Abstract
Introduction
Heterogeneity of outcomes in Alzheimer's disease (AD) clinical trials necessitates large sample sizes and contributes to study failures. This analysis determined whether mild-to-moderate AD populations could be enriched for cognitive decline based on apolipoprotein (APOE) ε4 genotype, family history of AD, and amyloid abnormalities.
Methods
Modeling estimated the number of randomized patients needed to detect a 2-point treatment difference on the AD Assessment Scale–Cognitive subscale using placebo data from three randomized, double-blind trials (ClinicalTrials.gov Identifiers: NCT01955161, NCT02006641, and NCT02006654).
Results
An 80% power to detect a 2-point treatment effect required the randomization of 148 amyloid-positive patients; 178 ε4 homozygous or amyloid-positive patients; and 231 ε4 homozygous, family history-positive, or amyloid-positive patients, compared with 1619 unenriched patients (per arm).
Discussion
Enrichment in mild-to-moderate AD clinical trials can be achieved using combinations of biomarkers/risk factors to increase the likelihood of observing potential treatment effects.
Keywords: Alzheimer's disease, Clinical trial, Enrichment, Power, Biomarkers
Highlights
-
•
APOE ɛ4, family history, and amyloid status can enrich mild-to-moderate AD samples.
-
•
Enrichment increases the likelihood of detecting a treatment effect on cognition.
-
•
Enrichment may reduce sample sizes in clinical trials of symptomatic drugs in AD.
1. Background
Developing new and more effective treatments for Alzheimer's disease (AD) is an urgent priority; however, there have been no new licensed pharmacologic therapies for 15 years [1]. The drive to develop disease-modifying treatments for people with preclinical AD is vital but has so far been unsuccessful and has led to a reduced focus on the treatment needs of people with symptomatic AD. The failure rate of AD drug development is close to 100%, attributed to lack of efficacy and excessive side effects with investigational agents, as well as challenges in trial execution, including a lack of decline in the placebo group [1]. Late-onset AD is a heterogeneous disease, possibly even multiple diseases with varying clinical profiles, and this heterogeneity will also impact on trial outcomes [2], [3], [4]. The high failure rate and cost of randomized controlled trials, driven by clinical heterogeneity and the related need for large sample sizes, have led to inefficiencies in randomized controlled trials over the last decade. As a consequence, there are currently just 112 agents in the AD treatment pipeline, 10-fold less than the number of agents in development for the treatment of cancer [5], [6].
Sample enrichment is recognized increasingly as a key component of AD clinical trial design for disease-modifying and symptomatic agents, to identify cohorts with biologically confirmed AD and more rapid decline and thereby to reduce required sample sizes and improve the chances of detecting a treatment effect. To date, most research has used neuroimaging approaches, notably positron emission tomography (PET) imaging (particularly with amyloid ligands), with the major goal of reliably identifying patients with prodromal AD/mild cognitive impairment who are likely to experience cognitive decline [7], [8], [9]. Cerebrospinal fluid (CSF) amyloid has also been used to enrich mild cognitive impairment populations [9].
Heterogeneity is also a major confounder in randomized controlled trials focusing on people with mild-to-moderate AD, but fewer studies have explored enrichment techniques in this population [8], [10]. The ongoing AD Neuroimaging Initiative 3, which aims to validate biomarkers for use in AD clinical trials, is providing useful data to improve enrichment designs in studies of mild AD dementia [11]. In contrast, among people with moderate AD dementia, enrichment techniques have been used only to identify special populations (e.g., enrichment for behavioral issues based on Neuropsychiatric Inventory [NPI] score [12]) and not specifically to identify populations with accelerated cognitive decline.
Amyloid-PET neuroimaging is not always feasible at clinical trial sites and may not be feasible in all people with moderate AD. Furthermore, enrichment work to date has not focused on moderate AD or symptomatic treatments. There is a need to broaden enrichment strategies to enable realistic, cost-effective recruitment across sites, even in locations where PET imaging is not routinely available, and to develop reliable approaches that are applicable to people with moderate AD.
Using data from placebo-treated patients in the multinational phase 3 clinical program of idalopirdine, this analysis explored whether enrichment, based on apolipoprotein E (APOE) ε4 genotype, family history of AD, and abnormal amyloid status (PET or CSF) can be used to identify a group of patients with mild-to-moderate AD who show more rapid and more consistent decline over 6 months. APOE ε4 carriage is a risk factor for developing late-onset AD, and at an earlier age, with homozygotes showing increased risk compared with heterozygotes [13]. Having a first-degree relative with AD is a risk factor for developing dementia [14], and amyloid PET and CSF profiles are biomarkers related to AD pathology [15], [16].
2. Methods
2.1. Study design and patient population
Data were pooled from three similarly designed, randomized, double-blind, placebo-controlled studies in mild-to-moderate AD: STARSHINE (ClinicalTrials.gov identifier NCT01955161), STARBEAM (NCT02006641), and STARBRIGHT (NCT02006654). The studies were conducted in 34 countries worldwide from October 2013 to January 2017. For a full description of the designs and outcomes of these studies, see the study by Atri et al. (2018) [17]. All studies were conducted in accordance with the International Conference on Harmonisation Good Clinical Practice Guideline and the Declaration of Helsinki. Local ethics committees approved all aspects of study design. Eligible patients or their legal representatives provided written informed consent before starting the studies.
Briefly, the studies included outpatients aged ≥50 years with a National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association (NINCDS-ADRDA) criteria diagnosis of probable AD [18], a Mini–Mental State Examination (MMSE) score of 12–22 at screening [19], and who had received a therapeutic and stable dose of a cholinesterase inhibitor (ChEI) for ≥4 months before screening (donepezil in STARSHINE and STARBEAM; any ChEI in STARBRIGHT). Patients were excluded if they were taking memantine, had an alternative cause of dementia, had serious non-AD central nervous system or somatic disorders, had clinically significant abnormalities (determined by laboratory testing), or were taking concomitant medications that would interfere with the safety and efficacy assessments.
This article presents results for only those patients randomized to placebo, taken in addition to their base ChEI treatment.
2.2. Outcomes
The primary outcome measure of each study was the AD Assessment Scale–Cognitive subscale (ADAS-Cog), scored from 0–70, where a higher score indicates more cognitive impairment [20]. Key secondary outcome measures were the AD Cooperative Study–Activities of Daily Living, 23-item version (ADCS-ADL23), scored from 0–78, where a higher score indicates less functional impairment [21], [22]; and the AD Cooperative Study–Clinical Global Impression of Change (ADCS-CGIC), a global rating scored at baseline from 1 (normal, not at all ill) to 7 (among the most extremely ill patients) and at follow-up from 1 (marked improvement) to 7 (marked worsening) [23], [24]. Other secondary outcomes included the NPI, scored from 0–144, where a higher score indicates more behavioral disturbance [25], and the MMSE, scored from 0–30, where a higher score indicates less cognitive impairment [19].
2.3. Statistical analysis
In this analysis, enrichment was performed using a selection of biomarkers/risk factors for AD, individually and in combination, to identify an enriched population of patients likely to experience more rapid cognitive decline.
The biomarkers/risk factors were prespecified by the coordinating investigators before conducting the analysis (but after review of the overall results of the three trials) and comprised (1) APOE ε4 carrier (“ε4+”) or homozygote (“ε4++”); (2) first-degree relative with AD (“FH+”); and (3) amyloid positivity (“A+”). APOE genotyping was scheduled in all patients at baseline. Family history of AD was reported by the patient/caregiver. Amyloid status was defined on the basis of amyloid PET or CSF profiles. There was no requirement for amyloid positivity in the idalopirdine program; patient medical histories were used, and only 10.4% of patients (258/2475) had such data at study entry. Owing to the small number of patients with amyloid PET or CSF data, these two biomarkers were grouped together. Both are measures of amyloid pathology, identify the same patient population [26], and have similar, high accuracy in identifying early AD [27].
The following combined biomarker/risk factor enrichment groups were defined, with patients counted a maximum of once per group: (1) confirmed APOE ε4 carrier, first-degree relative with AD, or amyloid positive (“ε4+/FH+/A+”); (2) confirmed APOE ε4 homozygous, first-degree relative with AD, or amyloid positive (“ε4++/FH+/A+”); and (3) confirmed APOE ε4 homozygous or amyloid positive (“ε4++/A+”).
Analyses were conducted in the full analysis set (FAS), defined as all randomized patients who took at least one dose of investigational medicinal product and had a valid baseline and post-baseline ADAS-Cog assessment (n = 2475; placebo FAS, n = 939). Baseline characteristics are presented using descriptive statistics. Changes from baseline in rating scale scores were analyzed using a restricted maximum likelihood–based mixed model for repeated measures approach. The model adjusted for MMSE stratum (12–18 or 19–22), ChEI therapy stratum (donepezil or rivastigmine/galantamine), and baseline score at each visit, as well as country as a fixed factor across visits, and study, with a study-by-visit interaction term. Finally, the model included a three-way interaction between enrichment group membership, treatment, and visit. A sensitivity analysis was performed by also adjusting for age (adding an age-by-week interaction to the model). The model is described in more detail in Supplementary Material. Testing was carried out for the least-square means estimate of contrasts across group membership in the three-way interaction term; P values were computed using a t-test with Kenward-Roger approximation to calculate denominator degrees of freedom. Testing for enrichment group contrasts of the three-way interaction term used “lsmeans” in “proc mixed” in SAS, version 9.4 (SAS Institute Inc). P values were tested at a significance level of 0.05 (two-sided) with no correction for multiple comparisons.
2.4. Study power modeling
Observed change in ADAS-Cog score in the pooled placebo group was used to estimate the power gains (amounting to sample size reduction) that could be achieved by enrichment. The model assumed that the average active treatment effect could not improve cognitive scores to above the baseline level after 24 weeks, and thus the observed treatment effect would be masked by a lack of decline in the placebo group. Consequently, the power to detect a statistically significant treatment effect depended on sufficient decline in the placebo group. The model assumed a maximum potential treatment effect of 2 points on the ADAS-Cog at week 24 and a standard deviation of 5.87 (as observed in a pooled mixed model for repeated measures analysis of the three idalopirdine trials). Power calculations assumed an active treatment arm versus placebo, evaluating treatment effects with a two-sample t-test at the 0.05 level. Power calculations were performed for the individual biomarker/risk factor groups and the combined enrichment groups. Power as a function of number of randomized patients was calculated assuming equal withdrawal rates in both treatment arms, matching the observed withdrawal in the corresponding placebo enrichment groups. In addition, assuming a randomized-to-screened ratio of 63.4% (average observed ratio in the three idalopirdine trials) and using the observed fraction of the total population that each enrichment group comprised, power as a function of number of screened patients was calculated.
3. Results
3.1. Individual biomarker/risk factor groups
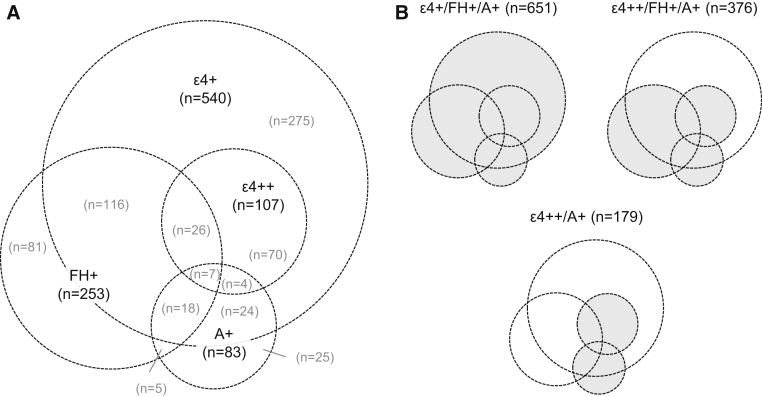
In the placebo FAS (n = 939), 540 patients (57.5%) were confirmed ε4+, 107 (11.4%) were confirmed ε4++, and 253 (26.9%) were known FH+. Eighty-three patients were confirmed A+ (8.8% of the placebo FAS, but 84.7% of the 98 patients who were tested). Fig. 1A shows the overlap between biomarker/risk factor groups.
Fig. 1.
Distribution of biomarkers/risk factors, (A) individually and (B) in combination, among patients receiving placebo. Abbreviations: A+, Amyloid positive (n = 98 tested); APOE, apolipoprotein E; ε4+, APOE ε4 carrier; ε4++, APOE ε4 homozygous; FH+, first-degree relative with Alzheimer's disease.
Considering baseline demographic and clinical characteristics (Table 1A), ε4++ patients were younger than APOE ε4 heterozygous (“ε4+−”) patients and noncarriers (“ε4−”). FH+ patients were more likely to be male than those without a first-degree relative with AD (“FH−”). A+ patients, compared with patients who were not positive/not tested (“A−”), were younger, had a higher level of education, and had a higher level of functioning.
Table 1.
Baseline demographic and clinical characteristics of patients receiving placebo
| A. Split by individual biomarker/risk factor status | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic |
APOE ε4 carriage∗ |
FH+† |
A+ |
||||
| ε4++ (n = 107) | ε4+− (n = 433) | ε4− (n = 374) | Yes (n = 253) | No (n = 680) | Yes (n = 83) | No or not tested (n = 856) | |
| Age, mean (SD), years | 70.9 (7.0) | 74.1 (6.9) | 74.4 (9.2) | 73.5 (7.3) | 74.0 (8.2) | 67.6 (7.6) | 74.4 (7.8) |
| Female, n (%) | 67 (62.6) | 278 (64.2) | 240 (64.2) | 148 (58.5) | 450 (66.2) | 54 (65.1) | 545 (63.7) |
| BMI, mean (SD), kg/m2 | 25.9 (4.3) | 26.1 (4.3) | 26.2 (4.8) | 26.2 (4.7) | 26.1 (4.5) | 25.5 (4.0) | 26.2 (4.6) |
| Education, mean (SD), years | 11.3 (3.8) | 11.1 (4.0) | 11.0 (4.4) | 11.1 (4.1) | 11.0 (4.3) | 12.2 (4.4) | 10.9 (4.2) |
| Time since AD diagnosis, mean (SD), years | 2.6 (2.1) | 2.2 (1.8) | 2.3 (1.9) | 2.2 (1.9) | 2.3 (1.9) | 2.0 (1.7) | 2.3 (1.9) |
| Prestudy treatment duration, mean (SD), years | 2.1 (2.0) | 1.7 (1.6) | 1.7 (1.7) | 1.7 (1.5) | 1.8 (1.8) | 1.8 (1.9) | 1.8 (1.7) |
| Screening MMSE, mean (SD) | 16.9 (3.1) | 17.6 (2.9) | 17.6 (2.9) | 17.9 (2.8) | 17.3 (3.0) | 17.3 (3.0) | 17.5 (2.9) |
| Screening MMSE stratum, n (%) | |||||||
| 19–22 | 42 (39.3) | 182 (42.0) | 157 (42.0) | 113 (44.7) | 271 (39.9) | 32 (38.6) | 356 (41.6) |
| 12–18 | 65 (60.7) | 251 (58.0) | 217 (58.0) | 140 (55.3) | 409 (60.1) | 51 (61.4) | 500 (58.4) |
| ADAS-Cog, mean (SD) | 26.4 (8.5) | 25.3 (7.9) | 26.2 (8.6) | 25.1 (8.3) | 26.1 (8.3) | 26.0 (8.9) | 25.8 (8.3) |
| ADCS-CGIC, mean (SD) | 3.8 (0.7) (n = 106) |
3.8 (0.7) (n = 431) |
3.8 (0.8) (n = 373) |
3.7 (0.7) (n = 252) |
3.8 (0.8) (n = 677) |
4.0 (0.8) | 3.8 (0.8) (n = 852) |
| ADCS-ADL23, mean (SD) | 56.2 (12.4) | 57.2 (12.5) | 54.1 (14.5) | 56.7 (13.3) | 55.4 (13.5) | 59.8 (11.9) | 55.3 (13.5) |
| NPI, mean (SD) | 10.0 (9.7) | 10.3 (11.3) | 9.9 (11.6) | 10.9 (10.8) | 9.7 (11.4) | 10.9 (12.4) | 9.9 (11.2) |
| B. Split by combined enrichment group status | ||||||
|---|---|---|---|---|---|---|
| ε4+/FH+/A+‡ |
ε4++/FH+/A+‡ |
ε4++/A+§ |
||||
| Enriched (n = 651) | Nonenriched (n = 266) | Enriched (n = 376) | Nonenriched (n = 541) | Enriched (n = 179) | Nonenriched (n = 737) | |
| Age, mean (SD), years | 73.3 (7.6) | 75.2 (8.8) | 72.2 (7.7) | 75.0 (8.0) | 69.5 (7.5) | 74.8 (7.8) |
| Female, No. (%) | 407 (62.5) | 181 (68.0) | 226 (60.1) | 362 (66.9) | 113 (63.1) | 473 (64.2) |
| BMI, mean (SD), kg/m2 | 26.0 (4.4) | 26.3 (4.9) | 25.9 (4.4) | 26.2 (4.6) | 25.7 (4.2) | 26.2 (4.6) |
| Education, mean (SD), years | 11.2 (4.1) | 11.0 (4.5) | 11.3 (4.1) | 11.0 (4.3) | 11.6 (4.1) | 11.0 (4.2) |
| Time since AD diagnosis, mean (SD), years | 2.3 (1.9) | 2.3 (1.9) | 2.3 (1.9) | 2.3 (1.9) | 2.3 (1.9) | 2.3 (1.9) |
| Prestudy treatment duration, mean (SD), years | 1.7 (1.6) | 1.8 (1.8) | 1.7 (1.6) | 1.8 (1.7) | 1.9 (1.9) | 1.7 (1.6) |
| Screening MMSE, mean (SD) | 17.5 (2.9) | 17.6 (2.9) | 17.5 (2.9) | 17.5 (2.9) | 17.0 (3.0) | 17.7 (2.9) |
| Screening MMSE stratum, n (%) | ||||||
| 19–22 | 269 (41.3) | 112 (42.1) | 156 (41.5) | 225 (41.6) | 66 (36.9) | 317 (43.0) |
| 12–18 | 382 (58.7) | 154 (57.9) | 220 (58.5) | 316 (58.4) | 113 (63.1) | 420 (57.0) |
| ADAS-Cog, mean (SD) | 25.7 (8.3) | 26.0 (8.2) | 25.7 (8.5) | 25.8 (8.1) | 26.4 (8.7) | 25.6 (8.2) |
| ADCS-CGIC, mean (SD) | 3.8 (0.8) (n = 648) |
3.8 (0.8) (n = 265) |
3.8 (0.8) (n = 375) |
3.8 (0.8) (n = 538) |
3.9 (0.8) (n = 178) |
3.8 (0.8) (n = 734) |
| ADCS-ADL23, mean (SD) | 56.6 (12.9) | 53.7 (14.5) | 56.7 (12.9) | 55.1 (13.8) | 57.3 (12.4) | 55.4 (13.7) |
| NPI, mean (SD) | 10.2 (10.8) | 9.6 (12.1) | 10.6 (10.8) | 9.6 (11.5) | 10.6 (11.2) | 9.9 (11.2) |
Abbreviations: A+, Amyloid positive (n = 98 tested); AD, Alzheimer's disease; ADAS-Cog, AD Assessment Scale–Cognitive subscale; ADCS-ADL23, AD Cooperative Study–Activities of Daily Living, 23-item version; ADCS-CGIC, AD Cooperative Study–Clinical Global Impression of Change; BMI, body mass index; APOE, apolipoprotein E; ε4+, APOE ε4 carrier; ε4++, APOE ε4 homozygous; ε4+−, APOE ε4 heterozygous; ε4−, APOE ε4 noncarrier; FH+, first-degree relative with AD; MMSE, Mini–Mental State Examination; NPI, Neuropsychiatric Inventory; SD, standard deviation.
25 patients were missing data for APOE ε4 allele count.
6 patients were missing data for first-degree relative with AD.
22 patients were missing data and could not be assigned.
23 patients were missing data and could not be assigned.
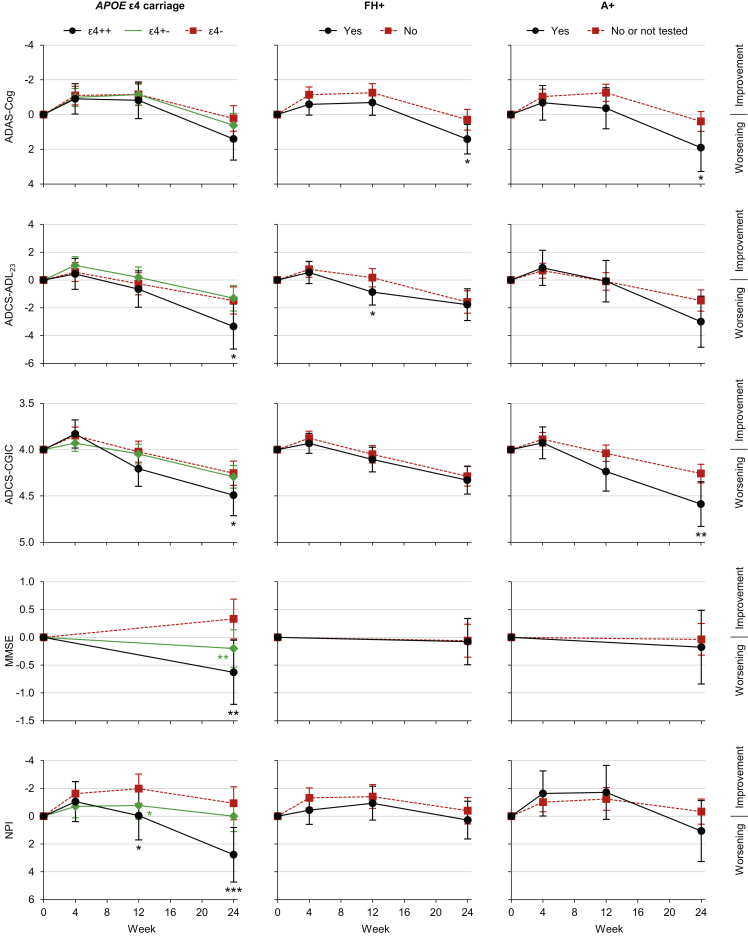
Adjusted mean changes from baseline in rating scale scores, split by biomarker/risk factor status, are shown in Fig. 2. “ε4+−” patients had a greater decline (nominal P < .05) than ε4− patients on the MMSE alone (mean difference at week 24: −0.53; 95% confidence limits: −0.92, −0.14; P = .007), whereas ε4++ patients had a greater decline than ε4− patients on the ADCS-ADL23 (−1.88; −3.60, −0.15; P = .033), ADCS-CGIC (0.24; 0.00, 0.47; P = .048), MMSE (−0.96, −1.56, −0.35; P = .002), and NPI (3.70; 1.61, 5.79; P < .001). FH+ patients had a greater decline than FH− patients on the ADAS-Cog (1.12; 0.25, 1.99; P = .012). Finally, A+ patients had a greater decline than A− patients on the ADAS-Cog (1.51; 0.13, 2.90; P = .032) and ADCS-CGIC (0.33; 0.08, 0.57; P = .008).
Fig. 2.
Mean score change from baseline among patients receiving placebo, split by individual biomarker/risk factor status. *P < .05, **P < .01, ***P < .001 versus corresponding group without the biomarker/risk factor. Error bars are 95% confidence intervals. Abbreviations: A+, Amyloid positive; AD, Alzheimer's disease; ADAS-Cog, AD Assessment Scale–Cognitive subscale; ADCS-ADL23, AD Cooperative Study–Activities of Daily Living, 23-item version; ADCS-CGIC, AD Cooperative Study–Clinical Global Impression of Change; APOE, apolipoprotein E; ε4++, APOE ε4 homozygous; ε4+−, APOE ε4 heterozygous; ε4−, APOE ε4 noncarrier; FH+, first-degree relative with AD; MMSE, Mini–Mental State Examination; NPI, Neuropsychiatric Inventory.
A sensitivity analysis adjusting for age revealed that differences in mean age at baseline did not account for the APOE findings (data not shown).
3.2. Combined enrichment groups
Of the placebo FAS, 651 patients (69.3%) were in the ε4+/FH+/A+ group, 376 (40.0%) were in the ε4++/FH+/A+ group, and 179 (19.1%) were in the ε4++/A+ group (patients counted once per group; see Fig. 1B for overlap).
In general, baseline demographic and clinical characteristics were similar between each enrichment group and the rest of the study population (Table 1B). The only clinically meaningful difference between groups was for age, with patients in the enrichment groups being 2–5 years younger than the rest of the study population.
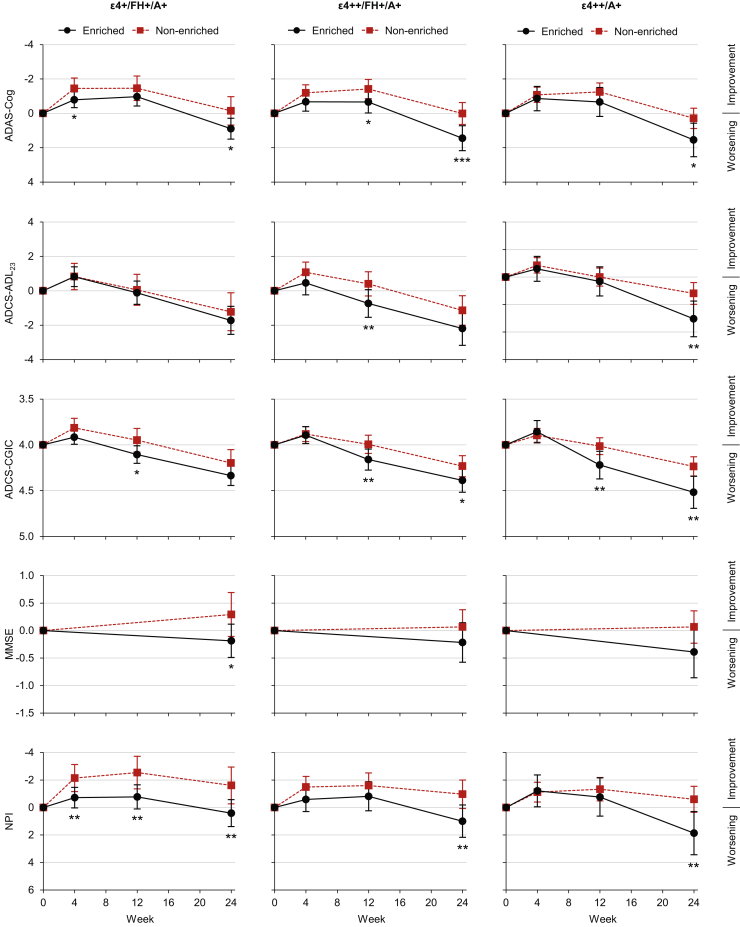
Each enrichment group was associated with an increased rate of decline across the majority of outcomes (Fig. 3). The ε4+/FH+/A+ group had a greater decline (nominal P < .05) than the rest of the study population on the ADAS-Cog (mean difference at week 24: 1.04; 95% confidence limits: 0.18, 1.90; P = .017), MMSE (−0.48; −0.88, −0.08; P = .020), and NPI (2.02; 0.62, 3.41; P = .005). The ε4++/FH+/A+ group had a greater decline than the rest of the study population on the ADAS-Cog (1.44; 0.65, 2.23; P < .001), ADCS-CGIC (0.15; 0.01, 0.30; P = .033), and NPI (1.96; 0.68, 3.24; P = .003). Finally, the ε4++/A+ group had a greater decline than the rest of the study population on the ADAS-Cog (1.26; 0.27, 2.25; P = .013), ADCS-ADL23 (−1.85; −3.17, −0.52; P = .006), ADCS-CGIC (0.28; 0.10, 0.46; P = .002), and NPI (2.46; 0.86, 4.06; P = .003). A sensitivity analysis adjusting for age revealed that differences in mean age at baseline did not account for the findings (data not shown).
Fig. 3.
Mean score change from baseline among patients receiving placebo, split by combined enrichment group status. *P < .05, **P < .01, ***P < .001 versus corresponding nonenriched group. Error bars are 95% confidence intervals. Abbreviations: A+, Amyloid positive; AD, Alzheimer's disease; ADAS-Cog, AD Assessment Scale–Cognitive subscale; ADCS-ADL23, AD Cooperative Study–Activities of Daily Living, 23-item version; ADCS-CGIC, AD Cooperative Study–Clinical Global Impression of Change; APOE, apolipoprotein E; ε4+, APOE ε4 carrier; ε4++, APOE ε4 homozygous; FH+, first-degree relative with AD; MMSE, Mini–Mental State Examination; NPI, Neuropsychiatric Inventory.
3.3. Power gains in enrichment groups
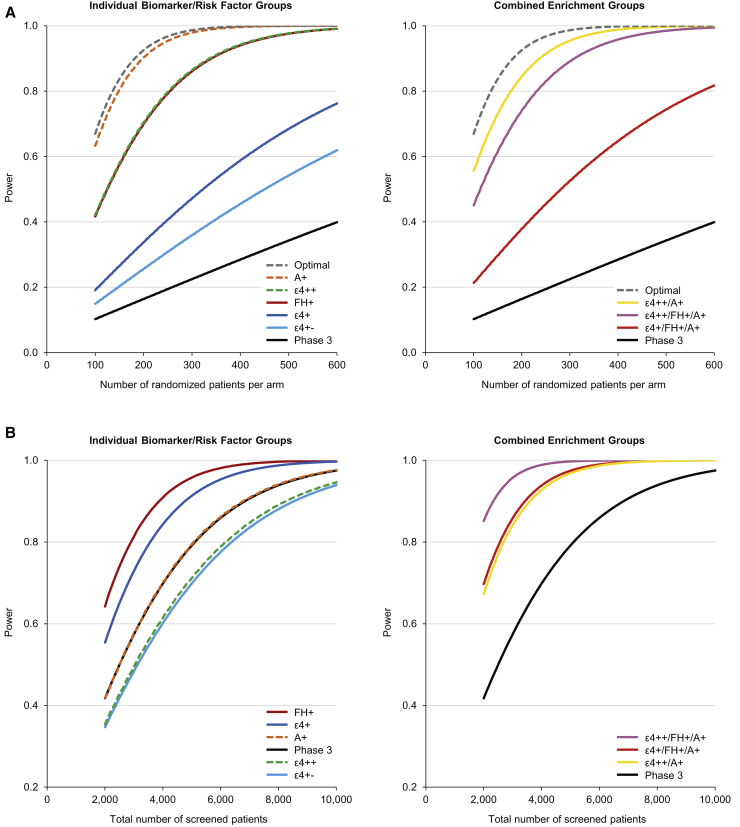
Data used for power modeling are given in Table A1 in Supplementary Material. Based on the number of randomized patients per treatment arm, all enrichment groups had an increased power to detect a treatment effect compared with the general phase 3 population (Fig. 4A). The greatest power gains were in the A+ group, followed by the ε4++/A+ group, then the ε4++/FH+/A+ group. An 80% power to detect a treatment effect required 148 A+ patients per arm, 178 ε4++/A+ patients per arm, 231 ε4++/FH+/A+ patients per arm, and 251 ε4++ patients per arm, compared with 1619 patients per arm in the general phase 3 population (Table A2 in Supplementary Material).
Fig. 4.
Estimated study powers to detect a treatment effect based on (A) randomized and (B) screened patients. Abbreviations: A+, amyloid positive; AD, Alzheimer's disease; ADAS-Cog, AD Assessment Scale–Cognitive subscale; APOE, apolipoprotein E; ε4+, APOE ε4 carrier; ε4++, APOE ε4 homozygous; ε4+−, APOE ε4 heterozygous; FH+, first-degree relative with AD; Optimal, optimal power curve (full 2-point treatment effect on the ADAS-Cog. no withdrawal); Phase 3, total phase 3 population.
Fig. 4B shows the achieved study powers based on the total number of screened patients, as opposed to randomized patients, thereby taking into account the fraction of the total population that each enrichment group represents. The combined enrichment groups, especially the ε4++/FH+/A+ group, were the most efficient in terms of power per patient screened.
4. Discussion
Recent studies show that around a quarter of patients entering clinical trials with a clinical diagnosis of mild AD do not have brain amyloidosis when studied with amyloid PET and therefore do not have the bioclinical syndrome of AD [8]. Non-AD patients included in AD trials will progress more slowly, reduce the power to detect a drug-placebo difference, and may lack the underlying pathophysiology that is the target of pharmacotherapy. The present analysis of a large clinical trial program explored several strategies to enrich trials by identifying participants more likely to have AD and who exhibit a more rapid rate of decline. The analysis showed that biomarkers/risk factors (individually and in combination) can be used to predict progression in mild-to-moderate AD and that such enrichment can increase the statistical power to detect a potential treatment effect of 2 points on the ADAS-Cog, thereby reducing the required sample size. Of the individual biomarkers/risk factors considered, ε4++ was associated with the most consistent decline across all outcomes (cognitive, functional, global, and behavioral) in the placebo group. Although ε4++ patients are known to have an earlier onset of AD [13] and were younger in the present analysis, sensitivity analyses showed that the APOE subgroup results were not driven by age. Modeling showed that, for ε4++ and A+ patients, 80% power to detect a treatment effect of 2 points on the ADAS-Cog could be achieved with samples that were approximately one-sixth and one-eleventh the size of the total randomized phase 3 population (i.e., an unenriched population). However, this must be balanced with the fact that the ε4++ group represented only 12.9% of randomized patients. Furthermore, although the majority of patients with amyloid PET or CSF data were A+ (84.7% in the placebo group), amyloid testing is expensive and not widely available at international clinical trial sites.
To overcome these recruitment, cost, and feasibility issues, larger enrichment groups were created that considered combinations of AD biomarkers/risk factors, thereby creating an enrichment approach that is applicable to all settings. The ε4++/A+ group showed the most consistent decline across all outcomes, and the biggest power gain, of the combined groups. However, this was still a relatively small group of patients (19.1%) partly because of the small proportion of patients who were tested for amyloid in this program. The balance between study power and screening success rate was optimized by the addition of FH+ to the enrichment process. Although APOE ε4 carriage is the major genetic risk factor for late-onset AD [28], recent research has shown that combined genetic architecture provides important predictive information beyond APOE [29] and that parental history of dementia contains additional predictive information beyond even polygenic risk [30]. In the present study, FH+ was used as a proxy for polygenic and environmental risk factors. Taking into account screening success rate and availability of amyloid testing, the ε4++/FH+/A+ group provided the most efficient enrichment strategy. This strategy also offers cost benefits because APOE genotyping is a low-cost and minimally invasive strategy and historic amyloid results and family history of AD can be obtained for free.
Overall, these analyses suggest that enrichment could impact substantially on the results of clinical trials of potentially symptomatic or disease-modifying treatments in mild-to-moderate AD, by delineating treatment arms as well as reducing the number of patients needed to detect a treatment effect. Whereas previous trials in mild AD have shown only a modest rate of decline among patients receiving placebo [31], making it difficult to demonstrate potential treatment benefits, use of enriched populations with increased rate of decline may provide a more efficient and effective basis to detect symptomatic treatment effects, under the assumption that a symptomatic treatment may not measurably improve stable patients or those with unknown underlying diagnosis.
It is worth noting that a large proportion of patients in the placebo FAS (30.7%) did not meet the criteria for any enrichment group. Patients not meeting enrichment group criteria were associated with a slower rate of decline.
Limitations of this analysis include that it was performed in studies not designed for this purpose. The enrichment groups were chosen based on limited biomarker/risk factor data, and the combined enrichment groups were not devised in a data-driven manner but were based on expert opinion informed by emerging data on AD diagnosis and prognosis. The analysis did not control for multiple comparisons. Only a minority of patients had data on amyloid status; the testing rationale of these patients is unknown, meaning that they might represent a special population. Future trials using APOE ε4 strategic enrichment should bear in mind that, due to the putative role of APOE in amyloid-β metabolism [32], APOE ε4 carriers may respond differently to treatment than noncarriers.
A limitation of the model used to evaluate power gains was the assumption that lack of placebo decline would directly mask an observed treatment effect. Although such assumptions are natural for disease-modifying agents that work to slow or stop cognitive decline, it is not given that such assumptions are generally true for symptomatic cognitive enhancers. The estimated rate of decline on the ADAS-Cog for patients with mild-to-moderate AD is approximately 5.5 points per year [33], and thus lack of decline (or an improvement) over the 6-month study period for this population suggests a considerable placebo response or study participation effect [34]. In the context of symptomatic cognitive enhancers, the power modeling assumptions were that the placebo/study participation effects seen for patients on placebo or active treatment would take the patients to their individual cognitive ceilings, thus leaving no window for the detection of the additional benefit of a symptomatic treatment.
In conclusion, there is a need to enrich populations in clinical trials of symptomatic treatments in AD. Enrichment may be achieved in mild-to-moderate AD using combinations of low-cost, minimally invasive biomarkers/risk factors, specifically APOE genotyping, historic amyloid test results, and family history of AD, to increase the power to detect treatment differences in clinical trials.
Research in Context.
-
1.
Systematic review: The authors searched PubMed for articles published from Jan 1, 1990, to Jun 1, 2018, using the terms: enrich* AND Alzheimer's disease AND clinical trial. Although enrichment based on neuroimaging and cerebrospinal fluid biomarkers is increasingly common in studies of prodromal AD, enrichment is less common in mild AD dementia. No studies that used enrichment techniques to identify populations with accelerated cognitive decline in moderate or severe AD dementia were identified.
-
2.
Interpretation: Whereas previous studies have relied on amyloid testing, an expensive technique that is not widely available at international clinical trial sites, we showed that enrichment can be achieved in mild-to-moderate AD using the following low-cost, minimally invasive strategies: APOE genotyping, historic amyloid test results, and family history of AD.
-
3.
Future directions: The article proposes a strategy for enrichment that should be tested in future clinical trials of symptomatic drugs in AD.
Acknowledgments
This work was supported by funding from H. Lundbeck A/S (Valby, Denmark). Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge Medical Communication Ltd (Cambridge, UK), and funded by H. Lundbeck A/S.
The authors acknowledge the contributions of the principal investigators in the original studies: the Idalopirdine Development Teams; the STAR Program Teams, operational partners, and site staff and investigators; and those individuals serving on the data and safety monitoring board from the respective trials. In addition, the authors express their gratitude for the commitment of the study participants and their caregivers; without their generous contributions and dedication, this research would not be possible.
Role of the funding source: The study sponsor was responsible for the design and conduct of the study; for the collection, management, analysis of data; and aided in the interpretation of the data. Medical writing to assist in the preparation of the manuscript was funded by the study sponsor. The study sponsor had a role in the review and approval of the manuscript and the decision to submit for publication because two of the coauthors were employed by the sponsor at the time of the study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
C.B. reported receiving research grants from Acadia and H. Lundbeck A/S and receiving honoraria from Acadia, Bristol-Myers Squibb, Heptares, Lilly, H. Lundbeck A/S, Novartis, Orion, Otsuka, and Roche. A.A. reported receiving honoraria for consulting, participating in independent data safety monitoring boards, providing educational lectures, programs, and materials, or serving on advisory boards for Allergan, the Alzheimer's Association, Axovant, Biogen, Grifols, Harvard Medical School Graduate Continuing Education, Lundbeck, Merck, Roche/Genentech, Sunovion, and Suven; receiving book royalties from Oxford University Press; and having institutional contracts or receiving investigational clinical trial–related funding from the American College of Radiology, AbbVie, Avid, Biogen, Lilly, Lundbeck, Merck, and vTV Therapeutics. N.B. and L.L.R. are full-time employees of H. Lundbeck A/S. J.L.C. reported serving as a consultant to Acadia, Actinogen, Adamas, Allergan, Alzheon, Avanir, Avid, Axsome, Bioasis, Biogen, Bracket, Denali, Diadem, EIP, Eisai, Genentech, Green Valley, Grifols, Hisun, Intra-Cellular Therapies, Kyowa Kirin, Lilly, H. Lundbeck A/S, MedAvante, Merck, Otsuka, Pain Therapeutics, Proclara, QR Pharma, Resverlogix, Roche, Samus, Suven, Takeda, and United Neuroscience; receiving research support from Avid and Teva; owning stock options in Adamas, Alzheon, MedAvante, Neurokos, Prana, QR Pharma, and Samus; and owning the copyright of the Neuropsychiatric Inventory and all its derivatives. He is supported by Keep Memory Alive and a COBRE grant from the NIGMS (P20GM109025). L.F. reported receiving honoraria for consulting, providing educational lectures, materials, and programs or serving on advisory boards for Avid–Eli Lilly & Co, Avraham Pharmaceuticals, Axon Neuroscience, Boehringer Ingelheim, GE Healthcare, H. Lundbeck A/S, Merck Sharp & Dohme, Novartis, Nutricia, Pfizer, Piramal Imaging, Schwabe Pharma, TAD Pharma, and Takeda. J.L.M. reported receiving honoraria for consulting, providing educational lectures, or serving on advisory boards for ABL, Axovant, Boehringer Ingelheim, Eli Lilly & Co, Fujirebio, GE Healthcare, H. Lundbeck A/S, Merck Sharp & Dohme, Novartis, Pfizer, Piramal Imaging, Roche, and Roche Diagnostics. P.N.T. reported receiving consulting fees from Abbott Laboratories, AbbVie, AC Immune, Auspex, Boehringer Ingelheim, Clintara, CME Inc, Corium, GliaCure, INSYS, and T3D; receiving consulting fees and research support from AstraZeneca, Avanir, Cognoptix, Lilly, H. Lundbeck A/S, Merck and Company, and Takeda; receiving research support from Elan, Functional Neuromodulation, Genentech, Novartis, Roche, Targacept, the National Institute on Aging, and the Arizona Department of Health Services; owning stock options in Adamas; and being listed as a contributor to a patent owned by the University of Rochester. No other disclosures were reported.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.04.001.
Supplementary Data
References
- 1.Cummings J.L., Morstorf T., Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au R., Piers R.J., Lancashire L. Back to the future: Alzheimer's disease heterogeneity revisited. Alzheimers Dement (Amst) 2015;1:368–370. doi: 10.1016/j.dadm.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yashin A.I., Fang F., Kovtun M., Wu D., Duan M., Arbeev K. Hidden heterogeneity in Alzheimer's disease: insights from genetic association studies and other analyses. Exp Gerontol. 2018;107:148–160. doi: 10.1016/j.exger.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komarova N.L., Thalhauser C.J. High degree of heterogeneity in Alzheimer's disease progression patterns. PLoS Comput Biol. 2011;7:e1002251. doi: 10.1371/journal.pcbi.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings J., Lee G., Ritter A., Zhong K. Alzheimer's disease drug development pipeline: 2018. Alzheimers Dement (N Y) 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pharmaceutical Research and Manufacturers of America Medicines in development for cancer 2018 report. http://phrma-docs.phrma.org/files/dmfile/2018_MID_Cancer.pdf Available at.
- 7.Yu P., Sun J., Wolz R., Stephenson D., Brewer J., Fox N.C., Coalition Against Major Diseases and the Alzheimer's Disease Neuroimaging Initiative Operationalizing hippocampal volume as an enrichment biomarker for amnestic MCI trials: effect of algorithm, test–retest variability, and cut point on trial cost, duration, and sample size. Neurobiol Aging. 2014;35:808–818. doi: 10.1016/j.neurobiolaging.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sevigny J., Suhy J., Chiao P., Chen T., Klein G., Purcell D. Amyloid PET screening for enrichment of early-stage Alzheimer disease clinical trials: experience in a Phase 1b clinical trial. Alzheimer Dis Assoc Disord. 2016;30:1–7. doi: 10.1097/WAD.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 9.Wolz R., Schwarz A.J., Gray K.R., Yu P., Hill D.L.G., Alzheimer's Disease Neuroimaging Initiative Enrichment of clinical trials in MCI due to AD using markers of amyloid and neurodegeneration. Neurology. 2016;87:1235–1241. doi: 10.1212/WNL.0000000000003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang T.S., Teng E., Elashoff D., Grill J.D., Alzheimer's Disease Neuroimaging Initiative Optimizing effect sizes with imaging enrichment and outcome choices for mild Alzheimer disease clinical trials. Alzheimer Dis Assoc Disord. 2017;31:19–26. doi: 10.1097/WAD.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Green R.C., Alzheimer's Disease Neuroimaging Initiative The Alzheimer's Disease Neuroimaging Initiative 3: continued innovation for clinical trial improvement. Alzheimers Dement. 2017;13:561–571. doi: 10.1016/j.jalz.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann N., Gauthier S., Boneva N., Lemming O.M., 10158 Investigators A randomized, double-blind, placebo-controlled trial of memantine in a behaviorally enriched sample of patients with moderate-to-severe Alzheimer's disease. Int Psychogeriatr. 2013;25:919–927. doi: 10.1017/S1041610213000239. [DOI] [PubMed] [Google Scholar]
- 13.Sando S.B., Melquist S., Cannon A., Hutton M.L., Sletvold O., Saltvedt I. APOE ε4 lowers age at onset and is a high risk factor for Alzheimer's disease; a case control study from central Norway. BMC Neurol. 2008;8:9. doi: 10.1186/1471-2377-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayeux R., Sano M., Chen J., Tatemichi T., Stern Y. Risk of dementia in first-degree relatives of patients with Alzheimer's disease and related disorders. Arch Neurol. 1991;48:269–273. doi: 10.1001/archneur.1991.00530150037014. [DOI] [PubMed] [Google Scholar]
- 15.Ossenkoppele R., Jansen W.J., Rabinovici G.D., Knol D.L., van der Flier W.M., van Berckel B.N.M., the Amyloid PET Study Group Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313:1939–1949. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R.J., the Amyloid Biomarker Study Group Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atri A., Frölich L., Ballard C., Tariot P.N., Molinuevo J.L., Boneva N. Effect of idalopirdine as adjunct to cholinesterase inhibitors on change in cognition in patients with Alzheimer disease: three randomized clinical trials. JAMA. 2018;319:130–142. doi: 10.1001/jama.2017.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Folstein M.F., Folstein S.E., McHugh P.R. “Mini–mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 21.Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M., The Alzheimer's Disease Cooperative Study An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1997;11:S33–S39. [PubMed] [Google Scholar]
- 22.Robert P., Ferris S., Gauthier S., Ihl R., Winblad B., Tennigkeit F. Review of Alzheimer's disease scales: is there a need for a new multi-domain scale for therapy evaluation in medical practice? Alzheimers Res Ther. 2010;2:24. doi: 10.1186/alzrt48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider L.S., Olin J.T., Doody R.S., Clark C.M., Morris J.C., Reisberg B., The Alzheimer's Disease Cooperative Study Validity and reliability of the Alzheimer's Disease Cooperative Study – Clinical Global Impression of Change. Alzheimer Dis Assoc Disord. 1997;11:S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 24.Guy W. National Institute of Mental Health; Rockville, MD: 1976. ECDEU Assessment Manual for Psychopharmacology, Revised. [Google Scholar]
- 25.Cummings J.L. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 26.Li Q.X., Villemagne V.L., Doecke J.D., Rembach A., Sarros S., Varghese S., AIBL Research Group Alzheimer's disease normative cerebrospinal fluid biomarkers validated in PET amyloid-β characterized subjects from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Alzheimers Dis. 2015;48:175–187. doi: 10.3233/JAD-150247. [DOI] [PubMed] [Google Scholar]
- 27.Palmqvist S., Zetterberg H., Mattsson N., Johansson P., Minthon L., Blennow K., Alzheimer's Disease Neuroimaging Initiative; Swedish BioFINDER Study Group Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85:1240–1249. doi: 10.1212/WNL.0000000000001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winblad B., Amouyel P., Andrieu S., Ballard C., Brayne C., Brodaty H. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 29.Desikan R.S., Fan C.C., Wang Y., Schork A.J., Cabral H.J., Cupples L.A. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med. 2017;14:e1002258. doi: 10.1371/journal.pmed.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Lee S.J., Wolters F.J., Ikram M.K., Hofman A., Ikram M.A., Amin A. The effect of APOE and other common genetic variants on the onset of Alzheimer's disease and dementia: a community-based cohort study. Lancet Neurol. 2018;17:434–444. doi: 10.1016/S1474-4422(18)30053-X. [DOI] [PubMed] [Google Scholar]
- 31.Seltzer B., Zolnouni P., Nunez M., Goldman R., Kumar D., Ieni J., Donepezil “402” Study Group Efficacy of donepezil in early-stage Alzheimer disease: a randomized placebo-controlled trial. Arch Neurol. 2004;61:1852–1856. doi: 10.1001/archneur.61.12.1852. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Q., Lee C.Y.D., Mandrekar S., Wilkinson B., Cramer P., Zelcer N. ApoE promotes the proteolytic degradation of Aß. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito K., Corrigan B., Zhao Q., French J., Miller R., Soares H., Alzheimer's Disease Neuroimaging Initiative Disease progression model for cognitive deterioration from Alzheimer's Disease Neuroimaging Initiative database. Alzheimers Dement. 2011;7:151–160. doi: 10.1016/j.jalz.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 34.McCarney R., Warner J., Iliffe S., van Haselen R., Griffin M., Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.