Abstract
Artesunate (ARS) induced significant reactive oxygen species (ROS) generation in HepG2, HeLa, and A549 lines. However, ARS induced ROS-dependent apoptosis in HeLa and A549 cell lines but ROS-independent apoptosis in HepG2 cells. A total of 200 μM hydrogen peroxide (H2O2) significantly induced cytotoxicity in HeLa cells, while H2O2 up to 300 μM did not induce cytotoxicity in HepG2 cells, further demonstrating the strong resistance of HepG2 cells to ROS. HeLa cells had much higher basic total glutathione (T-GSH) level than HepG2 cells, while the ratio of basic reduced glutathione (GSH)/oxidized glutathione (GSSG) in HepG2 cells was nearly twice than that in HeLa and A549 cells. Inhibition of glutathione markedly enhanced H2O2- or ARS-induced cytotoxicity in HeLa and A549 cell lines but modestly enhanced the cytotoxicity of H2O2 and even did not affect the cytotoxicity of ARS in HepG2 cells. Moreover, addition of GSH remarkably prevented H2O2- or ARS-induced cytotoxicity in HeLa and A549 cell lines, further indicating the involvement of GSH in scavenging ROS in the two cell lines. HepG2 cells exhibited higher catalase activity than HeLa cells, and inhibiting catalase activity by using 3-aminotriazole (3-AT, a specific inhibition of catalase) or catalase siRNA remarkably reduced the resistance of HepG2 cells to ROS, demonstrating the key roles of catalase for the strong resistance of HepG2 cells to ROS. Collectively, catalase activity instead of glutathione level dominates the resistance of cells to ROS.
Keywords: Catalase, Reactive oxygen species, Glutathione, Resistance, Hydrogen peroxide
Introduction
Reactive oxygen species (ROS) play an important role in numerous physiological and pathological processes (Niess et al. 1999). Various antitumor agents induce ROS-mediated cell apoptosis (Wang and Yi 2008; Fang et al. 2007; Cheng et al. 2013; Sun et al. 2011). Taxol and arsenic trioxide (As2O3) induce apoptosis through a ROS-dependent pathway in some cell lines (Sun et al. 2011; Lee 2015; Wang et al. 2004; Meshkini and Yazdanparast 2012; Woo et al. 2002). Our previous publications have reported that dihydroartemisinin (DHA), an artemisinin derivative, induced ROS-dependent apoptosis in A549 cells (Chen et al. 2013). However, Park et al. (2004) reported that taxol induced ROS-independent apoptosis in T lymphoblastic leukemia cells, and DHA induced ROS-independent apoptosis in HL-60 leukemia cells (Lu et al. 2008; Hou et al. 2004). Alía et al. (2005) found that HepG2 cells were resistant to hydrogen peroxide (H2O2, a ROS donor). We recently also found that artesunate (ARS) induced ROS-dependent apoptosis in Hep3B and A549 cells but ROS-independent apoptosis in HepG2 cells (Zhuo et al. 2012; Qin et al. 2015; Pang et al. 2016) .
Glutathione, one of the most important scavengers of ROS, is generally considered to be correlated with the resistance of cells to ROS. ROS-resistant cell lines showed high level of glutathione (Spitz et al. 1995; Yae and Tsuchihashi 2012). Spitz and coworkers (Spitz et al. 1995, 1988) found that H2O2-resistant OC-14 cells had a twofold glutathione level than H2O2-sensitive HA-1 cells. Cai et al. (2000) found that As2O3-sensitive cells had lower glutathione level than the As2O3-resistant cells. Furthermore, reducing intracellular glutathione can increase the sensitivity of cells towards ROS (Spitz et al. 1995; Kiesslich et al. 2005). Therefore, glutathione depletion is a potential strategy to sensitize drug-resistant tumor cells (Davison et al. 2003; Rudin et al. 2003; Schnelldorfer et al. 2000).
In addition to the glutathione level, catalase activity is also related to the resistance of cells to ROS. H2O2, a kind of ROS, can react with iron ion to produce hydroxyl radicals (.OH), one of the most active and most cytotoxic free radical (Madeo et al. 1999; Koppenol 2001). Catalase, a scavenger of H2O2, efficiently decomposes H2O2 to water and dioxygen to protect cells against H2O2 stress (Martins and English 2014). It was reported that H2O2-resistant cells exhibited high catalase activity (Spitz et al. 1992; Kasugai and Yamada 1992; Dejeans et al. 2012), and inhibition of catalase activity increased the sensitivity of the cells to H2O2 (Smith et al. 2007). CHOr cells, exhibiting more 4.3-fold catalase activity than CHOs cells, were more resistant to H2O2 than the CHOs cells (Keizer et al. 1998). Smith et al. (2007) found that glioma cells with high catalase activity were extremely resistant to oxidative stress, and inhibiting catalase activity significantly increased sensitivity of the glioma cells to H2O2.
We here evaluate the effect of intracellular glutathione and catalase on the resistance of cancer cells to ROS in HepG2, HeLa, and A549 cell lines. In contrast to the important role of ROS in ARS-induced apoptosis in HeLa and A549 cells, ROS did not participate in ARS-induced apoptosis in HepG2 cells. Moreover, HepG2 cells exhibited the strongest resistance to H2O2 among the three cell lines. Although HepG2 cells have much lower glutathione level than HeLa cells, this cell line exhibited higher catalase activity than HeLa cells. Furthermore, inhibiting catalase by using 3-AT or catalase siRNA significantly reduced the resistance of HepG2 cells to H2O2 or ARS-induced ROS. In contrast to the important role of glutathione for the resistance of HeLa cells to ROS, reducing intracellular glutathione level had less effect on the resistance of HepG2 cells H2O2 and even had no effect on the resistance of this cell line to ARS-induced ROS. These findings indicate that catalase activity instead of glutathione level play a key role for the resistance of cells to ROS.
Material and methods
Materials
ARS was from Holleypharm (Chongqing, China). Working solutions were prepared as described previously and diluting it with culture medium to different concentration before experiments (Pang et al. 2016). The final concentration of dimethylsulfoxide (Sigma, USA) was less than 1% in all experiments. 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA), N-acetyl-cysteine (NAC), L-buthionine-sulfoximine (BSO), and 3-aminotriazole (3-AT) were purchased from Sigma (St. Louis, USA). Hydrogen peroxide (H2O2) was purchased from Guangzhou chemical reagent factory (Guangzhou, China). Reduced glutathione (GSH) was from Jian Yang Biotechnology (Guangzhou, China). Dulbecco’s modified Eagle Medium (DMEM) was from Gibco (Carlsbad, California, USA). Fetal bovine serum (FBS) was from Sijiqing (Hangzhou, China).
Cell lines and cell culture
HepG2 cells and HeLa cells were obtained from the Department of Medicine, Jinan University, Guangzhou, China. A549 cells were purchased from Cell Bank of CAS (Shanghai, China). Cells were cultured in DMEM supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100 U/ml streptomycin). All cells were grown in a humidified incubator with 5% CO2 at 37 °C.
Cell viability assay
Cell viability was detected by Cell Counting Kit-8 (CCK-8, Beyotime Institute of Biotechnology, China) assay according to the manufacture’s protocol as described previously (Lu et al. 2009). Briefly, cells were seeded in 96-well plates for 24 h and subjected to the indicated treatment, and viable cells were assessed by absorbance measurements at 450 nm using an auto-microplate reader (infinite M200, Tecan, Austria).
Fashion of cells death detection
Fashion of cells death was analyzed by double staining with Hoechst 33258 and PI using fluorescence microscopy (Olympus, IX73, Hamburg, Japan). Cells were defined as apoptotic if they exhibited characteristic changes in apoptosis such as chromatin condensation and margination, nuclear fragmentation, and the appearance of apoptotic bodies, as visualized with Hoechst 33258 (Rogalska et al. 2011). Necrotic cells were defined as nuclear PI positive without condensed chromatic or apoptotic bodies. Cells without apoptotic or necrotic features were considered viable (Li et al. 2005). A minimum of 200 cells were counted for each treatment. The numbers of normal, necrotic, and apoptotic cells were calculated as a percentage of total cells. In brief, cells were grown on confocal dishes for 24 h. After being subjected to the indicated treatment, the cells were washed with PBS one or two times and incubated with 20 μg/ml Hoechst 33258 and 10 μg/ml PI for 20 min at 37 °C in the dark, respectively. Cells were then washed three times with PBS and visualized by Olympus fluorescence microscope.
Detection of intracellular ROS
Intracellular ROS generation was measured by flow cytometry (FCM) with an oxidation-sensitive probe DCF-DA, which is cleaved by non-specific esterases and converts to highly fluorescent DCF upon oxidation by ROS. Cells were seeded in 6-well plates for 24 h and treated with indicated treatment, and then the cells were stained with 20 μM DCF-DA in PBS for 30 min in the dark. Then cells were harvested and re-washed with PBS three times, oxidation-induced increase of DCF fluorescence was assayed by FCM subsequently.
Measurement of intracellular glutathione
Intracellular glutathione level was measured using GSH and GSSG Assay Kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instructions. Cell cultured in 6-well plates were subjected to the indicated treatments, and collected and washed with PBS two times. Total glutathione (T-GSH) was assayed using the 5,5-dithio-bis (2-nitrobenzoic) acid (DTNB)-GSSG reductase recycling. Oxidized disulfide (GSSG) was measured by measuring 5-thio-2-nitrobenzoic acid (TNB) produced from the reaction of reduced glutathione (GSH) with DTNB. The rate of TNB formation was measured at 412 nm by the auto-microplate reader. The concentrations of GSH were obtained by the GSSG levels from the T-GSH (GSH = T-GSH-2 × GSSG). Samples were normalized to protein concentration determined by using the BCA method.
Catalase activity assay
Catalase activity was assayed by using a catalase analysis kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instructions. Briefly, cells were cultured in 6-well plates and subjected to the indicated treatments. Then cells were lysed on ice for 30 min by RIPA lysis reagent. Cell lysates were treated with H2O2 for 3 min, and then the residual H2O2 was combined with a substrate to produce N-4-antipyryl-3-chloro-5-sulfonate-p-benzoquinonemonoimine, which has an absorption maximum at 520 nm. Catalase activity was determined by measuring the decomposition of H2O2 in an auto-microplate reader (infinite M200, Tecan, Austria). The protein concentration was measured with the BCA method.
Silencing of catalase with siRNA
Cells were cultured on 12-well plates (4 × 104/ well) and grown overnight to reach 60–70% confluence. Cells were transfected with serum-free DMEM containing non-specific (siCON) and catalase-specific siRNA (siCAT) (igeBio, Guangzhou, China) using lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s recommendations. The cells were collected 36 h after transfection, and processed in the following experiments. Changes in catalase expression were determined by western blot.
Western blotting analysis
Cells were collected and resuspended in ice-cold whole cell lysis buffer (Triton X-100 plus protease inhibitor cocktail). Equal amount of total protein, quantified by using the BCA method, was separated by SDS-PAGE electrophoresis and transferred on to polyvinylidene fluoride (PVDF) membranes according to standard techniques. Membranes were probed with the indicated primary antibodies (rabbit anti-catalase, 1:1000, CST, USA) overnight at 4 °C followed by 1-h incubation at room temperature with fluorescent secondary antibodies. Finally, the membranes were scanned using Odyssey Infrared Imaging System (LI-COR Biosciences, Nebraska, USA). GAPDH was used as loading control.
Statistics
Data were presented as mean ± SD from at least three independent experiments and analyzed using Student’s t test. Statistical and graphic analyses were done using the software SPSS19.0 (SPSS, Chicago) and Origin 8.0 (Origin Lab Corporation). P< 0.05 was defined as statistical significance.
Results
Roles of ROS in ARS-induced apoptosis in three cancer cell lines
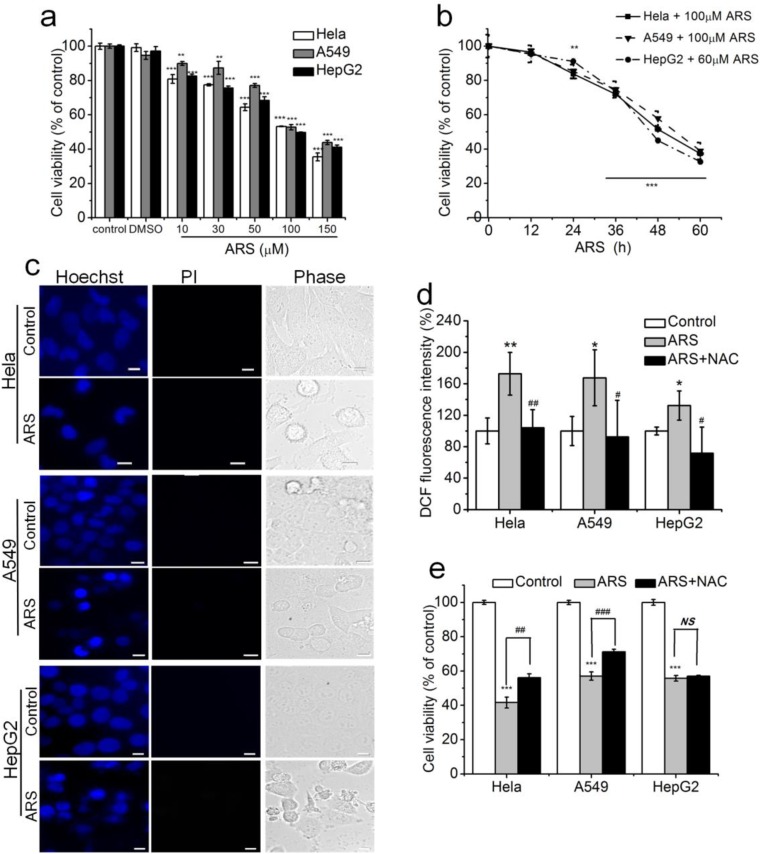
We firstly assessed the cytotoxicity of ARS in three kinds of cancer cell lines (HepG2, HeLa, A549) using CCK-8 assay. Treatment with different concentration (10–150 μM) of ARS for 48 h or 60/100 μM of ARS for different times (0–60 h) induced dose- and time-dependent cytotoxicity (Fig. 1a, b). A total of 60 μM ARS were adopted for HepG2 cells and 100 μM ARS were adopted for A549/HeLa cells in the following experiments without specific indication. We next examined the fashion of ARS-induced cell death by staining with Hoechst 33258 and PI. Microscopic imaging of cells showed apoptosis-related chromatin condensation and margination in ARS-treated cells (Fig. 1c). To explore whether ROS was involved in ARS-induced apoptosis, we detected intracellular ROS level by FCM analysis with DCF-DA staining. Our results showed that ARS induced significant increase of intracellular ROS and pretreatment with 10 mM NAC, a widely used ROS scavenger, completely neutralized ARS-induced ROS generation (Fig. 1d). Moreover, pretreatment with NAC significantly inhibited ARS-induced cytotoxicity in HeLa and A549 cells, but did not affect ARS-induced cytotoxicity in HepG2 cells (Fig. 1e). These data demonstrated that ARS induced ROS-dependent apoptosis in both HeLa and A549 cell lines but ROS-independent apoptosis HepG2 cell line.
Fig. 1.
Roles of ROS in ARS-induced apoptosis in three cancer cell lines. a and b ARS induced dose- (a) and time- (b) dependent cytotoxicity. Cells were treated with different concentrations of ARS (0–150 μM) for 48 h (a) or with 60 or 100 μM ARS for different times (0–60 h) (b). c ARS induced apoptosis. Cell treated with ARS for 48 h were stained with Hoechst 33258 and PI before imaging using fluorescent microscope. Scale bar, 10 μm. d ARS induced ROS generation. Cells were treated with ARS for 2 h in the presence or absence of 10 mM NAC before FCM analysis. e ARS induced ROS-dependent cytotoxicity in Hela and A549 cells but ROS-independent cytotoxicity in HepG2 cells. Cells were pre-incubated with 10 mM NAC for 2 h and then treated with ARS for 48 h before CCK-8 assay. Those results represent duplicates with three independent experiments. NS, no statistical significance. *P < 0.05, ** P< 0.01, and *** P< 0.001, compared with control; #P < 0.05, ##P < 0.01, and ###P < 0.001, compared with ARS treatment alone
Much stronger resistance of HepG2 cells to hydrogen peroxide (H2O2) than HeLa cells
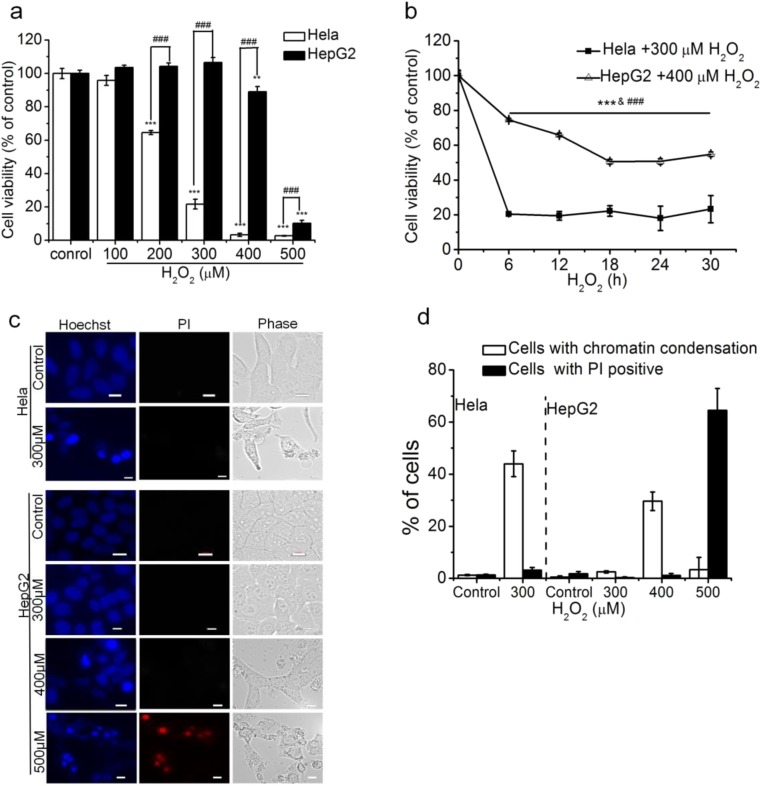
To compare the sensitivity of HeLa cells and HepG2 cells to ROS, we firstly used CCK-8 assay to assess H2O2-induced cytotoxicity in the two cell lines. After treatment with H2O2 for 24 h, CCK-8 assays showed that 200 μM H2O2 induced a significant cytotoxicity in HeLa cells, while 300 μM H2O2 did not induce cytotoxicity and even 400 μM H2O2 only induced a 10.99% of decrease in HepG2 cell viability (Fig. 2a), demonstrating the much stronger resistance of HepG2 cells to H2O2 than HeLa cells. We also exposed HeLa and HepG2 cells respectively to 300/400 μM H2O2 for different treatment times (6, 12, 18, 24, 30 h), and found that treatment with H2O2 for 6 h decreased cell viability to the lowest in HeLa cells but only induced a about 30% of decrease in cell viability in HepG2 cell (Fig. 2b), further demonstrating that HepG2 cells had much stronger resistance to H2O2 than HeLa cells. A total of 300 μM H2O2 were adopted for HeLa cells and 400 μM H2O2 were adopted for HepG2 and A549 cells in the following experiments without specific indication.
Fig. 2.
HepG2 cells have much stronger resistance to H2O2 than Hela cells. a and b H2O2 induced dose- (a) and time- (b) dependent cytotoxicity in HepG2 and Hela cells. Cells were exposed to various concentrations of H2O2 (0–500 μM) for 24 h (a) or to with 300/400 μM H2O2 for different times (0–30 h) (b). c H2O2 less than 400 μM induced apoptosis. Cells treated with 300, 400, or 500 μM H2O2 for 24 h before staining and imaging. Scale bar, 10 μm. d Statistical results of (c). A minimum of 200 cells were counted for each treatment in each experiment. The numbers of cells with chromatin concentration or PI positive were calculated as a percentage of total cells. Those results represent duplicates with three independent experiments. NS, no statistical significance. *P < 0.05, **P < 0.01, and ***P < 0.001, compared with control; #P < 0.05, ## P< 0.01, and ###P < 0.001, compared with HepG2 cells treated with H2O2
We next used Hoechst 33258 and PI staining to detect the fashion of H2O2-induced cell death and mortality in the two cancer cell lines. We found that after treatment with 300 μM for 24 h, 43.96% HeLa cells showed apoptosis-related chromatin condensation, while HepG2 cells were almost completely alive (Fig. 2c, d). In addition, we also examined the fashion of 500 μM H2O2–induced cell death in HepG2 cells by staining with Hoechst 33258 and PI, and found that 64.51% of HepG2 cells exhibited necrotic-related changes (PI positive) based on their morphological and staining characteristics (Fig. 2c, d). These data further demonstrated the strong resistance of HepG2 cells to H2O2.
Intracellular glutathione plays an important role for the sensitivity of HeLa and A549 cells to ROS
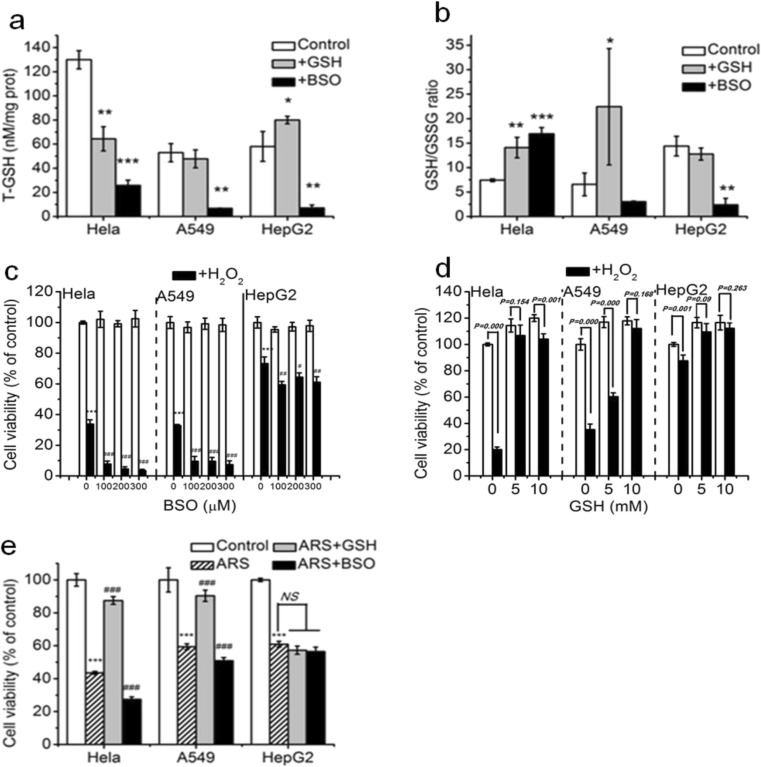
Intracellular glutathione exists mainly in two forms: reduced glutathione (GSH), the biological active form, and oxidized glutathione (GSSG) (Adeoye et al. 2017). GSH/GSSG ratio, often used as a representative maker of the cellular redox state, is the main redox couple that determines the antioxidative capacity of cells (Wu et al. 2004). We firstly detected T-GSH (total glutathione) level and GSH/GSSG ratio in the three cancer cell lines. To our surprise, the basic T-GSH level in HeLa cells was more twice than that of HepG2 cells, and the basic T-GSH level in A549 cells was similar to that of HepG2 cells (Fig. 3a). Treatment with BSO, a glutathione synthesis inhibitor, almost completely reduced the T-GSH level in the three cancer cell lines (Fig. 3a). Interestingly, treatment with GSH increased the T-GSH level in HepG2 but significantly reduced the T-GSH level in HeLa cells (Fig. 3a). As shown in Fig. 3b, the basic GSH/GSSG ratio in HepG2 cells was nearly twice than that in HeLa and A549 cells, and treatment with GSH increased the GSH/GSSG ratio in HeLa and A549 cells, while treatment with BSO increased the GSH/GSSG ratio in HeLa cells but reduced the GSH/GSSG ratio in A549 and HepG2 cells. These data implicate that the GSH/GSSG ratio instead of the T-GSH level is associated with the resistance of cells to ROS.
Fig. 3.
Intracellular glutathione plays an important role for the sensitivity of Hela and A549 cell lines to ROS. a and b Intracellular total glutathione (T-GSH) level (a) and GSH/GSSG ratio (b) in the absence or presence of GSH or BSO. Cells were treated with 100 μM BSO for 12 h and 5 mM GSH for 2 h, respectively, and then were detected the intracellular T-GSH level (a) and GSH/GSSG ratio (b) in the three cancer cell lines. c Inhibition of glutathione by BSO markedly enhanced H2O2-induced cytotoxicity in Hela and A549 cell lines but modestly enhanced cytotoxicity of H2O2 in HepG2 cells. d Addition of GSH remarkably prevented H2O2-induced cytotoxicity in Hela and A549 cell lines. Cells were pre-incubated with different concentration of BSO for 12 h (c) and GSH for 2 h (d), respectively, and then treated with H2O2 for 24 h before CCK-8 assay. e GSH played an important role in ARS-induced cytotoxicity in Hela and A549 cell lines but was not involved in ARS-induced cytotoxicity in HepG2 cells. Cells were pre-incubated with 5 mM GSH and 100 μM BSO, respectively, and then treated with ARS for 48 h. Those results represent duplicates with three independent experiments. NS, no statistical significance. *P < 0.05, **P < 0.01, and ***P < 0.001, compared with control; #P < 0.05, ##P < 0.01, and ###P < 0.001, compared with H2O2 or ARS treatment alone
We next assessed the effects of BSO (100~300 μM) on the H2O2-induced cytotoxicity. As shown in Fig. 3c, pretreatment with BSO larger than 100 μM almost completely eliminated the resistance of HeLa and A549 cells to H2O2, and only partially but significantly eliminated the resistance of HepG2 cells to H2O2. We also assessed the influence of GSH pretreatment on the cytotoxicity of H2O2, and found that in contrast to the significant protective effect of GSH on the H2O2-induced cytotoxicity in HeLa and A549 cell lines, GSH pretreatment only had a very modest protective effect to the H2O2-induced cytotoxicity in HepG2 cells (Fig. 3d), further demonstrating the important role of glutathione in H2O2- induced cytotoxicity in HeLa and A549 cells.
We finally assessed the effect of GSH and BSO respectively on the cytotoxicity of ARS. As shown in Fig. 3e, pretreatment with 5 mM GSH or 100 μM BSO did not affect the cytotoxicity of ARS in HepG2 cells, while pretreatment with GSH significantly reduced the cytotoxicity of ARS in both HeLa and A549 cell lines, and pretreatment with BSO significantly enhanced the cytotoxicity of ARS in the two cell lines, demonstrating that glutathione played an key role in ARS-induced cytotoxicity in both A549 cell and HeLa cell lines, but did not mediate the ARS-induced cytotoxicity in HepG2 cells.
Catalase activity dominates the resistance of cells to ROS
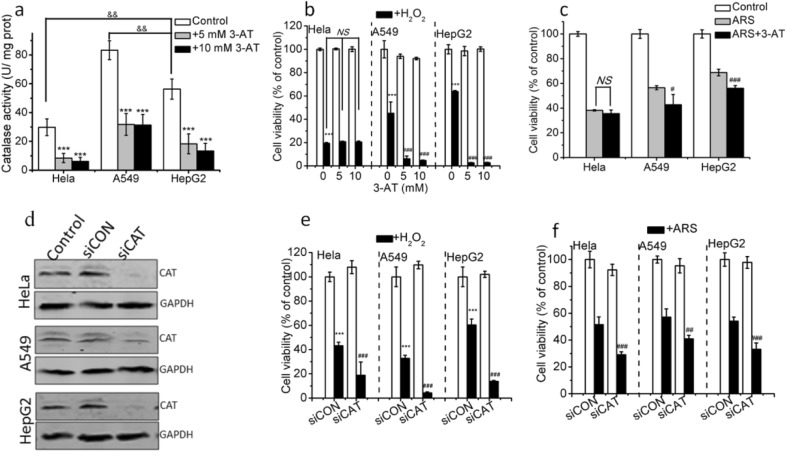
Catalase, another important antioxidant enzyme, protects cells by degrading H2O2 into H2O and O2 (Vetrano et al. 2005). To explore whether the resistance of cells to ROS is related to catalase activity, we firstly examined catalase activity in the presence or absence of 3-aminotriazole (3-AT, a catalase inhibitor). HepG2 and A549 cells had higher basic catalase activity than HeLa cells, and pretreatment with 5 or 10 mM 3-AT significantly inhibited catalase activity in the three cancer cell lines (Fig. 4a). We next assessed the effects of pretreatment with 3-AT on the cytotoxicity induced by H2O2, and found that pretreatment with 5 mM 3-AT almost completely counteracted the resistance of HepG2 cells and A549 cells to H2O2, but did not influence the cytotoxicity of H2O2 in HeLa cells (Fig. 4b), illustrating that catalase activity played a dominant role on the resistance of HepG2 and A549 cells instead of HeLa cells to H2O2. In addition, 3-AT pretreatment significantly enhanced the cytotoxicity of ARS in HepG2 cells and A549 cells but did not affect the ARS-induced cytotoxicity in HeLa cells (Fig. 4c), indicating that catalase activity does not participate in ARS-induced cytotoxicity in HeLa cells but plays an important role in ARS-induced cytotoxicity in HepG2 and A549 cells.
Fig. 4.
Dominant role of catalase in the resistance of cells to ROS. a Intracellular catalase activity in the presence or absence of 3-AT treatment. Cells were seeded into 6-well plates and treated with 5 or 10 mM 3-AT for 28 h before catalase activity detection. b and c Pretreatment with 3-AT significantly enhanced H2O2- (b) and ARS- (c)induced cytotoxicity in HepG2 and A549 cells instead of in Hela cells. Pretreatment with 3-AT for 4 h cells were treated with H2O2 for 24 h (b) or ARS for 48 h (c) before CCK-8 assay. d Western blotting analysis on the catalase expression in the three cell lines. Cells transfected with non-specific (siCON) and catalase-specific siRNA (siCAT) cultured for 48 h and catalase expression analyzed by Western blotting analysis, and the values were normalized to GAPDH. e and f Effect of silencing catalase on the cytotoxicity of H2O2 (e) and ARS (f). Cells were transfected with non-specific (siCON) and catalase-specific siRNA (siCAT) plasmids respectively before treatment with H2O2 for 24 h (e) and ARS (f) for 48 h. Cells transfected with siCON were used as negative control. Those results represent duplicates with three independent experiments. NS, no statistical significance. *P < 0.05, **P < 0.01, and ***P < 0.001, compared with control or siCON; #P < 0.05, ##P < 0.01, and ###P < 0.001, compared with H2O2 or ARS treatment alone; &P < 0.05, compared with HepG2 cells
In order to further verify the dominant role of catalase in the resistance of cells to ROS induced by H2O2 and ARS, we also used catalase siRNA (siCAT) as a catalase inhibition to assess the effect of inhibition of catalase on the cytotoxicity induced by H2O2 and ARS. Firstly, Western blotting was used to assess the efficiency of siCAT suppression, and 50 nM siCAT significantly inhibited catalase level in the three cancer cell lines. We next assessed the effects of silencing catalase with siCAT on the cytotoxicity induced by H2O2 and ARS. As shown in Fig. 4e and f, silencing catalase significantly enhanced the cytotoxicity of H2O2 and ARS in the three cell lines, further demonstrating the notion that catalase play a dominant role in the H2O2- and ARS-induced cytotoxicity.
Discussion
In this study, we evaluate the effect of intracellular glutathione and catalase on the resistance of cancer cells to ROS in HepG2, HeLa, and A549 cell lines. HepG2 cells showed the strongest antioxidant capacity, and HeLa cells exhibited the weakest antioxidant capacity. Intracellular catalase activity dominates the resistance of HepG2 cells to ROS, while intracellular glutathione dominates the sensitivity of HeLa cells to ROS, and only play a modest role for the resistance of HepG2 cells to ROS. In addition, both glutathione and catalase activity play an important role for the sensitivity of A549 to ROS.
Our data, that BSO pretreatment significantly enhances the cytotoxicity of H2O2 (Fig. 3c) and GSH pretreatment completely counteracts H2O2-induced cytotoxicity (Fig. 3d) in HeLa cells, indicate that intracellular glutathione plays an important role in mediating the sensitivity of HeLa cells to ROS, which was further verified by the fact that altering intracellular glutathione level by pretreatment with GSH or BSO significantly affected the sensitivity of both HeLa and A549 cell lines to ARS (Fig. 3e). It is seem reasonable that the ROS-resistant cells have higher level of glutathione than the ROS-sensitive cells (Arrick et al. 1982; Davison et al. 2003; Al-Qenaei et al. 2014). However, some studies showed that the level of glutathione in some ROS-resistant cells is indistinguishable from, or even lower than that of ROS-sensitive cells (Dejeans et al. 2012; Andreoli et al. 1992). Dejeans et al. (2012) found that ROS-resistant MCF-7 breast cancer cells and normal MCF-7 cells had equivalent level of glutathione. Although HepG2 had much lower T-GSH level than that of HeLa cells (Fig. 3a), HepG2 cells exhibited much stronger resistance than HeLa cells to H2O2 (Fig. 2a–d), indicating that the strong resistance of HepG2 cells to ROS may be independent of the high levels of glutathione. Moreover, in contrast to the significantly protective effect on H2O2-induced cytotoxicity in HeLa cells, GSH pretreatment had modest protective effect in HepG2 cells and BSO pretreatment also exhibited modest enhancement in the cytotoxicity of H2O2 in HepG2 cells than that of HeLa cells (Fig. 3c, d), further demonstrating that glutathione does not dominate the resistance of HepG2 cells to ROS, which was further verified by the fact that pretreatment with BSO and GSH did not affect the cytotoxicity of ARS in HepG2 cells (Fig. 3e).
Our observations suggest that the GSH/GSSG ratio instead of the T-GSH level may determine the antioxidant capacity of glutathione. Although GSH pretreatment decreased the T-GSH level of HeLa cells (Fig. 3a), this treatment significantly increased the GSH/GSSG ratio (Fig. 3b) and the resistance of HeLa cells to H2O2 (Fig. 3d). Moreover, GSH pretreatment remarkably enhanced the resistance of HeLa cells to ARS (Fig. 3e), which may be due to the increased GSH/GSSG ratio (Fig. 3b). Although the T-GSH level of HepG2 cells is much lower than that of HeLa cells, the GSH/GSSG ratio of HepG2 cells is nearly twice times than that of HeLa cells (Fig. 3a, b), which may determine the resistance of HepG2 cells to ROS and the sensitivity of HeLa cells to ROS (Fig. 2a–d). However, BSO pretreatment increased the GSH/GSSG ratio in HeLa cells (Fig. 3b) but significantly enhanced the cytotoxicity of both H2O2 and ARS (Fig. 3c, e), which may be due to the too low glutathione level by BSO pretreatment (Fig. 3a) to scavenge ROS.
Our data, that pretreatment with 3-AT significantly inhibits the catalase activity (Fig. 4a) and silencing catalase significantly decreased catalase level (Fig. 4d), and both inhibition completely counteract the resistance of HepG2 cells to H2O2 (Fig. 4b and e), support the view that catalase activity dominates the resistance of HepG2 cells to ROS. It was reported that ROS-resistant cells exhibited high catalase activity (Kasugai and Yamada 1992; Dejeans et al. 2012). Moreover, Lenehan and coworkers (1995) found that multidrug-resistant human leukemia cells (HL-60/AR) showed more 10-fold resistance to H2O2 than drug-sensitive HL-60 cells, and inhibition of catalase activity diminished the resistance of HL-60/AR to H2O2 by more than 80%. Pretreatment with 5 mM 3-AT or silencing catalase significantly not only inhibited catalase (Fig. 4a and d) in HepG2 and A549 cells but also enhanced their sensitivity to ARS (Fig. 4c), further demonstrating the key role of catalase activity for the resistance of both HepG2 and A549 cell lines to ROS.
Antioxidant action of glutathione redox cycle and catalase, two main intracellular antioxidant systems (Jafri 2014), may be associated with their levels and other regulating factors. The reaction rate constant between catalase and H2O2 to produce H2O is about ~ 106 M-l•s-l (Ogura and Yamazaki 1983), much higher than the 18~26 M-l•s-l between glutathione and H2O2 to produce H2O (Winterbourn and Metodiewa 1999) and the 63.0 M-l•s-l between iron ions and H2O2 to produce hydroxyl radical (•OH) (And and Gallard 1999), one of the strongest oxidants in nature. HepG2 cells exhibited higher catalase activity than HeLa cells (Fig. 4a), which was consistent with previous report that the catalase activity of HepG2 cells was more four times than that of HeLa cells (Duthie et al. 1997). Combining the fact that inhibiting catalase activity significantly enhanced the sensitivity of HepG2 cells to H2O2 and ARS (Fig. 4b, c), the strong resistance of HepG2 cells to H2O2 (Fig. 2a–d) and ARS-induced ROS-independent apoptosis in HepG2 cells (Fig. 1c–e) were consistent with our previous findings (Qin et al. 2015; Pang et al. 2016), which may be ascribed to the high catalase activity of this cell line to rapidly scavenge H2O2. In contrast, HeLa cells has too low catalase activity to effectively scavenge H2O2, thus most of H2O2 react with intracellular iron ions to produces a mass of hydroxyl radical (•OH) to kill cells. Our recent study demonstrated that Fenton reaction between iron ions and H2O2 produces •OH which dominates the SNP-induced induce apoptosis in chondrocyte (Quan et al. 2016), which may be the reason why HeLa cells showed more sensitivity to H2O2 (Fig. 2a–d) and ARS induced a ROS-dependent apoptosis in HeLa cells (Fig. 1c–e). The fact that pretreatment with GSH significantly enhances the antioxidant capacity of HeLa cells (Fig. 3b) can be explained as follows: the increased antioxidant capacity of glutathione competes with the iron ions to react with H2O2 to produce H2O.
However, the facts that A549 cells have higher basic catalase activity (Fig. 4a) but lower resistance to ROS (Fig. 1c–e) than HepG2 cells indicate that in addition to the high catalase activity, there are other factors to determine the resistance of cells to ROS. It was reported that H2O2 larger than 100 μM can lead to the irreversible inactivation of catalase (Lardinois et al. 1996). Our data that HepG2 cells have remarkable resistance to H2O2 within 300 μM (Fig. 2a–d) support a conjecture that HepG2 cells have a mechanism to maintain catalase activity. Glutathione peroxidases (GPx), a key antioxidant enzyme in glutathione redox cycle, can catalyze the translation of H2O2 to H2O by GSH (Gao et al. 2014). Inhibiting GPx activity can not only decrease the ability of catalase to decompose H2O2 to H2O (Sokolova et al. 2001) but also increase the sensitivity of mature OLs cells to H2O2 (Baud et al. 2004). It was reported that the GPx activity of HepG2 cells was 10,350 ± 85.21 U/mg protein (Ramyaa et al. 2014), much higher than the 3395.86 ± 1.16 U/mg protein in A549 cells (Kalaivani et al. 2014). Therefore, we here speculate that although A549 cells possessed the higher catalase activity than HepG2 cells, the low GPx activity in A549 cells does not prevent the H2O2-dependent inactivation of catalase, while the high GPx activity in HepG2 cells prevents the H2O2-depenednt inactivation of catalase, resulting in the sensitization of A549 cell line to ROS and the strong resistance of HepG2 cells to ROS (Fig. 1c–e).
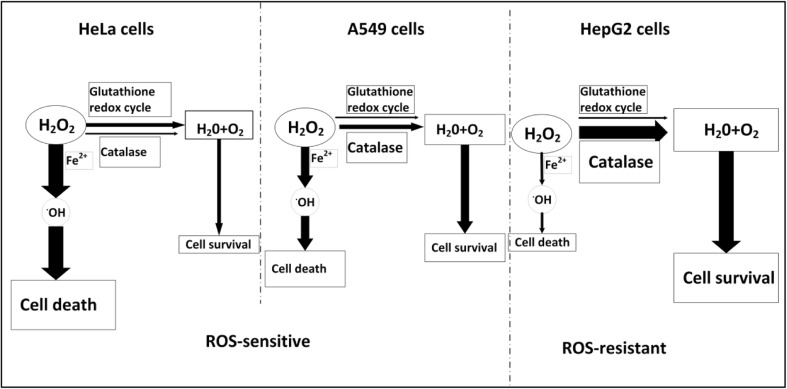
According to our data, we summarize the antioxidant mechanisms of the three cancer cell lines in Fig. 5. In HepG2 cells, catalase instead of glutathione redox cycle efficiently scavenges H2O2, thus dominating the strong resistance of HepG2 cells to ROS. HeLa cells exhibit a weak resistance to ROS due to the low catalase activity, in which catalase activity only partially scavenges H2O2 and glutathione redox cycle plays a modest role in scavenging H2O2. In A549 cells, both the glutathione redox cycle and catalase activity only play a modest role in scavenging H2O2, resulting in the high sensitivity of A549 cells to ROS. Collectively, catalase activity instead of glutathione level plays a key role for the resistance of cells to ROS.
Fig. 5.
Schematic diagram showing the resistance mechanism of the three cancer cell lines to ROS
Funding
This work was supported by the National Natural Science Foundation of China (Grants 61527825, 81572184, and 81471699) and the Natural Science Foundation of Guangdong Province (Grant 2014A030313378).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adeoye O, Olawumi J, Opeyemi A, Christiania O. Review on the role of glutathione on oxidative stress and infertility. Jbra Assist Reprod. 2017;22:61–66. doi: 10.5935/1518-0557.20180003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alía M, Ramos S, Mateos R, Bravo L, Goya L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2) J Biochem Toxic. 2005;19:119–128. doi: 10.1002/jbt.20061. [DOI] [PubMed] [Google Scholar]
- Al-Qenaei A, Yiakouvaki A, Reelfs O, Santambrogio P, Levi S, Hall ND, Tyrrell RM, Pourzand C. Role of intracellular labile iron, ferritin, and antioxidant defence in resistance of chronically adapted Jurkat T cells to hydrogen peroxide. Free Radic Biol Med. 2014;68:87–100. doi: 10.1016/j.freeradbiomed.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- And JDL, Gallard H. Catalytic decomposition of hydrogen peroxide by Fe(III) in homogeneous aqueous solution: mechanism and kinetic modeling. Environ Sci Technol. 1999;33:2726–2732. doi: 10.1021/es981171v. [DOI] [Google Scholar]
- Andreoli SP, Mallett C, Mcateer JA, Williams LV. Antioxidant defense mechanisms of endothelial cells and renal tubular epithelial cells in vitro: role of the glutathione redox cycle and catalase. Pediatr Res. 1992;32:360–365. doi: 10.1203/00006450-199209000-00023. [DOI] [PubMed] [Google Scholar]
- Arrick BA, Nathan CF, Griffith OW, Cohn ZA. Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem. 1982;257:1231–1237. [PubMed] [Google Scholar]
- Baud O, Greene AE, Li J, Wang H, Volpe JJ. Glutathione peroxidase–catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J Neurosci. 2004;24:1531–1540. doi: 10.1523/JNEUROSCI.3989-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Shen YL, Zhu Q, Jia PM, Yu Y, Zhou L, Huang Y, Zhang JW, Xiong SM, Chen SJ, Wang ZY, Chen Z, Chen GQ. Arsenic trioxide- induced apoptosis and differentiation are associated respectively with mitochondrial transmembrane potential collapse and retinoic acid signaling pathways in acute promyelocytic leukemia. Leukemia. 2000;14:262–270. doi: 10.1038/sj.leu.2401650. [DOI] [PubMed] [Google Scholar]
- Chen T, Chen M, Chen J. Ionizing radiation potentiates dihydroartemisinin- induced apoptosis of A549 cells via a caspase-8-dependent pathway. PLoS One. 2013;8:e59827. doi: 10.1371/journal.pone.0059827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R, Li C, Li C, Wei L, Li L, Zhang Y, Yao Y, Gu X, Cai W, Yang Z, Ma J, Yang X, Gao G. The artemisinin derivative artesunate inhibits corneal neovascularization by inducing ROS-dependent apoptosis in vascular endothelial cells. Invest Ophthalmol Vis Sci. 2013;54:3400–3409. doi: 10.1167/iovs.12-11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison K, Cote S, Mader S, Miller WH. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia. 2003;17:931–940. doi: 10.1038/sj.leu.2402876. [DOI] [PubMed] [Google Scholar]
- Dejeans N, Glorieux C, Guenin S, Beck R, Sid B, Rousseau R, Bisig B, Delvenne P, Buc Calderon P, Verrax J. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: implications for tumor recurrence. Free Radic Biol Med. 2012;52:993–1002. doi: 10.1016/j.freeradbiomed.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Duthie SJ, Johnson W, Dobson VL. The effect of dietary flavonoids on DNA damage (strand breaks and oxidised pyrimdines) and growth in human cells. Mutat Res. 1997;390:141–151. doi: 10.1016/S0165-1218(97)00010-4. [DOI] [PubMed] [Google Scholar]
- Fang J, Nakmura H, Iyer AK. Tumor-targeted induction of oxystress for cancer therapy. J Drug Target. 2007;15:475–486. doi: 10.1080/10611860701498286. [DOI] [PubMed] [Google Scholar]
- Gao F, Chen J, Ma T, Li H, Wang N, Li Z, Zhang Z, Zhou Y. The glutathione peroxidase gene family in Thellungiella salsuginea: genome-wide identification, classification, and gene and protein expression analysis under stress conditions. Int J Mol Sci. 2014;15:3319–3335. doi: 10.3390/ijms15023319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D, Uto T, Tong X, Takeshita T, Tanigawa S, Imamura I, Ose T, Fujii M. Involvement of reactive oxygen species- independent mitochondrial pathway in gossypol-induced apoptosis. Arch Biochem Biophys. 2004;428:179–187. doi: 10.1016/j.abb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Jafri MS. Mechanisms of myofascial pain. Int Sch Res Notices. 2014;156:4S10–4S14. doi: 10.1155/2014/523924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaivani P, Saranya S, Poornima P, Prabhakaran R, Dallemer F, Vijaya PV, Natarajan K. Biological evaluation of new nickel (II) metallates: synthesis, DNA/protein binding and mitochondrial mediated apoptosis in human lung cancer cells (A549) via ROS hypergeneration and depletion of cellular antioxidant pool. Eur J Med Chem. 2014;82:584–599. doi: 10.1016/j.ejmech.2014.05.075. [DOI] [PubMed] [Google Scholar]
- Kasugai I, Yamada M. High production of catalase in hydrogen peroxide- resistant human leukemia HL-60 cell lines. Leuk Res. 1992;16:173–179. doi: 10.1016/0145-2126(92)90129-U. [DOI] [PubMed] [Google Scholar]
- Keizer HG, Van RJ, Pinedo HM, Joenje H. Effect of endogenous glutathione, superoxide dismutases, catalase, and glutathione peroxidase on adriamycin tolerance of Chinese hamster ovary cells. Cancer Res. 1998;48:4493. [PubMed] [Google Scholar]
- Kiesslich T, Plaetzer K, Oberdanner CB, Berlanda J, Obermair FJ, Krammer B. Differential effects of glucose deprivation on the cellular sensitivity towards photodynamic treatment- based production of reactive oxygen species and apoptosis-induction. FEBS Lett. 2005;579(1):185–190. doi: 10.1016/j.febslet.2004.11.073. [DOI] [PubMed] [Google Scholar]
- Koppenol WH. The haber-weiss cycle—70 years later. Redox Rep. 2001;6:229–234. doi: 10.1179/135100001101536373. [DOI] [PubMed] [Google Scholar]
- Lardinois OM, Mestdagh MM, Rouxhet PG. Reversible inhibitionand irreversible inactivation of catalase in presence of hydrogen peroxide. Biochim Biophys Acta. 1996;1295:222–238. doi: 10.1016/0167-4838(96)00043-X. [DOI] [PubMed] [Google Scholar]
- Lee C. Overexpression of Tyro3 receptor tyrosine kinase leads to the acquisition of taxol resistance in ovarian cancer cells. Mol Med Rep. 2015;12:1485–1492. doi: 10.3892/mmr.2015.3542. [DOI] [PubMed] [Google Scholar]
- Lenehan PF, Gutierrez PL, Wagner JL, Milak N, Fisher GR, Ross DD. Ross resistance to oxidants associated with elevated catalase activity in HL-60 leukemia cells that overexpress multidrug-resistance protein does not contribute to the resistance to daunorubicin manifested by these cells. Cancer Chemother Pharmacol. 1995;35:377–386. doi: 10.1007/s002800050250. [DOI] [PubMed] [Google Scholar]
- Li L, Elkholy W, Rhodes CJ, Brubaker PL. Glucagon- like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia. 2005;48(7):1339–1349. doi: 10.1007/s00125-005-1787-2. [DOI] [PubMed] [Google Scholar]
- Lu JJ, Meng LH, Cai YJ, Chen Q, Tong LJ, Lin LP, Ding J. Dihydroartemisinin induces apoptosis in HL-60 leukemia cells dependent of iron and p38 mitogenactivated protein kinase activation but independent of reactive oxygen species. Cancer Biol Ther. 2008;7:1017–1023. doi: 10.4161/cbt.7.7.6035. [DOI] [PubMed] [Google Scholar]
- Lu YY, Chen TS, Qun JL, Pan WL, Sun L, Wei XB. Dihydroartemisinin (DHA) induces caspase-3-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. J Biomed Sci. 2009;16:16. doi: 10.1186/1423-0127-16-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Frohlich E, Ligr M, Grey M, Sigrist SJ, Wolf DH, Frohlich KU. Oxygen stress: a regulator of apoptosis in yeast. J Cell Biol. 1999;145:757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins D, English AM. Catalase activity is stimulated by H2O2 in rich culture medium and is required for H2O2 resistance and adaptation in yeast. Redox Biol. 2014;2:308–313. doi: 10.1016/j.redox.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshkini A, Yazdanparast R. Involvement of oxidative stress in taxol-induced apoptosis in chronic myelogenous leukemia K562 cells. Exp Toxicol Pathol. 2012;64:357–365. doi: 10.1016/j.etp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Niess AM, Dickhuth HH, Northoff H, Fehrenbach E. Free radicals and oxidative stress in exercise- immunological aspects. Exerc Immunol Rev. 1999;5:22–56. [PubMed] [Google Scholar]
- Ogura Y, Yamazaki I. Steady-state kinetics of the catalase reaction in the presence of cyanide. J Biomech. 1983;94(2):403–408. doi: 10.1093/oxfordjournals.jbchem.a134369. [DOI] [PubMed] [Google Scholar]
- Pang YL, Qin GQ, Wu LP, Wang XP, Chen TS. Artesunate induces ROS-dependent apoptosis via a Bax-mediated intrinsic pathway in Huh-7 and Hep3B cells. Exp Cell Res. 2016;347(2):251–260. doi: 10.1016/j.yexcr.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Park SJ, Wu CH, Gordon JD, Zhong X, Emami A, Safa AR. Taxol Induces Caspase-10-dependent Apoptosis. J Biol Chem. 2004;279(49):51057–51067. doi: 10.1074/jbc.M406543200. [DOI] [PubMed] [Google Scholar]
- Qin GQ, Wu LP, Liu YH, Pang YL, Zhao CB, Wu S, Wang XP, Chen TS. Artesunate induces apoptosis via a ROS-independent and Bax-mediated intrinsic pathway in HepG2 cells. Exp Cell Res. 2015;336(2):308–317. doi: 10.1016/j.yexcr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Quan YY, Qin GQ, Huang H, Liu XH, Wang XP, Chen TS. Dominant roles of Fenton reaction in sodium nitroprusside- induced chondrocyte apoptosis. Free Radic Biol Med. 2016;94:135–144. doi: 10.1016/j.freeradbiomed.2016.02.026. [DOI] [PubMed] [Google Scholar]
- Ramyaa P, Krishnaswamy R, Padma VV. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim Biophys Acta. 2014;1840:681–692. doi: 10.1016/j.bbagen.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Rogalska A, Gajek A, Szwed M, Jóźwia Z, Marczak A. The role of reactive oxygen species in WP 631-induced death of human ovarian cancer cells: a comparison with the effect of doxorubicin. Toxicol in Vitro. 2011;25(8):1712–1720. doi: 10.1016/j.tiv.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Rudin CM, Yang Z, Schumaker LM, Vanderweele DJ, Newkirk K, Egorin MJ, Zuhowski EG, Cullen KJ. Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer Res. 2003;63:312–318. [PubMed] [Google Scholar]
- Schnelldorfer T, Gansauge S, Gansauge F, Schlosser S, Beger HG, Nussler AK. Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer. 2000;89:1440–1447. doi: 10.1002/1097-0142(20001001)89:7<1440::AID-CNCR5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Smith PS, Zhao WL, Robbins ME. Inhibiting catalase activity sensitizes 36B10 rat glioma cells to oxidative stress. Free Radic Biol Med. 2007;42:787–797. doi: 10.1016/j.freeradbiomed.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Sokolova T, Gutterer JM, Hirrlinger J, Hamprecht B, Dringen R. Catalase in astroglia-rich primary cultures from rat brain: immunocytochemical localization and inactivation during the disposal of hydrogen peroxide. Neurosci Lett. 2001;297:129–132. doi: 10.1016/S0304-3940(00)01689-X. [DOI] [PubMed] [Google Scholar]
- Spitz DR, Li GC, McCormick ML, Sun Y, Oberley LW. Stable H2O2-resistant variants of Chinese hamster fibro- blasts demonstrate increases in catalase activity. Radiat Res. 1988;114:114–124. doi: 10.2307/3577149. [DOI] [PubMed] [Google Scholar]
- Spitz DR, Adams DT, Sherman CM, Roberts RJ. Mechanisms of cellular resistance to hydrogen peroxide, hyperoxia, and 4-hydroxy-2-nonenal toxicity: the significance of increased catalase activity in H2O2-resistant fibroblasts. Arch Biochem Biophys. 1992;292:221–227. doi: 10.1016/0003-9861(92)90071-4. [DOI] [PubMed] [Google Scholar]
- Spitz DR, Kinter MT, Roberts RJ. Contribution of increased glutathione content to mechanisms of oxidative stress resistance in hydrogen peroxide resistant hamster fibroblasts. J Cell Physiol. 1995;165(3):600–609. doi: 10.1002/jcp.1041650318. [DOI] [PubMed] [Google Scholar]
- Sun QL, Sha HF, Yang XH, Bao GL, Lu J, Xie YY. Comparative proteomic analysis of paclitaxel sensitive A549 lung adenocarcinoma cell line and its resistant counterpart A549-Taxol. J Cancer Res Clin Oncol. 2011;137(3):521–532. doi: 10.1007/s00432-010-0913-9. [DOI] [PubMed] [Google Scholar]
- Vetrano AM, Heck DE, Mariano TM, Mishin V, Laskin DL. Characterization of the oxidase activity in mammalian catalase. J Biol Chem. 2005;280(42):35372–35381. doi: 10.1074/jbc.M503991200. [DOI] [PubMed] [Google Scholar]
- Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther. 2008;7(12):1875–1884. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- Wang YF, Chen CY, Chung SF, Chiou YH, Lo HR. Involvement of oxidative stress and caspase activation in paclitaxel-induced apoptosis of primary effusion lymphoma cells. Cancer Chemother Pharmacol. 2004;54:322–330. doi: 10.1007/s00280-004-0831-0. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999;27:322–328. doi: 10.1016/S0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Woo SH, Park IC, Park MJ, Lee HC, Lee SJ. Arsenic trioxide induces apoptosis through a reactive oxygen species-dependent pathway and loss of mitochondrial membrane potential in HeLa cells. Int J Oncol. 2002;21:57. [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Yae T, Tsuchihashi K. Alternative splicing of CD44 mRNA by EsRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3(2):883. doi: 10.1038/ncomms1892. [DOI] [PubMed] [Google Scholar]
- Zhuo CJ, Pan WL, Wang XP, Chen TS. Artesunate induces apoptosis via a Bak-mediated caspase-independent intrinsic pathway in human lung adenocarcinoma cells. J Cell Physiol. 2012;227(12):3778–3786. doi: 10.1002/jcp.24086. [DOI] [PubMed] [Google Scholar]