Abstract
Cancer cells can escape the immune system by different mechanisms. The evasion of cancer cells from immune surveillance is prevented by immune checkpoint inhibitors, allowing the patient’s own immune system to attack their cancer. Immune checkpoint inhibitors have shown improvement in overall survival for melanoma, lung cancer and renal cell carcinoma in clinical trials. Unfortunately, not all patients respond to this therapy.
In cancer management, percutaneous ablation techniques are well established for both cure and local control of many tumour types. Cryoablation of the tumour tissue results in cell destruction by freezing. Contrary to heat-based ablative modalities, cryoablation induces tumour cell death by osmosis and necrosis. It is hypothesised that with necrosis, the intracellular contents of the cancer cells stay intact allowing the immune system to induce an immune-specific reaction. This immune-specific reaction can, in theory, also affect cancer cells outside the ablated tissue, known as the abscopal effect. Unfortunately, this effect is rarely observed, but when cryoablation is combined with immunotherapy, the effect of both therapies may be enhanced. Although several preclinical studies demonstrated a synergistic effect between cryoablation and immunotherapy, prospective clinical trials are needed to prove this clinical benefit for patients. In this review, we will outline the current evidence for the combination of cryoablation with immunotherapy to treat cancer.
Keywords: Cryoablation, Immunotherapy, Cancer, Immune checkpoint inhibitor
Key points
It is hypothesised that cell death by cryoablation leaves the intracellular contents of the cancer cells intact for the immune system to induce an immune-specific reaction also known as the abscopal effect.
Immunotherapy uses the immune system for treatment of the tumour, but not all patients respond to immunotherapy.
Combination of cryoablation with immunotherapy may enhance the effect of both therapies for better tumour destruction.
Introduction
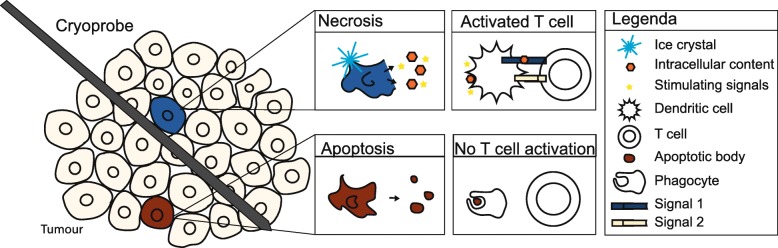
Cryoablation is a percutaneous ablation technique that uses extreme low temperatures for tumour destruction [1]. During cryoablation liquefied gas, such as nitrogen or argon, is passed through cryoprobes and expands into a gaseous state at the end of the probe to create temperatures as low as − 190 °C. Cytotoxic cell destruction is achieved at temperatures below − 20 °C. To ensure complete ablation of the tumour, a circumferential margin of 1 cm is needed [2]. After the freezing phase, a thawing phase follows by replacing the liquefied gas with helium or internally heating the needle (available in new systems). The whole process of freezing-thawing is repeated to obtain an effective ablation. Intra-procedure computed tomography (CT) identifies the ablated zone in real time as a low-density area which corresponds to the generated ice ball (Fig. 1). The procedure results in focal destruction of tumour tissue in a minimal invasive setting with reduced morbidity and mortality and represents a cost-effective alternative to surgery [3, 4]. However, surgery remains the gold standard in most tumour types with additional pathological assessment of margins [5, 6]. A possible advantage of cryoablation is that the intracellular contents of the damaged tumour cells are preserved and can be recognised by the immune system initiating a tumour-specific immune response (Fig. 2) [7, 8]. Recently, immune checkpoint inhibitors have been studied for treatment of melanoma, renal cell carcinoma (RCC) and non-small lung cancer (NSLC). The challenge of the immune checkpoint inhibitors is that only a minority of patients respond. Therefore, a combination treatment of cryoablation and immunotherapy, might be beneficial to enhance the effect of the immune checkpoint inhibitors (Fig. 3).
Fig. 1.
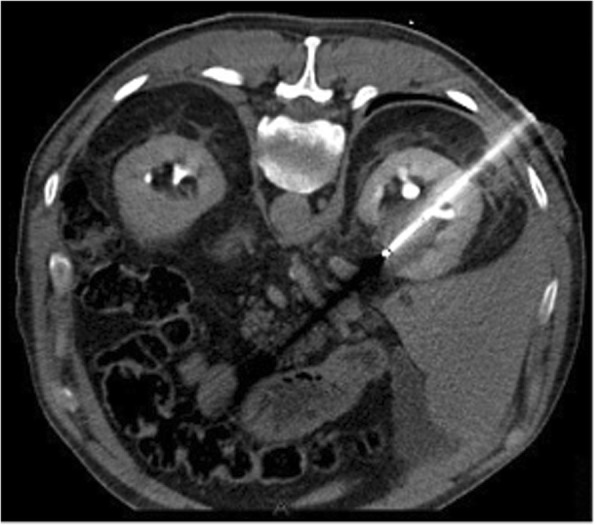
Cryoablation of a patient with stage IA renal cell carcinoma. The hypodense area around the needle corresponds with the generated ice ball in real time
Fig. 2.
One of the hypotheses of how cryoablation induces an immune response is in the way cell death is induced. Cryoablation induces cell death by both necrosis and apoptosis. Necrosis releases intra-cellular contents stimulating signals (among others danger signals) that may activate T cells for a specific immune response to the cryoablated tissue. Contrary, after cell death by apoptosis, only apoptotic bodies are released, without stimulating signals. Without these stimulating signals, T cells are not being activated. Therefore, apoptosis may lead to an immune-suppressing signal
Fig. 3.
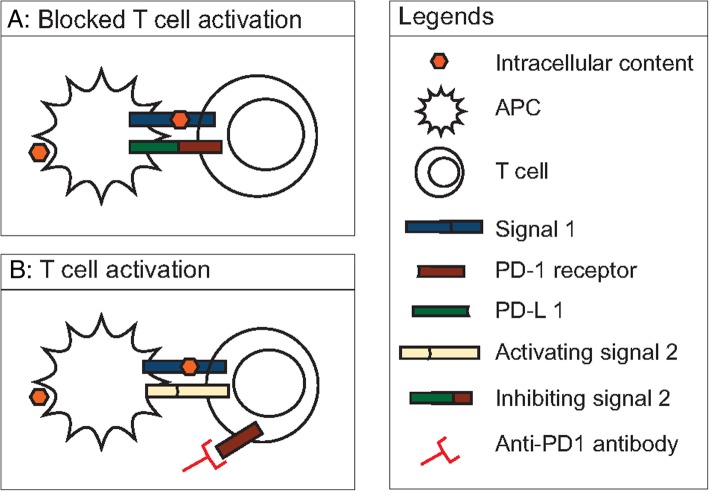
For T cell activation, both stimulatory signal 1 and 2 are needed. Signal 1 consists of the interaction between the major histocompatibility complex on the dendritic cell and the T cell receptor. Different combinations of interactions are possible for signal 2. One inhibiting signal 2 is the interaction between programmed death receptor 1 (PD-1) on T cell and programmed death ligand 1 (PD-L1) on tumour cells or antigen presenting cells. a T cell activation is blocked by an inhibiting signal 2 between PD-L1 and PD-1 receptor binding. b When an anti-PD1 antibody is used, the inhibiting signal of the T cell is blocked whereby an activation signal of the T cell is gathered and the T cell is activated
In the early 1970s, Shulman et al. reported the production of antibodies specifically against cryoablated tissue in rabbits [9, 10]. This immunogenic effect potentially resulted in a “bystander effect” with regression of tumours outside the primary ablation zone, known as the abscopal effect, firstly defined by Mole after radiotherapy [11, 12]. Unfortunately, this effect occurs infrequently, but when cryoablation is combined with other immunomodulatory therapy, this effect might be enhanced (Fig. 3) [13]. Here, we review the current evidence on the combination of cryoablation with immunomodulatory drugs (immunotherapy).
Material and method
A literature search in MEDLINE was performed for available publications between 2001 and 2017 about cryoablation, its effect on the immune system and the combination of cryoablation and immunotherapy. Only full text articles available in English were included. Keywords included cryoablation, immune system, immunotherapy, melanoma and cancer. Data collection included factors related to the synergy between cryoablation and the immune system, cryoablation and immunotherapy, effect of cryoablation on tumour tissue and cryoablation technique. For immunotherapy, all therapies which could theoretically enhance the anti-cancer immune response through either stimulation of the adaptive or innate immune system were included. Studies could be performed in patients and animals. Articles about combination of different ablation modalities were excluded.
A total of 45 relevant papers were identified and the most interesting and relevant studies were used for this review. To outline this paper, the studies were structured by tumour type and the current knowledge about the tumour type, cryoablation and immunotherapy was reviewed.
Results
Cryoablation and the immune system
Cryoablation may be synergistic with the immune system in the way cell death is induced. After ablation, the tumour remains, releasing various factors attracting the immune system. A proposed theory regarding the potential mechanisms of cryoablation and the immune system is the danger theory of Matzinger [14, 15]. This theory proposes that after cell death by necrosis, cells secrete danger signals. These danger signals can initiate an immune response. In addition, these signals can mature dendritic cells (DCs) to fully activate T cells which may lead to a specific immune response. Cryoablation induces cell death by necrosis in which intracellular contents are still preserved while DNA, RNA and heat shock protein (HSP), which can induce danger signals, are released [16]. On the other hand, the cells in the outer margin of cryoablated tissue die from apoptosis and do not release DNA, RNA and HSP, and with no danger signal, the DCs remain immature. Immature DCs may trigger immune suppressive signals that could lead to anergy (T cell inactivation) [17, 18]. Therefore, cryoablation can induce both an immunostimulatory and immunosuppressive response (Fig. 2).
In addition, cytokines are produced after cryoablation, and these can also influence an immune response. Again, both immunosuppressive and immunostimulatory cytokines may be released depending on the tumour tissue, age and freeze rate [19]. For cryoablation of liver tumours, when more than 20% of the liver volume is ablated, a systemic inflammatory response can occur due to a release of cytokines interleukine-6 (IL-6), IL-10 and tumour necrosis factor alpha (TNFα), which can have marked systemic effects [7, 20–22]. Therefore, cryoablation is not the preferred treatment since heat-based ablations are better established in this setting [23]. Yet, two clinical studies reported favourable outcomes on overall survival when cryoablation was combined with immunotherapy of allogenic natural killer (NK) cell infusion and dendritic cell cytokine-induced killer (DC-CIK) cells [24, 25].
Preclinical work from den Brok et al. confirms the effect of cryoablation on the immune system. This study reported that cryoablation creates an antigen depot resulting in maturation of DCs. The maturation of DCs led to a tumour-specific immune response protecting half of the mice against a new injection of similar tumour cells. When cryoablation was combined with an immune checkpoint inhibitor, anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) antibody, this anti-tumour effect was further enhanced with up to 80% of the mice becoming tumour free [26].
Clinical work by Takaki et al. evaluated peripheral blood after heath- and cold-based ablation modalities with pre-treatment (baseline) in different tumours showing no change in T cell subtypes (regulatory T cell (Tregs), T1 helper or Th2 helper cells) in all modalities, an elevation of cytotoxic T cells after heat-based ablative treatment was noted, and this was not identified after cryoablation [27]. In a group of hepatitis B-positive hepatocellular carcinoma patients, the presence of elevated programmed cell death protein 1 (PD-1) on T cells and programmed cell death ligand 1 (PD-L1) expression on tumour cells had a poor overall survival post cryoablation [28]. Theoretically, when combining cryoablation with a PD-1 inhibitor, such as nivolumab or pembrolizumab, the cryoablation induced adaptive immune resistance with upregulation of PD-L1 on tumour cells could be overcome, resulting in an effective anti-tumour T cell response. This potential synergy between cryoablation and anti-PD-1 may result in a more effective disease control; see Fig. 3 [29].
To enhance the immunogenicity effect of the cryoablation, different immunotherapies can be given as shown in Table 1. Most immunotherapy enhances the innate immunity, namely natural killer (NK) cell therapy, dendritic cells (DCs) and CpG oligonucleotide (CpG ODN). When cryoablation presents the contents of the tumour cells, the innate immune system can assimilate these contents and present it to T cells; an immune response specific to the tumour cells could be obtained. Table 2 is an overview exhibiting the most studied factors after cryoablation in the reviewed papers.
Table 1.
Summary of the major therapies with their mode of action used in combination with cryoablation in the reviewed papers. All therapies stimulate the immune system in a way and in combination with cryoablation an enhancement of this effect is hypothesized
| Therapy | Mode of action | Number of articles |
|---|---|---|
| CpG oligonucleotide (CpG ODN) | Is recognized by dendritic cells (DCs) and B cells. Activates T cells, natural killer (NK) cells, monocytes, neutrophils and plasma cell differentiation | 8 |
| Anti-cytotoxic T lymphocyte-associated protein 4 (anti CTLA-4) | Blocks the inhibitory receptor (CTLA-4) on the T cell and therefore activates the T cell for a specific immune response | 6 |
| Immature dendritic cells (DCs) | Phagocytosis of pathogens; antigen-presentation to other immune cells (among others T cells) | 4 |
| Natural killer (NK) cell therapy | Infusion with autologous NK cells to directly destroy tumour cells | 4 |
| Dendritic cell- cytokine induced killer (DC-CIK) | May act similarly to T cells or NK cells but is unrestricted to major histocompatibility complex | 3 |
| Granulocyte-macrophage colony-stimulating factor (GM-CSF) | A protein that functions as a cytokine and stimulates stem cells and can induce an immune cascade | 3 |
| Anti-programmed death-ligand 1 (PDL-1) | Blocks the receptor programmed death 1 on the tumour cell. This results in the activation of the T cell to induce a specific immune response | 1 |
Table 2.
Overview of the most studied factors of cryoablation and the immune system in the reviewed papers
| Most studied factors of cryoablation and the immune system | ||
|---|---|---|
| Mice | Total of 28 articles | |
| Survival | ↑ | 17/17 |
| Rechallenge of primary tumour | ↑ | 12/12 |
| Reduction in distant metastasis | ↑ | 10/10 |
| Cytokine release | ↑ | 16/17 |
| IFN-y release | ↑ | 16/17 |
| TNFα release | ↑ | 4/5 |
| IL-4 | ↑ | 1/7 |
| IL-10 | ↑ | 1/4 |
| Th1/Th2 cytokine ratio | ↑ | 10/0 |
| CD4+ infiltration | ↑ | 12/16 |
| CD8+ infiltration | ↑ | 14/19 |
| Treg | ↓ | 5/6 |
| NK cells | ↑ | 3/4 |
| Human | Total of 17 articles | |
| Survival | ↑ | 8/8 |
| Quality of Life | ↑ | 4/4 |
| Cytokine release | ↑ | 6/7 |
| IFN-y release | ↑ | 6/7 |
| TNFβ release | ↑ | 3/4 |
| IL-10 | ↓ | 3/5 |
| IL-4 | ↓ | 3/4 |
| IL-2 | ↑ | 3/5 |
| Th1/Th2 cytokine ratio | ↑ | 5/0 |
| CD4+ infiltration | ↑ | 5/8 |
| CD8+ infiltration | ↑ | 5/8 |
| Treg | ↓ | 2/2 |
| NK cells | ↑ | 4/4 |
Breast Cancer
Cryoablation was approved for the treatment of fibroadenomas for over a decade [30]. In 2016, the American College of Surgeons Oncology Group (ACOSOG) alliance considered cryoablation as an effective treatment for unifocal ductal cancer, with a success rate of complete tumour ablation of 92% after correction for multifocal disease [31]. Also, for the treatment of stage IV breast cancer, cryoablation is a safe and effective procedure to control the disease and debulks the tumour in the breast [32].
Although antibodies are part of the treatment for human epidermal growth factor receptor 2 (HER2)-positive breast cancers, no active forms of immunotherapy such as immune checkpoint inhibitors are currently approved for breast cancer. Recently, the interim analysis of the Impassion 130 study with the combination of nab-paclitaxel plus atezolizumab revealed an impressive 10 months of improvement in overall survival compared with chemotherapy alone in PD-L1-positive triple negative metastatic breast cancers [33]. Other immune checkpoint blocking antibodies, such anti-CTLA-4 antibody, are also under investigation [34].
One of the first reports confirming the immunogenicity of cryoablation in breast cancer was in a mammary mouse model. After cryoablation or surgery, mice were re-challenged with tumour cells and only 16% of the cryoablated exhibited tumour development compared to 86% of the surgically treated mice [35].
In a metastatic breast cancer mouse model, cryoablated mice treated with a high freeze rate (100% cryoablation cycle) showed an improvement in overall survival with significant reduction in the number of pulmonary metastasis compared to treatment with a low freeze rate (10% cryoablation cycle) or those treated with surgery [19]. When cryoablation was combined with injection of CpG ODN (a single-strand DNA molecule that acts as a toll-like receptor (TLR) agonist to stimulate and mature DCs) in mice, less tumour recurrence and secondary tumour growth were seen after the re-challenge compared to the mice that received cryoablation alone or surgery [36]. No significant difference was reported between CpG ODN alone or in combination with cryoablation groups, leaving the added role of cryoablation to CpG ODN injection undetermined regarding cytokine release and potential immune activation [36].
Other studies have investigated the combination of cryoablation with an immune checkpoint inhibitor (ipilimumab) 7 days before mastectomy in a group of patients with early-stage breast cancer. The pilot study, including 19 patients, showed that this approach was a safe option without delaying the mastectomy [37]. A post hoc analysis was performed to assess the possibility of T cell receptor sequencing as a biomarker for T cell response to cryoablation, where no specific T cell response was observed [38]. Additionally, a phase II trial is ongoing where cryoablation in combined with ipilimumab and nivolumab before breast surgery in triple negative breast cancer patients after taxane-based neoadjuvant chemotherapy [39].
In another study, recurrent HER2-positive breast cancer patients were treated with the combination of cryoablation, trastuzumab and natural killer (NK) cell therapy (intravenous infusion of allogenic NK cells). These patients displayed a significantly prolonged progression-free survival (PFS), significantly larger numbers of T cells and Th1 cytokines together with a significantly reduction in the number of circulation tumour cells in the peripheral blood compared to patients only treated with cryoablation alone or cryoablation and NK cell therapy. To note, PFS was not reached in the triple combination group, and the significantly prolonged PFS could be due to trastuzumab [40]. Niu et al. evaluated the use of cryoablation in combination with immunotherapy of DC-CIK cells in metastatic breast cancer patients versus chemotherapy or cryoablation alone. The group of patients that received multiple cryoablations (several sites) in combination with immunotherapy displayed a significantly longer median overall survival compared to the other groups [41].
Together, cryoablation before mastectomy is feasible and its combination with immunotherapy, consisting of NK cell therapy, DC-CIK or anti-CTLA-4 antibody, is safe and effective in different stages in breast cancer. These results encourage further investigation into the combination of cryoablation and immunotherapy for breast cancer patients. Two trials are currently open which combine cryoablation with immune checkpoint inhibitors (Table 3) [42, 43].
Table 3.
Overview of clinical trials of cryoablation and immunotherapy
| Cancer type | Tumour stage | Study phase | Study design | Therapy | End points | End date | Centre | Identifier |
|---|---|---|---|---|---|---|---|---|
| Breast cancer | Early/resectable disease | Pilot | Open label single arm | Pre-operative ipilimumab + nivolumab + cryoablation | Safety: number of adverse events, no secondary end points. | June 2019 | Memorial Sloan Kettering Cancer Center, USA | NCT02833233 |
| Resectable disease | II | Prospective randomized parallel interventional | Peri-operative cryoablation, ipilimumab nivolumab vs pre-operative care | Distant disease-free survival* | May 2021 | Cedars-Sinai Medical Center, USA | NTC03546686 | |
| Renal cell carcinoma | Metastatic disease | I | Open label single arm | Tremelimumab +/− cryoablation before surgery | Objective response rate by irRC* | March 2021 | MD Anderson, USA | NCT02626130 |
| Stage I | – | Prospective observational cohort | Immune response of cryoablation vs RFA vs LPN | Immune response: number of leucocytes in tissue samples post ablation. | June 2019 | UC Irvine, USA | NCT03409224 | |
| Prostate cancer | Localized disease | Basic science | Open label single arm | Immune response profile after total cryotherapy, focal cryotherapy, SBRT and radical prostatectomy | Evaluate change in blood cytokine profile | Sept 2019 | Winthrop University Hospital, USA | NCT03331367 |
| Metastatic disease | II | Open label single arm | Pembrolizumab and cryosurgery in combination with short term androgen ablation | Proportion of men with PSA < 0.6 ng/mL PD-1/PDL-1 expression | Nov 2018 | Sidney Kimmel Comprehensive Cancer Center, USA | NCT02489357 | |
| Stage I tm IIB | I | Prospective randomized open label clinical trial | GM-CSF after cryoablation | Change in B cell, T cells and PSA levels | Dec 2018 | University of Colorado Cancer Center, USA | NCT02250014 | |
| Castration resistant disease with positive lymph nodes | I | Open label single arm | Cryoablation plus intratumoural immature dendritic cells | Maximum tolerated dose* | April 2019 | Haukeland University Hospital, Norwegian | NCT02423928 | |
| Lung cancer | Stage IV | II | Open label single arm | Core needle biopsy and cryoablation added to continued treatment with immune checkpoint inhibitor | Response by RECIST* | March 2025 | Massachusetts General Hospital, USA | NCT03290677 |
| Melanoma | Stage III tm IV cutaneous melanoma | I + II | Open label single arm | Dendritic cell therapy after cryosurgery in combination with pembrolizumab |
Response by RECIST Clinical benefit* |
Oct 2022 | Mayo Clinic, USA | NCT03325101 |
|
Stage IV, HLA-A2 + no curative disease |
I | Open label single arm | Radiofrequency therapy + RFA/CA+ GM-CSF injection |
Level of immune response by heat shock protein and lymphocyte Response by RECIST* |
May 2018 | Mayo Clinic, USA | NCT00568763 | |
| Other | Metastatic Colorectal cancer | I/IIb | Open label single arm | Combining cryoablation with intra-lesional immunotherapy with AlloStim® with dose escalation |
Safety of increased frequency of dosing* Tumour response RECIST and histopathology HRQoL |
Feb 2018 | MD Anderson Medical Center, USA |
NB this study was first designed in BC |
| Palliative stetting of HCC or BTC | I/II | Open label single arm | Tremelimumab and durvalumab + RFA/CA/TACE | PFS* | Apr 2021 | National institutes of health clinical centre, USA | NCT02821754 | |
| Palliative stetting of HCC or BTC stage B and C | I | Clinical prospective non-randomised | Tremelimumab + RFA/CA/SBRT/TACE |
Response Rate Time to tumour progression Overall survival* |
Dec 2018 | National Institutes of Health Clinical Center, USA | NCT01853618 | |
| Other | Non-Hodgkin lymphoma | I/II | Open label single arm | Intratumoral DC therapy after cryosurgery and pembrolizumab |
Maximum tolerated dose* Complete responses Disease free survival rate Duration of response OS, PFS, HRQoL |
Feb 2021 | Mayo Clinic, USA | NCT03035331 |
|
1. Oesophageal cancer, 2. Tongue cancer, 3. Ovarian cancer, 4. Laryngeal cancer, 5. Pharyngeal cancer, 6. Cervical cancer 7. Tumours in transplanted livers 8. Sarcoma |
I/II | Open label single arm trial | Cryoablation and NK cell immunotherapy | Response, PFS, OS by RECIST* | Jul 2019 | Cancer Institute in Fuda Cancer Hospital, China | ||
|
1. Breast cancer, 2. Liver cancer, 3. Lung cancer, 4. Gastric cancer, 5. Colorectal cancer, 6. Pancreatic cancer 7. Kidney cancer |
II | Prospective randomized triple arm study | Activated CIK cells + anti-bispecific antibody with or without cryoablation vs conventional therapy |
Objective response rate PFS TTP* |
April 2021 | Fuda Cancer Hospital, China |
NTC03524261 NTC03484962 NTC03501056 NTC03554395 NTC03524274 NTC03509298 NTC03540199 |
RECIST response evaluation criteria in solid tumours, irRC immune-related response criteria, PFS progression-free survival, OS overall survival, CR complete response, PR partial response, HRQoL health-related quality of life, DSS disease specific survival, LHRH agonist luteinizing-hormone releasing-hormone agonist, TTP time tumour progression, RFA radiofrequency ablation, CA cryoablation, SBRT stereotactic body radiotherapy, DC dendritic cell, CIK cytokine-induced killer cells, NK natural killer, TACE transarterial chemo-embolisation, GM-CSF granulocyte-macrophage colony-stimulating factor, LPN laparoscopic partial nephrectomy, PSA prostate specific antigen, PD-1 programmed cell death protein, PDL-1 programmed cell death ligand
*Safety has been performed in all studies by number of adverse events
Renal cell carcinoma
In RCC, cryoablation is most frequently used to treat stage I cancer (ideally smaller than 4 cm taken as the largest diameter) in patients not eligible for surgical resection [44, 45]. With optimal patient selection, results similar to partial nephrectomy can be achieved [46].
Immunotherapy for RCC has been used for quite some time, and nivolumab, a PD-1 inhibitor, is already approved for the treatment of RCC [47, 48].
Two animal studies showed the favourable effect of cryoablation in the microenvironment of RCC and in the kidney. The first study used two mice models, one with and one without injected RCC to observe an inflammatory immune response after cryoablation in the tumour or healthy kidney tissue. An infiltration of neutrophils, macrophages and CD4+ and CD8+ T cells was reported after cryoablation whereby no difference was observed after cryoablation of normal kidney tissue or tumour tissue [49]. Another study compared cryoablation with surgery and showed decreased tumour growth after the re-challenge of the tumour cells with significantly more T cells in the peripheral blood after cryoablation [50].
Kato et al. showed that in half of the patients with T1 RCC, a significant increase in T cell receptor (TCR) B CD3 clonotypes of T-cells in post ablation tissue and blood was seen with a low diversity (TCR clones were not evenly distributed anymore) [51]. In another clinical study, two sessions of cryoablation of the pulmonary metastases, each combined with two Intratumoural injections of granulocyte-macrophage colony-stimulating factor (GM-CSF), resulted in higher levels of NK cells, Th1 cytokines and T and B cells in the peripheral blood compared to baseline [52]. Lin et al. showed similar effects of allogeneic NK cell immunotherapy combined with cryoablation in 60 advanced RCC patients, and this treatment combination resulted in more tumour responses and decrease in Hounsfield units count than cryoablation on its own [53].
To summarise, cryoablation of RCC elicits an immune response and can be safely combined with GM-CSF and NK cell therapy. Currently, one trial is ongoing investigating the synergy of cryoablation with anti-PD-1 therapy (tremelimumab), and another trial investigates the effect of ablation of the immune system [54, 55].
Prostate cancer
Cryoablation is currently being used to treat stage I prostate cancer. Cryoablation could also be considered as salvage treatment for local recurrence after radiation therapy. Future perspectives in prostate cancer shift towards a more targeted therapy where cryoablation may have an important role in prostate cancer [56].
Presently in prostate cancer, the only approved immunotherapy is sipuleucel-T (Provenge), a DC-based immunotherapy that sensitises dendritic cells with prostate antigens and is used as a therapeutic vaccine [57]. Other immunotherapies evaluated have failed to show improvement in overall survival [58–60]. Current developments are focusing on immunotherapy for the subgroup with defects in DNA-repair mechanisms, which include microsatellite instability and breast cancer gene mutations.
Waitz et al. reported a regression in secondary tumour growth with infiltration of CD4+ and CD8+ T cells and lower counts of Tregs in mice treated by cryoablation and anti-CTLA-4 antibodies [29]. Another study reported that the combination of cryoablation with anti-CTLA-4 antibodies reduced distant metastasis in mice together with a reduction in the number of Tregs; these were lowest on day 14 but returned to normal levels at day 21 [61]. Recently, combination therapy of androgen deprivation plus anti-PD-1, anti-CLTA-4 or placebo with or without cryoablation demonstrated a delay in distant tumour growth and decreased mortality in mice in the trimodal therapy groups [62].
Pre cryoablation tissue in high-risk Localised prostate cancer patients showed elevated numbers of Tregs compared to healthy volunteers. Numbers of Tregs decreased significantly after cryoablation in the prostate patients, and, conversely, 7 of 12 patients had an increase of suppressive function of Tregs measured by immunosuppressive assay of CD4+CD25+CD127− which was linked to the probability of recurrence of the cancer in 2 patients [63]. Another clinical study reported significantly higher cytokines (TNFα and IFN-y) levels, increased T cell response to autologous tumour tissue (IFN-y ELISPOT assay) after 4 weeks and higher cytotoxic activity of T cells after 4 and 8 weeks (measured by luciferase assay) after cryoablation in 20 high-risk prostate patients [64]. In patients with metastatic hormone refractory prostate cancer, a combination of cryoablation and GM-CSF showed a 70% decrease of PSA levels and a median time to progression of 18 months. No correlation was seen between the increase tumour-specific T cell responses in the peripheral blood and the increased cytolytic activity (measured by luciferase assay) after 4 and 8 weeks [65]. The addition of cryoablation to androgen deprivation therapy (ADT) in 30 prostate cancer patients with bone metastases significantly improved progression-free, cancer-specific and overall survival compared to 30 prostate patients only treated with ADT [66].
No clinical trials have been performed so far to evaluate a combination of anti-CTLA-4 antibodies with cryoablation therapy in humans. Only immunotherapy with GM-CSF has been investigated. Currently, a phase II trial of the combination of pembrolizumab and cryosurgery in stage IV prostate patients is ongoing. In addition, other trials are ongoing searching for the relation between cryoablation and the effect on the immune system [67–70].
Lung cancer
Percutaneous local ablative therapies are considered viable options for the treatment of stage IA non-small-cell lung carcinoma (NSCLC). Recurrent lesions after radiation therapy or surgery and metastatic lesions can be treated by means of ablation as well [71, 72].
Cryoablation of lung lesions is associated with lower pain levels and fewer complications in tumours located close to the chest wall and mediastinum or central lesions close to the hilum; however, no clinical randomised studies have been executed comparing the different percutaneous ablative therapies [73, 74].
NSCLC is a heterogeneous group of cancers which is known for high numbers of tumour-specific mutations that are linked with response to immunotherapy [75]. In recent years, several immunotherapies have been approved for the treatment of lung cancer, namely PD-1 checkpoint inhibitors nivolumab and pembrolizumab and the anti-CTLA-4 inhibitor ipilimumab.
Preclinical work in a mouse model revealed that Intratumoural injection of DCs with cryoablation elicits a Th1 response with higher levels of IFN-y and effector memory CD8+ T cells observed from spleen cells resulting in protection against secondary tumours and prolonged survival [76]. In another study, the addition of CpG ODN to the cryoablation plus DCs resulted in a significant reduction of new tumour growth, fewer metastasis development and a prolonged survival compared to all the therapies alone. A decrease in Tregs and increase in cytotoxic T cells were observed and linked to the better response in the combination group [77]. In another study, the same treatment combination showed a higher elevation of CD4+ and CD8+ T cells and IL-12, IFN-y and TNFα together with a delay in tumour growth and improved survival in mice treated with the combination therapy [78]. Takahashi et al. reported the greatest immune response (higher numbers of specific T cells and higher levels of stimulating cytokines) and the slowest tumour growth after two cycles of cryoablation compared to one or three cycles of cryoablation [79].
Clinical work combining cryoablation and allogenic intravenous NK cells showed an improvement in the quality of life and tumour response rates compared to cryoablation alone. Additional phase II/III trials must be conducted to reveal the potential benefits in larger patient groups before combination treatment is considered an alternative [80]. Another strategy, consisting of a combination of cryoablation with gefitinib, an inhibitor of epidermal growth factor receptor’s (EGFR) tyrosine kinase domain, showed significant improvement of overall response with a higher 1-year survival rate in patients treated with gefitinib and cryoablation compared to gefitinib alone [81]. Lastly, 166 metastatic NSCLC patients received either cryoablation alone, cryoablation followed by immunotherapy (DC-CIK) or chemotherapy or all three therapies. The survival of patients treated with cryoablation combined with chemo or immunotherapy was longer than treatment consisting of chemo or immunotherapy alone (18 and 17 months vs 8.5 and 12 months). The overall survival in patients that received the triple combination therapy (cryoablation, immunotherapy and chemotherapy) was significantly longer (27 months) compared to other groups [82].
These studies show positive results for the combination of cryoablation and stimulants to the immune system, NK cell therapy or DC-CIK, with improvement in survival. Clinical results are expected from a phase II study where cryoablation is combined with an immune checkpoint inhibitor [83, 84].
Melanoma
Cryoablation is used for the treatment of benign superficial lesions, such as actinic keratosis, but it is not indicated as a treatment for primary melanoma. Only in unresectable lesions with high metastatic load, cryoablation may diminish tumour load by ablation of the primary site [85, 86]. The treatment of melanoma metastasis can be performed with cryoablation to slow down the rate of tumour spread [87, 88]. In metastatic mouse models, combinations with different immunostimulants (including TLR 9 and CPG) have shown an enhanced effect of cryoablation for suppression of new tumour growth [26, 89–91]. A pilot study observed the induction of endogenous heat-shock protein after administration of GM-CSF and radiofrequency ablation or cryoablation in metastatic melanoma patients, with a small number of subjects demonstrating the combination therapy as a feasible and safe therapeutic option [92]. The combination of cryoablation and immunotherapy may be beneficial in the metastatic stetting to overcome the limitations of the immunotherapy. Currently, two trials are open combining immunotherapy and cryoablation (Table 3).
Conclusion
Cryoablation has proven to be successful for local control in various cancer types. Mostly, it is indicated for tumours at an early stage or those not eligible for surgery. The synergies of local ablative techniques together with systemic treatments are currently one of the most exciting developments of interventional oncology. Particularly, given that cryoablation provides a pool of antigens visible for the immune system to induce an immune-specific activation directed against the tumour cells. In this review, a total of 41 out of 45 publications show favourable effects for cryoablation when combined with other therapies, potentially enhancing the anti-cancer immune response. This emphasises the potential advantage of this ablation technique as an adjunct with immunotherapy.
RCC and NSCLC are the neoplasms where cryoablation and its synergy with immunotherapy are predominantly studied. Both, immunostimulants for the innate and adaptive immune system demonstrate feasibility and effectiveness. For the translation of the results seen in animals and small groups of patients, larger prospective trials need to be designed to first study safety and efficacy before moving into randomized controlled settings. In the next 5 years, several pilot, phase I and early II clinical trials currently in place will demonstrate whether or not the combination of cryoablation and the immune system will have a true beneficial effect. This benefit can come from two different approaches that is first the contribution of cryoablation to immunological systemic effect of immunotherapy. Second, the impact of immunotherapy to overcome the limitations of cryoablation related to the upregulation of PD-L1. The features of imaging and molecular biomarkers, such as PD-L1 and others will help to optimise the combination strategies.
Furthermore, the strategy of combining cryoablation with new therapeutic options (specifically DC vaccine, CAR T cells, oncolytic viruses and adoptive T cell transfer) will provide a broader scale of immune-boosting therapies that will hopefully translate into the clinics and may thereby provide even better outcomes when combined with cryoablation.
Abbreviations
- ACOSOG
American College of Surgeons Oncology Group
- ADT
Androgen deprivation therapy
- anti-CTLA-4
Anti-cytotoxic T lymphocyte-associated protein 4
- CpG ODN
CpG oligodeoxynucleotide
- CT
Computed tomography
- DC
Dendritic cell
- DC-CIK
Dendritic cell cytokine-induced killer
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- HSP
Heat shock protein
- IL
Interleukin
- NK
Natural killer
- NSCLC
Non-small cell lung carcinoma
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death ligand 1
- PFS
Progression-free survival
- RCC
Renal cell carcinoma
- TCR
T cell receptor
- TLR
Toll-like receptor
- TNFα
Tumour necrosis factor alpha
- Treg
Regulatory T cell
Authors’ contributions
BA and FG wrote the article. All other authors read, reviewed and contributed with their expertise in their different fields to the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cazzato RL, Garnon J, Ramamurthy N, et al. Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol. 2016;33:140. doi: 10.1007/s12032-016-0848-3. [DOI] [PubMed] [Google Scholar]
- 2.Baust JG, Gage AA. Progress toward optimization of cryosurgery. Technol Cancer Res Treat. 2004;3:95–101. doi: 10.1177/153303460400300202. [DOI] [PubMed] [Google Scholar]
- 3.Mues AC, Landman J. Results of kidney tumor cryoablation: renal function preservation and oncologic efficacy. World J Urol. 2010;28:565–570. doi: 10.1007/s00345-010-0552-4. [DOI] [PubMed] [Google Scholar]
- 4.Chehab M, Friedlander JA, Handel J, et al. Percutaneous cryoablation vs partial nephrectomy: cost comparison of T1a tumors. J Endourol. 2016;30:170–176. doi: 10.1089/end.2015.0183. [DOI] [PubMed] [Google Scholar]
- 5.van Amerongen MJ, Jenniskens SFM, van den Boezem PB, Fütterer JJ, de Wilt JHW (2017) Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases - a meta-analysis. HPB (Oxford) 19:749–756 [DOI] [PubMed]
- 6.Rivero JR, De La Cerda J 3rd, Wang H et al (2018) Partial nephrectomy versus thermal ablation for clinical stage T1 renal masses: systematic review and meta-analysis of more than 3,900 patients. J Vasc Interv Radiol 29:18–29 [DOI] [PubMed]
- 7.Jansen MC, van Hillegersberg R, Schoots IG, Levi M, Beek JF, Crezee H, van Gulik TM. Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model. Surgery. 2010;147(5):686–695. doi: 10.1016/j.surg.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 8.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Can. 2014;14(3):199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 9.Cooper AJ, Fraser JD, MacIver A. Host responses to cryoablation of normal kidney and liver tissue. Br J Exp Pathol. 1978;59:97–104. [PMC free article] [PubMed] [Google Scholar]
- 10.Shulman S, Brandt EJ, Yantorno C. Studies in cryo-immunology. II Tissue and species specificity of the autoantibody response and comparison with iso-immunization. Immunology. 1968;14:149–158. [PMC free article] [PubMed] [Google Scholar]
- 11.Ablin RJ. Cryoimmunotherapy. Br Med J. 1972;3:476. doi: 10.1136/bmj.3.5824.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 13.Soule E, Bandyk M, Matteo J (2018) Percutaneous ablative cryoimmunotherapy for micrometastaic abscopal effect: No complications. Cryobiology. 10.1016/j.cryobiol.2018.04.013 [DOI] [PubMed]
- 14.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 15.Skoberne M, Beignon AS, Bhardwaj N (2004) Danger signals: a time and space continuum. Trends Mol Med 10:251–257 [DOI] [PubMed]
- 16.Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 17.Chan JK, Roth J, Oppenheim JJ, et al. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12:1539–1546 [DOI] [PubMed]
- 19.Sabel MS, Su G, Griffith KA, Chang AE. Rate of freeze alters the immunologic response after cryoablation of breast cancer. Ann Surg Oncol. 2010;17:1187–1193. doi: 10.1245/s10434-009-0846-1. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad F, Gravante G, Bhardwaj N, et al. Changes in interleukin-1β and 6 after hepatic microwave tissue ablation compared with radiofrequency, cryotherapy and surgical resections. Am J Surg. 2010;200:500–506. doi: 10.1016/j.amjsurg.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Sadikot RT, James Wudel L, Jansen DE et al (2002) Hepatic cryoablation-induced multisystem injury: bioluminescent detection of NF-κB activation in a transgenic mouse model. J Gastrointest Surg 6:264–270. [DOI] [PubMed]
- 22.Seifert JK, France MP, Zhao J, et al. Large volume hepatic freezing: association with significant release of the cytokines interleukin-6 and tumor necrosis factor a in a rat model. World J Surg. 2002;26:1333–1341. doi: 10.1007/s00268-002-6139-5. [DOI] [PubMed] [Google Scholar]
- 23.Vroomen LGPH, Petre EN, Cornelis FH, Solomon SB, Srimathveeravalli G (2017) Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: what are the differences? Diagn Interv Imaging 98:609–617 [DOI] [PubMed]
- 24.Niu LZ, Li JL, Zeng JY et al (2013) Combination treatment with comprehensive cryoablation and immunotherapy in metastatic hepatocellular cancer. World J Gastroenterol 19:3473–3480 [DOI] [PMC free article] [PubMed]
- 25.Lin M, Liang S, Wang X, et al. Cryoablation combined with allogenic natural killer cell immunotherapy improves the curative effect in patients with advanced hepatocellular cancer. Oncotarget. 2017;8:81967–81977. doi: 10.18632/oncotarget.17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.den Brok MH, Sutmuller RP, Nierkens S et al (2006) Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 95:896–905 [DOI] [PMC free article] [PubMed]
- 27.Takaki H, Imai N, Thomas CT, et al. Changes in peripheral blood T-cell balance after percutaneous tumor ablation. Minim Invasive Ther Allied Technol. 2017;26:331–337. doi: 10.1080/13645706.2017.1310737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng Z, Shi F, Zhou L, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One. 2011;6:e23621. doi: 10.1371/journal.pone.0023621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waitz R, Solomon SB, Petre EN, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72:430–439. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bea VJ, Black D, Hunt K (2018) Percutaneous ablation in the treatment of breast cancer. In: Howard-McNatt M (eds) Changing Paradigms in the Management of Breast Cancer. Springer, Cham, pp 71–84
- 31.Simmons RM, Ballman KV, Cox C, et al. A phase II trial exploring the success of cryoablation therapy in the treatment of invasive breast carcinoma: results from ACOSOG (Alliance) Z1072. Ann Surg Oncol. 2016;23:2438–2445. doi: 10.1245/s10434-016-5275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pusceddu C, Melis L, Ballicu N, et al. Cryoablation of primary breast cancer in patients with metastatic disease: considerations arising from a single-centre data analysis. Biomed Res Int. 2017;2017:3839012. doi: 10.1155/2017/3839012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles D, Andre F, Gligorov J et al (2017) IMpassion131: phase III study comparing 1L atezolizumab with paclitaxel vs placebo with paclitaxel in treatment-naive patients with inoperable locally advanced or metastatic triple negative breast cancer (mTNBC). Ann Oncol 28(Suppl 5):105
- 34.Nakasone ES, Hurvitz SA, McCann KE. Harnessing the immune system in the battle against breast cancer. Drugs Context. 2018;7:212520. doi: 10.7573/dic.212520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabel MS, Nehs MA, Su G, Lowler KP, Ferrara JL, Chang AE (2005) Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat 90:97–104 [DOI] [PubMed]
- 36.Veenstra JJ, Gibson HM, Littrup PJ, et al. Cryotherapy with concurrent CpG oligonucleotide treatment controls local tumor recurrence and modulates HER2/neu immunity. Cancer Res. 2014;74:5409–5420. doi: 10.1158/0008-5472.CAN-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McArthur HL, Diab A, Page DB, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res. 2016;22:5729–5737. doi: 10.1158/1078-0432.CCR-16-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page DB, Yuan J, Redmond D, et al. Deep sequencing of T-cell receptor DNA as a biomarker of clonally expanded TILs in breast cancer after immunotherapy. Cancer Immunol Res. 2016;4:835–844. doi: 10.1158/2326-6066.CIR-16-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.(2018) US National Library of Medicine. NCT03546686. In: Clinical Trials.gov. https://clinicaltrials.gov/ct2/show/NCT03546686. Accessed 27 July 2018
- 40.Liang S, Niu L, Xu K, et al. Tumor cryoablation in combination with natural killer cells therapy and herceptin in patients with HER2-overexpressing recurrent breast cancer. Mol Immunol. 2017;92:45–53. doi: 10.1016/j.molimm.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Niu L, Mu F, Zhang C, et al. Cryotherapy protocols for metastatic breast cancer after failure of radical surgery. Cryobiology. 2013;67:17–22. doi: 10.1016/j.cryobiol.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 42.U.S. National Library of Medicine US National Library of Medicine. NCT02833233. In: ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02833233?term=cryoablation&cond=breast+cancer&rank=3. Accessed 16 June 2018
- 43.Increased Frequency of AlloStim(TM) Dosing in Combination With Cryoablation in Metastatic Breast Cancer Patients - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02018419?term=cryoablation&cond=breast+cancer&rank=6. Accessed 16 Mar 2018
- 44.Uhlig A, Hahn O, Strauss A et al (2017) Treatment for Localised T1a clear cell renal cell carcinoma: survival benefit for cryosurgery and thermal ablation compared to deferred therapy. Cardiovasc Intervent Radiol 10.1007/s00270-017-1816-9 [DOI] [PubMed]
- 45.Breen DJ, King AJ, Patel N, Lockyer R, Hayes M (2018) Image-guided cryoablation for sporadic renal cell carcinoma: three- and 5-year outcomes in 220 patients with biopsy-proven renal cell carcinoma. Radiology. 10.1148/radiol.2018180249 [DOI] [PubMed]
- 46.Xing M, Kokabi N, Zhang D, Ludwig JM, Kim HS (2018) Comparative effectiveness of thermal ablation, surgical resection, and active surveillance for T1a renal cell carcinoma: a surveillance, epidemiology, and end results (SEER)-Medicare-linked population study. Radiology 288:81–90 [DOI] [PubMed]
- 47.Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer. 2018;94:115–125. doi: 10.1016/j.ejca.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross K, Jones RJ (2017) Immune checkpoint inhibitors in renal cell carcinoma. Clin Sci (Lond) 131:2627–2642 [DOI] [PMC free article] [PubMed]
- 49.Matin SF, Sharma P, Gill IS, et al. Immunological response to renal cryoablation in an in vivo orthotopic renal cell carcinoma murine model. J Urol. 2010;183:333–338. doi: 10.1016/j.juro.2009.08.110. [DOI] [PubMed] [Google Scholar]
- 50.Kim HK, Pyun JH, Cho S, et al. Tumor-specific immunity induced by cryoablation in a murine renal cell carcinoma model. Korean J Urol. 2014;55:834–840. doi: 10.4111/kju.2014.55.12.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato T, Iwasaki T, Uemura M, et al. Characterization of the cryoablation-induced immune response in kidney cancer patients. Oncoimmunology. 2017;6:e1326441. doi: 10.1080/2162402X.2017.1326441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thakur A, Littrup P, Paul EN, Adam B, Heilbrun LK, Lum LG (2011) Induction of specific cellular and humoral responses against renal cell carcinoma after combination therapy with cryoablation and granulocyte-macrophage colony stimulating factor: a pilot study. J Immunother 34:457–467 [DOI] [PMC free article] [PubMed]
- 53.Lin M, Xu K, Liang S, et al. Prospective study of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced renal cell cancer. Immunol Lett. 2017;184:98–104. doi: 10.1016/j.imlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Pilot Study of Presurgical Tremelimumab With or Without Cryoablation in Patients With Metastatic Renal Cell Carcinoma (RCC) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02626130. Accessed 19 Mar 2018
- 55.US National Library of Medicine. NCT02843607. In: ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02843607. Accessed 27 July 2018
- 56.Tay KJ, Tsivian M, Polascik TJ (2017) Prostate cryotherapy. In: Hughes S (eds) Management Management of prostate cancer. Springer, Cham, pp 273–285
- 57.Schepisi G, Farolfi A, Conteduca V et al (2017) Immunotherapy for prostate cancer: where we are headed. Int J Mol Sci 18 10.3390/ijms18122627 [DOI] [PMC free article] [PubMed]
- 58.Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of Ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 59.Hansen AR, Massard C, Ott PA et al (2018) Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol 10.1093/annonc/mdy232 [DOI] [PubMed]
- 60.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li F, Guo Z, Yu H, et al. Anti-tumor immunological response induced by cryoablation and anti-CTLA-4 antibody in an in vivo RM-1 cell prostate cancer murine model. Neoplasma. 2014;61:659–671. doi: 10.4149/neo_2014_081. [DOI] [PubMed] [Google Scholar]
- 62.Benzon B, Glavaris SA, Simons BW et al (2018) Combining immune check-point blockade and cryoablation in an immunocompetent hormone sensitive murine model of prostate cancer. Prostate Cancer Prostatic Dis 10.1038/s41391-018-0035-z [DOI] [PMC free article] [PubMed]
- 63.Si TG, Wang JP, Guo Z (2013) Analysis of circulating regulatory T cells (CD4+CD25+CD127-) after cryosurgery in prostate cancer. Asian J Androl 15:461–465 [DOI] [PMC free article] [PubMed]
- 64.Si T, Guo Z, Hao X. Immunologic response to primary cryoablation of high-risk prostate cancer. Cryobiology. 2008;57:66–71. doi: 10.1016/j.cryobiol.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Si T, Guo Z, Hao X. Combined cryoablation and GM-CSF treatment for metastatic hormone refractory prostate cancer. J Immunother. 2009;32:86–91. doi: 10.1097/CJI.0b013e31818df785. [DOI] [PubMed] [Google Scholar]
- 66.Si T, Guo Z, Yang X, Zhang W, Xing W (2017) The oncologic results of cryoablation in prostate cancer patients with bone metastases. Int J Hyperthermia:1–5 [DOI] [PubMed]
- 67.US National Library of Medicine. NCT03331367. In: clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03331367?cond=NCT03331367&rank=1. Accessed 19 Mar 2018
- 68.US National Library of Medicine. NCT02489357. In: Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT02489357?cond=NCT02489357&rank=1. Accessed 19 Mar 2018
- 69.US National Library of Medicine. NCT02250014. In: clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT02250014?cond=NCT02250014&rank=1. Accessed 19 Mar 2018
- 70.US National Library of Medicine. NCT02423928. In: Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT02423928?cond=NCT02423928&rank=1. Accessed 19 Mar 2018
- 71.de Baere T, Tselikas L, Woodrum D, et al. Evaluating cryoablation of metastatic lung tumors in patients--safety and efficacy: the ECLIPSE trial--interim analysis at 1 year. J Thorac Oncol. 2015;10:1468–1474. doi: 10.1097/JTO.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 72.Moore W, Talati R, Bhattacharji P, Bilfinger T. Five-year survival after cryoablation of stage I non–small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol. 2015;26:312–319. doi: 10.1016/j.jvir.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Lee KS, Erinjeri JP (2017) Decision making in interventional oncology: ablative options in the lung. Semin Intervent Radiol 34:176–181 [DOI] [PMC free article] [PubMed]
- 74.Mouli SK, Kurilova I, Sofocleous CT, Lewandowski RJ. The role of percutaneous image-guided thermal ablation for the treatment of pulmonary malignancies. AJR Am J Roentgenol. 2017;209:740–751. doi: 10.2214/AJR.17.18368. [DOI] [PubMed] [Google Scholar]
- 75.Lyu GY, Yeh YH, Yeh YC, Wang YC (2018) Mutation load estimation model as a predictor of the response to cancer immunotherapy. NPJ Genom Med 3:12 [DOI] [PMC free article] [PubMed]
- 76.Machlenkin A, Goldberger O, Tirosh B, et al. Combined dendritic cell cryotherapy of tumor induces systemic antimetastatic immunity. Clin Cancer Res. 2005;11:4955–4961. doi: 10.1158/1078-0432.CCR-04-2422. [DOI] [PubMed] [Google Scholar]
- 77.Alteber Z, Azulay M, Cafri G, Vadai E, Tzehoval E, Eisenbach L (2014) Cryoimmunotherapy with local co-administration of ex vivo generated dendritic cells and CpG-ODN immune adjuvant, elicits a specific antitumor immunity. Cancer Immunol Immunother 63:369–380 [DOI] [PMC free article] [PubMed]
- 78.Zhang M, Yin T, Lu Y, Feng H. The application of cytidyl guanosyl oligodeoxynucleotide can affect the antitumor immune response induced by a combined protocol of cryoablation and dendritic cells in Lewis lung cancer model. Med Sci Monit. 2016;22:1309–1317. doi: 10.12659/MSM.898194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi Y, Izumi Y, Matsutani N, et al. Optimised magnitude of cryosurgery facilitating anti-tumor immunoreaction in a mouse model of Lewis lung cancer. Cancer Immunol Immunother. 2016;65:973–982. doi: 10.1007/s00262-016-1858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin M, Liang SZ, Wang XH et al (2017) Clinical efficacy of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced non-small cell lung cancer. Immunol Res 65:880–887 [DOI] [PubMed]
- 81.Gu XY, Jiang Z, Fang W. Cryoablation combined with molecular target therapy improves the curative effect in patients with advanced non-small cell lung cancer. J Int Med Res. 2011;39:1736–1743. doi: 10.1177/147323001103900516. [DOI] [PubMed] [Google Scholar]
- 82.Yuanying Y, Lizhi N, Feng M, et al. Therapeutic outcomes of combining cryotherapy, chemotherapy and DC-CIK immunotherapy in the treatment of metastatic non-small cell lung cancer. Cryobiology. 2013;67:235–240. doi: 10.1016/j.cryobiol.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 83.BrUOG 317:Nivolumab and Ablation For Patients With Advanced Non-Small Cell Lung Cancer Progressing After at Least One Prior Therapy For Metastatic Disease - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02469701. Accessed 5 Nov 2018
- 84.US National Library of Medicine. NCT03290677. In: clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03290677?term=cryoablation+and+immune&cond=Lung+Cancer&rank=1
- 85.Abramovits W, Graham G, Har-Shai Y, Strumia R (2016) Dermatological Cryosurgery and Cryotherapy. 1st ed. 2016 Springer
- 86.Tanaka S (2001) Cryosurgery for malignant melanoma. In: Korpan NN (eds) Basics of cryosurgery. Springer, Vienna, pp 289–293
- 87.Joosten JJ, Muijen GN, Wobbes T, Ruers TJ. In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: an experimental study. Cryobiology. 2001;42:49–58. doi: 10.1006/cryo.2001.2302. [DOI] [PubMed] [Google Scholar]
- 88.Rivoire M, De Cian F, Meeus P, Gignoux B, Fréring B, Kaemmerlen Pl (2000) Cryosurgery as a means to improve surgical treatment of patients with multiple unresectable liver metastases. Anticancer Res 20:3785–3790 [PubMed]
- 89.Kudo-Saito C, Fuwa T, Kawakami Y. Targeting ALCAM in the cryo-treated tumour microenvironment successfully induces systemic anti-tumour immunity. Eur J Cancer. 2016;62:54–61. doi: 10.1016/j.ejca.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 90.den Brok MH, Sutmuller RP, Nierkens S et al (2006) Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res 66:7285–7292 [DOI] [PubMed]
- 91.Nierkens S, den Brok MH, Sutmuller RP et al (2008) In vivo colocalization of antigen and CpG [corrected] within dendritic cells is associated with the efficacy of cancer immunotherapy. Cancer Res 68:5390–5396 [DOI] [PubMed]
- 92.Domingo-Musibay E, Heun JM, Nevala WK, et al. Endogenous heat-shock protein induction with or without radiofrequency ablation or cryoablation in patients with stage IV melanoma. Oncologist. 2017;22:1026–1e93. doi: 10.1634/theoncologist.2017-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]