Abstract
Diabetes develops due to deficient functional β cell mass, insulin resistance, or both. Yet, various challenges in understanding the mechanisms underlying diabetes development in vivo remain to be overcome owing to the lack of appropriate intravital imaging technologies. To meet these challenges, we have exploited the anterior chamber of the eye (ACE) as a novel imaging site to understand diabetes basics and clinics in vivo. We have developed a technology platform transplanting pancreatic islets into the ACE where they later on can be imaged non-invasively for long time. It turns out that the ACE serves as an optimal imaging site and provides implanted islets with an oxygen-rich milieu and an immune-privileged niche where they undergo optimal engraftment, rich vascularization and dense innervation, preserve organotypic features and live with satisfactory viability and functionality. The ACE technology has led to a series of significant observations. It enables in vivo microscopy of islet cytoarchitecture, function and viability in the physiological context and intravital imaging of a variety of pathological events such as autoimmune insulitis, defects in β cell function and mass and insulin resistance during diabetes development in a real-time manner. Furthermore, application of the ACE technology in humanized mice and non-human primates verifies translational and clinical values of the technology. In this article, we describe the ACE technology in detail, review accumulated knowledge gained by means of the ACE technology and delineate prospective avenues for the ACE technology.
Keywords: Autoimmune insulitis, Diabetes mellitus, Insulin resistance, In vivo imaging, Pancreatic islet, The anterior chamber of the eye
Abbreviations: ACE, The anterior chamber of the eye; Ad, Adenoviral; B6, C57BL/6; βFLUOMETRI, β Cell fluorescence metabolic transcriptional-response indicator; βIRB, β Cell insulin resistance biosensor; CaV, Voltage-gated Ca2+; [Ca2+]i, Cytosolic free calcium concentration; CFP, Cyan fluorescent protein; DC, Dendritic cell; GFP, Green fluorescent protein; HA, Influenza hemagglutinin; HFD, High fat diet; HFrD, High fructose diet; HSD, High sucrose diet; IEQ, Islet equivalent; iPSC, Induced pluripotent stem cell; KATP, Adenosine triphosphate-sensitive K+; MHC, Major histocompatibility complex; MIP, Mouse insulin promoter; ND, Normal diet; NOD, Non-obese diabetic; ob/ob, Homozygous for the obese spontaneous mutation; ob/+, Heterozygous for the obese spontaneous mutation; Rag2−/−, Recombination activating gene 2-deficient; RIP, Rat insulin promoter; SCID, Severe combined immunodeficiency mutation; STZ, Streptozotocin; TCR-HA, T cell receptor α/β chains specific for the MHC Class II I-Ed-restricted determinant site 1 of HA; Teff, Effector T; Tg, Transgenic; Treg, Regulatory T; T1D, Type 1 diabetes; T2D, Type 2 diabetes
1. Introduction
Diabetes is a heterogeneous disease characterized by hyperglycemia and caused by functional β cell mass deficiency, insulin resistance or both (American Diabetes Association, 2014; Danaei et al., 2011; DiMeglio, Evans-Molina, & Oram, 2018; Inzucchi, 2012; Katsarou et al., 2017). This heterogeneous disease involves multiple etiological factors and complex molecular and cellular pathogenesis, undergoes sophisticated dynamic progression and responds to different medications inconsistently (American Diabetes Association, 2014; Danaei et al., 2011; DiMeglio et al., 2018; Inzucchi, 2012; Katsarou et al., 2017; Nathan, 2015; Tahrani, Barnett, & Bailey, 2016; Tauschmann & Hovorka, 2018). Diabetes is mainly classified into type 1 and type 2 diabetes (T1D and T2D) (American Diabetes Association, 2014; Danaei et al., 2011; DiMeglio et al., 2018; Inzucchi, 2012; Katsarou et al., 2017; Nathan, 2015). T1D makes up about 5–10% of diagnosed cases of diabetes. It results from a T-cell mediated autoimmune attack on β cells as evidenced by the presence of an inflammatory infiltrate in the islets and a strong linkage with certain alleles of the major histocompatibility complex (MHC) class II genes. This T cell-mediated autoimmune disease is also imprinted with autoantibodies that react with islet cell autoantigens. Complex autoimmune events progressively drive autoimmune insulitis characterized by β cell destruction, eventually leading to the absolute loss of β cells and hyperglycemia onset (DiMeglio et al., 2018; Katsarou et al., 2017; Kopan et al., 2018; Pugliese, 2017). T2D accounts for about 90–95% of diagnosed cases of diabetes (American Diabetes Association, 2014; Danaei et al., 2011; Inzucchi, 2012; Nathan, 2015). The whole picture of T2D becomes more and more complex (American Diabetes Association, 2014; Danaei et al., 2011; Inzucchi, 2012; Nathan, 2015). Nevertheless, T2D can succinctly been deemed as a chronic hyperglycemic state caused by insulin resistance and/or inadequate functional β cell mass (American Diabetes Association, 2014; Danaei et al., 2011; Inzucchi, 2012; Nathan, 2015). Actually, T2D is a systemic degenerative disease that affects numerous organs and tissues and even the entire body and is also termed a lifestyle disease since adverse lifestyles act as a key driver of T2D on top of unhealthy inherited traits (Cornelis & Hu, 2012; Kligler, 2004). However, a variety of questions about diabetes remain to be solved owing to the lack of appropriate in vivo studies and the fact that in vitro findings cannot simply be extrapolated to in vivo situations (Halban et al., 2014; Katsarou et al., 2017; Leibiger, Caicedo, & Berggren, 2012; Rhodes, 2005; Weigert, Sramkova, Parente, Amornphimoltham, & Masedunskas, 2010). Among these questions, the in vivo dynamics of β cell architecture, function and viability concomitant with diabetes progression have since long been the most important and challenging (Halban et al., 2014; Rhodes, 2005). To meet this challenge, it is important to find ways to implement non-invasive, longitudinal experiments on pancreatic islets in live animals and humans at high-resolution.
The body's tissues/organs including islets behave differently in vivo versus in vitro (Barker, Leibiger, & Berggren, 2013; Leibiger & Berggren, 2017; Weigert et al., 2010). However, in vivo and in situ visualization of islets is not practical with non-invasive optical approaches since the islets are deeply embedded in the pancreas and covered by the opaque exocrine pancreas, other tissues and organs as well as the abdominal wall. This obstacle has complicated our understanding of the dynamic cytoarchitecture, function and viability of islets in vivo since the discovery of this micro-organ by Paul Langerhans in 1869 (Langerhans, 1869; Ramirez-Dominguez, 2016). Available knowledge shows that the anterior chamber of the eye (ACE) is the only optically accessible site in the body and equipped with the most suitable islet habitat iris where there are rich vasculature and autonomic nerve endings as well as an oxygen-rich milieu and an immune-privileged niche (Fig. 1) (Cunha-Vaz, 1979; Freddo, 1996; Hayreh & Scott, 1978; McDougal & Gamlin, 2015; Meek, 2009; Meek & Knupp, 2015; Sharifipour, Idani, Zamani, Helmi, & Cheraghian, 2013; Streilein, Wilbanks, Taylor, & Cousins, 1992; Zhou & Caspi, 2010). After careful consideration of the optical and biological features of the ACE, we have decided to take advantage of the ACE to establish a unique in vivo approach by combining intraocular islet transplantation and confocal/multiphoton microscopy, herein termed the ACE technology (Fig. 2) (Speier et al., 2008; Speier et al., 2008). We have succeeded in developing the nearly non-invasive technique for transplanting islets into the ACE and the ACE-based imaging technique for visualizing intraocular islets under healthy and diabetic conditions in a non-invasive, longitudinal and in vivo real-time manner (Abdulreda et al., 2011; Abdulreda & Berggren, 2013; Abdulreda, Caicedo, & Berggren, 2013; Abdulreda, Rodriguez-Diaz, Caicedo, & Berggren, 2016; Ali et al., 2016; Almaca et al., 2014; Avall et al., 2015; Diez et al., 2017; Faleo, Berggren, & Pileggi, 2014; Ilegems et al., 2013; Ilegems et al., 2015; Johansson et al., 2015; Juntti-Berggren, Ali, & Berggren, 2015; Lee et al., 2018; Leibiger et al., 2012; Leibiger & Berggren, 2017; Leibiger, Brismar, & Berggren, 2010; Miska et al., 2014; Nyqvist et al., 2011; Paschen et al., 2016; Paschen et al., 2018; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Schmidt-Christensen et al., 2013; Shalaly et al., 2016; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008; van Krieken et al., 2017). We and others have tackled a series of issues in the diabetes arena by employing the ACE technology (Fig. 2) (Abdulreda et al., 2011; Abdulreda et al., 2016; Almaca et al., 2014; Avall et al., 2015; Berclaz et al., 2016; Chen et al., 2016; Chmelova et al., 2015; Faleo et al., 2014; Juntti-Berggren et al., 2015; Lee et al., 2018; Miska et al., 2014; Mojibian et al., 2013; Paschen et al., 2016; Paschen et al., 2018; Perez et al., 2011; Schmidt-Christensen et al., 2013; van Krieken et al., 2017).
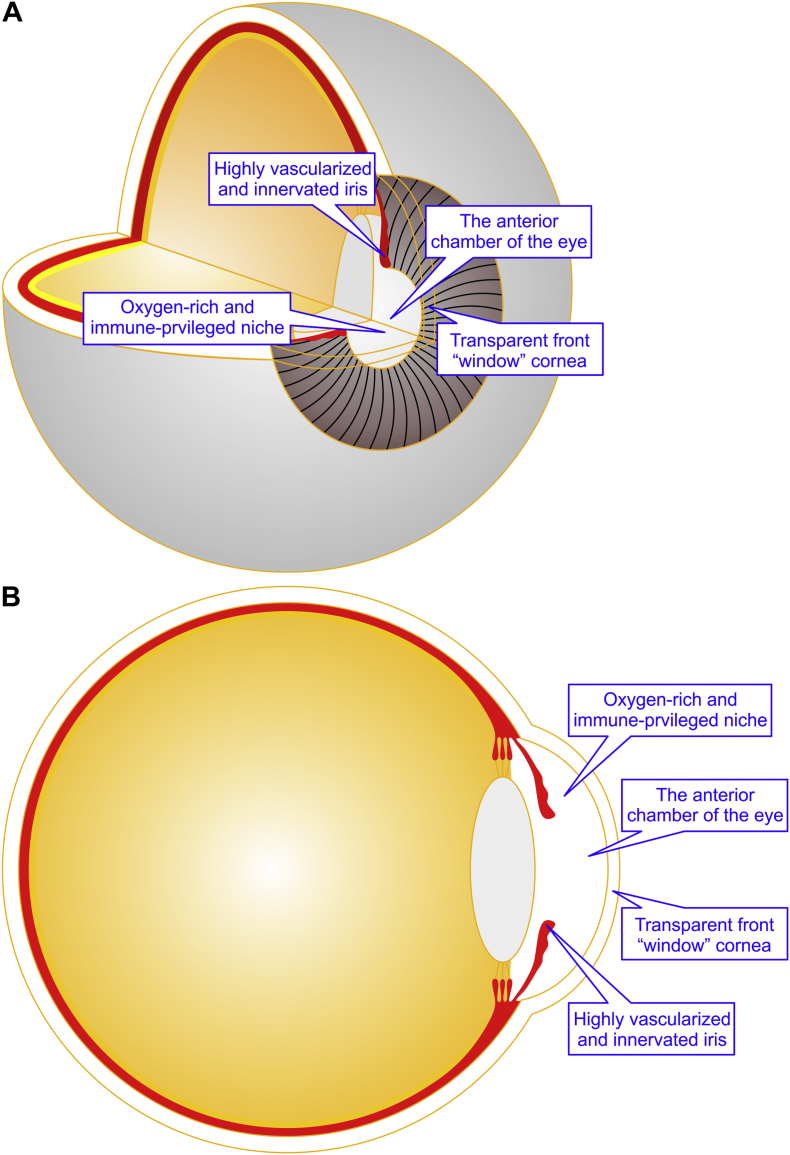
Fig. 1.
Schematic representation of the anterior chamber of the eye (ACE). The ACE is endowed with the transparent front “window” cornea, highly vascularized and innervated iris as well as an oxygen-rich milieu and an immune-privileged niche. (A) The 3/4 view of the eyeball. (B) The 1/2 sagital view of the eyeball.
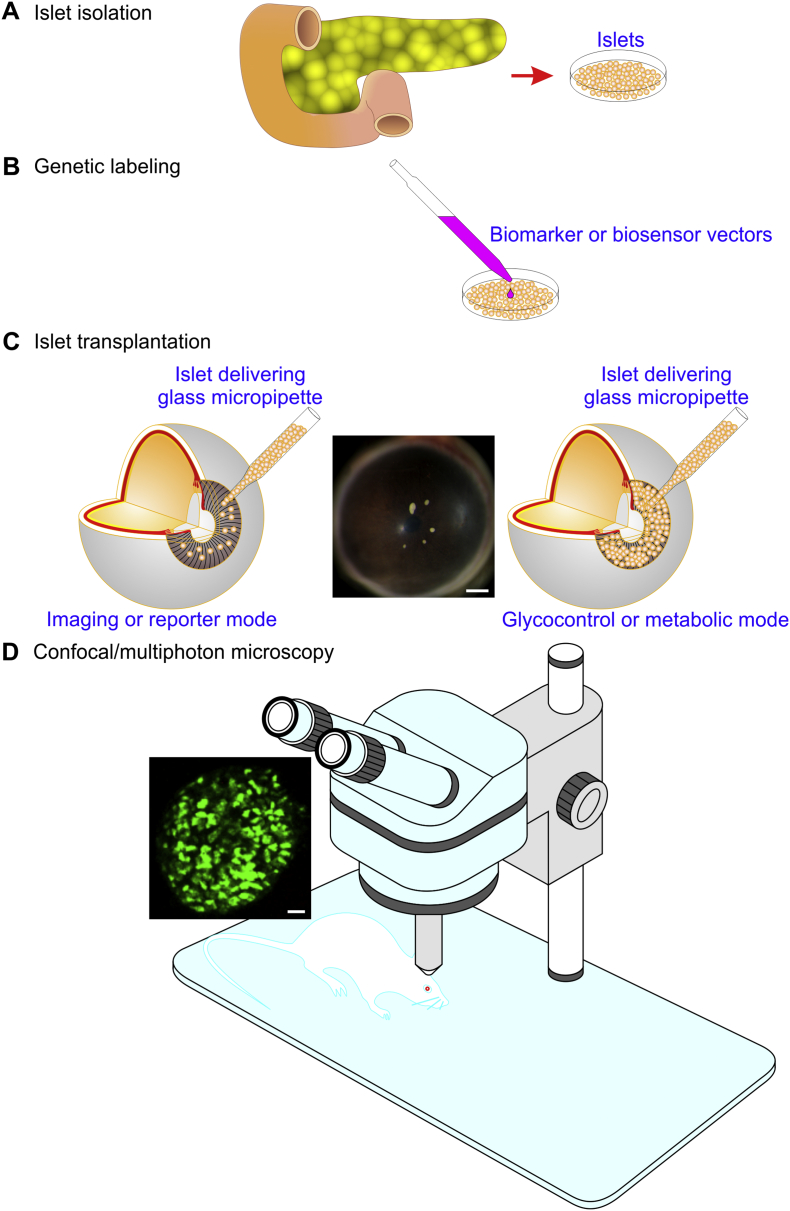
Fig. 2.
A scheme illustrating the practical procedures of the ACE technology. Typically, they include the folowing four steps. (A) Isolation of islets from the pancreas. (B) Genetic labeling of islets with biomarkers or biosensors. (C) Transplantation of islets into the ACE either in the imaging or reporter mode (left panel) or in the glycocontrol or metabolic mode (right panel). A stereomicroscopic photograph showing 5 B6 mouse islets implanted into the B6 mouse ACE (middle pannel). Calbriation bar = 500 μm. (D) Confocal/multiphoton microscopy of islets. A sample confocal image (left insert) showing a MIP-GFP islet engrafted on the MIP-GFP mouse ACE. Calibration bar = 20 μm. The ACE technology consists of two parts, namely the non-invasive technique for transplanting islets into the ACE including the first three steps and the ACE-based imaging technique of intraocular islets constituted by the last step.
In this article, we discuss the methodological details, merits and pitfalls of the ACE technology, review accumulated knowledge on islet biology and diabetes by using this technology and address the future directions of this technology.
2. The ACE technology
The ACE technology comprises two parts, namely transplantation of islets into the ACE and imaging of islets engrafted in the ACE (Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008). Their methodological details, merits and pitfalls are discussed in this section (Fig. 2, Fig. 3).
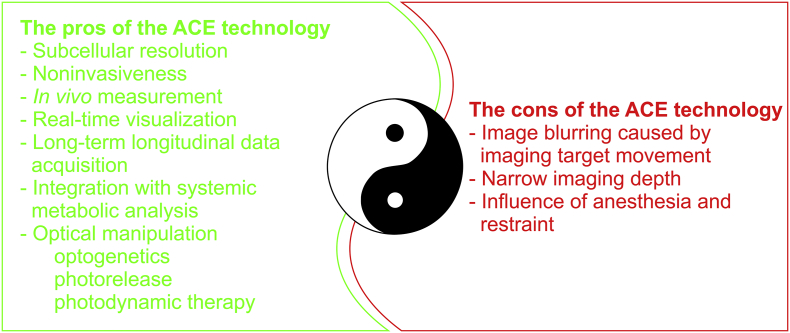
Fig. 3.
A scheme depicting the pros and cons of the ACE technology.
2.1. Technique for transplanting islets into the ACE
The technique for transplanting islets into the ACE mainly involves choosing donor-recipient pairs, determining the number of transplanted islets and implementing transplantation of islets into the ACE as detailed below (Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008).
2.1.1. Donor-recipient pairs in islet transplantation into the ACE
Our group has carried out transplantation of islets into the ACE of various animals including mice, rats, non-human primates and is undertaking clinical trials in humans (Berggren et al., unpublished data) (Abdulreda et al., 2011; Abdulreda et al., 2013; Abdulreda et al., 2016; Abdulreda & Berggren, 2013; Almaca et al., 2014; Avall et al., 2015; Diez et al., 2017; Faleo et al., 2014; Ilegems et al., 2013; Ilegems et al., 2015; Johansson et al., 2015; Juntti-Berggren et al., 2015; Lee et al., 2018; Leibiger et al., 2010; Leibiger et al., 2012; Leibiger & Berggren, 2017; Miska et al., 2014; Nyqvist et al., 2011; Paschen et al., 2016; Paschen et al., 2018; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Schmidt-Christensen et al., 2013; Shalaly et al., 2016; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008; van Krieken et al., 2017). We have used autologous, syngeneic, allogeneic and xenogeneic donor-recipient pairs in our research. Autologous islets have been implanted into the ACE of monkeys (Diez et al., 2017). These autologous islets survive well without experiencing immune rejection (Diez et al., 2017). Virtually, our group mostly uses syngeneic mouse models in our research (Ilegems et al., 2013; Ilegems et al., 2015; Johansson et al., 2015; Lee et al., 2018; Leibiger et al., 2012; Leibiger & Berggren, 2017; Nyqvist et al., 2011; Paschen et al., 2016; Paschen et al., 2018; Rodriguez-Diaz et al., 2012; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008; van Krieken et al., 2017). This transplantation setting allows islet grafts to be satisfactorily viable and to suffer from little immune rejection. It has helped us and others accumulate substantial new knowledge on islet biology and diabetes (Chen et al., 2016; Chmelova et al., 2015; Ilegems et al., 2013; Ilegems et al., 2015; Johansson et al., 2015; Lee et al., 2018; Leibiger et al., 2012; Leibiger & Berggren, 2017; Mojibian et al., 2013; Nyqvist et al., 2011; Paschen et al., 2016; Paschen et al., 2018; Rodriguez-Diaz et al., 2012; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008; van Krieken et al., 2017). Allogeneic islet transplantation into the mouse ACE has been conducted for longitudinally visualizing dynamic behavior of immune cells and β cells in transplanted mouse islets at cellular levels during allorejection (Abdulreda et al., 2011). Of note, for therapeutic transplantation in humans, nearly all cases belong to the allogeneic setting (Marzorati, Pileggi, & Ricordi, 2007; Shapiro et al., 2000; Shapiro et al., 2006; Shapiro, Pokrywczynska, & Ricordi, 2017). In fact, the xenogeneic transplantation setting is really useful especially for human islet research (Abdulreda et al., 2016). This transplantation setting offers great feasibility to study human islets in vivo without violating ethical standards. Surprisingly, the implanted human islets not only display satisfactory survival but also possess a sufficient ability to restore the normoglycemia in the recipient humanized mice rendered diabetic by streptozotocin (STZ) treatment (Abdulreda et al., 2016).
2.1.2. Methodological details of islet transplantation into the ACE
Transplantation of islets or other tissues/organs into the ACE includes the following steps (Fig. 2). The first step is to isolate and prepare islets or other tissues/organs to be transplanted (Fig. 2A) (Abdulreda et al., 2013; Kistler et al., 2014; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008). Currently, we focus on the islet as a microorgan. Generally, isolated islets without being trimmed or chopped are ready for transplantation since they are the suitable size to fit into the islet delivering glass micropipette. Usually, however, other types of naturally existing tissues/organs cannot be directly transplanted (Kistler et al., 2014). They have to be chopped and trimmed into pieces in a suitable size (Kistler et al., 2014). Furthermore, in vitro engineered tissues/organs such as stem cell-derived organoids including surrogate islets can be designed and produced in a suitable size for transplantation into the ACE.
Next, the isolated islets and appropriately-prepared tissue/organ pieces need to be genetically labeled with fluorescent protein biomarkers or biosensors (Fig. 2B) (Paschen et al., 2016; Paschen et al., 2018). Although various transgenic (Tg) mice expressing different fluorescent protein biomarkers or biosensors are available, quite a few types of donor tissues/organs especially isolated from humans need to be transduced with genetic vectors encoding fluorescent protein biomarkers or biosensors of interest (Paschen et al., 2016; Paschen et al., 2018; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008).
Finally, the genetically labeled islets or tissues/organs are transplanted into the ACE (Fig. 2C) (Kistler et al., 2014; Paschen et al., 2016; Paschen et al., 2018). They are gently aspirated into an islet-delivering micropipette, connected by tygon tubing to a threaded plunger syringe. The tip of the islet-delivering micropipette is beveled and lightly heat-polished. The final diameter of the micropipette tip opening typically ranges from 150 to 300 μm. Of course, if the size of transplanted tissue/organ pieces cannot be further reduced for some reasons, the tip opening of the tissue-delivering micropipette can be widen appropriately but not to a large extent. The large corneal hole can hardly be healed. Upon aspiration of islets into the micropipette, a tiny corneal hole is made by cautiously inserting the tip of an insulin syringe needle (29G) through the cornea of recipients normally anesthetized with isoflurane whose onset, depth, duration and termination are controllable. Subsequently, the tip of the glass micropipette preloaded with islets is carefully inserted into the corneal hole. Then immediately, preloaded islets are slowly injected into the ACE. Finally, the tip of the glass micropipette is cautiously extracted out of the corneal hole. Actually, careful and skillful transplantation of islets or other tissues/organs into the ACE can be nearly non-invasive without damaging any blood vessels (Fig. 2).
Of note, for in vivo microscopic characterization of a few transplants engrafted in the ACE, the above-described procedures are followed step by step. If there is no need to perform intravital microscopy of grafts in the ACE, the second step is not applicable.
2.1.3. Modes of transplantation of islets into the ACE
Generally speaking, we employ the ACE as a unique islet transplantation site aiming to image islet cytoarchitecture, function and viability and to control glycemic levels in live organisms. To meet these two aims, we have designed two modes of islet transplantation into the ACE (Fig. 2C).
First, we implant several to a couple of dozens of islets into the ACE where they are engrafted separately from each other to image their morphology and function by high-resolution (Fig. 2C). These separately-engrafted islets stay free of mechanical squeezing, physical interaction and opaque cover (Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008). Therefore, they keep their optical visibility optimal and their microarchitecture, function and viability more or less the same as their counterparts in the pancreas of recipients. Furthermore, these intraocular islets change their morphology, function and viability synchronously with in situ pancreatic islets (Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008). The structural and functional images of these transplanted islets faithfully represent or report the status of their counterparts in the pancreas. For these reasons, we coined the term “imaging or reporter mode” to describe this transplantation type (Fig. 2C) (Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008). For controlling glycemic levels in recipients, we transplant several dozens to 500 islets into the mouse ACE and ten-thousand islets into the monkey ACE (Fig. 2C) (Almaca et al., 2014; Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Speier, Nyqvist, Cabrera, et al., 2008). They squeeze each other aggregating together in the ACE. The aggregated islets significantly deform without spherical boundaries and are not suitable for microscopic imaging although their function and viability are intact. However, to our surprise, the ACE can accommodate sufficient islets to effectively restore normoglycemia although its space is limited in comparison to other transplantation sites (Almaca et al., 2014; Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Speier, Nyqvist, Cabrera, et al., 2008). It has been reported that 50 syngeneic islet equivalents (IEQs) engrafted in the ACE of STZ-induced diabetic C57BL/6 (B6) mice are enough to produce a significant anti-hyperglycemic activity and to ameliorate survival of these diabetic mice (Mojibian et al., 2013). However, at least 125–150 syngeneic IEQs suffice to normalize hyperglycemia (Mojibian et al., 2013; Nyqvist et al., 2011). This demonstrates that intraocular islets act as a powerful regulator of glucose homeostasis (Almaca et al., 2014; Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Speier, Nyqvist, Cabrera, et al., 2008). Therefore, we coined the term “glycocontrol or metabolic mode” to depict the transplantation type where a larger number of islets engrafted in the ACE are sufficient to maintain glucose homeostasis.
2.2. ACE-based imaging technique
We carry out confocal/multiphoton laser scanning microscopy of islets engrafted in the ACE of live recipients in the following order (Fig. 2D) (Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008). First, we anesthetize a recipient animal. Then, the anesthetized recipient is immobilized with a head holder that tilts the eye containing the engrafted islets to a proper orientation for microscopic imaging. Subsequently, the recipient eyeball is stabilized by using an eyeball holder. Thereafter, the anesthetized and immobilized recipient is placed under an upright Leica DMIRBE microscope equipped with a Leica TCS-SP2 confocal laser-scanner. Finally, the transplanted islets are illuminated with appropriate laser beams and resultant emissions are collected at appropriate wavelengths through an objective lens (Fig. 2D) (Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008).
A note of caution needs to be added concerning to anesthetic selection for different purposes of imaging experiments in the ACE platform. Some anesthetic agents significantly affect the parameter(s) of interest and/or have obvious pitfalls that interfere with or even prevent experimental implementation. For example, isoflurane not only impairs glucose-stimulated insulin secretion resulting in hyperglycemia, but also brings about irregular eyeball/iris movements (Speier, Nyqvist, Kohler, et al., 2008; Zuurbier, Keijzers, Koeman, Van Wezel, & Hollmann, 2008). Therefore, this anesthetic drug is used for the purpose of imaging the cytoarchitecture, light scattering, vascularization, innervation of intraocular islets in addition to executing transplantation of islets into the ACE. It is not suitable for monitoring events like [Ca2+]i responses to intravenous glucose injection and others requiring stable acquisition (Chen et al., 2016; Speier, Nyqvist, Cabrera, et al., 2008). In fact, a mixture of fluanisone, fentanyl and midazolam has been tested to be satisfactory for experiments where [Ca2+]i changes in intraocular islets are imaged following intravenous glucose injection (Chen et al., 2016; Speier, Nyqvist, Cabrera, et al., 2008).
2.3. Pros and cons of the ACE technology
The ACE technology brings a series of advantages over other imaging approaches like conventional non-invasive in vivo imaging modalities, invasive in vivo microscopy and in vitro microscopy (Fig. 3) (Abdulreda et al., 2013; Koo, Hamilton, & Williamson, 2006; Speier, Nyqvist, Kohler, et al., 2008; Weigert et al., 2010).
First, this technique is featured with non-invasiveness, which remove a great barrier for application of in vivo microscopy (Fig. 3) (Weigert et al., 2010). Actually, in vivo microscopic imaging could not be harnessed for two main reasons. On the one hand, only surface tissues/organs in the body such as some parts of the eye and the digestive, respiratory, urinary and reproductive tracts are accessible to in vivo microscopy or endoscopy non-invasively (Gastrointestinal Endoscopy Technology Committee, 2014; Villani et al., 2014). The rest of tissues/organs including pancreatic islets are not because of their anatomical locations. On the other hand, acute surgical operation causes local physical and chemical insults such as cutting damage and physical inflammation as well as systemic traumatic stress. The surgical insults and stress inevitably produce unwanted adverse effects on the tissues/organs of interest including pancreatic islets resulting in potential false interpretations of results. Furthermore, acute surgical operation is not suitable for longitudinal imaging. We remove the anatomical barrier and avoid the surgical influence by transplanting islets and other tissues/organs into the ACE equipped with a natural body window (Kistler et al., 2014; Speier, Nyqvist, Cabrera, et al., 2008). These transplanted islets survive satisfactorily on the iris and behave more or less the same way as their counterparts in situ in the pancreas (Kistler et al., 2014; Rodriguez-Diaz et al., 2012; Speier, Nyqvist, Cabrera, et al., 2008). Therefore, the ACE-based imaging technique has a great advantage allowing repetitive non-invasive in vivo imaging of most, if not all, of the body's tissues/organs including islets in the absence of local cutting damage and physical inflammation as well as systemic traumatic stress (Abdulreda et al., 2013; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008).
Second, the ACE technology enables transfer of in vitro microscopic research to in vivo levels (Fig. 3) (Abdulreda et al., 2013; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008). The highly viable and fully functional islets and other tissues/organs engrafted in the ACE become accessible to microscopy (Abdulreda et al., 2013; Almaca et al., 2014; Almaca, Weitz, Rodriguez-Diaz, Pereira, & Caicedo, 2018; Cohrs et al., 2017; Diez et al., 2017; Ilegems et al., 2013; Ilegems et al., 2015; Kemter et al., 2017; Kragl et al., 2016; Lee et al., 2018; Leibiger et al., 2010; Leibiger et al., 2012; Leibiger & Berggren, 2017; Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Speier, 2011; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008; van Krieken et al., 2017). Importantly, morphological and functional profiles detected in vivo reflect true physiological and pathological situations (Abdulreda et al., 2011; Abdulreda et al., 2013; Abdulreda et al., 2016; Abdulreda & Berggren, 2013; Ali et al., 2016; Almaca et al., 2014; Almaca et al., 2018; Avall et al., 2015; Bader et al., 2016; Berclaz et al., 2016; Borg et al., 2014; Chen et al., 2016; Chmelova et al., 2015; Cohrs et al., 2017; Diez et al., 2017; Faleo et al., 2014; Ilegems et al., 2013; Ilegems et al., 2015; Johansson et al., 2015; Juntti-Berggren et al., 2015; Kemter et al., 2017; Kragl et al., 2016; Lee et al., 2018; Leibiger et al., 2010; Leibiger et al., 2012; Leibiger & Berggren, 2017; Miska et al., 2014; Mojibian et al., 2013; Nyqvist et al., 2011; Paschen et al., 2016; Paschen et al., 2018; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Schmidt-Christensen et al., 2013; Shalaly et al., 2016; Speier, 2011; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008; van Krieken et al., 2017). In fact, the majority of previous findings in islet research were obtained from dispersed single islet cells including β cells and isolated islets in the absence of functional nerve endings, vascular networks and in vivo interstitial fluid. Moreover, these in vitro preparations also suffer from chemical, enzymatic and mechanical stresses during preparation. These harsh circumstances interfere with the cellular architecture, function and viability of islets. Undoubtedly, caution should be exercised when using these in vitro findings to explain in vivo situations (Barker et al., 2013; Leibiger et al., 2012; Weigert et al., 2010). We need to revisit the knowledge achieved on the basis of in vitro studies using advanced in vivo approaches such as the ACE technology.
Third, the ACE technology satisfactorily increases the resolution of non-invasive in vivo imaging of the body's tissues/organs (Fig. 3). Indeed, a series of non-invasive in vivo imaging modalities such as bioluminescence imaging, computer-assisted tomography, elastography, magnetic particle imaging, magnetic resonance imaging, positron emission tomography, photoacoustic imaging and ultrasonography have been developed and made great contribution to biomedical research and clinical diagnosis (Abdulreda et al., 2013; Koo et al., 2006; Speier, Nyqvist, Kohler, et al., 2008; Weigert et al., 2010). Unfortunately, they are inferior to the ACE-based imaging of cellular architecture, function and viability because of limitations in resolution (Abdulreda et al., 2013; Koo et al., 2006; Speier, Nyqvist, Kohler, et al., 2008; Weigert et al., 2010).
Forth, the ACE technology enables us to run repetitive and longitudinal experiments (Fig. 3) (Abdulreda et al., 2013; Speier, Nyqvist, Kohler, et al., 2008). Therefore, this approach prevails over invasive in vivo microscopy and in vitro microscopy (Abdulreda et al., 2013; Speier, Nyqvist, Kohler, et al., 2008; Weigert et al., 2010). It is well known that diabetes occurs following the dynamic progression of various pathogenic processes, many of which are reversible at their earlier stages (Taylor, 2013). Therefore, it is important to understand the temporal pattern of these processes for diabetes therapy, i.e. before β cell destruction and thereby reaching the point of no return.
Fifth, in combination with analysis of systemic metabolic parameters, the ACE-based microimaging technique can serve as a complementary systems approach to medicine and in particular diabetes by simultaneously monitoring multiple optical signals, encoding different morphological and functional parameters of islets engrafted in the ACE, and systemic metabolic parameters, such as blood glucose, insulin and C-peptide levels (Fig. 3) (Nielsen, 2017).
Sixth, the ACE technology not only offers non-invasive in vivo imaging platforms, but also a non-invasive in vivo optical manipulation site. The islets engrafted in the ACE are perfectly accessible to optogenetics, photorelease approach and photodynamic therapy (Fig. 3) (Kim, Adhikari, & Deisseroth, 2017; Kwiatkowski et al., 2018; Yang et al., 1999; Zucker, 2010). This turns the idea of non-invasive manipulation of the cellular activity and viability of intraocular islets into reality through various ways, e.g., optogenetic activation of heterologously-expressed light-sensing proteins, photorelease of caged Ca2+ and photoactivation of photosensitizers (Kim et al., 2017; Kwiatkowski et al., 2018; Yang et al., 1999; Zucker, 2010).
However, the ACE technology not only has merits but also pitfalls. The technical concerns about any non-invasive in vivo imaging approach focus on how clearly and deeply it can see. The ACE technology does not bring into full play the resolution capacity of confocal/multiphoton microscopy due to the movements of islet grafts caused by respiration, heartbeat, pupil constriction and dilation. These movements can substantially be reduced by adequately immobilizing the head and eyeball, appropriately adjusting the level of anesthesia and optically or pharmacologically controlling pupil constriction and dilation (Fig. 3). By taking these measures, the resolution of the technology can only reach crude subcellular levels allowing discrimination between the cytoplasm and nucleus of relatively small mammalian cells like pancreatic β cells (Fig. 3). The ACE technology cannot as of yet be applied to awake free-moving animals. The results obtained from anesthetized and restrained animals may contain potential pitfalls since anesthesia and physical restrainment may produce various side effects on imaged islets and systemic metabolism (Fig. 3) (Zuurbier et al., 2008).
3. Basic knowledge obtained by applying the ACE technology
The ACE technology and its developments have made us and others accumulate substantial new knowledge on the engraftment, survival, vascularization, innervation, blood glucose controllability, in vivo dynamics of cytosolic free calcium concentration ([Ca2+]i) in the β cell and functional β mass of transplanted islets as well as islet development (Abdulreda et al., 2013; Ali et al., 2016; Almaca et al., 2014; Almaca et al., 2018; Bader et al., 2016; Borg et al., 2014; Chen et al., 2016; Cohrs et al., 2017; Diez et al., 2017; Ilegems et al., 2013; Ilegems et al., 2015; Kemter et al., 2017; Kragl et al., 2016; Leibiger et al., 2010; Leibiger et al., 2012; Leibiger & Berggren, 2017; Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Speier, 2011; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008).
3.1. The settlement and integration of transplanted islets in the ACE
Islets implanted into the ACE exhibit satisfactory engraftment, rich vascularization and adequate innervation, demonstrating that they settle down and intimately integrate into the environment of the ACE of the recipient (Abdulreda et al., 2013; Almaca et al., 2014; Almaca et al., 2018; Bader et al., 2016; Borg et al., 2014; Cohrs et al., 2017; Diez et al., 2017; Ilegems et al., 2013; Ilegems et al., 2015; Kemter et al., 2017; Kragl et al., 2016; Lee et al., 2018; Leibiger et al., 2010; Leibiger et al., 2012; Leibiger & Berggren, 2017; Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Speier, 2011; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008; van Krieken et al., 2017).
3.1.1. Engraftment of transplanted islets in the ACE
Transplanted islets readily engraft on the iris where they survive and keep their cellular composition unaltered (Speier, Nyqvist, Cabrera, et al., 2008). Although islets can be transplanted into multiple alternative locations, such as hepatic sinusoids, renal subcapsules, intra-abdominal cavity, omental pouch, gastrointestinal wall, subcutaneous tissue, skeletal muscle, bone marrow, pancreas, spleen, lung, brain, testis and thymus, the favorite site is most likely to going be the ACE (Fiorina, Shapiro, Ricordi, & Secchi, 2008; McCall & Shapiro, 2014; Pepper, Gala-Lopez, Ziff, & Shapiro, 2013; Shapiro et al., 2000; Shapiro et al., 2006; Speier, Nyqvist, Cabrera, et al., 2008). Islets rapidly sit on and attach themselves to the iris a little while after being injected into the ACE (Speier, Nyqvist, Cabrera, et al., 2008). Thereafter, they gradually integrate with and fully engraft on the iris within about 4 weeks. Fully engrafted islets can reside separately or gather into either small clusters or a macroaggregate, depending on the number and layout of transplanted islets. The size of the contact interface between an engrafted islet and recipient iris can vary appreciably. Transplanted islets having smaller or larger contact areas with the iris remain spherical or become somewhat flatten. No matter how small or large the contact area is, islets appear to engraft and function equally well. Furthermore, there is no significant difference in the ratio of β cells to α cells between islets engrafted on the iris and those in the pancreas. Islets engrafted on the iris do not change their cellular composition (Speier, Nyqvist, Cabrera, et al., 2008).
3.1.2. Vascularization of transplanted islets in the ACE
The native in situ islet is a highly vascularized microorgan (Jansson et al., 2016; Nyman et al., 2008). The intraislet vascular network plays an important role in maintaining the extracellular milieu of islet cells through exchange between blood plasma and interstitial fluid (Jansson et al., 2016). It not only guarantees nutrient supply to and metabolic waste removal from islets, but also ensures timely transportation of hormonal factors like insulin and glucagon to and from islet cells (Weir & Bonner-Weir, 1990). Therefore, islet vasculature not only plays an important role in orchestration of the structural integrity and functional competence of islets, but also systemic metabolism and glucose homeostasis (Jansson et al., 2016; Mazier & Cota, 2017; Weir & Bonner-Weir, 1990). Furthermore, interactions of vascular endothelial cells with islet cells and in particular β cells are important for islet cell function (Mazier & Cota, 2017). Undoubtedly, it is of paramount necessity to monitor vascularization of transplanted islets in the ACE and anywhere in the recipient's body.
The ACE technology reveals that some functional blood vessels appear in islets engrafted on the iris, especially in their regions connected with the iris, 3 days after transplantation (Speier, Nyqvist, Cabrera, et al., 2008). Thereafter, these vessels progressively spread in islet grafts forming appreciable microvascular networks throughout engafted islets at week 2 posttransplantation. Afterwards, islet vascular density becomes greater and greater. It plateaus at week 4 after intraocular islet transplantation. From then on, intraislet microvascular networks are typically enriched with densely interweaving, highly tortuous and uniformly sized capillaries. On the contrary to their density and distribution, the diameter of newly formed intraislet vessels shrink from nearly twice the diameter of a red blood cell at day 3 to about the same diameter as a single one at day 14 posttransplantation and then remains constant (Speier, Nyqvist, Cabrera, et al., 2008). This indicates that maturation of newly formed vessels into capillaries occurs in islet grafts during this period of time (Speier, Nyqvist, Cabrera, et al., 2008).
The transplanted islets intimately integrate themselves with the iris. The highly vascularized iris of the host provides the foreign islets with most or even all of endothelial cells for islet revascularization, depending on whether they carry their own endothelial cells or not (Freddo, 1996; Hayreh & Scott, 1978; Nyqvist et al., 2011). Vascular endothelial cells remain in freshly isolated islets, but disappear in cultured islets. Nevertheless, both types of islets are well revascularized to the same extent over long periods of time due to the dominant contribution of host endothelial cells. However, the freshly isolated islets display a higher revascularization rate than the cultured islets. To our surprise, the freshly isolated islets take longer to reverse diabetes than the cultured islets do when transplanted into the ACE of mice rendered diabetic by STZ injection (Nyqvist et al., 2011). This means that donor islet's own endothelial cells participate in islet revascularization on a small scale and are mainly involved in early revascularization of islet grafts without either elevating the vascular density or improving the function of islet grafts. Furthermore, transmission electron microscopy reveals that blood vessels lined with donor and host endothelial cells in islet grafts exhibit a normal ultrastructure, which is more or less the same as that of islets residing in the pancreas and quite homogenous. Ultrastructural examination cannot discriminate between donor and host endothelial cells. Vascular endothelial and islet cells are slightly separated by a thin basement membrane. Intraislet capillary walls are composed of thin endothelial cell bodies with fenestrations covered by a diaphragm (Nyqvist et al., 2011).
3.1.3. Innervation of transplanted islets in the ACE
Immunofluorescence microscopy reveals that adequate sympathetic and parasympathetic innervations occur in mouse islet engrafted on the iris (Rodriguez-Diaz et al., 2012). The density of sympathetic and parasympathetic terminals in the islet grafts retrieved from the ACE is very similar to that in islets residing in the pancreas. It appears that reinnervation follows after revascularization during islet engraftment on the iris. Autonomic nerve endings sparsely appear in close proximity to the islet grafts at day 3 posttransplantation. Sympathetic terminals invade into the islet grafts, mostly surrounding blood vessels, within 15 days after transplantation and those adjacent to blood vessels expand between days 15 and 30 posttransplantation. Afterwards, sympathetic endings become the neighbor of islet cells and plateau around 90 days after transplantation by rising their density and complexity. Parasympathetic reinnervation similarly occurs in the islet grafts. It shows up near intraislet vessels at early stages and then in close vicinity to endocrine cells. However, parasympathetic terminals reinnervate the islet grafts somewhat more slowly than sympathetic ones do. In addition to the above in vitro visualization, in vivo characterization of parasympathetic reinnervation of islets engrafted on the iris has also been performed in Tg mice expressing green fluorescent protein (GFP) specifically in central and peripheral cholinergic neurons, including somas and processes of the parasympathetic nervous system. The combination of the ACE technology with these Tg mice reveals that GFP-positive cholinergic neurons and processes emerge in wild type islets engrafted on the iris in a similar way to those present in situ in the pancreas (Rodriguez-Diaz et al., 2012).
It is noteworthy that interspecies differences in parasympathetic innervation of native in situ islets remain in islet grafts on the iris as exemplified in B6 and 129 × 1 mice (also known as 129/SvJ mice, being an inbred strain and most commonly used to generate embryonic stem cells for making knockout mice) (Rodriguez-Diaz et al., 2012). When islets isolated from B6 or 129 × 1 mice are transplanted into the ACE of nude mice, the former islets are reinnervated on the iris as they are innervated in situ in the donor mice, whereas the latter islets only receive few cholinergic fibers like 129 × 1 mouse islets embedded in the pancreas. Furthermore, the mice carrying B6 mouse islet grafts display light-induced facilitation of blood glucose excursions, but those accommodating 129 × 1 mouse islet grafts do not. This reveals the importance of parasympathetic innervation in modulation of glucose-stimulated insulin secretion in addition to interspecies differences in parasympathetic innervation (Rodriguez-Diaz et al., 2012).
The ACE is not only equipped with the cornea as a transparent body window but also the unique light-sensitive control facility, i.e. iridic cholinergic parasympathetic/adrenergic sympathetic nerves (McDougal & Gamlin, 2015; Meek, 2009; Meek & Knupp, 2015). Both the sympathetic and parasympathetic nervous systems are involuntary. Therefore, the term autonomic nervous system is used to cover both the sympathetic and parasympathetic nervous systems. Nevertheless, the parasympathetic output to the iris sphincter muscle is easily controllable with light since it increases proportionally with light intensity detected by the retina (McDougal & Gamlin, 2015). In addition, the sympathetic output to the iris dilator muscles is negatively related to light strength sensed by the retina, but the sympathetic response to light is more slowly and less obviously than the light-induced parasympathetic response (McDougal & Gamlin, 2015). These responses are mediated by the two classical neurotransmitters acetylcholine and noradrenaline, which play an important role in regulation of islet hormone secretion (McDougal & Gamlin, 2015). Therefore, these light-sensitive features can be used to non-invasively manipulate hormone secretion from the innervated islets on the iris (Rodriguez-Diaz et al., 2012). STZ-treated mice transplanted with 300 islets either in the ACE or under the kidney capsule are normoglycemic. The former exhibit higher circulating insulin and glucagon and lower blood glucose levels than the latter when exposed to ambient light. Importantly, the former show a decrease in plasma insulin levels and an increase in blood glucose concentration, whereas the latter display no changes in glycemic levels when moved from a bright ambient to a dark one. The former reduce their blood glucose concentration but the latter still cannot alter this metabolic parameter in response to reexposure to ambient light. The light exposure-induced effects are mainly attributed to action of acetylcholine released from iridic parasympathetic endings on muscarinic receptors of engrafted islet cells. Glucose tolerance tests illustrate that the ambient light facilitates blood glucose clearance in mice transplanted with 300 islets in the ACE, but not under the kidney capsule. The effect is ablated by the muscarinic receptor antagonist atropine and mimicked by the muscarinic receptor agonist pilocarpine (Rodriguez-Diaz et al., 2012). These findings demonstrate that the iridic parasympathetic pathway not only send branches into islet grafts on the iris but also modulate the function of the engrafted islets (Rodriguez-Diaz et al., 2012).
It is worthwhile to note that human islets are innervated by autonomic nerves differently from mouse ones. Few parasympathetic fibers are present and some sympathetic terminals distribute in the human islet where sympathetic axons preferentially innervate vascular smooth muscles and rarely contact endocrine cells. Such an autonomic innervation pattern remains in human islets engrafted on the iris and is not likely to mediate the effect of ambient light on insulin secretion detected in mouse intraocular islets (Rodriguez-Diaz et al., 2012).
3.2. Function of transplanted islets in the ACE
The most important thing to consider when predicting the clinical value of implantation of islets into the ACE is whether the implantation is able to maintain glucose homeostasis in recipients. On the other hand, cellular functions of islets critically rely on [Ca2+]i signaling in islet cells including β cells (Berggren et al., 2004; Cabrera et al., 2008; Yang et al., 2014; Yang and Berggren, 2005a, Yang and Berggren, 2006). Therefore, we have selected the effectiveness of transplanted islets in the ACE for normalizing glycemic levels and in vivo dynamics of β cell [Ca2+]i as priorities in the development phase of the ACE technology (Almaca et al., 2014; Mojibian et al., 2013; Nyqvist et al., 2011; Paschen et al., 2018; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Speier, Nyqvist, Cabrera, et al., 2008).
3.2.1. Glycemic control by islets engrafted in the ACE
In fact, 300 mouse islets engrafted in the ACE of mice rendered diabetic by STZ injection fully restore normoglycemia post-transplantation and keep the recipients nondiabetic before removal of the graft-bearing eye (Speier, Nyqvist, Cabrera, et al., 2008). Random glycemic levels of these mice rise up to over 500 mg/dl after STZ injection, drop to about 100 mg/dl within 2 weeks post-transplantation and stay around 100 mg/dl for >200 days (Speier, Nyqvist, Cabrera, et al., 2008). The recipients immediately suffer from diabetes again after removal of the graft-bearing eye and their random blood glucose returns back to high levels similar to those observed in STZ-induced diabetes before islet transplantation. These intraocular islets also effectively reverse glucose intolerance in the STZ-treated mice. They make the STZ-treated mice remove blood glucose as efficiently as intact control mice during intraperitoneal glucose tolerance tests. Time courses of blood glucose concentrations following intraperitoneal glucose injection start with an initial value of about 100 mg/dl at time point 0, peak at around 400 mg/dl in 20 min and gradually drop to more or less the same value as the initial one at the 120-min time point. This is the first demonstration of effective glycemic control by islets engrafted in the ACE (Speier, Nyqvist, Cabrera, et al., 2008). Later, 300 mouse islets engrafted in the mouse ACE have been confirmed to reliably and efficiently normalize blood glucose levels in recipients, indicating these intraocular islet grafts contain an optimal β cell mass for glycemic normalization (Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018). Furthermore, it has been found that STZ-induced diabetic mice become normoglycemic following transplantation of 150 syngeneic islets into their ACE (Nyqvist et al., 2011). A titration with 25, 50, 75, 125 mouse islets implanted into the mouse ACE has revealed that at least 125 intraocular islets can restore normoglycemia in recipient mice (Mojibian et al., 2013). These two studies verify that the marginal mouse islet mass transplanted into the mouse ACE for glycemic normalization is about 125–150 mouse islets (Mojibian et al., 2013; Nyqvist et al., 2011). However, the marginal human islet mass engrafted in the humanized mouse ACE for glycemic normalization is 1000 human islet IEQs (500 human IEQs in each ACE) (Abdulreda et al., 2016). In fact, 200 intraocular islets even from 18-month-old mice are sufficient to normalize hyperglycemia, but need longer time to fully function than those from 2 months old mice (Almaca et al., 2014). Moreover, A STZ-induced diabetic baboon transplanted with 20,000 allogeneic IEQs and then 18,000 IEQs on day 292 post-transplantation in the ACE displays reduced fluctuations in fasting blood glucose, corroborating that the transplanted islets ameliorate glucose homeostasis (Perez et al., 2011). Importantly, comparison of effectiveness for glycemic control reveals that 50 intraocular mouse islets can produce a hypoglycemic effect equivalent to that yielded by ≥200 renal subcapsular mouse islets (Mojibian et al., 2013). The findings demonstrate that islets engrafted in the ACE not only normalize blood glucose levels but also do so more effectively than those at other transplantation sites (Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Speier, Nyqvist, Cabrera, et al., 2008).
3.2.2. [Ca2+]i signaling in transplanted islets in the ACE
The physiological and pathological roles of [Ca2+]i have been extensively studied in dispersed single islet cells and isolated islets under non-physiological in vitro conditions (Berggren et al., 2004; Cabrera et al., 2008; Yang et al., 2014; Yang and Berggren, 2005a, Yang and Berggren, 2006). Such conditions inevitably interfere with [Ca2+]i and corresponding signaling pathways in these in vitro preparations. Obviously, in vivo investigation of [Ca2+]i signaling in islet cells is inevitably needed under different physiological and pathophysiological conditions.
Owing to the important roles of [Ca2+]i in physiology and pathology of β cells and other islet cells, β cell [Ca2+]i dynamics in islets engrafted on the iris has been characterized during the development of the ACE technology (Berggren et al., 2004; Cabrera et al., 2008; Speier, Nyqvist, Cabrera, et al., 2008; Yang et al., 2014). Within islet grafts loaded with the Ca2+ indicators Fluo-4 and Fura-Red, β cells display a robust [Ca2+]i response to systemic administration of the sulfonylurea compound glibenclamide, which closes adenosine triphosphate-sensitive K+ (KATP) channels resulting in plasma membrane depolarization, voltage-gated Ca2+ (CaV) channel opening, Ca2+ influx and [Ca2+]i rise in β cells (Speier, Nyqvist, Cabrera, et al., 2008; Yang et al., 2007; Yang et al., 2014). Such a [Ca2+]i response begins within 30–40 s after injection of glibenclamide into the tail vein. It is characterized by a rapid initial rise followed by a sustained increase. Furthermore, β cells in different regions of the islet graft exhibit simultaneous [Ca2+]i increases with a similar pattern in response to stimulation with glibenclamide. This synchronized [Ca2+]i response reflects that the β cells in different regions of the islet graft are tightly coupled. Moreover, [Ca2+]i responses in the same islet cells within the islet graft can be repeatedly registered at different time points (Speier, Nyqvist, Cabrera, et al., 2008). β Cell [Ca2+]i dynamics of Tg mouse islets, whose β cells specifically express the Ca2+ sensor protein GCaMP3 (Tg GCaMP3), engrafted in the ACE following injection of high glucose into the tail vein. After overnight-fasting, mouse β cell [Ca2+]i is relatively stable before glucose injection, quickly rises to its peak after intravenous glucose injection and then gradually falls down to a lower plateau with or without oscillations (Chen et al., 2016). Interestingly, intraocular islets display significantly quicker [Ca2+]i responses to glucose stimulation than isolated islets in vitro. The former show the initial [Ca2+]i peak within <1 min after intravenous bolus injection of glucose, whereas the latter need to be exposed to glucose for 3–4 min to produce their first [Ca2+]i transient (Chen et al., 2016; Jing et al., 2005; Salunkhe et al., 2016). It is interesting to figure out the causes of this difference. Undoubtedly, the ACE technology serves as a powerful approach to understand in vivo islet physiology as exemplified by in vivo dynamics of β cell [Ca2+]i (Chen et al., 2016; Speier, Nyqvist, Cabrera, et al., 2008).
3.3. β Cell mass and insulin secretory capacity of transplanted islets in the ACE
Establishment of the ACE technology primarily aims at imaging the in vivo dynamics of β cell mass and function due to the utmost importance of functional β cell mass in maintenance of glucose homeostasis and in development of diabetes (DiMeglio et al., 2018; Halban et al., 2014; Katsarou et al., 2017; Meier & Bonadonna, 2013; Pugliese, 2017; Rhodes, 2005). β Cells are endowed with unique light scattering properties which the ACE technology can detect without fluorescence or genetic labeling. Islets emit light scattering signals mainly depending on zinc-insulin crystals within insulin secretory granules (Ilegems et al., 2015). By employing the ACE technology, Chmelova et al. and Ilegems et al. have independently characterized the light scattering properties of islets engrafted in the ACE (Chmelova et al., 2015; Ilegems et al., 2015). By measuring light scattering signals, the ACE technology enables quantification of the in vivo dynamics of both intraocular islet volumes and insulin granule density. The former and latter reflect real-time β cell mass and insulin secretory capacity, respectively, in different metabolic situations, such as fasting state or hyperglycemia. This approach prevails over other methods because of higher resolution and sensitivity as well as no artificial influences (Alanentalo et al., 2010; Cline, Zhao, Jakowski, Soeller, & Treadway, 2011; Hara et al., 2003; Ilegems et al., 2013; Ilegems et al., 2015; Lamprianou et al., 2011; Reiner et al., 2011; van Krieken et al., 2017; Villiger et al., 2009; Virostko et al., 2013).
Notably, the volume and density of light scattering signals emitted from intraocular islets roughly reflect but do not necessarily equate insulin secretory capability. First, these signals are not solely contributed by zinc-insulin crystal-containing secretory granules but also other cellular components in intraocular islets. Second, not all these secretory granules are release-competent in response to glucose stimulation. In fact, only 50 out of a total of about 10,000 insulin secretory granules per β cell are primed and situated in the immediately releasable pool for instantaneous exocytosis upon glucose stimulation (Barg, Eliasson, Renstrom, & Rorsman, 2002; Rorsman & Renstrom, 2003). For the ACE platform, imaging actual insulin secretion is of outmost importance. Currently, there are two basic kinds of in vitro imaging modalities for measurement of actual insulin secretion. One is using extracellular insulin-sensitive fluorescent dyes such as a fluorescent zinc probe and the other is employing genetically encoded fluorescent indicators for imaging insulin released from β cells (Li D et al., 2011; Suzuki T et al., 2017). We are adopting these in vitro imaging modalities into our ACE platform to intravitally image insulin secreted from intraocular islets.
3.4. Pancreatic islet development in the ACE
The ACE technology makes non-invasive in vivo cellular imaging of the developing pancreas practical. Pancreatic buds from 10.5-day mouse embryos carrying the GFP gene driven by a mouse insulin promoter (MIP-GFP) can now be transplanted into mouse ACE. The ACE technology reveals that the transplanted bud undergoes satisfactory engraftment, rich vascularization and progressive growth (Ali et al., 2016). Sparse GFP-positive cells emerge on day 4 after transplantation. Thereafter, more and more GFP-positive cells appear and aggregate into expanding cell clusters throughout the transplanted bud. In vitro immunofluorescence labeling verifies that the pancreatic progenitor cells in the engrafted bud well differentiates into exocrine and endocrine cells, expressing amylase and the islet hormones insulin, glucagon and somatostatin, respectively. The pancreatic bud engrafted on the iris does not differ from the native in situ developing pancreas in their cytoarchitecture. In addition, in vitro insulin secretion assay demonstrates that β cells originated in the transplanted pancreatic bud retrieved from the ACE show a similar insulin-secretory response as native fetal β cells, but predominate over in vitro cultured buds in this regard. This work demonstrates that the ACE suffices to provide suitable development environments for growth, differentiation and function of pancreatic buds. E10.5 mouse pancreatic buds possess adequate intrinsic signals to drive differentiation into specialized pancreatic exocrine and endocrine cells. Hence, the ACE technology is suitable for monitoring in vivo pancreatic islet development at cellular resolution in a non-invasive, longitudinal manner (Ali et al., 2016).
4. Diabetes knowledge gained by using the ACE technology
The ACE technology in conjunction with other approaches has offered great feasibility for executing a variety of insurmountable tasks such as real-time intravital imaging of autoimmune insulitis, functional β cell mass and cellular insulin resistance as well as islet allorejection (Chmelova et al., 2015; Paschen et al., 2016; Paschen et al., 2018). This technology has satisfactorily been employed for intravitally evaluating rejuvenation of aged islets and antidiabetic drugs in human islets (Abdulreda et al., 2016; Almaca et al., 2014). It has also successfully been used in non-human primates to lay the foundation for its clinical application for treating diabetes (Diez et al., 2017; Perez et al., 2011). The findings gained using the ACE technology are discussed below.
4.1. In vivo dynamics of islet allorejection in the ACE
Upon establishment, the ACE technology has been used to characterize in vivo behavior of effector T cells in DBA/2 (H-2d) mouse islets transplanted into the ACE of MHC-mismatched B6 (H-2b) mice where activated and memory T lymphocytes express GFP (Abdulreda et al., 2011). These T lymphocytes appear in three different shapes: round, elongated and ruffled. Round cells surround islet grafts in the early phase of transplantation. Elongated ones move long distances at a mean instantaneous velocity of about 3.5 μm/min. Ruffled ones gather together in islet grafts and travel along a complex trajectory. Interestingly, the proportion and density of ruffled cells increase during acute rejection. In vivo immunolabeling with fluorophore-conjugated antibodies verfies that GFP-labeled T cells infiltrating into islet grafts are activated and consist of ≥80% CD8+ and < 10% CD4+ cells. Interestingly, 70% GFP-labeled T cells contact apoptotic islet cells. Interestingly, the former also carry lysotracker-labeled lytic granules. Furthermore, abundant infiltration of GFP-labeled T cells into allogeneic islets occur in parallel with islet destruction. Unlike syngeneic islet grafts, allogeneic ones in the ACE of STZ-induced diabetic mice quickly lose their action against hyperglycemia due to acute rejection. Moreover, GFP-labeled T cells move with significantly higher velocities within the islets during acute rejection than before. Admittedly, islet grafts in the ACE undergo allorejection somewhat slowly in comparison to those under the kidney capsule consistent with the immune privilege of the ACE (Abdulreda et al., 2011).
In addition, the suitability and competence of the ACE technology in measuring in vivo dynamic changes of effector T cells in islet allografts have been further proven by acutely manipulating these T cells. Injection of TAK-779, a specific antagonist of the chemokine receptors CCR5 and CXCR3, into the ACE converts the majority of the highly dynamic T cells into stationary round ones within 10 min. The effect is effectively reversed by subsequent injection of CXCL9/CXCL10, the natural ligands to CXCR3, into the same ACE. Furthermore, systemic administration of TAK-779 delays initial infiltration of T cells into islet allografts and acute rejection. The treatment reduces the relative proportion of the ruffled cells in islet grafts, and significantly slows down overall T-cell velocity (Abdulreda et al., 2011).
The above findings confirm that the ACE technology is optimal for intravital cellular imaging of immune responses to allogeneic islets in a longitudinal and non-invasive fashion (Abdulreda et al., 2011). This approach to problems related to islet allograft rejection will significantly benefit clinical islet transplantation that almost exclusively belong to allotransplantation (Shapiro et al., 2017).
4.2. In vivo dynamics of immune and islet cells in the ACE during T1D insulitis
T1D results from the autoimmune-mediated destruction of β cells, namely autoimmune insulitis (DiMeglio et al., 2018; Katsarou et al., 2017; Kopan et al., 2018; Pugliese, 2017). In vitro evidence suggests that this β cell-specific autoimmune process comprises dynamic immune cell infiltration, multiplex immune cell interplay, immune cell-β cell interaction and progressive loss of β cell mass and function (DiMeglio et al., 2018; Katsarou et al., 2017; Morgan, 2017; Pugliese, 2017). The ACE technology has revealed in vivo dynamics of these autoimmune events at cellular levels (Chmelova et al., 2015; Miska et al., 2014; Mojibian et al., 2013; Schmidt-Christensen et al., 2013).
4.2.1. Autoimmune insulitis in islets implanted into the ACE
The immune privilege of the ACE and separation of intraocular islets from the local pancreatic immune milieu raise a concern whether autoimmune insulitis in the pancreas can be recapitulated in syngeneic islet grafts in the ACE of the non-obese diabetic (NOD) mice, an animal model of human T1D. Several groups including ours have independently addressed this concern by employing several recipient NOD mouse substrains and other mouse strains in combination with corresponding immune cell donor mice (Chmelova et al., 2015; Miska et al., 2014; Mojibian et al., 2013; Schmidt-Christensen et al., 2013). The recipient mouse strains include NOD, NOD bearing severe combined immunodeficiency mutation (NOD SCID), NOD recombination activating gene 2-deficient (NOD Rag2−/−), RIP-HA mice whose β cells specifically express influenza hemagglutinin (HA) under control of the rat insulin promoter (RIP) and MIP-GFP/RIP-HA mice generated by crossing MIP-GFP mice with RIP-HA mice (Chmelova et al., 2015; Miska et al., 2014; Mojibian et al., 2013; Schmidt-Christensen et al., 2013). The immune cell donor mouse strains involve NOD whose CD11c+ cells express GFP (NOD CD11c-GFP), NOD whose Foxp3+ cells express GFP (NOD Foxp3-GFP), NOD whose β cell-specific CD4+ BDC2.5 effector T (Teff) cells express cyan fluorescent protein (CFP) (NOD CD4+ BDC2.5 Teff-CFP), NOD whose β cell-specific CD4+ BDC2.5 regulatory T (Treg) cells express GFP (NOD CD4+ BDC2.5 Treg-GFP) and TCR-HA mice expressing T cell receptor α/β chains specific for the MHC Class II I-Ed-restricted determinant site 1 of HA (TCR-HA) (Chmelova et al., 2015; Miska et al., 2014; Mojibian et al., 2013; Schmidt-Christensen et al., 2013).
Stereomicroscopic observation by Mojibian et al. has revealed that syngeneic islets transplanted into the ACE are prone to autoimmune attack in both recipient NOD and NOD.SCID mice (Mojibian et al., 2013). Accelerated by adoptive transfer of splenocytes from NOD mice with new onset diabetes, marginal and significant autoimmune destruction occur in intraocular islets at 2 and 4 weeks, respectively, after the adoptive transfer. During the autoimmune destruction, intraocular islets progressively display ruffled borders, noticeable holes, increased transparency and decreased size. Importantly, insulitis patterns are similar and insulitis scores are correlated between intraocular and in situ pancreatic islets. This demonstrates that intraocular islets can serve as a mirror of in situ pancreatic islets during autoimmune destruction (Mojibian et al., 2013).
Schmidt-Christensen et al. have investigated the autoimmune insulitis in syngeneic islets transplanted into the ACE of recipient NOD Rag2−/− mice following adaptive transfer of CD11c+ and Foxp3+ cells from NOD CD11c-GFP and NOD Foxp3-GFP, respectively (Schmidt-Christensen et al., 2013). The results demonstrate that the adoptively transferred CD11c+ cells infiltrate and accumulate in islet grafts at 2 weeks resulting in β cell disappearance. The adoptively transferred Foxp3+ cells appear in intraocular islets similarly to the adoptively transferred CD11c+ cells. However, there is no immune cell infiltration in syngeneic islet grafts in the ACE of control B6 Rag2−/− mice reconstituted with spleen cells from B6 Foxp3-GFP reporter mice. These findings verify that the autoimmune insulitis selectively occur in NOD Rag2−/− rather than B6 Rag2−/− islets following adaptive transfer of corresponding immune cells (Schmidt-Christensen et al., 2013). Moreover, CD11c+ or Foxp3+ cell infiltration into intraocular islets mirrors autoimmune insulitis in the recipient pancreas (Schmidt-Christensen et al., 2013).
By employing adoptive transfer of β cell-specific CD4+ BDC2.5 Teff cells alone or together with CD4+ BDC2.5 Treg cells purified from NOD CD4+ BDC2.5 Teff-CFP and NOD CD4+ BDC2.5 Treg-GFP mice, Miska et al. have characterized autoimmune insulitis in NOD SCID mouse islets engrafted in the ACE of syngeneic recipients (Miska et al., 2014). The adoptively transferred T cells interplay with each other and interact with β cells regardless of the immune privilege of the ACE. The β cell-specific CD4+ BDC2.5 Teff cells destroy islets engrafted in the ACE as they did in in situ pancreatic islets. Co-transferred CD4+ BDC2.5 Treg cells produce similar protection of CD4+ BDC2.5 Teff cell-mediated destruction in islets engrafted on the iris and in those located within the recipient pancreas. These observations demonstrate that the above transplantation setting is technically sound for non-invasive, intravital, longitudinal and cellular imaging of T cell-mediated pathogenesis of T1D (Miska et al., 2014).
In recent work by Chmelova et al., they have found that RIP-HA mouse islets implanted into the ACE of syngeneic recipients serve as a reliable mirror of in situ pancreatic islets during the onset and remission of autoimmune diabetes following adoptive transfer of CD4+ T cells from TCR-HA mice and treatment with Anti-CD3 mAb (Chmelova et al., 2015). They have also generated MIP-GFP/RIP-HA mice by crossing MIP-GFP mice with RIP-HA mice (Chmelova et al., 2015). In such crossbred mice, β cell-specific expression of both HA and GFP not only allows reliable induction of autoimmune insulitis but also microscopic quantification of β cell mass. This enables intravital and non-invasive imaging of dynamic changes in β cell mass and insulin-secretory function during autoimmune insulitis in intraocular islets. They have demonstrated that adoptive transfer of preactivated CD4+ T cells isolated from TCR-HA mice reliably induces autoimmune insulitis in MIP-GFP/RIP-HA+ islets engrafted in the ACE of RIP-HA− recipient mice. Abundant preactivated CD4+ T cells appear in the vasculature of the engrafted islets and their associated iris 3 days after adoptive transfer. Following that, the adoptive transfer rapidly reduces the β cell-specific GFP signal from intraocular islets. Only about 1% of this GFP signal is detectable 17 days after adoptive transfer. Massive CD45 cells infiltrate into the engrafted islets killing most of the β cells. In addition to reduced β cell mass, insulin-secretory function drops dramatically as evidenced by a drastic decline in the islet backscatter signal during autoimmune insulitis. Meanwhile, the islet vasculature undergoes reorganization along with autoimmune destruction of β cells (Chmelova et al., 2015). Furthermore, the β cell mass and insulin-secretory function of intraocular islets change in syngeneic NOD.SCID recipients similarly as in syngeneic MIP-GFP/RIP-HA recipients during autoimmune insulitis (Chmelova et al., 2015). These findings verify that in vivo dynamics of β cell mass and function can be microimaged in intraocular islets during autoimmune insulitis without interruption by the immune privilege of the ACE (Chmelova et al., 2015).
The above results verify that autoimmune insulitis can indeed be reproduced in the ACE of several recipient NOD mouse substrains and other mouse strains following adoptive transfer of immune cells from corresponding donor mice (Chmelova et al., 2015; Miska et al., 2014; Mojibian et al., 2013; Schmidt-Christensen et al., 2013). Strikingly, the critical events of autoimmune insulitis including the behavior of different immune cells and the impairment of β cell mass and function can be microscopically quantified in a non-invasive, longitudinal and intravital manner (Chmelova et al., 2015; Miska et al., 2014; Mojibian et al., 2013; Schmidt-Christensen et al., 2013). Especially noteworthy is that the ACE loses its immune privilege when engrafted with syngeneic islets in the above mouse models (Chmelova et al., 2015; Miska et al., 2014; Mojibian et al., 2013; Schmidt-Christensen et al., 2013). This is due to vascularization of transplanted islets that then become accessible to the immune system of the recipients (Mojibian et al., 2013). Actually, Schmidt-Christensen et al. have found that lymphatic vessel endothelial hyaluronan receptor 1 appear in eyes engrafted with islets but not in intact contralateral eyes and Foxp3+ cells are rarely but surely extravasated from blood vessels into the islet parenchyma (Schmidt-Christensen et al., 2013). These results provide evidence that the immune privilege of the mouse ACE transplanted with syngeneic islets were broken due to lymphatic neogenesis and vascularization (Schmidt-Christensen et al., 2013).
4.2.2. Foxp3+ Treg and Cd11c+ cell infiltration into islets engrafted in ACE
Foxp3+ Treg cells are potential candidates to control autoimmune diseases including insulitis in T1D, but in vivo dynamics of recruited Foxp3+ cells in islets has remained mysterious until we developed the ACE technology (Long & Buckner, 2011; Tang & Bluestone, 2008). This technology has now unraveled this mystery in syngeneic islet grafts in the ACE of NOD mice adoptively transferred with spleen cells from NOD mice carrying Foxp3+ cells genetically labeled with GFP (Schmidt-Christensen et al., 2013). Three-dimensional tracking has disclosed that Foxp3+ cells exhibit round, ruffled and elongated profiles in the engrafted islets as activated and memory T lymphocytes do (Abdulreda et al., 2011; Schmidt-Christensen et al., 2013). Round and elongated Foxp3+ cells mostly appear around the main inflammatory site. In fact, they belonged to the same population of Foxp3+ cells, being elongated during moving likely to serve as inflammation inspectors and turning into a rounded shape at rest. Ruffled Foxp3+ cells invade into the main infiltration. They are intimately bound up with the main inflammatory activity, probably reflecting their destruction of β cells, interactions of Treg cells with their target leucocytes and their activation of Teff cells (Schmidt-Christensen et al., 2013).
CD11c+ cells have been recognized to be a key cellular regulator of autoimmune insulitis (Turley, Poirot, Hattori, Benoist, & Mathis, 2003). However, the intravital trajectory of CD11c+ cells during autoimmune insulitis has long been a conundrum. Dynamics of CD11c+ cells has been visualized in NOD Rag2−/− islets engrafted in the ACE of NOD Cd11c-GFP mice whose Cd11c+ cells express GFP (Schmidt-Christensen et al., 2013). These GFP-labeled Cd11c+ cells comprise a mixture of immune cells including CD11c+ F4/80− dendritic cell (DC)-like cells and CD11c+ F4/80+ macrophages. CD11c+ F4/80− DC-like cells mainly infiltrate into islet grafts, whereas CD11c+ F4/80+ macrophages surround them. Most CD11c+ cells carrying ramified protrusions reside in the infiltrated regions of islet grafts. These DCs are repeatedly stretching and pulling back their dendrites, and are likely to probe their surroundings in line with their likely role in antigen presentation. A minority of CD11c+ cells are small mobile and round and located in the periphery of engrafted islets. Their specific role is ambiguous (Schmidt-Christensen et al., 2013).
4.2.3. CD4+ Teff, CD4+ Treg, CD8+ Teff and CD11c+ cell behavior in the ACE engrafted with islets
Dynamic immune cell interactions within islets have been believed to take center stage in autoimmune insulitis, but never been characterized in vivo (DiMeglio et al., 2018; Katsarou et al., 2017; Morgan, 2017; Pugliese, 2017). To fill the gap, Miska et al. have monitored self-antigen-specific T cell behavior in the mouse ACE engrafted with syngeneic islets following adoptive transfer of self-antigen-specific T cells including CD4+ Teff, CD4+ Treg and CD8+ Teff cells genetically labeled with fluorescence proteins (Miska et al., 2014). The antigen-specific CD4+ Teff cells invade into islet grafts and contact with their target β cells. Correspondingly, apoptotic signals occur in the interface between CD4+ Teff cells and β cells and on β cells. The direct contact between antigen-specific CD4+ Teff cells and their target β cells may account for CD4+ Teff cell killing of their target β cells in damaged islet grafts, regardless of the absence of CD8+ Teff cells (Miska et al., 2014).
In the ACE co-transplanted with islets of Tg mice whose β cells specifically express ovalbumin (ovalbumin+ β cells) and those whose β cells do not express ovalbumin (ovalbumin− β cells), CD8+ OT1 Tg Teff cells, which specifically recognize ovalbumin, selectively kill ovalbumin+ β cells but not adjacent ovalbumin− β cells. Interestingly, the adjacent ovalbumin− β cells instead significantly and quickly grow at the interface of immune destruction. Such growth results from cell replication rather than hypertrophy and only occurs to ovalbumin− islets in close juxtaposition with damaged ovalbumin+ islets, but not to evidently separate ones. The quick replication of ovalbumin− β cells in an inflammatory milieu may be used in a positive way for islet regeneration and diabetes therapy (Miska et al., 2014).
Treg cells orchestrate complex mechanisms to control immune effector function at the target tissue (Josefowicz, Lu, & Rudensky, 2012). The contact-dependent Treg suppression occurs in vitro, but is uncorroborated in vivo in nonlymphoid target tissues such as islets (Hagness et al., 2012; Mempel et al., 2006; Nakamura, Kitani, & Strober, 2001; Tang et al., 2006). Miska et al. have now revealed that most CD4+ Teff cells directly contact CD4+ Treg cells with reduced motility in protected islets engrafted in the ACE of NOD SCID mice reconstituted with antigen-specific CD4+ Treg and Teff cells (Miska et al., 2014). These findings verify that the contact-dependent CD4+ Treg suppression operates in islet grafts in the ACE (Miska et al., 2014).
The homeostasis of antigen-presenting DCs critically takes part in autoimmune damage of pancreatic islets (Dissanayake et al., 2011). However, involvement of DCs in interactions between Treg cells and Teff cells is not known especially in vivo. The ACE technology enables in vivo dynamic microimaging of direct contact-based interactions among CD4+ Treg, CD4+ Teff cells and CD11c+ DCs in CD4+ Treg cell-protected islet grafts in the ACE (Miska et al., 2014). In fact, direct contact-based interactions between CD4+ Treg and CD4+ Teff cells exist with or without CD11c+ DCs, the latter being in the majority. Indeed, the stable interaction of DCs with Treg cells and Teff cells has been shown in in vitro draining lymph nodes. However, such interactions are not involved in stable Treg-Teff cell contact (Tang et al., 2006). Therefore, it appears that DCs do not obligate contact-based Treg-Teff cell interaction. The functional relevance of rare Treg cell-Teff cell-DC interaction remains to be addressed and should not be simply extrapolated from in vitro findings (Miska et al., 2014).
In damaged islet grafts where CD4+ Teff cells predominate over CD4+ Treg cells, CD4+ Treg and CD4+ Teff cells interact with each other in a persistent manner as seen in protected islet grafts. However, the ratio of CD4+ Treg cells to CD4+ Teff cells and the interaction index, i.e., the number of CD4+ Treg-CD4+ Teff cell interaction pairs divided by the number of CD4+ Teff cells, significantly decrease in damaged islet grafts in comparison to protected ones. These decreases reflect reduced CD4+ Treg cell protection and thus precipitates immune damage rather than protection. In fact, islet grafts can undergo either immune protection or damage in the ACE of NOD SCID mice reconstituted with the same mixture of CD4+ BDC2.5 Treg cells and CD4+ BDC2.5 Teff cells in the same batch of experiments. Correspondingly, these mice either suffer from diabetes or stay away from the disease. This happens because the imbalance of CD4+ Teff cells versus CD4+ Treg cells in islet grafts appears in some mice but not in others. Indeed, CD4+ Teff cell density and CD4+ Treg/CD4+ Teff cell ratio increases and decreases, respectively, in damaged islet grafts when diabetes occurs in mice. Importantly, these islet grafts not only suffer infiltration of CD4+ Treg and CD4+ Teff cells, but also show a significant reduction in both CD4+ Treg cell density and CD4+ Treg/CD4+ Teff cell ratio prior to the occurrence of immune damage and the onset of diabetes. This emphasizes that early CD4+ Treg cell recruitment to the inflammatory site is more important than late CD4+ Teff infiltration into the same site in controlling the fate of islet grafts and the onset of diabetes. Furthermore, after acute depletion of CD4+ Treg cells, CD4+ Teff cells released from depleted interaction partners are able to recover their aggressiveness (Miska et al., 2014).
The evidence that CTLA4 functionally regulates both Treg and Teff cells suggests that CTLA4 may be involved in CD4+ Treg-CD4+ Teff cell interaction (Teft, Kirchhof, & Madrenas, 2006; Wing et al., 2008). The possible involvement is verified by blocking CTLA4 with anti-CTLA4-antibody. CTLA4 blockade has no immediate effect on CD4+ Treg-CD4+ Teff cell interaction, but does gradually decrease CD4+ Treg-CD4+ Teff interaction pairs and their interaction time. Furthermore, CTLA4 blockade increases the motility of CD4+ Treg and CD4+ Teff cells. The findings suggest that CTLA4 positively influences CD4+ Treg-CD4+ Teff cell interaction through reducing the motility of CD4+ Treg and CD4+ Teff cells (Miska et al., 2014).
4.2.4. β Cell mass/function dynamics in islets engrafted in the ACE during autoimmune insulitis
Data suggest that β cell mass and function undergo complex changes during autoimmune insulitis progression and remission (DiMeglio et al., 2018; Katsarou et al., 2017; Pugliese, 2017). The true dynamics of these critical events in T1D cannot be visualized directly without adequate approaches. By using the ACE technology, Chmelova et al. have got this task done satisfactorily (Chmelova et al., 2015). They have microscopically characterized β cell mass/function dynamics by measuring β cell-specific GFP and light scattering signals, as readouts of β cell mass and function, emitted from MIP-GFP/RIP-HA+ islets engrafted in the ACE of RIP-HA+ recipient mice reconstituted with preactivated TCR-HA+ T cells. In this setting, the adoptive transfer induces autoimmune insulitis in both intraocular islets and those in the pancreas resulting in hyperglycemia. Initial insulitis rapidly induces significant β cell destruction and marginal β cell degranulation, but not hyperglycemia indicating that an autoimmune attack not only reduces β cell mass, but also impairs β cell function prior to hyperglycermia. Subsequently, hyperglycemia appears. At such a moment, immediate intervention of autoimmune infiltration with anti-CD3 mAbs timely ceases β cell destruction but not β cell degranulation. This brings about a hyperglycemic phase where β cells are quantitatively reduced and functionally exhausted. Thereafter, β cells are continuously degranulated and becomes almost empty of insulin-secretory granules, whereas GFP signal-derived β cell volume significantly increases almost in parallel with hyperglycermia. Finally, β cells become regranulated along with euglycemia restoration. Of note, meanwhile, the increased GFP signal-derived β cell volume unexpectedly dropped back to the basal level. However, this happened due to reduced expression of GFP resulting from decreased insulin promotor activity in MIP-GFP β cells without being related to apoptosis. Therefore, this decline phase of GFP signal-derived β cell volume does not reflect decreased β cell mass. This is verified by analysis of total islet volume as discussed below (Chmelova et al., 2015).
To induce autoimmune infiltration in either of intraocular islets or those situated in the pancreas, different tansplantation settings are chosen by combing RIP-HA+ or RIP-HA− donor islets with RIP-HA+ or RIP-HA− recipient mice (Chmelova et al., 2015). By this way, distinct roles of autoimmune infiltration and hyperglycemia on β cell mass and function are characterized, i.e. adoptive transfer of preactivated HA-specific T cells destroys RIP-HA+ islets without influencing RIP-HA− islets. Selective autoimmune infiltration in MIP-GFP/RIP-HA+ donor islets engrafted in the RIP-HA− recipient ACE not only destroys, but also degranulates β cells in these islet grafts in the absence of hyperglycermia. Subsequent intervention with Anti-CD3 mAb rapidly blocks not only further destruction but also degranulation of β cells. Of note, the immune intervention does not induce an increase of GFP signal-derived β cell volume, which occurs in the presence of both autoimmune infiltration and hyperglycermia. In contrast, following adoptive transfer of preactivated HA-specific T cells into the ACE of RIP-HA+ recipients, consequent hyperglycemia rapidly depletes insulin granules and increases β cell volume in GFP/RIP-HA− islet grafts in the absence of autoimmune infiltration. The effects disappear following glycemic normalization by eliminating autoimmune attack with Anti-CD3 mAb antibody. These findings demonstrate that either autoimmune infiltration or hyperglycemia degranulates β cells impairing insulin secretory capacity independently of each other, whereas hyperglycemia alone is responsible for increased islet β cell volume following treatment with Anti-CD3 mAb antibody (Chmelova et al., 2015).
Interestingly, total islet volume measurements reveal that transient hyperglycemia induced by combining initial adoptive transfer with T cells and subsequent immune intervention brings about a continuous elevation of β cell mass that sustains even after restoration of euglycemia (Chmelova et al., 2015). This event is mainly attributed to glucose-induced β cell proliferation since a remarkable increase of β cell number, a mild enlargement of β cell size and an unaltered incidence of β cell apoptosis are observed (Chmelova et al., 2015).
Furthermore, glucose intolerance not only appears during the onset period of adoptive transfer-induced diabetes but also lasts for additional two weeks after immune intervention-induced remission of the disease before it recovers to its basal level (Chmelova et al., 2015). Importantly, such a glucose intolerance changes in antiparallel with the granular density of β cells in intraocular islet grafts and plasma insulin levels during the onset and remission of the autoimmune diabetes. It is worthwhile to notice that β cells in intraocular islets do not undergo further replication but cumulative regranulation during remission of the autoimmune diabetes. Apparently, amelioration of glucose intolerance subsequent to the immune intervention-induced remission of adoptive transfer-induced diabetes critically relies on the functionality of individual β cells and in particular the insulin secretory capacity rather than the number of these cells. These findings pinpoint the importance of restoration of glucose homeostasis by relieving the metabolic burden of β cells to regain their function (Chmelova et al., 2015).
Chmelova et al. have also visualized the morphological and functional plasticity of human islets transplanted into the ACE of RIP-HA+ mice adoptively reconstituted with CD4+ T cells isolated from TCR-HA mice and treated with anti-CD3 mAb (Chmelova et al., 2015). Human islets are reasonably engrafted and vascularized on the iris of RIP-HA+ mice even in this xenotransplantation setting. The engrafted human islets behave somewhat differently from the mouse intraocular islets in response to transient hyperglycemia induced by adoptive transfer of CD4+ T cells and treatment with anti-CD3 mAb. The adoptive transfer does not influence the morphology and viability of the transplanted human islets, but effectively induces autoimmune insulitis and β cell destruction in in situ endogenous islets of the recipient mice resulting in autoimmune diabetes exhibiting severe hyperglycemia. Subsequent treatment with anti-CD3 mAbs efficiently induces the remission of autoimmune diabetes by immediately halting β cell destruction in recipient mice. Surprisingly, the backscatter signal from the transplanted human islets drastically increases just before onset of hyperglycemia following the adoptive transfer probably due to predominant involvement of human α cells. Thereafter, this signal drops to a nadir of about 60% and recovers once the recipient mice become normoglycemic. In response to hyperglycemia, human β cell mass, reflected by total islet volume, just undergoes a moderate, delayed and transient increase peaking at about 125% after two weeks of hyperglycemia onset. This can be explained by the fact that human β cells undergo relatively low glucose-induced replication in comparison to mouse β cells or by other factors such as age and species. The findings suggest that human β cells display a similar functional plasticity as mouse β cells during transient hyperglycemia, but only transiently undergo a slight but significant morphological change during transient hyperglycemia (Chmelova et al., 2015).
4.3. In vivo alterations in β cell function, mass and insulin resistance in the ACE during T2D development
Insulin resistance and functional β cell mass deficiency serve as key players in T2D development, but their relative contribution varies not only among individual patients but also during the course of the disease (American Diabetes Association, 2014; Danaei et al., 2011; Inzucchi, 2012). Analysis of systemic glucose disposal points out that individuals with T2D progressively develop insulin resistance in their hepatocytes, skeletal muscles, adipocytes and even pancreatic β cells and gradually lose their β cell function and mass throughout the progression from the prediabetes stage to overt T2D (American Diabetes Association, 2014; Avall et al., 2015; Danaei et al., 2011; Halban et al., 2014; Inzucchi, 2012; Meier & Bonadonna, 2013; Rhodes, 2005; Samuel & Shulman, 2016; Weir, Laybutt, Kaneto, Bonner-Weir, & Sharma, 2001). Indisputably, it is of upmost importance to visualize in real-time changes in these pathogenic events non-invasively, intravitally, longitudinally and microscopically. The ACE technology together with other methodologies have microscopically visualized in vivo dynamic profiles of β cell [Ca2+]i, function, mass and insulin resistance in islets engrafted in the ACE during T2D development (Chen et al., 2016; Paschen et al., 2016; Paschen et al., 2018).
4.3.1. β Cell [Ca2+]i dynamics and mass in islets engrafted in the ACE during development of HFD-induced prediabetes
By applying the ACE technology in Tg GCaMP3, MIP-GFP and B6 mice, Chen et al., have characterized β cell [Ca2+]i and mass of mouse islets during progression to prediabetes induced by 17-week high fat diet (HFD) feeding from the initial euglycemic state through the compensatory phase of insulin resistance (Chen et al., 2016). HFD feeding increases the integrated basal level of [Ca2+]i significantly after 8 weeks and decreases the glucose-induced initial peak and integrated glucose-stimulated level of [Ca2+]i significantly after 1 and 4 weeks. In vivo backscatter and GFP imaging shows a gradual rise in β cell mass of intraocular islets following HFD exposure, reaching a significant level at 4 weeks and doubling in size after 16 weeks. As expected, HFD-fed mice gradually gain body weight, exhibit elevations in glycemic levels, plasma insulin concentrations and insulin secretory response to glucose and suffer from glucose intolerance and prediabetes. Furthermore, in vivo β cell [Ca2+]i efficacy, derived by dividing glucose-stimulated plasma insulin by the product of glucose-stimulated [Ca2+]i per minute and fold changes in islet mass at corresponding time points, significantly rises after 1 week of HFD treatment due to activation of Epac signaling. In vivo β cell functional index, obtained by dividing glucose-stimulated plasma insulin by fold changes in β cell mass at corresponding time points, does the same. These findings demonstrate that the altered β cell [Ca2+]i dynamics and efficacy prevail over increased β cell mass to compensate for insulin resistance and prediabetes (Chen et al., 2016). Intriguingly, 2-week normal diet (ND) refeeding suffices to restore the 17-week HFD feeding-induced defects except increased body weight and β cell mass and thereby reverses prediabetes (Chen et al., 2016).
4.3.2. β Cell insulin resistance of islets engrafted in the ACE
Recently, the dynamics of β cell insulin resistance in ACE-engrafted islets whose β cells specifically express insulin resistance biosensor (βIRB) has been demonstrated (Paschen et al., 2016). Application of βIRB in T2D mice homozygous (ob/ob) for the obese spontaneous mutation verifies that this biosensor enables non-invasive microscopic evaluation of the dynamics of in vivo β cell insulin resistance. The ob/ob mice exhibit short-lasting hyperinsulinemia, hyperglycemia and systemic insulin resistance that reaches its peak and drops back to normal when aged 3 and 10 months, respectively. As mentioned earlier, 1-month time is required for full vascularization and innervation of the transplanted islets on the recipient mouse iris. For a best fit to these time frames, syngeneic islets transduced with adenoviral (Ad)-RIP-βIRB are transplanted into the ACE of age-matched littermate recipient B6, ob/+ and ob/ob mice at the age of two months. After one month, in vivo β cell insulin resistance is assessed by microscopically quantifying the ratio of nuclear to cytosolic FoxO1(H215R)-GFP signal in engrafted islets for an 8-month period. As expected, significantly more insulin resistant β cells are present in Ad-RIP-βIRB-transduced islets engrafted in the ACE of 3-month old ob/ob mice than in those of age-matched ob/+ mice. By 10 months of age, the percentage of insulin resistant β cells in Ad-RIP-βIRB-transduced islets implanted in ob/ob mouse ACE significantly drops to a level similar to that in age-matched ob/+ mice. This dynamics of in vivo β cell insulin resistance occurs in phase with that of systemic insulin resistance as validated by glucose and insulin tolerance tests. In contrast, the percentage of insulin resistant β cells keeps stable and remains significantly lower in the experimental settings of ob/+ and B6 mice than in that of ob/ob mice. Furthermore, immunocytochemistry and immunoblot analysis confirm that β cell insulin resistance detected in Ad-RIP-βIRB-transduced islets engrafted in the ob/ob mouse ACE also happens in in situ pancreatic islets of the same recipients mice. These findings verify that the ACE technology is suitable for monitoring the in vivo dynamics of cellular insulin resistance, as exemplified in β cells, in any types of cells if they are transducible by Ad-RIP-βIRB and engraftable in the ACE (Paschen et al., 2016).
More recent work has characterized β cell insulin resistance, functional β cell mass and β cell [Ca2+]i during diet-induced diabetes by combing the AEC technology, expression of βIRB, β cell fluorescence metabolic transcriptional-response indicator (βFLUOMETRI) and GCaMP3 reporter in β cells or islet cells and analysis of systemic metabolism (Paschen et al., 2018). Diabetes-prone male B6 islets expressing these biosensors engrafted in the ACE of syngeneic recipients gradually suffer from β cell insulin resistance, consequently lose functional β cell mass, but display no change in β cell [Ca2+]i within 8-week exposure to a combination of HFD and high sucrose diet (HSD) (HFD-HSD). Analysis of systemic metabolism shows that these recipient mice develop T2D as evidenced by obesity, insulin and glucose intolerances, and noncompensatory insulin release, but not pyruvate intolerance reflecting liver insulin resistance. Importantly, substitution of HFD-HSD by normal chow diet can satisfactorily reverse both the intraocular β cell defects and the systemic diabetic phenotypes, indicating a great plasticity of functional β cell mass during development of prediabetes under dietary stress. Interestingly, β cell insulin resistance develops only following exposure to HFD-HSD feeding but not to treatment with HSD, HFD or HFD plus high fructose diet (HFrD) (HFD-HFrD), suggesting lipotoxicity together with sucrose overload is accounted for the development of β cell insulin resistance in this context. These findings provide evidence that different types of unhealthy diets can induce diabetes through distinct pathogenic mechanisms such as β cell, liver, muscle and fat insulin resistance (Paschen et al., 2018). The ACE technology enables defining a critical time point where β cell insulin resistance and functional β cell mass can be reversed which is important for T2D prevention.
4.4. Rejuvenation of aged islets engrafted in the ACE of young recipients
Age is one of the main risk factors for developing T2D (Niccoli & Partridge, 2012). This is at least partially due to age-associated alterations of islet function and structure (Chang & Halter, 2003; Dillberger, 1994; Gumbiner et al., 1989). However, whether they are intrinsic or extrinsic still remains a matter of debate. To circumvent this issue, Almaca et al. have evaluated the functional recovery, proliferation, vascularization, blood flow, fibrosis and inflammation of young (2 months old) and aged islets (18 months old) implanted into the ACE of young and old mice with STZ-induced diabetes by employing the ACE technology in conjunction with other approaches (Almaca et al., 2014).
The engrafted aged islets surprisingly reverse diabetes in most of the young diabetic recipients and the engrafted young islets expectedly enable all of the young diabetic recipients to become normoglycemic during the first 3 months after transplantation. However, only half of the aged diabetic recipients grafted with aged islets become normoglycemic 6 months post-transplant. Indeed, the young diabetic recipients grafted with aged islets display a decrease in blood glucose disposal in comparison to those with young islets before 7 months post-transplant but after that period the former do not differ from the latter anymore in glucose tolerance. Moreover, they are indistinguishable in terms of plasma insulin levels, grow and gain body weight at a similar rate within 9 months after transplantation. Interestingly, the aged islets engrafted in the ACE of young recipient mice proliferate more actively than the young islets in the same host environment. This is likely attributed to higher blood glucose levels in the early stage of transplantation of aged islets in young recipients. More interestingly, the transplanted aged and young islets are gradually vascularized to a similar extent although the former display a less dense vasculature consisting of larger blood vessels with fewer branches and accommodating faster and more turbulent blood flow in comparison to the latter in the first month after transplantation. Importantly, increases in the fibrosis marker laminin and macrophage density in in situ aged pancreatic islets did not prevail in the aged islets grafts 11 months after transplantation (Almaca et al., 2014).
Undoubtedly, prolonged exposure to the environment in a young organism can revitalize aged islets by reversing the age-associated inflammation and fibrosis of islet vasculature (Almaca et al., 2014). Such revitalized islets can adequately secrete insulin and adaptively proliferate in response to high glucose burden, thereby governing glucose homeostasis (Almaca et al., 2014). The age-associated defects of islet function and structure appear to be extrinsic and reversible (Almaca et al., 2014).
4.5. Appraisals of antidiabetic drugs in the humanized mouse ACE engrafted with human islets
The ACE of humanized immunodeficient mice has been used as a human islet transplantation and imaging site for appraisals of antidiabetic drugs (Abdulreda et al., 2016). The antidiabetic drug liraglutide has been appraised in human islets transplanted into the ACE of immunedeficient athymic nude mice who suffer from STZ-induced diabetes (Abdulreda et al., 2016; Sfairopoulos, Liatis, Tigas, & Liberopoulos, 2018). Each mouse transplanted with 1000 human IEQs (500 human IEQs in each ACE) is subjected to systemic treatment with liraglutide, All liraglutide-treated mice restore normoglycemia in about two weeks, whereas only 70% of control mice treated with saline become normoglycemic in almost double the time. During the initial treatment period with liraglutide, no adverse systemic side effects occur. Unexpectedly, however, prolonged treatment with liraglutide do not further improve the function of the human islets, but instead progressively deteriorate them as evidenced by gradual loss of glycemic control and impaired insulin release from the intraocular human islets. Moreover, the intraocular human islets retrieved after 250 days post-transplantation still show a typical distribution of α and β cells without a sign of cell death. These results may suggest that long-term treatment with liraglutide progressively deteriorates the transplanted human islets even though it initially and temporarily boosts their function. These observations indicate that long-term use of incretin mimetics can exhaust β cells in T2D patients leading to compromised glucose homeostasis due to excessive stimulation of overburdened β cells. (Abdulreda et al., 2016).
4.6. Preclinical application of the ACE technology in non-human primates
Indeed, the ACE of humanized mice can accommodate human islets for in vivo investigation of their physiological processes and pathological changes (Abdulreda et al., 2016). Despite of this, the complexity and pitfalls associated with this human-mouse chimera are not negligible (Walsh et al., 2017). Hence, non-human primates are admittedly deemed to be the ultimate preclinical and translational research model due to their close phylogenetic relationship to humans (Contreras, Smyth, Curiel, & Eckhoff, 2004; Pound, Kievit, & Grove, 2014). It is therefore important to extend the application of the ACE technology to non-human primates.
4.6.1. Intravital imaging of macaque islets transplanted into the autorecipient ACE
Recently, the ACE technology has been applied to the clinically relevant human surrogate macaque (Diez et al., 2017). Macaque islets transplanted into the ACE of partially-pancreatectomized autorecipients attract some iris pigment epithelial cells on top of them within 4 days post-transplantation, but are still visible. They are readily engrafted on the iris. Their vascularization initially occurs between 4 and 10 days after islet transplantation. Large-diameter microvessels first appear and then progressively branch into smaller microvessels in islet grafts. The vascularization of macaque islets engrafted on the macaque iris resembles that in in situ macaque islets and mouse islets transplanted into mouse ACE. Furthermore, α, β and δ cells intermingle with one another. TH-positive sympathetic fibers innervate vascular networks throughout islet grafts retrieved after a post-transplantation period of up to 210 days in a similar manner as observed in in situ macaque islets. In all, the cytoarchitecture, vascularization and innervation of the engrafted islets are appropriately preserved verifying that the macaque ACE provides autologous islets with an adequate niche comparable to the pancreas in macagues. This experimental setting using a clinically relevant human surrogate definitely brings more benefits to basic, translational and clinical research on human health and disease than that using rodents (Diez et al., 2017).
4.6.2. Preclinical transplantation of baboon islets into the baboon ACE
To extend the ACE technology to clinical applications, the preclinical model where the ACE of a STZ-induced diabetic baboon is transplanted with allogeneic islets has been successfully established (Perez et al., 2011). The baboon is subjected to a first transplantation of 20,000 IEQs, pancreatectomy on day 256 after the first transplantation to ablate possible insulin secretion from residual endogenous islets escaping from STZ treatment and a second transplantation of 18,000 IEQs. Most of the transplanted islets aggregate together on the iris of the recipient. They are well engrafted and richly vascularized. Their cytoarchitecture, insulin- and glucagon-immunocytochemical profiles remain unchanged. <15% of intraocular islets disappear during the 357-day observation period. A series of clinical variables verify that islet grafts function well, thereby ameliorating blood glucose homeostasis. The recipient baboon requires less exogenous insulin and shows a gradual decrease in HbA1c and reduced fluctuations in fasting blood glucose. Importantly, C-peptide levels increase in both the plasma and the aqueous humor, but are higher in the latter than in the former after islet transplantation. C-peptide levels rise in response to challenges with either glucose or glucagon. The effect is abolished after removal of the transplanted eye.
Furthermore, thorough eye examinations of both the transplanted eye and the contralateral non-transplanted eye show that the transplantation of 38,000 baboon IEQs into a baboon ACE produced no major adverse effects on eye function and structure. The recipient has no behavioral signs indicative of being blind. Neither mechanical defects nor immune/inflammatory responses occur in the transplanted eye or the contralateral non-transplanted eye. The cornea, conjunctiva, aqueous humor, optic nerve and retina are normal. Our data suggest that the ACE can be safely used as a clinical islet transplantation site for treating diabetes (Perez et al., 2011).
5. Prospective avenues for the ACE technology
The ACE technology brings us significantly closer to understanding the role of islets in the maintenance of glucose homeostasis and the development of diabetes. However, the present state-of-the-art for the ACE technology still leaves room for improvement, and current knowledge gained using this technology is still limited.
Undoubtedly, various interesting aspects in the diabetes arena await us to advance by employing the ACE technology. Perhaps clinical application of the ACE technology in patients with diabetes is one of the most challenging objectives. The ACE technology demonstrates that both mouse and human islets engrafted in the ACE act as the powerful regulators of glucose homeostasis in mice and non-human primates (Almaca et al., 2014; Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Speier, Nyqvist, Cabrera, et al., 2008). This because the ACE offers intraocular islets an immune-privileged niche, oxygen-rich milieu and metabolic stress-reducing environment where they undergo rich vascularization and dense innervation, survive better and become functionally stronger (Abdulreda et al., 2013; Almaca et al., 2014; Almaca et al., 2018; Bader et al., 2016; Borg et al., 2014; Cohrs et al., 2017; Cunha-Vaz, 1979; Diez et al., 2017; Freddo, 1996; Hayreh & Scott, 1978; Ilegems et al., 2013; Ilegems et al., 2015; Kemter et al., 2017; Kragl et al., 2016; Lee et al., 2018; Leibiger et al., 2010; Leibiger et al., 2012; Leibiger & Berggren, 2017; McDougal & Gamlin, 2015; Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Shalaly et al., 2016; Sharifipour et al., 2013; Speier, 2011; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008; Streilein et al., 1992; van Krieken et al., 2017; Zhou & Caspi, 2010). The findings in mice and non-human primates spur us to see what will happen if human islets are transplanted into the ACE of patients with diabetes. In fact, we are performing clinical trials to evaluate the human ACE as a new transplantation site for treating diabetes (Berggren et al., unpublished data). We expect that the ACE as a transplantation site for diabetes therapy in human should prevail over others such as hepatic sinusoids because of the same biological merits as described for rodents and non-human primates (Cunha-Vaz, 1979; Freddo, 1996; Hayreh & Scott, 1978; McDougal & Gamlin, 2015; Sharifipour et al., 2013; Streilein et al., 1992; Zhou & Caspi, 2010). Of note, it is too early to judge whether this is true or not. The conclusion whether or not the human ACE is the optimal clinical islet transplantation site for treating diabetes should be made by comparing the success rate of the aforementioned clinical trials to those applying other sites such as the portal vein in terms of graft survival, function, and insulin independence when necessary data are available. Furthermore, caution should be exercised when using the human ACE as a clinical islet transplantation site. The islet receiving capacity of the ACE to reverse diabetes is significantly lower than other sites. The purity, viability and functionality of human islet preparations, obtained through currently used procedures, vary significantly from batch to batch. They are less uniform in size and more irregular in shape than rodent islet preparations (Ricordi, Lacy, Finke, Olack, & Scharp, 1988; Yamamoto et al., 2009). The currently used procedures for human islet isolation should be improved to obtain high-quality human islets with few or no exocrine tissues to maximize the number of islets implanted into the limited space of the ACE.
It is well known that uncontrolled diabetes as a systemic degenerative disease progressively leads to a series of diabetes complications due to hyperglycemia-induced damages to peripheral nerves and microvascular networks throughout the entire body. In fact, these complications are the real culprit responsible for compromising the patients' life quality and depriving their lives (Forbes & Cooper, 2013; Harcourt, Penfold, & Forbes, 2013). Diabetic neuropathy and vasculopathy have attracted great attention of scientists and clinicians. They could use the ACE technology as new research and diagnostic tools to image pathological changes of peripheral nerves and microvascular networks in the iris or tissues/organs engrafted on it.
Immunodeficient mice bearing human-derived tissues as a humanized mouse model have been proven to be useful in the development of new drugs or treatment regimens (Walsh et al., 2017). The humanized mouse model turns a series of ethically impossible ideas into reality and impressively contributes to in vivo evaluation of the efficacy and toxicology of potential new drugs before they move into clinical trials (Walsh et al., 2017). The humanized mouse model in combination with the ACE technology can be applied for intravital assessment of therapeutic and toxicological effects of new antidiabetic drugs in islets and other relevant tissues like liver, skeletal muscle and adipose tissue.
The ACE technology needs a special confocal/multiphoton microscope equipped with a high-performance, continuous autofocus function in combination with a servomechanism-driven target-chasing system. Another limitation of this technology is the imaging depth and working distance. This makes the ACE technology inferior to conventional non-invasive in vivo imaging modalities like CT, MRI and PET whose penetration depth has no limit (Koo et al., 2006).
Currently, the ACE technology can only be applied to anesthetized, restrained animals. General anesthesia and physical restraining can produce various side effects, especially on imaged islets and systemic metabolism (Zuurbier et al., 2008). We are currently solving these challenges in upgrading this technology for research on awake and freely-moving animals.
Clinical islet transplantation represents a promising therapy for diabetes, but falls into a dilemma due to the scarce human islet availability and allogeneic immune rejection of transplanted islets (Marzorati et al., 2007; McCall & Shapiro, 2014; Pagliuca & Melton, 2013; Shapiro et al., 2000; Shapiro et al., 2006; Shapiro et al., 2017). Fortunately, induced pluripotent stem cell (iPSC) technology offers promising solutions to this dilemma (Takahashi et al., 2007; Yu et al., 2009). Together with the organoid approach, iPSC technology can create immune rejection-free human iPSC-derived surrogate islets from the patient's own somatic cells (Maehr et al., 2009; Millman et al., 2016; Pagliuca et al., 2014; Rezania et al., 2014). This potentially removes the need for donated pancreases and immunosuppressive regimens. However, human iPSC-derived surrogate islets, which secrete notably less insulin following glucose exposure even at quite high concentrations, have not been used to treat patients with diabetes since they cannot mature in vitro into authentic pancreatic islets (Pagliuca et al., 2014; Rezania et al., 2014). It is clear that generation of an unlimited amount of human iPSC-derived surrogate islets is not a difficult task. However, currently there is no way to fully differentiate human iPSC-derived surrogate islets in vitro into authentic islets with the exquisite glucose sensitivity and delicate insulin secretory responsiveness. Nevertheless, human iPSC-derived surrogate islets can normalize blood glucose levels to a certain extent in STZ-induced diabetic immunodeficient mice several months post-transplantation (Pagliuca et al., 2014; Rezania et al., 2014). Obviously, the transplanted human iPSC-derived surrogate islets can only execute their anti-hyperglycemic action in vivo until they differentiate to a certain stage of maturation. As of yet, the basics and underlying mechanisms of the in vivo maturation of the transplanted human iPSC-derived surrogate islets has remained a conundrum due to the lack of appropriate in vivo approaches. The ACE technology allows visualization of in vivo differentiation of the same stem cell-derived organoids including human iPSC-derived surrogate islets non-invasively, longitudinally and repeatedly. In fact, we are mechanistically dissecting the in vivo maturation of human iPSC-derived surrogate islets using the ACE technology to establish effective target- or mechanism-based approaches to direct and fine-tune this process for diabetes therapy.
Islets consisting of electrically excitable endocrine cells critically rely on complex electrical events to govern their function and in particular islet hormone secretion through a variety of ion channels (Berggren et al., 2004; Shi et al., 2014; Yang et al., 1999; Yang et al., 2007; Yang et al., 2014; Yang et al., 2015; Yang and Berggren, 2005a, Yang and Berggren, 2005b, Yang and Berggren, 2006). However, we are still ignorant of how these ion channels behave in vivo due to anatomical barriers to in situ pancreatic islets for patch clamp experiments. Most, if not all, of the electrophysiological studies on these ion channels have been performed with dispersed single islet cells and isolated islets which are deprived of functional nerve endings, vascular networks and in vivo interstitial fluid and experience different types of harmful stress during preparation (Yang & Berggren, 2006). Direct translation of findings obtained from these in vitro preparations to what happens in vivo can inevitably introduce biases and errors (Barker et al., 2013; Leibiger et al., 2012; Weigert et al., 2010). Therefore, it is important to find a way to intravitally characterize ion channels in islets under healthy and diabetic conditions. The ACE technology opens up the possibility to study ion channels in islets in vivo.
6. Conclusions
Challenged by the inaccessibility of islets in situ and spurred by the importance of functional β cell mass and insulin sensitivity in health and diabetes, we have established the ACE technology (Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008). This technology allows in vivo microscopy of islet cytoarchitecture, function and viability in physiological situations and functional β cell mass deficiency, cellular insulin resistance and autoimmune insulitis under diabetic conditions in a non-invasive, longitudinal and real-time manner (Abdulreda et al., 2011; Abdulreda et al., 2013; Abdulreda et al., 2016; Abdulreda & Berggren, 2013; Ali et al., 2016; Almaca et al., 2014; Almaca et al., 2018; Avall et al., 2015; Bader et al., 2016; Berclaz et al., 2016; Borg et al., 2014; Chen et al., 2016; Chmelova et al., 2015; Cohrs et al., 2017; Diez et al., 2017; Faleo et al., 2014; Ilegems et al., 2013; Ilegems et al., 2015; Johansson et al., 2015; Juntti-Berggren et al., 2015; Kemter et al., 2017; Kragl et al., 2016; Lee et al., 2018; Leibiger et al., 2010; Leibiger et al., 2012; Leibiger & Berggren, 2017; Miska et al., 2014; Mojibian et al., 2013; Nyqvist et al., 2011; Paschen et al., 2016; Paschen et al., 2018; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Schmidt-Christensen et al., 2013; Shalaly et al., 2016; Speier, 2011; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008; van Krieken et al., 2017).
The ACE serves as an optimal imaging site and provides implanted islets with the special nursery bed, i.e., the iris, where they undergo engraftment, rich vascularization and dense innervation, keep their cellular composition unaltered and survive in an oxygen-rich milieu and immune-privileged niche (Abdulreda et al., 2013; Almaca et al., 2014; Almaca et al., 2018; Bader et al., 2016; Borg et al., 2014; Cohrs et al., 2017; Cunha-Vaz, 1979; Diez et al., 2017; Freddo, 1996; Hayreh & Scott, 1978; Ilegems et al., 2013; Ilegems et al., 2015; Kemter et al., 2017; Kragl et al., 2016; Lee et al., 2018; Leibiger & Berggren, 2017; Leibiger et al., 2010; Leibiger et al., 2012; McDougal & Gamlin, 2015; Meek, 2009; Meek & Knupp, 2015; Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2018; Rodriguez-Diaz et al., 2012; Shalaly et al., 2016; Sharifipour et al., 2013; Speier, 2011; Speier, Nyqvist, Cabrera, et al., 2008; Speier, Nyqvist, Kohler, et al., 2008; Streilein et al., 1992; van Krieken et al., 2017; Zhou & Caspi, 2010). Importantly, islets engrafted in the ACE are able to control glucose homeostasis in recipients, whose endogenous β cells are depleted by STZ treatment (Almaca et al., 2014; Mojibian et al., 2013; Nyqvist et al., 2011; Perez et al., 2011; Rodriguez-Diaz et al., 2012; Rodriguez-Diaz et al., 2018; Speier, Nyqvist, Cabrera, et al., 2008). Furthermore, the ACE technology reveals that intraocular islets display characteristic [Ca2+]i responses to systemic administration of high glucose (Chen et al., 2016). Moreover, this technology enables in vivo visualization of dynamic changes in functional β mass of islets engrafted into the ACE and development of pancreatic buds implanted into the ACE at cellular resolution in a non-invasive, longitudinal and real-time manner (Ali et al., 2016; Chmelova et al., 2015; Paschen et al., 2018).
The ACE technology has enabled unique intravital observations on in vivo dynamics of immune cell infiltration, multiplex immune cell interplay, immune cell-β cell interaction and β cell mass/function during the onset and remission of autoimmune diabetes (Chmelova et al., 2015; Miska et al., 2014; Mojibian et al., 2013; Schmidt-Christensen et al., 2013). Novel in vivo investigations visualize the dynamic profiles of β cell [Ca2+]i/function/mass and insulin resistance in islets engrafted in the ACE during T2D development (Chen et al., 2016; Paschen et al., 2016; Paschen et al., 2018). They also reveal the rejuvenation of aged islets in the ACE of young mice rendered diabetic by STZ injection, T cell behavior in intraocular islets during allorejection and compromised hypoglycemic activity of human islets in the humanized mouse ACE following long-term treatment with the antidiabetic drug liraglutide (Abdulreda et al., 2011; Abdulreda et al., 2016; Almaca et al., 2014). Furthermore, application of the ACE technology in non-human primates demonstrates that macague islets are well engrafted in the macague ACE where they preserve their cytoarchitecture, vascularization and innervation, and show a characteristic local blood flow (Diez et al., 2017). Importantly, it also verifies that 38,000 baboon IEQs engrafted in an allogeneic baboon ACE rendered diabetic by STZ treatment are able to ameliorate hyperglycemia and have no major adverse effects on eye function and structure, suggesting that the human ACE can be safely used as a clinical islet transplantation site for treating diabetes (Perez et al., 2011).
Remarkable advances in the development and application of the ACE technology motivate us to delineate some prospective avenues, such as its application in antidiabetic drug discovery and clinical diabetes practice. Hence, we expect that the ACE technology will greatly contribute to the prevention and cure of diabetes.
Disclosure of potential conflicts of interest
P.-O.B. is the cofounder and CEO of BioCrine AB. S.-N.Y. is a consultant to Biocrine AB.
Acknowledgments
Our own research discussed in this review was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2010992), funded by the Ministry of Science, ICT & Future Planning (2016K1A1A2912722), R&D Project of the Ministry for Health & Welfare Affairs, Republic of Korea (HI12C0135), Korea Health Industry Development Fund (HI16C2131), the Swedish Diabetes Association, Funds of Karolinska Institutet, The Swedish Research Council, Novo Nordisk Foundation, The Family Erling-Persson Foundation, Strategic Research Program in Diabetes at Karolinska Institutet, The ERC-2013-AdG 338936-BetaImage, The Family Knut and Alice Wallenberg Foundation, Skandia Insurance Company, Ltd., Diabetes and Wellness Foundation, The Bert von Kantzow Foundation, Lee Kong Chian School of Medicine, Nanyang Technological University, Imperial College London and The Stichting af Jochnick Foundation.
Contributor Information
Shao-Nian Yang, Email: shao-nian.yang@ki.se.
Per-Olof Berggren, Email: per-olof.berggren@ki.se.
References
- Abdulreda M.H., Berggren P.O. Islet inflammation in plain sight. Diabetes, Obesity and Metabolism. 2013;15(3 Supplement):105–116. doi: 10.1111/dom.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulreda M.H., Caicedo A., Berggren P.O. Transplantation into the anterior chamber of the eye for longitudinal, non-invasive in vivo imaging with single-cell resolution in real-time. Journal of Visualized Experiments. 2013;73 doi: 10.3791/50466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulreda M.H., Faleo G., Molano R.D., Lopez-Cabezas M., Molina J., Tan Y.…Berggren P.O. High-resolution, noninvasive longitudinal live imaging of immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):12863–12868. doi: 10.1073/pnas.1105002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulreda M.H., Rodriguez-Diaz R., Caicedo A., Berggren P.O. Liraglutide compromises pancreatic β cell function in a humanized mouse model. Cell Metabolism. 2016;23(3):541–546. doi: 10.1016/j.cmet.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanentalo T., Hornblad A., Mayans S., Karin Nilsson A., Sharpe J., Larefalk A.…Holmberg D. Quantification and three-dimensional imaging of the insulitis-induced destruction of β-cells in murine type 1 diabetes. Diabetes. 2010;59(7):1756–1764. doi: 10.2337/db09-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Y., Diez J., Selander L., Zheng X., Edlund H., Berggren P.O. The anterior chamber of the eye is a transplantation site that supports and enables visualisation of beta cell development in mice. Diabetologia. 2016;59(5):1007–1011. doi: 10.1007/s00125-016-3883-x. [DOI] [PubMed] [Google Scholar]
- Almaca J., Molina J., Arrojo E.D.R., Abdulreda M.H., Jeon W.B., Berggren P.O.…Nam H.G. Young capillary vessels rejuvenate aged pancreatic islets. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(49):17612–17617. doi: 10.1073/pnas.1414053111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaca J., Weitz J., Rodriguez-Diaz R., Pereira E., Caicedo A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metabolism. 2018;27(3):630–644. doi: 10.1016/j.cmet.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1 Supplement):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- Avall K., Ali Y., Leibiger I.B., Leibiger B., Moede T., Paschen M.…Juntti-Berggren L. Apolipoprotein CIII links islet insulin resistance to β-cell failure in diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(20):E2611–E2619. doi: 10.1073/pnas.1423849112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader E., Migliorini A., Gegg M., Moruzzi N., Gerdes J., Roscioni S.S.…Lickert H. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature. 2016;535(7612):430–434. doi: 10.1038/nature18624. [DOI] [PubMed] [Google Scholar]
- Barg S., Eliasson L., Renstrom E., Rorsman P. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse β-cells. Diabetes. 2002;51:S74–S82. doi: 10.2337/diabetes.51.2007.s74. [DOI] [PubMed] [Google Scholar]
- Barker C.J., Leibiger I.B., Berggren P.O. The pancreatic islet as a signaling hub. Advances in Biological Regulation. 2013;53(1):156–163. doi: 10.1016/j.jbior.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Berclaz C., Schmidt-Christensen A., Szlag D., Extermann J., Hansen L., Bouwens A.…Holmberg D. Longitudinal three-dimensional visualisation of autoimmune diabetes by functional optical coherence imaging. Diabetologia. 2016;59(3):550–559. doi: 10.1007/s00125-015-3819-x. [DOI] [PubMed] [Google Scholar]
- Berggren P.O., Yang S.N., Murakami M., Efanov A.M., Uhles S., Kohler M.…Flockerzi V. Removal of Ca2+ channel β3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell. 2004;119(2):273–284. doi: 10.1016/j.cell.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Borg D.J., Weigelt M., Wilhelm C., Gerlach M., Bickle M., Speier S.…Hommel A. Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngeneic mouse model. Diabetologia. 2014;57(3):522–531. doi: 10.1007/s00125-013-3109-4. [DOI] [PubMed] [Google Scholar]
- Cabrera O., Jacques-Silva M.C., Speier S., Yang S.N., Kohler M., Fachado A.…Berggren P.O. Glutamate is a positive autocrine signal for glucagon release. Cell Metabolism. 2008;7(6):545–554. doi: 10.1016/j.cmet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A.M., Halter J.B. Aging and insulin secretion. American Journal of Physiology - Endocrinology and Metabolism. 2003;284(1):E7–E12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- Chen C., Chmelova H., Cohrs C.M., Chouinard J.A., Jahn S.R., Stertmann J.…Speier S. Alterations in β-cell calcium dynamics and efficacy outweigh islet mass adaptation in compensation of insulin resistance and prediabetes onset. Diabetes. 2016;65(9):2676–2685. doi: 10.2337/db15-1718. [DOI] [PubMed] [Google Scholar]
- Chmelova H., Cohrs C.M., Chouinard J.A., Petzold C., Kuhn M., Chen C.…Speier S. Distinct roles of β-cell mass and function during type 1 diabetes onset and remission. Diabetes. 2015;64(6):2148–2160. doi: 10.2337/db14-1055. [DOI] [PubMed] [Google Scholar]
- Cline G.W., Zhao X., Jakowski A.B., Soeller W.C., Treadway J.L. Islet-selectivity of G-protein coupled receptor ligands evaluated for PET imaging of pancreatic beta-cell mass. Biochemical and Biophysical Research Communications. 2011;412(3):413–418. doi: 10.1016/j.bbrc.2011.07.077. [DOI] [PubMed] [Google Scholar]
- Cohrs C.M., Chen C., Jahn S.R., Stertmann J., Chmelova H., Weitz J.…Speier S. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology. 2017;158(5):1373–1385. doi: 10.1210/en.2016-1184. [DOI] [PubMed] [Google Scholar]
- Contreras J.L., Smyth C.A., Curiel D.T., Eckhoff D.E. Nonhuman primate models in type 1 diabetes research. ILAR Journal. 2004;45(3):334–342. doi: 10.1093/ilar.45.3.334. [DOI] [PubMed] [Google Scholar]
- Cornelis M.C., Hu F.B. Gene-environment interactions in the development of type 2 diabetes: Recent progress and continuing challenges. Annual Review of Nutrition. 2012;32:245–259. doi: 10.1146/annurev-nutr-071811-150648. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J. The blood-ocular barriers. Survey of Ophthalmology. 1979;23(5):279–296. doi: 10.1016/0039-6257(79)90158-9. [DOI] [PubMed] [Google Scholar]
- Danaei G., Finucane M.M., Lu Y., Singh G.M., Cowan M.J., Paciorek C.J.…Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- Diez J.A., Arrojo E.D.R., Zheng X., Stelmashenko O.V., Chua M., Rodriguez-Diaz R.…Berggren P.O. Pancreatic islet blood flow dynamics in primates. Cell Reports. 2017;20(6):1490–1501. doi: 10.1016/j.celrep.2017.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillberger J.E. Age-related pancreatic islet changes in Sprague-Dawley rats. Toxicologic Pathology. 1994;22(1):48–55. doi: 10.1177/019262339402200107. [DOI] [PubMed] [Google Scholar]
- DiMeglio L.A., Evans-Molina C., Oram R.A. Type 1 diabetes. Lancet. 2018;391(10138):2449–2462. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake D., Hall H., Berg-Brown N., Elford A.R., Hamilton S.R., Murakami K.…Ohashi P.S. Nuclear factor-κB1 controls the functional maturation of dendritic cells and prevents the activation of autoreactive T cells. Nature Medcine. 2011;17(12):1663–1667. doi: 10.1038/nm.2556. [DOI] [PubMed] [Google Scholar]
- Faleo G., Berggren P.O., Pileggi A. Intravital imaging of cytotoxic T lymphocytes. Methods in Molecular Biology. 2014;1186:121–129. doi: 10.1007/978-1-4939-1158-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorina P., Shapiro A.M., Ricordi C., Secchi A. The clinical impact of islet transplantation. American Journal of Transplantation. 2008;8(10):1990–1997. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiological Reviews. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- Freddo T.F. Ultrastructure of the iris. Microscopy Research and Technique. 1996;33(5):369–389. doi: 10.1002/(SICI)1097-0029(19960401)33:5<369::AID-JEMT1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gastrointestinal Endoscopy Technology Committee Confocal laser endomicroscopy. Gastrointestinal Endoscopy. 2014;80(6):928–938. doi: 10.1016/j.gie.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Polonsky K.S., Beltz W.F., Wallace P., Brechtel G., Fink R.I. Effects of aging on insulin secretion. Diabetes. 1989;38(12):1549–1556. doi: 10.2337/diab.38.12.1549. [DOI] [PubMed] [Google Scholar]
- Hagness M., Henjum K., Landskron J., Brudvik K.W., Bjornbeth B.A., Foss A.…Aandahl E.M. Kinetics and activation requirements of contact-dependent immune suppression by human regulatory T cells. Journal of Immunology. 2012;188(11):5459–5466. doi: 10.4049/jimmunol.1101367. [DOI] [PubMed] [Google Scholar]
- Halban P.A., Polonsky K.S., Bowden D.W., Hawkins M.A., Ling C., Mather K.J.…Weir G.C. β-Cell failure in type 2 diabetes: Postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37(6):1751–1758. doi: 10.2337/dc14-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Wang X., Kawamura T., Bindokas V.P., Dizon R.F., Alcoser S.Y.…Bell G.I. Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. American Journal of Physiology - Endocrinology and Metabolism. 2003;284(1):E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- Harcourt B.E., Penfold S.A., Forbes J.M. Coming full circle in diabetes mellitus: From complications to initiation. Nature Reviews Endocrinology. 2013;9(2):113–123. doi: 10.1038/nrendo.2012.236. [DOI] [PubMed] [Google Scholar]
- Hayreh S.S., Scott W.E. Fluorescein iris angiography. I. Normal pattern. Archives of Ophthalmology. 1978;96(8):1383–1389. doi: 10.1001/archopht.1978.03910060137009. [DOI] [PubMed] [Google Scholar]
- Ilegems E., Dicker A., Speier S., Sharma A., Bahow A., Edlund P.K.…Berggren P.O. Reporter islets in the eye reveal the plasticity of the endocrine pancreas. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(51):20581–20586. doi: 10.1073/pnas.1313696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilegems E., van Krieken P.P., Edlund P.K., Dicker A., Alanentalo T., Eriksson M.…Berggren P.O. Light scattering as an intrinsic indicator for pancreatic islet cell mass and secretion. Scientific Reports. 2015;5:10740. doi: 10.1038/srep10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzucchi S.E. Clinical practice. Diagnosis of diabetes. The New England Journal of Medicine. 2012;367(6):542–550. doi: 10.1056/NEJMcp1103643. [DOI] [PubMed] [Google Scholar]
- Jansson L., Barbu A., Bodin B., Drott C.J., Espes D., Gao X.…Carlsson P.O. Pancreatic islet blood flow and its measurement. Upsala Journal of Medical Sciences. 2016;121(2):81–95. doi: 10.3109/03009734.2016.1164769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X., Li D.Q., Olofsson C.S., Salehi A., Surve V.V., Caballero J.…Renstrom E. CaV2.3 calcium channels control second-phase insulin release. The Journal of Clinical Investigation. 2005;115(1):146–154. doi: 10.1172/JCI22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson U., Ria M., Avall K., Dekki Shalaly N., Zaitsev S.V., Berggren P.O.…Hedhammar M. Pancreatic islet survival and engraftment is promoted by culture on functionalized spider silk matrices. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz S.Z., Lu L.F., Rudensky A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annual Review of Immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntti-Berggren L., Ali Y., Berggren P.O. The pancreatic β-cell in deadly encounter with apolipoprotein CIII. Cell Cycle. 2015;14(17):2715–2716. doi: 10.1080/15384101.2015.1064677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsarou A., Gudbjornsdottir S., Rawshani A., Dabelea D., Bonifacio E., Anderson B.J.…Lernmark A. Type 1 diabetes mellitus. Nature Reviews Disease Primers. 2017;3:17016. doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- Kemter E., Cohrs C.M., Schafer M., Schuster M., Steinmeyer K., Wolf-van Buerck L.…Wolf E. INS-eGFP transgenic pigs: A novel reporter system for studying maturation, growth and vascularisation of neonatal islet-like cell clusters. Diabetologia. 2017;60(6):1152–1156. doi: 10.1007/s00125-017-4250-2. [DOI] [PubMed] [Google Scholar]
- Kim C.K., Adhikari A., Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nature Reviews Neuroscience. 2017;18(4):222–235. doi: 10.1038/nrn.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler A.D., Caicedo A., Abdulreda M.H., Faul C., Kerjaschki D., Berggren P.O.…Fornoni A. In vivo imaging of kidney glomeruli transplanted into the anterior chamber of the mouse eye. Scientific Reports. 2014;4:3872. doi: 10.1038/srep03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligler B. The role of the optimal healing environment in the care of patients with diabetes mellitus type II. The Journal of Alternative and Complementary Medicine. 2004;10(1 Supplement):S223–S229. doi: 10.1089/1075553042245926. [DOI] [PubMed] [Google Scholar]
- Koo V., Hamilton P.W., Williamson K. Non-invasive in vivo imaging in small animal research. Cellular Oncology. 2006;28(4):127–139. doi: 10.1155/2006/245619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan C., Tucker T., Alexander M., Mohammadi M.R., Pone E.J., Lakey J.R.T. Approaches in immunotherapy, regenerative medicine, and bioengineering for type 1 diabetes. Fronttiers in Immunology. 2018;9:1354. doi: 10.3389/fimmu.2018.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragl M., Schubert R., Karsjens H., Otter S., Bartosinska B., Jeruschke K.…Lammert E. The biomechanical properties of an epithelial tissue determine the location of its vasculature. Nature Communications. 2016;7:13560. doi: 10.1038/ncomms13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Krieken P.P., Dicker A., Eriksson M., Herrera P.L., Ahlgren U., Berggren P.O.…Ilegems E. Kinetics of functional β cell mass decay in a diphtheria toxin receptor mouse model of diabetes. Scientific Reports. 2017;7(1):12440. doi: 10.1038/s41598-017-12124-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski S., Knap B., Przystupski D., Saczko J., Kedzierska E., Knap-Czop K.…Kulbacka J. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomedicine and Pharmacotherapy. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- Lamprianou S., Immonen R., Nabuurs C., Gjinovci A., Vinet L., Montet X.C.…Meda P. High-resolution magnetic resonance imaging quantitatively detects individual pancreatic islets. Diabetes. 2011;60(11):2853–2860. doi: 10.2337/db11-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langerhans P. Gustav Lange; Berlin: 1869. Beitrage zur mikroscopischen anatomie der bauchspeichel druse, Inaugural-dissertation. [Google Scholar]
- Lee K., Kim J., Kohler M., Yu J., Shi Y., Yang S.N.…Berggren P.O. Blocking Ca2+ channel β3 subunit reverses diabetes. Cell Reports. 2018;24(4):922–934. doi: 10.1016/j.celrep.2018.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibiger I.B., Berggren P.O. Intraocular in vivo imaging of pancreatic islet cell physiology/pathology. Molecular Metabolism. 2017;6(9):1002–1009. doi: 10.1016/j.molmet.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibiger I.B., Brismar K., Berggren P.O. Novel aspects on pancreatic beta-cell signal-transduction. Biochemical and Biophysical Research Communications. 2010;396(1):111–115. doi: 10.1016/j.bbrc.2010.02.174. [DOI] [PubMed] [Google Scholar]
- Leibiger I.B., Caicedo A., Berggren P.O. Non-invasive in vivo imaging of pancreatic β-cell function and survival - a perspective. Acta Physiologica. 2012;204(2):178–185. doi: 10.1111/j.1748-1716.2011.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Chen S., Bellomo E.A., Tarasov A.I., Kaut C., Rutter G.A., Li W.H. Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR) Proceedings of the National Academy of Sciences of the United States of America. 2011;108(52):21063–21068. doi: 10.1073/pnas.1109773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.A., Buckner J.H. CD4+FOXP3+ T regulatory cells in human autoimmunity: More than a numbers game. Journal of Immunology. 2011;187(5):2061–2066. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr R., Chen S., Snitow M., Ludwig T., Yagasaki L., Goland R.…Melton D.A. Generation of pluripotent stem cells from patients with type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(37):15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzorati S., Pileggi A., Ricordi C. Allogeneic islet transplantation. Expert Opinion on Biological Therapy. 2007;7(11):1627–1645. doi: 10.1517/14712598.7.11.1627. [DOI] [PubMed] [Google Scholar]
- Mazier W., Cota D. Islet endothelial cell: Friend and foe. Endocrinology. 2017;158(2):226–228. doi: 10.1210/en.2016-1925. [DOI] [PubMed] [Google Scholar]
- McCall M., Shapiro A.M. Islet cell transplantation. Seminars in Pediatric Surgery. 2014;23(2):83–90. doi: 10.1053/j.sempedsurg.2014.03.006. [DOI] [PubMed] [Google Scholar]
- McDougal D.H., Gamlin P.D. Autonomic control of the eye. Comprehensive Physiology. 2015;5(1):439–473. doi: 10.1002/cphy.c140014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek K.M. Corneal collagen-its role in maintaining corneal shape and transparency. Biophysical Reviews. 2009;1(2):83–93. doi: 10.1007/s12551-009-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek K.M., Knupp C. Corneal structure and transparency. Progress in Retinal and Eye Research. 2015;49:1–16. doi: 10.1016/j.preteyeres.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J.J., Bonadonna R.C. Role of reduced β-cell mass versus impaired β-cell function in the pathogenesis of type 2 diabetes. Diabetes Care. 2013;36(2 Supplement):S113–S119. doi: 10.2337/dcS13-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel T.R., Pittet M.J., Khazaie K., Weninger W., Weissleder R., von Boehmer H.…von Andrian U.H. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25(1):129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Millman J.R., Xie C., Van Dervort A., Gurtler M., Pagliuca F.W., Melton D.A. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nature Communications. 2016;7:11463. doi: 10.1038/ncomms11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska J., Abdulreda M.H., Devarajan P., Lui J.B., Suzuki J., Pileggi A.…Chen Z. Real-time immune cell interactions in target tissue during autoimmune-induced damage and graft tolerance. Journal of Experimental Medicine. 2014;211(3):441–456. doi: 10.1084/jem.20130785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojibian M., Harder B., Hurlburt A., Bruin J.E., Asadi A., Kieffer T.J. Implanted islets in the anterior chamber of the eye are prone to autoimmune attack in a mouse model of diabetes. Diabetologia. 2013;56(10):2213–2221. doi: 10.1007/s00125-013-3004-z. [DOI] [PubMed] [Google Scholar]
- Morgan N.G. Bringing the human pancreas into focus: New paradigms for the understanding of type 1 diabetes. Diabetic Medicine. 2017;34(7):879–886. doi: 10.1111/dme.13365. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Kitani A., Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. Journal of Experimental Medicine. 2001;194(5):629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D.M. Diabetes: Advances in diagnosis and treatment. The Journal of the American Medical Association. 2015;314(10):1052–1062. doi: 10.1001/jama.2015.9536. [DOI] [PubMed] [Google Scholar]
- Niccoli T., Partridge L. Ageing as a risk factor for disease. Current Biology. 2012;22(17):R741–R752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Nielsen J. Systems biology of metabolism: A driver for developing personalized and precision medicine. Cell Metabolism. 2017;25(3):572–579. doi: 10.1016/j.cmet.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Nyman L.R., Wells K.S., Head W.S., McCaughey M., Ford E., Brissova M.…Powers A.C. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. The Journal of Clinical Investigation. 2008;118(11):3790–3797. doi: 10.1172/JCI36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyqvist D., Speier S., Rodriguez-Diaz R., Molano R.D., Lipovsek S., Rupnik M.…Berggren P.O. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes. 2011;60(10):2571–2577. doi: 10.2337/db10-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca F.W., Melton D.A. How to make a functional β-cell. Development. 2013;140(12):2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H.…Melton D.A. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen M., Moede T., Leibiger B., Jacob S., Bryzgalova G., Leibiger I.B.…Berggren P.O. Non-invasive cell type selective in vivo monitoring of insulin resistance dynamics. Scientific Reports. 2016;6:21448. doi: 10.1038/srep21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen M., Moede T., Valladolid-Acebes I., Leibiger B., Moruzzi N., Jacob S.…Berggren P.O. Diet-induced beta-cell insulin resistance results in reversible loss of functional β-cell mass. FASEB Journal. 2018;33(1):204–218. doi: 10.1096/fj.201800826R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper A.R., Gala-Lopez B., Ziff O., Shapiro A.M. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clinical and Developmental Immunology. 2013;2013:352315. doi: 10.1155/2013/352315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez V.L., Caicedo A., Berman D.M., Arrieta E., Abdulreda M.H., Rodriguez-Diaz R.…Berggren P.O. The anterior chamber of the eye as a clinical transplantation site for the treatment of diabetes: A study in a baboon model of diabetes. Diabetologia. 2011;54(5):1121–1126. doi: 10.1007/s00125-011-2091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound L.D., Kievit P., Grove K.L. The nonhuman primate as a model for type 2 diabetes. Current Opinion in Endocrinology, Diabetes, and Obesity. 2014;21(2):89–94. doi: 10.1097/MED.0000000000000043. [DOI] [PubMed] [Google Scholar]
- Pugliese A. Autoreactive T cells in type 1 diabetes. The Journal of Clinical Investigation. 2017;127(8):2881–2891. doi: 10.1172/JCI94549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Dominguez M. Historical background of pancreatic islet isolation. Advances in Experimental Medicine and Biology. 2016;938:1–9. doi: 10.1007/978-3-319-39824-2_1. [DOI] [PubMed] [Google Scholar]
- Reiner T., Thurber G., Gaglia J., Vinegoni C., Liew C.W., Upadhyay R.…Weissleder R. Accurate measurement of pancreatic islet β-cell mass using a second-generation fluorescent exendin-4 analog. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):12815–12820. doi: 10.1073/pnas.1109859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A.…Kieffer T.J. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature Biotechnology. 2014;32(11):1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- Rhodes C.J. Type 2 diabetes-a matter of β-cell life and death? Science. 2005;307(5708):380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- Ricordi C., Lacy P.E., Finke E.H., Olack B.J., Scharp D.W. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Diaz R., Molano R.D., Weitz J.R., Abdulreda M.H., Berman D.M., Leibiger B.…Berggren P.O. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metabolism. 2018;27(3):549–558. doi: 10.1016/j.cmet.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R., Speier S., Molano R.D., Formoso A., Gans I., Abdulreda M.H.…Caicedo A. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(52):21456–21461. doi: 10.1073/pnas.1211659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46(8):1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- Salunkhe V.A., Mollet I.G., Ofori J.K., Malm H.A., Esguerra J.L., Reinbothe T.M.…Vikman J. Dual effect of rosuvastatin on glucose homeostasis through improved insulin sensitivity and reduced insulin secretion. eBioMedicine. 2016;10:185–194. doi: 10.1016/j.ebiom.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel V.T., Shulman G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. The Journal of Clinical Investigation. 2016;126(1):12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Christensen A., Hansen L., Ilegems E., Fransen-Pettersson N., Dahl U., Gupta S.…Holmberg D. Imaging dynamics of CD11c+ cells and Foxp3+ cells in progressive autoimmune insulitis in the NOD mouse model of type 1 diabetes. Diabetologia. 2013;56(12):2669–2678. doi: 10.1007/s00125-013-3024-8. [DOI] [PubMed] [Google Scholar]
- Sfairopoulos D., Liatis S., Tigas S., Liberopoulos E. Clinical pharmacology of glucagon-like peptide-1 receptor agonists. Hormones. 2018 doi: 10.1007/s42000-018-0038-0. [DOI] [PubMed] [Google Scholar]
- Shalaly N.D., Ria M., Johansson U., Avall K., Berggren P.O., Hedhammar M. Silk matrices promote formation of insulin-secreting islet-like clusters. Biomaterials. 2016;90:50–61. doi: 10.1016/j.biomaterials.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Shapiro A.M., Lakey J.R., Ryan E.A., Korbutt G.S., Toth E., Warnock G.L.…Rajotte R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. The New England Journal of Medicine. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- Shapiro A.M., Pokrywczynska M., Ricordi C. Clinical pancreatic islet transplantation. Nature Reviews Endocrinology. 2017;13(5):268–277. doi: 10.1038/nrendo.2016.178. [DOI] [PubMed] [Google Scholar]
- Shapiro A.M., Ricordi C., Hering B.J., Auchincloss H., Lindblad R., Robertson R.P.…Lakey J.R. International trial of the Edmonton protocol for islet transplantation. The New England Journal of Medicine. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- Sharifipour F., Idani E., Zamani M., Helmi T., Cheraghian B. Oxygen tension in the aqueous humor of human eyes under different oxygenation conditions. Journal of Ophthalmic and Vision Research. 2013;8(2):119–125. [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Yang G., Yu J., Yu L., Westenbroek R., Catterall W.A.…Yang S.N. Apolipoprotein CIII hyperactivates β cell CaV1 channels through SR-BI/β1 integrin-dependent coactivation of PKA and Src. Cellular and Molecular Life Sciences. 2014;71(7):1289–1303. doi: 10.1007/s00018-013-1442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speier S. Experimental approaches for high-resolution in vivo imaging of islet of Langerhans biology. Current Diabetes Reports. 2011;11(5):420–425. doi: 10.1007/s11892-011-0207-x. [DOI] [PubMed] [Google Scholar]
- Speier S., Nyqvist D., Cabrera O., Yu J., Molano R.D., Pileggi A.…Berggren P.O. Noninvasive in vivo imaging of pancreatic islet cell biology. Nature Medicine. 2008;14(5):574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speier S., Nyqvist D., Kohler M., Caicedo A., Leibiger I.B., Berggren P.O. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nature Protocols. 2008;3(8):1278–1286. doi: 10.1038/nprot.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J.W., Wilbanks G.A., Taylor A., Cousins S. Eye-derived cytokines and the immunosuppressive intraocular microenvironment: A review. Current Eye Research. 1992;11(1 Supplement):41–47. doi: 10.3109/02713689208999510. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Kanamori T., Inouye S. Quantitative visualization of synchronized insulin secretion from 3D-cultured cells. Biochemical and Biophysical Research Communications. 2017;486(4):886–892. doi: 10.1016/j.bbrc.2017.03.105. [DOI] [PubMed] [Google Scholar]
- Tahrani A.A., Barnett A.H., Bailey C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nature Reviews Endocrinology. 2016;12(10):566–592. doi: 10.1038/nrendo.2016.86. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K.…Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tang Q., Adams J.Y., Tooley A.J., Bi M., Fife B.T., Serra P.…Bluestone J.A. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nature Immunology. 2006;7(1):83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Bluestone J.A. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nature Immunology. 2008;9(3):239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauschmann M., Hovorka R. Technology in the management of type 1 diabetes mellitus - current status and future prospects. Nature Endocrinology. 2018;14(8):464–475. doi: 10.1038/s41574-018-0044-y. [DOI] [PubMed] [Google Scholar]
- Taylor R. Type 2 diabetes: Etiology and reversibility. Diabetes Care. 2013;36(4):1047–1055. doi: 10.2337/dc12-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teft W.A., Kirchhof M.G., Madrenas J. A molecular perspective of CTLA-4 function. Annual Review of Immunology. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- Turley S., Poirot L., Hattori M., Benoist C., Mathis D. Physiological β cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. Journal of Experimental Medicine. 2003;198(10):1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani E., Baudouin C., Efron N., Hamrah P., Kojima T., Patel S.V.…Dogru M. In vivo confocal microscopy of the ocular surface: From bench to bedside. Current Eye Research. 2014;39(3):213–231. doi: 10.3109/02713683.2013.842592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger M., Goulley J., Friedrich M., Grapin-Botton A., Meda P., Lasser T.…Leitgeb R.A. In vivo imaging of murine endocrine islets of Langerhans with extended-focus optical coherence microscopy. Diabetologia. 2009;52(8):1599–1607. doi: 10.1007/s00125-009-1383-y. [DOI] [PubMed] [Google Scholar]
- Virostko J., Radhika A., Poffenberger G., Dula A.N., Moore D.J., Powers A.C. Bioluminescence imaging reveals dynamics of β cell loss in the non-obese diabetic (NOD) mouse model. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0057784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh N.C., Kenney L.L., Jangalwe S., Aryee K.E., Greiner D.L., Brehm M.A.…Shultz L.D. Humanized mouse models of clinical disease. Annual Review of Pathology. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert R., Sramkova M., Parente L., Amornphimoltham P., Masedunskas A. Intravital microscopy: A novel tool to study cell biology in living animals. Histochemistry and Cell Biology. 2010;133(5):481–491. doi: 10.1007/s00418-010-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G.C., Bonner-Weir S. Islets of Langerhans: The puzzle of intraislet interactions and their relevance to diabetes. The Journal of Clinical Investigation. 1990;85(4):983–987. doi: 10.1172/JCI114574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G.C., Laybutt D.R., Kaneto H., Bonner-Weir S., Sharma A. β-Cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001;50:S154–S159. doi: 10.2337/diabetes.50.2007.s154. [DOI] [PubMed] [Google Scholar]
- Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z.…Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Horiguchi A., Ito M., Nagata H., Ichii H., Ricordi C.…Miyakawa S. Quality control for clinical islet transplantation: Organ procurement and preservation, the islet processing facility, isolation, and potency tests. Journal of Hepato-Biliary-Pancreatic Surgery. 2009;16(2):131–136. doi: 10.1007/s00534-009-0064-z. [DOI] [PubMed] [Google Scholar]
- Yang G., Shi Y., Yu J., Li Y., Yu L., Welling A.…Yang S.N. CaV1.2 and CaV1.3 channel hyperactivation in mouse islet β cells exposed to type 1 diabetic serum. Cellular and Molecular Life Sciences. 2015;72(6):1197–1207. doi: 10.1007/s00018-014-1737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.N., Tang Y.G., Zucker R.S. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. Journal of Neurophysiology. 1999;81(2):781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Yang S.N., Berggren P.O. β-Cell CaV channel regulation in physiology and pathophysiology. American Journal of Physiology. Endocrinology and Metabolism. 2005;288(1):E16–E28. doi: 10.1152/ajpendo.00042.2004. [DOI] [PubMed] [Google Scholar]
- Yang S.N., Berggren P.O. CaV2.3 channel and PKCλ: New players in insulin secretion. The Journal of Clinical Investigation. 2005;115(1):16–20. doi: 10.1172/JCI23970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.N., Berggren P.O. The role of voltage-gated calcium channels in pancreatic β-cell physiology and pathophysiology. Endocrine Reviews. 2006;27(6):621–676. doi: 10.1210/er.2005-0888. [DOI] [PubMed] [Google Scholar]
- Yang S.N., Larsson O., Branstrom R., Bertorello A.M., Leibiger B., Leibiger I.B.…Berggren P.O. Syntaxin 1 interacts with the LD subtype of voltage-gated Ca2+ channels in pancreatic β cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(18):10164–10169. doi: 10.1073/pnas.96.18.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.N., Shi Y., Yang G., Li Y., Yu J., Berggren P.O. Ionic mechanisms in pancreatic β cell signaling. Cellular and Molecular Life Sciences. 2014;71(21):4149–4177. doi: 10.1007/s00018-014-1680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.N., Wenna N.D., Yu J., Yang G., Qiu H., Yu L.…Berggren P.O. Glucose recruits KATP channels via non-insulin-containing dense-core granules. Cell Metabolism. 2007;6(3):217–228. doi: 10.1016/j.cmet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Yu J., Hu K., Smuga-Otto K., Tian S., Stewart R., Slukvin I.I.…Thomson J.A. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Caspi R.R. Ocular immune privilege. F1000 Biology Reports. 2010;2 doi: 10.3410/B2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. Photorelease techniques for raising or lowering intracellular Ca2+ Methods in Cell Biology. 2010;99:27–66. doi: 10.1016/B978-0-12-374841-6.00002-5. [DOI] [PubMed] [Google Scholar]
- Zuurbier C.J., Keijzers P.J., Koeman A., Van Wezel H.B., Hollmann M.W. Anesthesia's effects on plasma glucose and insulin and cardiac hexokinase at similar hemodynamics and without major surgical stress in fed rats. Anesthesia and Analgesia. 2008;106(1):135–142. doi: 10.1213/01.ane.0000297299.91527.74. [DOI] [PubMed] [Google Scholar]