Abstract
Commiphora spp., Burseraceae family and their resinous matter, myrrh, are used in Mesopotamian medicine as fragrance or antiinsectant. Based on in vitro, leaves, bark, and resin methyl alcohol extract of C. myrrha showed similar inhibitory effects of 17.00, 26.00, and 29.33% for acetylcholinesterase (AChE) as compared to eserine, respectively. The ADMET properties and putative anticholinesterase activity of phytochemicals of myrrh were computationally predicted using in silico tools. Phytochemicals of C. myrrha had acceptable binding affinity (BA) towards principal sites of AChE ranging from − 5.8 (m-cresol) to − 10.5 (abietic acid) kcal/mol. In this regard, all terpenoid compounds (25 out of 28) of myrrh were dual inhibitors since they hydrophobically interacted with both catalytic triad and peripheral anionic site (PAS) of AChE while alpha-terpineol, elemol, and eugenol employed hydrogen bonds with AChE. Cuscohygrine as a pyrrolidine alkaloid has been docked with AChE through hydrogen bonds with PAS and through hydrophobic interactions with catalytic triad thereby we initially proposed it as dual inhibitor of AChE. M-cresol as a methylphenol has been loosely docked with AChE via hydrogen bond and would be a hit molecule for further drug synthesis. This study not only confirmed archaeopharmacological applications of myrrh as antiinsectant or nootropics but also offered an array of terpenoid compounds, cuscohygrine, and m-cresol as a good starting point for hit-to-lead-to-drug optimization phase in synthesis of phyto-nootropics and ecofriendly insecticides.
Keywords: Myrrh, Acetylcholinesterase, Nootropics, Insecticides, Terpenes, Sesquiterpenes, Cuscohygrine
Background
Commiphora myrrha (Nees), Engler, Burseraceae family, is small deciduous tree with many branches and thorny trunk, small leaves, yellow–red flowers and pointed fruits which grows about 3 m high (Ben-Yehoshua et al. 2012). Myrrh is reddish-brown granular excreta consists of resin, gum, and aromatic volatile oils which discharge into cavities of bark when it is wounded and dry there as unequal masses or tear (Ben-Yehoshua et al. 2012). The Arabic word murr, means bitter, is name known in the market of spices (Ben-Yehoshua et al. 2012). Myrrh consists of gum (40–60%), resins (23–40%), volatile oils (2–8%) and a bitter substance (10–25%) (El Ashry et al. 2003; Ben-Yehoshua et al. 2012).
Decoding remained ancient tablets showed that myrrh has been used in ancient Mesopotamian medicine and orthodox medicine also acknowledges the therapeutic powers of many Mesopotamian herbal extracts (Bertman 2003). In this line, many species in the Burseraceae family are woody perennial trees or shrubs with fragrant resins in the leaves and/or stems. From a pharmacognostic perspective, such resins are known to repel herbivores, and some resinous extracts have insect repellent and insecticidal properties (El Ashry et al. 2003). In this continuum, major insecticides industrialized till now act on nervous system of insects through following targets; acetylcholinesterase (AChE, EC 3.1.1.7), main enzyme involved in the transmission of nerve impulse (organophosphorus and carbamates), voltage-gated sodium channels involved in action potential (pyrethroids and DDT), and cholinergic receptor involved in synapses (neonicotinoids; Singh et al. 2017).
In both insects and animals, acetylcholine (ACh) is produced at the presynaptic terminal through combination of acetyl-CoA and choline by the action of enzyme choline acetyltransferase (EC 2.3.1.6) and stored as synaptic vesicles. During neurotransmission, ACh is released from the nerve terminal to the synaptic cleft and binds to ACh receptors on the post-synaptic membrane. Acetylcholinesterase exists in both the presynaptic and postsynaptic membranes that catalyzes breakdown ACh to acetate and choline that way has been ending the synaptic activity at nicotinic and muscarinic cholinergic receptors in the post-synaptic membrane (e.g., Čolović et al. 2013). ACh has a remarkable task in enhancing memory and learning operations. Any long-term disturbances in the central cholinergic system may lead to neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), dementia with Lewy bodies formation (Parent and Baxter 2004; Suganthy et al. 2009).
The studies conducted on natural or synthetic AChE inhibitors showed that cholinesterase inhibitors are commonly used as insecticidal or nootropic agents to improve memory in patients suffering from amnesia. It has been recognized that many phytocompounds can inhibit AChE may enhance cognitive activity or improve signs of any related diseases. The herbal medication working in the brain to reinforce memory and learning are called “nootropic herbs” or “phyto-nootropics” and their secluded ingredients referred as smart drugs (Karimi et al. 2017).
Commiphora myrrha (Nees), Engler, is the species of choice for producing myrrh essential oil used in aromatherapy in Mesopotamia (Watt and Sellar 1996). The aim of this study was to investigate the possible nootropic or insecticidal effects of C. myrrha with a focus on its putative AChE inhibitors by using in silico molecular docking to elucidate mechanisms of its traditional uses.
Methods
Plant extraction
Firstly, whole plant specimens were purchased from Sultanate of Oman, their leaves, bark, and resin were ground and frozen at −20 °C for 3 days. The resulting powders were extracted with methyl alcohol (MA) for 7 days with sporadic shaking. All extracts were filtered and concentrated using a rotary evaporator at 37 °C and were finally obtained as semisolids; then weighed and stored in a refrigerator in airtight vials.
In vitro ChE inhibitory effect
All chemicals were obtained from Sigma-Aldrich (St. Louis, MA, USA) and used as received. The ChE inhibition activity was performed according to the Ellman’s method (Ellman et al. 1961) with slight modifications. The reaction mixture contained Na2HPO4 buffer (1000 μL; 50 mM; pH 7.7), test compound (100 μL), and enzyme (100 μL). The reaction mixtures were mixed and pre-read at 412 nm then pre-incubated for 10 min at 37 °C. The reaction was initiated by the addition of 100 μL of substrate (acetylthiocholine iodide for AChE or butyrylthiocholine chloride for BChE), followed by the addition of 100 μL DTNB (5,5′-Dithiobis [2- Nitrobenzoic Acid]). After 30 min of incubation at 37 °C, absorbance was measured at 412 nm using UV–VIS Spectrophotometer (Biotech, Germany). All experiments were carried out thrice with their respective controls. The percent inhibition was calculated by the help of following equation: Inhibition (%) = (absorbance of negative control − absorbance of test compound)/(absorbance of negative control) × 100; where, control = total enzyme activity without inhibitor; test = activity in the presence of test compound. The inhibition percentage of extract has been reported in proportion to eserine as a canonical reversible ChE inhibitor.
ADMET assay
The drug discovery is a complex and very expensive endeavor that briefly including the choice of disease, the identification of the target and the clinical evaluation. The prediction of ADMET (absorption, distribution, metabolism, excretion and toxicity) properties is the most important and initial phase of drug discovery. The reported chemical compositions of C. myrrha was selected based on binding affinity (BA) lesser than − 5 kcal/mol from reliable work of eminent scientist, Prof. Lumír O. Hanuš, from Hebrew University, Israel (Hanuš et al. 2005). The structures of the main known bioactive compounds of C. myrrha were retrieved from ChemSpider (http://www.chemspider.com) and PubChem (https://pubchem.ncbi.nlm.nih.gov) databases. Afterward their canonical SMILES formats were submitted to AdmetSAR Database (http://lmmd.ecust.edu. Cn/admetsar1/) and intentionally predicted parameters including blood–brain barrier (BBB) penetration, human gastrointestinal tract (GIT) absorption, distribution, subcellular localization, metabolism via CYP450 2C9, and cardiotoxicity endpoint like human ether-a-go-go-related gene (HERG) inhibition have been selected as druglikeness or leadlikness fitness (Cheng et al. 2012).
Molecular docking simulation
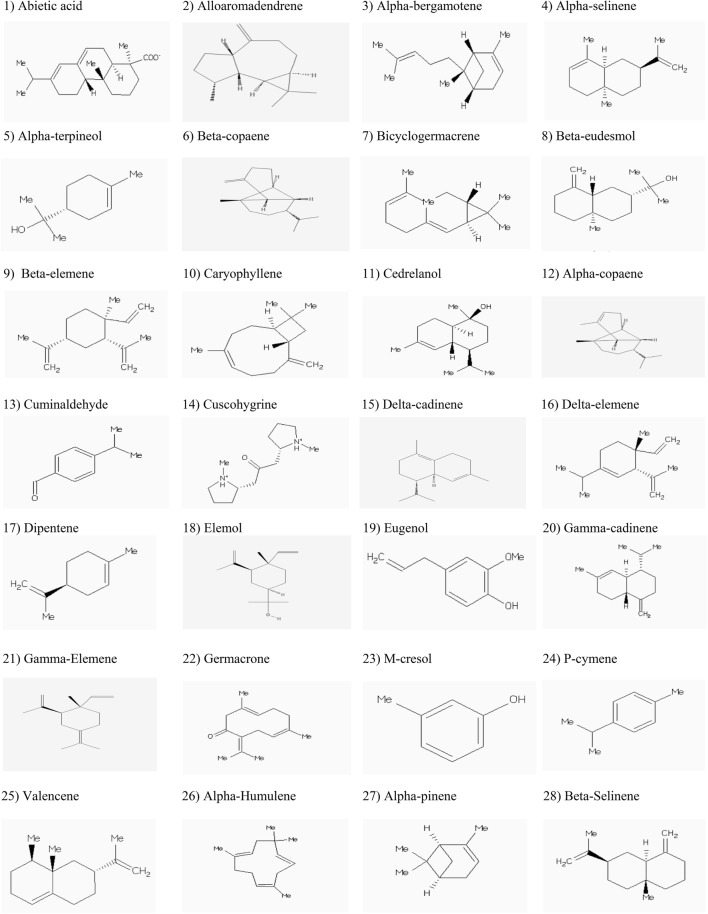
For docking, crystal structure of protein target was retrieved from Protein Data Bank (PDB) (http://www.RCSB.org) (Berman et al. 2002). The PDB format of target protein, AChE (1EVE: Pacific electric ray (Torpedo californica) [TaxId: 7787], has been edited, optimized and trimmed in Molegro Virtual Docker (Thomsen and Christensen 2006) and Chimera 1.8.1 (http://www.rbvi.ucsf.edu/chimera) before submission to PyRx software version 0.8 ((Dallakyan and Olson 2015). The number of each amino acid has been reported in edited 1EVE is Torpedo’s number minus one. The structures of the major known bioactive compounds of C. myrrha were retrieved from PubChem databases (https://pubchem.ncbi.nlm.nih.gov) and ZINC database ver. 12.0 (http://zinc.docking.org/) and ChemSpider databases (http://www.chemspider.com; Fig. 1). After accomplishment of in silico molecular docking, the results have been shown as BA (kcal/mol) values. The more negative BA reflects the best pose and BA of ligand to target protein in its binding sites. The selected structure of ligand and target protein were combined in Molegro Virtual Docker (Thomsen and Christensen 2006) or Chimera 1.8.1 (http://www.rbvi.ucsf.edu/chimera) and their graphical interface has been analyzed with LigPlot+ software (Laskowski and Swindells 2011).
Fig. 1.
The phytochemicals that have been selected in Commiphora myrrha (Nees), Engler, Burseraceae (Hanuš et al. 2005)
Results and discussion
The inhibitory effect of herbal medicines on AChE activity may share in regulating cognitive performance and promoting cholinergic function in people with neurological diseases or may mediate insecticidal property. More specifically, major subsites of AChE are the oxyanion hole (OH), the anionic subsite (AS) and the acyl pocket (AP), however, the hydrolysis operation of ACh occurs in peripheral anionic site (PAS) (Ordentlich et al. 1998; Houghton Peter et al. 2006; Johnson and Moore 2006). The OH consists of Gly118, Gly119, and Ala201 and AP is built by Trp233, Phe288, Phe290, and Phe331. The AS contains Trp84, Phe330, and Glu199. The PAS consists of five residues including Tyr70, Asp72, Tyr121, Trp279, and Tyr334. The catalytic or acylation site of AChE composed of Ser200, His440, and Glu327 residues lies deep within the molecule at the base of a narrow 20 Å deep gorge, lined predominantly with aromatic residues (Ordentlich et al. 1998; Houghton Peter et al. 2006; Johnson and Moore 2006).
In this study, AChE was selected as target protein because neurodegenerative disorders, such as AD, and insecticidal effects are often characterized by the disintegration of the cholinergic system. Thus, many therapeutic regimens were aimed to support this system by inhibition of AChE which increase the concentration of ACh in synapses (Holzgrabe et al. 2007). More interestingly, the occupation of PAS of AChE is able to stop the formation of the amyloid plaque led to the development of bivalent ligands that occupy both active and peripheral site (Holzgrabe et al. 2007).
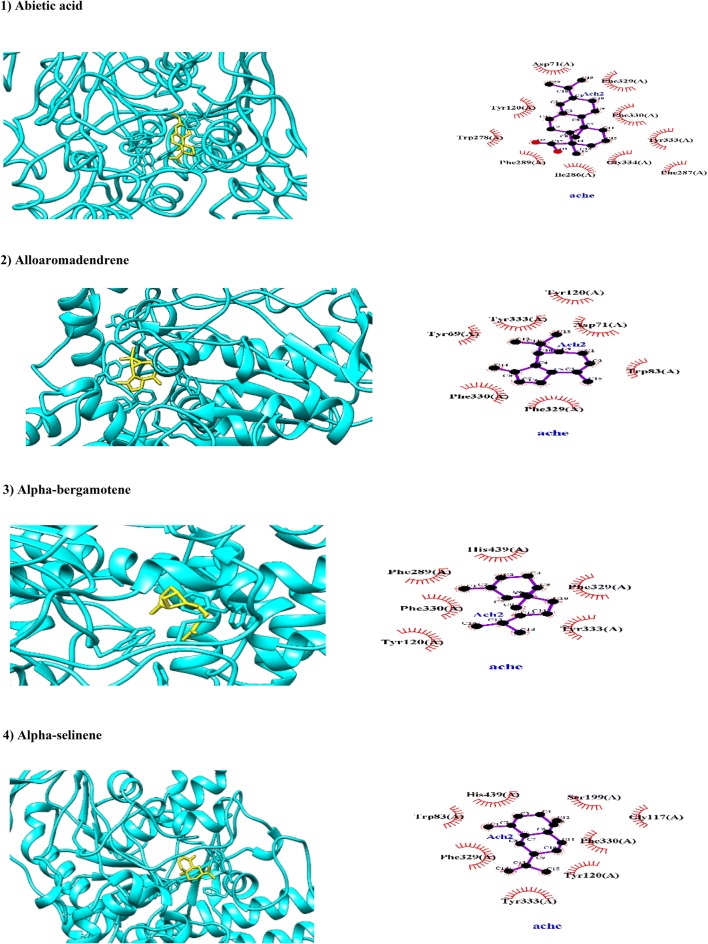
Based on in vitro results obtained in this study, leaves, bark, and resin extract of C. myrrha showed inhibitory effects of 17.00, 26.00, and 29.33% for AChE, respectively. In present study, phytocompounds were selected of C. myrrha which showed acceptable BA with AChE. In this continuum, abietic acid is a diterpenoid found in C. myrrha and its anti-AChE activity as a therapeutic candidate for AD and dementia has been reported experimentally (Ramnath et al. 2015). Among phytochemicals of C. myrrha, abietic acid has been docked with AChE with the most negative BA of -10.2 kcal/mol through hydrophobic interactions by Asp71, Tyr120, Tyr278, and Trp278 in PAS and by Phe330 and Phe287 in AP (Fig. 2). Since abietic acid has been interacted with both PAS and catalytic site, it is a bifunctional agent. In this line, PAS at the mouth of well-known gorge of AChE is implicated in promoting aggregation of beta amyloid (Aβ) peptide responsible for neurodegeneration in AD (Haviv et al. 2007). The insecticidal effects of abietic acid and its congeners have been reported (Xie et al. 1993). Based on ADMET properties, abietic acid was suitable in the evaluation of drug likeness standard where it can be passed BBB and absorbed by GIT while it inhibits HERG (Table 1).
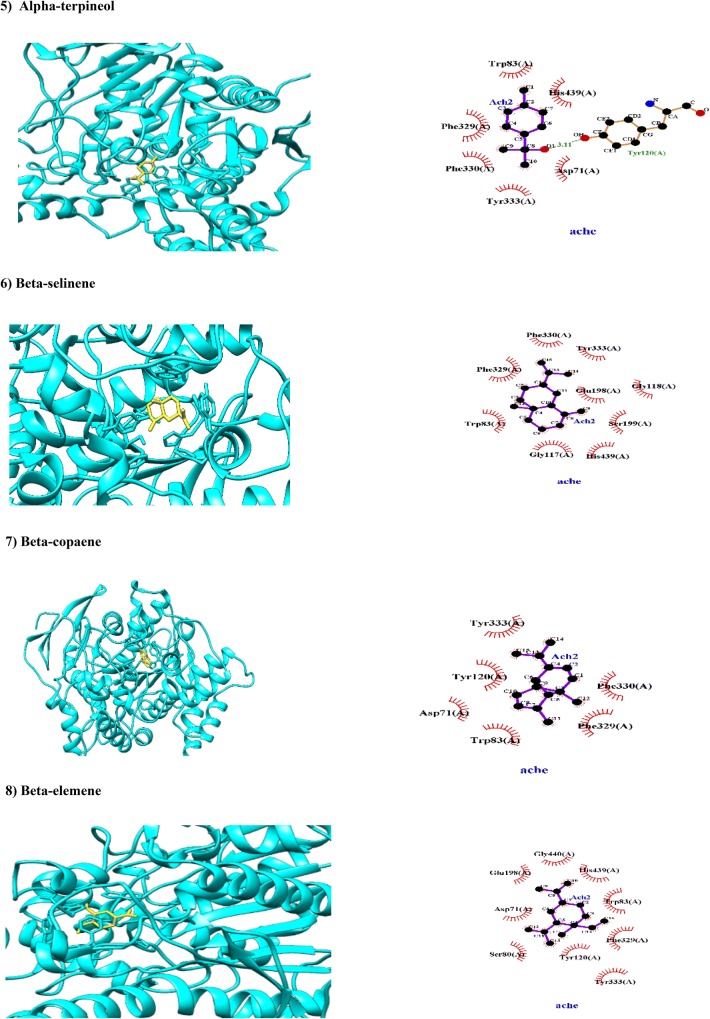
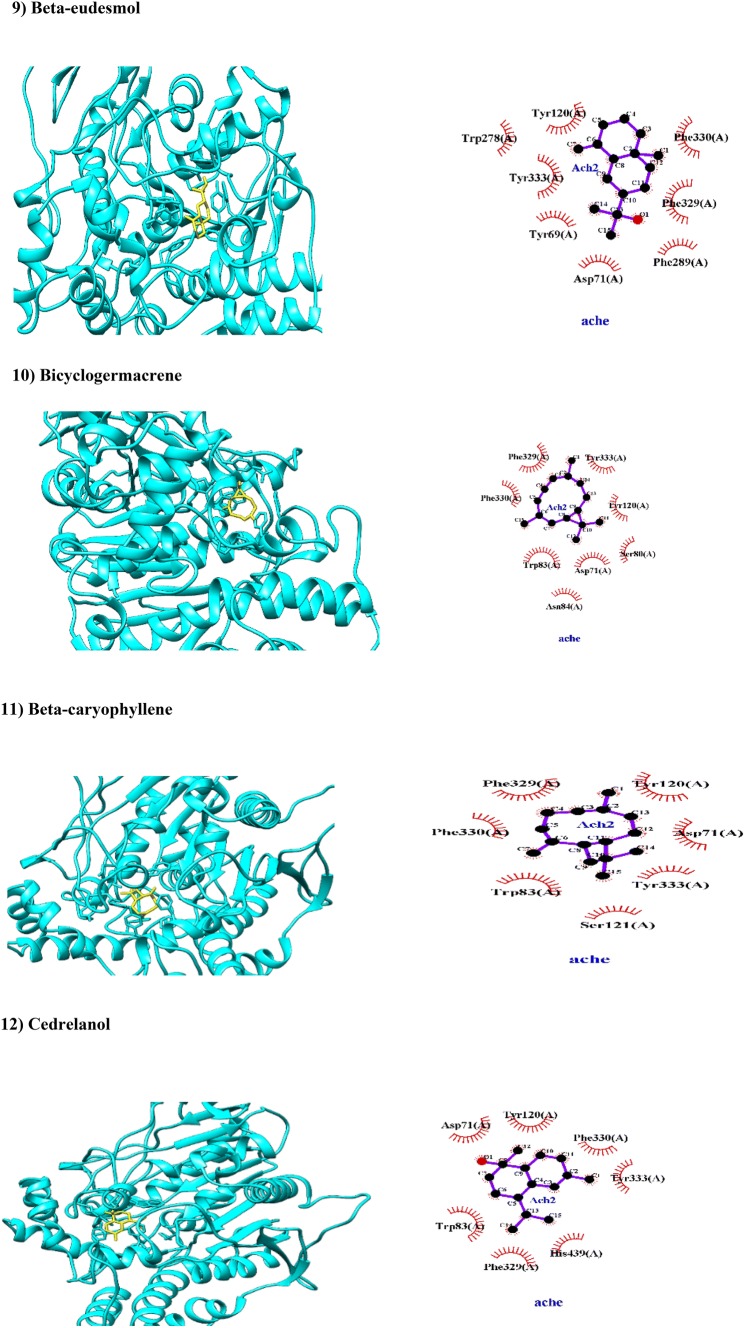
Fig. 2.
Molecular docking of phytochemicals of Commiphora myrrha (Nees; in yellow color) against AChE (PDB code 1EVE; in cyan color). Hydrogen bonds are indicated by dashed lines between the atoms involved, while hydrophobic interactions are symbolized by a semicircle with spokes radiating towards the ligand atoms they contact
Table 1.
In silico ADMET properties of phytochemicals of Commiphora myrrha (Nees) by admetSAR
| Compound | BBB | HIA | CYP inhibition/substrate | Subcellular localization | HERG Inhibition |
|---|---|---|---|---|---|
| Abietic acid | 0.9581 | 0.9974 | Non-substrate/inhibitor | Mitochondria | Weak |
| Alloaromadendrene | 0.9725 | 0.9925 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Alpha-bergamotene | 0.9493 | 0.9920 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Alpha-selinene | 0.9731 | 0.9942 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Alpha-terpineol | 0.9568 | 0.9941 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Beta-eudesmol | 0.9596 | 0.9950 | Non-substrate/inhibitor | Lysosome | Weak |
| Beta-copaene | 0.9435 | 0.9963 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Beta-elemene | 0.9725 | 0.9861 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Bicyclogermacrene | 0.9676 | 0.9965 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Caryophyllene | 0.9536 | 0.9926 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Cedrelanol | 0.9455 | 1.0000 | Non-substrate/non-inhibitor | Lysosome | Weak |
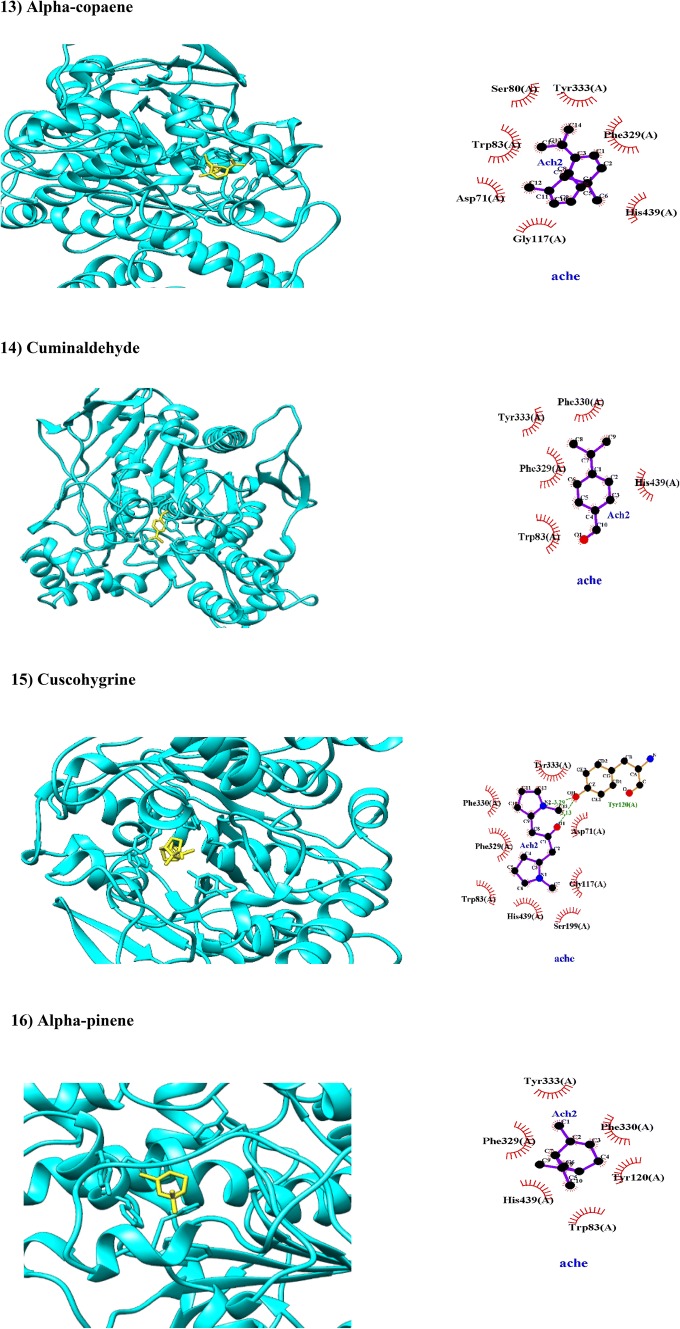
| Alpha-copaene | 0.9435 | 0.9963 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Cuminaldehyde | 0.9764 | 1.0000 | Non-substrate/non-inhibitor | Mitochondria | Weak |
| Cuscohygrine | 0.9940 | 0.9712 | Non-substrate/non-inhibitor | Mitochondria | Weak |
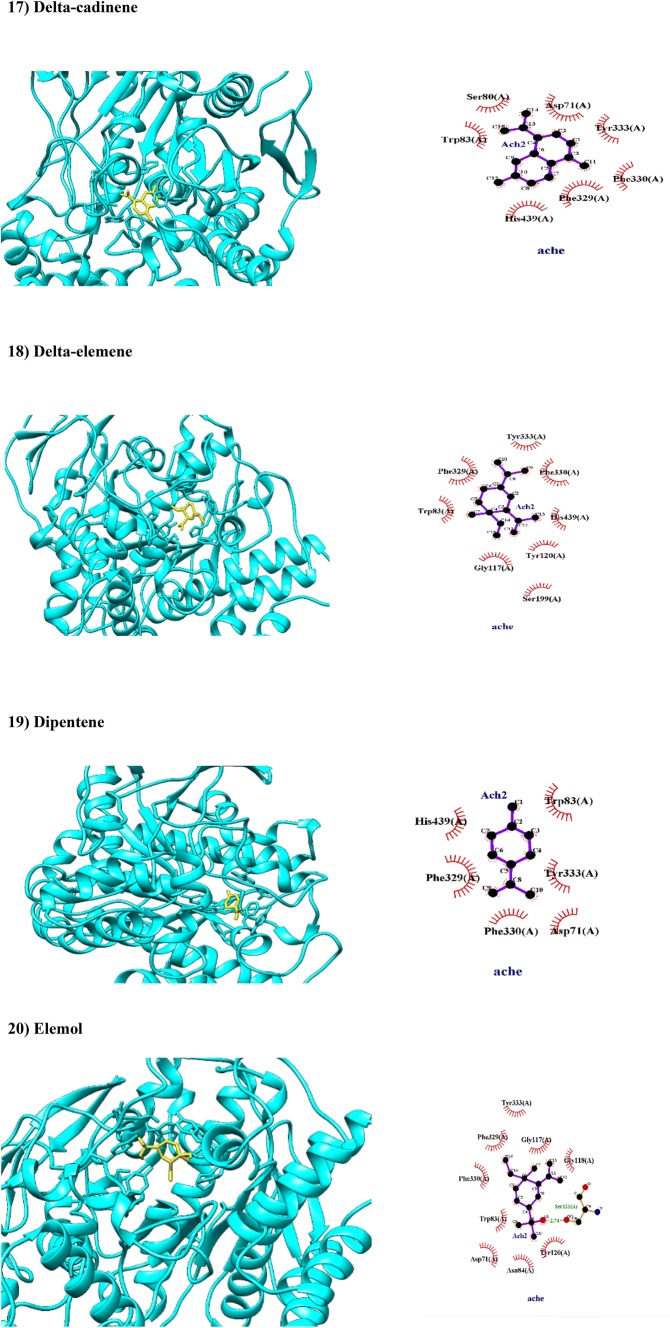
| Delta-cadinene | 0.9460 | 1.0000 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Delta-elemene | 0.9664 | 0.9873 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Dipentene | 0.9444 | 0.9887 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Elemole | 0.9545 | 0.9879 | Non-substrate/non-inhibitor | Lysosome | Weak |
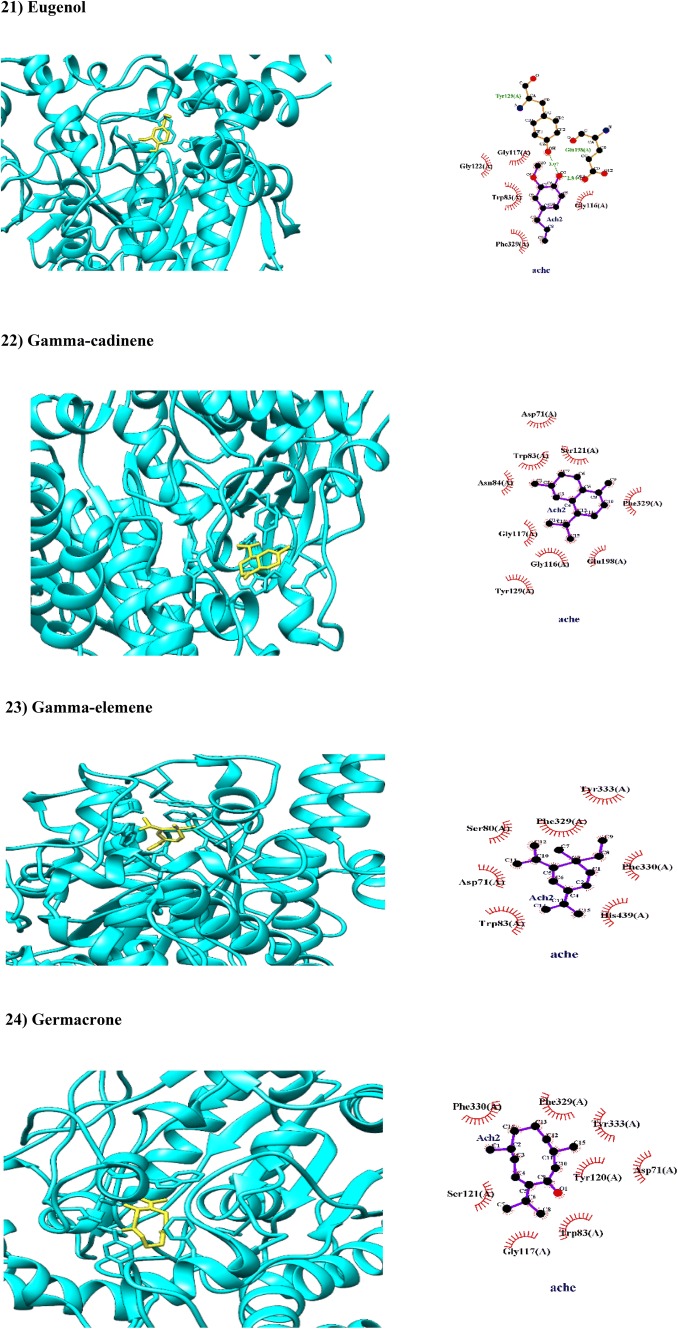
| Eugenol | 0.8736 | 0.9832 | Non-substrate/non-inhibitor | Mitochondria | Weak |
| Gamma-cadinene | 0.9304 | 0.9968 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Gamma-elemene | 0.9609 | 0.9898 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Germacrone | 0.9300 | 1.0000 | Non-substrate/non-inhibitor | Mitochondria | Weak |
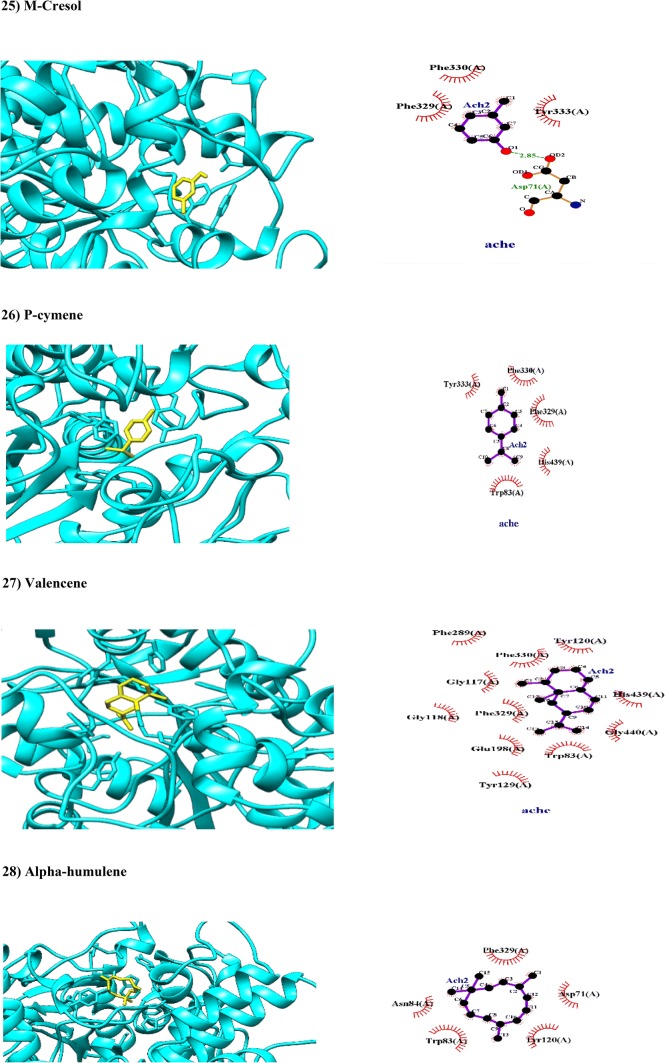
| M-cresol | 0.8911 | 0.9960 | Non-substrate/non-inhibitor | Mitochondria | Weak |
| P-cymene | 0.9677 | 0.9960 | Non-substrate/non-inhibitor | Lysosome | Weak |
| Valencene | 0.9796 | 0.9961 | Non-substrate/non-inhibitor | Lysosome | Weak |
| α-Humulene | 0.9733 | 0.9972 | Non-substrate/non-inhibitor | Lysosome | Weak |
| α-Pinene | 0.8959 | 0.9964 | Non-substrate/non-inhibitor | Lysosome | Weak |
| β-Selinene | 0.9732 | 0.9947 | Non-substrate/non-inhibitor | Lysosome | Weak |
BBB blood brain barrier, HIA human intestinal absorption, CYP2C9 cytochrome P450, HERG Human Ether-a-go-go-related gene
Alloaromadendrene belongs to terpenoids (Jesionek et al. 2018). In present study, alloaromadendrene has been hydrophobically docked with AChE with BA of − 9.1 kcal/mol through Phe329 residue which involved in hydrophobic interactions of anti-Alzheimer E2020 (Aricept) drug with AChE (1EV1; Kryger et al. 1999; Fig. 2). The alloaromadendrene, humulene, and caryophyllene (vide infra) are active sesquiterpenoid compounds against Southeast Asian termites (Neotermes spp.; Messer et al. 1990). Alloaromadendrene has accepted ADMET properties to be considered as lead-like compound, however experimental data about its biological activities are limited (Table 1).
Alpha-bergamotene belongs to bicyclic monoterpenoids found in many plants like Zingiber officinale and C. myrrha (Tung et al. 2017). It has been docked with AChE with BA of − 7.8 kcal/mol through hydrophobic interactions with amino acid residues in AP (Phe289 and Phe330) and PAS (Tyr333 and Tyr120; Fig. 2). More surprisingly, alpha-bergamotene also hydrophobically interacts with His439 residue which involved in hydrophobic interactions of anti-Alzheimer E2020 (Aricept) drug with AChE (1EV1; Kryger et al. 1999; Fig. 2). Alpha-bergamotene has been interacted with both PAS and catalytic site as a bifunctional agent. The ADMET properties of alpha-bergamotene showed that it can be transferred through BBB and orally absorbed from GIT while it is weak inhibitor of HERG (Table 1). To the best of our knowledge, there is no information about its biological and pharmacological effects of alpha-bergamotene.
Alpha- and beta-selinene are sesquiterpenoid compounds in C. myrrha (Gitau 2015). Our results showed that, α- and β-selinene have been hydrophobically docked with AChE with BA of − 8.7 and − 9.1 kcal/mol, respectively. In this line, alpha-selinene has been docked with AChE through hydrophobic interactions with amino acid residues of PAS (Tyr333) and AP (Phe330), OH (Gly117) and catalytic AS (Trp83). More interestingly, alpha-selinene interacted hydrophobically with two cardinal amino acid residues which are involving in acylation site of AChE (His439 and Ser199; Haviv et al. 2007). Beta-selinene has hydrophobic interactions with amino acid residues in both PAS (Tyr333 and Asp71) and acylation site (Phe330 and His439). Alpha- and beta-selinene can be considered as bivalent inhibitors of AChE. Based on ADMET results, these two isomers can be absorbed from GIT and crossed BBB (Table 1).
Alpha-terpineol is a monocyclic monoterpene alcohol found in the essential oils of several resiniferous plant species as C. myrrha and Boswellia ameero (Table 1; Murthy et al. 2016). In a seminal review, various biological effects of alpha-terpineol have been reviewed (Khaleel et al. 2018). Alpha-terpineol has been docked with AChE with BA − 7 kcal/mol via hydrogen bonding with Tyr120 and several hydrophobic interactions with amino acid residues of PAS (Tyr333 and Asp71) and catalytic triad (Trp83 and His439; Fig. 2). Insect repellency and insecticidal activity of alpha-terpineol have been reported previously (Liu et al. 2013). Based on ADMET results, alpha-terpineol can be absorbed from GIT and crossed BBB (Table 1).
Beta-eudesmol is a sesquiterpenoid alcohol found in C. myrrha. This study showed that beta-eudesmol has been docked with BA of − 8.9 kcal/mol through several hydrophobic interactions with PAS (Tyr120, Tyr278, Tyr 333, Tyr69 and Asp71; Fig. 2) and AP (Phe289; Fig. 2). Based on ADMET properties, beta-eudesmol can be absorbed from GIT and crossed BBB (Table 1), however, it inhibits CYP2C9 and HERG (Table 1). It has been reported that like phencyclidine, beta-eudesmol blocked the nicotinic ACh receptor channel in both open and closed conformations, and accelerated the desensitization of the nicotinic ACh receptor (Kimura et al. 1991), therefore more studies are requested to clarify activity of beta-eudesmol in cholinergic systems.
Alpha- and beta-copaene are tricyclic sesquiterpenes found in volatile organic contents of C. myrrha and Artemisia (Jyotshna et al. 2015). This study showed that alpha- and beta-copaene have been docked with AChE with BA of − 8.8 and − 9.1 kcal/mol, respectively. In this regard, alpha-copaene interacted hydrophobically with amino acid residues in catalytic triad (Trp83, Gly117 and His439, Tyr333) and PAS (Asp71 and Tyr333) while beta-copaene interacted hydrophobically with amino acid residues of PAS (Tyr120, Tyr333, Asp71, Phe329 and Phe330) and solely with Trp83 residues in catalytic site (Fig. 2). Our ADMET assays showed that alpha-copaene and beta-copaene can be absorbed orally and crossed BBB while they are week inhibitor of HERG (Table 1).
Beta-, delta- and gamma-elemene are assumed anti-neoplastic sesquiterpenoids found in an array of medicinal plants like C. myrrha (Wang et al. 2012). In present study, beta-, delta- and gamma-elemene have been docked with AChE with BA of − 8.4, − 8.4, − 8.8 kcal/mol, respectively. Beta-elemene has hydrophobic interactions with amino acid residues in catalytic triad (His439, Trp83, Phe329 and Tyr333) and PAS (Asp71, Trp83, Ser80, Tyr120, Phe329 and Tyr333; Fig. 2). Delta-elemene has also hydrophobic interactions with amino acid residues in catalytic acylation site (Ser199 and His439), AP (Phe330), AS (Trp83), OH (Gly117) and PAS (Tyr333 and Tyr120; Fig. 2). Gamma-elemene has hydrophobic interactions with amino acid residues in catalytic triad (Trp83, His439 and Phe330) and PAS (Tyr333 and Asp71; Fig. 2). In sum, all three structural isomers of elemenes reported here employed His439 residue to interact with AChE and this interesting finding suggests their applications in aromatherapy for AD. In consistent with experimental data (Shamsizadeh et al. 2017), our ADMET prediction showed that elemenes can be crossed from GIT and BBB (Table 1).
Bicyclogermacrene is a sesquiterpenoid found in C. myrrha. This study also showed that it produced acceptable BA of − 8.7 kcal/mol with AChE through hydrophobic interactions in AP (Phe330) and AS (Trp83) and PAS (Asp71, Tyr120 and Tyr333). Bicyclogermacrene also interacted hydrophobically with Phe329 which is an aromatic residue at the midpoint of the gorge acts as swinging gate in the catalytic site of AChE (Kryger et al. 1999). Based on ADMET properties, bicyclogermacrene is suitable in the evaluation of drug-likeness standard where it can be crossed GIT and BBB (Table 1). This is the first report that computationally mentions to biological activity of bicyclogermacrene.
Beta-caryophyllene also known as dietary phytocannabinoid is a sesquiterpene found in essential oils of many plants such as C. myrrha and Cannabis sativa L. Previous experimental studies have been reported that essential oil containing beta-caryophyllene has an anti-AChE effect (e.g., Yu et al. 2011). Beta-caryophyllene has been docked with AChE via hydrophobic interactions with suitable BA of − 8.6 kcal/mol; Table 2). It has several hydrophobic interactions with amino acid residues of AP (Phe330) and PAS (Tyr333, Asp71 and Tyr120) and catalytic anionic subsite (Trp83; Fig. 2) as a bifunctional inhibitor. In a seminal work, anti-AChE and anti-beta-secretase activities of beta-caryophyllene has been reported previously (Murata et al. 2015). Beta-caryophyllene can cross GIT and BBB based on our computational evidences (Table 1) and experimental findings (Murata et al. 2015).
Table 2.
In silico molecular docking of phytochemicals of Commiphora myrrha (Nees) against AChE (PDB code 1EVE)
| Ligand | Binding affinity (kcal/mol) | RMSD/upper bound | RMSD/lower bound |
|---|---|---|---|
| Abietic acid | − 10.2 | 0.807 | 0.793 |
| Alloaromadendrene | − 9.1 | 4.912 | 1.104 |
| Alpha-selinene | − 8.7 | 2.827 | 1.848 |
| Alpha-terpineol | − 7.0 | 4.935 | 2.472 |
| Beta-eudesmol | − 8.9 | 6.674 | 3.419 |
| Beta-copaene | − 9.1 | 5.147 | 1.052 |
| Beta-elemene | − 8.4 | 5.358 | 1.649 |
| Bicyclogermacrene | − 8.7 | 4.612 | 1.590 |
| Caryophyllene | − 8.6 | 4.242 | 1.146 |
| Cedrelanol | − 8.5 | 4.739 | 2.126 |
| Alpha-copaene | − 8.8 | 3.949 | 2.153 |
| Cuminaldehyde | − 7.2 | 2.877 | 2.417 |
| Cuscohygrine | − 7.4 | 6.079 | 0.290 |
| Delta-cadinene | − 9.2 | 4.041 | 0.869 |
| Delta-elemene | − 8.4 | 4.955 | 1.543 |
| Dipentene (limonene) | − 7.1 | 1.985 | 0.422 |
| Elemol | − 8.4 | 2.095 | 1.527 |
| Eugenol | − 6.7 | 5.484 | 4.016 |
| Gamma-cadinene | − 8.6 | 4.256 | 1.736 |
| Gamma-elemene | − 8.8 | 5.566 | 1.669 |
| Germacrone | − 9.3 | 3.229 | 1.670 |
| m-cresol | − 5.8 | 2.386 | 0.649 |
| p-cymene | − 7.5 | 1.537 | 0.084 |
| Valencene | − 8.7 | 2.720 | 1.821 |
| Alpha-humulene | − 9.0 | 4.558 | 1.410 |
| Alpha-pinene | − 6.6 | 4.202 | 1.947 |
| Beta-selinene | − 9.1 | 5.311 | 1.464 |
| Alpha-bergamotene | − 7.8 | 5.508 | 1.797 |
RMSD root mean-square deviation is the measure of the average distance between the atoms
Cedrelanol also known as T-cadinol belongs to sesquiterpenes. The present study showed that cedrelanol has been docked with an acceptable BA of − 8.5 kcal/mol to amino acid residues of PAS (Tyr120, Asp71 and Tyr333) and catalytic triad (Trp83, His439 and Phe330; Fig. 2), through hydrophobic interactions. Moreover, ADMET assessment showed that cedrelanol can cross GIT and BBB (Table 1). The antimicrobial (Claeson et al. 1992) and inhibitory effect on cholera toxin-induced intestinal hypersecretion (Claeson et al. 1991) of the sesquiterpene T-cadinol isolated from scented myrrh (resin of Commiphora guidottii Chiov., Burseraceae) were reported.
Cuminaldehyde or 4-isopropylbenzaldehyde is found mainly in cumin (Cuminum cyminum L.; Ebada 2017) and C. myrrha. It has inhibitory effect on alpha-synuclein which gathered in Lewy bodies of sporadic PD and dementia (Morshedi et al. 2015). This study showed that cuminaldehyde has been docked with AChE in both catalytic triad (Phe330, Trp83 and His439) and PAS (Tyr333) with BA of − 7.2 kcal/mol through hydrophobic interactions (Fig. 2). Based on ADMET properties, cuminaldehyde has ability to penetrate BBB and cross GIT while it weak HERG inhibitor (Table 1).
Cuscohygrine is a pyrrolidine alkaloid found in several plants such as C. myrrha and coca leaves (Rubio et al. 2017). An in silico study showed that cuscohygrine found in Withania somnifera can act as nicotinic ACh receptor agonist and may mediate anti-AD effects of Withania somnifera based formulation in traditional ayurvedic medicines (Remya et al. 2016). In this study, cuscohygrine has a BA of − 7.4 kcal/mol with AChE through two hydrogen bonds with amino acid Tyr120 in PAS with a bond length of 3.29 and 3.13 Å (Fig. 2) and through hydrophobic interactions with (Tyr333 and Asp71). Moreover, several amino acid residues of AChE took part in hydrophobic interactions of catalytic triad including AS (Trp83), AP (Phe330), OH (Gly117) and acylation site (His439 and Ser199) with cuscohygrine (Fig. 2). Therefore, cuscohygrine can be proposed as a bivalent ligand of AChE. Based on ADMET properties, cuscohygrine has ability to penetrate BBB and cross GIT while it weak HERG inhibitor (Table 1).
Delta- and gamma-cadinene are bicyclic sesquiterpenes found in essential oils of many plants such as C. myrrha and Monodora myristica (Owokotomo et al. 2015). Present study showed that delta- and gamma-cadinene have been hydrophobically docked with BA of − 9.2, − 8.6 kcal/mol, respectively. In this regard, delta-cadinene interacted with amino acid residues in catalytic triad (Trp83, His439, Phe330) and PAS (Tyr333, Asp71) of AChE, while gamma-cadinene interacted with amino acid residues of OH (Gly117), AS (Trp83), acylation site (Glu198) in catalytic triad and Asp71 in PAS of AChE (Fig. 2). These two isomers can be absorbed from GIT and transported across BBB (Table 1).
Dipentene or limonene is a stable monocyclic monoterpenoid found in various volatile oils of plants like Citrus limon (L.) Burm (Zahi et al. 2015) and C. myrrha. Both in vitro (Zahi et al. 2015) and in silico (Ali et al. 2017) investigations reported antibacterial effects of limonene based products. In the present study, limonene has been docked with catalytic triad (His439, Phe330 and Trp83) and PAS (Asp71 and Tyr333) of AChE via hydrophobic interactions (Fig. 2) with moderate BA of -7.1 kcal/mol (Table 1). Limonene has potential as a nontoxic, natural pesticide for insect pests on tolerant plants (Hollingsworth 2005). Dipentene is a weak HERG inhibitor which distributed in lysosome and can be crossed GIT and BBB (Table 1).
Elemol is sesquiterpene alcohol found in essential oil of various plants like Mentha species (Odimegwu et al. 2013). Data from previous reports confirm that elemol has anti-AChE activity (Miyazawa et al. 1998) with unknown mechanism. In present study, elemol has been docked with AChE (BA of − 8.4 kcal/mol) through hydrogen bonds with hydroxyl group of Ser121 and through hydrophobic interactions with PAS (Tyr333, Asp71 and Tyr120; Fig. 2) and OH of catalytic triad (Gly117 and Gly118; Fig. 2) and AP (Phe330; Fig. 2) and catalytic anionic subsite (Trp83; Fig. 2). Elemol can be absorbed from GIT, crossed BBB, and localized in lysosome while it is a week inhibitor of HERG (Table 1).
Eugenol is a monoterpenoid with various therapeutic effects (Pramod et al. 2010) found in C. myrrha. In this study, Tyr129 and Glu198 as crucial amino acid residue in acylation site of AChE interacted with eugenol via hydrogen bindings with length 3.07 and 2.8 Å while Gly117 and Trp83 residues in catalytic triad interacted hydrophobically with eugenol (BA − 6.7 kcal/mol; Fig. 2). In consistent with our in silico finding, experimental findings showed anti-ChE activity of eugenol (Dohi et al. 2009). Eugenol can be absorbed from GIT, crossed BBB, and localized in mitochondria while it is a week inhibitor of HERG (Table 1).
Germacrone is a sesquiterpenoid found in C. myrrha. In this study, germacrone has been hydrophobically docked with amino acid residues in PAS (Tyr120, Asp71 and Tyr333) and catalytic triad (Trp83, His439 and Phe330) of AChE with reliable BA of − 9.3 kcal/mol. Germacrone can be absorbed from GIT, crossed BBB, and localized in mitochondria while it is a week inhibitor of HERG (Table 1). In contrast to our in silico finding, anti-ChE activity of germacrone was not approved in experimental investigation (Haritakun et al. 2016).
M-cresol belong to methylphenols (Mogey and Young 1949). In present study, m-cresol has been loosely docked with AChE (BA − 5.8 kcal/mol; Table 2). The Asp71 residue of PAS interacted with m-cresol with hydrogen bond while Tyr333 residue of PAS and Phe330 residue of AP interacted hydrophobically with m-cresol (Fig. 2). The ADMET properties showed m-cresol can be crossed BBB and GIT and distributed into mitochondria (Table 1). Due to its low molecular weight and its tendency to dock with AChE, m-cresol would be a good “hit” to synthetize potent inhibitors of AChE.
P-cymene is a monoterpene possesses anti-hyperalgesic and anti-inflammatory properties (de Santana et al. 2015). In present study, p-cymene has been docked with AChE in both PAS (Tyr333) and catalytic triad (His439, Phe330 and Trp83) with BA of − 7.5 kcal/mol through hydrophobic interactions (Fig. 2). The ADMET properties of p-cymene showed that it can be absorbed from GIT and transferred through BBB while it tends to be localized in lysosome (Table 1).
Valencene is a sesquiterpene hydrocarbon found in C. myrrha and orange fruits (Sharon-Asa et al. 2003). In present study, valencene has been docked with AChE with BA (− 8.7 kcal/mol; Table 2) in catalytic site including OH (Gly117 and Gly118), AP (Phe289), AS (Trp83), and catalytic acylation site (Glu198 and His439) through hydrophobic interactions (Fig. 2). Based on ADMET properties, valencene has ability to pass BBB and GIT (Table 1).
Alpha-humulene is a sesquiterpene found in C. myrrha and medicinal plants like Humulus lupulus, Cannabis sativa, and Ginseng (Gitau 2015). In a pioneered work, it has been reported that alpha-humulene contained herbal extracts have insecticidal effects through inhibition of AChE (Lee and Ahn 2013). In this study, alpha-humulene has been docked with more acceptable BA (− 9.0 kcal/mol) through hydrophobic interactions with amino acid residues in PAS (Tyr120 and Asp71) and catalytic triad (Trp83) involved in AChE (Fig. 2). Based on ADMET properties, alpha-humulene has ability to pass BBB and GIT (Table 1).
Alpha-pinene is a monoterpenoid possesses anti-inflammatory, anti-nociceptive and anti-AChE activities (Him et al. 2008; Picollo et al. 2008; Zarred et al. 2017). In consistent to our findings, alpha-pinene has been docked with AChE with BA of − 6.6 kcal/mol via hydrophobic interactions in PAS (Tyr333, Tyr120) and AP (Phe330). More specifically, alpha-pinene also interacted with His439 residue in catalytic or acylation site of AChE (Fig. 2), therefore it would be considered as a bivalent inhibitor of AChE. Based on ADMET properties, alpha-pinene can be absorbed orally and passed through BBB without considerable side effects (Table 1).
Conclusions
Myrrh has been used in Mesopotamia as fragrance and antiinsectant as confirmed by our in vitro findings. Computationally, compounds which dock with AChE have a potential to be considered as nootropics or antiinsectans. The majority compounds in C. myrrha showed hydrophobic interactions with amino acid residues that belong to both catalytic triad and PAS of AChE, therefore as bivalent ligands may be candidates for preclinical investigations of translational model of AD or amnesia and/or examined for their insecticidal potentials. In this regard, abietic acid, germacrone, cadinene, alloaromadendrene, beta-copaene, beta-selinene, and alpha-humulene showed BA ≤ − 9 kcal/mol. Beta-eudesmol, alpha-copaene, gamma-elemene, alpha-selinene, bicyclogermacrene, valencene, caryophyllene, gamma-cadinene, cedrelanol, beta-elemene, delta-elemene, elemol showed − 9.0 < BA ≤ − 8.4 kcal/mol. Alpha-bergamotene, p-cymene, cuminaldehyde, dipentene, alpha-terpineol, eugenol, alpha-pinene, m-cresol showed − 8.0 < BA ≤ − 5.8 kcal/mol. All compounds reported here showed acceptable ADMET while they were weak inhibitor of HERG therefore may lead to cardiotoxicity in large doses. In this essence, abietic acid and beta-eudesmol showed CYP inhibition. To sum up, our results computationally approved traditional application of C. myrrha as antiinsectant or nootropic agent, however more in silico and/or experimental studies are requested to achieve more evidences.
Acknowledgements
This paper emanates from MSc thesis of first author submitted to Department of Biology, Faculty of Science, Razi University 67149-67346, Kermanshah, Iran. This study was supported by intramural fund and first and second authors paid the fee of in silico investigation.
Author contributions
BAH and NY gathered data of Mesopotamian Medicine, authenticated plants, and carried out the experiments. BAH and IK analyzed data and carried out the molecular docking work. BAH and IK prepared the manuscript while all authors have read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali SE, Chehri K, Karimi N, Karimi I. In vitro antibacterial activity of Citrus limon (L.) Burm against gentamicin-resistant Escherichia coli complemented with in silico molecular docking of its major phytochemicals with ribosome recycling factor. Basic Appl Pharm Pharmacol. 2017;1(1):1–6. [Google Scholar]
- Ben-Yehoshua S, Borowitz C, Hanuš LO. Spices: frankincense, myrrh, and balm of Gilead: ancient spices of Southern Arabia and Judea. In: Janick J, editor. Horticultural Reviews. New York, USA: Wiley; 2012. pp. 45–50. [Google Scholar]
- Berman HM, Battistuz T, Bhat TN, Bluhm WF, Bourne PE, Burkhardt K, Fagan P. The protein data bank. Acta Crystallogr D Biol Crystallogr. 2002;58:899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- Bertman S. Handbook to life in Ancient mesopotamia. New York: Infobase Publishing History; 2003. pp. 258–305. [Google Scholar]
- Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G, Lee PW, Tang Y. Admetsar: a comprehensive source and free tool for assessment of chemical admet properties. J Chem Inf Model. 2012;52:3099–3105. doi: 10.1021/ci300367a. [DOI] [PubMed] [Google Scholar]
- Claeson P, Andersson R, Samuelsson G. T-cadinol: a pharmacologically active constituent of scented myrrh: introductory pharmacological characterization and high field 1H- and 13C-NMR data. Planta Med. 1991;57:352–356. doi: 10.1055/s-2006-960116. [DOI] [PubMed] [Google Scholar]
- Claeson P, Rådström P, Sköld O, Nilsson Å, Höglund S. Bactericidal effect of the sesquiterpene T-cadinol on Staphylococcus aureus. Phytother Res. 1992;6:94–98. [Google Scholar]
- Čolović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol. 2015;1263:243–450. doi: 10.1007/978-1-4939-2269-7_19. [DOI] [PubMed] [Google Scholar]
- de Santana MF, Guimarães AG, Chaves DO, Silva JC, Bonjardim LR, de Lucca Júnior W, Ferro JN, Barreto Ede O, dos Santos FE, Soares MB, Villarreal CF, Quintans Jde S, Quintans-Júnior LJ. The anti-hyperalgesic and anti-inflammatory profiles of p-cymene: evidence for the involvement of opioid system and cytokines. Pharma Biol. 2015;53:1583–1590. doi: 10.3109/13880209.2014.993040. [DOI] [PubMed] [Google Scholar]
- Dohi S, Terasaki M, Makino M. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J Agric Food Chem. 2009;57:4313–4318. doi: 10.1021/jf804013j. [DOI] [PubMed] [Google Scholar]
- Ebada ME. Cuminaldehyde: a potential drug candidate. J Pharmacol Clin Res. 2017;2:555–585. [Google Scholar]
- El Ashry ES, Rashed N, Salama OM, Saleh A. Components, therapeutic value and uses of myrrh. Pharmazie. 2003;58:163–168. [PubMed] [Google Scholar]
- Ellman GL, Courtney DK, Andreas V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Gitau WJ (2015) Evaluation of the composition, physico-chemical characteristics, surfactant and anti-microbial potential of Commiphora abyssinica gum resin. Doctoral dissertation, University of Nairobi
- Hanuš LO, Řezanka T, Dembitsky VM, Moussaieff A. Myrrh—commiphora chemistry. Biomed Papers. 2005;149:3–28. doi: 10.5507/bp.2005.001. [DOI] [PubMed] [Google Scholar]
- Haritakun W, Taworn W, Suebsakwong P. Acetylcholinesterase inhibitory activity of a sesquiterpenoid from Curcuma aromatic rhizomes. SDU Res J. 2016;9:51–61. [Google Scholar]
- Haviv H, Wong DM, Silman I, Sussman JL. Bivalent ligands derived from Huperzine A as acetylcholinesterase inhibitors. Curr Top Med Chem. 2007;7:375–387. doi: 10.2174/156802607779941215. [DOI] [PubMed] [Google Scholar]
- Him A, Özbek H, Turel I. Antinociceptive activity of alpha-pinene and fenchone. PhOL. 2008;3:363–369. [Google Scholar]
- Hollingsworth RG. Limonene, a citrus extract, for control of mealybugs and scale insects. J Econ Entomol. 2005;98:772–779. doi: 10.1603/0022-0493-98.3.772. [DOI] [PubMed] [Google Scholar]
- Holzgrabe U, Kapková P, Alptüzün V, Scheiber J, Kugelmann E. Targeting acetylcholinesterase to treat neurodegeneration. Expert Opin Ther Targets. 2007;11:161–179. doi: 10.1517/14728222.11.2.161. [DOI] [PubMed] [Google Scholar]
- Houghton Peter J, Ren Yuhao, Howes Melanie-Jayne. Acetylcholinesterase inhibitors from plants and fungi. Nat Prod Rep. 2006;2:181–199. doi: 10.1039/b508966m. [DOI] [PubMed] [Google Scholar]
- Jesionek A, Poblocka-Olech L, Zabiegala B, Bucinski A, Krauze-Baranowska M, Luczkiewicz M. Validated HPTLC method for determination of ledol and alloaromadendrene in the essential oil fractions of Rhododendron tomentosum plants and in vitro cultures and bioautography for their activity screening. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1086:63–72. doi: 10.1016/j.jchromb.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Johnson G, Moore SW. The peripheral anionic site of acetylcholinesterase: structure, functions and potential role in rational drug design. Curr Pharm Des. 2006;12:217–225. doi: 10.2174/138161206775193127. [DOI] [PubMed] [Google Scholar]
- Jyotshna Srivastava N, Singh B, Chanda D, Shanker K. Chemical composition and acetylcholinesterase inhibitory activity of Artemisia maderaspatana essential oil. Pharm Biol. 2015;53:1677–1683. doi: 10.3109/13880209.2014.1001405. [DOI] [PubMed] [Google Scholar]
- Karimi I, Zahraminoosh SH, Najafi A, Becker LA. Nootropic effects of quince leaf (Cydonia oblonga miller.) decoct in mice: a neurobehavioral approach complemented with kinetics and molecular docking studies of encephalic acetyl cholinesterase inhibition. J Bioinfo Proteomics Rev. 2017;3:1–7. [Google Scholar]
- Khaleel C, Tabanca N, Buchbauer G. α-Terpineol, a natural monoterpene: a review of its biological properties. Open Chem. 2018;16:349–361. [Google Scholar]
- Kimura M, Nojima H, Muroi M, Kimura I. Mechanism of the blocking action of beta-eudesmol on the nicotinic acetylcholine receptor channel in mouse skeletal muscles. Neuropharmacology. 1991;30(8):835–841. doi: 10.1016/0028-3908(91)90117-t. [DOI] [PubMed] [Google Scholar]
- Kryger G, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure. 1999;7:297–307. doi: 10.1016/s0969-2126(99)80040-9. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Swindells MB. Ligplot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Lee DC, Ahn YJ. Laboratory and simulated field bioassays to evaluate larvicidal activity of Pinus densiflora hydrodistillate, its constituents and structurally related compounds against Aedes albopictus, Aedes aegypti and Culex pipiens pallens in relation to their inhibitory effects on acetylcholinesterase activity. Insects. 2013;4:217–229. doi: 10.3390/insects4020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XC, Li YP, Li HQ, Deng ZW, Zhou L, Liu ZL, Du SS. Identification of repellent and insecticidal constituents of the essential oil of Artemisia rupestris L. aerial parts against Liposcelis bostrychophila Badonnel. Molecules. 2013;18:10733–10746. doi: 10.3390/molecules180910733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer A, McCormick K, Sunjaya, Hagedorn HH, Tumbel F, Meinwald J. Defensive role of tropical tree resins: antitermitic sesquiterpenes from Southeast Asian Dipterocarpaceae. J Chem Ecol. 1990;16:3333–3352. doi: 10.1007/BF00982102. [DOI] [PubMed] [Google Scholar]
- Miyazawa M, Watanabe H, Umemoto K, Kameoka H. Inhibition of acetylcholinesterase activity by essential oils of Mentha species. J Agri Food Chem. 1998;46:3431–3434. [Google Scholar]
- Mogey GA, Young PA. The antagonism of curarizing activity by phenolic substances. Br J Pharmacol Chemother. 1949;4:359–365. doi: 10.1111/j.1476-5381.1949.tb00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedi D, Aliakbari F, Tayaranian-Marvian A, Fassihi A, Pan-Montojo F, Pérez-Sánchez H. Cuminaldehyde as the major component of Cuminum cyminum, a natural aldehyde with inhibitory effect on alpha-synuclein fibrillation and cytotoxicity. J Food Sci. 2015;80(10):H2336–H2345. doi: 10.1111/1750-3841.13016. [DOI] [PubMed] [Google Scholar]
- Murata K, Matsumura S, Yoshioka Y, Ueno Y, Matsuda H. Screening of β-secretase and acetylcholinesterase inhibitors from plant resources. J Nat Med. 2015;69:123–129. doi: 10.1007/s11418-014-0859-3. [DOI] [PubMed] [Google Scholar]
- Murthy KSR, Reddy MC, Rani SS, Pullaiah T. Bioactive principles and biological properties of essential oils of Burseraceae: a review. J Pharmacogn Phytochem. 2016;5:247–258. [Google Scholar]
- Odimegwu JI, Odukoya O, Yadav RK, Chanotiya CS, Ogbonnia S, Sangwan NS. A new source of elemol rich essential oil and existence of multicellular oil glands in leaves of the Dioscorea species. Sci World J. 2013 doi: 10.1155/2013/943598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordentlich A, Barak D, Kronman C, Ariel N, Segall Y, Velan B, Shafferman A. Functional characteristics of the oxyanion hole in human acetylcholinesterase. J Biol Chem. 1998;273:19509–19517. doi: 10.1074/jbc.273.31.19509. [DOI] [PubMed] [Google Scholar]
- Owokotomo IA, Ekundayo O, Abayomi TG, Chukwuka AV. In-vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxicol Rep. 2015;2:850–857. doi: 10.1016/j.toxrep.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory. Learn Memory. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picollo MI, Toloza AC, Cueto GM, Zygadlo J, Zerba E. Anticholinesterase and pediculicidal activities of monoterpenoids. Fitoterapia. 2008;79(4):271–278. doi: 10.1016/j.fitote.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Pramod K, Ansari SH, Ali J. Eugenol: a natural compound with versatile pharmacological actions. Nat Prod Commun. 2010;5(12):1999–2006. [PubMed] [Google Scholar]
- Ramnath MG, Thirugnanasampandan R, Sadasivam M, Mohan PS. Antioxidant, antibacterial and antiacetylcholinesterase activities of abietic acid from Isodon wightii (Bentham) H. Hara. Free Rad Antiox. 2015;5:1–5. [Google Scholar]
- Remya C, Dileep KV, Variayr EJ, Sadasivan C. An in silico guided identification of nAChR agonists from Withania somnifera. Front Life Sci. 2016;9(3):201–213. [Google Scholar]
- Rubio NC, Thurmann D, Krumbiegel F, Pragst F. Behaviour of hygrine and cuscohygrine in illicit cocaine production establishes their use as markers for chewing coca leaves in contrast with cocaine abuse. Drug Test Anal. 2017;9(2):323–326. doi: 10.1002/dta.1972. [DOI] [PubMed] [Google Scholar]
- Shamsizadeh A, Roohbakhsh A, Ayoobi F, Moghaddamahmadi A (2017) The Role of natural products in the prevention and treatment of multiple sclerosis. In: Nutrition and Lifestyle in Neurological Autoimmune Diseases Multiple Sclerosis. Academic Press, pp 249–260
- Sharon-Asa L, Shalit M, Frydman A, Bar E, Holland D, Or E, Lavi U, Lewinsohn E, Eyal Y. Citrus fruit flavor and aroma biosynthesis: isolation, functional characterization, and developmental regulation of Cstps1, a key gene in the production of the sesquiterpene aroma compound Valencene. Plant J. 2003;36(5):664–674. doi: 10.1046/j.1365-313x.2003.01910.x. [DOI] [PubMed] [Google Scholar]
- Singh KD, Labala RK, Devi TB, Singh NI, Chanu HD, Sougrakpam S, Nameirakpam BS, Sahoo D, Rajashekar Y (2017) Biochemical efficacy, molecular docking and inhibitory effect of 2, 3-dimethylmaleic anhydride on insect acetylcholinesterase. Sci Rep 7, Article number: 12483 [DOI] [PMC free article] [PubMed]
- Suganthy N, Pandian SK, Devi KP. Cholinesterase inhibitors from plants: possible treatment strategy for neurological disorders-a review. Int J Biomed Pharmaceut Sci. 2009;3:87–103. [Google Scholar]
- Thomsen R, Christensen MH. Moldock: a new technique for high-accuracy molecular docking. J Med Chem. 2006;49(11):3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- Tung BT, Thu DK, Thu NTK, Hai NT. Antioxidant and acetyl-cholinesterase inhibitory activities of ginger root (Zingiber officinale Roscoe) extract. J Complement Integr Med. 2017 doi: 10.1515/jcim-2016-0116. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhao Z, Yun-ting S, Zeng Z, Zhan X, Li C, Xie T. A review of medicinal plant species with elemene in China. Af J Pharm Pharmacol. 2012;6(44):3032–3040. [Google Scholar]
- Watt M, Sellar W. Frankincense and myrrh: through the ages, and a complete guide to their use in herbalism and aromatherapy today. Saffron Walden: C. W. Daniel Co.; 1996. [Google Scholar]
- Xie Y, Isman MB, Feng Y, Wong A. Diterpene resin acids: major active principles in tall oil against Variegated cutworm, Peridroma saucia (Lepidoptera: Noctuidae) J Chem Ecol. 1993;19(6):1075–1084. doi: 10.1007/BF00987370. [DOI] [PubMed] [Google Scholar]
- Yu Z, Wang B, Yang F, Sun Q, Yang Z, Zhu L. Chemical composition and anti-acetyl cholinesterase ctivity of flower essential oils of Artemisia annua at different flowering stage. Iran J Pharm Res. 2011;10(2):265–271. [PMC free article] [PubMed] [Google Scholar]
- Zahi MR, Liang H, Yuan Q. Improving the antimicrobial activity of d-limonene using a novel organogel-based nanoemulsion. Food Control. 2015;50:554–559. [Google Scholar]
- Zarred K, Laarif A, Ben Hamouda A, Chaieb I, Mediouni-Ben Jemaa J. Anticholinesterase Potential of monoterpenoids on the whitefly Bemisia tabaci and their kinetic studies. JAST. 2017;19(3):643–652. [Google Scholar]