Abstract
Pancreatectomy might confer a survival benefit in patients with metastatic tumors of the pancreas (MTPs); however, the optimal treatment for MTP has not been established. We reviewed six patients with MTP undergoing pancreatectomy and discussed the clinical features, surgical treatment, and survival. The sites of primary cancer included renal cell carcinoma (RCC) (n = 5; 83.3%) and rectal cancer (n = 1; 16.7%). The median interval between the resection of the primary site and the development of MTP was 157 months (range, 16–180 months). Three (60.0%) of the five cases of MTP-originating RCC and a MTP-originating rectal cancer, biopsy was performed under endoscopic ultrasonography guidance and MTP was pathologically diagnosed. All patients with MTP originating from RCC have remained alive for 3, 13, 18, 18, and 113 months without recurrence after pancreatectomy. In contrast, the patient with MTP originating from rectal cancer developed multiple liver metastases at 7 months after pancreatectomy, and then underwent chemotherapy. A preoperative pathological diagnosis using biopsy under endoscopic ultrasonography guidance was indispensable for the treatment of MTP. Pancreatectomy for MTP conferred a survival benefit in patients with metastatic RCC, whereas a combination of pancreatectomy and chemotherapy might be necessary to improve the prognosis of patients with metastatic colorectal cancer.
Keywords: Pancreatic metastasis, Pancreatic resection, Secondary tumors, Renal cell carcinoma, Colorectal cancer
Introduction
Metastatic tumors of the pancreas (MTPs) account for less than 5% of pancreatic malignancies diagnosed in living patients [1, 2]. Renal cell carcinoma (RCC) is the most common primary tumor to cause MTP [3], followed by sarcoma, colorectal cancer (CRC), ovarian cancer, and melanoma [4–6]. Several reports have indicated that pancreatectomy might confer a survival benefit in patients with MTP [4, 6], however, the optimal treatment for MTP has not established due to the rarity of the disease [4, 5]. A certain number of retrospective case series and reviews suggest that pancreatectomy confers a survival benefit in patients with MTP originating from RCC; however, it is difficult to provide clear evidence. Furthermore, due to the small number of cases in which pancreatectomy has been performed for patients with MTP originating from CRC, we could not find any recommendations for its treatment. In the present study, we retrospectively reviewed six cases of MTP—five originating from RCC and one originating from rectal cancer—in which pancreatectomy was performed and the clinical features, surgical treatment, and survival were discussed.
Materials and Methods
Clinical Data Collection
We retrospectively reviewed the database of Saitama Medical Center, Jichi Medical University, between February 2006 and June 2017. During this period, six patients underwent pancreatectomy for MTP. The primary sites of the six patients included RCC (n = 5) and rectal cancer (n = 1). The demographic and clinical variables that were collected included gender, age, site, and histology of the primary tumor; interval between surgery of the primary site and MTP; location and size of MTP; characteristics on enhanced abdominal CT or enhanced ultrasonography; surgical procedure; presence of lymph node metastasis; morbidity; postoperative hospital stay; and overall survival time after pancreatectomy. These data were retrospectively collected by a medical chart review and the clinical features, surgical treatment, and survival were assessed. This study was reviewed and approved by the Institutional Review Board of Jichi Medical University.
Indications for Pancreatic Resection for MTP
The indications for pancreatectomy in patients with MTP were as follows: (1) the primary tumor was resected; (2) no other synchronous metastatic lesions (other than MTP) are present; (3) pancreatectomy has the potential to achieve curative resection; and (4) the patient can tolerate pancreatic surgery.
Surgical Procedure
The type of pancreatectomy, which included pancreatoduodenectomy, distal pancreatectomy, and total pancreatectomy, was selected based on the preoperative findings. When we found tumor infiltration to the portal vein or superior mesenteric vein intraoperatively, combined resection was performed to achieve a curative resection. We also performed regional lymph node dissection, because previous studies have reported that metastasis to the regional lymph nodes was detected in 20–30% of patients with MTP [7, 8].
Results
Between February 2006 and June 2017, 308 patients underwent pancreatic resection for pancreatic neoplasms at our hospital, and the incidence of MTPs in resected pancreatic neoplasms was 1.9%. Table 1 shows the clinical features of six patients who underwent pancreatectomy for MTP. This series included two men and four women; the median age was 69 years (range, 52–79 years). The sites of the primary cancer included RCC (n = 5; 83.3%) of RCC and rectal cancer (n = 1; 16.7%). The median of interval between the resection of the primary site and MTP was 157 months (range, 16–180 months). In one case, MTP was found after a patient presented with back pain. None of the other five patients showed symptoms and MTP was found by routine follow-up CT or incidentally detected during surveillance for another disease. The median size of MTPs, which occurred in various locations, was 28.5 mm (range, 11–35 mm). One patient, whose primary tumor was RCC, harbored multiple MTPs.
Table 1.
The demographic and clinical variables of the six patients
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Gender | Female | Male | Female | Female | Female | Male |
| Age | 69 | 72 | 73 | 79 | 71 | 52 |
| Site of primary tumor | RCC (lt.) | RCC (rt.) | RCC (lt.) | RCC (lt.) | RCC (lt.) | Rectal cancer |
| Pathological diagnosis of primary tumor | N/A | Clear cell carcinoma | Clear cell carcinoma | Clear cell carcinoma | N/A | Well-differentiated adenocarcinoma |
| Pathological stage of primary tumor | N/A | T1bN0M0 | T1bN0M0 | T2 | N/A | T3N2aM0 |
| Interval from primary site resection | 12 y | 1 y and 4 m | 14 y and 2 m | 6 y and 3 m | 15 y | 6 y and 4 m |
| Location of pancreatic tumor | Tail | Tail | Head/body/tail | Head | Tail | Head |
| Tumor size | 35 mm | 26 mm | 22/34/11 mm | 31 mm | 16 mm | 33 mm |
| Vascularity | Hyper | Hyper | N/A | Hyper | Hyper | Ring-enhanced |
| Surgical procedure | DP | DP | TP | PD | DP | PD |
| Lymph node metastasis | Negative | Negative | Negative | Negative | Negative | Negative |
| Morbidity | Peptic ulcer | – | – | – | – | Intra-abdominal abscess |
| Prognosis | Alive | Alive | Alive | Alive | Alive | Dead |
| Survival time after pancreatectomy | 9 y and 5 m | 1 y and 6 m | 1 y and 6 m | 1 y and 1 m | 3 m | 2 y and 5 m |
RCC renal cell carcinoma, lt left side, rt. right side, N/A not available, y year(s), m month(s), DP distal pancreatectomy, TP total pancreatectomy, PD pancreatoduodenectomy
The clinical features of the five patients with MTP originating from RCC were as follows. One primary RCC was right-sided; four primary RCCs were left-sided. Pathological examinations of the primary RCC tumors revealed that two cases were stage I (according to the eighth edition of the Union of International Cancer Control) [9]; the results of the pathological examinations were not available for the other three cases. Four (80.0%) of the five cases involving MTP originating from RCC showed strong enhancement on CT (Fig. 1) or ultrasonography (Fig. 2). The other one did not undergo enhanced CT or ultrasonography due to chronic renal dysfunction. In three (60.0%) of the five cases of MTP originating RCC, biopsy was performed under endoscopic ultrasonography guidance and MTP originating from RCC was pathologically diagnosed.
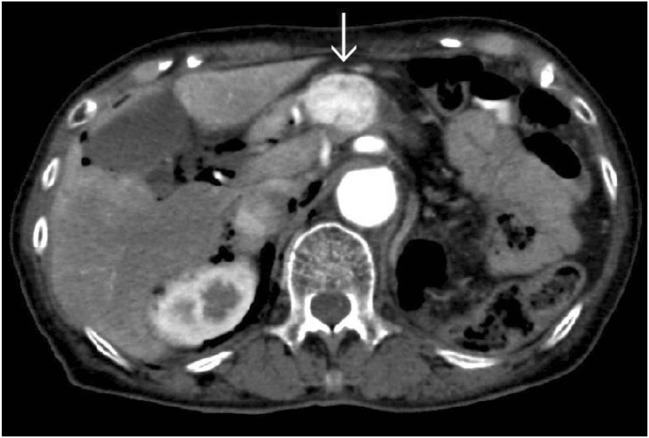
Fig. 1.
Abdominal enhanced computed tomography (CT) showed a hypervascular mass of 35 mm in size located from the pancreatic body to the pancreatic head, the primary lesion of which was renal cell carcinoma (Case 4 in Table 1). Arrow: tumor
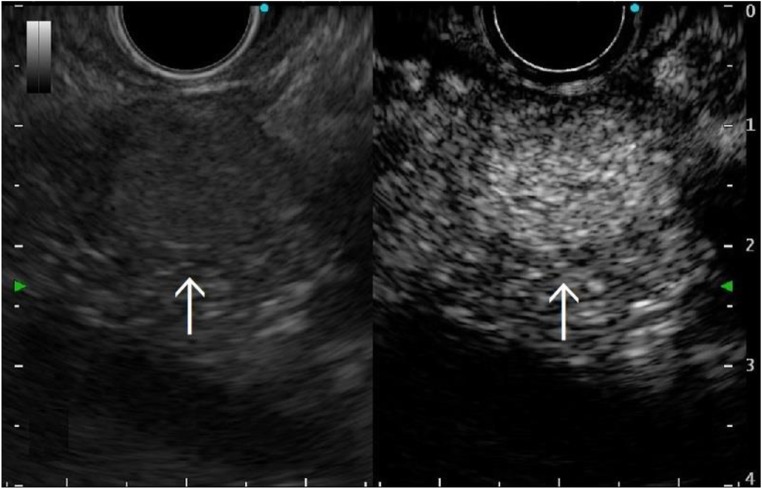
Fig. 2.
Abdominal enhanced ultrasonography showed a hypervascular mass of 16 mm in size located in the pancreatic tail, the primary lesion of which was renal cell carcinoma (Case 5 in Table 1). Arrow: tumor
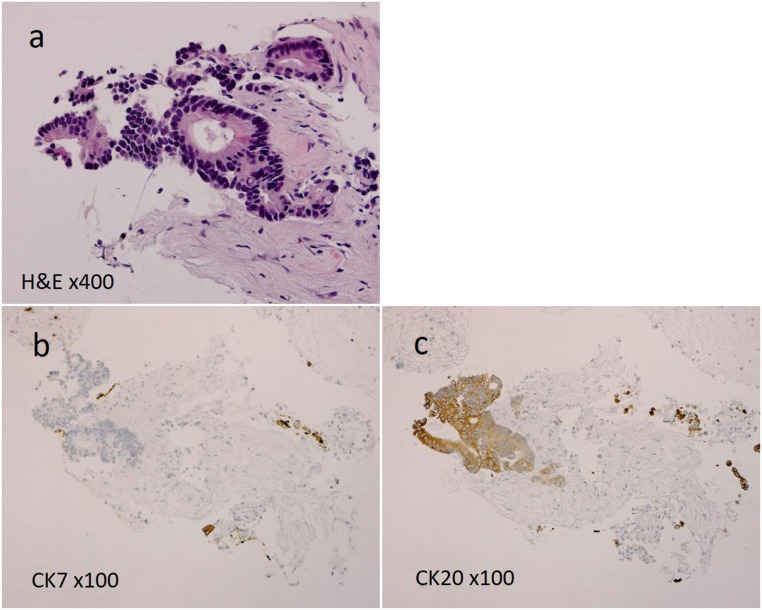
The brief clinical course of the patient with MTP originating from rectal cancer was as follows. The patient was a man aged in his 50s who underwent resection for rectal cancer. The tumor was pathologically diagnosed as stage IIIB (T3, N2a, M0) according to the eighth edition of the UICC [9]. He underwent resection for metachronous lung metastases at 36 and 40 months after primary resection, after which a pancreatic head mass associated with ring enhancement was detected by abdominal CT (Fig. 3). A biopsy was performed under endoscopic ultrasonography guidance and found a well-differentiated adenocarcinoma (Fig. 4a). Immunohistochemistry (IHC) showed that cells were negative for cytokeratin (CK) 7 (Fig. 4b) and positive for CK 20 (Fig. 4c), and the final diagnosis was a metachronous MTP originating from RCC. The interval between primary resection and the appearance of the pancreatic head mass was 6 years. Prior to pancreatectomy for MTP, he had not received any chemotherapy.
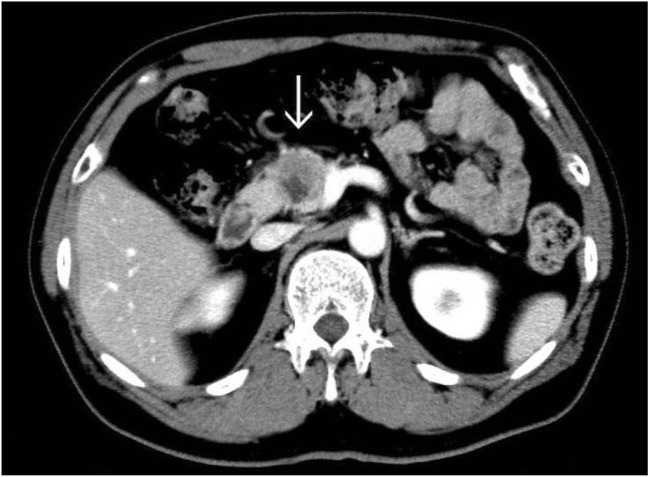
Fig. 3.
Abdominal enhanced CT showed a ring-enhanced mass of 33 mm in size that contained necrotic tissue and which was located in the pancreatic head. The primary lesion was rectal cancer (Case 6 in Table 1). Arrow: tumor
Fig. 4.
Pathological study found a well-differentiated adenocarcinoma (Fig. 4a), and tumor cells were negative for CK 7 (Fig. 4b) and positive for CK 20 (Fig. 4c) immunohistochemically
The surgical procedures for MTP included distal pancreatectomy (n = 3), pancreatoduodenectomy (n = 2), and total pancreatectomy (n = 1). The pathological examinations revealed no lymph node metastasis in six cases. Postoperative complications occurred in two patients (duodenal ulcer and intra-abdominal abscess). The median postoperative hospital stay was 30 days (range, 11–44 days).
At the time of writing, all of the patients with MTP originating from RCC remain alive without recurrence at 3, 13, 18, 18, and 113 months, respectively, after pancreatectomy (the median postoperative observation period was 18 months; range, 3–113 months); none of these patients received chemotherapy. In contrast, the patient with MTP originating from rectal cancer developed multiple liver metastases at 7 months after pancreatectomy. He received chemotherapy for 22 months as treatment for liver metastasis.
Discussion
Pancreatectomy for MTP is uncommon and the optimal treatment for MTP remains to be established. Recent reports show that the morbidity and mortality rates of pancreatectomy have improved [10–12], and the number of retrospective case series and reviews on pancreatectomy for MTP is increasing. In these reports, similarly to our series, the most common primary cancer of MTP patients who underwent pancreatectomy was RCC (58.6–73.9%), and followed by sarcoma, CRC, ovarian cancer, and melanoma [4–6, 13]. The median interval between resection at the primary site and MTP was 65.9–122.4 months [4–6, 14], and the interval tended to be longer in cases of RCC than colorectal cancer (108 months vs 24 months) [5]. In this series, the median interval between the resection at the primary site and MTP of RCC was 157 months, and despite the limited number of the cases, it seems longer in comparison to previous reports. Thus, when RCC patients present pancreatic tumors after resection, it is important to evaluate the possibility of MTP, even when the interval is ≥ 10 years.
Patients with MTP originating from RCC showed hypervascular tumors on enhanced CT, MRI, and ultrasonography, which resembled pancreatic neuroendocrine tumors and clear cell adenocarcinoma of the pancreas, similarly to our cases [15]. Clear cell adenocarcinoma is extremely rare and has been reported in only a few cases [16]; it is therefore important to distinguish among these entities, as the surgical strategies for MTP originating from RCC and pancreatic neuroendocrine tumors are different. The recommended surgical procedures for pancreatic neuroendocrine tumors differ according to the tumor size, and include enucleation, partial resection with or without lymph node dissection, and standard pancreatectomy with lymph node dissection [17, 18]. When the tumor size is small, observation is acceptable [17, 18]. Conversely, pancreatectomy has been shown to improve of survival outcomes of patients with MTP originating from RCC [2, 4–6, 19, 20]. Several recent studies have suggested that therapy with tyrosine kinase inhibitors (TKIs) for MTP originating from RCC had long-term efficacy comparable to surgery, but the disease-free survival was better with the surgical approach [21, 22]. We also demonstrated that all five of our patients with MTP originating from RCC are currently alive without recurrence after pancreatectomy. Thus, an appropriate diagnosis and surgical treatment are important for patients with MTP originating from RCC. As mentioned, we have recently diagnosed MTP originating from RCC based on biopsy under endoscopic ultrasonography guidance; this technique may be useful for distinguishing MTP originating from RCC from other types of hypervascular tumors, such as pancreatic neuroendocrine tumors. When it is pathologically difficult to distinguish between MTP originating from RCC and other entities, including ductal adenocarcinoma with clear cell appearance, clear cell endocrine pancreatic tumor, solid serous adenoma of the pancreas, clear cell variant of solid pseudopapillary tumor of the pancreas, and perivascular epithelioid cell tumor, determining the CD10 and PAX8 expression on IHC is useful for diagnosing MTP originating from RCC [15]. In addition, CT scan could not distinguish MTP originating from CRC and primary pancreatic adenocarcinoma in this series and IHC for biopsy specimens collected under endoscopic ultrasonography guidance was particularly useful to diagnose, as same as a previous report [23]. Previous studies reported that IHC found that 92% of pancreatic adenocarcinomas were positive for CK 7 versus 5% of colorectal carcinoma [24, 25]. In our case, tumor cells were negative for CK 7 and positive for CK 20 and the preoperative diagnosis was a MTP originating from CRC. When biopsy specimens collected under endoscopic ultrasonography is limited, IHC might not be feasible, but IHC may be recommended for patient who associates pancreatic mass and past history of colorectal cancer to make a precise diagnosis and appropriate therapeutic strategy.
In the treatment of metastasized CRC, it was well known that hepatectomy improved the prognosis of patients with metastatic liver tumors, and surgical resection is still considered the only curative option for patients with liver metastasis [26]. Kato et al. reported that the 5-year survival rate of CRC patients with liver metastasis who were treated by hepatectomy was significantly higher (32.9%) than that of those who did not undergo hepatectomy (3.4%) [27]. Similarly, pancreatectomy might confer a survival benefit in cases of MTP originating from CRC; however, the reported recurrence and survival rates after pancreatectomy for MTP originating from non-RCC have differed in previous studies [13, 28, 29], and the impact of pancreatectomy on MTP originating from CRC has been unclear due to the small number of cases available. Hung et al. reported that the median survival time and 5-year survival rate after pancreatectomy for MTP originating from CRC were 24.0 months and 24.6%, respectively [5]. On the other hand, the median survival time of patients with unresectable CRC has been prolonged to approximately 30 months with chemotherapy [30]. In the present series, the patient with MTP originating from rectal cancer has remained alive for 8 years and 9 months after primary resection, three resections of metastasis (two resections of lung metastasis and one resection of MTP), and subsequent chemotherapy. A recent meta-analysis demonstrated that the use of chemotherapy for patients with resectable colorectal liver metastases who underwent resection with curative intent was a worthwhile strategy that improved both recurrence-free survival and overall survival [31]. Thus, our case might also suggest that a combination of pancreatectomy and chemotherapy is needed to improve the prognosis of patients with MTP originating from colorectal cancer, if it is feasible for the patient.
In conclusion, a precise pathological diagnosis using a biopsy under endoscopic ultrasonography guidance is indispensable for the treatment of MTP, and IHC is useful for making a pathological diagnosis in some cases. Pancreatectomy may confer a survival benefit in patients with MTP originating from RCC, even in the era of therapy with TKIs for recurred RCC. However, a combination of pancreatectomy and chemotherapy might be necessary to improve the prognosis of patients with MTP originating from colorectal cancer.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Institutional Review Board Statement
This study was reviewed and approved by the Ethics Committee of Saitama Medical Center, Jichi Medical University.
Informed Consent Statement
Patients were not required to give their informed consent for inclusion in this retrospective study because we used anonymous clinical data and no individuals could be identified from the data. We announced this study on our institution’s website with an explanation about the patients’ right to refuse inclusion in the present study and about the study’s publication.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444:527–535. doi: 10.1007/s00428-004-0987-3. [DOI] [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, Nakeeb A, Lillemoe KD. Renal cell carcinoma metastatic to the pancreas: results of surgical management. J Gastrointest Surg. 2001;5:346–351. doi: 10.1016/S1091-255X(01)80060-3. [DOI] [PubMed] [Google Scholar]
- 3.Wente MN, Bergmann F, Fröhlich BE, Schirmacher P, Büchler MW, Friess H. Pancreatic metastasis from gastric carcinoma: a case report. World J Surg Oncol. 2004;2:43. doi: 10.1186/1477-7819-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler H, Redmond CE, Heneghan HM, Swan N, Maguire D, Traynor O, Hoti E, Geoghegan JG, Conlon KC. Pancreatectomy for metastatic disease: a systematic review. Eur J Surg Oncol. 2014;40:379–386. doi: 10.1016/j.ejso.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Hung JH, Wang SE, Shyr YM, Su CH, Chen TH, Wu CW. Resection for secondary malignancy of the pancreas. Pancreas. 2012;41:121–129. doi: 10.1097/MPA.0b013e31821fc8f2. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney AD, Wu MF, Hilsenbeck SG, Brunicardi FC, Fisher WE. Value of pancreatic resection for cancer metastatic to the pancreas. J Surg Res. 2009;156:189–198. doi: 10.1016/j.jss.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Reddy S, Edil BH, Cameron JL, Pawlik TM, Herman JM, Gilson MM, Campbell KA, Schulick RD, Ahuja N, Wolfgang CL. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol. 2008;15:3199–3206. doi: 10.1245/s10434-008-0140-7. [DOI] [PubMed] [Google Scholar]
- 8.Strobel O, Hackert T, Hartwig W, Bergmann F, Hinz U, Wente MN, Fritz S, Schneider L, Büchler MW, Werner J. Survival data justifies resection for pancreatic metastases. Ann Surg Oncol. 2009;16:3340–3349. doi: 10.1245/s10434-009-0682-3. [DOI] [PubMed] [Google Scholar]
- 9.Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. eighth. Chichester: Wiley; 2017. [Google Scholar]
- 10.Le Borgne J, Partensky C, Glemain P, Dupas B, de Kerviller B. Pancreaticoduodenectomy for metastatic ampullary and pancreatic tumors. Hepatogastroenterology. 2000;47:540–544. [PubMed] [Google Scholar]
- 11.Hiotis SP, Klimstra DS, Conlon KC, Brennan MF. Results after pancreatic resection for metastatic lesions. Ann Surg Oncol. 2002;9:675–679. doi: 10.1007/BF02574484. [DOI] [PubMed] [Google Scholar]
- 12.Søreide JA, Sandvik OM, Søreide K. Improving pancreas surgery over time: performance factors related to transition of care and patient volume. Int J Surg. 2016;32:116–122. doi: 10.1016/j.ijsu.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 13.Madkhali AA, Shin SH, Song KB, Lee JH, Hwang DW, Park KM, Lee YJ, Kim SC. Pancreatectomy for a secondary metastasis to the pancreas: a single institution experience. Medicine (Baltimore) 2018;97:e 12653. doi: 10.1097/MD.0000000000012653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fikatas P, Klein F, Andreou A, Schmuck RB, Pratschke J, Bahra M. Long term survival after surgical treatment of renal cell carcinoma metastasis within the pancreas. Anticancer Res. 2016;36:4273–4278. [PubMed] [Google Scholar]
- 15.Cheng SK, Chuah KL. Metastatic renal cell carcinoma to the pancreas : a review. Arch Pathol Lab Med. 2016;140:598–602. doi: 10.5858/arpa.2015-0135-RS. [DOI] [PubMed] [Google Scholar]
- 16.Sun PJ, Yu YH, Cui XJ. Primary clear cell adenocarcinoma of the pancreas: a case report and literature update. Onco Targets Ther. 2018;16(11):8197–8200. doi: 10.2147/OTT.S183054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT, Vienna Consensus Conference participants ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–171. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsutsumi K, Ohtsuka T, Mori Y, Fujino M, Yasui T, Aishima S, Takahata S, Nakamura M, Ito T, Tanaka M. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J Gastroenterol. 2012;47:678–685. doi: 10.1007/s00535-012-0540-0. [DOI] [PubMed] [Google Scholar]
- 19.Z'graggen K, Fernández-del Castillo C, Rattner DW, Sigala H, Warshaw AL. Metastases to the pancreas and their surgical extirpation. Arch Surg. 1998;133:413–419. doi: 10.1001/archsurg.133.4.413. [DOI] [PubMed] [Google Scholar]
- 20.Nakeeb A, Lillemoe KD, Cameron JL. The role of pancreaticoduodenectomy for locally recurrent or metastatic carcinoma to the periampullary region. J Am Coll Surg. 1995;180:188–192. [PubMed] [Google Scholar]
- 21.Grassi P, Verzoni E, Mariani L, De Braud F, Coppa J, Mazzaferro V, Procopio G. Prognostic role of pancreatic metastases from renal cell carcinoma: results from an Italian center. Clin Genitourin Cancer. 2013;11:484–488. doi: 10.1016/j.clgc.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Santoni M, Conti A, Partelli S, Porta C, Sternberg CN, Procopio G, Bracarda S, Basso U, De Giorgi U, Derosa L, Rizzo M, Ortega C, Massari F, Iacovelli R, Milella M, Di Lorenzo G, Buti S, Cerbone L, Burattini L, Montironi R, Santini D, Falconi M, Cascinu S. Surgical resection does not improve survival in patients with renal metastases to the pancreas in the era of tyrosine kinase inhibitors. Ann Surg Oncol. 2015;22:2094–2100. doi: 10.1245/s10434-014-4256-7. [DOI] [PubMed] [Google Scholar]
- 23.Stoltz A, Barnoud R, Plok V, Ducerf C, Baulieux J, Mabrut JY. A pancreatic metastasis from a colon cancer. Clin Res Hepatol Gastroenterol. 2011;35:586–589. doi: 10.1016/j.clinre.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Matsubara N, Baba H, Okamoto A, Kurata M, Tsuruta K, Funata N, Ashizawa K. Rectal cancer metastasis to the head of the pancreas treated with pancreaticoduodenectomy. J Hepato-Biliary-Pancreat Surg. 2007;14:590–594. doi: 10.1007/s00534-007-1219-4. [DOI] [PubMed] [Google Scholar]
- 25.Palmowski M, Hacke N, Satzl S, Klauss M, Wente MN, Neukamm M, Kleeff J, Hallscheidt P. Metastasis to the pancreas: characterization by morphology and contrast enhancement features on CT and MRI. Pancreatology. 2008;8:199–203. doi: 10.1159/000128556. [DOI] [PubMed] [Google Scholar]
- 26.Al Bandar MH, Kim NK. Current status and future perspectives on treatment of liver metastasis in colorectal cancer (review) Oncol Rep. 2017;37:2553–2564. doi: 10.3892/or.2017.5531. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Yasui K, Hirai T, Kanemitsu Y, Mori T, Sugihara K, Mochizuki H, Yamamoto J. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 2003;46:S22–S31. doi: 10.1097/01.DCR.0000089106.71914.00. [DOI] [PubMed] [Google Scholar]
- 28.Crippa S, Angelini C, Mussi C, Bonardi C, Romano F, Sartori P, Uggeri F, Bovo G. Surgical treatment of metastatic tumors to the pancreas: a single center experience and review of the literature. World J Surg. 2016;30:1536–1542. doi: 10.1007/s00268-005-0464-4. [DOI] [PubMed] [Google Scholar]
- 29.Jarufe N, McMaster P, Mayer AD, Mirza DF, Buckels JA, Orug T, Tekin K, Bramhall SR. Surgical treatment of metastases to the pancreas. Surgeon. 2005;3:79–83. doi: 10.1016/S1479-666X(05)80066-6. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K, Japanese Society for Cancer of the Colon and Rectum Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araujo RL, Gönen M, Herman P. Chemotherapy for patients with colorectal liver metastases who underwent curative resection improves long-term outcomes: systematic review and meta-analysis. Ann Surg Oncol. 2015;22:3070–3078. doi: 10.1245/s10434-014-4354-6. [DOI] [PubMed] [Google Scholar]