Abstract
Phosphatidylcholine (PC) is a major cell membrane constituent and precursor of important second messengers. In Leishmania parasites, PC synthesis can occur via the choline branch of the Kennedy pathway, the N-methylation of phosphatidylethanolamine (PE), or the remodeling of exogenous phospholipids. To investigate the role of de novo PC synthesis in Leishmania major, we focused on the cholinephosphate cytidylyltransferase (CPCT) which catalyzes the formation of CDP-choline, a key intermediate in the choline branch of the Kennedy pathway. Without CPCT, L. major parasites cannot incorporate choline into PC, yet the CPCT-null mutants contain similar levels of PC and PE as wild type parasites. Loss of CPCT does not affect the growth of parasites in complete medium or their virulence in mice. These results suggest that other mechanisms of PC synthesis can compensate the loss of CPCT. Importantly, CPCT-null parasites exhibited severe growth defects when ethanolamine and exogenous lipids became limited or when they were co-cultured with certain bacteria that are known to be members of sandfly midgut microbiota. These findings suggest that Leishmania employ multiple PC synthesis pathways to utilize a diverse pool of nutrients, which may be crucial for their survival and development in the sandfly.
Subject terms: Parasite biology, Phospholipids, Pathogens, Membrane lipids
Introduction
Leishmaniases are a group of neglected tropical diseases transmitted through the bite of female phlebotomine sandflies1. The causative agents are protozoan parasites of the genus Leishmania which alternate between flagellated promastigotes colonizing the midgut of sandflies and non-flagellated amastigotes residing in the macrophages of mammals. Without a safe vaccine, disease management primarily depends on vector control and drugs2. Discoveries that reveal fundamental insights into Leishmania biology can lead to new drug targets, better treatments, and improved vector control strategies.
The plasma membrane of Leishmania parasites contains a combination of glycerophospholipids, sphingolipids, and ergostane-based sterols3–6. Besides being membrane components, these lipids play important roles in the anchoring of glycoconjugates and the formation of ordered membrane microdomains or lipid rafts5,7–9. Leishmania parasites are capable of synthesizing these lipids de novo. Enzymes involved in the biosynthesis of sphingolipids and sterols are often crucial for stress response and virulence10–12. In addition to de novo synthesis, Leishmania parasites also acquire lipids from the media (for promastigotes) or host (for amastigotes)12–16.
As in most eukaryotes, glycerophospholipids constitute the most abundant class of lipids in Leishmania4,5. The physical nature and function of glycerophospholipids are dictated by the charge of the head group, and the length and saturation of the fatty acyl chains that are attached to the glycerol backbone. The most abundant glycerophospholipid in Leishmania are phosphatidylcholine (PC) which constitutes 30–35% of total cellular lipids5,17. Because of its positively charged head group, PC is a membrane-forming phospholipid that is more abundant on the outer leaflet of the plasma membrane18,19. In mammalian cells, PC also functions as the precursor of several signaling molecules such as diacylglycerol, phosphatidic acid, and lyso-phospholipid20.
In many eukaryotes including Leishmania, the de novo synthesis of PC starts with the phosphorylation of choline by choline kinase21,22; the resulting choline phosphate (choline-P) is then converted into CDP-choline by cholinephosphate cytidylyltransferase (CPCT); and finally, the enzyme choline/ethanolamine phosphotransferase (C/EPT) conjugates CDP-choline and diacylglycerol (DAG) into PC (Fig. 1). A similar pathway is responsible for the de novo synthesis of phosphatidylethanolamine (PE), as ethanolamine (EtN) is phosphorylated by ethanolamine kinase (EK), and the resulting ethanolamine phosphate (EtN-P) is converted into CDP-EtN by ethanolaminephosphate cytidylyltransferase (EPCT). In Leishmania, CDP-EtN is utilized to synthesize either plasmenylethanolamine (PME)23 or 1,2-diacyl-PE (Fig. 1). These two biosynthetic routes, collectively known as the Kennedy pathway24, is responsible for the production of the majority of PC and PE in many mammalian cell types and Trypanosoma brucei, a kinetoplastid parasite closely related to Leishmania species22,25–28.
Figure 1.
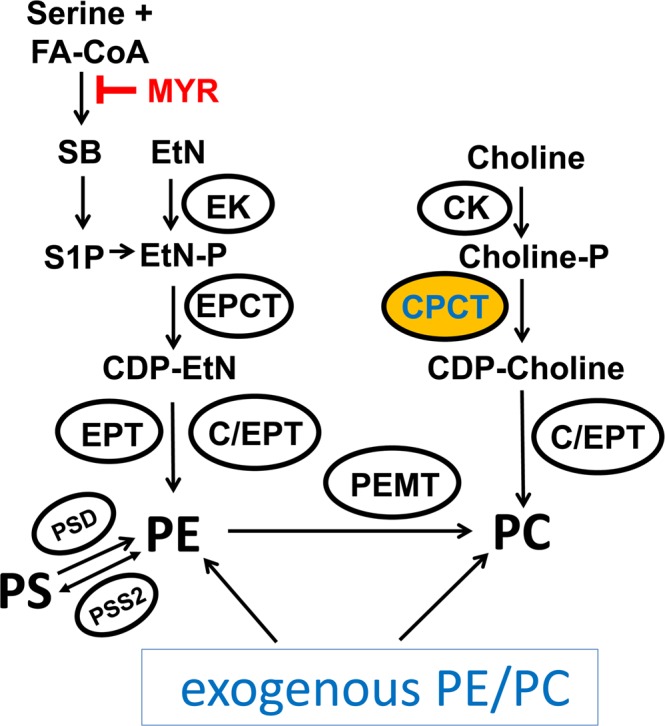
Predicted PE and PC synthesis in Leishmania. EK: ethanolamine kinase; EPCT: ethanolaminephosphate cytidylyltransferase; EPT: ethanolamine phosphotransferase; CK: choline kinase; CPCT: cholinephosphate cytidylyltransferase; C/EPT: choline/ethanolamine phosphotransferase; PEMT: phosphatidylethanolamine N-methyltransferase; PSD: phosphatidylserine decarboxylase; PSS2: phosphatidylserine synthase 2. EtN: ethanolamine; EtN-P: ethanolamine phosphate; PE: phosphatidylethanolamine; Choline-P: choline phosphate; PC: phosphatidylcholine; PS: phosphatidylserine; MYR: myriocin; S1P: sphingosine-1-phosphate; FA-CoA: fatty acyl-CoA.
In Leishmania, the sphingoid base metabolism can effectively convert serine into EtN-P which is then incorporated into PE10,29 (Fig. 1). Genomes of Leishmania species contain orthologs of phosphatidylserine synthase 2 and phosphatidylserine decarboxylase, suggesting that these parasites could generate PE through phosphatidylserine (Fig. 1). It is known that Leishmania parasites can also synthesize PC through the N-methylation of PE using S-adenosine-methionine as the methyl donor23,30. This PE N-methylation pathway is the predominant PC synthesis route in hepatocytes31 and Saccharomyces cerevisiae32, but is absent in Trypanosoma brucei33,34. Additionally, Leishmania can directly take up lipids including glycerophospholipids from the host or media and then remodel them into parasite-specific lipids35–38. So why would Leishmania parasites retain multiple, seemingly redundant PC synthesis pathways (Fig. 1)? What is the relative contribution of each pathway to the overall PC production during the promastigote and amastigote stages of Leishmania? And, does each mechanism favor the synthesis of particular PC species (variants in fatty acyl chain length and saturation)? Addressing these questions will provide novel insights into the physiological role of PC synthesis in Leishmania and may facilitate the development of new treatments.
In this study, we generated a CPCT-null mutant (cpct−) in Leishmania major, the etiological agent of cutaneous leishmaniasis in the old world39. CPCT is generally considered the rate-limiting enzyme in the de novo synthesis of PC34,40,41. In malaria parasites, CPCT is a vital enzyme for PC synthesis and a potential therapeutic target42–44. While L. major cpct− mutants failed to incorporate choline into PC, they retained a similar level and composition of PC as wild type (WT) parasites when cultivated in complete media. Deletion of CPCT did not affect the proliferation of promastigotes in complete media or their virulence in mice. These findings indicate that Leishmania parasites can compensate the loss of de novo PC synthesis through other mechanisms such as PE N-methylation and lipid salvage. Importantly, cpct− promastigotes did show significant growth reduction under starvation conditions when EtN and exogenous lipids became limited. Retaining the choline branch of the Kennedy pathway may allow Leishmania parasites to survive in the sandfly midgut when they must compete for nutrients with other microorganisms.
Results
Targeted deletion and cellular localization of CPCT in L. major
To explore the impact of de novo PC synthesis from choline in L. major, we focused on a CPCT ortholog (Tritrypdb ID: LmjF.18.1330, 592 amino acids) which is expected to catalyze the production of CDP-choline from CTP and choline-P (Fig. 1). L. major CPCT has six predicted transmembrane helices and no obvious N-terminal signal sequence. The endogenous CPCT alleles were deleted from L. major WT parasites and the resulting cpct− mutants were confirmed by Southern blot (Fig. 2 and Fig. S1). The null mutant was then complemented with a plasmid to restore CPCT expression (cpct−/+CPCT).
Figure 2.
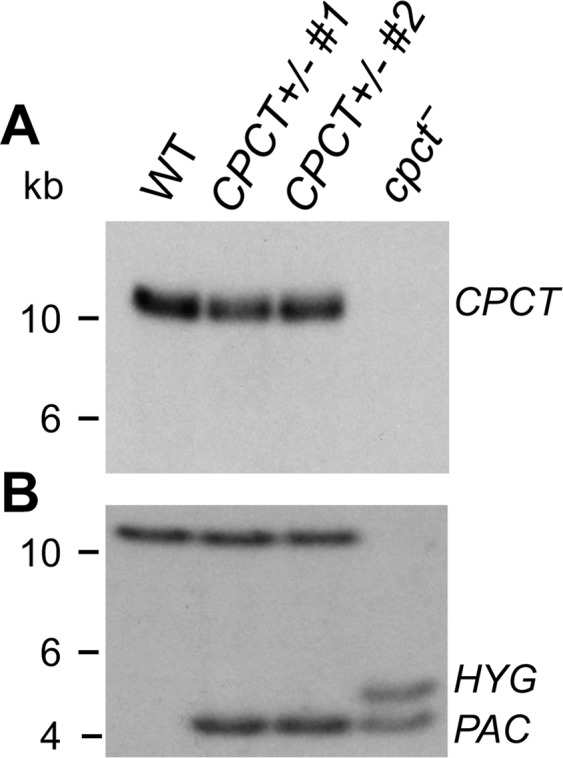
Southern blot confirms the targeted deletion of CPCT. Genomic DNA samples from WT, CPCT+/−, and cpct− parasites were digested with restriction enzyme SacII and separated on agarose gels. Blots were probed with radiolabeled DNA fragments recognizing the ORF (A) or an upstream flanking region of CPCT. (B) The replacement of CPCT alleles by PAC and HYG is indicated. Full-size, unedited blots and loading controls are presented in Supplementary Fig. S1.
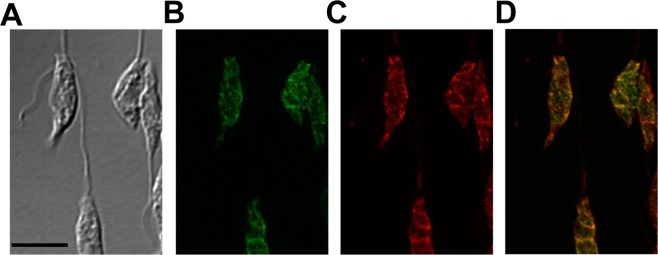
In mammalian cells and yeast, CPCT is reported to relocate from nucleoplasm/cytoplasm to nuclear membrane and endoplasmic reticulum (ER) in response to the need for PC synthesis45,46. To examine the cellular localization of L. major CPCT, GFP-fusion proteins were introduced into the cpct− mutant (Fig. S2). In fluorescence microscopy, both GFP-CPCT and CPCT-GFP exhibited a diffused, membranous pattern resembling the bulk ER (Fig. 3 and data not shown). Quantative analysis of GFP-CPCT showed ~85% overlap (by JaCOP Image J analysis of 30 randomly selected cells, Table S1) with the ER marker BiP47,48. Thus, CPCT is mainly located in the ER (Fig. 3 and Table S1).
Figure 3.
Endoplasmic Reticulum (ER) localization of CPCT in L. major. Log phase promastigotes of cpct−/+GFP-CPCT were labeled with rabbit anti-T. brucei BiP antiserum followed by a goat anti-rabbit IgG-Texas Red antibody and subjected to confocal immunofluorescence microscopy. (A) Phase contrast; (B) GFP fluorescence; (C) Anti-BiP staining; (D) Merge of B and C. Scale bar: 5 µm. The overlap between BiP and GFP-CPCT was determined by the JaCOP Image J analysis of 30 cells (Table S1).
Cpct− mutants cannot synthesize PC from choline but can incorporate EtN into PE and PC
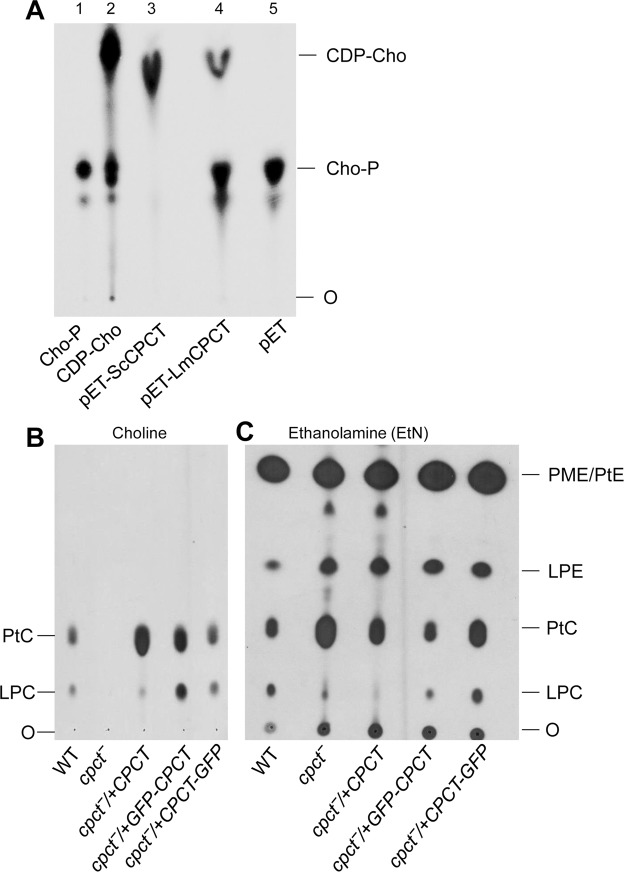
To determine whether CPCT is required for the synthesis of CDP-choline, E. coli lysates containing recombinant L. major CPCT (LmCPCT) or S. cerevisiae CPCT (ScCPCT) were incubated in the presence of CTP and radiolabeled choline-P and the products were examined by thin layer chromatography (TLC). As shown in Fig. 4A and Fig. S3A, lysate containing ScCPCT could efficiently catalyze the formation of CDP-choline whereas lysate from empty pET vector had no activity. By comparison, LmCPCT conferred a low but clearly detectable level of CPCT activity (~26% of ScCPCT, Fig. 4A and Fig. S3A). It is not clear whether this result reflects the intrinsic difference between ScCPCT and LmCPCT in specific activity, or their ability to be functionally expressed in E. coli.
Figure 4.
CPCT is required for incorporating choline to PC. (A) Lane 1: [14C]-Choline-P. Lane 2: [14C]-CDP-choline. Lane 3–5: cell lysates from E. coli transformed with pET-ScCPCT, pET-LmCPCT, or pET only were incubated with [14C]-labeled choline-P, followed by TLC analysis as described in METHODS. (B,C) Log phase Leishmania promastigotes were cultivated in the presence of [3H]-labeled choline (B) or [3H]-labeled EtN (C). Total lipids were extracted after 48 hours and analyzed by TLC. O: origin of loading; LPC: lyso-phosphatidylcholine; LPE: lyso-phosphatidylethanolamine. Full-size, unedited images are presented in Supplementary Fig. S4.
In agreement with this finding, L. major WT parasites but not cpct− mutants could incorporate [3H]-choline into PC including 1,2-diacyl-PC (PtC) and lyso-PC (LPC, a hydrolytic product of PtC49) (Fig. 4B and Fig. S3B). Complementation of cpct− with CPCT, GFP-CPCT or CPCT-GFP led to robust assimilation of choline into PtC and LPC (Fig. 4B and Fig. S3B). These findings indicate that CPCT is solely responsible for generating PC from choline in L. major. As we reported previously23, WT parasites were able to incorporate [3H]-EtN into PE (PME + 1,2-diacyl-PE + lyso-PE or LPE49) and PC (Fig. 4C and Fig. S3C); while similar results were observed with the cpct− mutants, we detected a 1.5–1.9-fold increase in the incorporation of [3H]-EtN into PC (Fig. 4C and Fig. S3C), which could be a compensatory response to the loss of PC synthesis from choline.
Cpct− mutants contain normal levels of PC and PE when grown in the complete medium
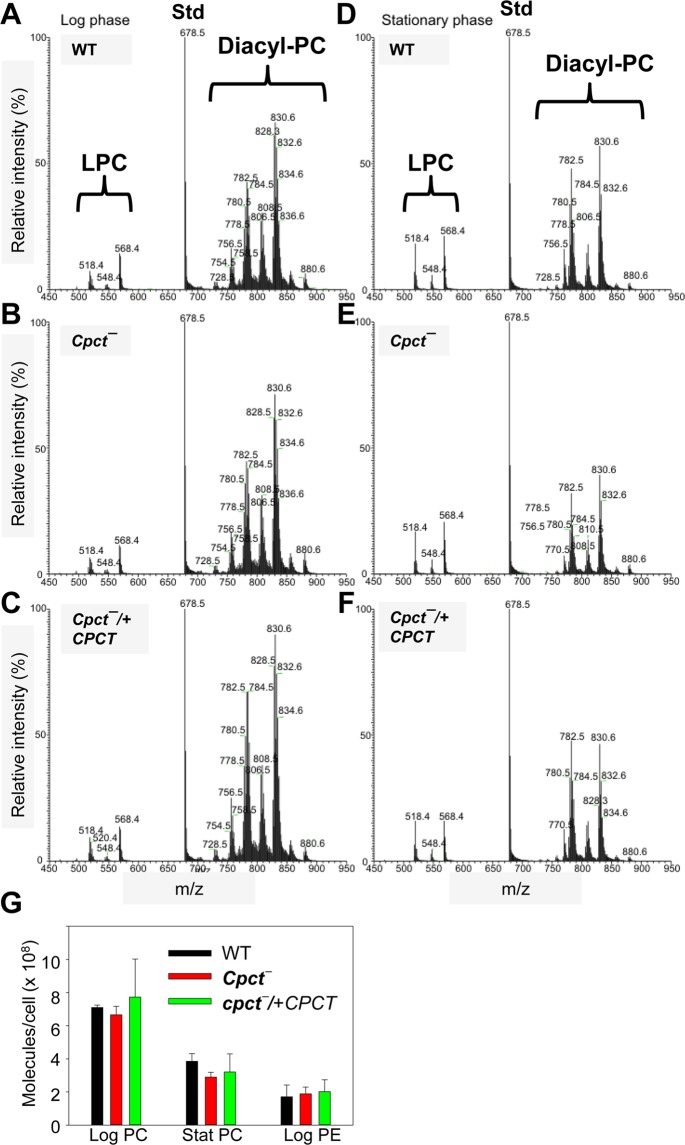
To examine whether the choline branch of Kennedy pathway plays a major role in the overall PC production in L. major, we extracted total lipids from promastigotes cultured in the complete M199 medium (M199 medium with 10% fetal bovine serum or FBS and other supplements)50. The composition of PC was assessed using electrospray ionization mass spectrometry (ESI/MS) in the positive-ion mode. As shown in Fig. 5A–F, in log phase and stationary phase, the LPC and diacyl-PC species in cpct− mutants closely resembled those found in WT and cpct−/+CPCT parasites. Through comparison with a PC standard, we estimated the overall abundance of PC in log phase cpct− mutants to be 6.5–7.1 × 108 molecules/cell, which was close to the average values in log phase WT and cpct−/+CPCT parasites (Fig. 5G). During the stationary phase, cpct− mutants contained 2.6–3.1 × 108 PC molecules/cell, whereas WT and cpct−/+CPCT parasites had 3–4 × 108 molecules/cell (Fig. 5G; the difference between WT and cpct− is not statistically significant). The decrease of PC abundance in stationary phase is consistent with the global lipid remodeling when promastigotes transition from replicative procyclics to infectious metacyclics10,51.
Figure 5.
Cpct− mutants contain normal levels of PC. Total lipids from log phase (A–C) and stationary phase (D–F) promastigotes were examined by ESI/MS in the positive ion mode. (A,D): WT; (B,E): cpct−; (C,F): cpct−/+CPCT. Peaks corresponding to LPC and diacyl-PC are indicated. Std: PC standard (14:0/14:0-PC) for quantitation. (G) Abundance of PC (diacyl-PC + LPC) and PE in L. major promastigotes were determined and averaged from three independent experiments. Error bars represent standard deviations from three biological repeats.
In addition to PC, we examined the cellular levels of PE in log phase promastigotes. As summarized in Fig. 5G, no significant difference was detected between WT, cpct− and cpct−/+CPCT parasites (1.7–2.1 × 108 PE molecules/cell). Thus, the choline branch of Kennedy pathway is not required for bulk phospholipid synthesis when L. major parasites were cultured in the complete medium. These results suggest that Leishmania promastigotes can compensate the loss of CPCT through PE N-methylation and/or lipid uptake followed by remodeling to meet the demand of PC synthesis.
Role of CPCT in Leishmania differentiation and growth under EtN-limiting conditions
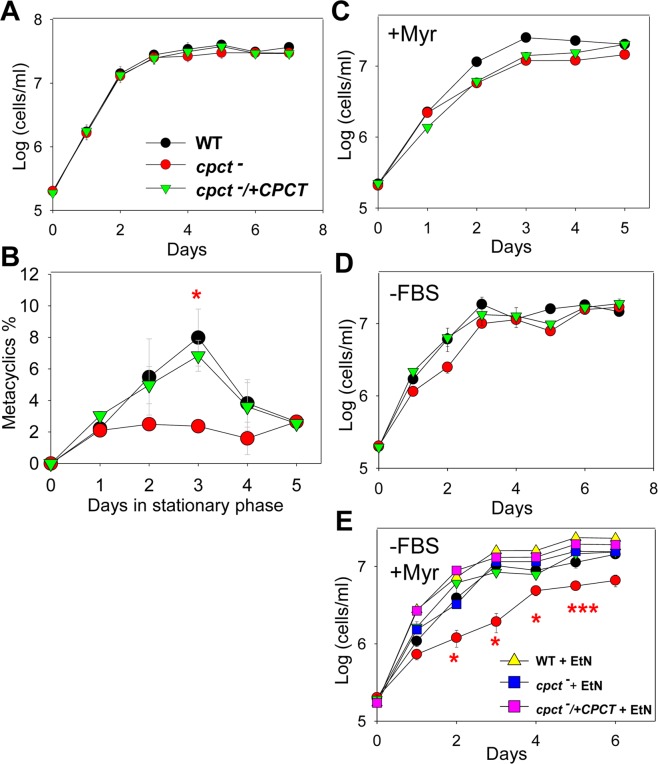
When cultivated in the complete medium, cpct− mutants grew and replicated at a similar rate as WT and cpct−/+CPCT parasites (Fig. 6A). These mutants produced less metacyclics (the infective form to mammals)52 than WT and add-back parasites during the stationary phase (Fig. 6B). In L. major, formation of metacyclics (metacyclogenesis) is associated with the modification of lipophosphoglycan (LPG)53. Western blot analysis revealed that LPG in stationary phase cpct− was of normal abundance, but migrated slightly slower than that from WT and cpct−/+CPCT parasites (Fig. S4), suggesting minor structural alteration of LPG.
Figure 6.
CPCT-null mutants proliferate normally in the complete medium but show significant growth delay under EtN-limiting conditions. Promastigotes were cultivated under various conditions and culture densities were determined daily using a hemocytometer in A, C-E. In B, percentages of metacyclics during stationary phase were determined daily as described in METHODS. Culture conditions: (A,B) complete M199 medium; (C) complete M199 medium + 4 μM of myriocin; (D) lipid-free M199 medium (no FBS); and (E) lipid-free M199 + 4 μM of myriocin with or without 250 μM of EtN. Error bars represent standard deviations from three independent experiments (*p < 0.05; ***p < 0.001).
To further examine the contribution of CPCT in vitro, we monitored the growth of cpct− mutants under various EtN-limiting conditions. When promastigotes were inoculated in complete M199 medium in the presence of myriocin, which inhibits the conversion of serine into EtN-P (Fig. 1), no major growth defects were observed in the cpct− mutants (Fig. 6C). This suggests that uptake of exogenous PE and/or PC is sufficient to compensate the loss of CPCT (Fig. 1). Similarly, in a lipid-free M199 medium (M199 without FBS but with 0.4% fatty acid free bovine serum albumin), cpct− mutants proliferated nearly as well as WT and cpct−/+CPCT parasites showing a slight but not statistically significant delay (Fig. 6D). This suggests that in the absence of lipid uptake, the serine-to-EtN conversion is largely sufficient for PC synthesis (Fig. 1). However, when inoculated in lipid-free M199 (no exogenous lipids available to uptake) containing myriocin (inhibiting serine-to-EtN conversion), cpct− mutants grew significantly slower than WT and cpct−/+CPCT parasites (Fig. 6E). Finally, supplementation of EtN rescued the growth of cpct− to WT levels under this condition (Fig. 6E). Together, these results suggest that in comparison to WT and cpct−/+CPCT parasites, cpct− mutants are more dependent on PE N-methylation and the uptake of exogenous lipids for PC synthesis.
Cpct− mutants do not have virulence defects in mice
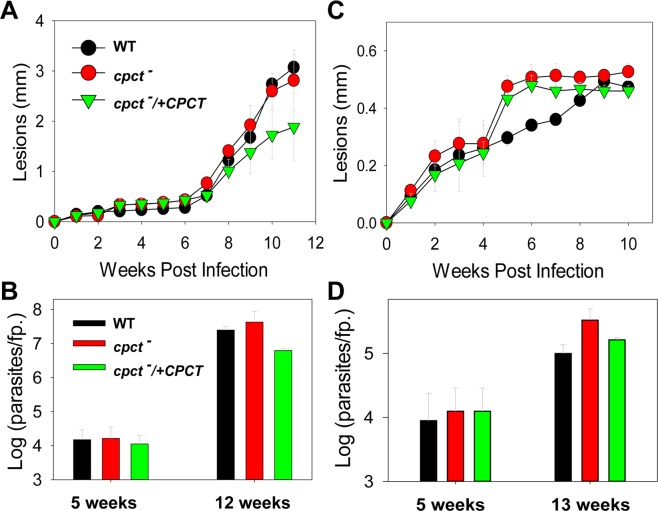
To study the role of de novo PC synthesis in Leishmania virulence, mice were infected subcutaneously in the footpad with late stationary phase WT, cpct−, and cpct−/+CPCT promastigotes. In both BALB/c mice (susceptible to L. major; Fig. 7A) and C57BL/6 mice (resistant to L. major; Fig. 7C)54, cpct− mutants induced lesions of similar sizes over time as WT and cpct−/+CPCT parasites. Limiting dilution assay was performed at 5, 12, or 13 weeks post infection to determine parasite burden (Fig. 7C-D). No significant difference was observed between WT and cpct− parasites. Therefore, although CPCT affects metacyclogenesis in vitro (Fig. 6B), it is not required for the virulence of L. major, suggesting that amastigotes can fulfill their need for PC synthesis through lipid salvage or PE N-methylation (Fig. 1).
Figure 7.
Cpct− mutants are fully virulent. BALB/c mice (A,B) and C57BL/6 mice (C,D) were infected with stationary phase promastigotes as described in METHODS. Footpads lesions were measured weekly and plotted in (A,C). Limiting dilution assay was performed at the indicated times to determine parasite burden (B,D). Error bars represent standard deviations (5 mice per group).
Cpct− mutants show growth delay when co-cultured with certain bacteria
In the midgut of sandfly, Leishmania promastigotes need to proliferate and differentiate in the presence of a community of microorganisms55–59. The interaction between parasites and sandfly microbiota has significant impact on Leishmania differentiation and transmission55,60,61. While PC is the most abundant phospholipid in eukaryotes, most bacteria synthesize PE as a major membrane lipid and have a high demand for EtN62–65. Here we examined whether CPCT was required when L. major promastigotes were co-cultured with Serratia marcescens or Enterobacter cloacae, two Gram-negative bacteria that have been identified in the midgut of several sandfly species59,66,67. In this experiment, promastigotes (1.0 × 106 cells/ml) and bacteria (50 cells/ml) were co-cultured in lipid-free M199 media and the density of Leishmania parasite was determined after 24 hours. As shown in Fig. 8, all parasites grew slower in the presence of S. marcescens presumably due to nutrient competition and waste/toxin production from the bacteria. For WT parasites, a 37% reduction was observed during S. marcescens co-culture, while cpct− and cpct−/+CPCT mutants displayed 61% and 45% reduction respectively (Fig. 8, lipid free M199 vs. S. marcescens). Thus, losing CPCT seems to reduce parasites’ fitness under a competitive condition. Importantly, addition of EtN largely restored the replication of cpct− to levels close to WT and cpct−/+CPCT parasites (Fig. 8, S. marcescens vs. S. marcescens + EtN). By comparison, a less significant effect on Leishmania growth was observed during co-culture with Enterobacter cloacae, although cpct− mutants still replicated slower than WT and add-back parasites, and EtN supplementation improved their growth (Fig. 8, E. cloacae vs. E. cloacae + EtN). Together, these findings suggest that the choline branch of the Kennedy pathway allows Leishmania to be less dependent on EtN, which may be a limiting nutrient in the sandfly (Fig. 1).
Figure 8.
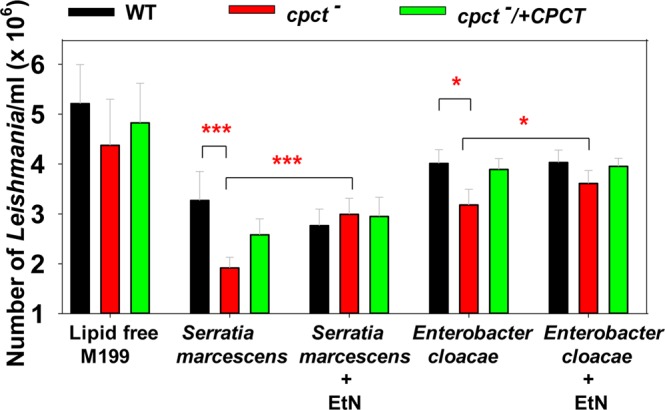
Cpct− mutants show significant growth delay when co-cultured with bacteria and the growth delay can be rescued by EtN supplementation. Effects of Serratia marcescens and Enterobacter cloacae on the growth of cpct− mutants were determined in a co-culture experiment. L. major promastigotes (1 × 106 cells/ml) were incubated in lipid-free M199 medium alone or with Serratia marcescens (50 bacteria/ml) or Enterobacter cloacae (50 bacteria/ml). EtN (250 μM) was included as indicated. The number of promastigotes/ml was recorded using a hemocytometer after 24 h. Results were averaged from three independent experiments with triplicates. Error bars represent standard deviations from three biological repeats (*p < 0.05; ***p < 0.001) based on one way Anova relative to WT.
Discussion
In this study, we characterized an ER-localized CPCT that is responsible for the incorporation of choline into PC in L. major. Deletion of CPCT had no obvious impact on the cellular levels of PC or PE when promastigotes were cultivated in complete M199 media (Fig. 6). Cpct− mutants replicated normally and did not show any defects in morphology when they were able to generate PC through PE N-methylation and/or lipid salvage. We observed a 2–4 folds reduction in metacyclogenesis during late stationary phase (Fig. 6B), which may be related to the minor alteration of LPG in cpct− (Fig. S4)53,68. Importantly, the fact that of cpct− mutants are fully virulent and replicative mice (Fig. 7) suggests that the de novo synthesis of PC is not required during the mammalian stage of L. major.
Meanwhile, the proliferation of cpct− mutants was severely reduced when they were cultivated in a lipid-free medium (which eliminated the uptake, degradation and remodeling of exogenous lipids) containing myriocin (which blocked the conversion of serine into EtN-P via sphingoid base metabolism) (Fig. 6). These findings indicate that the choline branch of the Kennedy pathway is dispensable when parasites can synthesize PC from EtN, serine, or exogenous lipids (Fig. 1).
The fact that cpct− mutants can still grow (albeit at a reduced rate) and synthesize PC in lipid-free M199 containing myriocin (Fig. 6D) suggests that residue amount of EtN/EtN-P (which may be converted from serine after myriocin loses efficacy) is sufficient to sustain a low level of PC synthesis and cell proliferation. Alternatively, parasites may generate PE/EtN from serine via the activity of phosphatidylserine synthase 2 and phosphatidylserine decarboxylase69 (Fig. 1). These two enzymes are essential for the optimal growth and mitochondrial function of procyclic Trypanosoma brucei, and their exact roles in Leishmania have yet to be determined69.
Since cpct− mutants are fully replicative and virulent in mice (Fig. 7), we postulate that the ability to synthesize PC from choline is dispensable during the mammalian stage when amastigotes reside within the phagolysosome of macrophages and have access to lipids, amino acids, sugars and amino sugars70–72. Meanwhile, it is of interest to determine if CPCT contributes to the proliferation of Leishmania promastigotes in the midgut of sandfly, where they must compete for nutrients with the resident microbiota55,73 (Fig. 8). While the exact composition of carbon source in the sandfly gut is not well defined, the diet of female sandfly consists of blood meals and nectar, which is digested by the hydrolytic enzymes from the sandfly and microbiota. Because EtN or PE is an important source of carbon and nitrogen for many bacterial species64,65, these nutrients may be limited in the sandfly gut. In contrast, choline is not an essential nutrient for many bacteria which mainly synthesize PE, phosphatidylglycerol (PG), and cardiolipin as their membrane phospholipids74. Retaining the choline branch of the Kennedy pathway may allow Leishmania to synthesize PC from choline when EtN/exogenous phospholipid is limited. In support of this hypothesis, cpct− mutants showed a significant growth delay when co-cultured in a lipid-free medium with Serratia marcescens or Enterobacter cloacae, two bacteria found in both field-captured and lab-reared sandflies, and the supplementation of EtN largely restored the proliferation of cpct− mutants (Fig. 8). The reason why E. cloacae has less inhibitory effect on Leishmania than S. marcescens may involve a combination of factors including their rates on EtN consumption, nutrient depletion and toxin production.
In summary, L. major promastigotes may retain the choline branch of the Kennedy pathway to survive nutrient-limiting conditions. Such metabolic flexibility may improve their competitive fitness in the sandfly midgut.
Methods
Materials
Ethanolamine (EtN) hydrochloride ([1-3H], 40–60 Ci/mmol) and [methyl-14C] phosphorylcholine (50–60 mCi/mmol) were purchased from American Radiolabeled Chemicals (St. Louis, MO). Choline chloride [methyl-14C] (50–60 mCi/mmol) and cytidine diphosphocholine [methyl-14C] (50–60 mCi/mmol) were purchased from Perkin Elmer, Inc (Waltham, MA). Lipid standards for mass spectrometry including 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (16:0/16:0-PE) and 1,2-dimyristoyl-sn-glycerol-3-phosphocholine (14:0/14:0-PC) were purchased from Avanti Polar Lipids (Alabaster, AL). Fatty acid free, low endotoxin bovine serum albumin was purchased from Sigma-Aldrich (St. Louis, MO). Antisera for mouse-anti-alpha tubulin, rabbit-anti-GFP, goat-anti-rabbit-IgG-HRP, and goat-anti-mouse-IgG-HRP were purchased from Life Technologies (Carlsbad, CA). All other reagents were purchased from VWR International (Radnor, PA) or Thermo Fisher Scientific (Hampton, NH) unless specified otherwise.
Molecular cloning
With the exception of cloning Saccharomyces cerevisiae CPCT into pET vector (described below), CPCT refers to L. major CPCT in this study. To facilitate CPCT assay, the ORF of Saccharomyces cerevisiae CPCT (YGR202C) was amplified from yeast genomic DNA by PCR using primers 5′-GTACTGGGATCCATGGCAAACCCAACAACAGGGAAGTCC-3′/5′- GATCATGGATCCCAGTTCGCTGATTGTTTCTTCTTC-3′. The resulting 1280 bp DNA fragment was digested with BamHI and ligated into E. coli expression vector pET23a + to generate pET-ScCPCT. The ORF of L. major CPCT was cloned into pET23a + to generate pET23a-LmCPCT. These pET constructs were transformed into E. coli BL21 (DE3) competent cells.
The open reading frame (ORF) of L. major CPCT (LmjF18.1330) was amplified by PCR from L. major WT genomic DNA using primers 5′-GTACTGGGATCCACCATGCCGCATCTGCTTGAGCAC-3′/5′-GATCAGGGATCCTCATGCCCCGTCCTTCACC-3′. The resulting 1.8 kb DNA fragment was digested with BamHI and ligated into pXG175, generating pXG1-CPCT. To study localization, the CPCT ORF was amplified and cloned into pXG1-GFP’ or pXG1-‘GFP to generate pXG1-GFP-CPCT or pXG1-CPCT-GFP, respectively.
To generate CPCT knockout constructs, the upstream and downstream sequences of CPCT ORF (~1 kb each) were amplified using primers 5′-GATCATGAGCTCAACGAACGGTAGCCCGCATATTG-3′/5′-GTCAGAACTAGTGATCTAGGATCCGGCAGACTCGTGTGTGTATG-3′ and 5′-GTCAGTGGATCCGGGGAGATGCCGGCGTCTTC-3′/5′-GTACGAAAGTCCAACGCCATCAACTATGCG-3′, respectively. The amplified upstream and downstream sequences were cloned in tandem into the cloning vector pUC18. Subsequently, genes conferring resistance to hygromycin (HYG) and puromycin (PAC) were cloned between these regions to generate pUC18-KO-CPCT: HYG and pUC18-KO-CPCT: PAC.
All the molecular constructs were verified by restriction enzyme digestion and DNA sequencing.
Leishmania promastigote culture and genetic manipulation
Unless specified otherwise, L. major LV39 clone 5 (Rho/Su/59/P) promastigotes were cultured at 26 °C in M199 media with or without 10% heat-inactivated FBS and other supplements50. Cell density was determined using a hemocytometer. Percentages of metacyclics in stationary phase culture were determined as previously described68. To investigate the functions of CPCT, the endogenous CPCT alleles were deleted from L. major LV39 wild type (WT) parasites by two consecutive rounds of targeted replacement as described50 using linearized knockout constructs from pUC18-KO-CPCT:PAC and pUC18-KO-CPCT:HYG. The resulting cpct− mutants (∆CPCT::PAC/∆CPCT::HYG) were confirmed by Southern blot, where genomic DNA from promastigotes was digested with SacII, separated on a 0.7% agarose gel, transferred to a nitrocellulose membrane, and probed with [32P]-labeled DNA fragments corresponding to the ORF or an upstream region of CPCT. Results of Southern blot were visualized by autoradiography. To restore CPCT expression, pXG1-CPCT was transfected into cpct− mutants and referred to as cpct−/+CPCT (∆CPCT::PAC/∆CPCT::HYG/+pXG1-CPCT). For localization studies, pXG1-GFP-CPCT or pXG1-CPCT-GFP was introduced into cpct− to generate cpct−/+GFP-CPCT or cpct−/+CPCT-GFP, respectively.
To examine cell growth under various nutrient-limiting conditions, we prepared M199 media that are supplemented with either 10% FBS (complete medium), 0.4% fatty acid free bovine serum albumin (lipid-free M199 medium, which contains 23–24 μM of serine but no EtN), 0.4% bovine serum albumin plus 4 μM of myriocin, or 0.4% bovine serum albumin plus 4 μM of myriocin and 250 μM of EtN. At low densities (<1.0 × 106 cells/ml), cells were concentrated 10-fold before counting, and at high densities (>1 × 107 cells/ml), cells were passed through a 27 ½ G needle three times to break up clumps before counting. To measure the percentage of dead cells parasites were labeled with 5.6 µg/ml propidium iodide followed by flow cytometry.
Metabolic labeling, CPCT assay and thin layer chromatography (TLC)
To examine the incorporation of choline and EtN into phospholipids, promastigotes were inoculated into complete M199 media at 2.0 × 105 cells/ml and labeled with 1 µCi/ml of choline chloride [methyl-14C] or 1 µCi/ml of EtN hydrochloride [1-3H]. Total lipids were extracted after 48 hours and dissolved in chloroform:methanol (1:2, v/v) at 2.0 × 109 cells/ml. The incorporation of EtN or choline into Leishmania lipids was determined by scintillation counting, followed by one dimensional TLC (each lane contained lipid from 1.0 × 107 cells) performed in a solvent made of methyl acetate:1-propanol:chloroform:methanol:0.9% KCl (25:25:25:10:9 by volume). The TLC plates were sprayed with EN3HANCE (Perkin Elmer, for 3H-EtN labeling) and exposed to autoradiography film. To quantify the incorporation of [3H]-EtN into PC over total [3H]-EtN incorporation into PE + PC, TLC images were subjected to densitometry analysis by Image J and results from two experiments were averaged.
To examine the activity of recombinant CPCT, E. coli BL21 (DE3) transformants containing pET23-LmCPCT, pET23-ScCPCT, or empty pET23a + vector were grown in LB media until OD600 nm reached 0.6 and induced with 1 mM of IPTG for 3 hours. Bacteria were resuspened in a lysis buffer (Tris HCl 30 mM at pH7.4, 5% glycerol, 1 mM EDTA, and 1 x protease inhibitor) and sonicated on ice. Protocol of CPCT assay was adapted from a previous report76. Briefly, E. coli lysate containing 60 μg of bacterial protein was incubated in a 20 μl reaction mix containing 25 mM of MgCl2, 5 mM of CTP, and 0.1 mM of phosphorylcholine [methyl-14C] for 30 minutes at 30 °C. Reaction mix (10 μl each) was then analyzed by TLC using silica-60 plates and a solvent made of ethanol:0.5% NaCl:25% ammonium hydroxide (10:10:1 by volume). Radioactive signals were detected using a Personal Molecular Imager (Bio-Rad).
Fluorescence microscopy
Promastigotes expressing GFP-CPCT or CPCT-GFP were attached to poly-L-lysine coated coverslips, fixed with 3.7% formaldehyde, and then permeabilized on ice with ethanol. Incubation with rabbit anti-T. brucei BiP antiserum (1:1000) was performed at room temperature for 40 minutes. After washing, coverslips were incubated with a goat anti-rabbit-Texas Red (1:2000) antiserum for 40 minutes. An Olympus Fluoview FV3000 Laser Scanning Confocal Microscope was used to visualize the expression and localization of GFP-CPCT. To quantify the overlap between GFP-CPCT and anti-BiP staining, 30 randomly selected cells were analyzed using Image J JACoP (Just Another Colocalization Plugin)48.
Lipidomic analysis
In this study, all choline glycerophospholipids including 1,2-diacyl-phosphatidylcholine (PtC) and 1-acyl-phosphatidylcholine (lyso-PC) are referred to as PC. Similarly, all ethanolamine glycerophospholipids including plasmenylethanolamine (PME), 1,2-diacyl-phosphatidylethanolamine (PtE) and 1-acyl-phosphatidylethanolamine (lyso-PE) are referred to as PE.
To analyze composition and quantity of glycerophospholipids, promastigote lipids were extracted (1 × 108 cells per sample) using the Bligh-Dyer approach77 and dissolved in a mixture of chloroform:methanol (1:2, v/v). Lipid standards were added to cell lysates prior to lipid extraction (5.0 × 107 molecules/cell for 14:0/14:0-PC and 1.0 × 108 molecules/cell for 16:0/16:0-PE; these lipids are not present in L. major promastigotes). Lipid samples were analyzed by electrospray ionization mass spectrometry (ESI/MS) as described23. Quantitation of PC and PE was performed using precursor ion scan of [m/z]+ 184 (positive ion mode) and [m/z]− 196 (negative ion mode), respectively. All lipidomic analyses were performed three times.
Western blot
Whole cell lysates from Leishmania promastigotes were resolved by SDS-PAGE, followed by immunoblotting with rabbit anti-GFP antibody (1:1000), anti-LPG monoclonal antibody WIC79.3 (1:1000) or monoclonal antibody against tubulin (1:5000), followed by appropriate secondary antibodies as previously described78. Signals from Western blot were quantified using a FluorChem E system (Protein Simple).
Mouse infection
Use of mice in this study was approved by the Animal Care and Use Committee at
Texas Tech University (US PHS Approved Animal Welfare Assurance NO. A3629-01). BALB/c and C57BL/6 mice (female, 8 weeks old) were purchased from Charles River Laboratories International. Mice were housed and cared for in the facility operated by the Animal Care and Resources Center at Texas Tech University adhering to the Guide for the Care and Use of Laboratory Animals (the 8th Edition, NRC 2011) for animal husbandry. To maintain virulence, promastigotes were injected into the footpads of BALB/c mice and recovered after 3–4 weeks to start low passage in vitro cultures. To assess virulence, day 3 stationary phase promastigotes (cultured for less than five passages after recovery from mice) were resuspended in DMEM and injected into the left hind footpads of mice (1.0 × 106 cells per mouse, 5 mice per group). Lesion sizes were measured weekly using a Vernier caliper and parasite loads were determined by limiting dilution assay79.
Leishmania co-culture with bacteria
Serratia marcescens (ATCC #13880) and Enterobacter cloacae (Carolina Biological # 155032) were cultured in LB media at 37 °C until OD600 nm reached 0.6. Bacteria were counted manually using a hemocytometer and diluted in lipid-free M199. For Leishmania bacteria co-culture, promastigotes were inoculated into lipid-free M199 at 1.0 × 106 cells/ml with 50 bacteria/ml in 12-well plates (in the presence or absence of 250 μM of EtN) and incubated at 27 °C. Parasites cultured in the absence of bacteria were included as a control. Concentration of promastigotes was determined using a hemocytometer after 24 hours.
Statistical analysis
Unless otherwise specified, all experiments (in Figs 5–8) were repeated three times and each biological repeat contained 2–3 technical repeats. Differences among experimental groups were determined by the unpaired Student’s t test (for two groups) or one way ANOVA (for three to four groups) using Sigmaplot 11.0 (Systat Software Inc, San Jose, CA). P values indicating statistical significance were grouped into values of <0.05 (*), <0.01 (**) and <0.001 (***).
Supplementary information
Acknowledgements
We thank Dr. Jay Bangs (University at Buffalo, SUNY) for providing the rabbit anti-T. brucei BiP antiserum. This work was supported by the US National Institutes of Health (AI099380 for KZ and P41-GM103422, P60-DK20579, P30-DK56341 for the Biomedical Mass Spectrometry Resource at Washington University in St. Louis, MO, USA) and by the TTU CISER Howard Hughes Medical Institute Undergraduate Science Education Program.
Author Contributions
K.Z. designed the study. S.M., M.C.P. and F.H. performed the experiments. S.M., M.C.P. and K.Z. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44086-6.
References
- 1.Alvar J, et al. Leishmaniasis worldwide and global estimates of its incidence. PloS one. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croft SL, Olliaro P. Leishmaniasis chemotherapy–challenges and opportunities. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17:1478–1483. doi: 10.1111/j.1469-0691.2011.03630.x. [DOI] [PubMed] [Google Scholar]
- 3.Zheng L, et al. Profiling of lipids in Leishmania donovani using hydrophilic interaction chromatography in combination with Fourier transform mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:2074–2082. doi: 10.1002/rcm.4618. [DOI] [PubMed] [Google Scholar]
- 4.Wassef MK, Fioretti TB, Dwyer DM. Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids. 1985;20:108–115. doi: 10.1007/BF02534216. [DOI] [PubMed] [Google Scholar]
- 5.Zhang, K. & Beverley, S. M. Phospholipid and sphingolipid metabolism in Leishmania. Molecular and biochemical parasitology170, 55–64. PMCID: 2815228. (2010). [DOI] [PMC free article] [PubMed]
- 6.Goad LJ, Holz GG, Jr., Beach DH. Sterols of Leishmania species. Implications for biosynthesis. Molecular and biochemical parasitology. 1984;10:161–170. doi: 10.1016/0166-6851(84)90004-5. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson MA. The surface glycoconjugates of trypanosomatid parasites. Philos Trans R Soc Lond B Biol Sci. 1997;352:1295–1302. doi: 10.1098/rstb.1997.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denny PW, Field MC, Smith DF. GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida. FEBS Lett. 2001;491:148–153. doi: 10.1016/S0014-5793(01)02172-X. [DOI] [PubMed] [Google Scholar]
- 9.Yoneyama KA, Tanaka AK, Silveira TG, Takahashi HK, Straus AH. Characterization of Leishmania (Viannia) braziliensis membrane microdomains, and their role in macrophage infectivity. Journal of lipid research. 2006;47:2171–2178. doi: 10.1194/jlr.M600285-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang K, et al. Redirection of sphingolipid metabolism toward de novo synthesis of ethanolamine in Leishmania. EMBO J. 2007;26:1094–1104. doi: 10.1038/sj.emboj.7601565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang O, Hsu FF, Xu W, Pawlowic M, Zhang K. Sphingosine kinase A is a pleiotropic and essential enzyme for Leishmania survival and virulence. Molecular microbiology. 2013;90:489–501. doi: 10.1111/mmi.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W, Hsu FF, Baykal E, Huang J, Zhang K. Sterol Biosynthesis Is Required for Heat Resistance but Not Extracellular Survival in Leishmania. PLoS pathogens. 2014;10:e1004427. doi: 10.1371/journal.ppat.1004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Kai, Hsu Fong-Fu, Scott David A., Docampo Roberto, Turk John, Beverley Stephen M. Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Molecular Microbiology. 2005;55(5):1566–1578. doi: 10.1111/j.1365-2958.2005.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali HZ, Harding CR, Denny PW. Endocytosis and Sphingolipid Scavenging in Leishmania mexicana Amastigotes. Biochemistry research international. 2012;2012:691363. doi: 10.1155/2012/691363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, O. et al. Degradation of host sphingomyelin is essential for Leishmania virulence. PLoS pathogens5(12), e1000692. PMCID: 2784226. (2009). [DOI] [PMC free article] [PubMed]
- 16.Bouazizi-Ben Messaoud H, Guichard M, Lawton P, Delton I, Azzouz-Maache S. Changes in Lipid and Fatty Acid Composition During Intramacrophagic Transformation of Leishmania donovani Complex Promastigotes into Amastigotes. Lipids. 2017;52:433–441. doi: 10.1007/s11745-017-4233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beach DH, Holz GG, Jr., Anekwe GE. Lipids of Leishmania promastigotes. J Parasitol. 1979;65:201–216. doi: 10.2307/3280147. [DOI] [PubMed] [Google Scholar]
- 18.Nickels JD, Smith JC, Cheng X. Lateral organization, bilayer asymmetry, and inter-leaflet coupling of biological membranes. Chem Phys Lipids. 2015;192:87–99. doi: 10.1016/j.chemphyslip.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 19.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nature reviews. Molecular cell biology. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Exton JH. Phosphatidylcholine breakdown and signal transduction. Biochimica et biophysica acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 21.Pulido SA, et al. Insights into the phosphatidylcholine and phosphatidylethanolamine biosynthetic pathways in Leishmania parasites and characterization of a choline kinase from Leishmania infantum. Comparative biochemistry and physiology. Part B, Biochemistry & molecular biology. 2017;213:45–54. doi: 10.1016/j.cbpb.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Gibellini F, Smith TK. The Kennedy pathway–De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB life. 2010;62:414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- 23.Pawlowic MC, Hsu FF, Moitra S, Biyani N, Zhang K. Plasmenylethanolamine synthesis in Leishmania major. Molecular microbiology. 2016;101:238–249. doi: 10.1111/mmi.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy EP. The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. The Journal of biological chemistry. 1956;222:185–191. [PubMed] [Google Scholar]
- 25.Gibellini F, Hunter WN, Smith TK. The ethanolamine branch of the Kennedy pathway is essential in the bloodstream form of Trypanosoma brucei. Molecular microbiology. 2009;73:826–843. doi: 10.1111/j.1365-2958.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Signorell A, Rauch M, Jelk J, Ferguson MA, Butikofer P. Phosphatidylethanolamine in Trypanosoma brucei is organized in two separate pools and is synthesized exclusively by the Kennedy pathway. The Journal of biological chemistry. 2008;283:23636–23644. doi: 10.1074/jbc.M803600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Signorell A, et al. Perturbation of phosphatidylethanolamine synthesis affects mitochondrial morphology and cell-cycle progression in procyclic-form Trypanosoma brucei. Molecular microbiology. 2009;72:1068–1079. doi: 10.1111/j.1365-2958.2009.06713.x. [DOI] [PubMed] [Google Scholar]
- 28.Cui Z, Vance DE. Expression of phosphatidylethanolamine N-methyltransferase-2 is markedly enhanced in long term choline-deficient rats. The Journal of biological chemistry. 1996;271:2839–2843. doi: 10.1074/jbc.271.5.2839. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K. Sphingolipids are essential for differentiation but not growth in Leishmania. The EMBO Journal. 2003;22(22):6016–6026. doi: 10.1093/emboj/cdg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bibis SS, Dahlstrom K, Zhu T, Zufferey R. Characterization of Leishmania major phosphatidylethanolamine methyltransferases LmjPEM1 and LmjPEM2 and their inhibition by choline analogs. Molecular and biochemical parasitology. 2014;196:90–99. doi: 10.1016/j.molbiopara.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridgway ND, Vance DE. Specificity of rat hepatic phosphatidylethanolamine N-methyltransferase for molecular species of diacyl phosphatidylethanolamine. The Journal of biological chemistry. 1988;263:16856–16863. [PubMed] [Google Scholar]
- 32.Kodaki T, Yamashita S. Yeast phosphatidylethanolamine methylation pathway. Cloning and characterization of two distinct methyltransferase genes. The Journal of biological chemistry. 1987;262:15428–15435. [PubMed] [Google Scholar]
- 33.Rifkin MR, Strobos CA, Fairlamb AH. Specificity of ethanolamine transport and its further metabolism in Trypanosoma brucei. The Journal of biological chemistry. 1995;270:16160–16166. doi: 10.1074/jbc.270.27.16160. [DOI] [PubMed] [Google Scholar]
- 34.Smith TK, Butikofer P. Lipid metabolism in Trypanosoma brucei. Molecular and biochemical parasitology. 2010;172:66–79. doi: 10.1016/j.molbiopara.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawlowic MC, Zhang K. Leishmania parasites possess a platelet-activating factor acetylhydrolase important for virulence. Molecular and biochemical parasitology. 2012;186:11–20. doi: 10.1016/j.molbiopara.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castanys-Munoz E, Alder-Baerens N, Pomorski T, Gamarro F, Castanys S. A novel ATP-binding cassette transporter from Leishmania is involved in transport of phosphatidylcholine analogues and resistance to alkyl-phospholipids. Molecular microbiology. 2007;64:1141–1153. doi: 10.1111/j.1365-2958.2007.05653.x. [DOI] [PubMed] [Google Scholar]
- 37.Parodi-Talice A, et al. The overexpression of a new ABC transporter in Leishmania is related to phospholipid trafficking and reduced infectivity. Biochimica et biophysica acta. 2003;1612:195–207. doi: 10.1016/S0005-2736(03)00131-7. [DOI] [PubMed] [Google Scholar]
- 38.Henriques C, Atella GC, Bonilha VL, de Souza W. Biochemical analysis of proteins and lipids found in parasitophorous vacuoles containing Leishmania amazonensis. Parasitology research. 2003;89:123–133. doi: 10.1007/s00436-002-0728-y. [DOI] [PubMed] [Google Scholar]
- 39.Croft SL, Coombs GH. Leishmaniasis–current chemotherapy and recent advances in the search for novel drugs. Trends in parasitology. 2003;19:502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Mages F, Rey C, Fonlupt P, Pacheco H. Kinetic and biochemical properties of CTP:choline-phosphate cytidylyltransferase from the rat brain. European journal of biochemistry / FEBS. 1988;178:367–372. doi: 10.1111/j.1432-1033.1988.tb14459.x. [DOI] [PubMed] [Google Scholar]
- 41.Post M, Batenburg JJ, Schuurmans EA, Van Golde LM. The rate-limiting step in the biosynthesis of phosphatidylcholine by alveolar type II cells from adult rat lung. Biochimica et biophysica acta. 1982;712:390–394. doi: 10.1016/0005-2760(82)90357-5. [DOI] [PubMed] [Google Scholar]
- 42.Wein S, et al. Contribution of the precursors and interplay of the pathways in the phospholipid metabolism of the malaria parasite. Journal of lipid research. 2018;59:1461–1471. doi: 10.1194/jlr.M085589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dechamps S, et al. The Kennedy phospholipid biosynthesis pathways are refractory to genetic disruption in Plasmodium berghei and therefore appear essential in blood stages. Molecular and biochemical parasitology. 2010;173:69–80. doi: 10.1016/j.molbiopara.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Contet A, et al. Plasmodium falciparum CTP:phosphocholine cytidylyltransferase possesses two functional catalytic domains and is inhibited by a CDP-choline analog selected from a virtual screening. FEBS Lett. 2015;589:992–1000. doi: 10.1016/j.febslet.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Cornell RB, Northwood IC. Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization. Trends in biochemical sciences. 2000;25:441–447. doi: 10.1016/S0968-0004(00)01625-X. [DOI] [PubMed] [Google Scholar]
- 46.Haider A, et al. PCYT1A Regulates Phosphatidylcholine Homeostasis from the Inner Nuclear Membrane in Response to Membrane Stored Curvature Elastic Stress. Developmental cell. 2018;45:481–495 e488. doi: 10.1016/j.devcel.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bangs JD, Uyetake L, Brickman MJ, Balber AE, Boothroyd JC. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. Journal of cell science. 1993;105(Pt 4):1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- 48.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. Journal of microscopy. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 49.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. The Journal of biological chemistry. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 50.Kapler GM, Coburn CM, Beverley SM. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990;10:1084–1094. doi: 10.1128/MCB.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao C, Wilson ME. Dynamics of sterol synthesis during development of Leishmania spp. parasites to their virulent form. Parasites & vectors. 2016;9:200. doi: 10.1186/s13071-016-1470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 53.Sacks DL, Brodin TN, Turco SJ. Developmental modification of the lipophosphoglycan from Leishmania major promastigotes during metacyclogenesis. Molecular and biochemical parasitology. 1990;42:225–233. doi: 10.1016/0166-6851(90)90165-I. [DOI] [PubMed] [Google Scholar]
- 54.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nature reviews. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 55.Kelly, P. H. et al. The Gut Microbiome of the Vector Lutzomyia longipalpis Is Essential for Survival of Leishmania infantum. mBio8, 10.1128/mBio.01121-16 (2017). [DOI] [PMC free article] [PubMed]
- 56.Dillon RJ, el Kordy E, Shehata M, Lane RP. The prevalence of a microbiota in the digestive tract of Phlebotomus papatasi. Ann Trop Med Parasitol. 1996;90:669–673. doi: 10.1080/00034983.1996.11813102. [DOI] [PubMed] [Google Scholar]
- 57.Li K, et al. Diversity of bacteriome associated with Phlebotomus chinensis (Diptera: Psychodidae) sand flies in two wild populations from China. Scientific reports. 2016;6:36406. doi: 10.1038/srep36406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monteiro CC, et al. Bacterial diversity of the American sand fly Lutzomyia intermedia using high-throughput metagenomic sequencing. Parasites & vectors. 2016;9:480. doi: 10.1186/s13071-016-1767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sant’Anna MR, et al. Investigation of the bacterial communities associated with females of Lutzomyia sand fly species from South America. PloS one. 2012;7:e42531. doi: 10.1371/journal.pone.0042531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sant’Anna MR, et al. Colonisation resistance in the sand fly gut: Leishmania protects Lutzomyia longipalpis from bacterial infection. Parasites & vectors. 2014;7:329. doi: 10.1186/1756-3305-7-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Louradour Isabelle, Monteiro Carolina Cunha, Inbar Ehud, Ghosh Kashinath, Merkhofer Richard, Lawyer Phillip, Paun Andrea, Smelkinson Margery, Secundino Nagila, Lewis Michael, Erram Dinesh, Zurek Ludek, Sacks David. The midgut microbiota plays an essential role in sand fly vector competence for Leishmania major. Cellular Microbiology. 2017;19(10):e12755. doi: 10.1111/cmi.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geiger O, Gonzalez-Silva N, Lopez-Lara IM, Sohlenkamp C. Amino acid-containing membrane lipids in bacteria. Prog Lipid Res. 2010;49:46–60. doi: 10.1016/j.plipres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Lara IM, Geiger O. Bacterial lipid diversity. Biochimica et biophysica acta. 2017;1862:1287–1299. doi: 10.1016/j.bbalip.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Garsin DA. Ethanolamine utilization in bacterial pathogens: roles and regulation. Nature reviews. Microbiology. 2010;8:290–295. doi: 10.1038/nrmicro2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaval, K. G. & Garsin, D. A. Ethanolamine Utilization in Bacteria. mBio9, 10.1128/mBio.00066-18 (2018). [DOI] [PMC free article] [PubMed]
- 66.Akhoundi M, et al. Diversity of the bacterial and fungal microflora from the midgut and cuticle of phlebotomine sand flies collected in North-Western Iran. PloS one. 2012;7:e50259. doi: 10.1371/journal.pone.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gouveia C, Asensi MD, Zahner V, Rangel EF, Oliveira SM. Study on the bacterial midgut microbiota associated to different Brazilian populations of Lutzomyia longipalpis (Lutz & Neiva) (Diptera: Psychodidae) Neotropical entomology. 2008;37:597–601. doi: 10.1590/S1519-566X2008000500016. [DOI] [PubMed] [Google Scholar]
- 68.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 69.Farine L, et al. Phosphatidylserine synthase 2 and phosphatidylserine decarboxylase are essential for aminophospholipid synthesis in Trypanosoma brucei. Molecular microbiology. 2017;104:412–427. doi: 10.1111/mmi.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naderer T, et al. Intracellular Survival of Leishmania major Depends on Uptake and Degradation of Extracellular Matrix Glycosaminoglycans by Macrophages. PLoS pathogens. 2015;11:e1005136. doi: 10.1371/journal.ppat.1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naderer T, McConville MJ. The Leishmania-macrophage interaction: a metabolic perspective. Cellular microbiology. 2008;10:301–308. doi: 10.1111/j.1462-5822.2007.01096.x. [DOI] [PubMed] [Google Scholar]
- 72.De Cicco NN, et al. LDL uptake by Leishmania amazonensis: involvement of membrane lipid microdomains. Exp Parasitol. 2012;130:330–340. doi: 10.1016/j.exppara.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 73.Fraihi W, et al. An integrated overview of the midgut bacterial flora composition of Phlebotomus perniciosus, a vector of zoonotic visceral leishmaniasis in the Western Mediterranean Basin. PLoS neglected tropical diseases. 2017;11:e0005484. doi: 10.1371/journal.pntd.0005484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parsons JB, Rock CO. Bacterial lipids: metabolism and membrane homeostasis. Prog Lipid Res. 2013;52:249–276. doi: 10.1016/j.plipres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ha DS, Schwarz JK, Turco SJ, Beverley SM. Use of the green fluorescent protein as a marker in transfected Leishmania. Molecular and biochemical parasitology. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 76.Tsukagoshi Y, Nikawa J, Hosaka K, Yamashita S. Expression in Escherichia coli of the Saccharomyces cerevisiae CCT gene encoding cholinephosphate cytidylyltransferase. J Bacteriol. 1991;173:2134–2136. doi: 10.1128/jb.173.6.2134-2136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 78.Zhang K, Barron T, Turco SJ, Beverley SM. The LPG1 gene family of Leishmania major. Mol. Biochem. Parasitol. 2004;136:11–23. doi: 10.1016/j.molbiopara.2004.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.