Abstract
The complex spatial and temporal organization of neural activity in the brain is important for information-processing that guides behavior. Hence, revealing the real-time neural dynamics in freely-moving animals is fundamental to elucidating brain function. Miniature fluorescence microscopes have been developed to fulfil this requirement. With the help of GRadient INdex (GRIN) lenses that relay optical images from deep brain regions to the surface, investigators can visualize neural activity during behavioral tasks in freely-moving animals. However, the application of GRIN lenses to deep brain imaging is severely limited by their availability. Here, we describe a protocol for GRIN lens coating that ensures successful long-term intravital imaging with commercially-available GRIN lenses.
Keywords: Grin lens, Coating, Toxic, Neurodegeneration
Introduction
To understand how neural circuits respond during particular behaviors, many intravital imaging methods have been developed using fluorescent neural activity reporters that measure intracellular Ca2+ concentrations [1]. However, even with multiphoton microscopy, the depth of imaging is limited to ~1 mm [2–6] due to light-scattering within brain tissue. To overcome this limitation, investigators use GRadient INdex (GRIN) lenses to relay optical images from one end of a GRIN lens implanted into a deep brain region to the other end above the brain surface. Combining this technology with the recently-developed miniature fluorescence microscopes [7–9], neural activity can be recorded from anywhere in the brain in freely-moving animals. These advanced techniques pave the way to unraveling the neural encoding mechanisms underlying the control of animal behavior, including social learning and memory, movement, and maternal care [7, 10, 11].
Miniature fluorescence microscopes can be obtained in a number of ways; for instance, they can be purchased from Inscopix (Palo Alto, CA, USA) and other commercial sources, or constructed based on information on the internet (e.g., http://miniscope.org/index.php/Main_Page; https://github.com/giovannibarbera/miniscope_v1.0). GRIN lenses that are suitable for implantation into brain tissue, however, are difficult to procure. The fabrication of GRIN lenses requires single- or multi-step ion exchange within special glass. To date, most commercial GRIN lenses with a high numerical aperture (≥ 0.5) are made of thallium-containing glass and salt melts [12]. Consequently, these lenses may leach toxic residues that cause neurodegeneration in the vicinity of lens implantation. Therefore, most commercial GRIN lenses are not suitable for implantation into brain tissue for longitudinal imaging studies. The only proven non-toxic GRIN lens is offered by GrinTech (Jena, Germany) which uses a proprietary technology with special glass and silver and lithium ion-exchange. Therefore, acquiring a non-toxic GRIN lens may be difficult due to limited sources and quantities. Developing new coating technology that allows the use of commercially-available GRIN lenses for brain imaging is important. Here, we describe a custom coating method that completely prevents the toxic side-effects of most commercial GRIN lenses. The coated lens we describe is non-toxic, biocompatible, and offers excellent imaging performance at minimal cost, thereby enhancing access to GRIN lens technology. Our coating method could potentially be applied to other optics or implantable devices to minimize toxicity and maximize biocompatibility.
Materials and Methods
Animals
All procedures were performed in accordance with the guidelines of the Animal Care and Use Committee, University of Science and Technology of China, and the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, USA. Transgenic mice expressing Cre recombinase under the control of the dopamine D1 receptor promoter (D1-Cre, FK150 line, C57BL/6J congenic, Gensat, RRID: MMRRC_036916-UCD) or dopamine D2 receptor promoter (D2-Cre, ER44 line, C57BL/6J congenic, Gensat, RRID: MMRRC_032108-UCD) were used in the experiments. All animals were group-housed in a temperature-controlled environment with a 12:12-h light/dark cycle and were given access to food and water ad libitum.
The GRIN Lens Coating
The GRIN lenses used in our experiment were 1-mm diameter, 0.46 pitch SELFOC lenses (ILW-100-P0460-055-NC) purchased from Go!Foton (Somerset, NJ, USA). The Terms and Conditions of Sale for Go!Foton lenses states that there may be material toxicity for in vivo application; this was confirmed by our experiments in vitro and in vivo.
We used a parylene-C coating to prevent possible leaching of toxic material from the GRIN lens. Parylene-C is an FDA-approved polymer safe for use within the human body. The coating was performed by VSI Parylene (Broomfield, CO, USA). The coating technique used by VSI Parylene exploited the unique characteristics of a vapor deposition process to deposit parylene molecule-by-molecule onto the GRIN lens surface to a 10-μm thickness. There was no parylene-C coating on the top end of the GRIN lens (Fig. 1A). The parylene-C coating was transparent and optic-friendly.
Fig. 1.
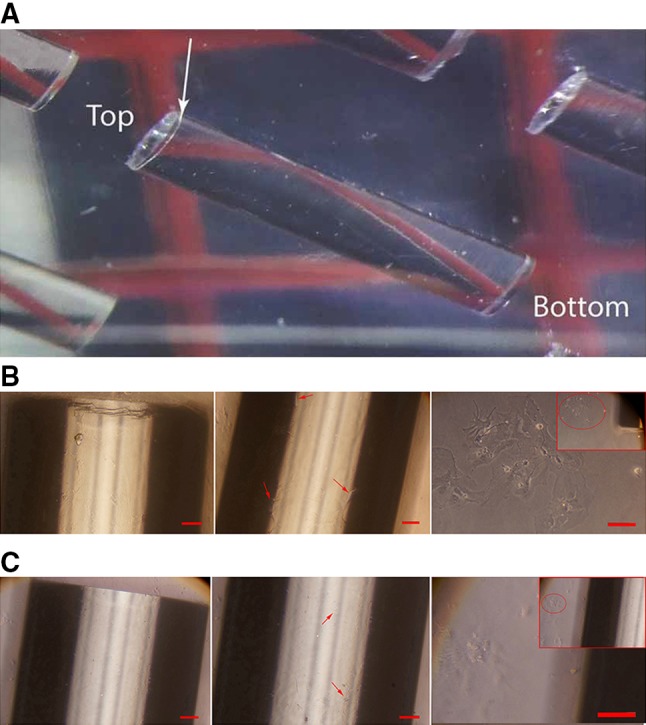
Images of primary rat hippocampal neurons after 15 days in culture with coated and uncoated lenses. A Parylene-C-coated 1-mm diameter Go!Foton lenses. There was no parylene-C coating on the top end of the GRIN lens (arrow:coating interface). B Hippocampal neurons cultured with a coated lens maintained normal morphology. Left, coated lens; middle, neurons on the surface of lens (arrows); right, neurons near the lens. C Neurons cultured with an uncoated lens were characterized by the complete loss of neurites and changes in the cytoplasm. Left, uncoated lens; middle, neurons on the surface of lens (arrows); right, neurons near the lens. Scale bars, 100 μm.
Measurement of the Toxicity of GRIN Lenses
Eight uncoated and 8 coated Go!Foton GRIN lenses were immersed for 20 days in 5 mL phosphate-buffered saline (PBS) at room temperature with the top end of the lens above the solution. The solutions were then sent to the Public Experimentation Center (University of Science and Technology of China) for measurement of the thallium ion concentration by inductively-coupled plasma-atomic emission spectrometry (ICP-AES; Optima 7300 DV, Perkin-Elmer Corp., Waltham, MA, USA). All samples were tested three times and blind to the operator in the Public Experimentation Center.
Hippocampal Neuronal Culture with GRIN Lenses
Rats were anesthetized with pentobarbital sodium(350 mg/kg, i.p.). Hippocampi were removed from rats on embryonic day 18 (without distinguishing sex differences) and treated with trypsin for 15 min at 37°C, followed by washing and gentle trituration. The original and coated GRIN lenses were each placed on a poly-L-lysine-coated coverslip in 35-mm Petri dishes. Next, the dissociated cells were plated on the side surface of the lens and near the lens at a density of 40,000–60,000 cells/mL and maintained in incubators at 37°C under 5% CO2. The culture medium was NeuroBasal (Invitrogen, Carlsbad, CA, USA) supplemented with 5% heat-inactivated bovine calf serum (Thermo Fisher, Waltham, MA, USA) plus 5% heat-inactivated fetal bovine serum (Thermo Fisher), 1× Glutamax (Invitrogen), and 1× B27 (Invitrogen). Twenty-four hours after plating, half of the medium was replaced with serum-free medium. Subsequently, one-third of the culture medium was replaced with fresh medium twice a week. To prevent overgrowth of glial cells, the cultures were treated with cytosine arabinoside (Sigma-Aldrich, St. Louis, MO, USA) at various stages. After 15 days of co-culture, neurons were imaged under an inverted microscope (Olympus, Tokyo, Japan).
Animal Surgery
Mice were anaesthetized with ketamine/xylazine (ketamine:100 mg/kg and xylazine:15 mg/kg). Adeno-Associated Virus (AAV) expressing the Ca2+ indicator GCaMP6f, AAV1.CAG.Flex.GCaMP6f.WPRE.SV40 (University of Pennsylvania Vector Core), was injected into the dorsal striatum of mice at the stereotactic coordinates A/P(Anterior/Posterior), − 0.93 mm; M/L(Medial/Lateral), + 1.8 mm; D/V(Dorsal/Ventral), − 3.46 mm, at a 30° from the vertical to caudate. One week after AAV injection, a 1-mm-diameter GRIN lens was implanted into the brain directly above the dorsal striatum under using ketamine (100 mg/kg)/xylazine (15 mg/kg) anesthesia. Two weeks after the implantation, a custom miniScope base was mounted on the mouse’s head under anesthesia with 2% isoflurane in oxygen at a flow rate of 0.4 L/min. Typically, data acquisition was performed 3–4 weeks after implantation. GRIN lenses with similar parameters from GrinTech (Jena, Germany) were used as controls.
Data Acquisition
Open field tests were done during the light phase. First, the miniScope was mounted onto the mouse’s head under anesthesia with 2% isoflurane in oxygen at a flow rate of 0.4 L/min. Next, the mouse was allowed to recover from anesthesia in the home cage for 30 min. We conducted 6 sessions (5 min/session) of the open field test for each mouse in a 34 cm × 40 cm × 20 cm (length × width × height) chamber with a 5-min interval between imaging sessions. The GCaMP6f fluorescence signal and the video of mouse behavior were recorded simultaneously.
Data Analysis
The Ca2+ images and videos of behavior were processed and analyzed using custom scripts in MATLAB. Here, we only present the GCaMP6f fluorescence signals. Averages were compared across groups and analyzed using one-way ANOVA and Tukey’s post-hoc test.
Results
The Two-Step Coating Strategy for Go!Foton GRIN Lenses Eliminates Toxicity In Vitro
To determine whether the Go!Foton GRIN lens leached thallium ion into the solution, we soaked 8 uncoated lenses in 5 mL PBS for 20 days at room temperature. ICP-AES showed that 6.6 mg/L thallium was found in the solution, demonstrating that thallium ion in the lens can dissolve into the saline. Thallium and its compounds are often highly toxic. The federal maximum contaminant level for thallium in drinking water is 0.5 μg/L according to the US Environmental Protection Agency (https://www.epa.gov/sites/production/files/2015-06/documents/ny_hh_255_w_09241998.pdf). A previous study showed that 48-h exposure to 100 μg/L thallium induces the complete loss of neurites in cultured hippocampal neurons [13]. Next, we cultured hippocampal neurons in the presence of uncoated lenses for 15 days. Neurons around the lens showed abnormal morphology, such as shrinkage and rounding of somata with fewer neurites (Fig. 1C), suggesting toxicity of the GRIN lens. To prevent potential toxic materials from leaching, we coated Go!Foton GRIN lenses with a 10-μm layer of parylene-C, excluding the very top end (Fig. 1A). This treatment effectively prevented >99% of thallium leaching. To improve tissue compatibility, we further coated the parylene-C passivated GRIN lens with fibronectin, an extracellular matrix glycoprotein with a high molecular weight, for 2 h. We then co-cultured neurons with the coated lens for 15 days and found that neurons grew with normal morphology on the coating layer (Fig. 1B). These findings demonstrate that this coating method can prevent the leaching of toxic ingredients from the Go!Foton GRIN lens.
The Two-Step Coating Strategy for Go!Foton GRIN Lenses Eliminates Toxicity In Vivo
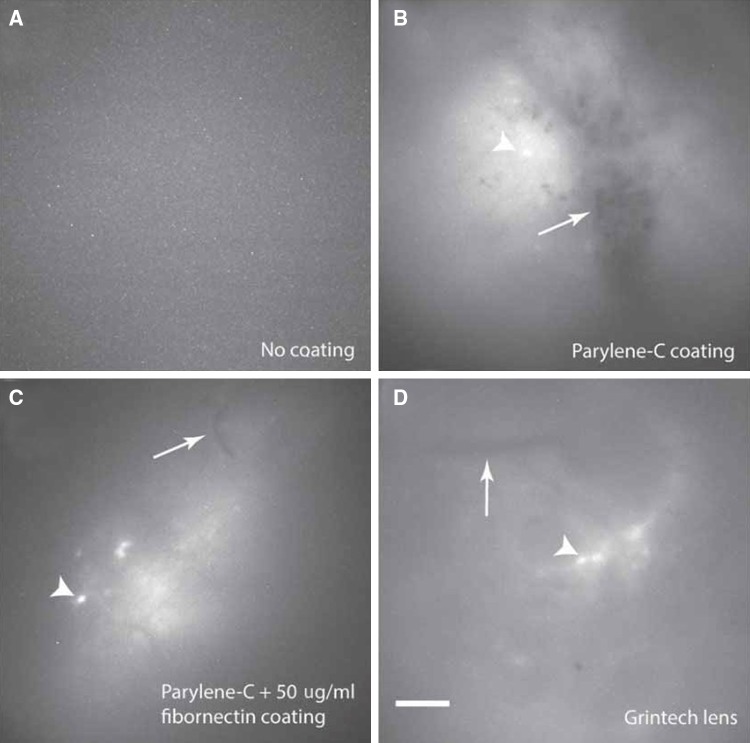
We next investigated the quality of Ca2+ imaging during the open field test after the implantation of GRIN lenses with different coating strategies. As expected, original Go!Foton lenses were clearly toxic, similar to the results in vitro. We implanted a GRIN lens into the dorsal striatum in 26 mice, of which 24 mice had no signal and 2 mice had very poor signal. No blood vessels were observed, and no active neurons, as indicated by GCaMP6f, were detected under the lens (Fig. 2A). With the parylene-C coating on the lens, active neurons labeled with GCaMP6f were detectable (Fig. 2B). However, hemorrhages also became apparent (Fig. 2B). After the implantation of a parylene-C-coated lens into the dorsal striatum of 18 mice and into the prefrontal cortex of 4 mice, all mice except one with prefrontal cortex implantation had hemorrhages, especially during the first week after surgery, most likely due to the hydrophobic property of parylene-C. To eliminate the parylene-C-induced hemorrhaging, we further coated parylene-C passivated GRIN lenses with fibronectin. After fibronectin coating, blood vessels without hemorrhage were clearly visible under the lens, and active neurons were easily detected (Fig. 2C). This demonstrated that fibronectin coating greatly improved tissue compatibility of parylene-C-coated lenses. Moreover, the Ca2+ image quality with the double-coated Go!Foton lenses was comparable to that with the GrinTech lens (Fig. 2D).
Fig. 2.
Two steps of coating enable successful in vivo imaging using Go!Foton lenses. A Neither active neurons nor blood vessels were observed under a Go!Foton lens without coating. B Active neurons (arrowhead) and blood clots (arrow) were observed under a GRIN lens coat with parylene-C alone. C Both active neurons (arrowhead) and blood vessels (arrow) were observed under GRIN lenses coated with both parylene-C and 50 μg/mL fibronectin. D Both active neurons (arrowhead) and blood vessels (arrow) were observed under a GrinTech lens. Scale bar, 100 μm.
Imaging Performance After Parylene-C and Fibronectin Double-Coating
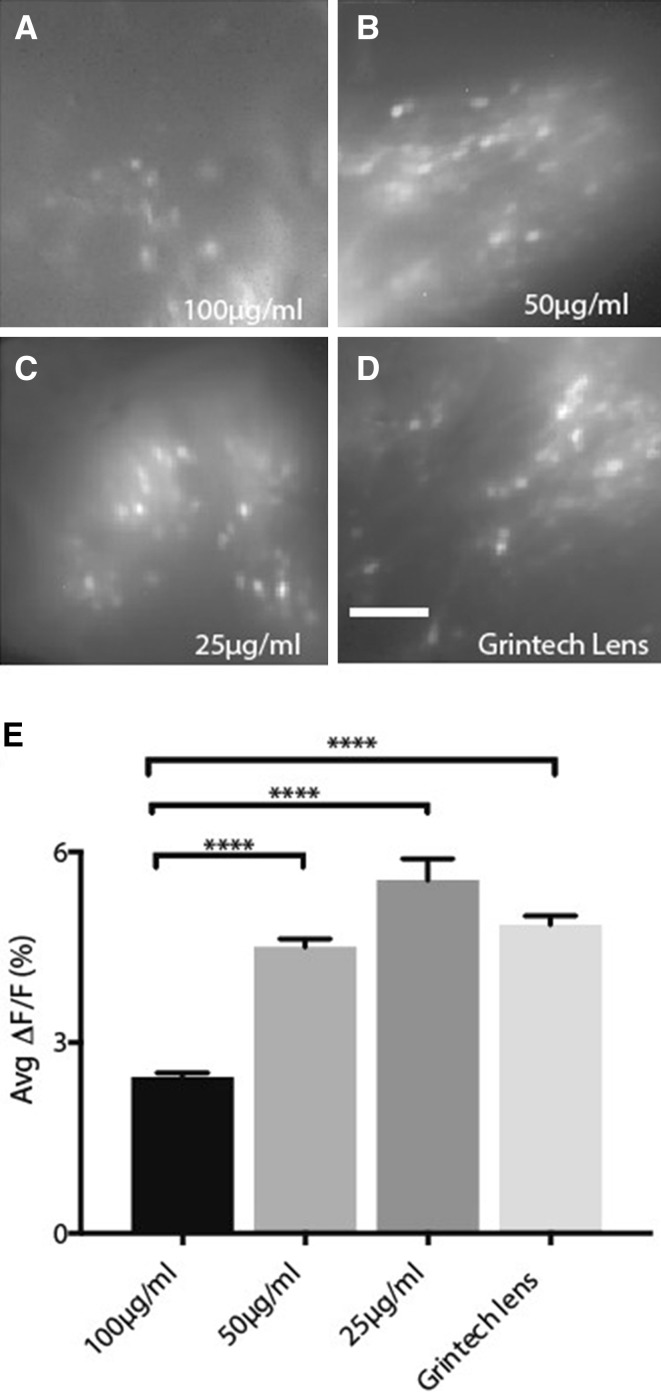
To optimize the fibronectin coating conditions, we compared the quality of fluorescent images taken by miniScope from freely-behaving mice via the GrinTech lens (Fig. 3D) versus the coated Go!Foton lens coated with parylene-C plus different concentrations of fibronectin: parylene-C + 100 μg/mL fibronectin (Fig. 3A), parylene-C + 50 μg/mL fibronectin (Fig. 3B), and parylene-C + 25 μg/mL fibronectin (Fig. 3C). We readily identified active neurons as indicated by GCaMP6f fluorescence in all four groups. We then compared the average ∆F/F of Ca2+ transients from all active neurons in each group (Fig. 3E). Our results showed that the 25 μg/mL and 50 μg/mL fibronectin-coated Go!Foton GRIN lenses and GrinTech lens displayed comparable fluorescent signal levels. As the fibronectin concentration increased, the chance of hemorrhage decreased, but image quality and contrast were also reduced, as seen using the 100 μg/mL fibronectin-coated GRIN lens, possibly due to promotion of the proliferation of glial cells by fibronectin. Last, we implanted 37 mice with two-step coated GRIN lenses with a 40 μg/mL fibronectin coating. No angiorrhexis was observed.
Fig. 3.
Comparison of imaging performance. A–C Representative standard deviation projection images from miniScope recording via GRIN lenses coated with parylene-C and fibronectin at 100 μg/mL (Group 1) (A), 50 μg/mL (Group 2) (B), and 25 μg/mL (Group 3) (C). D Representative standard deviation projection image from miniScope recording via a GrinTech GRIN lens (Group 4). E Average ∆F/F of all the neurons from each group. Group 1: 2.46% ± 0.06%, 96 neurons from two mice; Group 2: 4.50% ± 0.13%, 224 neurons from two mice; Group 3: 5.55% ± 0.34%, 100 neurons from two mice; Group 4: 4.85% ± 0.14%, 366 neurons from three mice. ****P < 0.0001, one-way ANOVA and Tukey’s post hoc test. Histogram bars represent the mean value for all neurons, with error bars representing SEM. Scale bar, 100 μm.
Discussion
The GRIN lens is one of the most important components for intravital imaging in deep brain regions. For long-term recording in freely-moving animals the commercial or custom microscopes, GRIN lenses need to be implanted into the brain and remain over months. Thus, their toxicity and bio-compatibility must be considered. Leached toxic ingredients from the implanted conventional thallium-containing GRIN lenses cause cell damage in the vicinity of lenses. Due to the limited working distance of single-photon imaging, nearby cell death prevents the recording of neural activity using miniature fluorescence microscopes in freely-moving animals. To eliminate GRIN lens toxicity, Bocarsly et al. used an optical guide cannula with a coverslip bottom, placing a GRIN lens inside the implanted cannula during imaging experiments [14]. Our coating method with a 10-μm parylene-C layer served a similar purpose to avoid direct contact between the GRIN lens and the imaged brain tissue. However, the outer diameter of an optical guide cannula is normally hundreds of microns larger than that of a GRIN lens, and its implantation inevitably causes unnecessary brain tissue damage. Our coating method only slightly increased the GRIN lens diameter by ~2%, minimizing any extra tissue damages. In addition, the cost of coating is about one dollar per GRIN lens, much cheaper than the cost of a guide cannula.
It is worth noting that parylene-C is optically transparent, chemically inert, and non-biodegradable, making it an excellent material for insulating implantable biomedical devices [15]. However, the surface of parylene-C has been shown to be hydrophobic [16], and easily induces angiorrhexis and thrombosis after implantation. Our second coating step with fibronectin that overlaid the parylene-C greatly enhanced the contact compatibility of the parylene-C-coated GRIN lens with brain tissue. Fibronectin-treated surfaces have cell adhesion levels comparable to polystyrene, a commercially-available material suitable for tissue culture [16, 17]. We also optimized a range of fibronectin concentrations that were suitable for parylene-C coated GRIN lenses to maintain image quality. Our two-step coating method for Go!Foton GRIN lenses could be applied to most commercial sources of GRIN lenses or other implanted optics/devices to improve brain tissue compatibility with minimal added cost. It will also facilitate the development of custom-made miniature fluorescence microscopes.
Conclusions
We developed a GRIN lens-coating method that allows the use of any commercial GRIN lens for in vivo neuroscience research with excellent imaging quality. This method rivals the output from the market leader in non-toxic GRIN lenses and could be applied to other optics or devices to enhance compatibility for long-term brain implantation at a fraction of the cost.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31871054 and 31371112) and the Intramural Research Program of the National Institute on Drug Abuse, NIH, USA.
Conflict of interest
The authors declare no competing financial interests or conflicts of interest.
Contributor Information
Yupeng Yang, Email: yangyp@ustc.edu.cn.
Lifeng Zhang, Email: lfzhang916@hotmail.com.
References
- 1.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 3.Lechleiter JD, Lin DT, Sieneart I. Multi-photon laser scanning microscopy using an acoustic optical deflector. Biophys J. 2002;83:2292–2299. doi: 10.1016/S0006-3495(02)73989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung JC, Mehta AD, Aksay E, Stepnoski R, Schnitzer MJ. In vivo mammalian brain imaging using one- and two-photon fluorescence microendoscopy. J Neurophysiol. 2004;92:3121–3133. doi: 10.1152/jn.00234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levene MJ, Dombeck DA, Kasischke KA, Molloy RP, Webb WW. In vivo multiphoton microscopy of deep brain tissue. J Neurophysiol. 2004;91:1908–1912. doi: 10.1152/jn.01007.2003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Zhang L, Liang B, Schroeder D, Zhang ZW, Cox GA, et al. Hyperactive somatostatin interneurons contribute to excitotoxicity in neurodegenerative disorders. Nat Neurosci. 2016;19:557–559. doi: 10.1038/nn.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbera G, Liang B, Zhang L, Gerfen CR, Culurciello E, Chen R, et al. Spatially compact neural clusters in the dorsal striatum encode locomotion relevant information. Neuron. 2016;92:202–213. doi: 10.1016/j.neuron.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534:115–118. doi: 10.1038/nature17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, et al. Miniaturized integration of a fluorescence microscope. Nat Methods. 2011;8:871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353:1536–1541. doi: 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang YY, Yamaguchi T, Song SC, Tritsch NX, Lin D. A hypothalamic-midbrain pathway essential for driving maternal behaviors. Neuron. 2018;98:192–207. doi: 10.1016/j.neuron.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tagantsev DK, Lipovskii AA, Schultz PC, Tatarintsev BV. Phosphate glasses for GRIN structures by ion exchange. J Non Cryst Solids. 2008;354:1142–1145. doi: 10.1016/j.jnoncrysol.2006.11.030. [DOI] [Google Scholar]
- 13.Colombaioni L, Onor M, Benedetti E, Bramanti E. Thallium stimulates ethanol production in immortalized hippocampal neurons. PLoS One. 2017;12:e0188351. doi: 10.1371/journal.pone.0188351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bocarsly ME, Jiang WC, Wang C, Dudman JT, Ji N, Aponte Y. Minimally invasive microendoscopy system for in vivo functional imaging of deep nuclei in the mouse brain. Biomed Opt Express. 2015;6:4546–4556. doi: 10.1364/BOE.6.004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeb GE, Walker AE, Uematsu S, Konigsmark BW. Histological reaction to various conductive and dielectric films chronically implanted in the subdural space. J Biomed Mater Res. 1977;11:195–210. doi: 10.1002/jbm.820110206. [DOI] [PubMed] [Google Scholar]
- 16.Chang TY, Yadav VG, De Leo S, Mohedas A, Rajalingam B, Chen CL, et al. Cell and protein compatibility of parylene-C surfaces. Langmuir. 2007;23:11718–11725. doi: 10.1021/la7017049. [DOI] [PubMed] [Google Scholar]
- 17.Delivopoulos E, Ouberai MM, Coffey PD, Swann MJ, Shakesheff KM, Welland ME. Serum protein layers on parylene-C and silicon oxide: effect on cell adhesion. Colloids Surf B Biointerfaces. 2015;126:169–177. doi: 10.1016/j.colsurfb.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]