptoms were not ideal as predictors except for cough.
Our meta-analysis showed that individual clinical history and symptoms do not have adequate discriminatory power except cough to diagnose pneumonia among adults in primary care setting. This is consistent with previous study showing that no clinical symptoms is sufficient on its own for diagnosis of radiographic pneumonia among children under five years old14. Consistent with the previous analyses, cough was a poorly specific indicator of pneumonia, assuming that patients visiting to clinic with symptoms of cough would unlikely to have pneumonia14,36. However, there is likely an overestimation of cough because it was part of the inclusion criteria for most of the studies. Thus, likely resulting in cough having a good pooled negative likelihood ratio and high diagnostic odds ratio in our study, Respiratory rate (one of the criteria to classify pneumonia) was one of the two most useful predictors among the clinical signs, beside temperature ≥38 °C based on diagnostic odds ratio. Fast breathing had highest specificity, therefore it might be useful clinically to identify patients without fast breathing would be unlikely to have pneumonia. There was evidence that an adult with a respiratory rate of over 20 per minute is probably unwell and an adult with a respiratory rate of over 24 breaths per minute is likely to be critically ill37. Pyrexia was the next most useful predictor, and followed by tachycardia. These findings are similar to the clinical decision rule of a published study9, that ordered CXR only for patients with at least one abnormal vital signs (i.e. temperature greater than 37.8 °C, respiratory rate greater than 20 breaths per minute, or pulse rate greater than 100 beats per minute). Consistent with other studies7,11, auscultation sounds such as crackles was shown as predictor of pneumonia in our study. Moreover, the predictors in our findings were also found in Heckerling clinical decision rules for pulmonary infiltrates. The rule identified five key predictors for pneumonia: temperature greater than 37.8 °C, pulse rate greater than 100 beats per minute, crackles, decreased breath sounds, and absence of asthma11. In addition, fever, tachycardia and crackles were observed to be useful as part of the predictions models externally validated for pneumonia in primary care38.
In our results, biomarker such as PCT and CRP were the strongest predictors among all variables tested and had significant discriminating power than clinical signs and symptoms for pneumonia. PCT, a marker of sepsis, strongly correlated with bacteria load39 and the severity of infection40. In addition, elevated PCT levels point towards bacterial infection rather than viral infection41. There is some evidence that PCT >0.25 ng/ml reflects a typical bacterial aetiology42. This evidence is in line with our result demonstrating that PCT > 0.25 ng/ml was able to predict pneumonia. On the other hand, PCT has been most frequently studied with regard to its prognostic value and correlation with disease severity42,43, in patients with pneumonia. Notably, PCT has been regarded as a prognostic rather than diagnostic factor in adult patients with community-acquired pneumonia42. CRP is a widely used point of care test in ambulatory care. CRP has been studied as a screening device for inflammation, and as a marker of bacterial infection44,45. Our result revealed that the diagnostic role of CRP > 20 mg/l has value in ruling in pneumonia. This finding is similar to previous systematic reviews showing that pneumonia is ruled out if CRP below 20 mg/l44,46. Moreover, it also seems that CRP test cannot be used as a stand-alone diagnostic test for pneumonia. Current evidences have shown that adding CRP value to basic signs and symptoms models in diagnosing pneumonia improved diagnostic discrimination of adult patients in primary care10,47.
There are a number of limitations in this review. Firstly, there was heterogeneity among the selected studies in terms of inclusion criteria, chest radiograph (interpretation criteria and lack of blinding), inconsistencies in the reporting of clinical features across different studies and the prevalence of pneumonia. Sensitivity and specificity values are highly dependent on the prevalence of the pnemonia in the respective population of different studies. Moreover, the time between potential exposure to infection and the point when the test gives an accurate result was not clearly reported in the studies. Bivariate random effects model was used to account for heterogeneity between the studies. Secondly, a small number of variables did not allow for meta-analysis to be conducted to investigate the tests’ accuracies. Thirdly, it is possible that there are some relevant studies which were not published, resulting in potential publication bias. In addition, only studies published in English were included in our review which may have resulted in limited generalizability. Finally, our findings may also have limited applicability in low- or middle-income countries, since all the selected studies except one study24 were conducted in high income countries. Moreover, the study only focused on the predictive nature of the variables singly and potentially, performance of the variables may be improved but likely to a limited extent with more than one clinical signs and symptoms as covariables in the model.
The findings of this review suggest that individual clinical symptom (cough) and clinical signs (pyrexia, tachycardia, tachypnea, and crackles) are associated to pneumonia but limited as a single predictor for diagnosis of radiographic pneumonia. The combination of these clinical features in decision rule might indeed enhance the overall diagnostic performance of individual symptoms and signs. Future high quality and large-scale case-control studies using the clinical data relevant to the population of interest is necessary to assess the combination with the clinical features identified in this review, and to propose a practical scoring system to aid clinical judgement for ordering of CXR to confirm pneumonia. Moreover, the combination of these clinical features together with molecular biomarkers is likely to further add value to the overall diagnostic accuracy.
Supplementary information
Acknowledgements
We thanked Dr. Jeremiah Chng and Ms Christine Gao from Biodefence Centre of the Singapore Armed Forces for their guidance in this area of interest. The work was supported by Centre of Infectious Disease Epidemiology & Research under Saw Swee Hock School of Public Health, an operational research program funded Singapore Ministry of Defence (grant number N-608-000-065-001). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Author Contributions
Conceptualization: Junxiong Pang, Tha Pyai Htun. Data Curation: Tha Pyai Htun. Formal Analysis: Tha Pyai Htun, Yinxiaohe Sun. Investigation: Junxiong Pang, Tha Pyai Htun, Yinxiaohe Sun. Methodology: Junxiong Pang, Tha Pyai Htun, Yinxiaohe Sun. Project Administration: Junxiong Pang, Hui Lan Chua. Supervision: Junxiong Pang. Validation: Tha Pyai Htun, Yinxiaohe Sun, Hui Lan Chua. Visualization: Tha Pyai Htun, Yinxiaohe Sun. Writing – Original draft: Tha Pyai Htun, Yinxiaohe Sun. Writing – Review & Editing: Junxiong Pang, Tha Pyai Htun, Yinxiaohe Sun, Hui Lan Chua.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44145-y.
References
- 1.Colin, M. et al. Global and Regional Causes of Death: Patterns and Trends, 2000–15 (2017).
- 2.Cillóniz, C., Cardozo, C. & García-Vidal, C. Epidemiology, pathophysiology, and microbiology of communityacquired pneumonia. Annals of Research Hospitals, 2(1) (2018).
- 3.Levy ML, et al. Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK. Prim Care Respir J. 2010;19(1):21–7. doi: 10.4104/pcrj.2010.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandell LA, et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clinical Infectious Diseases. 2007;44(Supplement_2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch T, et al. A Systematic Review on the Diagnosis of Pediatric Bacterial Pneumonia: When Gold Is Bronze. Plos One. 2010;5(8):e11989. doi: 10.1371/journal.pone.0011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niederman MS. Imaging for the Management of Community-Acquired Pneumonia: What to Do if the Chest Radiograph Is Clear. CHEST. 2018;153(3):583–585. doi: 10.1016/j.chest.2017.09.045. [DOI] [PubMed] [Google Scholar]
- 7.AI_Mulhim FA, et al. Clinical prediction rule for pulmonary infiltrates. Saudi Medical Journal. 1998;19(3):306–312. [PubMed] [Google Scholar]
- 8.Diehr P, et al. Prediction of pneumonia in outpatients with acute cough–a statistical approach. Journal of Chronic Diseases. 1984;37(3):215–225. doi: 10.1016/0021-9681(84)90149-8. [DOI] [PubMed] [Google Scholar]
- 9.Gennis P, et al. Clinical criteria for the detection of pneumonia in adults: Guidelines for ordering chest roentgenograms in the emergency department. Journal of Emergency Medicine. 1989;7(3):263–268. doi: 10.1016/0736-4679(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 10.Graffelman AW, et al. Can history and exam alone reliably predict pneumonia? J Fam Pract. 2007;56(6):465–70. [PubMed] [Google Scholar]
- 11.Heckerling PS, et al. CLinical prediction rule for pulmonary infiltrates. Annals of Internal Medicine. 1990;113(9):664–670. doi: 10.7326/0003-4819-113-9-664. [DOI] [PubMed] [Google Scholar]
- 12.Moore Michael, Stuart Beth, Little Paul, Smith Sue, Thompson Matthew J., Knox Kyle, van den Bruel Anne, Lown Mark, Mant David. Predictors of pneumonia in lower respiratory tract infections: 3C prospective cough complication cohort study. European Respiratory Journal. 2017;50(5):1700434. doi: 10.1183/13993003.00434-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singal BM, Hedges JR, Radack KL. Decision rules and clinical prediction of pneumonia: Evaluation of low-yield criteria. Annals of Emergency Medicine. 1989;18(1):13–20. doi: 10.1016/S0196-0644(89)80304-X. [DOI] [PubMed] [Google Scholar]
- 14.Rambaud-Althaus C, et al. Clinical features for diagnosis of pneumonia in children younger than 5 years: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2015;15(4):439–450. doi: 10.1016/S1473-3099(15)70017-4. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiting PF, et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Annals of Internal Medicine. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 17.Decks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ-British Medical Journal-International Edition. 2001;323(7305):157–162. doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. The Lancet. 2005;365(9469):1500–1505. doi: 10.1016/S0140-6736(05)66422-7. [DOI] [PubMed] [Google Scholar]
- 19.Ebell MH, White LL, Casault T. A systematic review of the history and physical examination to diagnose influenza. J Am Board Fam Pract. 2004;17(1):1–5. doi: 10.3122/jabfm.17.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Arends LR, et al. Bivariate Random Effects Meta-Analysis of ROC Curves. Medical Decision Making. 2008;28(5):621–638. doi: 10.1177/0272989X08319957. [DOI] [PubMed] [Google Scholar]
- 21.R Development Core Team, R., A language and enviorment for stastical computing: Vienna, Austria (2016).
- 22.European network for health technology assessment Meta-analysis of diagnostic test accuracy studies. (2014).
- 23.Reitsma JB, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology. 2005;58(10):982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Ebrahimzadeh A, et al. Clinical and Laboratory Findings in Patients With Acute Respiratory Symptoms That Suggest the Necessity of Chest X-ray for Community-Acquired Pneumonia. Iran J Radiol. 2015;12(1):e13547. doi: 10.5812/iranjradiol.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flanders SA, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. American Journal of Medicine. 2004;116(8):529–535. doi: 10.1016/j.amjmed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Holm A, et al. Aetiology and prediction of pneumonia in lower respiratory tract infection in primary care. Br J Gen Pract. 2007;57(540):547–54. [PMC free article] [PubMed] [Google Scholar]
- 27.Holm A, et al. Procalcitonin versus C-reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care. Br J Gen Pract. 2007;57(540):555–60. [PMC free article] [PubMed] [Google Scholar]
- 28.Hopstaken RM, et al. Contributions of symptoms, signs, erythrocyte sedimentation rate, and C-reactive protein to a diagnosis of pneumonia in acute lower respiratory tract infection. Br J Gen Pract. 2003;53(490):358–64. [PMC free article] [PubMed] [Google Scholar]
- 29.Hopstaken RM, Cals JW, Dinant GJ. Accuracy of lipopolysaccharide-binding protein (LBP) and fibrinogen compared to C-reactive protein (CRP) in differentiating pneumonia from acute bronchitis in primary care. Prim Care Respir J. 2009;18(3):227–30. doi: 10.4104/pcrj.2009.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melbye H, Straume B, Brox J. Laboratory tests for pneumonia in general practice: the diagnostic values depend on the duration of illness. Scand J Prim Health Care. 1992;10(3):234–40. doi: 10.3109/02813439209014067. [DOI] [PubMed] [Google Scholar]
- 31.Melbye H, et al. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care. 1988;6(2):111–7. doi: 10.3109/02813438809009300. [DOI] [PubMed] [Google Scholar]
- 32.Moberg AB, et al. Community-acquired pneumonia in primary care: clinical assessment and the usability of chest radiography. Scand J Prim Health Care. 2016;34(1):21–7. doi: 10.3109/02813432.2015.1132889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nolt BR, et al. Vital-sign abnormalities as predictors of pneumonia in adults with acute cough illness. The American Journal of Emergency Medicine. 2007;25(6):631–636. doi: 10.1016/j.ajem.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Steurer J, et al. A decision aid to rule out pneumonia and reduce unnecessary prescriptions of antibiotics in primary care patients with cough and fever. BMC Med. 2011;9:56. doi: 10.1186/1741-7015-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Vugt SF, et al. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: diagnostic study. Bmj. 2013;346:f2450. doi: 10.1136/bmj.f2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K, et al. Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community-acquired pneumonia. Cochrane Database Syst Rev. 2012;10:Cd009175. doi: 10.1002/14651858.CD009175.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cretikos MA, et al. Respiratory rate: the neglected vital sign. Medical Journal of Australia. 2008;188(11):657–659. doi: 10.5694/j.1326-5377.2008.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 38.Schierenberg A, et al. External Validation of Prediction Models for Pneumonia in Primary Care Patients with Lower Respiratory Tract Infection: An Individual Patient Data Meta-Analysis. PLoS One. 2016;11(2):e0149895. doi: 10.1371/journal.pone.0149895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller F, et al. Procalcitonin Levels Predict Bacteremia in Patients With Community-Acquired Pneumonia: A Prospective Cohort Trial. Chest. 2010;138(1):121–129. doi: 10.1378/chest.09-2920. [DOI] [PubMed] [Google Scholar]
- 40.Chan Y-L, et al. Procalcitonin as a marker of bacterial infection in the emergency department: an observational study. Critical Care. 2003;8(1):R12. doi: 10.1186/cc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christ-Carin, M. Procalcitonin in bacterial infections-hype, hope, more or less? Swiss medical weekly, 135(3132) (2005). [DOI] [PubMed]
- 42.Berg P, Lindhardt BØ. The role of procalcitonin in adult patients with community-acquired pneumonia—a systematic review. Dan Med J. 2012;59(3):A4357. [PubMed] [Google Scholar]
- 43.Liu D, et al. Prognostic value of procalcitonin in pneumonia: A systematic review and meta-analysis. Respirology. 2016;21(2):280–288. doi: 10.1111/resp.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meili M, et al. Management of patients with respiratory infections in primary care: procalcitonin, C-reactive protein or both? Expert Review of Respiratory Medicine. 2015;9(5):587–601. doi: 10.1586/17476348.2015.1081063. [DOI] [PubMed] [Google Scholar]
- 45.Simon L, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clinical Infectious Diseases. 2004;39(2):206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 46.Falk G, Fahey T. C-reactive protein and community-acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies. Family Practice. 2008;26(1):10–21. doi: 10.1093/fampra/cmn095. [DOI] [PubMed] [Google Scholar]
- 47.Minnaard MC, et al. The added diagnostic value of five different C-reactive protein point-of-care test devices in detecting pneumonia in primary care: A nested case-control study. Scand J Clin Lab Invest. 2015;75(4):291–5. doi: 10.3109/00365513.2015.1006136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.