Abstract
Zinc-α2-glycoprotein (ZAG), encoded by the AZGP1 gene, is a major histocompatibility complex I molecule and a lipid-mobilizing factor. ZAG has been demonstrated to promote lipid metabolism and glucose utilization, and to regulate insulin sensitivity. Apart from adipose tissue, skeletal muscle, liver, and kidney, ZAG also occurs in brain tissue, but its distribution in brain is debatable. Only a few studies have investigated ZAG in the brain. It has been found in the brains of patients with Krabbe disease and epilepsy, and in the cerebrospinal fluid of patients with Alzheimer disease, frontotemporal lobe dementia, and amyotrophic lateral sclerosis. Both ZAG protein and AZGP1 mRNA are decreased in epilepsy patients and animal models, while overexpression of ZAG suppresses seizure and epileptic discharges in animal models of epilepsy, but knowledge of the specific mechanism of ZAG in epilepsy is limited. In this review, we summarize the known roles and molecular mechanisms of ZAG in lipid metabolism and glucose metabolism, and in the regulation of insulin sensitivity, and discuss the possible mechanisms by which it suppresses epilepsy.
Keywords: Zinc-α2-glycoprotein, Metabolism, Glucose, Lipid, Insulin sensitivity, Neuron
Zinc-α2-Glycoprotein (ZAG)
ZAG is a 42-kDa secretory protein expressed in many animal species and is encoded by AZGP1 gene, which is located on chromosome 7q22.1 [1, 2]. The AZGP1 gene is 9.7 kb long and its organization and nucleotide sequence are highly similar to the first four exons of major histocompatibility complex (MHC) I genes, but the AZGP1 gene does not have sequences encoding transmembrane and cytoplasmic domains [1, 3]. In accordance with the AZGP1 gene, the structure of ZAG protein is also very similar to MHC I molecules. The ZAG protein contains 3 domains, among which the α1 and α2 domains form a groove-like structure, and the α3 domain adopts a fold resembling immunoglobulin constant domains [4]. The groove-like α1–α2 superdomains of ZAG can bind to ligands and thus determine its specificity for its ligands, while the α3 domain links the α1–α2 superdomains and light chain of ZAG [4]. Unlike the MHC I proteins, the light chain of ZAG is a prolactin-induced protein [4].
ZAG binds to zinc and fatty-acids. It has been reported that ZAG has 2 strong and 15 weak binding sites for zinc, and zinc binding to these sites influences the binding of ZAG to fatty-acids and β-adrenergic receptors [5]. Zinc binding to the weak binding sites is considered to induce the oligomerization and precipitation of ZAG [6]. In the groove in ZAG formed by the α1–α2 superdomains, there are at least 2 sites that bind to the dansylated C11 fatty-acids 11-(dansylamino)undecanoic acid or 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-hexadecanoic acid. And the binding of ZAG to the latter is competitively inhibited by zinc [6]. Interestingly, polyethylene glycol has also been identified as a ligand of ZAG. Polyethylene glycol displaces fatty-acids from ZAG binding sites, indicating that ZAG can bind to hydrophobic molecules [7]. However, the effects of these ligands on the structure and function of ZAG need further investigation.
The Role of ZAG in Metabolism and Insulin Sensitivity
Lipid Metabolism
ZAG is known to be a lipid-mobilizing adipokine [8]. In humans, the ZAG level in serum has been shown to be positively associated with serum triglycerides and adipocyte fatty-acid-binding protein levels, and negatively associated with high-density lipoprotein-cholesterol levels [9]. In rats, AZGP1 mRNA is detectable in white and brown adipose tissues just 1 day after birth, and decreases rapidly thereafter in brown adipose tissue and after weaning in white adipose tissue, accompanied by an increase of adipose tissue amount [10]. ZAG treatment also reduces adipose tissue in rats by increasing lipolysis and the utilization of non-esterified fatty-acids; the plasma triglyceride level is decreased [11]. In vitro, ZAG stimulates the proliferation of 3T3-L1 pre-adipocytes and inhibits their differentiation [12]. ZAG treatment has also been found to increase the levels of adipose triglyceride lipase and hormone-sensitive lipase, which contribute to lipolysis [11]. While overexpressing ZAG in hepatocytes promotes the lipolysis and β-oxidation of fatty-acids, it also inhibits palmitic acid-induced lipogenesis and lipid accumulation in hepatocytes [13]. On the contrary, knockdown of ZAG expression significantly promotes lipogenesis and increases the lipid level in hepatocytes [13, 14]. All these studies suggest an important role of ZAG in lipolysis.
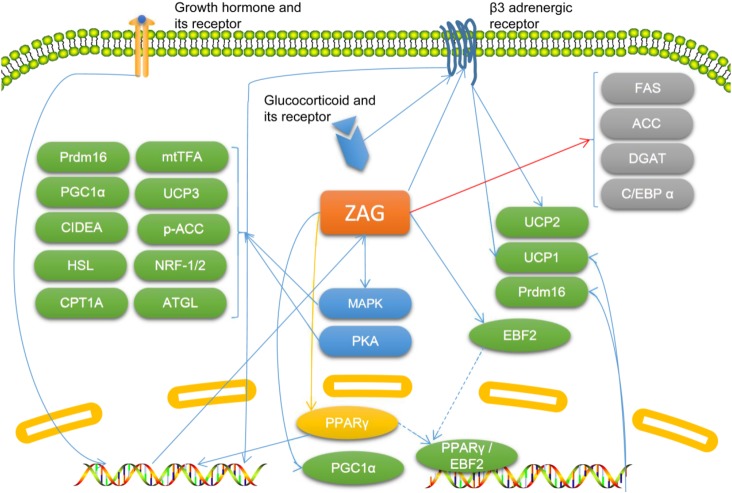
The mechanism by which ZAG influences lipid metabolism has not been clearly established, but increasing evidence shows that ZAG is involved in lipid metabolism in several ways. ZAG promotes the browning of white adipose tissue, and thus increase lipolysis [15]. The promoting effect of ZAG on white adipose tissue browning may be due to its stimulation of the expression of peroxisome proliferator-activated receptor γ (PPARγ) and early B cell factor 2, which may enhance the binding of these molecules to the promoter of PR/SET domain 16 (Prdm16) and the promoter of uncoupling protein (UCP) 1, while both Prdm16 and UCP1 are known to convert white adipose precursors into brown-like adipocytes and promote energy consumption [15]. In brown adipose tissue, ZAG enhances the expression of PPARγ and its coactivator 1α, and thus increases the expression of UCP1, which increases energy expenditure [15]. Interestingly, ZAG expression in SGBS (Simpson-Golabi-Behmel syndrome) adipocytes is enhanced by the PPARγ agonist rosiglitazone [16]. The ZAG-induced browning of white adipose tissue may also be mediated by PKA and p38 mitogen-activated protein kinase (MAPK) signaling, by which ZAG may enhance the expression of many lipolysis-related molecules, including UCP-1, Prdm16 and CIDEA, PPARγ coactivator 1α, nuclear respiratory factor-1/2, mitochondrial transcription factor A, adipose triglyceride lipase, hormone-sensitive lipase, carnitine palmitoyltransferase 1A, and p-acyl-CoA carboxylase [17]. The induction of UCP1 and UCP2 by ZAG is dose-dependent and via β3 adrenergic receptors, while the induction of UCP3 by ZAG is meditated by MAPK [11, 18]. Therefore, the increase in body temperature and decrease in body weight and adipose tissue induced by ZAG may be partly attributed to its promotional effect on UCP expression, which leads to increased energy expenditure [11, 15, 18]. The expression of ZAG is up-regulated by growth hormone, while silencing ZAG expression abolishes the lipolytic effect of growth hormone on adipocytes [19, 20]. Overexpression of ZAG also decreases the mRNA level of fatty-acid synthase (FAS), acetyl-CoA carboxylase, and acyl-coenzyme A: diacylglycerol transferase, and increases the mRNA level of hormone-sensitive lipase; these effects result in inhibition of lipogenesis and enhancement of lipolysis [21]. However, ZAG has also been reported to promote the proliferation of 3T3-L1 pre-adipocytes and inhibit their differentiation by inhibiting the expression of PPARγ and CCAAT-enhancer-binding protein α, and inhibit the activity of the promoter of FAS [12]. Therefore, ZAG has a pro-lipolysis effect, but the mechanism by which it influences lipid metabolism is controversial and needs further study (Fig. 1).
Fig. 1.
The known molecular mechanisms by which zinc-α2-glycoprotein (ZAG) participates in lipid metabolism. Molecules colored in green are known to be upregulated by ZAG directly or indirectly, and those colored in grey are known to be downregulated by ZAG directly or indirectly. The molecule colored in yellow (PPARγ) is affected by ZAG but the results are controversial. Blue arrows indicate promotional effects, red arrow indicates inhibitory effects, and yellow arrow indicates controversial effects. ACC, acetyl-CoA carboxylase; ATGL, adipose triglyceride lipase; C/EBPα, CAAT-enhancer-binding proteins α; CIDEA, cell death-inducing DNA fragmentation factor alpha-like effector A; CPT1A, carnitine palmitoyltransferase 1A; DGAT, acyl-coenzyme A: diacylglycerol transferase; EBF2, early B cell factor 2; FAS, fatty-acid synthase; HSL, hormone-sensitive lipase; MAPK, mitogen-activated protein kinase; mtTFA, mitochondrial transcription factor A; NRF-1/2, nuclear respiratory factor-1/2; PGC1α, PPARγ coactivator 1α; PKA, protein kinase A; PPARγ, peroxisome proliferator-activated receptor γ; Prdm16, PR/SET domain 16; UCP, uncoupling protein.
Glucose Metabolism
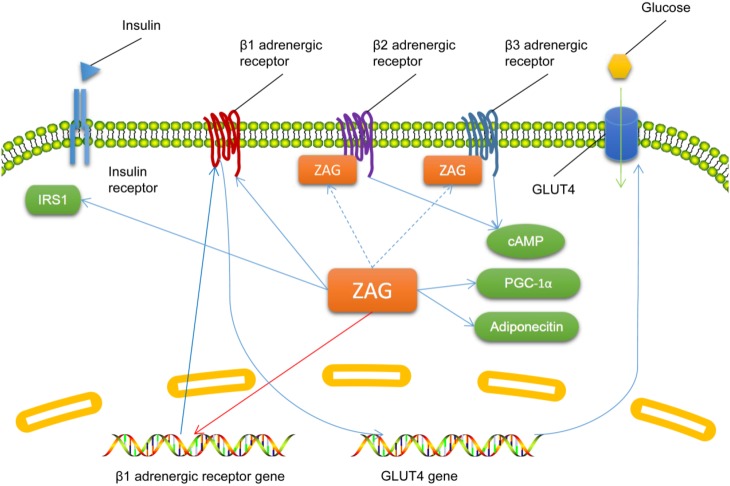
There has been little research on the effect of ZAG on glucose metabolism. It has been shown that intravenous administration of ZAG to mice decreases fasting blood glucose and improves glucose tolerance without changing the plasma insulin level 30 min after oral administration of glucose [22]. ZAG increases urinary glucose excretion and decreases the maximal plasma glucose and insulin levels in oral glucose tolerance tests in mice, as well as promoting the transfer of glucose into skeletal muscle and adipocytes [23]. These effects of ZAG on glucose metabolism are attenuated by the non-specific β adrenergic receptor antagonist, propranolol [23], indicating a role of β adrenergic receptors in ZAG-regulated glucose metabolism. ZAG binds to β2 and β3 but not β1 adrenergic receptors and activates them both in white and brown adipose tissues, which leads to an increase of cyclic adenosine monophosphate; it also inhibits the expression of β1 adrenergic receptor mRNA [20, 22]. However, ZAG has also been reported to decrease the circulating glucose level and increase the basal glucose intake into adipocytes and the expression of glucose transporter 4 (GLUT4) via β1 adrenergic receptors, while the plasma glucose level has also been found not to be associated with the plasma ZAG level [11, 24]. Silencing AZGP1 expression in adipocytes also decreases the expression of adiponectin, insulin receptor substrate 1 (IRS1), GLUT4, and PGC1α [25], indicating its role in glucose uptake, insulin action, mitochondrial biogenesis, and lipid oxidation. ZAG may promote glucose utilization, storage, and excretion, and β adrenergic receptors may play important roles in ZAG-regulated glucose metabolism, but the specific mechanism by which ZAG influences glucose metabolism needs further investigation (Fig. 2).
Fig. 2.
The known molecular mechanisms by which zinc-α2-glycoprotein (ZAG) participates in glucose metabolism. Descriptions of molecules and arrows in different colors are as in Fig. 1. cAMP, cyclic adenosine monophosphate; GLUT4, glucose transporter 4; IRS, insulin receptor substrate; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1α.
Insulin Resistance (IR)
ZAG has been associated with IR. In the subcutaneous adipose tissue of adults, a higher ZAG level is an independent predictor of better adipose tissue and whole-body insulin sensitivity [25]. Circulating ZAG levels in children, patients with polycystic ovary syndrome, and patients with type 2 diabetes mellitus are negatively associated with IR [26–28]. Similarly, a negative association between the AZGP1 mRNA level in adipose tissue and IR has also been verified [29]. Some researchers recommend a circulating ZAG index [] to identify IR, as it is negatively associated with IR and shows favorable diagnostic efficacy (sensitivity 88% and specificity 91%) [30]. However, it has also been reported that serum ZAG levels are positively associated with IR [9], and ZAG has also been reported to induce IR in adipocytes [24]. Interestingly, in cultured liver HPG2 cells, ZAG is not associated with IR, and insulin does not affect ZAG expression either [31]. Therefore, controversial results have been found in different studies on the relationship between ZAG and IR, but the majority of reports support a negative association between ZAG and IR.
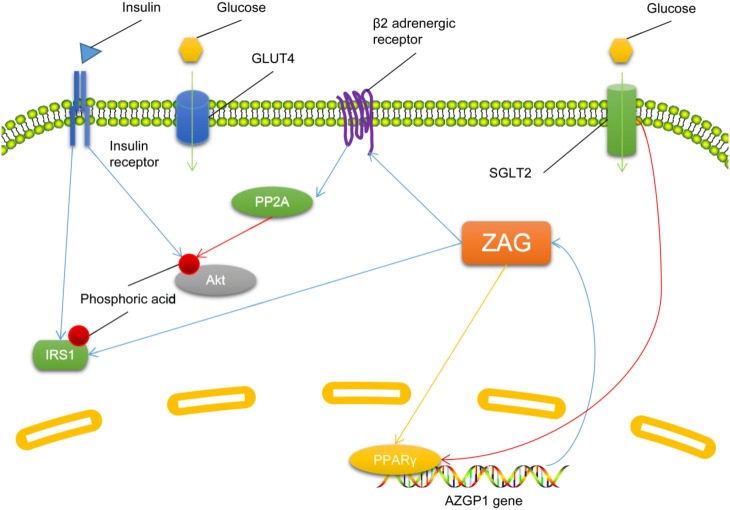
The mechanism by which ZAG influences IR is not clear, and there are few reports on this topic. In type 2 diabetes mellitus patients, treatment with dapagliflozin, an antagonist of sodium-dependent glucose transporter 2 (SGLT2), increases serum ZAG and alleviates their IR [32]. The effect of dapagliflozin on ZAG and IR can be abolished by a PPARγ inhibitor (GW9662) [32], indicating that inhibiting SGLT2 or activating PPARγ increases ZAG and alleviates IR, and negatively associates ZAG with IR. However, ZAG has also been reported to induce IR in adipocytes by increasing the activity of protein phosphatase 2 (PP2A) in a β2 adrenergic receptor activation-dependent manner [24]. And ZAG also increases the phosphorylation of IRS1 at the Ser307 residue in gastrocnemius muscle, thus contributing to IR [33]. The limited research and the controversial findings make it difficult to outline the role of ZAG in IR; further study is needed (Fig. 3).
Fig. 3.
Molecular mechanisms underlying the regulation of insulin sensitivity by zinc-α2-glycoprotein (ZAG). Descriptions of molecules and arrows in different colors are as in Fig. 1. GLUT4, glucose transporter 4; IRS, insulin receptor substrate; PP2A, protein phosphatase 2A; PPARγ, peroxisome proliferator-activated receptor γ; SGLT2, sodium-dependent glucose transporter 2.
Other Molecular Mechanisms Involving ZAG
Besides its role in metabolism and insulin sensitivity, ZAG has also been shown to be involved in many other molecular mechanisms.
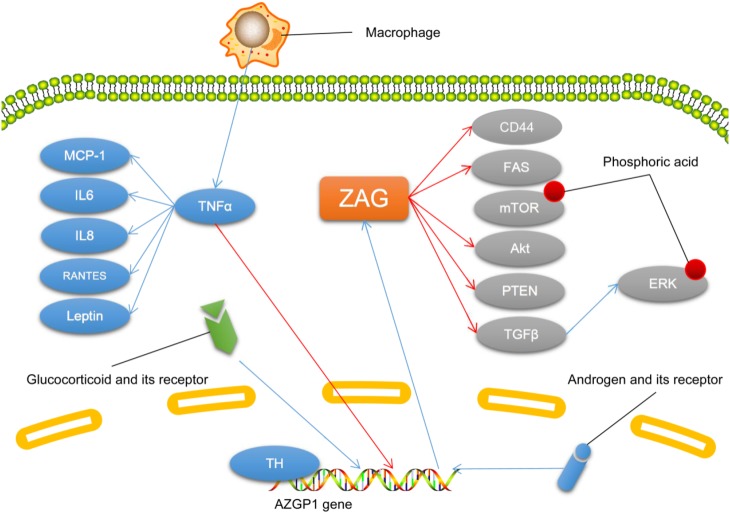
In both types of adipose tissue in mice, cultured human adipocytes, and SGBS pre-adipocytes, tumor necrosis factor α (TNFα) treatment decreases ZAG expression [6, 16, 34, 35]. And in patients receiving hemodialysis, the plasma ZAG level is negatively associated with plasma TNFα [36]. It has been reported that macrophage-treated human adipocytes show a significant decrease in ZAG expression, while TNFα treatment induces a similar decrease accompanied by a significant increase in inflammatory factors including interleukin 6 (IL6), leptin, IL8, monocyte chemoattractant protein 1, and RANTES (regulated upon activation, normal T cell expressed and presumably secreted protein) [35]. These findings suggest that macrophage-related inflammation plays important roles in the downregulation of ZAG expression, and TNFα is central in the molecular mechanism of such inflammation.
In malignant tumors such as ductal adenocarcinoma of the pancreas and hepatocellular carcinoma, ZAG acts as a tumor suppressor. It inhibits invasion and induces the mesenchymal-to-epithelial transdifferentiation of tumor cells by inhibiting the tumor growth factor β (TGFβ)-mediated extracellular regulated protein kinase (ERK) signaling pathway [37, 38]. ZAG has also been shown to have an antifibrotic effect in fibrosis of the kidney and heart induced by chronic kidney disease or heart stress via the negative regulation of TGFβ [39]. In LoVo cells, overexpression of ZAG inhibits the proliferation of tumor cells and their invasion, also leading to a reduction of FAS and phosphorylated mammalian target of rapamycin (p-mTOR), thus inhibiting the activity of the mTOR pathway and endogenous FAS-regulated fatty-acid synthesis [40]. On the contrary, ZAG increases the mTOR level in mouse gastrocnemius muscle [33]. ZAG also inhibits the proliferation, invasion, and metastasis of hepatocellular carcinoma both in vivo and in vitro by regulating the PTEN (phosphate and tension homology deleted on chromosome ten)/Akt and CD44 (cluster of differentiation 44) pathways [41].
ZAG expression is induced by hormones, including thyroid hormone, androgen, and glucocorticoid. In cultured hepatocytes, thyroid hormone induces ZAG expression dose-dependently, and there is a thyroid hormone-binding site in the proximal promoter of AZGP1 [42]. In prostate cancer cells, AZGP1 mRNA is induced by androgen stimulation, and this effect is inhibited by GATA-2 [43]. And in adipose tissue, dexamethasone induces a 6-fold increase in ZAG expression [44] (Fig. 4).
Fig. 4.
The molecular mechanisms associated with zinc-α2-glycoprotein (ZAG). Descriptions of molecules and arrows in different colors are as in Fig. 1. CD, cluster of differentiation; ERK, extracellular regulated protein kinases; FAS, fatty acid synthase; IL, interleukin; MCP-1, monocyte chemoattracctant protein; mTOR, mammalian target of rapamycin; PTEN, phosphate and tension homology deleted on chromsome ten; RANTES, regulated upon activation, normal T cell expressed and presumably secreted protein; TGFβ, tumor growth factor β; TH, thyroid hormone; TNFα, tumor necrosis factor α.
Presence and Distribution of ZAG in Brain
Only a few studies have investigated the presence and distribution of ZAG in brain, and their results are contradictory. In brain tissue from patients with Krabbe disease, ZAG has been found in astrocytes and extracellular matrix surrounding capillaries, but not in neurons [45]. However, in brain tissue from epilepsy patients, ZAG was reported to be expressed in neurons but not astrocytes [46]. Besides, the former study did not identify ZAG in brain tissue from 7- to 25-month-old controls, while the latter study identified ZAG in the neurons of adult controls with brain trauma. This difference could be attributed to the different ages and disease conditions of the controls in these studies [46]. The synthesis of ZAG in brain may be age-dependent as it is for many other proteins [47–49]. While it is possible that ZAG is synthesized in the brain of adults but not infants, this hypothesis needs to be tested in further research.
In addition, ZAG has also been found in the cerebrospinal fluid of patients with Alzheimer disease, amyotrophic lateral sclerosis, and frontotemporal lobe dementia, and is considered a potential biomarker for these diseases [50–52]. But the role of ZAG in these diseases has not yet been clarified.
The source of ZAG in the brain is still debatable. Maślińska and colleagues speculated that, in brain tissue from patients with Krabbe disease, ZAG came from the plasma through a damaged blood-brain barrier [45], but in epilepsy patients, AZGP1 mRNA and ZAG protein were both identified in brain tissues from patients and rats, and the authors speculated that ZAG may be synthesized in the brain, probably in neurons [46, 53]. In Alzheimer disease, the ZAG level in cerebrospinal fluid is not associated with its level in plasma [51], so the source of ZAG in cerebrospinal fluid remains controversial.
The Role and Mechanism of ZAG in CNS Diseases
Some studies have investigated the role and mechanism of ZAG in CNS diseases, most concerning epilepsy. In brain tissue from epilepsy patients, AZGP1 mRNA and ZAG protein are decreased, and in brain from epileptic rat models, decreased AZGP1 mRNA and ZAG protein in neurons have also been identified [46, 50]. And in epileptic rat models, the decrease of AZGP1 mRNA and ZAG protein exacerbates as seizures worsen [46]. Overexpressing ZAG in epileptic rat models has been found to alleviate seizures and epileptic discharges in the electroencephalogram [54]. Interestingly, liraglutide, a drug for diabetes which increases circulating ZAG, has been shown to suppress epilepsy in animal models and the comorbidity of epilepsy [55–57]. However, whether ZAG can alleviate epilepsy in the clinic is still unknown, and the mechanism by which ZAG alleviates epilepsy needs further investigation.
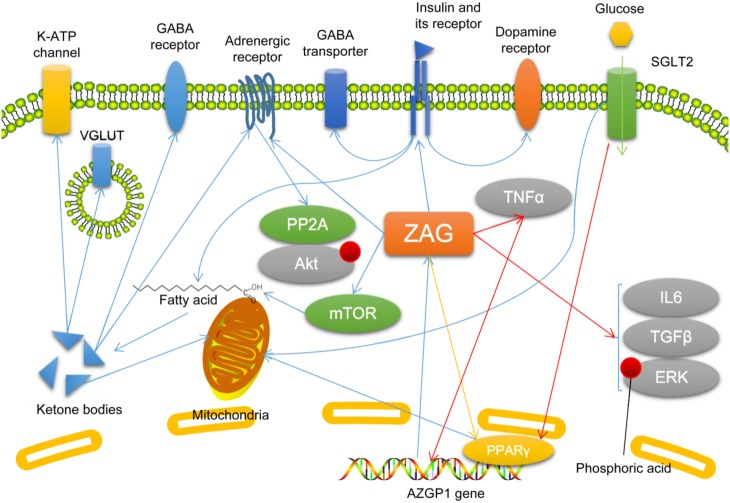
It is possible that ZAG suppresses epilepsy or seizures via several potential mechanisms. As a lipid-mobilizing factor, ZAG may affect the metabolism of fatty-acids in neurons. The traditional opinion is that neurons cannot use fatty-acids directly as fuel. However, it has recently been demonstrated that neurons can use fatty-acids for energy, and under either hypoxia or normal conditions, most of the fatty-acids consumed by neurons are metabolized into ketone bodies rather than CO2 and water [58]. Astrocytes are also able to metabolize fatty-acids and produce ketone bodies, which are also considered to have a neuroprotective effect [59]. Meanwhile, ketone bodies have been shown to regulate the γ-aminobutyric acid (GABA) signaling pathway, vesicular glutamate transporter, norepinephrine signaling, potassium-adenosine triphosphate channels, the tricarboxylic acid cycle, and the mitochondrial permeability transition, and may exert an antiepileptic effect by these mechanisms [60]. ZAG expression is regulated by SGLT2 and PPARγ [31], while both are known to participate in epilepsy [61–63]. And both SGLT2 and PPARγ are known to participate in fatty-acid oxidation and ketogenesis [63, 64]. ZAG is known to promote fatty-acid metabolism [11], and in neurons, the catabolism of fatty-acids mainly produces ketone bodies [58]. Although whether SGLT2 and PPARγ regulate ketogenesis via ZAG is unclear, it is possible that ZAG affects ketogenesis by regulating fatty-acid metabolism in neurons. However, as there is no research on the effect of ZAG on ketogenesis, this hypothesis needs to be tested. Besides, ZAG affects the activity of mTOR [33, 40], which is known to participate in fatty-acid oxidation [40] and epilepsy [65, 66]. Therefore, it is possible for ZAG to promote fatty-acid metabolism and ketogenesis in neurons and astrocytes, and thus have a neuroprotective effect. However, no studies have investigated the effect of ZAG on fatty-acid metabolism and ketone body production in neurons and astrocytes.
The regulation of insulin sensitivity is another potential mechanism by which ZAG may act in epilepsy. Receptors for insulin are distributed widely in brain, and via these receptors, insulin modulates synaptic plasticity by regulating the release, uptake, and degradation of neurotransmitters [67, 68]. Evident IR occurs in patients with chronic epilepsy [69, 70], while in patients with type 1 diabetes mellitus who lack insulin, the risk of epilepsy is 3-fold that in normal controls [71]. And the H1085H C > T polymorphism of the insulin receptor gene is associated with drug resistance in refractory epilepsy patients [72]. Insulin recruits GABA receptors to the postsynaptic membrane and dendritic membrane, regulating the electrophysiological activity of neurons via GABA receptor-dependent and independent mechanisms [73, 74]. Insulin also regulates the expression and activity of dopamine transporters and GABA transporters, and plays a role in the antiepileptic mechanism of ketogenic diets [75]. A decrease of insulin receptors on membrane also leads to a reduction of dendrites in neurons [76]. All these findings indicate the involvement of insulin sensitivity in epilepsy. ZAG influences insulin sensitivity, but its specific role in the regulation of insulin sensitivity is still controversial based on current studies. ZAG has been positively associated with insulin sensitivity of the whole body and adipose tissue [25], and it has also been found to induce IR in adipocytes [24]. MHC I molecules are known to reduce insulin sensitivity by binding to insulin receptors [77]. As a member of MHC I molecules [3], it is possible that ZAG binds to insulin receptors and affect insulin sensitivity, but there is no direct evidence. As described above, SGLT2, PPARγ, PP2A, and Akt are involved in ZAG-related IR [31, 32]. Therefore, it is possible that ZAG participates in epilepsy by regulating insulin sensitivity.
Overexpressing ZAG suppresses the seizure-induced increase in inflammatory factors such as TNFα, IL6, and TGFβ, and the seizure-induced phosphorylation of ERK is decreased [54]. In addition, ZAG can be suppressed by TNFα [35]. Considering that these inflammatory factors have pro-epileptic effects [78–84], ZAG may suppress epilepsy by inhibiting neuroinflammation. Moreover, TNFα is known to suppress ZAG expression [35], while overexpression of ZAG inhibits TNFα expression [54], so there may be a circuit between ZAG and TNFα. Due to the limited number of reports on the interaction between ZAG and TNFα, it is difficult to dissect the specific mechanism by which these two molecules affect each other and the function of this potential circuit. Based on the literature, TNFα may inhibit ZAG expression by inhibiting PPARγ [35], because PPARγ activation increases ZAG expression [16]. The potential circuit between ZAG and TNFα indicates a possible interaction between inflammation and metabolic dysfunction, but more investigations are needed to confirm or deny its existence and function (Fig. 5).
Fig. 5.
Possible molecular mechanisms by which zinc-α2-glycoprotein (ZAG) participates in epilepsy. Descriptions of molecules and arrows in different colors are as in Fig. 1. ERK, extracellular regulated protein kinases; GABA, γ-aminobutyric acid; IL, interleukin; mTOR, mammalian target of rapamycin; PP2A, protein phosphatase 2A; PPARγ, peroxisome proliferator-activated receptor γ; SGLT2, sodium-dependent glucose transporter 2; TGFβ, tumor growth factor β; TNFα, tumor necrosis factor α; VGLUT, vesicular glutamate transporter.
Conclusion
ZAG is involved in lipid metabolism, glucose metabolism, and the regulation of insulin sensitivity. It may also participate in inflammation. ZAG is present in brain, but its distribution is still controversial. There are few studies on the role of ZAG in brain diseases, but it may suppress epilepsy by promoting ketogenesis, improving insulin sensitivity, and inhibiting neuroinflammation. The specific mechanism by which ZAG functions in epilepsy and other brain diseases, such as Alzheimer disease, needs further investigation. It is possible that research on ZAG in the CNS may discover new mechanisms of metabolic dysfunction in CNS diseases, especially considering the effectiveness of a ketogenic diet in many of these diseases. Furthermore, research on ZAG may also provide a means of reducing the adverse effects of a ketogenic diet, or develop new therapies with less adverse effects than a ketogenic diet. Besides, ZAG may regulate insulin sensitivity in brain, potentially participating in the mechanisms of CNS diseases with insulin signaling pathway dysfunction, and thus may provide new therapeutic targets for these diseases. In addition, ZAG may underlie an interaction between metabolic dysfunction and inflammation, and research on this may improve our understanding of this interaction. Finally, the physiological and pathophysiological roles of ZAG in the CNS are not clear, and research in this area, especially on regulation of the immune response, electrophysiology, and metabolism may be promising.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (81771391, 81401073), Chongqing Municipal Public Health Bureau, Chongqing People’s Municipal Government (20142026), and the Program for Innovative Research Team of Chongqing Kuanren Hospital, China.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Freije JP, Fueyo A, Uría JA, Velasco G, Sánchez LM, López-Boado YS, et al. Human Zn-alpha 2-glycoprotein: complete genomic sequence, identification of a related pseudogene and relationship to class I major histocompatibility complex genes. Genomics. 1993;18:575–587. doi: 10.1016/S0888-7543(05)80359-3. [DOI] [PubMed] [Google Scholar]
- 2.Pendás AM, Matilla T, Uría JA, Freije JP, Fueyo A, Estivill X, et al. Mapping of the human Zn-alpha 2-glycoprotein gene (AZGP1) to chromosome 7q22 by in situ hybridization. Cytogenet Cell Genet. 1994;66:263–266. doi: 10.1159/000133708. [DOI] [PubMed] [Google Scholar]
- 3.Uría JA, Fueyo A, Balbín M, Velasco G, Pendás AM, López-Otín C. Alternative splicing gives rise to two novel long isoforms of Zn-alpha 2-glycoprotein, a member of the immunoglobulin superfamily. Gene. 1996;169:233–236. doi: 10.1016/0378-1119(95)00727-X. [DOI] [PubMed] [Google Scholar]
- 4.Hassan I, Ahmad F. Structural diversity of class I MHC-like molecules and its implications in binding specificities. Adv Protein Chem Struct Biol. 2011;83:223–270. doi: 10.1016/B978-0-12-381262-9.00006-9. [DOI] [PubMed] [Google Scholar]
- 5.Kumar AA, Hati D, Thaker TM, Miah L, Cunningham P, Domene C, et al. Strong and weak zinc binding sites in human zinc-α2-glycoprotein. FEBS Lett. 2013;587:3949–3954. doi: 10.1016/j.febslet.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Zahid H, Miah L, Lau AM, Brochard L, Hati D, Bui TT, et al. Zinc-induced oligomerization of zinc α2 glycoprotein reveals multiple fatty acid-binding sites. Biochem J. 2016;473:43–54. doi: 10.1042/BJ20150836. [DOI] [PubMed] [Google Scholar]
- 7.Delker SL, West AP, Jr, McDermott L, Kennedy MW, Bjorkman PJ. Crystallographic studies of ligand binding by Zn-alpha2-glycoprotein. J Struct Biol. 2004;148:205–213. doi: 10.1016/j.jsb.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Marrades MP, Martínez JA, Moreno-Aliaga MJ. ZAG, a lipid mobilizing adipokine, is downregulated in human obesity. J Physiol Biochem. 2008;64:61–66. doi: 10.1007/BF03168235. [DOI] [PubMed] [Google Scholar]
- 9.Yeung DC, Lam KS, Wang Y, Tso AW, Xu A. Serum zinc-alpha2-glycoprotein correlates with adiposity, triglycerides, and the key components of the metabolic syndrome in Chinese subjects. J Clin Endocrinol Metab. 2009;94:2531–2536. doi: 10.1210/jc.2009-0058. [DOI] [PubMed] [Google Scholar]
- 10.Tzanavari T, Bing C, Trayhurn P. Postnatal expression of zinc-alpha2-glycoprotein in rat white and brown adipose tissue. Mol Cell Endocrinol. 2007;279:26–33. doi: 10.1016/j.mce.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Russell ST, Tisdale MJ. Studies on the anti-obesity activity of zinc-α2-glycoprotein in the rat. Int J Obes (Lond) 2011;35:658–665. doi: 10.1038/ijo.2010.193. [DOI] [PubMed] [Google Scholar]
- 12.Zhu HJ, Ding HH, Deng JY, Pan H, Wang LJ, Li NS, et al. Inhibition of preadipocyte differentiation and adipogenesis by zinc-α2-glycoprotein treatment in 3T3-L1 cells. J Diabetes Investig. 2013;4:252–260. doi: 10.1111/jdi.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao X, Li H, Qi X, Wang Y, Xu C, Liu G, et al. Zinc alpha2 glycoprotein alleviates palmitic acid-induced intracellular lipid accumulation in hepatocytes. Mol Cell Endocrinol. 2017;439:155–164. doi: 10.1016/j.mce.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Choi JW, Liu H, Mukherjee R, Yun JW. Downregulation of fetuin-B and zinc-α2-glycoprotein is linked to impaired fatty acid metabolism in liver cells. Cell Physiol Biochem. 2012;30:295–306. doi: 10.1159/000339065. [DOI] [PubMed] [Google Scholar]
- 15.Elattar S, Dimri M, Satyanarayana A. The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. FASEB J. 2018;32:4727–4743. doi: 10.1096/fj.201701465RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao Y, Bing C, Hunter L, Jenkins JR, Wabitsch M, Trayhurn P. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Lett. 2005;579:41–47. doi: 10.1016/j.febslet.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 17.Xiao XH, Qi XY, Wang YD, Ran L, Yang J, Zhang HL, et al. Zinc alpha2 glycoprotein promotes browning in adipocytes. Biochem Biophys Res Commun. 2018;496:287–293. doi: 10.1016/j.bbrc.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 18.Sanders PM, Tisdale MJ. Effect of zinc-alpha2-glycoprotein (ZAG) on expression of uncoupling proteins in skeletal muscle and adipose tissue. Cancer Lett. 2004;212:71–81. doi: 10.1016/j.canlet.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Balaz M, Ukropcova B, Kurdiova T, Gajdosechova L, Vlcek M, Janakova Z, et al. Adipokine zinc-α2-glycoprotein regulated by growth hormone and linked to insulin sensitivity. Obesity (Silver Spring) 2015;23:322–328. doi: 10.1002/oby.20856. [DOI] [PubMed] [Google Scholar]
- 20.Balaž M, Ukropcova B, Kurdiova T, Vlcek M, Surova M, Krumpolec P, et al. Improved adipose tissue metabolism after 5-year growth hormone replacement therapy in growth hormone deficient adults: The role of zinc-α2-glycoprotein. Adipocyte. 2014;4:113–122. doi: 10.4161/21623945.2014.973772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong FY, Zhang SJ, Deng JY, Zhu HJ, Pan H, Li NS, et al. Zinc-alpha2-glycoprotein is involved in regulation of body weight through inhibition of lipogenic enzymes in adipose tissue. Int J Obes (Lond) 2009;33:1023–1030. doi: 10.1038/ijo.2009.141. [DOI] [PubMed] [Google Scholar]
- 22.Wargent ET, O’Dowd JF, Zaibi MS, Gao D, Bing C, Trayhurn P, et al. Contrasts between the effects of zinc-α2-glycoprotein, a putative β3/2-adrenoceptor agonist and the β3/2-adrenoceptor agonist BRL35135 in C57Bl/6 (ob/ob) mice. J Endocrinol. 2013;216:157–168. doi: 10.1530/JOE-12-0402. [DOI] [PubMed] [Google Scholar]
- 23.Russell ST, Tisdale MJ. Role of β-adrenergic receptors in the anti-obesity and anti-diabetic effects of zinc-α2-glycoprotien (ZAG) Biochim Biophys Acta. 2012;1821:590–599. doi: 10.1016/j.bbalip.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Ceperuelo-Mallafré V, Ejarque M, Duran X, Pachón G, Vázquez-Carballo A, Roche K, et al. Zinc-α2-glycoprotein modulates AKT-dependent insulin signaling in human adipocytes by activation of the PP2A phosphatase. PLoS One. 2015;10:e0129644. doi: 10.1371/journal.pone.0129644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balaz M, Vician M, Janakova Z, Kurdiova T, Surova M, Imrich R, et al. Subcutaneous adipose tissue zinc-α2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity (Silver Spring) 2014;22:1821–1829. doi: 10.1002/oby.20764. [DOI] [PubMed] [Google Scholar]
- 26.Barraco GM, Luciano R, Manco M. Zinc-α2 -glycoprotein is associated with insulin resistance in children. Obesity (Silver Spring) 2015;23:5–6. doi: 10.1002/oby.20948. [DOI] [PubMed] [Google Scholar]
- 27.Mracek T, Ding Q, Tzanavari T, Kos K, Pinkney J, Wilding J, et al. The adipokine zinc-alpha2-glycoprotein (ZAG) is downregulated with fat mass expansion in obesity. Clin Endocrinol (Oxf) 2010;72:334–341. doi: 10.1111/j.1365-2265.2009.03658.x. [DOI] [PubMed] [Google Scholar]
- 28.Lai Y, Chen J, Li L, Yin J, He J, Yang M, et al. Circulating zinc-α2-glycoprotein levels and insulin resistance in polycystic ovary syndrome. Sci Rep. 2016;6:25934. doi: 10.1038/srep25934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian M, Liang Z, Liu R, Li K, Tan X, Luo Y, et al. Effects of sitagliptin on circulating zinc-α2-glycoprotein levels in newly diagnosed type 2 diabetes patients: a randomized trial. Eur J Endocrinol. 2016;174:147–155. doi: 10.1530/EJE-15-0637. [DOI] [PubMed] [Google Scholar]
- 30.Qu C, Zhou X, Yang G, Li L, Liu H, Liang Z. The natural logarithm of zinc-α2-glycoprotein/HOMA-IR is a better predictor of insulin sensitivity than the product of triglycerides and glucose and the other lipid ratios. Cytokine. 2016;79:96–102. doi: 10.1016/j.cyto.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Liao X, Wang X, Li H, Li L, Zhang G, Yang M, et al. Sodium-glucose cotransporter 2 (SGLT2) inhibitor increases circulating zinc-Α2-glycoprotein levels in patients with type 2 diabetes. Sci Rep. 2016;6:32887. doi: 10.1038/srep32887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selva DM, Lecube A, Hernández C, Baena JA, Fort JM, Simó R. Lower zinc-alpha2-glycoprotein production by adipose tissue and liver in obese patients unrelated to insulin resistance. J Clin Endocrinol Metab. 2009;94:4499–4507. doi: 10.1210/jc.2009-0758. [DOI] [PubMed] [Google Scholar]
- 33.Russell ST, Tisdale MJ. Mechanism of attenuation of skeletal muscle atrophy by zinc-alpha2-glycoprotein. Endocrinology. 2010;151:4696–4704. doi: 10.1210/en.2010-0532. [DOI] [PubMed] [Google Scholar]
- 34.Mracek T, Gao D, Tzanavari T, Bao Y, Xiao X, Stocker C, et al. Downregulation of zinc-{alpha}2-glycoprotein in adipose tissue and liver of obese ob/ob mice and by tumour necrosis factor-alpha in adipocytes. J Endocrinol. 2010;204:165–172. doi: 10.1677/JOE-09-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao D, Trayhurn P, Bing C. Macrophage-secreted factors inhibit ZAG expression and secretion by human adipocytes. Mol Cell Endocrinol. 2010;325:135–142. doi: 10.1016/j.mce.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Leal VO, Lobo JC, Stockler-Pinto MB, Farage NE, Abdalla DS, Leite M, Jr, et al. Is zinc-α2-glycoprotein a cardiovascular protective factor for patients undergoing hemodialysis? Clin Chim Acta. 2012;413:616–619. doi: 10.1016/j.cca.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Kong B, Michalski CW, Hong X, Valkovskaya N, Rieder S, Abiatari I, et al. AZGP1 is a tumor suppressor in pancreatic cancer inducing mesenchymal-to-epithelial transdifferentiation by inhibiting TGF-β-mediated ERK signaling. Oncogene. 2010;29:5146–5158. doi: 10.1038/onc.2010.258. [DOI] [PubMed] [Google Scholar]
- 38.Xu MY, Chen R, Yu JX, Liu T, Qu Y, Lu LG. AZGP1 suppresses epithelial-to-mesenchymal transition and hepatic carcinogenesis by blocking TGFβ1-ERK2 pathways. Cancer Lett. 2016;374:241–249. doi: 10.1016/j.canlet.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Sörensen-Zender I, Bhayana S, Susnik N, Rolli V, Batkai S, Baisantry A, et al. Zinc-α2-Glycoprotein Exerts Antifibrotic Effects in Kidney and Heart. J Am Soc Nephrol. 2015;26:2568–2659. doi: 10.1681/ASN.2014050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang L, Tian X, Lu Y, Jia M, Wu P, Huang P. Alpha-2-glycoprotein 1(AZGP1) regulates biological behaviors of LoVo cells by down-regulating mTOR signaling pathway and endogenous fatty acid synthesis. PLoS One. 2014;9:e99254. doi: 10.1371/journal.pone.0099254. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Tian H, Ge C, Zhao F, Zhu M, Zhang L, Huo Q, et al. Downregulation of AZGP1 by Ikaros and histone deacetylase promotes tumor progression through the PTEN/Akt and CD44 s pathways in hepatocellular carcinoma. Carcinogenesis. 2017;38:207–217. doi: 10.1093/carcin/bgw125. [DOI] [PubMed] [Google Scholar]
- 42.Simó R, Hernández C, Sáez-López C, Soldevila B, Puig-Domingo M, Selva DM. Thyroid hormone upregulates zinc-α2-glycoprotein production in the liver but not in adipose tissue. PLoS One. 2014;9:e85753. doi: 10.1371/journal.pone.0085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Böhm M, Locke WJ, Sutherland RL, Kench JG, Henshall SM. A role for GATA-2 in transition to an aggressive phenotype in prostate cancer through modulation of key androgen-regulated genes. Oncogene. 2009;28:3847–3856. doi: 10.1038/onc.2009.243. [DOI] [PubMed] [Google Scholar]
- 44.Russell ST, Tisdale MJ. The role of glucocorticoids in the induction of zinc-alpha2-glycoprotein expression in adipose tissue in cancer cachexia. Br J Cancer. 2005;92:876–881. doi: 10.1038/sj.bjc.6602404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maślińska D, Laure-Kamionowska M, Maślińska S. Crosstalk in human brain between globoid cell leucodystrophy and zinc-a-2-glycoprotein (ZAG), a biomarker of lipid catabolism. Folia Neuropathol. 2013;51:312–318. doi: 10.5114/fn.2013.39720. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Wang T, Liu X, Wei X, Xu T, Yin M, et al. Neuronal zinc-α2-glycoprotein is decreased in temporal lobe epilepsy in patients and rats. Neuroscience. 2017;357:56–66. doi: 10.1016/j.neuroscience.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 47.Chow ML, Pramparo T, Winn ME, Barnes CC, Li HR, Weiss L, et al. Age-dependent brain gene expression and copy number anomalies in autism suggest distinct pathological processes at young versus mature ages. PLoS Genet. 2012;8:e1002592. doi: 10.1371/journal.pgen.1002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuwaki T, Asakura R, Suzuki M, Yamanouchi K, Nishihara M. Age-dependent changes in progranulin expression in the mouse brain. J Reprod Dev. 2011;57:113–119. doi: 10.1262/jrd.10-116S. [DOI] [PubMed] [Google Scholar]
- 49.Prüss H, Grosse G, Brunk I, Veh RW, Ahnert-Hilger G. Age-dependent axonal expression of potassium channel proteins during development in mouse hippocampus. Histochem Cell Biol. 2010;133:301–312. doi: 10.1007/s00418-009-0668-z. [DOI] [PubMed] [Google Scholar]
- 50.Brettschneider J, Mogel H, Lehmensiek V, Ahlert T, Süssmuth S, Ludolph AC, et al. Proteome analysis of cerebrospinal fluid in amyotrophic lateral sclerosis (ALS) Neurochem Res. 2008;33:2358–2363. doi: 10.1007/s11064-008-9742-5. [DOI] [PubMed] [Google Scholar]
- 51.Hu Y, Hosseini A, Kauwe JS, Gross J, Cairns NJ, Goate AM, et al. Identification and validation of novel CSF biomarkers for early stages of Alzheimer’s disease. Proteomics Clin Appl. 2007;1:1373–1384. doi: 10.1002/prca.200600999. [DOI] [PubMed] [Google Scholar]
- 52.Hansson SF, Puchades M, Blennow K, Sjögren M, Davidsson P. Validation of a prefractionation method followed by two-dimensional electrophoresis-Applied to cerebrospinal fluid proteins from frontotemporal dementia patients. Proteome Sci. 2004;2:7. doi: 10.1186/1477-5956-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Ou S, Xu T, Liu S, Yuan J, Huang H, et al. New differentially expressed genes and differential DNA methylation underlying refractory epilepsy. Oncotarget. 2016;7:87402–87416. doi: 10.18632/oncotarget.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Wang T, Liu X, Wen Y, Xu T, Yu X, et al. Overexpression of zinc-α2-glycoprotein suppressed seizures and seizure-related neuroflammation in pentylenetetrazolkindled rats. J Neuroinflammation. 2018;15:92. doi: 10.1186/s12974-018-1132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang M, Liu R, Li S, Luo Y, Zhang Y, Zhang L, et al. Zinc-α2-glycoprotein is associated with insulin resistance in humans and is regulated by hyperglycemia, hyperinsulinemia, or liraglutide administration: cross-sectional and interventional studies in normal subjects, insulin-resistant subjects, and subjects with newly diagnosed diabetes. Diabetes Care. 2013;36:1074–1082. doi: 10.2337/dc12-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koshal P, Kumar P. Neurochemical modulation involved in the beneficial effect of liraglutide, GLP-1 agonist on PTZ kindling epilepsy-induced comorbidities in mice. Mol Cell Biochem. 2016;415:77–87. doi: 10.1007/s11010-016-2678-1. [DOI] [PubMed] [Google Scholar]
- 57.Koshal P, Kumar P. Effect of liraglutide on corneal kindling epilepsy induced depression and cognitive impairment in mice. Neurochem Res. 2016;41:1741–1750. doi: 10.1007/s11064-016-1890-4. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi S, Iizumi T, Mashima K, Abe T, Suzuki N. Roles and regulation of ketogenesis in cultured astroglia and neurons under hypoxia and hypoglycemia. ASN Neuro 2014, 6. pii: 1759091414550997. [DOI] [PMC free article] [PubMed]
- 59.Guzmán M, Blázquez C. Ketone body synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot Essent Fatty Acids. 2004;70:287–292. doi: 10.1016/j.plefa.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78:77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melo IS, Santos YM, Costa MA, Pacheco AL, Silva NK, Cardoso-Sousa L, et al. Inhibition of sodium glucose cotransporters following status epilepticus induced by intrahippocampal pilocarpine affects neurodegeneration process in hippocampus. Epilepsy Behav. 2016;61:258–268. doi: 10.1016/j.yebeh.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 62.Auvin S. Fatty acid oxidation and epilepsy. Epilepsy Res. 2012;100:224–228. doi: 10.1016/j.eplepsyres.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 63.Taha AY, Burnham WM, Auvin S. Polyunsaturated fatty acids and epilepsy. Epilepsia. 2010;51:1348–1358. doi: 10.1111/j.1528-1167.2010.02654.x. [DOI] [PubMed] [Google Scholar]
- 64.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 65.Lorigados Pedre L, Morales Chacón LM, Orozco Suárez S, Pavón Fuentes N, Estupiñán Díaz B, Serrano Sánchez T, et al. Inflammatory mediators in epilepsy. Curr Pharm Des. 2013;19:6766–6772. doi: 10.2174/1381612811319380009. [DOI] [PubMed] [Google Scholar]
- 66.Zhu F, Kai J, Chen L, Wu M, Dong J, Wang Q, et al. Akt Inhibitor Perifosine Prevents Epileptogenesis in a Rat Model of Temporal Lobe Epilepsy. Neurosci Bull. 2018;34:283–290. doi: 10.1007/s12264-017-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J, Spéder P, Brand AH. Control of brain development and homeostasis by local and systemic insulin signalling. Diabetes Obes Metab. 2014;16(Suppl 1):16–20. doi: 10.1111/dom.12337. [DOI] [PubMed] [Google Scholar]
- 68.Gralle M. The neuronal insulin receptor in its environment. J Neurochem. 2017;140:359–367. doi: 10.1111/jnc.13909. [DOI] [PubMed] [Google Scholar]
- 69.Hermann BP, Sager MA, Koscik RL, Young K, Nakamura K. Vascular, inflammatory, and metabolic factors associated with cognition in aging persons with chronic epilepsy. Epilepsia. 2017;58:e152–e156. doi: 10.1111/epi.13891. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Liu G, He M, Shen L, Shen D, Lu Y, et al. Increased insulin receptor expression in anterior temporal neocortex of patients with intractable epilepsy. J Neurol Sci. 2010;296:64–68. doi: 10.1016/j.jns.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Dafoulas GE, Toulis KA, Mccorry D, Kumarendran B, Thomas GN, Willis BH, et al. Type 1 diabetes mellitus and risk of incident epilepsy: a population-based, open-cohort study. Diabetologia. 2017;60:258–261. doi: 10.1007/s00125-016-4142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Che F, Fu Q, Li X, Gao N, Qi F, Sun Z, et al. Association of insulin receptor H1085H C > T, insulin receptor substrate 1 G972R and insulin receptor substrate 2 1057G/A polymorphisms with refractory temporal lobe epilepsy in Han Chinese. Seizure. 2015;25:178–180. doi: 10.1016/j.seizure.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 73.Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, et al. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 74.Kovacs P, Hajnal A. In vivo electrophysiological effects of insulin in the rat brain. Neuropeptides. 2009;43:283–293. doi: 10.1016/j.npep.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Figlewicz DP. Endocrine regulation of neurotransmitter transporters. Epilepsy Res. 1999;37:203–210. doi: 10.1016/S0920-1211(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 76.Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58:708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dixon-Salazar TJ, Fourgeaud L, Tyler CM, Poole JR, Park JJ, Boulanger LM. MHC class I limits hippocampal synapse density by inhibiting neuronal insulin receptor signaling. J Neurosci. 2014;34:11844–11856. doi: 10.1523/JNEUROSCI.4642-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levy N, Milikovsky DZ, Baranauskas G, Vinogradov E, David Y, Ketzef M, et al. Differential TGF-β signaling in glial subsets underlies IL-6-mediated epileptogenesis in mice. J Immunol. 2015;195:1713–1722. doi: 10.4049/jimmunol.1401446. [DOI] [PubMed] [Google Scholar]
- 79.Bar-Klein G, Cacheaux LP, Kamintsky L, Prager O, Weissberg I, Schoknecht K, et al. Losartan prevents acquired epilepsy via TGF-β signaling suppression. Ann Neurol. 2014;75:864–875. doi: 10.1002/ana.24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nateri AS, Raivich G, Gebhardt C, Da Costa C, Naumann H, Vreugdenhil M, et al. Activation causes epilepsy by stimulating NMDA receptor activity. EMBO J. 2007;26:4891–4901. doi: 10.1038/sj.emboj.7601911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Itoh T, Kaibuchi K, Masuda T, Yamamoto T, Matsuura Y, Maeda A, et al. A protein factor for ras p21-dependent activation of mitogenactivated protein (MAP) kinase through MAP kinase kinase. Proc Natl Acad Sci U S A. 1993;90:975–979. doi: 10.1073/pnas.90.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 83.Probert L, Akassoglou K, Pasparakis M, Kontogeorgos G, Kollias G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1995;92:11294–11298. doi: 10.1073/pnas.92.24.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen L, Liu X, Wang H, Qu M. Gastrodin attenuates pentylenetetrazole-induced seizures by modulating the mitogen-activated protein kinase-associated inflammatory responses in mice. Neurosci Bull. 2017;33:264–272. doi: 10.1007/s12264-016-0084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]