Dear Editor,
Human prion diseases consist of sporadic, genetic/familial, and acquired forms. The familial form accounts for 5%–15% of all human prion diseases, including familial Creutzfeldt–Jacob disease (fCJD), Gerstmann–Sträussler–Scheinker syndrome, and fatal familial insomnia (FFI) [1–3]. All genetic prion diseases are directly associated with mutations (point-mutation or insertion) in the PRNP gene located on human chromosome 20 and encodes prion protein (PrP). So far, >55 mutations in the PRNP gene have been described [4]. Some PRNP mutations and their related genetic prion diseases have been reported worldwide, while others show clear region- or ethnicity-associated features.
Since 2006, China has conducted a Creutzfeldt–Jacob disease surveillance program [5, 6], and 15 types of genetic prion disease have been identified. Among these, T188K-fCJD is frequently found among Chinese, but rarely reported in other countries, including Japan and Korea [7]. Clinically, T188K-fCJD is difficult to distinguish from sporadic CJD (sCJD). Cerebrospinal fluid (CSF) 14-3-3 positivity and MRI abnormality are frequent, whereas periodic sharp and wave complexes (PSWCs) in the EEG are unusual. The clinical survival time of T188K-fCJD patients is shorter than those with sCJD [7]. As a rare subtype of fCJD, the reactive feature of T188K-fCJD real-time quaking-induced conversion (RT-QuIC), which is a in vitro amplification technology for detection of the abnormal form of prion protein (PrPSc), in the CSF remains unsettled. To reveal the pattern of CSF RT-QuIC, we compared 25 samples from patients with T188K-fCJD with 24 samples from D178N-FFI patients and 16 samples from E200K-fCJD patients, two relatively common genetic prion diseases in China.
The median onset age of the patients with T188K-fCJD was 58 years (range, 40–85), comparable with that of E200K-fCJD patients (56 years; range, 44–68), but significantly older than that of D178N-FFI patients (49 years; range, 24–70). The gender distributions (male/female) were 15/10 in the T188K-fCJD, 10/14 in the D178N-FFI, and 7/9 in E200K-fCJD groups. PSWCs were noted in only 16% (4/25) of the T188K-fCJD cases, markedly lower than in the E200K-fCJD cases (56.25%, 9/16). None of the D178N-FFI cases showed PSWCs. sCJD-associated abnormalities [high signal in caudate/putamen and/or a high symmetrical or asymmetrical cortical signal in diffusion-weighted imaging (DWI)] on MRI were found in 76% (19/25) of T188K-fCJD cases, 8.33% (2/24) of D178N-FFI cases, and 93.75% (15/16) of E200K-fCJD cases. Positive 14-3-3 protein in the CSF (by Western blot) was found in 76% (19/25) of T188K-fCJD cases, remarkably more than in D178N-FFI cases (20.83%, 5/24) and fewer than in E200K-fCJD cases (100%, 16/16) (Table S1). In addition, all enrolled cases were homozygotic for methionine at codon 129 (M129M) and for glutamic acid at codon 219 (E219E). This revealed that, besides the clinical manifestations, these three subtypes of genetic prion disease have distinctively different demographic and laboratory features.
The stored CSF samples from the patients with the three subtypes of genetic prion disease were subjected to the established RT-QuIC assay using full-length recombinant hamster PrP protein (HaPrP23-231) as the substrate [7] (details in supplementary materials). Among the 25 CSF samples from T188K-fCJD cases, 13 (52%) were positive in RT-QuIC. The median lag phase was 20.3 h post-reaction (range, 5.1 h–65 h) and the median peak thioflavin-T (ThT) value was 70000 relative fluorescence units (rfu; range, 25000–110000) (Table S1, Fig. S1). Most (10/13) occurred before 25 h post-reaction and at >60000 rfu. A higher ratio of E200K-fCJD patients (62.5%, 10/16) showed positive reactions in CSF RT-QuIC. Meanwhile, the positive reactions appeared markedly early (median, 6.1 h post-reaction; range, 2.8 h–16 h), and the median peak ThT fluorescence value was high (90500 rfu; range, 43000–190000) (Table S1, Fig. S1). Only 4 (16.7%) out of 24 D178N-FFI cases were RT-QuIC-positive, with clearly longer lag phases (median, 31 h post-reaction; range, 10–45) and lower peak ThT values (median 51500 rfu; range, 42500–62500) (Table S1, Fig. S1).
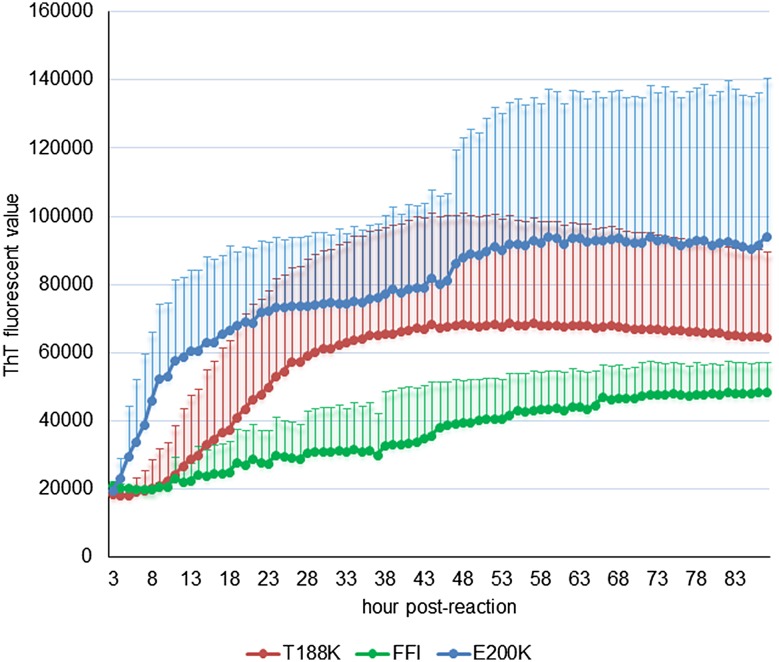
We also compared the real-time conversion features of the CSF samples from cases of the three diseases in RT-QuIC. As shown in Fig. 1, compared with the averaged conversion curve of E200K-fCJD that increased rapidly in the first 10 h post-reaction and continued to increase and remain at a high ThT value (>85000 rfu), the averaged conversion curve of T188K-fCJD increased relatively late, reaching a peak at 40 h post-reaction (~65000 rfu). The positive reaction of D178N-FFI samples appeared very late and remained at a remarkably low ThT value (~45000 rfu). The data here indicated a RT-QuIC reactive pattern in T188K-fCJD distinct from those of E200K-fCJD and D178N-FFI.
Fig. 1.
Averaged reaction curves of CSF RT-QuIC of the three genetic prion diseases. The data from positive samples, 13 T188K-fCJD, 10 E200K-fCJD, and 4 D178N-FFI samples, were separately averaged and are presented as mean ± SD. X-axis, hours post-reaction; Y-axis, ThT fluorescence values.
CSF 14-3-3 positivity, PSWCs in the EEG, and specific abnormalities on MRI are the important items for the diagnosis of probable sCJD. However, the appearances of abnormalities in these items in genetic prion diseases vary greatly. Among the 65 cases of genetic prion diseases, 40 (61.5%) were positive for CSF 14-3-3, 13 (20.0%) showed PSWCs, and 36 (55.4%) displayed specific MRI abnormalities. To determine the association of the results of CSF RT-QuIC with these three items, we statistically evaluated the RT-QuIC reactivity of the samples with the results of CSF 14-3-3, EEG, and MRI. As shown in Table 1, the reactivity of CSF RT-QuIC was positively correlated with positive results for CSF 14-3-3 (P = 0.005), EEG (P = 0.008), and MRI (P = 0.008) in the context of all tested cases, showing that patients with the three genetic prion diseases who showed CSF 14-3-3 positivity, PSWCs, and sCJD-associated abnormalities on MRI had a high probability of being positive in CSF RT-QuIC. No significant correlation was found between the RT-QuIC reactivity and the two separate MRI abnormalities, a high signal in the caudate or putamen (P = 0.217) and a high symmetrical or asymmetrical cortical signal in DWI (P = 0.062).
Table 1.
Correlation of reactivity of CSF RT-QuIC with CSF 14-3-3, EEG, and MRI.
| RT-QuIC | P value | ||
|---|---|---|---|
| Negative (n) | Positive (n) | ||
| CSF 14-3-3 | |||
| Negative (n) | 20 | 5 | 0.005* |
| Positive (n) | 18 | 22 | |
| EEG | |||
| Negative (n) | 25 | 13 | 0.008* |
| Positive (n) | 3 | 10 | |
| MRI | |||
| Negative (n) | 19 | 5 | 0.008* |
| Positive (n) | 16 | 20 | |
*P-value < 0.05 was considered statistically significant
Our study has revealed distinct profiles of CSF RT-QuIC among the three most frequently-encountered genetic prion diseases in China. E200K-fCJD is the most commonly reported fCJD in many countries and is the third commonest genetic prion disease in China [8]. Clinically, Chinese patients with E200K-fCJD were more like typical sCJD patients with very high positivity rates for the EEG, MRI, and CSF 14-3-3, but have less of a disease-associated family history [8]. On the contrary, only a few of the CSF samples from D178N-FFI patients were positive in RT-QuIC, with very low ThT values and a long lag phase. FFI has completely different clinical manifestations and examination results, often with an early onset and a disease-associated family history [8, 9]. In line with another study, the different genetic prion diseases displayed different reactive profiles in CSF RT-QuIC assays, which may reflect different pathogenesis due to different point mutations in PRNP [10].
The reactivity of CSF RT-QuIC may vary with the type of PrPSc in the brain and may also reflect the amount of PrPSc released into the CSF [11]. Usually, large PrPSc deposits are detectable in the brains of E200K-fCJD patients, while E200K-M129M cases show PrP type 1 and E200K-V129V cases show PrP type 2 [12, 13], which may contribute to the higher positivity ratios in RT-QuIC. D178N-FFI cases have been described worldwide. Deposits of PrPSc in the brains of FFI cases are usually very limited and its resistance to proteinase K is usually markedly weak [14–17]. This limited distribution of PrPSc in the brains of FFI patients may be directly associated with their low positivity ratios in CSF RT-QuIC. T188K-fCJD is more prevalent in Chinese [1, 8]. Unfortunately, only a few T188K-fCJD cases have been subjected to neuropathological assays for confirmation of PrPSc deposits in the brain [18]. The association of the RT-QuIC reactivity of T188K-fCJD samples with PrPSc deposition needs further neuropathological study.
We also found that the CSF RT-QuIC reactions were positively correlated with the abnormal findings on EEG, MRI, and CSF 14-3-3. Based on our results, E200K-fCJD has very high ratios of CSF 14-3-3 positivity, specific abnormalities on MRI, and PSWCs on EEG. T188K-fCJD has high ratios of CSF 14-3-3 positivity and specific abnormalities on MRI, but very low ratios of PSWCs. D178N-FFI has very low ratios of CSF 14-3-3 and abnormalities on MRI, with no PSWCs. It seems that for a special subtype of human genetic prion disease, the higher the positivity rate of sCJD-associated abnormalities on these items, the more the positive reactivity in CSF RT-QuIC. The appearance of multiple sCJD-associated abnormalities on the EEG, MRI and CSF 14-3-3 may represent more extensive damage of the brain, so that PrPSc may be more likely to be released into the CSF. On the other hand, the presence of specific abnormalities in the EEG, MRI and CSF 14-3-3 in a patient with a genetic prion disease may reflect a clinical process more like typical sCJD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by National Science and Technology Major Project of China (2017ZX10104001002005), the National Natural Science Foundation of China (81630062, 81301429, and 81572048), National Key R&D Program (2016YFC1202700) and State Key Laboratory of Infectious Disease Prevention and Control (SKLID) Program of China (2012SKLID102 and 2015SKLID503).
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Qi Shi, Email: shiqi76@126.com.
Xiao-Ping Dong, Email: dongxp238@sina.com.
References
- 1.Chen C, Dong XP. Epidemiological characteristics of human prion diseases. Infect Dis Poverty. 2016;5:47. doi: 10.1186/s40249-016-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prusiner SB. The prion diseases. Brain Pathol. 1998;8:499–513. doi: 10.1111/j.1750-3639.1998.tb00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong BH, Kim YS. Genetic studies in human prion diseases. J Korean Med Sci. 2014;29:623–632. doi: 10.3346/jkms.2014.29.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao C, Shi Q, Tian C, Chen C, Han J, Zhou W, et al. The epidemiological, clinical, and laboratory features of sporadic Creutzfeldt-Jakob disease patients in China: surveillance data from 2006 to 2010. PLoS One. 2011;6:e24231. doi: 10.1371/journal.pone.0024231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Q, Gao C, Zhou W, Zhang BY, Chen JM, Tian C, et al. Surveillance for Creutzfeldt-Jakob disease in China from 2006 to 2007. BMC Public Health. 2008;8:360. doi: 10.1186/1471-2458-8-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Q, Zhou W, Chen C, Xiao K, Wang Y, Gao C, et al. Rare genetic Creutzfeldt-Jakob disease with T188K mutation: analysis of clinical, genetic and laboratory features of 30 Chinese patients. J Neurol Neurosurg Psychiatry. 2017;88:889–890. doi: 10.1136/jnnp-2016-314868. [DOI] [PubMed] [Google Scholar]
- 8.Shi Q, Zhou W, Chen C, Zhang BY, Xiao K, Zhang XC, et al. The Features of Genetic Prion Diseases Based on Chinese Surveillance Program. PLoS One. 2015;10:e0139552. doi: 10.1371/journal.pone.0139552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortelli P, Gambetti P, Montagna P, Lugaresi E. Fatal familial insomnia: clinical features and molecular genetics. J Sleep Res. 1999;8(Suppl 1):23–29. doi: 10.1046/j.1365-2869.1999.00005.x. [DOI] [PubMed] [Google Scholar]
- 10.Cramm M, Schmitz M, Karch A, Zafar S, Varges D, Mitrova E, et al. Characteristic CSF prion seeding efficiency in humans with prion diseases. Mol Neurobiol. 2015;51:396–405. doi: 10.1007/s12035-014-8709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orru CD, Groveman BR, Raymond LD, Hughson AG, Nonno R, Zou W, et al. Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains. PLoS Pathog. 2015;11:e1004983. doi: 10.1371/journal.ppat.1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MO, Cali I, Oehler A, Fong JC, Wong K, See T, et al. Genetic CJD with a novel E200G mutation in the prion protein gene and comparison with E200K mutation cases. Acta Neuropathol Commun. 2013;1:80. doi: 10.1186/2051-5960-1-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capellari S, Parchi P, Russo CM, Sanford J, Sy MS, Gambetti P, et al. Effect of the E200K mutation on prion protein metabolism. Comparative study of a cell model and human brain. Am J Pathol 2000, 157: 613–622. [DOI] [PMC free article] [PubMed]
- 14.Gambetti P, Parchi P, Petersen RB, Chen SG, Lugaresi E. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: clinical, pathological and molecular features. Brain Pathol. 1995;5:43–51. doi: 10.1111/j.1750-3639.1995.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 15.Shi XH, Han J, Zhang J, Shi Q, Chen JM, Xia SL, et al. Clinical, histopathological and genetic studies in a family with fatal familial insomnia. Infect Genet Evol. 2010;10:292–297. doi: 10.1016/j.meegid.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki K, Doh-ura K, Wakisaka Y, Tomoda H, Iwaki T. Fatal familial insomnia with an unusual prion protein deposition pattern: an autopsy report with an experimental transmission study. Neuropathol Appl Neurobiol. 2005;31:80–87. doi: 10.1111/j.1365-2990.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 17.Parchi P, Petersen RB, Chen SG, Autilio-Gambetti L, Capellari S, Monari L, et al. Molecular pathology of fatal familial insomnia. Brain Pathol. 1998;8:539–548. doi: 10.1111/j.1750-3639.1998.tb00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roeber S, Grasbon-Frodl EM, Windl O, Krebs B, Xiang W, Vollmert C, et al. Evidence for a pathogenic role of different mutations at codon 188 of PRNP. PLoS One. 2008;3:e2147. doi: 10.1371/journal.pone.0002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.