Abstract
Sparse labeling of neurons contributes to uncovering their morphology, and rapid expression of a fluorescent protein reduces the experiment range. To achieve the goal of rapid and sparse labeling of neurons in vivo, we established a rapid method for depicting the fine structure of neurons at 24 h post-infection based on a mutant virus-like particle of Semliki Forest virus. Approximately 0.014 fluorescent focus-forming units of the mutant virus-like particle transferred enhanced green fluorescent protein into neurons in vivo, and its affinity for neurons in vivo was stronger than for neurons in vitro and BHK21 (baby hamster kidney) cells. Collectively, the mutant virus-like particle provides a robust and convenient way to reveal the fine structure of neurons and is expected to be a helper virus for combining with other tools to determine their connectivity. Our work adds a new tool to the approaches for rapid and sparse labeling of neurons in vivo.
Electronic supplementary material
The online version of this article (10.1007/s12264-019-00362-z) contains supplementary material, which is available to authorized users.
Keywords: Semliki Forest virus, Mutant virus-like particle, Rapid labeling, Sparse labeling, Neuronal morphology
Introduction
A single neuron can project long-range from one region to other regions in the brain, which receives orders and sends information to execute different tasks [1–3]. The morphology and connectivity of neurons are important foundations of neuronal function. A tool for sparse labeling of neurons is a prerequisite to map their morphology, and this can contribute to elucidating their working mechanisms and discovering new functions. To achieve this goal, several labeling technologies have been developed, such as transgenic animals [4–7] and virus-based approaches [8–11]. These tools have clearly promoted the development of neuroscience [12–16]. However, animal-based methods are time-consuming and labor-intensive. Adeno-associated virus (AAV)-based tools are convenient approaches, but AAV can only hold < 4.5 kb of a foreign gene and needs a long time for protein expression [17]. Lentivirus also needs a long time for protein expression [18, 19]. Therefore, new tools with the properties of easy preparation, less time for protein expression, and a high cloning capacity are still needed in the neuroscience community.
To achieve these aims, it has been found that the mutant virus-like particle (VLP) of Semliki Forest virus (SFV) might be a candidate. The SFV is a positive-strand RNA virus, which belongs to the Alphavirus genus of the Togaviridae family. SFV can infect neurons and cause encephalitis [20]. The vectors based on SFV, such as VLP [21, 22], have been developed to rapidly express foreign genes at high levels in vitro and in vivo [22–28]. In addition, the insertion capacity of the vector is up to 6.5 kb [26].
Here, we found that mutant VLP rapidly and sparsely labeled neurons in vivo, and visualized the fine structure of neurons in a sparse manner at 24 h post-infection based on the virus. Our work adds a new tool to the approaches for rapid and sparse labeling of neurons in vivo.
Materials and Methods
All procedures in this study were approved by the Animal Care and Use Committee at the Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences. All the experiments with viruses were performed in a Biosafety Level 2 laboratory and animal facilities.
Construction of the SFV-Enhanced Green Fluorescent Protein (EGFP) Replicon
To construct a clone of the SFV-EGFP replicon, an SFV4 replicon (kindly donated by Dr. Markus U. Ehrengruber, Department of Biology, Kantonsschule Hohe Promenade, Zurich, Switzerland) was used for engineering. The EGFP gene was amplified with the primer pairs 5’-CACAGAATTCTGATTGGATCCACCATGGTGAGCAAGGGCGAGGAG-3′ and 5′-GGATCGACTAGTGAACTCGAGTTACTTGTACAGCTCGTCCATGCC-3′ and inserted into the replicon with BamHI and XhoI using In-Fusion HD Cloning kits (Takara), and the plasmid was verified by DNA sequencing.
Virus Production
The SFV-EGFP replicon and mutant helper RNAs were separately transcribed in vitro from the NruI and SpeI linearized cDNA plasmids using an SP6 mMACHINE kit (Ambion) according to the manufacturer’s specifications. Then the RNAs were co-transfected into BHK21 (baby hamster kidney) cells using DMRIE-C reagent (Invitrogen). Briefly, 10 μL DMRIE-C was added to a tube containing 1 mL Opti-MEM I medium, then 10 μg of SFV replicon RNA and 10 μg of helper RNA were added to the tube and immediately transfected into 8×105 BHK-21 cells in 6-well plates.
Cells were washed three times using 1 mL Dulbecco’s modified Eagle’s medium (DMEM) with 2% fetal bovine serum (FBS) at 4 h post-transfection (hpt), then 1 mL medium (DMEM with 2% FBS) was added to the well and cells cultured in 5% CO2 at 37 °C, and the supernatants were collected every 24 h until 4 days post-transfection. The culture medium containing the virus was filtered using a 0.22 μm filter and divided into two parts. One part was aliquoted and the other part was treated with α-chymotrypsin (final concentration 500 μg/mL) for 25 min at 37 °C, and aprotinin (final concentration 250 μg/mL) was added to stop the reaction. The virus was then aliquoted, titered, and stored at –80 °C.
SFV VLP Infects Neurons In Vivo
All procedures were approved by the Animal Care and Use Committees at the Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences. All the experiments with viruses were performed in a Biosafety Level 2 laboratory and animal facility. Eight-week-old male C57BL/6 mice (20 g–25 g) were used for virus injection. Animals were anesthetized with chloral hydrate (400 mg/kg), and placed in a stereotaxic apparatus (68030, 68025; RWD Life Sciences Inc., San Diego, CA). The skull above the targeted areas was thinned with a dental drill (90+102; STRONG, Guangdong, China) and removed carefully. Microinjections were made through a 10 μL syringe (Hamilton, Reno, NV) connected to a glass micropipette with 10 μm–15 μm diameter tip. The indicated volume of virus was stereotaxically microinjected into the target region. The micropipette was left in place for 10 min following injection to minimize diffusion. After the indicated times, mice were deeply anesthetized with an overdose of chloral hydrate and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. The brains were removed and post-fixed overnight in 4% paraformaldehyde before being cut into 30 μm sections which were stained with DAPI and imaged using a TCS SP8 confocal microscope (Leica).
Determining the Entry Site of SFV VLP Infecting Neurons
A microfluidic chamber was prepared as described in a previous study [29]. Briefly, a microfluidic device was bonded to a poly-D-lysine-coated glass coverslip to form the chamber. Dissociated neurons from embryonic mouse cortex (E16) were prepared as described previously. Approximately 300,000 cells were plated into one well of the chamber, designated the somal part. Neuronal axons extended along the microgroove to the axonal part within 2 weeks.
To analyze the soma-mediated infection of a neuron, the SFV VLP was loaded into the somal part and the EGFP signals were detected at the indicated time points. To determine the axon-mediated infection of a neuron, the SFV VLP was loaded into the axonal part and the EGFP signal located in the somal part was imaged at the indicated time points.
Immunohistochemical Assays In Vitro and In Vivo
To stain neurons in vitro, the neuron cultures and staining procedures were as in a previous study [28]. Briefly, neurons were isolated and cultured in medium (Neurobasal medium, penicillin-streptomycin-glutamine, and B27 supplement) on 24-well plates in 5% CO2 at 37 °C. Infected neurons were fixed in 4% paraformaldehyde at room temperature for 30 mins. Then the cells were incubated with Cy3-conjugated rabbit antibody against NeuN (1:200; ABN78C3 Merck Millipore,) and visualized using a fluorescence microscope (IX73, Olympus).
To stain neurons in vivo, the protocol was as in a previous study [28]. Briefly, the post-fixed brain was cut into 40-μm coronal sections and the fixed sections were immunostained with the Cy3-conjugated rabbit antibody against NeuN (1:100; ABN78C3; Merck Millipore).
To stain Purkinje cells, fixed sagittal sections 80 μm thick were immunostained with a mouse monoclonal antibody against calbindin-D28k (Proteintech) and amplified with a Cy3-conjugated anti-mouse secondary antibody (Merck). Imaging was performed using a TCS SP8 confocal microscope (Leica).
Results
Mutant SFV VLP Infects Neurons In Vitro and In Vivo
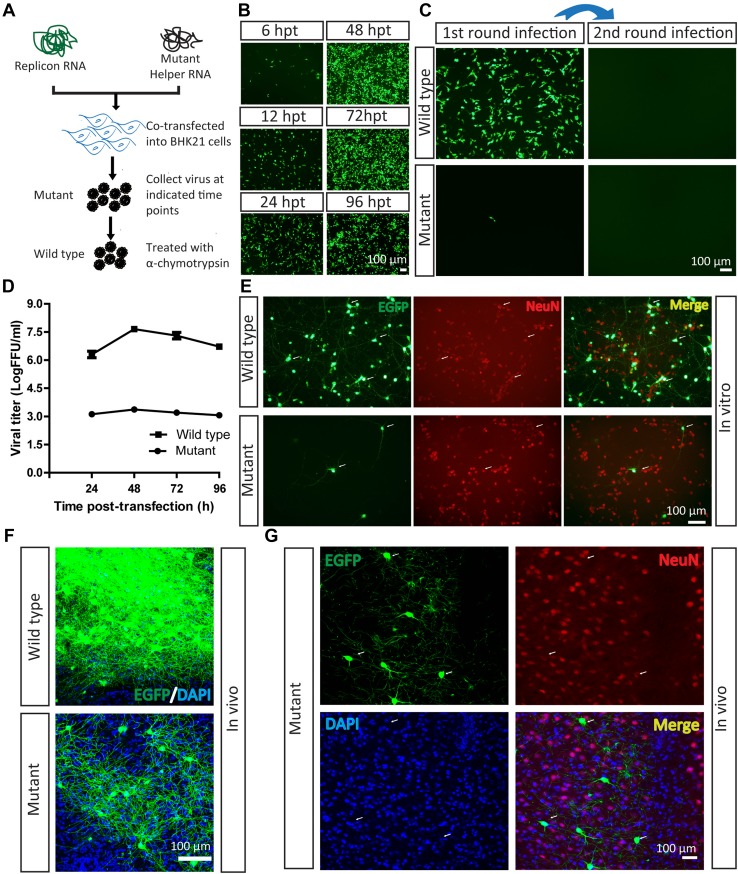
Virus-based tools are used to deliver genes into cells via the interaction of a virus envelope protein with receptors on the cell surface [30]. A previous study has shown that a three amino-acid mutation in the SFV E3 protein fully blocks the interaction and results in the loss of infectivity [22]. Here, the mutant VLP of SFV with the EGFP reporter was prepared by co-transfection of the replicon and mutant helper RNAs into BHK21 cells [21, 22] (Fig. 1A). EGFP signals were detected at 6 hpt, and were enhanced with time (Fig. 1B). The supernatant, containing mutant SFV VLP, was collected at 48 hpt and loaded into wells in which BHK21 cells were being cultured. Only a few EGFP-positive BHK21 cells were found (Fig. 1C). This result is not consistent with previous reports, which showed that mutant VLP is non-infectious [22]. The current result indicates that the three amino-acid mutation in SFV E3 does not fully eliminate the ability of the virus to enter cells, but significantly reduce its infection efficiency. When the mutant VLP was treated with α-chymotrypsin to produce wild-type SFV VLP, the infection efficiency significantly increased (Fig. 1C). The α-chymotrypsin was toxic to neurons in vitro and in vivo (data not shown). The amount of VLP increased from 6 h to 48 h (Fig. 1B), with a peak value of ~104 FFU (fluorescent-focus forming units)/mL for the mutant and ~107 FFU/mL for the wild-type (Fig. 1D). We then investigated the ability of mutant VLP to infect neurons in vitro and in vivo. The mutant VLP (2.3 × 102 FFU, tested on BHK21 cells) was loaded into plate wells containing neurons. The EGFP signals were imaged at 24 hpi and co-localized with the neuronal marker NeuN (Fig. 1E), which confirmed that the mutant VLP did infect neurons in vitro. In addition, the wild-type VLP (4 × 104 FFU) also infected neurons in vitro (Fig. 1E), consistent with previous reports [28, 31, 32]. Therefore, we speculated that these two viruses can infect neurons in vivo. Equal volumes of mutant and wild-type VLP were then separately injected into the ventral posteromedial nucleus of the thalamus (VPM) of the mouse brain at the following coordinates: −1.70 mm posterior to bregma, −1.40 mm lateral to the midline, and −3.65 dorsoventral relative to bregma, and the EGFP signals were imaged at 24 hpi. Interestingly, the wild-type VLP was found in a large number of labeled neurons, while the mutant virus was transduced into fewer neurons (Fig. 1F). The EGFP signals at 24 hpi were co-localized with the neuronal marker NeuN (Fig. 1G). These results demonstrated that the mutant VLP is able to sparsely label neurons in vivo.
Fig. 1.
Mutant SFV VLP infects neurons in vitro and in vivo. A Work flow for preparation of SFV VLP. B Virus production. Replicon and mutant helper RNAs were co-transfected into BHK21 cells. The EGFP signals were first detected at 6 hpi, and became enhanced with time. C Mutant SFV VLP had lower entry efficiency with a single round of infection. The mutant SFV VLP was treated with α-chymotrypsin to restore infectivity and prepare the wild-type virus, then it was loaded into a BHK21 well and EGFP was observed at 24 hpi. Fresh BHK21 cells were infected with culture medium collected from cells after the first infection at 24 hpi, but no EGFP signal was detected. In addition, the mutant virus also infected BHK21 cells, and fresh BHK21 cells were infected by culture medium collected from cells after the first infection at 24 hpi, but no EGFP signal was detected. D Growth curves of the mutant and wild-type viruses. The mutant and wild-type virus (treated with α-chymotrypsin) were titered in BHK21 cells. E Wild-type and mutant SFV VLP delivered EGFP into neurons in vitro. Cultured neurons were separately infected with mutant and wild-type viruses. After 24 hpi, the EGFP signals were imaged. The neurons were stained with antibody conjugated to Cy3 against neuron-specific protein NeuN. F Wild-type (100 nL, 6 × 107 FFU/mL) and mutant viruses (100 nL, 1.2 × 104 FFU/mL) were each injected into the VPM region of mouse brain. After 24 hpi, brain sections were prepared. The wild-type virus labeled many neurons and the mutant virus labeled a few neurons. These images are representative of 15 sections (n = 3 mice). G Mutant virus was injected into the VPM region. After 24 hpi, sections were prepared. The EGFP signals co-localized with signals of the neural marker NeuN (arrows). These images are representative of 15 sections (n = 3 mice).
Mutant SFV VLP Infects Neurons Anterogradely
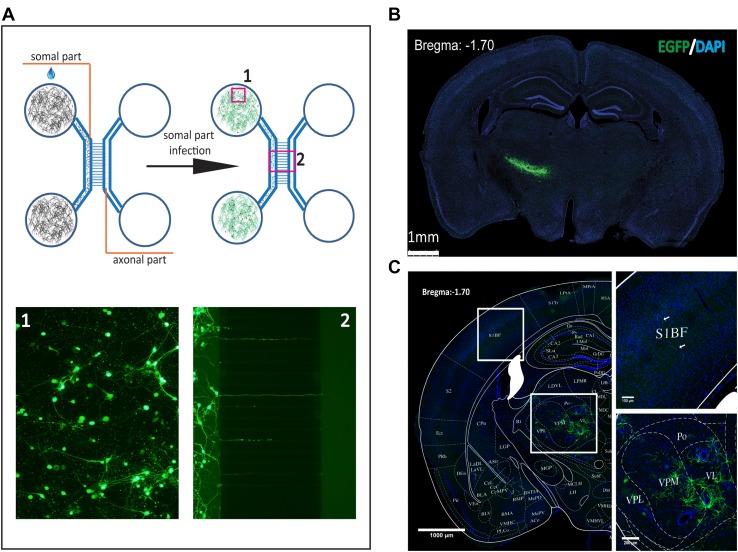
The entry site of SFV VLP was determined using the microfluidic chamber, which is a platform for testing the entry site when a virus infects a neuron [29]. Neurons were cultured in the somal parts of the chamber and axons extended into the axonal parts of the chamber within 2 weeks (Fig. 2A). The mutant VLP (23 FFU) was loaded into either the somal or the axonal chamber, and EGFP signals were detected in the axonal and somal chambers at 24 hpi after the virus was added to the somal chamber, while no EGFP signal was detected when the virus was added to the axonal chamber (data not shown). These results indicate that the mutant virus is able to enter neurons using a non-axonal pathway. However, these results cannot exclude the possibility that the mutant virus enters neurons through axon terminals when a high titer of virus is applied. Therefore, in order to adequately estimate the characteristics of the viral infection of neurons, the wild-type VLP (5 × 105 FFU) was added to the somal chamber, and EGFP signals were evident in the axonal and somal chambers at 24 hpi (Fig. 2A). When the wild-type virus was added to the axonal chamber, no EGFP signals were found in the axonal and somal chambers, even using a higher titer of virus (data not shown). These results demonstrate that SFV VLP enters neurons non-axonally and is anterogradely transported in vitro. Furthermore, we tested the entry site of the virus on neurons in vivo. When the wild-type VLP was injected into the VPM, it locally infected neurons in the injection site (Fig. 2B). Rare EGFP signals were detected in the contralateral subiculum when the virus was injected into the subiculum (data not shown). In addition, when the mutant VLP was injected into the VPM, EGFP signals were observed in the VPM region and primary sensory cortex at 24 hpi (Fig. 2C). These results indicated that SFV VLP is anterogradely transported, but with limited retrograde uptake, similar to the Sindbis virus that belongs to the same family [33].
Fig. 2.
Entry site of SFV VLP infection of neurons. A The microfluidic chamber was made of polydimethylsiloxane (Dow Corning, Midland, MI) and used to determine the efficiency of anterograde axonal spread. Neurons were plated in the somal part (1), and axons extended to the axonal part (2) along the microgrooves at 10 days post-culture. To test the non-axonal entry site, the wild-type virus was loaded into the somal part and the EGFP signal was imaged at 24 hpi. To test the axonal entry site, the wild-type virus was loaded into the axonal part and the EGFP signal was detected at 24 hpi (data not shown). B, C Entry site of SFV VLP infection of neurons in vivo. Wild-type (B) and mutant (C) SFV VLP were injected into the VPM region, then sections were prepared at 24 hpi and the EGFP signal was imaged. These images are representative of 15 sections (n = 3 mice). VPM, ventral posteromedial thalamic nucleus; S1BF, primary somatosensory cortex, barrel field.
Rapid and Sparse Labeling of Neurons by Mutant SFV VLP In Vivo
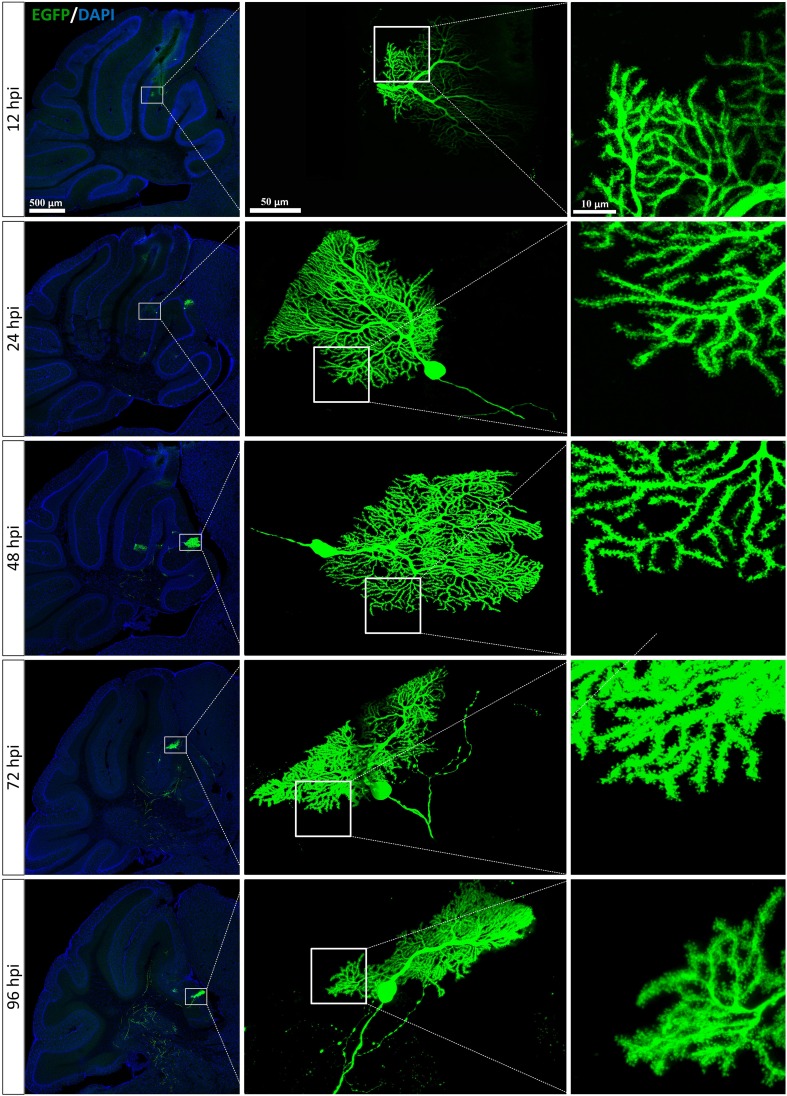
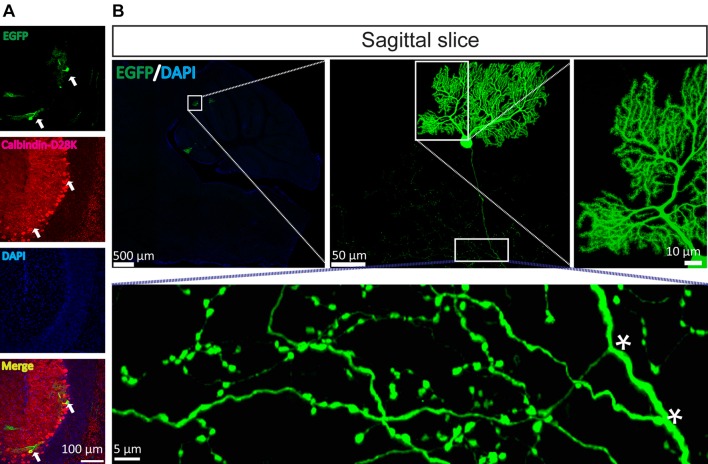
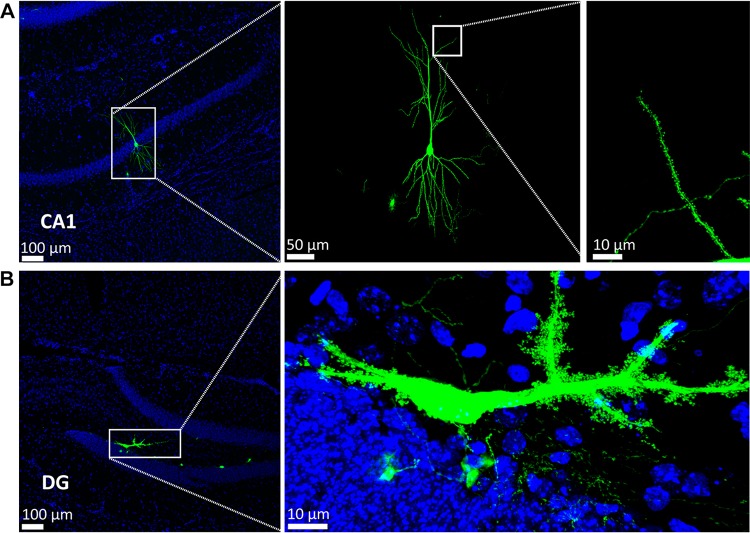
Based on the above data (Fig. 1F), we speculated that the mutant VLP may be a powerful tool for rapid and sparse labeling of neurons to display fine neuronal morphology. Thus, the cerebellar cortex was selected as a model region to test the ability of the mutant VLP to rapidly and sparsely label neurons in vivo. First, to characterize the time course of the mutant VLP labeling, mutant VLP (0.69 FFU) was injected into the third cerebellar lobule (3Cb) region at the following coordinates: 6.40 mm posterior to bregma, 0.5 mm lateral to the midline, 2.30 dorsoventral relative to bregma. Mice were sacrificed at 12 hpi, 24 hpi, 48 hpi, 72 hpi, and 96 hpi. EGFP signals were detected in the 3Cb at 12 hpi, and increased with time (Fig. 3). Each section containing the 3Cb had several EGFP-positive neurons, and the best time for clearly observing neuronal morphology was between 24 hpi and 48 hpi. The EGFP signals were clearly imaged and co-localized with the Purkinje cell marker calbindin-D28k (Fig. 4A). In sagittal sections (80 μm thick), the Purkinje cells were clearly labeled. The dendritic tree was apparent and the axon was long, with many branches (Fig. 4B and Supplementary Movie S1). Furthermore, the mutant SFV VLP also sparsely labeled hippocampal pyramidal neurons in the CA1 region [34] (Fig. 5A) and mossy cells in the dentate gyrus region [35–37] (Fig. 5B) based on the morphological characteristics of the neurons. Taken together, these results indicated that the mutant VLP is a candidate for rapid and sparse labeling of neurons in vivo.
Fig. 3.
Time course of SFV VLP infection of neurons. The mutant virus was injected into the 3Cb region of mouse brain and sections were prepared at 12 hpi, 24 hpi, 48 hpi, 72 hpi, and 96 hpi). These images are representative of 15 sections at the indicated time points (n = 3 mice at each time point).
Fig. 4.
Mutant SFV VLP rapidly and sparsely labels Purkinje neurons in vivo. To sparsely label Purkinje cells, the mutant virus was injected into the third cerebellar lobule (3Cb) region. The mice were sacrificed at 24 hpi and sagittal and coronal sections were prepared. A The EGFP signals were co-localized with the Purkinje cell marker calbindin-D28k (arrows). B The fine structure of the Purkinje cells was apparent, including the dendritic tree, long axon, and axonal branches (asterisks). These images are representative of 15 sections (n = 3 mice).
Fig. 5.
Mutant SFV VLP rapidly and sparsely labels other types of neurons in vivo. To test whether the mutant virus sparsely labels other neurons, the virus was injected into the CA1 field of the hippocampus (CA1, A) and dentate gyrus (DG, B). After 24 hpi, sections were prepared and the EGFP signals were imaged. Hippocampal pyramidal neurons (A) and mossy cells (B) were labeled.
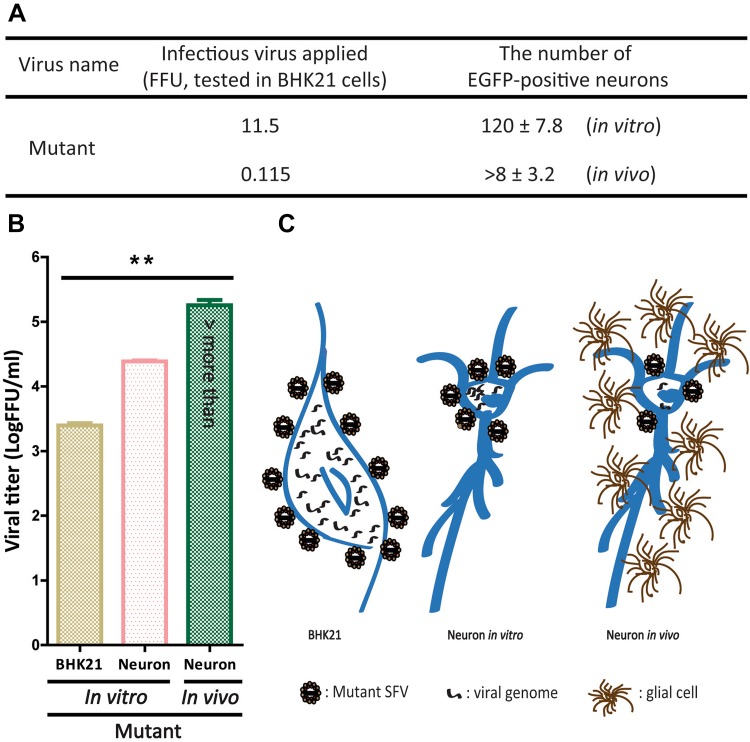
Mutant SFV VLP Has Higher Infection Efficiency in Neurons In Vivo
When we analyzed the infection efficiency of mutant VLP in BHK21 cells and neurons, the mutant VLP (11.5 FFU, tested on BHK21 cells) infected and stained 120 ± 7.8 EGFP-positive neurons in vitro (Fig. 6A), and the mutant VLP at 0.115 FFU injected into the target region of mouse brain (– 1.70 from bregma) stained 8 ± 3.2 EGFP-positive neurons (Fig. 6A). These results showed that the infection efficiency in BHK21 cells was lower than in neurons in vitro and in vivo, and the infection efficiency in neurons in vivo was higher than in vitro. These results demonstrate that 0.096 FFU and ~0.014 FFU of the mutant VLP can infect a neuron in vitro and in vivo, respectively. In addition, we found that the viral titer differed among these cells. The order of the titer was: neurons in vivo > neurons in vitro > BHK21 cells (Fig. 6B). These results also indicated that the infection efficiency is different in these cells. We speculated that the difference between neurons in vitro and in vivo was due to (i) interactions between glial cells and neurons [38, 39], and (ii) the micro-environment around neurons [40]. Previous reports have shown that neurons co-cultured with glial cells display a significantly increased transfection efficiency [38]. In addition, the discrepancy between BHK21 cells and neurons might have been due to (i) BHK21 cells are larger than neurons, so each BHK21 cell needs more viral genome when a virus infects it, and (ii) many alphavirus receptors have been identified, such as the high-affinity laminin receptor [41], α1β1 integrin [42], heparan sulfate [43], DC-SIGN and L-SIGN [44], and Mxra8 [45], so the efficiency of receptors that mediate the entry of virus into cells might be different. Based on the data, we proposed models for VLP infection of BHK21 cells and neurons in vitro and in vivo (Fig. 6C). However, the precise mechanism of infection in different cells needs to be determined in future.
Fig. 6.
Mutant SFV VLP enters BHK21 cells and neurons in vitro and neurons in vivo with different infection efficiency. A Three mice were tested and 15 sections were analyzed to determine the infection efficiency of the mutant virus on neurons in vivo. B Differences in infection efficiency of the mutant virus in different cells (n = 3; **P < 0.01, one-way ANOVA; error bars indicate standard error of the mean from the three independent experiments). C Models of mutant virus infection of BHK21 cells, neurons in vitro, and neurons in vivo.
Discussion
Mutant E3 Does Not Fully Eliminate Infectivity, but Significantly Reduces Infection Efficiency
Twenty years ago, Liljestrom and Garoff developed the replication-deficient SFV vector for protein expression in vitro and in vivo [21]. To enhance the safety of the tool, three amino-acids in SFV E3 were mutated to eliminate its infectivity [22]. The mutant SFV VLP has to be treated with α-chymotrypsin to restore the infectivity [22]. However, in this study, we found that the three amino-acid mutation did not fully eliminate the infectivity of SFV, rather it significantly reduced the infection rate (Fig. 1). Furthermore, we found that the mutant SFV VLP could be used for rapid and sparse labeling of neurons in vivo (Figs. 4 and 5). Therefore, the mutant SFV VLP is a convenient tool for preparation without treatment with more reagents (such as α-chymotrypsin). In addition, wild-type SFV VLP (treated with α-chymotrypsin) also rapidly and sparsely labeled neurons in vivo, but their morphology was less intact due to the toxicity of α-chymotrypsin (data not shown).
Mutant SFV VLP as a Tool for Rapid and Sparse Labeling of Neurons In Vivo
To sparsely label neurons in vivo, several methods have been developed. Feng et al. constructed a transgenic mouse in which different fluorescent proteins were selectively expressed in wild-type neurons [46]. Badea et al. generated a transgenic mouse to directly and sparsely label neurons using a CreER(T)/loxP strategy [6]. Zhang et al. and Barski et al. prepared Pcp2-cre mice to specifically express Cre recombinase in Purkinje cells, and these were crossed with transgenic mice containing a Cre-activated cassette to establish transgenic lines for analyzing the function and morphology of neurons [47–50]. However, animal-based approaches are time-consuming and labor-intensive. Virus-based methods are convenient and are complementary to the use of transgenic animals.
Gibson and Ma developed a method for sparsely labeling neurons by controlling the titer of AAV [8, 9]. In addition, a mixture of AAV expressing Cre recombinase and Cre-dependent AAV sparsely labels neurons in vivo [51]. Lentivirus can also be used for the sparse mapping of neurons in vivo [52]. Combination of Pcp2-cre mice with Cre-dependent virus also specifically expresses target protein in Purkinje cells [47, 48]. AAV has an insertion capacity < 4.5 kb and usually needs > 21 days for protein expression in vivo [10, 17]. Lentivirus has an insertion capacity up to 10 kb and usually needs > 7 days for protein expression in vivo [18, 53]. The mutant VLP rapidly and sparsely labeled neurons in vivo at 24 hpi (Figs. 4 and 5), and the insertion capacity is up to 6.5 kb [26]. In this study, we found that ~0.014 FFU of mutant VLP can infect a neuron in vivo (Fig. 3). Recombinant AAV and lentivirus usually need a higher titer of the virus to infect a neuron [26]. The discrepancy is that the SFV contains a replicase, while recombinant AAV and lentivirus do not. Therefore, the preparation protocol for the mutant SFV is convenient because it is not necessary to prepare high titer of the virus. AAV and lentivirus can express protein using a cell-type-specific promoter. The current version of the SFV-based tool cannot express protein using a cell-type-specific promoter because the genome of SFV is RNA.
Taken together, the mutant SFV VLP can serve as a tool to rapidly and sparsely label neurons for morphological studies and neural circuit tracing in combination with trans-synaptic tools.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Dr. Markus U. Ehrengruber (Department of Biology, Kantonsschule Hohe Promenade, Zurich, Switzerland) for providing the SFV replicon and helper cDNA clones. This work was supported by the National Natural Science Foundation of China (31771197,31830035 and 91732304), the National Basic Research Development Program (973 Program) of China (2015CB755600), the Strategic Priority Research Program (B), Chinese Academy of Sciences, China (XDBS01030200), and the Major Research Plan of the National Natural Science Foundation of China (91632303).
Conflict of interest
All authors declare that they have no conflict of interest.
Contributor Information
Fan Jia, Email: jiafan@wipm.ac.cn.
Fuqiang Xu, Email: fuqiang.xu@wipm.ac.cn.
References
- 1.Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6:297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- 2.Jefferis GS, Livet J. Sparse and combinatorial neuron labelling. Curr Opin Neurobiol. 2012;22:101–110. doi: 10.1016/j.conb.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Carletti B, Williams IM, Leto K, Nakajima K, Magrassi L, Rossi F. Time constraints and positional cues in the developing cerebellum regulate Purkinje cell placement in the cortical architecture. Dev Biol. 2008;317:147–160. doi: 10.1016/j.ydbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/S0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 5.Rotolo T, Smallwood PM, Williams J, Nathans J. Genetically-directed, cell type-specific sparse labeling for the analysis of neuronal morphology. PLoS One. 2008;3:e4099. doi: 10.1371/journal.pone.0004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badea TC, Hua ZL, Smallwood PM, Williams J, Rotolo T, Ye X, et al. New mouse lines for the analysis of neuronal morphology using CreER(T)/loxP-directed sparse labeling. PLoS One. 2009;4:e7859. doi: 10.1371/journal.pone.0007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141:536–548. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson DA, Ma L. Mosaic analysis of gene function in postnatal mouse brain development by using virus-based Cre recombination. J Vis Exp 2011. [DOI] [PMC free article] [PubMed]
- 9.Gibson DA, Tymanskyj S, Yuan RC, Leung HC, Lefebvre JL, Sanes JR, et al. Dendrite self-avoidance requires cell-autonomous slit/robo signaling in cerebellar purkinje cells. Neuron. 2014;81:1040–1056. doi: 10.1016/j.neuron.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosch MK, Nerbonne JM, Ornitz DM. Dual transgene expression in murine cerebellar Purkinje neurons by viral transduction in vivo. PLoS One. 2014;9:e104062. doi: 10.1371/journal.pone.0104062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goenawan H, Hirai H. Modulation of lentiviral vector tropism in cerebellar Purkinje cells in vivo by a lysosomal cysteine protease cathepsin K. J Neurovirol. 2012;18:521–531. doi: 10.1007/s13365-012-0134-x. [DOI] [PubMed] [Google Scholar]
- 12.Nassi JJ, Cepko CL, Born RT, Beier KT. Neuroanatomy goes viral! Front Neuroanat. 2015;9:80. doi: 10.3389/fnana.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim EJ, Jacobs MW, Ito-Cole T, Callaway EM. Improved monosynaptic neural circuit tracing using engineered rabies virus glycoproteins. Cell Rep. 2016;15:692–699. doi: 10.1016/j.celrep.2016.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo L, Anderson DJ. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron. 2011;72:938–950. doi: 10.1016/j.neuron.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, et al. Pseudorabies virus expressing enhanced green fluorescent protein: A tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci U S A. 2000;97:9264–9269. doi: 10.1073/pnas.97.16.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, Callaway EM. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron. 2011;71:617–631. doi: 10.1016/j.neuron.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takayama K, Torashima T, Horiuchi H, Hirai H. Purkinje-cell-preferential transduction by lentiviral vectors with the murine stem cell virus promoter. Neurosci Lett. 2008;443:7–11. doi: 10.1016/j.neulet.2008.07.058. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Qian X, Qin C, Lin Y, Wu H, Chang L, et al. Phosphofructokinase-1 negatively regulates neurogenesis from neural stem cells. Neurosci Bull. 2016;32:205–216. doi: 10.1007/s12264-016-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazakerley JK. Pathogenesis of Semliki Forest virus encephalitis. J Neurovirol. 2002;8(Suppl 2):66–74. doi: 10.1080/135502802901068000. [DOI] [PubMed] [Google Scholar]
- 21.Liljestrom P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology. 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 22.Berglund P, Sjoberg M, Garoff H, Atkins GJ, Sheahan BJ, Liljestrom P. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Biotechnology. 1993;11:916–920. doi: 10.1038/nbt0893-916. [DOI] [PubMed] [Google Scholar]
- 23.Andrell J, Tate CG. Overexpression of membrane proteins in mammalian cells for structural studies. Mol Membr Biol. 2013;30:52–63. doi: 10.3109/09687688.2012.703703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blasey HD, Lundstrom K, Tate S, Bernard AR. Recombinant protein production using the Semliki Forest Virus expression system. Cytotechnology. 1997;24:65–72. doi: 10.1023/A:1007974121182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrengruber MU, Lundstrom K, Schweitzer C, Heuss C, Schlesinger S, Gahwiler BH. Recombinant Semliki Forest virus and Sindbis virus efficiently infect neurons in hippocampal slice cultures. Proc Natl Acad Sci U S A. 1999;96:7041–7046. doi: 10.1073/pnas.96.12.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrengruber MU, Hennou S, Bueler H, Naim HY, Deglon N, Lundstrom K. Gene transfer into neurons from hippocampal slices: comparison of recombinant Semliki Forest virus, adenovirus, adeno-associated virus, lentivirus, and measles virus. Mol Cell Neurosci. 2001;17:855–871. doi: 10.1006/mcne.2001.0982. [DOI] [PubMed] [Google Scholar]
- 27.Lundstrom K. Alphaviruses in gene therapy. Viruses. 2015;7:2321–2333. doi: 10.3390/v7052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia F, Miao H, Zhu X, Xu F. Pseudo-typed Semliki Forest virus delivers EGFP into neurons. J Neurovirol. 2017;23:205–215. doi: 10.1007/s13365-016-0486-8. [DOI] [PubMed] [Google Scholar]
- 29.Liu WW, Goodhouse J, Jeon NL, Enquist LW. A microfluidic chamber for analysis of neuron-to-cell spread and axonal transport of an alpha-herpesvirus. PLoS One. 2008;3:e2382. doi: 10.1371/journal.pone.0002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casasnovas JM. Virus-receptor interactions and receptor-mediated virus entry into host cells. Subcell Biochem. 2013;68:441–466. doi: 10.1007/978-94-007-6552-8_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato Y, Shiraishi Y, Furuichi T. Cell specificity and efficiency of the Semliki Forest virus vector- and adenovirus vector-mediated gene expression in mouse cerebellum. J Neurosci Methods. 2004;137:111–121. doi: 10.1016/j.jneumeth.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Lundstrom K, Abenavoli A, Malgaroli A, Ehrengruber MU. Novel Semliki Forest virus vectors with reduced cytotoxicity and temperature sensitivity for long-term enhancement of transgene expression. Mol Ther. 2003;7:202–209. doi: 10.1016/S1525-0016(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 33.Kebschull JM, Garcia da Silva P, Zador AM. A new defective helper RNA to produce recombinant Sindbis virus that infects neurons but does not propagate. Front Neuroanat 2016, 10: 56. [DOI] [PMC free article] [PubMed]
- 34.Beaudoin GM, 3rd, Lee SH, Singh D, Yuan Y, Ng YG, Reichardt LF, et al. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc. 2012;7:1741–1754. doi: 10.1038/nprot.2012.099. [DOI] [PubMed] [Google Scholar]
- 35.GoodSmith D, Chen X, Wang C, Kim SH, Song H, Burgalossi A, et al. Spatial representations of granule cells and mossy cells of the dentate gyrus. Neuron. 2017;93(677–690):e675. doi: 10.1016/j.neuron.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribak CE, Seress L, Amaral DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- 37.Scharfman HE, Schwartzkroin PA. Electrophysiology of morphologically identified mossy cells of the dentate hilus recorded in guinea pig hippocampal slices. J Neurosci. 1988;8:3812–3821. doi: 10.1523/JNEUROSCI.08-10-03812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majumdar D, Gao Y, Li D, Webb DJ. Co-culture of neurons and glia in a novel microfluidic platform. J Neurosci Methods. 2011;196:38–44. doi: 10.1016/j.jneumeth.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer K, Kaspar BK. Glia-neuron interactions in neurological diseases: testing non-cell autonomy in a dish. Brain Res. 2017;1656:27–39. doi: 10.1016/j.brainres.2015.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Namba T, Funahashi Y, Nakamuta S, Xu C, Takano T, Kaibuchi K. Extracellular and intracellular signaling for neuronal polarity. Physiol Rev. 2015;95:995–1024. doi: 10.1152/physrev.00025.2014. [DOI] [PubMed] [Google Scholar]
- 41.Wang KS, Kuhn RJ, Strauss EG, Ou S, Strauss JH. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J Virol. 1992;66:4992–5001. doi: 10.1128/jvi.66.8.4992-5001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.La Linn M, Eble JA, Lubken C, Slade RW, Heino J, Davies J, et al. An arthritogenic alphavirus uses the alpha1beta1 integrin collagen receptor. Virology. 2005;336:229–239. doi: 10.1016/j.virol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Gardner CL, Ebel GD, Ryman KD, Klimstra WB. Heparan sulfate binding by natural eastern equine encephalitis viruses promotes neurovirulence. Proc Natl Acad Sci USA. 2011;108:16026–16031. doi: 10.1073/pnas.1110617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J Virol. 2003;77:12022–12032. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang R, Kim AS, Fox JM, Nair S, Basore K, Klimstra WB, et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature. 2018;557:570–574. doi: 10.1038/s41586-018-0121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/S0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 47.Guo C, Witter L, Rudolph S, Elliott HL, Ennis KA, Regehr WG. Purkinje cells directly inhibit granule cells in specialized regions of the cerebellar cortex. Neuron. 2016;91:1330–1341. doi: 10.1016/j.neuron.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witter L, Rudolph S, Pressler RT, Lahlaf SI, Regehr WG. Purkinje cell collaterals enable output signals from the cerebellar cortex to feed back to Purkinje cells and interneurons. Neuron. 2016;91:312–319. doi: 10.1016/j.neuron.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–98. doi: 10.1002/1526-968X(200011/12)28:3/4<93::AID-GENE10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 50.Zhang XM, Ng AH, Tanner JA, Wu WT, Copeland NG, Jenkins NA, et al. Highly restricted expression of Cre recombinase in cerebellar Purkinje cells. Genesis. 2004;40:45–51. doi: 10.1002/gene.20062. [DOI] [PubMed] [Google Scholar]
- 51.Li M, Liu F, Jiang H, Lee TS, Tang S. Long-term two-photon imaging in awake macaque monkey. Neuron. 2017;93(1049–1057):e1043. doi: 10.1016/j.neuron.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 52.Sawada Y, Kajiwara G, Iizuka A, Takayama K, Shuvaev AN, Koyama C, et al. High transgene expression by lentiviral vectors causes maldevelopment of Purkinje cells in vivo. Cerebellum. 2010;9:291–302. doi: 10.1007/s12311-010-0161-1. [DOI] [PubMed] [Google Scholar]
- 53.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.