Abstract
Our aim was to study the accuracy of CT scan in predicting the peritoneal cancer index (PCI) and the impact of neoadjuvant chemotherapy (NACT), abdominal region, disease volume, and primary tumor site on it. This was a prospective single-center study that included patients undergoing cytoreductive surgery ± HIPEC. The CT-PCI was calculated and compared to the surgical PCI. The accuracy of CT-PCI in predicting the surgical PCI and the difference between the two was evaluated. From January 2018 to August 2018, 50 patients were included. The median CT PCI was 6 (range 0–35) and median surgical PCI was 17 (range 2–35). CT-PCI was more than the surgical PCI in 12 (24%), less in 23 (46%), and same in 15 (30%) with an accuracy of 30%. The highest accuracy was in region 10 and lowest in region 3. It was 15% in patients with ovarian cancer, 30% in PMP, 21% in patients receiving NACT, 35% in high-volume disease, and 42.1% in low volume disease. The CT and surgical PCI varied significantly in patients with ovarian cancer (p < 0.001), following NACT (p = 0.01) and those with moderate volume disease (p < 0.001). CT has a low accuracy in predicting the surgical PCI in both high and low volume disease. The CT-PCI can differ significantly from the surgical PCI in patients with ovarian cancer and in patients who have received NACT for peritoneal disease. The impact of NACT on accuracy of CT-PCI in non-ovarian peritoneal metastases should be evaluated further.
Keywords: CT-PCI, Ovarian cancer, Peritoneal metastases
Introduction
The peritoneal cancer index (PCI) developed by Paul Sugarbaker is one of the most important prognostic variables in peritoneal surface oncology used both for selecting patients for cytoreductive surgery (CRS) and for predicting the benefit of the procedure [1]. Though a surgical evaluation remains the gold standard for evaluating the disease extent, it is often not possible to do so without performing a through exploration as laparoscopy if often limited by both tumor and adhesions [2, 3]. Hence, imaging remains a vital tool for therapeutic decision making. An abdominal CT scan is the most common imaging modality used [4]. CT scan has shown poor accuracy in detecting nodules less than 0.5 cm in size due to poor enhancement which makes it difficult to distinguish tumor nodules from the surrounding soft tissue [5, 6]. MRI has shown that a greater accuracy in some studies and the combination of post-contrast study with diffusion-weighted imaging has shown to correlate better with the surgical PCI [7, 8]. To perform these MRI studies, bowel preparation and fasting is needed and the study time is longer than it takes for a CT scan. More importantly, when patients are referred for surgery with one or more scans already performed, performing another scan has the risks of repeated contrast administration and an additional cost, especially if the surgical decision is unlikely to change. Peritoneal metastases arising from the same or different primary tumors do not have a similar morphological appearance and can present as thickening or enhancement of the peritoneum, tumor nodules or masses arising from the peritoneum, mesentery or omentum [8]. In patients who have received chemotherapy, the evaluation becomes more difficult. We performed this study to determine the accuracy of CT scan in predicting the PCI and the impact of prior chemotherapy, abdominal region, and primary tumor site on it.
Methods
This was a prospective observational single center study carried out from January 2018 to June 2018. Patients scheduled to undergo cytoreductive surgery with or without HIPEC for PM from various primary sites were included in the study. Patients who were excluded from CRS and HIPEC at the time of a diagnostic laparoscopy scheduled in the same sitting were also included. Patients in whom a CRS was not planned were not included. Institutional permission was obtained for the study.
Surgical Intervention
Patients are selected for CRS and HIPEC based on current recommendations which include completely resectable peritoneal disease, absence of unresectable distant metastases, and PCI within recommended cut-off for each primary site [9]. For pseudomyxoma peritonei (PMP), peritoneal mesothelioma, and primary/interval CRS for ovarian cancer, there is no PCI cut-off over which surgery is not offered. For non-mucinous colorectal tumors, a predicted PCI of 15–17 was used as a cut-off [9]. For uncommon indications, the decision was individualized [10]. A thorough exploration of the abdominal cavity was performed employing an incision from the xiphoid to the pubis. The goal of surgery was to obtain a CC-0/1 resection for which standard peritonectomy procedures and visceral resections were performed [11]. For patients undergoing interval CRS and salvage CRS (for recurrent ovarian cancer), complete removal of the parietal peritoneum was performed. HIPEC was performed by the coliseum technique using standard drug regimens for each primary site [12].
CT-Scan
All patients had a CT scan of the lower thorax, abdomen, and pelvis from the carina to mid-thigh on dual energy 256 slice GE CT scanner with Gemstone imaging, within 15 days of the surgical procedure. Though the lower limit is usually until the pubic symphysis, we extend the study to the mid-thigh to cover the inguinal regions which may have metastatic disease in patients with pelvic malignancies. If the CT was performed at another institution, all the images were reviewed at our center, and if the study was not satisfactory, the scan was repeated. For patients who had a PET scan, if a detailed CT evaluation was not performed along with it, another CT was performed. A 256-slice dual source with a maximum slice thickness of 5 mm and minimum slice thickness reconstruction up to 0.6 mm was used. Both oral and IV contrast and as when required per-rectal contrast was administered.
Evaluation of Peritoneal Disease
Sugarbaker’s peritoneal cancer index was used to quantify the disease extent and volume. It divides the abdomen into 13 regions (0–12), giving each region a score based on the lesion size and number of lesions and the PCI representing the sum of these scores (range 0–39) [13]. The structures in each region of the PCI were defined using the PROMISE application [14]. In addition, the peritoneal lesions were classified as thickened peritoneum, enhancement, or tumor nodules/masses. Presence of fluid without the presence of peritoneal lesions was not considered as disease. In the case of mucinous ascites, the fluid density was recorded in houndsfield units (HU). The peritoneal disease was classified as low volume (PCI-0-9), moderate volume (PCI-10-19), or high volume (PCI of 20 or above) [7]. All scans were reported by two senior radiologists jointly. Scans done at other institutions were reviewed jointly and reported.
The surgical PCI was recorded similarly. If any region could not be evaluated on laparoscopy or laparotomy, the region was marked as “not evaluated.” Such patients were still included in the study.
Statistical Analysis
A comparison between the radiological and surgical PCI was made. The score in each region was compared and marked as same, less, or more. Accuracy was considered to be the proportion of patients with “same” CT and surgical PCI. Accuracy was determined for the overall PCI score and the score for each region separately. The impact of disease volume, primary tumor site, and neoadjuvant chemotherapy (NACT) on the accuracy was determined.
The CT-PCI and surgical PCI were compared using the paired t test reporting two-tailed p values. A p value of < 0.05 was considered significant.
Results
From January 2018 to August 2018, 50 patients were included in the study. The primary tumor site was ovary in 20 (40%) patients, colorectal in 4 (8%), appendix in 10 (20%), mesothelioma in 4 (8%), stomach in 1 (2%), and rare primary in 11 (22%) patients. Forty-five patients underwent CRS with or without HIPEC and five underwent diagnostic laparoscopy alone. Overall, a diagnostic laparoscopy was performed in 13 (26%). Thirty-six/45 patients underwent HIPEC. The reason for omission was uncertain indication in 3, high risk of morbidity in 3, and patient refusal in 3. The median PCI and a CC-0/1 resection were obtained in 43/45patients. The median CT PCI was 6 (range 0–35) and the median surgical PCI was 17 (range 2–35).
Comparison of Surgical and Radiological PCI
The CT-PCI was more than the surgical PCI in 12 (24%), less in 23 (46%), and the same in 15 (30%) resulting in an accuracy of 30%. Looking at each region of the abdominal cavity, the accuracy ranged from 60 to 80%. It was 60–70% for the upper regions (1–3), 66–70% for the lower regions (5–7), and 60–80% for the small bowel regions (9–12). The highest accuracy was seen in region 10 and the lowest in region 3 (Table 1). Taking all patients together, the surgical and radiological PCI differed significantly on the paired t test analysis (p = 0.001).
Table 1.
Accuracy of CT-PCI in different abdominal regions
| CT-PCI versus surgical PCI (n = 50) | Accuracy | ||||||
|---|---|---|---|---|---|---|---|
| Region | Same | Less | More | All patients | Post-NACT | Ovarian cancer | PMP |
| 0 | 37 | 8 | 5 | 74% | 65.2% | 75% | 70% |
| 1 | 34 | 13 | 3 | 68% | 57.1% | 60% | 90% |
| 2 | 35 | 12 | 3 | 70% | 47.8% | 60% | 80% |
| 3 | 30 | 15 | 5 | 60% | 43.4% | 45% | 60% |
| 4 | 36 | 11 | 3 | 72% | 56.5% | 65% | 70% |
| 5 | 35 | 12 | 3 | 70% | 57.1% | 50% | 60% |
| 6 | 34 | 14 | 2 | 68% | 43.4% | 40% | 90% |
| 7 | 33 | 13 | 4 | 66% | 57.1% | 55% | 60% |
| 8 | 31 | 12 | 7 | 62% | 34.7% | 50% | 60% |
| 9 | 41 | 6 | 3 | 62% | 34.7% | 75% | 80% |
| 10 | 40 | 8 | 2 | 80% | 73.9% | 70% | 60% |
| 11 | 33 | 15 | 2 | 66% | 65.2% | 65% | 50% |
| 12 | 33 | 12 | 5 | 66% | 60.8% | 65% | 50% |
Impact of Disease Volume
There were 19 patients with low volume disease, 11 with moderate and 20 with high volume disease. The CT-PCI was less in 7 (35%), more in 6 (30%), and same in 7 (35%) patients with high volume disease. It was less in 5 (26.3%), more in 6 (31.5%) and same in 8 (42.1%) patients with low volume disease and less in all 11 patients with moderate volume disease. All patients with moderate volume disease were ovarian cancer patients who had received NACT for peritoneal disease. The accuracy was 35% for high volume, 0% for moderate volume, and 42.1% for low volume disease. On the paired t test analysis, a significant difference was not seen in patients with low volume disease in any of the regions or overall (p = 0.35). For moderate volume, there was a significant difference overall (p = 0.001) and in all regions except 0, 9, and 10. For high volume disease, a significant difference was seen in regions 1 and 11 (Table 2) with no difference overall (p = 0.13).
Table 2.
Difference between the CT-PCI and surgical PCI in different abdominal regions
| Patient subgroup | CT-PCI vs surgical PCI | p values on the paired t test for each abdominal region | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| All patients (50) | 0.01 | 0.37 | 0.01 | 0.003 | 0.01 | 0.14 | 0.08 | 0.11 | 0.04 | 0.78 | 1.35 | 0.44 | 0.01 | 0.50 |
| Ovarian cancer (20) | < 0.001 | 0.04 | 0.004 | 0.003 | 0.001 | 0.008 | 0.007 | 0.023 | 0.05 | 0.07 | 0.163 | 0.015 | 0.008 | 0.18 |
| Other primary sites (30) | 0.52 | 0.44 | 0.40 | 0.25 | 1.00 | 0.60 | 0.48 | 0.64 | 0.74 | 0.05 | 0.48 | 0.71 | 0.03 | 0.25 |
| Post-NACT (23) | 0.001 | 0.70 | 0.38 | 0.05 | 0.001 | 1.84 | 0.05 | 0.69 | 0.03 | 0.08 | 1.86 | 1.03 | 0.004 | 0.07 |
| No NACT (27) | 0.35 | 0.18 | 0.13 | 0.21 | 0.49 | 0.57 | 0.71 | 1.0 | 0.64 | 0.04 | 0.37 | 0.21 | 0.05 | 0.60 |
| Low-volume disease (19) | 0.35 | 0.77 | 1.00 | 0.33 | 0.33 | 0.33 | 0.33 | 0.25 | 0.68 | 0.26 | 0.33 | 0.33 | 0.33 | 0.33 |
| Moderate volume (11) | < 0.0001 | 0.08 | 0.01 | 0.03 | < 0.001 | 0.02 | 0.01 | 0.001 | 0.01 | 0.02 | 0.16 | 0.08 | 0.01 | 0.03 |
| High-volume disease (20) | 0.13 | 1.00 | 0.04 | 0.25 | 0.78 | 0.21 | 0.49 | 0.10 | 0.50 | 0.60 | 0.17 | 0.17 | 0.02 | 0.84 |
Patients Who Had Received Neoadjuvant Chemotherapy
There were 23 (46%) who received NACT before CRS. The primary tumor site was ovary in 13 (interval 8, recurrent 5), rare primaries in 7, colorectal in 1, mesothelioma 1 in, and stomach in 1. In these patients, the CT-PCI was less in 13 (5.5%) patients, and more and same as the surgical PCI in 5 (21.7%) patients each. Thus, the overall accuracy was 21.7%. In various regions, the accuracy ranged from 34.7 to 73.9%. It was the lowest for regions 8 and 9 at 34.7% and highest in region 10 (73.9%). In patients who did not receive NACT, it was less in 10 (37.0%) patients, more in 7 (26.0%), and same in 10 (37.0%). The CT and surgical PCI did not differ significantly in patients who did (p = 0.70) or did not receive NACT (p = 0.18). It significantly differed in regions 3, 7, and 11 in the NACT group and only in region 8 in patients who did not receive NACT.
Patients with Ovarian Cancer
Of the 20 patients with ovarian cancer, 8 had interval CRS, 7 had CRS for recurrent disease, and 5 had primary CRS. The median CT-PCI was 6 (range 2–27), and the median surgical PCI was 16 (range 2–27). The CT-PCI was less than the surgical PCI in 13 patients (65.0%), more in 4 (20.0%), and same in 3 (15.0%). The accuracy in determining the PCI was 15.0%. In eight patients undergoing interval CRS, the CT-PCI was less than the surgical PCI in seven and more in one with an accuracy of 0%. In seven patients undergoing surgery for recurrent ovarian cancer, it was less in six and more in one with an accuracy of 0%. In patients undergoing primary CRS, it was the same in three/five patients (60%) and more in one and less in one. The accuracy ranged from 40% in region 6 to 75% in regions 0 and 9. A significant difference was seen in the radiological and surgical PCI (p < 0.0001) and in all regions except 7, 9, and 12.
PMP Arising from Appendiceal Primary Tumor
For the 10 patients with PMP, the median CT PCI was 27 (range 0–35) and the median surgical PCI was also 27 (range 2–35). A CC-0 resection was obtained in one patient and CC-1 in seven. The CT-PCI was more than the surgical PCI in four (40.0%), less in three (30%), and same in three (30%). The overall accuracy was 30%. The accuracy in the upper and lower regions was the highest reaching 90% in regions 1 and 6 and lowest in the small bowel regions, and 50% in regions 11 and 12.
Discussion
The findings of this study concur with previous findings which show a low accuracy of a CT scan in predicting the surgical PCI. The additional significant findings are a low accuracy in predicting the surgical PCI in patients undergoing interval and salvage CRS as well as patients who received NACT and a variation in the accuracy between different primary tumors (both in terms of overall accuracy and that in each region). The CT and surgical PCI varied significantly in patients with ovarian cancer (p < 0.001), following NACT (p = 0.01) and those with moderate volume disease (p < 0.001). The prediction of operability was high with 5 patients being excluded on laparoscopy and 43/45 (95.5%) patients undergoing a CC-0/1 resection.
This study differs from other such studies as it does not consider the presence or absence of disease in a particular region, but the PCI score (total and in each region) determines the accuracy [15]. The surgical and CT-PCI can differ in terms of the presence or absence of disease in the region, the size of the tumor nodule, or the finding of additional nodules. It can be higher or lower than the surgical PCI both of which would be considered inaccurate. The morphology of PM may have a bearing. Peritoneal disease presents as diffuse thickening and plaques which may be difficult to detect on a CT scan especially after the administration of chemotherapy. Though active disease should undergo contrast enhancement, it may not be the case in all patients as demonstrated by our results. We have not characterized the lesions morphologically during surgery; hence, it is not possible to defend this statement with our results. However, the proportion of patients with ovarian cancer in this study is high (40%). Most of these patients received one or more lines of systemic chemotherapy. There is another study of 43 patients undergoing interval or salvage CRS after systemic chemotherapy that showed in accuracy of around 70–75% [15]. In this study, the factor determining accuracy was the presence or absence of disease.
Looking at each region, there was a lower accuracy in the upper and lower regions compared to regions 9 and 12. The correlation also varied significantly in these regions (Fig. 1). The small bowel is less commonly involved in ovarian cancer compared to other peritoneal regions. In this study, a complete removal of the parietal peritoneum was performed in ovarian cancer patients which reduces the chances of missing residual disease. Particularly, in the upper abdomen, mobilization of the liver is often required to detect residual disease.
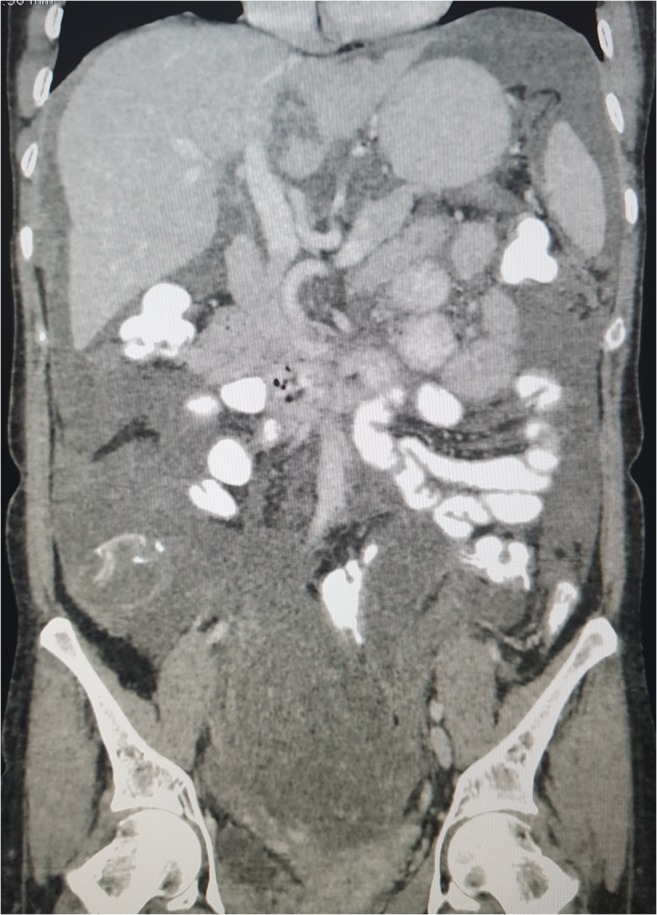
Fig. 1.
CT scan following NACT for first recurrence in ovarian cancer. The surgical PCI was 16 and CT-PCI 3. Except for the two lesions (b, c), the rest of the scan was normal (a)
The difference in median PCI on CT and surgery in our study was high (6 versus 16), which is in contrast to the study by Mazzei et al. [15]. Though the number of patients is small, the CT-PCI and surgical PCI were concordant in 60% of the patients undergoing primary CRS.
The prognostic impact of PCI has been shown in colorectal cancer, PMP, peritoneal mesothelioma, and recurrent ovarian cancer [16]. It has not been clearly demonstrated in patients undergoing frontline therapy for ovarian cancer. One study showed that PCI has an impact on survival; another study did not [17–19].
A low accuracy was seen in patient who had received NACT as well with an accuracy of only 21.7% compared to 37% in patients who did not receive NACT (p = 0.46). Sixty-five percent of the patients in this group were ovarian cancer patients. It would be interesting to know if the same inaccuracy is seen in patients with colorectal PM, but there was only patient with colorectal PM who had NACT in this study. Though a low accuracy of CT has been shown in these patients, the impact of NACT has not been evaluated [20].
In PMP arising from appendiceal tumors, despite the high median PCI, the accuracy was only 30%. The disease was overestimated in 40% and underestimated in 30%. In contrast to the overall results and those in ovarian cancer patients, accuracy in the upper abdominal and lower regions reached 90%. The accuracy in determining the PCI in regions 11 and 12 was only 50%. In patients with extensive disease especially those with large deposits, due to proximity of adjacent viscera, it may be difficult to judge their involvement (Fig. 2). However, even if involvement is present, it does not mean that should be scored separately. For example, infiltration of the small bowel loops by a pelvic peritoneal deposit is not scored as a small bowel deposit. There may be some reactionary thickening which could be mistakenly scored as a deposit. The same was seen in some patients with low volume disease and large tumor deposits (Fig. 3). Some tumors like granulosa cell tumors can form large deposits. These PM arise in one region of the peritoneum and extend into another. On imaging, it may seem that more than one region is involved leading to a falsely high PCI.
Fig. 2.
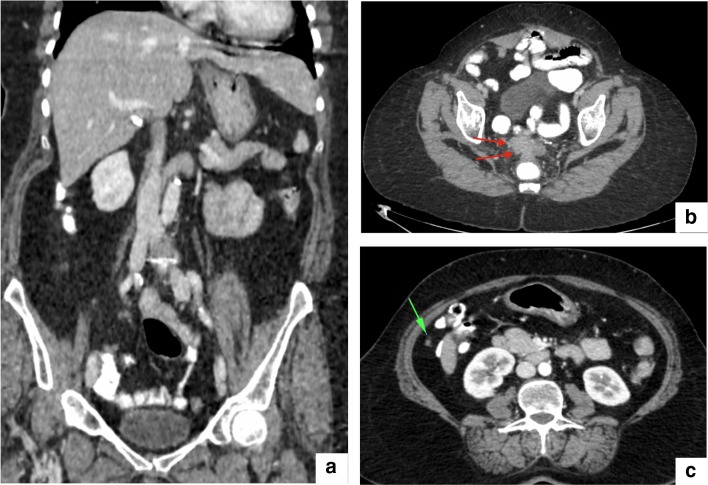
CT scan of a patient with high grade PMP. CT overestimated the PCI 3, 4, 5, 8–12 leading to a CT-PCI of 31 compared to the surgical PCI 24
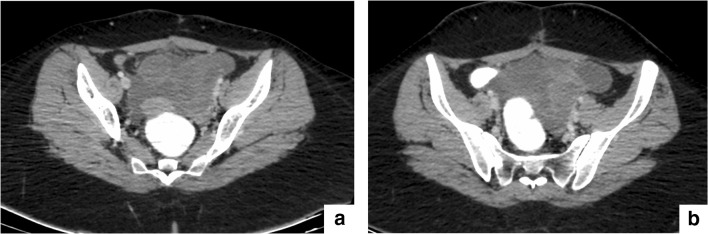
Fig. 3.
a and b: Large pelvic deposit with ascites in a patient with recurrent granulosa cell tumor leading to a falsely high PCI
Imaging is needed to rule out unresectable tumor and in this regard, CT performed well. There are two concerns in such accurate determination of the radiological PCI. The absolute value of PCI is useful in selecting the line of treatment (colorectal PM with a high PCI may be referred for chemotherapy first), in planning the surgery (based on disease distribution-expected visceral resections, the need for performing a stoma) and in counseling patients about the expected benefit. In most cases, it would not change the decision to operate, but the overall planning is better and misadventures can be minimized. Second is the need to perform additional imaging. The benefit of MRI has been shown in colorectal and appendiceal tumors. However, CT would still be useful for determining operability, as it can more accurately determine ureteric involvement with the help of delayed imaging—CT intravenous pyelography phase (performed after 5–7 min of delay) and portal vascular infiltration (portal venous phase—50–55 s).
Performing an additional contrast MRI has the theoretical risk of nephropathy. Laparoscopy may be preferable to additional imaging, especially in ovarian cancer [21]. When contrast cannot be administered due to underlying renal dysfunction, non-contrast MRI provides better soft tissue characterization than non-contrast CT. Diffusion-weighted MRI alone has shown a superior accuracy to CT in predicting the surgical PCI in both colorectal and ovarian cancer [22, 23]. The role of diffusion-weighted MRI as a single imaging modality needs further evaluation.
This study is limited by its small numbers and including PM from various primary sites. Though surgical PCI is considered the standard for comparison, the pathological PCI would be most accurate, but it has not been evaluated here.
The strengths are that all surgeries were performed systematically by the same surgeon who had performed over 150 such procedures and the CT reporting was done by two senior radiologists, experienced in reporting PM.
Conclusions
CT has a low accuracy in predicting the surgical PCI in patients with both high and low volume disease. The CT-PCI differs significantly from the surgical PCI in patients with ovarian cancer and in patients who have received neoadjuvant chemotherapy for peritoneal disease. The impact of neoadjuvant chemotherapy on accuracy of CT-PCI in non-ovarian peritoneal metastases should be evaluated further.
Funding information
The authors received no funding for this project.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhatt A, Mehta S. Cytoreductive surgery for peritoneal metastases: principles and techniques. In: Bhatt A, editor. Management of peritoneal metastases—cytoreductive surgery, HIPEC and beyond. Singapore: Springer; 2018. [Google Scholar]
- 2.Passot G, Dumont F, Goéré D, Arvieux C, Rousset P, Regimbeau JM, Elias D, Villeneuve L, Glehen O, BIG-RENAPE Surgery Working Group Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE) Br J Surg. 2018;105(6):663–667. doi: 10.1002/bjs.10723. [DOI] [PubMed] [Google Scholar]
- 3.Seshadri RA, Hemanth Raj E. Diagnostic laparoscopy in the pre-operative assessment of patients undergoing cytoreductive surgery and HIPEC for peritoneal surface malignancies. Indian J Surg Oncol. 2016;7(2):230–235. doi: 10.1007/s13193-015-0486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duhr CD, Kenn W, Kickuth R, Kerscher AG, Germer CT, Hahn D, W Pelz JO. Optimizing of preoperative computed tomography for diagnosis in patients with peritoneal carcinomatosis. World J Surg Oncol. 2011;9:171. doi: 10.1186/1477-7819-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coakley FV, Choi PH, Gougoutas CA, Pothuri B, Venkatraman E, Chi D, Bergman A, Hricak H. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology. 2002;223:495–500. doi: 10.1148/radiol.2232011081. [DOI] [PubMed] [Google Scholar]
- 6.Koh JL, Tan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009;16:327–333. doi: 10.1245/s10434-008-0234-2. [DOI] [PubMed] [Google Scholar]
- 7.Low RN, Barone RM, Lacey C, Sigeti JS, Alzate GD, Sebrechts CP. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology. 1997;204:513–520. doi: 10.1148/radiology.204.2.9240546. [DOI] [PubMed] [Google Scholar]
- 8.Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2015;22:1708–1715. doi: 10.1245/s10434-014-4041-7. [DOI] [PubMed] [Google Scholar]
- 9.Sugarbaker PH. Preoperative assessment of cancer patients with peritoneal metastases for complete cytoreduction. Indian J Surg Oncol. 2016;7:295–302. doi: 10.1007/s13193-016-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt A, Seshadri RA. Rare indications for Cytoreductive surgery and Hyperthermic intraperitoneal chemotherapy. In: Bhatt A, editor. Management of peritoneal metastases—cytoreductive surgery, HIPEC and beyond. Singapore: Springer; 2018. [Google Scholar]
- 11.Mehta SS, Bhatt A, Glehen O. Cytoreductive surgery and Peritonectomy procedures. Indian J Surg Oncol. 2016;7(2):139–151. doi: 10.1007/s13193-016-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Speeten K, Lemoine L. HIPEC methodology, comparison of techniques, and drug regimens: is there a need for standardization? In: Bhatt A, editor. Management of Peritoneal Metastases- Cytoreductive Surgery, HIPEC and beyond. Singapore: Springer; 2018. [Google Scholar]
- 13.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 14.Villeneuve L, Thivolet A, Bakrin N, Mohamed F, Isaac S, Valette PJ, Glehen O, Rousset P, Abba J, Abboud K, Arvieux C, Balagué G, Barrau V, Rejeb HB, Bereder JM, Bibeau F, Bouzard D, Brigand C, Carrère S, Carretier M, de Chaisemartin C, Chassang M, Chevallier A, Courvoisier T, Dartigues P, Delroeux D, Desolneux G, Dohan A, Dromain C, Dumont F, Durand-Fontanier S, Elias D, Eveno C, Evrard S, Fay O, Ferron G, Geffroy D, Gilly FN, Fontaine J, Goasguen N, Ghouti L, Goéré D, Guilloit JM, Guyon F, Heyd B, Kaci R, Karoui M, Kianmanesh R, Labbé C, Lacroix J, Lang-Averous G, Laverriere MH, Lefevre J, Lelong B, Leroux A, Dico RL, Loi V, Lorimier G, Marchal F, Mariani A, Mariani P, Mariette C, Meeus P, Mery E, Messager M, Msika S, Nadeau C, Ortega-Deballon P, Passot G, Petorin C, Peyrat P, Pezet D, Piessen G, Pirro N, Pocard M, Poizat F, Porcheron J, Pourcher G, Quenet F, Rat P, Regimbeau JM, Rousselot P, Sabbagh C, Svrcek M, Tetreau R, Thibaudeau E, Tuech JJ, Valmary-Degano S, Vaudoyer D, Velasco S, Verriele-Beurrier V, Wernert R, Zinzindohoue F. A new internet tool to report peritoneal malignancy extent. PeRitOneal MalIgnancy stage evaluation (PROMISE) application. Eur J Surg Oncol. 2016;42(6):877–882. doi: 10.1016/j.ejso.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Mazzei MA, Khader L, Cirigliano A, Cioffi Squitieri N, Guerrini S, Forzoni B, Marrelli D, Roviello F, Mazzei FG, Volterrani L. Accuracy of MDCT in the preoperative definition of peritoneal Cancer index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC) Abdom Imaging. 2013;38(6):1422–1430. doi: 10.1007/s00261-013-0013-9. [DOI] [PubMed] [Google Scholar]
- 16.Bakrin N, Bereder JM, Decullier E, Classe JM, Msika S, Lorimier G, Abboud K, Meeus P, Ferron G, Quenet F, Marchal F, Gouy S, Morice P, Pomel C, Pocard M, Guyon F, Porcheron J, Glehen O, FROGHI (FRench Oncologic and Gynecologic HIPEC) Group Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of566 patients. Eur J Surg Oncol. 2013;39:1435–1443. doi: 10.1016/j.ejso.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Llueca A, Escrig J. MUAPOS working group (multidisciplinary unit of abdominal pelvic oncology surgery). Prognostic value of peritoneal cancer index in primary advanced ovarian cancer. Eur J Surg Oncol. 2018;44(1):163–169. doi: 10.1016/j.ejso.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Elzarkaa AA, Shaalan W, Elemam D, Mansour H, Melis M, Malik E, Soliman AA. Peritoneal cancer index as a predictor of survival in advanced stage serous epithelial ovarian cancer: a prospective study. J Gynecol Oncol. 2018;29(4):e47. doi: 10.3802/jgo.2018.29.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasimli K, Braicu EI, Richter R, Chekerov R, Sehouli J. Prognostic and predictive value of the peritoneal Cancer index in primary advanced epithelial ovarian Cancer patients after complete Cytoreductive surgery: study of tumor Bank ovarian Cancer. Ann Surg Oncol. 2015;22(8):2729–2737. doi: 10.1245/s10434-014-4329-7. [DOI] [PubMed] [Google Scholar]
- 20.Dromain C, Leboulleux S, Auperin A, Goere D, Malka D, Lumbroso J, Schumberger M, Sigal R, Elias D. Staging of peritoneal carcinomatosis: enhanced CT vs. PET/CT. Abdom Imaging. 2008;33:87–93. doi: 10.1007/s00261-007-9211-7. [DOI] [PubMed] [Google Scholar]
- 21.Laparoscopy and Computed Tomography Imaging in Advanced Ovarian Tumors a roadmap for prediction of optimal Cytoreductive surgery. Gynecol Minim Invasive Ther. 2018;7(2):66–69. doi: 10.4103/GMIT.GMIT_1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espada M, Garcia-Flores JR, Jimenez M, Alvarez-Moreno E, De Haro M, Gonzalez-Cortijo L, et al. Diffusion-weighted magnetic resonance imaging evaluation of intra-abdominal sites of implants to predict likelihood of suboptimal cytoreductive surgery in patients with ovarian carcinoma. Eur Radiol. 2013;23:2636–2642. doi: 10.1007/s00330-013-2837-7. [DOI] [PubMed] [Google Scholar]
- 23.van’t Sant I, van Eden WJ, Engbersen MP, Kok NFM, Woensdregt K, Lambregts DMJ, Shanmuganathan S, Beets-Tan RGH, Aalbers AGJ, Lahaye MJ. Diffusion-weighted MRI assessment of the peritoneal cancer index before cytoreductive surgery. Br J Surg. 2018;24:491–498. doi: 10.1002/bjs.10989. [DOI] [PubMed] [Google Scholar]