Abstract
Stem cells from human exfoliated deciduous teeth (SHEDs) are a promising source for tissue engineering and stem cell transplantation. However, long-term in vitro culture and expansion lead to the loss of stemness of SHEDs, compromising their therapeutic benefits. Hypoxia plays an essential role in controlling the stem cell behavior of mesenchymal stem cells (MSCs). Thus, this study aimed to investigate the effects of cobalt chloride (CoCl2), a hypoxia-mimetic agent, on the stem cell marker expression and osteogenic differentiation of SHEDs. SHEDs were cultured with or without 50 or 100 μM CoCl2. Their proliferation, apoptosis, stem cell marker expression, migration ability, and osteogenic differentiation were examined. Culture with 50 and 100 μM CoCl2 increased the hypoxia-inducible factor-1 alpha (HIF-1α) protein levels in a dose-dependent manner in SHEDs without inducing significant cytotoxicity. This effect was accompanied by an increase in the proportion of STRO-1+ cells. CoCl2 significantly increased the expression of stem cell markers (OCT4, NANOG, SOX2, and c-Myc) in a dose-dependent manner. The migration ability was also promoted by CoCl2 treatment. Furthermore, SHEDs cultured in osteogenic medium with CoCl2 showed a dose-dependent reduction in alkaline phosphatase (ALP) activity and calcium deposition. The expression of osteogenic-related genes was also suppressed by CoCl2, especially in the 100-μM CoCl2 group. In conclusion, CoCl2 increased the expression of stem cell markers and inhibited the osteogenic differentiation of SHEDs. These findings may provide evidence supporting the use of in vitro hypoxic environments mimicked by CoCl2 in assisting the clinical application of SHEDs.
Keywords: Cobalt chloride, Dental pulp, Deciduous teeth, Stem cells, Stemness, Osteogenic differentiation
Introduction
Stem cell-based therapies have increasingly become the ideal therapeutic approach to cure numerous degenerative diseases. Among the many types of cells that can be used, stem cells from human exfoliated deciduous teeth (SHEDs) have attracted significant attention. SHEDs are derived from the dental pulp of young patients and can differentiate into cells of multilineages, including osteogenic, chondrogenic, adipogenic, neural, hepatic, myogenic, and endothelial lineages (Miura et al. 2003; Rosa et al. 2016). Compared with human adult dental pulp stem cells (DPSCs) and human adult periodontal ligament stem cells (PDLSCs), SHEDs are more immature and present greater proliferation rates and better differentiation potential (Koyam et al. 2009; Miura et al. 2003). Moreover, because exfoliated deciduous teeth are usually discarded, SHEDs can be obtained less invasively with fewer ethical concerns than MSCs derived from other tissues (Huang et al. 2009). Therefore, SHEDs have been considered a promising cell source for tissue engineering and stem cell transplantation.
However, the clinical use of SHEDs for tissue engineering still faces many challenges. One of the challenges is the expansion of sufficient amounts of stem cells from clinically limited tissues. Therefore, long-term in vitro culture to generate the required cell numbers is needed, although this process results in replicative senescence and impaired proliferation (Bork et al. 2010). Thus, numerous attempts have been made to positively influence stem cell behavior and improve the efficiency of stem cell-based therapies.
Stem cells reside within a unique microenvironment called the stem cell niche, which is regulated by cellular and acellular factors (Moore and Lemischka 2006). Low oxygen tension is a critical environmental factor of the stem cell niche (Mohyeldin et al. 2010). In arterial blood, the oxygen tension is approximately 14%, while in a variety of other tissues, such as bone marrow and brain tissue, the oxygen tension ranges from 1 to 7% (Chow et al. 2001; Nombela-Arrieta and Silberstein 2014). Although dental pulp is a highly vascularized tissue, the oxygen concentration in dental pulp is low. A previous study found approximately 3% oxygen in the pulp tissue of rats (Yu et al. 2002). Moreover, many causes, such as trauma and caries, can lead to much lower oxygen tension in the pulp tissue (Rombouts et al. 2017).
However, current culture conditions contain much higher oxygen tension than physiologic conditions. It has been shown that ambient oxygen tension (20% oxygen) can lead to the loss of primitive stem cell characteristics by inducing premature senescence, DNA damage, chromosomal aberrations, and metabolic changes (Fehrer et al. 2007; Kim et al. 2016). Hypoxia has been demonstrated to play an essential role in the maintenance of stem cell properties such as self-renewal, survival, and multipotency. Culture under low oxygen concentrations enhanced the proliferation and expression of stem cell markers in MSCs (Berniakovich and Giorgio 2013; Kim et al. 2016). Low oxygen concentrations enhanced the expression of some pluripotency markers, trophic factors, and immunomodulatory factors as well as the secretome trophic effect in DPSCs (Ahmed et al. 2016). SHEDs were also able to maintain higher mRNA expression of the pluripotency markers within 7 days when cultured in hypoxic conditions (Werle et al. 2018).
Unfortunately, it is difficult to simulate physiologic hypoxia in the cultural environment in vitro. Hypoxic conditions achieved using the hypoxia chambers available in the laboratory are expensive and inconvenient. Moreover, it is sometimes difficult to control and maintain steady oxygen tension. Thus, creating hypoxia by chemical agents is a more attractive method.
Hypoxia-inducible factor-1 alpha (HIF-1α) has been shown to mediate the response to hypoxia. Under normoxia, HIF-1α is rapidly degraded because of the activation of prolyl-hydroxylases (PHDs). Under hypoxic conditions, PHDs are inhibited, which leads to the accumulation of HIF-1α and initiates the transcription of downstream genes involved in metabolism, erythropoiesis, and angiogenesis (Semenza 2012). CoCl2, a widely used hypoxia-mimicking agent in vivo and in vitro, mimics hypoxia by preventing the degradation of HIF-1α (Ji et al. 2006). Previous studies found that CoCl2 increased stem cell marker mRNA expression and inhibited osteogenic differentiation in two less primitive dental-derived cells, i.e., human dental pulp cells (hDPCs) (Laksana et al. 2017) and human periodontal ligament cells (hPDLCs) (Osathanon et al. 2015).
Although several studies have focused on the influence of hypoxia on dental-derived stem cell stemness, the impact of hypoxia on SHED behavior is still a matter of discussion. Moreover, information is not available on the influence of hypoxia induced by CoCl2 on stem cell marker expression and osteogenic differentiation of SHEDs. In the present study, the effects of CoCl2 on the proliferation, apoptosis, migration, stem cell marker expression, and osteogenic differentiation of SHEDs were investigated.
Materials and methods
Cell isolation, culture, and characterization
The entire study was approved by the Ethics Committee of Sun Yat-sen University. All individuals who participated in this study provided informed consent. SHEDs were isolated from healthy exfoliated deciduous teeth of children between 6 and 12 years old. Briefly, the pulp tissues were removed from the teeth and washed with sterile phosphate-buffered saline (PBS) supplemented with 1% penicillin/streptomycin (P/S; Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA). The tissues were cut into approximately 1 × 1 mm pieces and enzymatically digested with 3 mg/ml type I collagenase (Sigma-Aldrich) and 4 mg/ml of dispase II (Roche) at 37 °C for 30 min. The cells were then cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 20% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (P/S; Gibco) at 37 °C in 5% CO2. When confluent, cells were detached by 0.25% trypsin-EDTA (Gibco) and subcultured at a 1:3 ratio. The medium was changed every 3 days. Cells from passages 3–5 were used in this study.
The isolated cells were characterized using flow cytometry. The expression of mesenchymal cell markers (CD73, CD90, and CD105) and hematopoietic cell markers (CD34, CD45) were examined. Cells were stained with PE-conjugated anti-CD73 antibody (BD Biosciences Pharmingen), PerCP-CyTM5.5-conjugated anti-CD90 antibody (BD Biosciences Pharmingen), PE-conjugated anti-CD105 antibody (BD Biosciences Pharmingen), FITC-conjugated anti-CD34 antibody (BD Biosciences Pharmingen), and PE-conjugated anti-CD45 antibody (BD Biosciences Pharmingen). The stained cells were analyzed using a CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA).
To evaluate the differentiation capacity, osteogenic induction was performed. In brief, cells were cultured in osteogenic medium (OM), which was DMEM supplemented with 10% FBS, 10 mmol/l sodium β-glycerophosphate (Sigma-Aldrich), 10−8 mol/l dexamethasone (Sigma-Aldrich), and 50 mg/ml l-ascorbic acid (Sigma-Aldrich). After 14 days of culture, calcium deposits were assessed by Alizarin red staining. Cells cultured in basal medium served as controls.
Cell viability assay
The viability of SHEDs was evaluated using a Cell Counting Kit-8 (CCK-8; Dojindo, Tokyo, Japan). SHEDs were seeded into 96-well plates at a concentration of 5 × 103 cells/well and then treated with CoCl2 at concentrations of 0, 50, and 100 μM. After culturing for 1, 3, 5, and 7 days, 100 μl of serum-free DMEM containing 10 μl of CCK-8 solution was added to each well. After incubation at 37 °C for 2 h, the optical density (OD) at 450 nm was detected with a microplate reader (Tecan, Infinite f200 PRO, Switzerland).
Flow cytometric analysis to quantify STRO-1+ cells
The expression levels of STRO-1, a marker for mesenchymal progenitor populations, were analyzed using a CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA) as previously described. SHEDs were treated with or without 50 or 100 μM CoCl2 for 3 days. Then, the cells were harvested with trypsin-EDTA and resuspended in the wash buffer. After incubation with a Dylight 650 conjugated anti-STRO-1 antibody (Novus Biologicals, USA) in the dark at 4 °C for 1 h, the cells were washed twice with PBS and then analyzed by flow cytometry. The data were analyzed with FlowJo 10 (Treestar, Ashland, OR, USA).
Cell apoptosis assay
The rates of SHED apoptosis were determined with an Annexin V-FITC/PI double staining apoptosis detection kit (Nanjing Jiancheng Biotechnology Institute, Nanjing, China). SHEDs were seeded into 6-well plates at a concentration of 2 × 105 cells/well and then treated with or without 50 or 100 μM CoCl2 for 3 days. The cells were collected, washed, and resuspended in 500-μl binding buffer. Next, 5 μl FITC-Annexin V and 5 μl PI were added to the cell suspension. The cells were incubated at room temperature for 10 min. Then, the samples were analyzed by flow cytometry. The data analysis was performed using CytExpert software.
Real-time quantitative polymerase chain reaction (qRT-PCR)
The expression of an antiapoptosis gene (e.g., Bcl-2), stemness genes (e.g., OCT4, NANOG, SOX2, and c-Myc), osteogenic genes (e.g., ALP, RUNX2, and COLI), hypoxia-inducible factor (HIF)-related genes (e.g., HIF-2α and VEGF), and β-catenin was detected by a qRT-PCR analysis. Briefly, SHEDs were seeded on 6-well plates at a density of 2 × 105/well and treated with or without 50 or 100 μM CoCl2. To assess the expression of osteogenic genes, the cells were incubated in OM with or without 50 or 100 μM CoCl2. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. One microgram of mRNA was converted into complementary DNA using the PrimeScript™ RT Master Mix (Takara, Japan). qRT-PCR was performed on a Light Cycler 480 Detection System (Roche, Sweden) with a SYBR PCR Master Mix kit (Roche, Indianapolis, IN, USA). β-Actin was used as an internal control. The primer sequences are shown in Table 1.
Table 1.
qRT-PCR primer sequences
| Gene | Primer sequences (5′-3′) |
|---|---|
| Bcl-2 |
F: GAGGATTGTGGCCTTCTTTG R:GCCGGTTCAGGTACTCAGTC |
| OCT4 |
F:TGGGGGTTCTATTTGGGAAGG R:GATCTGCTGCAGTGTGGGT |
| NANOG |
F:CCAGCCTTTACTCTTCCTACCA R:GCTGATTAGGCTCCAACCATAC |
| SOX2 |
F: GGATAAGTACACGCTGCCCG R: ATGTGCGCGTAACTGTCCAT |
| c-Myc |
F: GCTGCTTAGACGCTGGATTT R:CCTCCTCGTCGCAGTAGAAA |
| ALP |
F:CCTCCTCGGAAGACACTCTG R:GCAGTGAAGGGCTTCTTGTC |
| RUNX2 |
F: CCACTGAACCAAAAAGAAATCCC R: GAAAACAACACATAGCCAAACGC |
| COLI |
F: CGATGGATTCCAGTTCGAGTATG R: TGTTCTTGCAGTGGTAGGTGATG |
| VEGF |
F:CGCAGCTACTGCCATCCAAT R:GTGAGGTTTGATCCGCATAATCT |
| HIF-2α |
F:GTCTCTCCACCCCATGTCTC R:GGTTCTTCATCCGTTTCCAC |
| β-Catenin |
F:AAGTTCTTGGCTATTACGACA R:ACAGCACCTTCAGCACTCT |
| β-Actin |
F:CATGTACGTTGCTATCCAGGC R:CTCCTTAATGTCACGCACGAT |
Western blot analysis
To confirm the hypoxia-mimetic effects, the expression of HIF-1α protein was detected by western blot analysis. In parallel with the β-catenin gene analysis, the β-catenin protein levels were also detected. Briefly, SHEDs were washed twice in PBS and lysed in radioimmunoprecipitation assay (RIPA) buffer (KeyGen BioTECH, Nanjing, China) supplemented with 1 mmol/l protease inhibitor cocktail (CWBIO, Beijing, China). Whole cell lysates were separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; CWBIO) and transferred onto polyvinylidene fluoride (PDVF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% fat-free milk in TBST (10 mmol/l TrisHCl, 50 mmol/l NaCl, 0.25% Tween 20) for 1 h at room temperature and subsequently incubated with primary antibodies (anti-HIF-1α, anti-β-catenin, anti-β-Actin [1:1000; Cell Signaling Technology, Inc., Danvers, MA, USA]). The membranes were then treated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies. Bands were detected with an enhanced chemiluminescence kit (Millipore).
Wound healing assay
SHEDs were seeded in 6-well plates at a density of 2 × 105 cells/well. When the cells formed a confluent monolayer, wounds were produced by scratching with a 200-μl pipette tip across the center of the plate. The medium was changed to FBS-free medium with or without 50 or 100 μM CoCl2. After 24 h, the wells were photographed under an inverted microscope (Zeiss, Oberkochen, Germany). The width of the scratches was compared, and the number of cells migrating into the scratches was counted by an experimenter blinded to the experimental conditions.
Transwell assay
Transwell assays were conducted using 24-well transwell plates (pore size 8 μm, polycarbonate membrane, Corning, catalog no. 3422). SHEDs were seeded into the upper chamber of inserts (4 × 104 cells/well) containing FBS-free medium with or without 50 or 100 μM CoCl2. DMEM containing 10% FBS was added to the lower chambers of the inserts. After incubation for 24 h, the cells remaining on the upper side of the filter were removed with cotton swabs, and the filters were fixed in formaldehyde for 30 min and then stained with 0.1% crystal violet for 15 min. Cells that migrated to the lower surface were observed under a microscope. Five random fields (× 100) of cells were counted by an experimenter blinded to the experimental conditions. Each assay was repeated three times.
Osteogenic differentiation
SHEDs were seeded on 6-well plates at a density of 2 × 105/well. When the cells reached confluency, they were treated with OM with or without 50 or 100 μM CoCl2 for 7–14 days. The OM was replaced every 3 days. On day 7 and day 14, ALP activity was detected using an Alkaline Phosphatase Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol. On day 14, the cells were fixed in 4% paraformaldehyde and stained in Alizarin red solution (Cyagen Biosciences Inc., Guangzhou, China) to detect calcium deposits. Furthermore, calcium deposits were quantified by destaining the deposits with 10% cetylpyridinium chloride solution for 30 min. The absorbance was measured at 562 nm by a microplate reader. To assess the expression of osteogenic genes, total RNA was extracted using TRIzol reagent (Invitrogen) on day 7, and a qRT-PCR assay was performed.
Statistical analyses
All the data are shown as the mean ± standard deviation from triplicate independent experiments. Statistical analyses were performed using one-way analysis of variance (ANOVA) and the Bonferroni method was used for multiple comparisons. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA).
Results
Characterization of SHEDs
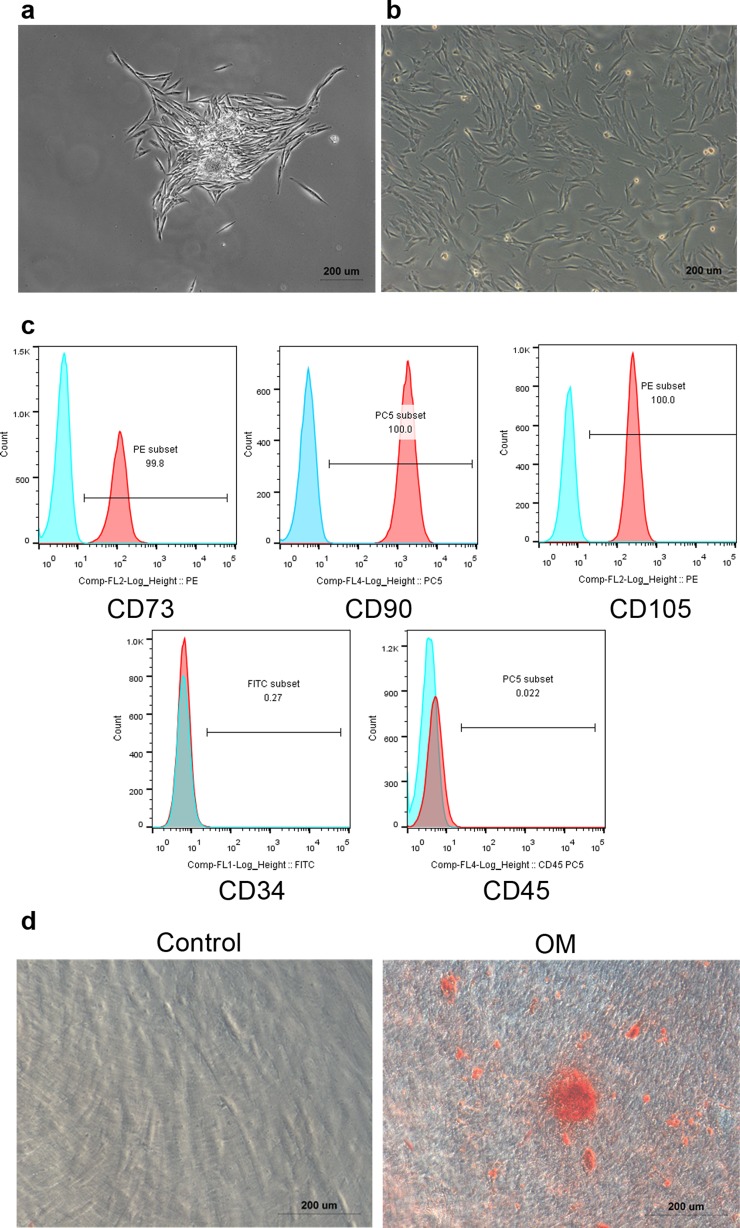
SHEDs showed a typical fibroblastic morphology in primary culture (Fig. 1a) and after being passaged (Fig. 1b). SHEDs were positive for mesenchymal cell markers (CD73, CD90, and CD105), but negative for hematopoietic cell markers (CD34, CD45) (Fig. 1c). When SHEDs were cultured under osteogenic culture conditions, we observed calcium deposits (Fig. 1d), confirming that the isolated SHEDs had the ability to differentiate into osteogenic lineages. These data suggest that SHEDs exhibit mesenchymal stem cell characteristics.
Fig. 1.
Characterization of SHEDs. a Primary culture of SHEDs under a microscope (× 50). b 3rd passage of SHEDs under a microscope (× 50). c Flow cytometry analysis of surface markers of SHEDs. SHEDs were positive for mesenchymal cell markers (CD73, CD90, and CD105), but negative for hematopoietic cell markers (CD34, CD45). d SHEDs were cultured in the absence (control) or in the presence of OM for 14 days and then stained with Alizarin red
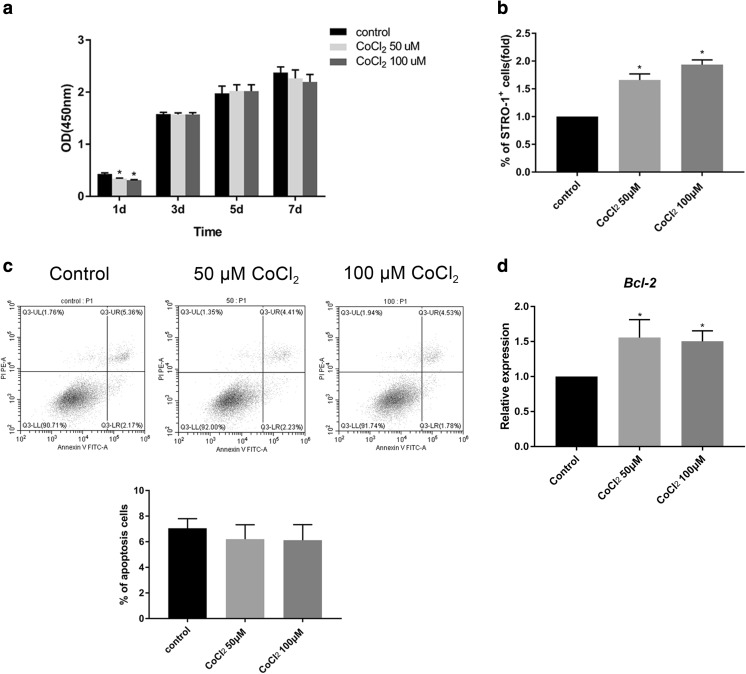
CoCl2 did not induce significant cytotoxicity in SHEDs
SHED viability was evaluated by CCK-8 assay. The cells were cultured in culture medium with or without 50 or 100 μM CoCl2 for 1, 3, 5, and 7 days. The results showed that the viability of cells was suppressed by 50 and 100 μM CoCl2 at the start of culture. However, there was no significant difference between the control and the CoCl2-treated cells on days 3, 5, and 7 (Fig. 2a). Next, because SHEDs likely do not include a homogeneous cell population, the proportion of progenitor cells was investigated by analyzing the percentage of the cells expressing STRO-1, a mesenchymal progenitor cell marker. The flow cytometry results showed that the proportion of STRO-1+ cells was significantly higher in the cells cultured with CoCl2 (Fig. 2b). The rate of apoptotic cells was also determined by flow cytometry. The results showed that after 3 days, CoCl2 did not increase the rate of apoptotic cells (Fig. 2c). Moreover, the mRNA expression of the antiapoptosis gene Bcl-2 was significantly increased in the CoCl2-treated groups compared with that of the control group (Fig. 2d). These data revealed that 50 or 100 μM CoCl2 did not induce cytotoxicity in SHEDs.
Fig. 2.
The cytotoxicity of CoCl2 was measured. a Cell viability of SHEDs was determined using a CCK-8 assay after incubation with or without 50 or 100 μM CoCl2 for 1, 3, 5, and 7 days. The absorbance was measured at 450 nm. b The percentage of STRO-1+ cells in SHEDs after incubation with or without 50 or 100 μM CoCl2 for 3 days. The data are shown as relative fold changes compared with the control. c Apoptosis of SHEDs was determined with Annexin V-FITC/PI double staining after incubation with or without 50 or 100 μM CoCl2 for 3 days. Flow cytometric contour plots and the percentage of apoptotic cells are shown. d The mRNA expression of Bcl-2 was examined by qRT-PCR. The data are shown as relative gene expression compared with the internal control. Bar graphs represent the mean ± SD of triplicate independent experiments.*P < 0.05 vs. control
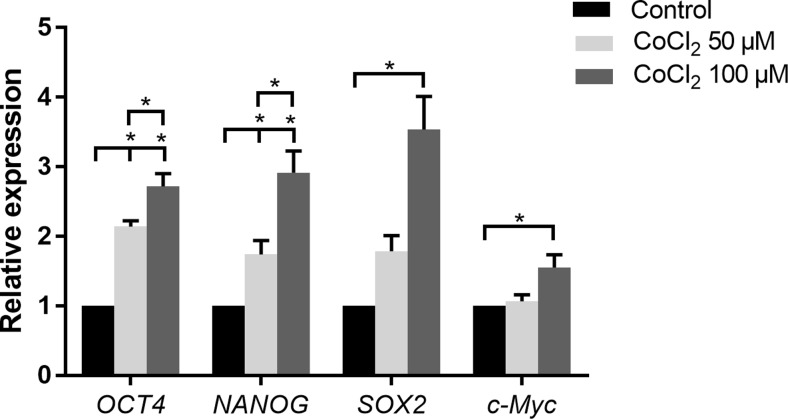
CoCl2 increased stem cell marker expression
The effects of CoCl2 on mRNA expression of the stem cell markers OCT4, NANOG, SOX2, and c-Myc after the cells were cultured with or without 50 or 100 μM CoCl2 for 3 days were evaluated. The results revealed that CoCl2 significantly induced the mRNA expression of OCT4 and NANOG compared with the control in a dose-dependent manner. SOX2 and c-Myc mRNA were significantly higher in cells treated with 100 μM CoCl2. However, a significant increase in the expression of SOX2 and c-Myc was not observed between the 50 μM CoCl2 group and the control group (Fig. 3).
Fig. 3.
Effects of CoCl2 on stem cell marker expression of SHEDs. The mRNA expression of OCT4, NANOG, SOX2, and c-Myc was assessed by qRT-PCR. The data are shown as relative gene expression compared with the internal control. Bar graphs represent the mean ± SD of triplicate independent experiments. *P < 0.05 vs. control
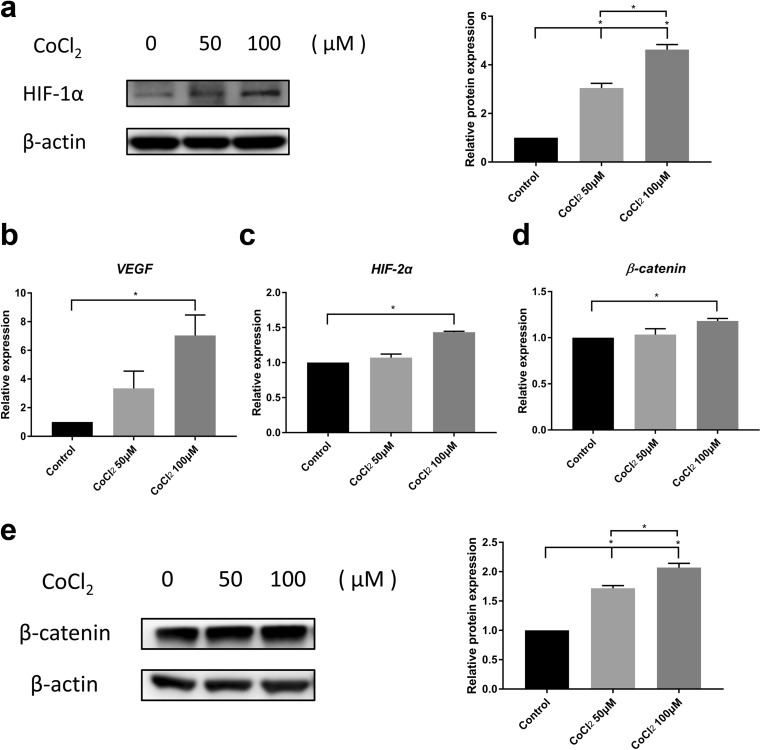
CoCl2 induced hypoxia-inducible factor-related genes and β-catenin in SHEDs
After treatment with 50 μM or 100 μM CoCl2, the expression of HIF-1α in SHEDs was determined by western blotting (Fig. 4a). The level of HIF-1α protein was enhanced in response to CoCl2 treatment in a dose-dependent manner, confirming its hypoxic effect. The mRNA expression of VEGF, a downstream gene of HIF-1α, was enhanced after 100 μM CoCl2 treatment, indicating activation of the HIF-1α pathway (Fig. 4b). Similarly, HIF-2α, a homologous protein of HIF-1α, also showed a significant increase in mRNA expression in the 100 μM CoCl2 group (Fig. 4c). Next, we examined the expression of β-catenin, which is important in stem cell proliferation and survival. Interestingly, we found that β-catenin mRNA was increased in the SHEDs treated with 100 μM CoCl2 (Fig. 4d), and β-catenin protein levels were increased upon the CoCl2 treatment in a dose-dependent manner (Fig. 4e).
Fig. 4.
CoCl2 induced HIF-related genes and β-catenin in SHEDs. a Western blots of HIF-1α protein levels in SHEDs treated with 50 or 100 μM CoCl2 for 12 h. β-Actin levels are shown as loading controls. b–d The mRNA expression of HIF-2α, VEGF, and β-catenin was evaluated in SHEDs cultured with or without CoCl2 (50 μM and 100 μM) for 3 days. e The relative protein expression of β-catenin in SHEDs. β-Actin levels are shown as loading controls. Bar graphs represent the mean ± SD of triplicate independent experiments. *P < 0.05 vs. control
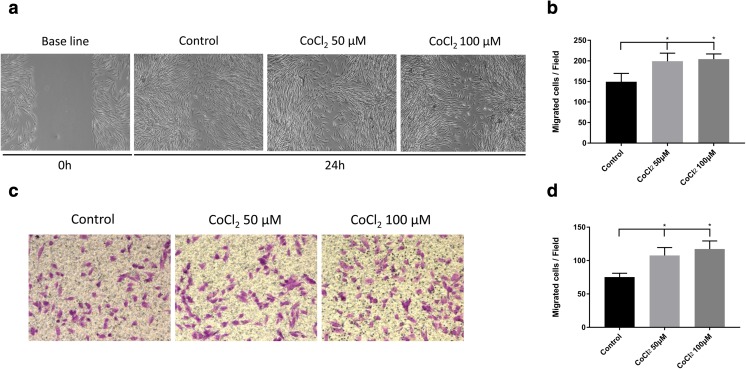
CoCl2 enhanced SHED migration
To detect the effects of CoCl2 on the migration of SHEDs, scratch wound healing and transwell assays were conducted. The wound healing assay results showed that 50 and 100 μM CoCl2 both significantly increased the number of SHED cells migrating toward the wound at 24 h (Fig. 5a, b). Similarly, the transwell assay results showed that the migration capability of the CoCl2-treated groups increased significantly compared with that of the control group (Fig. 5c, d). However, there was no significant difference between the 50 μM CoCl2 group and the 100 μM CoCl2 group. Taken together, these results indicate that CoCl2 can promote SHED migration in vitro.
Fig. 5.
Effects of CoCl2 on SHED migration. a Representative image of SHED scratch wound healing with or without 50 or 100 μM CoCl2 treatment (× 100). Cellular migration was monitored for 24 h. b Quantification of cells migrating into the scratches in the scratch wounding healing assay. c Representative image of migrating SHEDs treated with or without 50 or 100 μM CoCl2 in the transwell assay (× 100). d Quantification of the migrated cells in the transwell assay. *P < 0.05 vs. control
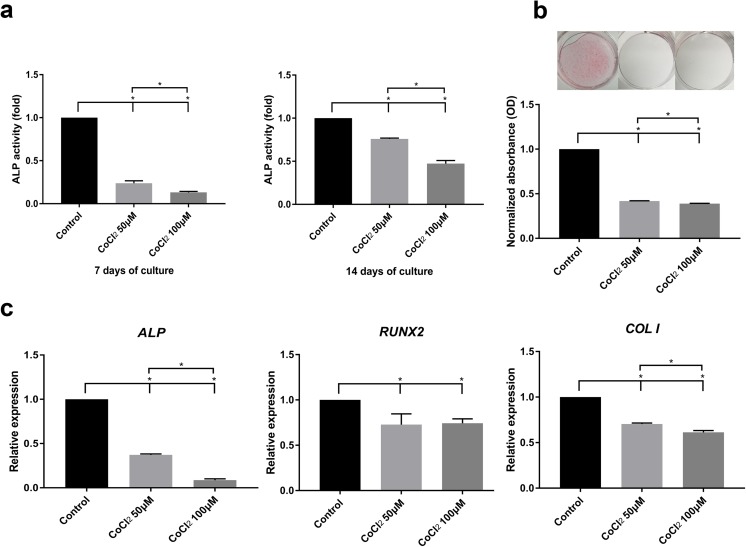
CoCl2 inhibited osteogenic differentiation
To examine the effect of CoCl2 on osteogenic differentiation, SHEDs were cultured in OM with or without 50 or 100 μM CoCl2. CoCl2 caused a dose-dependent decrease in ALP activity at both 7 and 14 days (Fig. 6a). Consistent with the ALP activity results, SHEDs cultured with CoCl2 exhibited a diminished capacity to form calcium deposits at 14 days (Fig. 6b). Furthermore, the mRNA expression of osteogenic markers was assessed by RT-qPCR. Consistent with the above results, the mRNA expression levels of ALP, RUNX2, and COLI were all significantly reduced in SHEDs cultured with CoCl2 (Fig. 6c). These findings suggest that CoCl2 inhibited the osteogenic differentiation of SHEDs.
Fig. 6.
CoCl2 decreased the osteogenic differentiation of SHEDs. a SHEDs cultured in osteogenic medium with 50 μM or 100 μM CoCl2 for 7 or 14 days showed an inhibition of ALP activity. b Deposition of calcium exhibited by Alizarin red staining. SHEDs cultured in OM with either 50 or 100 μM CoCl2 for 14 days showed reduced mineral deposition. Data were expressed as the fold change compared with the control. c After 7 days of osteogenic induction, the mRNA expression of ALP, RUNX2, and COLI was examined by RT-qPCR. The data are shown as relative gene expression compared with the internal control. Bar graphs represent the mean ± SD of triplicate independent experiments. *P < 0.05 vs. control
Discussion
High-quality stem cells are necessary for tissue regeneration and stem cell therapies. However, long-term in vitro culture leads to a loss of cell stemness, which strongly limits the clinical use of MSCs. Therefore, maintaining the stemness of MSCs, particularly with regard to their proliferation, migration, and differentiation, is important before allowing these cells to enter the clinical testing stage. Many researchers have developed various strategies, including preconditioning, genetic modification, and optimization of MSC culture conditions, to improve cell behavior in vitro culture (Hu and Li 2018; Yu et al. 2016; Zhao et al. 2016). Among these strategies, the incubation of cells under hypoxic conditions is one available method to induce stem cell proliferation and improve the behavior of several types of MSCs. Creating hypoxia by adding chemical agents, such as CoCl2, to the culture medium is an attractive option.
SHEDs represent an ideal source for tissue engineering and stem cell transplantation. Although they share many similar characteristics with DPSCs, SHEDs are unique due to their higher proliferative rates, stronger differentiation capacities, easily accessible source, and fewer ethical concerns (Koyam et al. 2009; Miura et al. 2003). These specific properties lead to diverse responses to hypoxia. To date, the effect of hypoxia on the behavior of SHEDs is still unclear. Thus, the aim of this study was to investigate the effects of hypoxia induced by CoCl2 on the stem cell marker expression and osteogenic differentiation of SHEDs. Our studies showed that CoCl2 induced the expression of stem cell markers, promoted migration, and inhibited the osteogenic differentiation of SHEDs.
Our study demonstrated that CoCl2 treatment activated HIF-1α pathways in SHEDs. Therefore, CoCl2 effectively mimicked the hypoxic conditions in SHEDs. However, CoCl2 is a chemical agent. To prevent potential toxicity induced by CoCl2, we performed a careful cytotoxicity test. Our CCK-8 assay showed that 50 or 100 μM CoCl2 inhibited SHED proliferation at the start of treatment. Nevertheless, there was no significant difference among these groups for treatments longer than 1 day. One possible explanation for this phenomenon is that an initial period of adaptation exists, as there is evidence that MSCs exposed to 5% O2 initially show lower proliferation but grow fast thereafter (Grayson et al. 2010). Other studies have also shown that MSCs establish redox homeostasis and, thus, adapt to hypoxic conditions (Boyette et al. 2014).
Hypoxia has been shown to have dual effects on MSC apoptosis. Severe and prolonged hypoxia may induce apoptosis, whereas acute and mild hypoxia induce adaptation and survival (Greijer and van der Wall 2004). Although previous studies have reported that dental-derived cells such as DPSCs and hPDLCs have high cell viability when cultured in 100 μM CoCl2 (Osathanon et al. 2015; Teti et al. 2018), contradictory findings have also been reported, namely, that 100 μM CoCl2 induced cell death (Laksana et al. 2017). These conflicting results may be due to variations in cell types and experimental conditions. In the present study, we showed that CoCl2 did not induce negative cell growth or apoptosis, consistent with previous studies on MSCs in which hypoxia (1% O2) reduced cell apoptosis compared with normoxia (Kim et al. 2016).
Previous reports have indicated that stem cells isolated from the pulp may not be a single-cell type but rather a heterogeneous stem cell population (Sloan and Waddington 2010). STRO-1 is an early MSC marker to identify clonogenic stromal cell progenitors (Kawashima 2012). Previous studies found that STRO-1+ cells possess higher proliferation and multipotentiality than cells negative for this MSC marker (Xuechao et al. 2009; Yang et al. 2007). Moreover, the STRO-1 expression ranges from 0.02 to 9.56% in human pulp stem cell cultures and increases under hypoxic condition (Sakdee et al. 2009; Werle et al. 2016). Our study showed that CoCl2 increased the expression of STRO-1, which is consistent with previous reports and suggests that CoCl2 might enhance the proportion of progenitor cells among isolated SHEDs in this study.
OCT4, NANOG, and SOX2 are stemness-related genes essential for the major properties of stem cells, and they are critical in maintaining a pluripotent state and self-renewal. Previous studies have shown that the overexpression of OCT4, NANOG, and SOX2 promoted proliferation and prevented spontaneous differentiation, whereas the downregulation of these genes led to a loss of differentiation and senescence (Basu-Roy et al. 2010; Han et al. 2014; Tsai et al. 2012). c-Myc is a transcription factor that generates induced pluripotent stem cells and has a unique role in cell proliferation and differentiation (Liu et al. 2013). Our results showed that CoCl2 upregulated the gene expression of OCT4, NANOG, SOX2, and c-Myc. This finding suggests that the CoCl2 treatment might have positive effects on the stemness of SHEDs via the activation of OCT4-, NANOG-, SOX2- and c-Myc-related signaling pathways.
SHEDs, capable of multilineage differentiation, are a unique type of MSCs. Previous studies have shown that hypoxia can affect the osteogenic differentiation of MSCs, although the reported results are conflicting. Although 5% of oxygen promoted the mineralization of bone marrow mesenchymal stem cells (BMSCs) (Sheehy et al. 2012), a recent study showed that 2% of oxygen inhibited the osteogenic differentiation of BMSCs (Zhang et al. 2017). This discrepancy may be due to the variations in oxygen concentration. The duration of hypoxia has also been suggested to affect the osteogenic differentiation of MSCs (Boyette et al. 2014). In the present study, osteogenic-related gene expression, ALP activity, and calcium deposition were all reduced under hypoxic conditions mimicked by CoCl2. This result corresponds with previous studies that reported the inhibitory effect of CoCl2 on osteogenic differentiation in hDPCs (Laksana et al. 2017) and hPDLCs (Osathanon et al. 2015). However, it was found that CoCl2 upregulated osteogenic gene expression in BMSCs (Yu et al. 2018). Differences in cell types may contribute to such disparities. Nevertheless, it is probable that CoCl2 inhibit the osteogenic differentiation of SHEDs without maintaining their stemness for example by differentiation to the other lineages. Therefore, further experiments are needed to verify the effect of hypoxia on the multilineage differentiation of SHEDs.
The migration capacity of MSCs together with stemness is critical for repairing damaged tissues. The stromal-derived factor-1 alpha (SDF-1α)/CXCR4 axis plays a critical role in regulating the trafficking of MSCs toward the target tissue (Cencioni et al. 2012). Previous studies have suggested that hypoxia and hypoxia-mimetic agents, such as CoCl2 or deferoxamine (DFX), may increase the migration of MSCs by increasing the expression of CXCR4 (Li et al. 2017; Wang et al. 2017). Our study also found that CoCl2 treatment enhanced SHED migration. Our results imply that SHEDs treated with CoCl2 might better migrate and home to the injured area, and thus, CoCl2 may improve the therapeutic effect of SHEDs in stem cell-based therapies.
Previous studies have shown that hypoxia can induce pluripotency and modulate reprogramming in somatic cells by stabilizing HIF-1α (López-Iglesias et al. 2015). Consistent with previous data, our findings showed that CoCl2 induced higher expression of HIF-1α protein. We also found that 100 μM CoCl2 significantly increased the mRNA expression of HIF-2α, a homologous protein of HIF-1α. Both HIF-1α and HIF-2α have been reported to induce the expression of genes controlling cell proliferation, self-renewal, and pluripotency (Cummins 2012). Moreover, hypoxia has been shown to stimulate the activation of other signaling pathways involved in stemness preservation, such as the Wnt/β-catenin, Notch, and Hedgehog signaling pathways (Landor and Lendahl 2017; Zhao et al. 2018). In the current study, we observed the upregulation of β-catenin. The Wnt/β-catenin signaling pathway regulates the transcription of an array of genes related to cellular proliferation and survival (cyclin D1, c-Myc, etc.) (Kahn 2014). The variable activation level of Wnt signaling under hypoxic conditions induced multiple cell changes (Li et al. 2016). In our study, the increased expression of HIF-related genes and β-catenin by CoCl2 treatment might partially explain the effect of CoCl2 on SHEDs, although the associations between hypoxia and these signaling pathways remain to be elucidated.
In our study, we observed that the mRNA expression levels of Bcl-2 and c-Myc were both increased in SHEDs cultured with CoCl2. Because of the well-known role of c-Myc and Bcl-2 in the regulation of apoptosis and tumorigenesis (Alarcon et al. 1996), safety concerns remain regarding the effect of increased expression of c-Myc and Bcl-2, which may stimulate cancer/tumor development, whereas cancer-suppressing effects of Bcl-2 have also been reported (Slape et al. 2012). Moreover, tumor formation by MSCs may be affected by many factors, such as the methods used for cell culture and differentiation, the site of transplantation, and the host background (Kyoko et al. 2009). Therefore, firm conclusions cannot be reached based on our study. However, this finding should be taken into consideration in the application of CoCl2 to maintain stem cells during in vitro expansion.
In conclusion, CoCl2 treatment increased the expression of stem cell markers and inhibited the osteogenic differentiation of SHEDs. The findings presented here may provide beneficial evidence for the use of the in vitro hypoxic environment mimicked by CoCl2 in assisting the clinical application of SHEDs. The use of CoCl2 in maintaining the undifferentiated state of SHEDs in the laboratory as well as the mechanism underlying the effect of CoCl2 on the stemness and differentiation of SHEDs needs further investigation.
Acknowledgements
We are grateful to the healthy donors for participating in this study.
Funding information
This work was supported by the National Natural Science Foundation of China (No. 81472526 and No. 81873711), the Natural Science Foundation of Guangdong Province (No. 2014A030313126), and the Guangdong Science and Technology Project (No. 2016A020215094 and No. 2016A020216007).
Compliance with ethical standards
The entire study was approved by the Ethics Committee of Sun Yat-sen University. All individuals who participated in this study provided informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yijing Chen and Qi Zhao contributed equally to this work.
Contributor Information
Dongsheng Yu, Phone: +86-208-384-4247, Email: yudsh@mail.sysu.edu.cn.
Wei Zhao, Phone: +86-208-386-2553, Email: zhaowei3@mail.sysu.edu.cn.
References
- Ahmed NE, Murakami M, Kaneko S, Nakashima M. The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs) Sci Rep. 2016;6:35476. doi: 10.1038/srep35476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon RM, Rupnow BA, Graeber TG, Knox SJ, Giaccia AJ. Modulation of c-Myc activity and apoptosis in vivo. Cancer Res. 1996;56:4315–4319. [PubMed] [Google Scholar]
- Basu-Roy U, Ambrosetti D, Favaro R, Nicolis SK, Mansukhani A, Basilico C. The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ. 2010;17:1345–1353. doi: 10.1038/cdd.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berniakovich I, Giorgio M. Low oxygen tension maintains multipotency, whereas normoxia increases differentiation of mouse bone marrow stromal cells. Int J Mol Sci. 2013;14:2119–2134. doi: 10.3390/ijms14012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork S, Pfister S, Witt H, Horn P, Korn B, Ho AD, Wagner W. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell. 2010;9:54–63. doi: 10.1111/j.1474-9726.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette LB, Creasey OA, Guzik L, Lozito T, Tuan RS. Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl Med. 2014;3:241–254. doi: 10.5966/sctm.2013-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc Res. 2012;94:400–407. doi: 10.1093/cvr/cvs132. [DOI] [PubMed] [Google Scholar]
- Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. I. Krogh’s model. Biophys J. 2001;81:675–684. doi: 10.1016/S0006-3495(01)75732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP. HIF-2 alpha - a mediator of stem cell altruism? Stem Cell Res Ther. 2012;3:52. doi: 10.1186/scrt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gülly C, Gaßner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2010;207:331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S-M et al (2014) Enhanced proliferation and differentiation of Oct4-and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp Mol Med 46. 10.1038/emm.2014.28 [DOI] [PMC free article] [PubMed]
- Hu C, Li L. Preconditioning influences mesenchymal stem cell properties invitro and invivo. J Cell Mol Med. 2018;22:1428–1442. doi: 10.1111/jcmm.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GTJ, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z, Yang G, Shahzidi S, Tkacz-Stachowska K, Suo Z, Nesland JM, Peng Q. Induction of hypoxia-inducible factor-1 alpha overexpression by cobalt chloride enhances cellular resistance to photodynamic therapy. Cancer Lett. 2006;244:182–189. doi: 10.1016/j.canlet.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima N. Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? Arch Oral Biol. 2012;57:1439–1458. doi: 10.1016/j.archoralbio.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Kim DS, Ko YJ, Lee MW, Park HJ, Park YJ, Kim DI, Sung KW, Koo HH, Yoo KH. Effect of low oxygen tension on the biological characteristics of human bone marrow mesenchymal stem cells. Cell Stress Chaperones. 2016;21:1089–1099. doi: 10.1007/s12192-016-0733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyam N, Okubo Y, Nakao K, Bessho K. Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Surg. 2009;67:501–506. doi: 10.1016/j.joms.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Kyoko M, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- Laksana K, Sooampon S, Pavasant P, Sriarj W. Cobalt chloride enhances the stemness of human dental pulp cells. J Endod. 2017;43:760–765. doi: 10.1016/j.joen.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Landor SKJ, Lendahl U. The interplay between the cellular hypoxic response and notch signaling. Exp Cell Res. 2017;356:146–151. doi: 10.1016/j.yexcr.2017.04.030. [DOI] [PubMed] [Google Scholar]
- Li C-T, Liu J-X, Yu B, Liu R, Dong C, Li S-J. Notch signaling represses hypoxia-inducible factor-1 alpha-induced activation of Wnt/beta-catenin signaling in osteoblasts under cobalt-mimicked hypoxia. Mol Med Rep. 2016;14:689–696. doi: 10.3892/mmr.2016.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jaiswal PK, Makhoul G, Jurakhan R, Selvasandran K, Ridwan K, Cecere R. Hypoxia modulates cell migration and proliferation in placenta-derived mesenchymal stem cells. J Thoracic Cardiovasc Surg. 2017;154:543–552.e3. doi: 10.1016/j.jtcvs.2017.03.141. [DOI] [PubMed] [Google Scholar]
- Liu L, Wei X, Huang R, Ling J, Wu L, Xiao Y. Effect of bone morphogenetic protein-4 on the expression of Sox2, Oct-4, and c-Myc in human periodontal ligament cells during long-term culture. Stem Cells Dev. 2013;22:1670–1677. doi: 10.1089/scd.2012.0548. [DOI] [PubMed] [Google Scholar]
- López-Iglesias P, Alcaina Y, Tapia N, Sabour D, Arauzo-Bravo MJ, Sainz de la Maza D, Berra E, O’Mara AN, Nistal M, Ortega S, Donovan PJ, Schöler HR, de Miguel MP. Hypoxia induces pluripotency in primordial germ cells by HIF1α stabilization and Oct4 deregulation. Antioxid Redox Signal. 2015;22:205–223. doi: 10.1089/ars.2014.5871. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao MR, Lu B, Fisher LW, Robey PG, Shi ST. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Silberstein LE. The science behind the hypoxic niche of hematopoietic stem and progenitors. Hematology Am Soc Hematol Educ Program. 2014;2014:542–547. doi: 10.1182/asheducation-2014.1.542. [DOI] [PubMed] [Google Scholar]
- Osathanon T, Vivatbutsiri P, Sukarawan W, Sriarj W, Pavasant P, Sooampon S. Cobalt chloride supplementation induces stem-cell marker expression and inhibits osteoblastic differentiation in human periodontal ligament cells. Arch Oral Biol. 2015;60:29–36. doi: 10.1016/j.archoralbio.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Rombouts C, Giraud T, Jeanneau C, About I. Pulp vascularization during tooth development, regeneration, and therapy. J Dent Res. 2017;96:137–144. doi: 10.1177/0022034516671688. [DOI] [PubMed] [Google Scholar]
- Rosa V, Dubey N, Islam I, Min KS, Nor JE. Pluripotency of stem cells from human exfoliated deciduous teeth for tissue engineering. Stem Cells Int. 2016;2016:5957806–5957806. doi: 10.1155/2016/5957806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakdee JB, White RR, Pagonis TC, Hauschka PV. Hypoxia-amplified proliferation of human dental pulp cells. J Endod. 2009;35:818–823. doi: 10.1016/j.joen.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy EJ, Buckley CT, Kelly DJ. Oxygen tension regulates the osteogenic, chondrogenic and-endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun. 2012;417:305–310. doi: 10.1016/j.bbrc.2011.11.105. [DOI] [PubMed] [Google Scholar]
- Slape CI, Jesslyn S, Jowett JBM, Aplan PD, Andreas S, Jane SM, Curtis DJ. Inhibition of apoptosis by BCL2 prevents leukemic transformation of a murine myelodysplastic syndrome. Blood. 2012;120:2475–2483. doi: 10.1182/blood-2012-05-430736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan A, Waddington R. Dental pulp stem cells: what, where, how? Int J Paediatr Dent. 2010;19:61–70. doi: 10.1111/j.1365-263X.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- Teti G, Focaroli S, Salvatore V, Mazzotti E, Ingra’ L, Mazzotti A, Falconi M. The hypoxia-mimetic agent cobalt chloride differently affects human mesenchymal stem cells in their chondrogenic potential. Stem Cells Int. 2018;2018(2018-3-13):2018. doi: 10.1155/2018/3237253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C-C, Su P-F, Huang Y-F, Yew T-L, Hung S-C. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol Cell. 2012;47:169–182. doi: 10.1016/j.molcel.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu TT, Jiang L, Rong D, Zhu YQ. Deferoxamine-induced migration and odontoblast differentiation via ROS-dependent autophagy in dental pulp stem cells. Cell Physiol Biochem. 2017;43:2535–2547. doi: 10.1159/000484506. [DOI] [PubMed] [Google Scholar]
- Werle SB, Chagastelles P, Pranke P, Casagrande L. The effects of hypoxia on in vitro culture of dental-derived stem cells. Arch Oral Biol. 2016;68:13–20. doi: 10.1016/j.archoralbio.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Werle SB, Chagastelles P, Pranke P, Casagrande L. Hypoxia upregulates the expression of the pluripotency markers in the stem cells from human deciduous teeth. Clin Oral Investig. 2018;23:199–207. doi: 10.1007/s00784-018-2427-9. [DOI] [PubMed] [Google Scholar]
- Xuechao Y, X Frank W, van den Beucken JJJP, Zhuan B, Mingwen F, Jansen JA. Hard tissue formation of STRO-1-selected rat dental pulp stem cells in vivo. Tissue Eng A. 2009;15:367. doi: 10.1089/ten.tea.2008.0133. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang W, Van dDJ, Walboomers XF, Bian Z, Fan M, Jansen JA. Multilineage potential of STRO-1+ rat dental pulp cells in vitro. J Tissue Eng Regen Med. 2007;1:128–135. doi: 10.1002/term.13. [DOI] [PubMed] [Google Scholar]
- Yu CY, Boyd NM, Cringle SJ, Alder VA, Yu DY. Oxygen distribution and consumption in rat lower incisor pulp. Arch Oral Biol. 2002;47:529–536. doi: 10.1016/S0003-9969(02)00036-5. [DOI] [PubMed] [Google Scholar]
- Yu Y, Bi CS, Wu RX, Yin Y, Zhang XY, Lan PH, Chen FM. Effects of short-term inflammatory and/or hypoxic pretreatments on periodontal ligament stem cells: in vitro and in vivo studies. Cell Tissue Res. 2016;366:311–328. doi: 10.1007/s00441-016-2437-3. [DOI] [PubMed] [Google Scholar]
- Yu X, Wan Q, Cheng G, Cheng X, Zhang J, Pathak JL, Li Z. CoCl2 , a mimic of hypoxia, enhances bone marrow mesenchymal stem cells migration and osteogenic differentiation via STAT3 signaling pathway. Cell Biol Int. 2018;42:1321–1329. doi: 10.1002/cbin.11017. [DOI] [PubMed] [Google Scholar]
- Zhang P, Ha N, Dai Q, Zhou S, Yu C, Jiang L. Hypoxia suppresses osteogenesis of bone mesenchymal stem cells via the extracellular signal-regulated 1/2 and p38-mitogen activated protein kinase signaling pathways. Mol Med Rep. 2017;16:5515–5522. doi: 10.3892/mmr.2017.7276. [DOI] [PubMed] [Google Scholar]
- Zhao L, Liu X, Zhang Y, Liang X, Ding Y, Xu Y, Fang Z, Zhang F. Enhanced cell survival and paracrine effects of mesenchymal stem cells overexpressing hepatocyte growth factor promote cardioprotection in myocardial infarction. Exp Cell Res. 2016;344:30–39. doi: 10.1016/j.yexcr.2016.03.024. [DOI] [PubMed] [Google Scholar]
- Zhao D, Liu L, Chen Q, Wang F, Li Q, Zeng Q, Huang J, Luo M, Li W, Zheng Y, Liu T. Hypoxia with Wharton’s jelly mesenchyma stem cell coculture maintains stemness of umbilical cord blood-derived CD34(+) cells. Stem Cell Res Ther. 2018;9:158. doi: 10.1186/s13287-018-0902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]