Abstract
Purpose: To analyze the genus profile of isolated pathogens and antibiotic susceptibility trends of microbial keratitis over nine years at a large referral eye center in southern China.
Methods: Data of corneal specimens from January 2010 to August 2018 of patients clinically diagnosed with infectious keratitis were obtained from the center’s microbiology database. Results with positive cultures along with antibiotic susceptibility were reviewed and analyzed.
Results: We collected and reviewed 7,229 specimens, including 3,092 with positive cultures. Among them, 1,630 (52.72%) were bacterial, 1781 (57.60%) were fungal, and 319 (10.32%) were coinfected. A significant decreasing trend was observed in the isolates of Gram-positive cocci (r =−0.711, P=0.032), among which the proportion of coagulase-negative staphylococcus (CNS) was also reduced (r =−0.883, P=0.002). In contrast, an increasing trend in the proportion of Gram-negative bacilli was observed (r=0.661, P=0.053). The susceptibility rates of Gram-positive cocci to cephalosporins were near 90%, which was relatively high compared to fluoroquinolones. Fluoroquinolones represented the antibiotics to which Gram-negative bacilli were the most susceptible. Their susceptibility to moxifloxacin was 78.79%. The overall performance of aminoglycosides and vancomycin was both around 70%. The susceptibility of Gram-positive cocci to several antibiotics including levofloxacin (r=−0.717, P=0.03), tobramycin (r= −0.933, P<0.001), cefazolin (r= −0.964, P<0.001), ceftazidime (r=−0.929, P=0.003), chloramphenicol (r=−0.929, P=0.003), and cefuroxime (r=−0.829, P=0.042) decreased over time. The susceptibility of Gram-negative bacilli to ofloxacin increased over time (r=0.854, P=0.004), whereas that to cefazolin (r=−0.833, P=0.005) and chloramphenicol (r=−0.886, P=0.019) decreased over time.
Conclusion: From 2010 to 2018 in Zhongshan Ophthalmic Center, most isolates from infectious keratitis were Gram-positive cocci (mainly CNS), which decreased over time, with an increase in Gram-positive bacilli. More than half of the antibiotics showed reducing trend of susceptibilities, and the antibiotic resistance situation in southern China was not encouraging.
Keywords: infectious keratitis, bacterial keratitis, antibiotic resistance, time trend
Introduction
Microbial keratitis is an ophthalmic emergency, which can cause irreversible sight loss if not treated promptly.1 Owing to the delay associated with the results of specimen culturing, which remains the mainstay of pathogenic diagnosis of infectious keratitis, the treatment of microbial keratitis usually starts with empiric antibiotic treatment, based on clinical features of the corneal lesions and previous knowledge of the antibacterial spectrum of antibiotics.2
However, the distribution of pathogens not only varies by region but also evolves rapidly over time, depending on various risk factors.3–6 More importantly, microorganisms can gradually develop resistance following exposure to antibiotics, thereby lowering the success rate of empiric antimicrobial treatment.7
Therefore, it is necessary to update the pathogenic and antibiotic susceptibility trends associated with microbial keratitis, to provide a regional reference for initial treatment before a thorough microbiological examination. In light of this, we collected the laboratory results of infectious keratitis from January 2010 to August 2018 at the largest referral eye institute in southern China, to analyze the time trends of pathogen proportions and antibiotic susceptibilities.
Materials and methods
Chronological results of microbiological examinations of patients who had been clinically diagnosed with infectious keratitis were collected from January 2010 to August 2018. The data were obtained anonymously from the microbiology database, with the omission of personal information, and the operation of sample collection and clinical care. The study was approved by the Ethics Committee of Zhongshan Ophthalmic Center. All protocols and interpretation of the results were conducted according to the Clinical and Laboratory Standards Institute guidelines.8
All specimens were obtained from corneal lesions via scraping with a platinum spatula. Smear staining of the specimens was optional and was performed according to the orders of the clinicians. The specimens were then inoculated into bacterial or fungal media.9–11 In addition to blood agar, chocolate agar, brain heart infusion broth, thioglycolate (broth), Sabouraud agar, sheep blood agar, and potato glucose agar, anaerobic blood agar has been included among the list of media since 2017. All bacterial colonies were subjected to species identification using the VITEK 2 compact automated system (BioMérieux, Marcy l’Etoile, France). Antibiotics, including cephalosporins (cefazolin, ceftazidime, and cefuroxime sodium); fluoroquinolones (ofloxacin, levofloxacin, and moxifloxacin); aminoglycosides (tobramycin and neomycin); chloramphenicol; and vancomycin, were used for the susceptibility tests. Not every antibiotic was tested for identical quantity of specimens, because some antibiotics were suspended for one or several months, owing to various practical reasons. Vancomycin and moxifloxacin were not tested until 2017. The genera of fungi were identified by experienced microbiological technicians, according to colony characteristics, as well as microscopic characteristics of the hyphae and spores.
Statistical analysis
All analyses were performed with commercially available software (SPSS 16.0; SPSS Inc., Chicago, IL, USA). All results of culture-positive specimens were analyzed. The incubation results and susceptibility data are presented as categorical variables, expressed as percentages. Thus, differences between groups were compared using the chi-squared test. To determine the time trends of bacterial proportions and antibiotic susceptibility, the Spearman’s rank correlation coefficient was used.12–14 Gram-negative cocci and Gram-positive bacilli were omitted from the analysis, as the annual sample sizes were too small. A two-tailed Student’s t-test was used to determine statistical significance, which was set at P<0.05.
Results
A total of 7,229 specimens were collected and reviewed. Positive cultures with species identification were achieved in 3,092 (42.77%) specimens. Of the 3,092 positive cultures, 1,630 (52.72%) were positive for bacterial species, 1,781 (57.60%) were positive for fungal species, and 319 (10.32%) were co-infected with both bacteria and fungi. Otherwise, no Acanthamoeba species were isolated from specimen cultures; however, amoeba cysts were detected by smear staining in five specimens.
The genus distribution of bacteria and fungi identified in the present study are presented in Tables 1 and 2, respectively. The most common bacteria isolated were Gram-positive cocci (n=1139, 69.88%), the majority of which were coagulase-negative staphylococci (CNS) (n=885, 54.29%), the most common of which was Staphylococcus epidermidis (n=493, 30.25%). The second most frequently detected bacteria was Pseudomonas aeruginosa (n=182, 11.17%).
Table 1.
Genus distribution of bacteria isolated from 2010 to 2018 in Zhongshan Ophthalmic Center
| Genus | Specimens, n (%) |
|---|---|
| Gram-positive cocci | 1139 (69.88) |
| Coagulase-negative staphylococci (CNS) | 885 (54.29) |
| Staphylococcus epidermidis | 493 (30.25) |
| Other CNS | 392 (24.05) |
| Streptococci | 80 (4.91) |
| Staphylococcus aureus | 49 (3.01) |
| Kocuria spp. | 35 (2.15) |
| Micrococci | 24 (1.47) |
| Enterococci | 19 (1.17) |
| Tetracocci | 13 (0.80) |
| Klebsiella spp. | 11 (0.67) |
| Other Gram-positive cocci | 23 (1.41) |
| Gram-negative bacilli | 399 (24.48) |
| Pseudomonas aeruginosa | 182 (11.17) |
| Acinetobacter spp. | 43 (2.64) |
| Other Pseudomonas spp. | 52 (3.19) |
| Serratia spp. | 17 (1.04) |
| Escherichia coli | 13 (0.80) |
| Burkholderia spp. | 14 (0.86) |
| Enteric bacilli | 10 (0.61) |
| Other Gram-negative bacilli | 68 (4.17) |
| Gram-positive bacilli | 82 (5.03) |
| Bacilli subtilis | 16 (0.98) |
| Corynebacterium | 15 (0.92) |
| Propionibacterium acnes | 7 (0.43) |
| Other Gram-positive bacilli | 44 (2.70) |
| Gram-negative cocci | 10 (0.61) |
| Total | 1630 (100.00) |
Table 2.
Genus distribution of fungi isolated from 2010 to 2018 in Zhongshan Ophthalmic Center
| Genus | Specimens, n (%) |
|---|---|
| Filamentous fungi | 1690 (94.89) |
| Fusarium sp. | 728 (40.88) |
| Aspergillus sp. | 376 (21.11) |
| Mucor | 185 (10.39) |
| Helminthosporium | 149 (8.37) |
| Curvularia | 85 (4.77) |
| Penicillium sp. | 79 (4.44) |
| Alternaria sp. | 20 (1.12) |
| Other filamentous fungi | 68 (3.82) |
| Non-filamentous fungi | 91 (25.11) |
| Candida sp. | 70 (3.93) |
| Zymoid epiphyte | 19 (1.07) |
| Yeast | 2 (0.11) |
| Total | 1781 (100.00) |
The most frequently detected fungal organism was Fusarium species (n=728, 40.88%), followed by Aspergillus species (n=376, 21.11%) and Mucor (n=185, 10.39%). Among 319 dual-positive specimens, the most common bacterial component was Staphylococcus epidermidis (n=137, 42.95%), and the most frequent fungus detected was Fusarium species (n=118, 36.99%) (Table 3).
Table 3.
Frequency of dual-cultured organisms from 2010 to 2018 in Zhongshan Ophthalmic Center
| Specimens, n (%) | Fusarium sp. | Aspergillus sp. | Other filamentous fungi | Non-filamentous fungi | Total |
|---|---|---|---|---|---|
| Staphylococcus epidermidis | 41 (12.85) | 39 (12.23) | 49 (15.36) | 8 (2.51) | 137 (42.95) |
| Other CNS | 29 (29.09) | 10 (3.13) | 23 (7.21) | 0 (0.00) | 62 (19.44) |
| Other bacteria | 29 (29.09) | 12 (3.76) | 46 (14.42) | 0 (0.00) | 87 (27.27) |
| Pseudomonas aeruginosa | 19 (5.96) | 7 (2.19) | 7 (2.19) | 0 (0.00) | 33 (10.34) |
| Total | 118 (36.99) | 68 (21.32) | 125 (39.18) | 8 (2.51) | 319 (100.00) |
Abbreviation: CNS, coagulase negative staphylococcus.
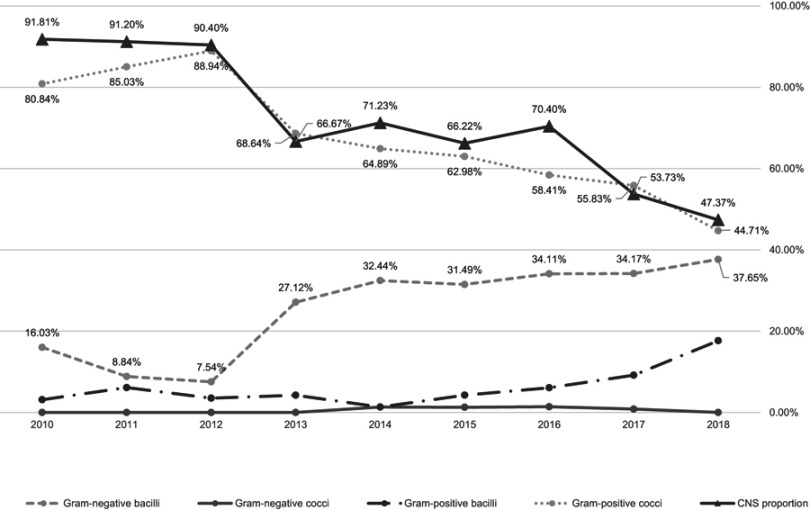
As shown in Figure 1, there was a significant trend of decline in the number of isolates of Gram-positive cocci (r =−0.711, P=0.032) recovered during the study period. Moreover, the proportion of CNS among the Gram-positive cocci also presented a similar trend of decline (r =−0.883, P=0.002). In contrast, we observed an increasing trend in the percentage of recovered Gram-negative bacilli (r =0.661, P=0.053).
Figure 1.
Time trends of bacteria isolated from 2010 to 2018 in Zhongshan Ophthalmic Center. There was a significant decreasing trend in the isolates of Gram-positive cocci (r=−0.711, P=0.032) from 2010 to 2018. Meanwhile, the proportion of coagulase-negative staphylococcus (CNS) among the Gram-positive cocci also presented a similar decreasing trend (r=−0.883, P=0.002). On the other hand, a statistically suggestive increasing trend in the percentage of recovered Gram-negative bacilli was observed (r=0.661, P=0.053).
Detailed susceptibility rates of isolated bacteria are presented in Table 4. The susceptibility of Gram-positive cocci to cephalosporins was close to 90%, which was relatively high in comparison to fluoroquinolones. Gram-negative bacilli showed the greatest susceptibility to fluoroquinolones, with a rate of 78.79% to moxifloxacin. However, Gram-negative bacilli showed low sensitivity to cephalosporins, especially cefazolin. The overall sensitivity to aminoglycosides was around 70%. In addition, sensitivity to vancomycin was also around 70%, which showed no considerable differences from the overall sensitivity to other antibiotics.
Table 4.
Overall susceptibility rate of isolated bacteria to different antibiotics in Zhongshan Ophthalmic Center
| Susceptibility rate | Gram-positive cocci | Gram-negative bacilli | Gram-negative cocci | Gram-positive bacilli | Total specimens |
|---|---|---|---|---|---|
| Levoflaxacin | 62.79% (697/1110) |
75.27% (283/376) |
50% (4/8) |
71.43% (55/77) |
66.14% (1039/1571) |
| Tobramycin | 64.92% (692/1066) |
71.09% (268/377) |
66.67% (6/9) |
51.22% (42/82) |
65.71% (1008/1534) |
| Neomycin | 77.89% (775/995) |
71.00% (191/269) |
62.50% (5/8) |
80.33% (49/61) |
76.52% (1020/1333) |
| Cefazolin | 89.60% (741/827) |
21.25% (75/353) |
75.00% (3/4) |
47.62% (20/42) |
68.43% (839/1226) |
| Ceftazidime | 85.13% (704/827) |
50.71% (179/353) |
50% (2/4) |
45.24% (19/42) |
73.74% (904/1226) |
| Ofloxacin | 70.11% (624/890) |
58.22% (124/213) |
42.86% (3/7) |
75.36% (52/69) |
68.11% (803/1179) |
| Chloramphenicol | 69.57% (599/861) |
72.09% (129/179) |
75% (3/4) |
81.58% (31/38) |
70.43% (762/1082) |
| Cefuroxime | 90.94% (572/629) |
67.94% (89/131) |
75% (3/4) |
84.09% (37/44) |
86.76% (701/808) |
| Moxifloxacin | 60.22% (112/186) |
78.79% (26/33) |
/ | 66.67% (4/6) |
63.11% (142/225) |
| Vancomycin | 72.49% (137/189) |
64.71% (22/34) |
/ | 83.33% (5/6) |
71.62% (164/229) |
Notes: The numerator in the parenthesis represents the number of specimens sensitive to the listed antibiotic, and the denominator represents the number of specimens that underwent susceptibility test with the listed antibiotics.
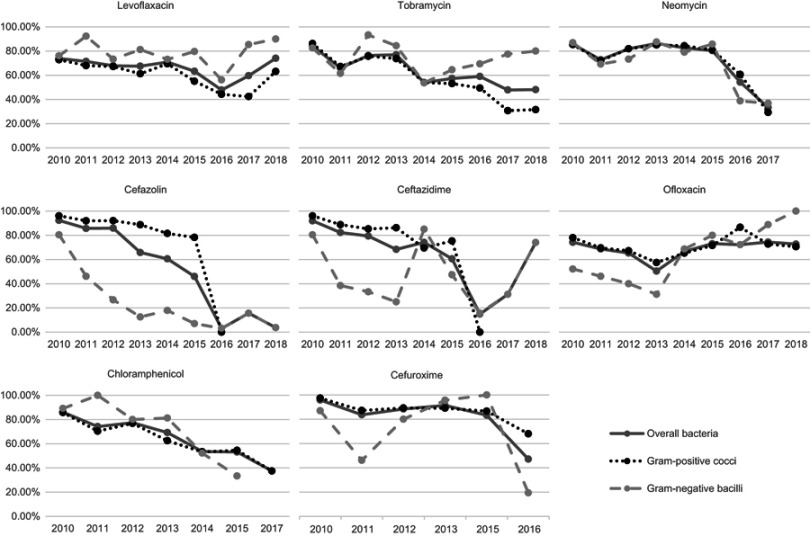
As presented in Figure 2, most antibiotics manifested a roughly similar trend of decline in bacterial susceptibility, with the exception of ofloxacin. Some data were absent for neomycin, chloramphenicol and cefuroxime, as susceptibility tests were suspended for some periods. The Spearman’s rank correlation coefficients (r) representing time trends in antibiotic susceptibility are summarized in Table 5.
Figure 2.
Time trends of bacterial susceptibilities from 2010 to 2018 in Zhongshan Ophthalmic Center. Most of the antibiotics manifested decreasing trends of susceptibility, except ofloxacin. Some data were absent for neomycin, chloramphenicol, and cefuroxime, as susceptibility tests were suspended for some periods. Time trend analysis of Gram-negative cocci and Gram-positive bacilli were omitted due to small sample sizes.
Table 5.
Spearman rank correlation coefficients of antibiotic susceptibility by year
| r Value | Overall bacteria | Gram-positive cocci | Gram-negative bacilli |
|---|---|---|---|
| Levoflaxacin | −0.333 | −0.717 * | −0.083 |
| Tobramycin | −0.850 ** | −0.933 * | −0.167 |
| Neomycin | −0.619 | −0.690 | −0.524 |
| Cefazolin | −0.933 ** | −0.964 ** | −0.833 ** |
| Ceftazidime | −0.783 * | −0.929 ** | −0.200 |
| Ofloxacin | 0.317 | 0.233 | 0.817 ** |
| Chloramphenicol | −0.946 ** | −0.929 ** | −0.886 * |
| Cefuroxime | −0.771 | −0.829 * | −0.029 |
Notes: Time trend analyses of Gram-negative cocci and Gram-positive bacilli were not performed due to small sample sizes. *P<0.05, **P<0.01.
Susceptibility of Gram-positive cocci to several antibiotics declined from 2010 to 2018, including levofloxacin (r =−0.717, P=0.03), tobramycin (r =−0.933, P<0.001), cefazolin (r =−0.964, P<0.001), ceftazidime (r =−0.929, P=0.003), chloramphenicol (r =−0.929, P=0.003), and cefuroxime (r =−0.829, P=0.042). The susceptibility rates of Gram-negative bacilli to levofloxacin and tobramycin showed no significant changes. Susceptibility to ofloxacin increased from 2010 to 2018 (r =0.854, P=0.004). Susceptibility rates to cefazolin (r =−0.833, P=0.005) and chloramphenicol (r =−0.886, P=0.019) both showed a decline over the same period.
Discussion
Even though new technologies, such as polymerase chain reaction, in-vivo confocal microscopy, and next-generation sequencing, have been applied in some studies and have even been used in some clinical institutions, the pathogenic diagnosis of infectious keratitis by specimen culturing remains the mainstay, owing to the various practical limitations of new technologies.15–17 As culture is a time-consuming job, the time trends of pathogens and antibiotic sensitivity remain important for effective guidance in the initial treatment of infectious keratitis in practice.
Organism distribution and time trends of microbial proportions
In the present study, fungi accounted for slightly more than half of the isolated organisms, which is consistent with the findings of our previous study (58.14%).9 Although the main etiologies of microbial keratitis are diverse worldwide, reports of fungal proportions are fairly consistent among various studies in northern China and other developing regions in Asia (46.21~81.95%).18–21 The populations of contact lens wearers are much higher in developed regions. As a result, contact lens-related bacterial keratitis is more commonly detected in these regions than in other developing countries.21–23
Gram-positive cocci comprise the predominant bacteria detected in most studies of bacterial keratitis, including the present study.18,21,24–26 The majority of Gram-positive cocci are CNS, which are opportunistic pathogens that commonly colonize the healthy ocular surface and usually cause impairment under conditions of trauma or hypoimmunitiy. A recent study of the ocular surface microbiome provided relevant evidence via sequencing of the samples harvested from the inferior bulbar conjunctiva and reported that Staphylococcus epidermidis was recovered from 73% of the healthy subjects.27 In the present study, CNS was more commonly detected in coinfected specimens, manifesting the opportunistic character of CNS. Therefore, positive culture results with CNS do not necessarily determine the etiology of infectious keratitis, and a preponderance of Gram-positive cocci should be interpreted with caution.
In the present study, we discovered a declining shift in CNS isolation, which might lead to a similar trend in Gram-positive cocci. Sun and co-authors reported a nearly opposite shifting trend in bacterial proportions in northern China from 2006 to 2015.13 Diversity in the trends of bacterial shifts among different regions are not uncommon. Many studies have demonstrated that topical antibiotic usage might alter the populations of conjunctival microflora, especially those of opportunistic pathogens, which can also be influenced by long-term usage of contact lenses or environmental pollution.28–30 Nevertheless, the increasing trend in the proportion of Gram-negative bacilli recovered in the present study is notable, as these species usually cause more severe consequences, owing to their comparatively higher levels of antibiotic resistance and strong virulence.31
Profiles and time trends of bacterial susceptibilities to antibiotics
Antibiotic resistance is a worldwide challenge. Overall susceptibility rates show compromise at some level, both in overall bacterial and in species-specific tests. Gram-positive cocci showed the highest susceptibility rates to cephalosporins (close to 90%). These results are higher than those of a report from northern China, in which susceptibility rates to ceftazidime were less than 50% from 2006 to 2015.13 However, the susceptibility rate of Gram-positive cocci to vancomycin in the present study were considerably low compared to other reports from northern China and other regions (around 90~100%).2,12,13 Vancomycin is considered the last antibiotic effective for Gram-positive bacterial infections. Even though no data has been presented regarding methicillin-resistant Gram-positive cocci in the present study, as we did not routinely test for methicillin or oxacillin, there is no need to emphasize the burden of antibiotic resistance burden revealed by prominent vancomycin resistance.
Gram-negative bacteria were most susceptible to fluoroquinolones and aminoglycosides in the present study. However, ofloxacin, which has been classified as a first-line antibiotic for the treatment of bacterial keratitis in the UK,22 was merely half as effective. Furthermore, most of the susceptibility rates determined in the present study were lower than those of other reports worldwide.3,12,22,32,33 These low susceptibility rates might be associated with the increasing trend of Gram-negative bacilli infections, as antibiotic-resistant keratitis is more difficult to treat.
More than half of the antibiotics evaluated in the present study yielded a trend of reducing susceptibility, which was not comparable with the results of other reports on keratitis. In northern China, the susceptibility of bacterial keratitis showed no decline from 2006 to 2015.13 Lalitha et al33 and Tan et al22 have also reported that antibiotic resistance rates were generally stable over a decade in southern India and the UK, respectively. However, antibiotic resistance is not an independent issue restricted to the discipline of ophthalmology. Antibiotic susceptibilities reported in different studies from the same region might corroborate each other. A recent study from Guangzhou showed an increasing trend of multidrug-resistant bacteria in neonatal invasive infections.34 Another study conducted in Guangzhou also reported the high and continuously increasing resistance rates among Gram-positive bacteria.35 Furthermore, the need for antibiotic usage in agricultural and animal farming is growing along with economic growth in China. Zhang et al36 reported that 92,700 tons of antibiotics were consumed in China in 2013 alone, with approximately even distribution between animals and humans. Considerable usage of antibiotics in China has become a huge burden to the ecological environment. Studies have demonstrated that antibiotic resistance is substantially increased in various aspects of the environment, such as water, soil, plants, and animals.37–40 Furthermore, antibiotic resistance genes might flow and accumulate along the food chain.41,42 The urgent need for antibiotic control has long drawn the attention of health management officials. However, with the establishment of a nationwide surveillance system aimed at gathering information regarding antibiotic resistance,43 and policies aimed at reducing the abuse of antibiotics, the control of antibiotic resistance remains a long, arduous, uphill struggle.
Our study had potential limitations. First, as a referral hospital-based study, the results might have been affected by sampling bias. Second, the lack of Acanthamoeba-specific media is a limitation that cannot be ignored, as Acanthamoeba keratitis is an important type of refractory keratitis. Moreover, we did not present the clinical characteristics of the pathogens or analyze the relationship between the pathogens and clinical outcomes. Nonetheless, we have added one more piece to the puzzle of the current situation regarding antibiotic susceptibility, by providing a general overview of microbial keratitis at a large referral eye center in southern China.
Conclusions
During the period from 2010 to 2018, the proportion of Gram-positive cocci (mainly CNS) isolated from cases of keratitis comprised the majority, yet showed a trend of decline, whereas Gram-negative bacilli are suspected to be increasing in number at the largest eye center in southern China. More than half of the antibiotics under investigation yielded a reducing trend of susceptibility. Thus, antibiotic resistance in southern China remains a considerable challenge, and the urgent implementation of more policies regarding its control is warranted.
Acknowledgments
This study was supported by grants from Fundamental Research Funds of the State Key Laboratory of Ophthalmology (30306020240020130, 3030902113030), and a grant from the Natural Science Foundation of Guangdong Province (2018A030313585).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Limberg MB. A review of bacterial keratitis and bacterial conjunctivitis. Am J Ophthalmol. 1991;112(4 Suppl):2S–9S. [PubMed] [Google Scholar]
- 2.Lin A, Rhee MK, Akpek EK, et al. Bacterial keratitis preferred practice pattern(R). Ophthalmology. 2019;126(1):P1–P55. doi: 10.1016/j.ophtha.2018.10.018 [DOI] [PubMed] [Google Scholar]
- 3.Sun X, Deng S, Li R, et al. Distribution and shifting trends of bacterial keratitis in north China (1989-98). Br J Ophthalmol. 2004;88(2):165–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong J, Chen J, Sun X, et al. Paediatric bacterial keratitis cases in Shanghai: microbiological profile, antibiotic susceptibility and visual outcomes. Eye (Lond). 2012;26(12):1571–1578. doi: 10.1038/eye.2012.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walkden A, Fullwood C, Tan SZ, et al. Association between season, temperature and causative organism in microbial keratitis in the UK. Cornea. 2018;37(12):1555–1560. doi: 10.1097/ICO.0000000000001748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997;81(11):965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SJ, Toma HS. Ophthalmic antibiotics and antimicrobial resistance a randomized, controlled study of patients undergoing intravitreal injections. Ophthalmology. 2011;118(7):1358–1363. doi: 10.1016/j.ophtha.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 8.NCCLS CaLSIF. Performance standards for antimicrobial susceptibility testing: 16th Informational supplement;.Vol. 25. [Google Scholar]
- 9.Lin L, Lan W, Lou B, et al. Genus distribution of bacteria and fungi associated with keratitis in a large eye center located in Southern China. Ophthalmic Epidemiol. 2017;24(2):90–96. doi: 10.1080/09286586.2016.1254250 [DOI] [PubMed] [Google Scholar]
- 10.Long C, Liu B, Xu C, Jing Y, Yuan Z, Lin X. Causative organisms of post-traumatic endophthalmitis: a 20-year retrospective study. BMC Ophthalmol. 2014;14:34. doi: 10.1186/1471-2415-14-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan F, Yang Y, Yuan Z, Zheng Y, Cheng Z, Lin X. Clinical features and visual acuity outcomes in culture-positive endogenous fungal endophthalmitis in Southern China. J Ophthalmol. 2017;2017:3483497. doi: 10.1155/2017/3483497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Camarena JC, Graue-Hernandez EO, Ortiz-Casas M, et al. Trends in microbiological and antibiotic sensitivity patterns in infectious keratitis: 10-year experience in Mexico City. Cornea. 2015;34(7):778–785. doi: 10.1097/ICO.0000000000000428 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Wang ZQ, Sun XG. [Etiological analysis and in vitro drug sensitivity of bacterial keratitis in northern China in the period of 2006-2015]. Zhonghua Yan Ke Za Zhi. 2017;53(9):662–667. doi: 10.3760/cma.j.issn.0412-4081.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 14.Lichtinger A, Yeung SN, Kim P, et al. Shifting trends in bacterial keratitis in Toronto: an 11-year review. Ophthalmology. 2012;119(9):1785–1790. doi: 10.1016/j.ophtha.2012.03.031 [DOI] [PubMed] [Google Scholar]
- 15.Chidambaram JD, Prajna NV, Palepu S, et al. In vivo confocal microscopy cellular features of host and organism in bacterial, fungal, and acanthamoeba keratitis. Am J Ophthalmol. 2018;190:24–33. doi: 10.1016/j.ajo.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inata K, Miyazaki D, Uotani R, et al. Effectiveness of real-time PCR for diagnosis and prognosis of varicella-zoster virus keratitis. Jpn J Ophthalmol. 2018;62(4):425–431. doi: 10.1007/s10384-018-0604-7 [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Ohashi Y. Utility of real-time PCR analysis for appropriate diagnosis for keratitis. Cornea. 2013;32(Suppl 1):S71–S76. doi: 10.1097/ICO.0b013e3182a2c79f [DOI] [PubMed] [Google Scholar]
- 18.Pan XJ, Jiang T, Zhu H, Liu PP, Zhou ZY, Mao AJ. Corneal infection in Shandong peninsula of China: a 10-year retrospective study on 578 cases. Int J Ophthalmol. 2016;9(1):53–57. doi: 10.18240/ijo.2016.01.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943–1948. doi: 10.1016/j.ophtha.2006.05.035 [DOI] [PubMed] [Google Scholar]
- 20.Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi PR. Aetiological diagnosis of microbial keratitis in South India - a study of 1618 cases. Indian J Med Microbiol. 2002;20(1):19–24. [PubMed] [Google Scholar]
- 21.Khor WB, Prajna VN, Garg P, et al. The Asia cornea society infectious keratitis study: a prospective multicenter study of infectious Keratitis in Asia. Am J Ophthalmol. 2018;195:161–170. doi: 10.1016/j.ajo.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 22.Tan SZ, Walkden A, Au L, et al. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye (Lond). 2017;31(8):1229–1236. doi: 10.1038/eye.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ung L, Bispo PJ, Shanbhag SS, Gilmore MS, Chodosh J. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2018. doi: 10.1016/j.survophthal.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Termote K, Joe AW, Butler AL, et al. Epidemiology of bacterial corneal ulcers at tertiary centres in Vancouver. B.C. Can J Ophthalmol. 2018;53(4):330–336. doi: 10.1016/j.jcjo.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 25.Jeng BH, Gritz DC, Kumar AB, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128(8):1022–1028. doi: 10.1001/archophthalmol.2010.144 [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93(10):1319–1324. doi: 10.1136/bjo.2008.151167 [DOI] [PubMed] [Google Scholar]
- 27.Wen X, Miao L, Deng Y, et al. The influence of age and sex on ocular surface microbiota in healthy adults. Invest Ophthalmol Vis Sci. 2017;58(14):6030–6037. doi: 10.1167/iovs.17-22957 [DOI] [PubMed] [Google Scholar]
- 28.Liesegang TJ. Contact lens-related microbial keratitis: part II: pathophysiology. Cornea. 1997;16(3):265–273. [PubMed] [Google Scholar]
- 29.Golofit-Szymczak M, Gorny RL, Lawniczek-Walczyk A, Cyprowski M, Stobnicka A. Bacterial and fungal aerosols in the work environment of cleaners. Med Pr. 2015;66(6):779–791. doi: 10.13075/mp.5893.00349 [DOI] [PubMed] [Google Scholar]
- 30.Montan PG, Setterquist H, Marcusson E, Rylander M, Ransjo U. Preoperative gentamicin eye drops and chlorhexidine solution in cataract surgery. Experimental and clinical results. Eur J Ophthalmol. 2000;10(4):286–292. doi: 10.1177/112067210001000403 [DOI] [PubMed] [Google Scholar]
- 31.Adler A, Friedman ND, Marchaim D. Multidrug-resistant gram-negative bacilli: infection control implications. Infect Dis Clin North Am. 2016;30(4):967–997. doi: 10.1016/j.idc.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 32.Hsu HY, Ernst B, Schmidt EJ, Parihar R, Horwood C, Edelstein SL. Laboratory results, epidemiologic features, and outcome analyses of microbial keratitis: a 15-year review from St. Louis. Am J Ophthalmol. 2019;198:54–62. doi: 10.1016/j.ajo.2018.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lalitha P, Manoharan G, Karpagam R, et al. Trends in antibiotic resistance in bacterial keratitis isolates from South India. Br J Ophthalmol. 2017;101(2):108–113. doi: 10.1136/bjophthalmol-2016-308487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao K, Guan X, Zeng L, et al. An increasing trend of neonatal invasive multidrug-resistant group B streptococcus infections in southern China, 2011-2017. Infect Drug Resist. 2018;11:2561–2569. doi: 10.2147/IDR.S178717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z, Xie J, Peters BM, et al. Longitudinal surveillance on antibiogram of important Gram-positive pathogens in Southern China, 2001 to 2015. Microb Pathog. 2017;103:80–86. doi: 10.1016/j.micpath.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 36.Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol. 2015;49(11):6772–6782. doi: 10.1021/acs.est.5b00729 [DOI] [PubMed] [Google Scholar]
- 37.Verraes C, Van Boxstael S, Van Meervenne E, et al. Antimicrobial resistance in the food chain: a review. Int J Environ Res Public Health. 2013;10(7):2643–2669. doi: 10.3390/ijerph10072643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greig J, Rajic A, Young I, Mascarenhas M, Waddell L, LeJeune J. A scoping review of the role of wildlife in the transmission of bacterial pathogens and antimicrobial resistance to the food Chain. Zoonoses Public Health. 2015;62(4):269–284. doi: 10.1111/zph.12147 [DOI] [PubMed] [Google Scholar]
- 39.Zou S, Xu W, Zhang R, Tang J, Chen Y, Zhang G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: impacts of river discharge and aquaculture activities. Environ Pollut. 2011;159(10):2913–2920. doi: 10.1016/j.envpol.2011.04.037 [DOI] [PubMed] [Google Scholar]
- 40.Hu Y, Gao GF, Zhu B. The antibiotic resistome: gene flow in environments, animals and human beings. Front Med. 2017;11(2):161–168. doi: 10.1007/s11684-017-0531-x [DOI] [PubMed] [Google Scholar]
- 41.Hu Y, Yang X, Li J, et al. The bacterial mobile resistome transfer network connecting the animal and human microbiomes. Appl Environ Microbiol. 2016;82(22):6672–6681. doi: 10.1128/AEM.01802-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiao M, Ying GG, Singer AC, Zhu YG. Review of antibiotic resistance in China and its environment. Environ Int. 2018;110:160–172. doi: 10.1016/j.envint.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 43.Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect. 2016;22(Suppl 1):S9–S14. doi: 10.1016/j.cmi.2016.01.001 [DOI] [PubMed] [Google Scholar]