Abstract
Background
This systematic review was performed to investigate the effects of Chinese herbal nedicine (CHM) on cognitive function and activity of daily living (ADL) in individuals with Alzheimer's disease.
Methods
Five electronic databases (Medline, Embase, Cinahl, PsycArticles, and CNKI) were searched from inception to January 2019. Randomized controlled trials (RCTs) assessing the effect of CHM on cognition and activity of daily living in adults with Alzheimer's disease were included. We pooled the effects size using the Comprehensive Meta-Analysis Software. Cochrane risk of bias tool was used to evaluate the study quality.
Results
Twenty-five RCTs (1855 individuals with AD) were included in this review. Overall findings of this meta-analysis indicated that CHM improved the cognitive function (SMD = 0.66, 95% CI [0.44, 0.89], I2 = 77.9%, p < 0.001) and ADL (SMD = 0.38, 95% CI [0.25, 0.49], I2 = 35.3%, p < 0.001) compared with conventional drugs. No publication biases were observed on both cognitive function and ADL.
Conclusion
CHM may have potential effects for improving cognitive function and ADL for individuals with AD compared with conventional drug therapies. However, the evidence is limited because of high risk of bias of the included trials.
Keywords: Alzheimer's disease, Chinese herbal medicine, Cognitive function, Meta-analysis
1. Introduction
Alzheimer's disease (AD), as the most common form of dementia, is a neurodegenerative disorder, and it has multiple symptoms, such as cognitive impairment, activity limitation, depression, and even loss of independence in older adults.1, 2 With the aggravated aging problem, a huge number of people are living with AD, which has represented a significant challenge for health issue around the world.3 The World Alzheimer's Disease Report 2016 indicated that total 47 million populations were diagnosed dementia, and the estimated number will be increased to 131 million by 2050.4 In today's United States, there are approximately 5.7 million adults who are suffering from the AD, and one of 10 people aged 65 years and over has AD according to the national statistic.1 In South Korea, the Korea Ministry of Health and Welfare reported that Korean older people with dementia were about 0.5 million in 2012, and the predictable number is expected to increase to 2.7 million by 2050.5
A variety of drugs for treatment AD have been applied to the clinic in the past decades. The conventional medicines are commonly aimed at declining the AD progression, such as increasing the level of acetylcholine (Donepezil, Galantamine, and Rivastigmine), and antagonist of the N-methyl-d-aspartate (NMDA) receptor by the Memantine.3 However, a recent meta-analysis study revealed that the Memantine drug had smaller effects on dementia,6 and some conventional medicines contributed to being small effects on cognitive function and ADL for people with AD in long-term treatment.7, 8 Additionally, previous studies reported that cholinesterase inhibitors and the Memantine drug have different levels of side effects for patients with AD.9, 10 Some patients even stopped taking these drugs.
Chinese herbal medicine (CHM) has been used to protect memory.11 A growing body of evidence suggested that the improvements of cognitive function and ADL in patients with AD may be attributed to the CHM (multi-target intervention effects) to some extent.12, 13, 14, 15 Some studies reported that CHM have similar effect on cognition and activity of daily living (ADL) improvement compared with conventional drug therapy.16, 17 Although previous literature review studies18, 19, 20, 21 showed several types of CHM may benefit their cognitive function, most of them included several types of dementia together (e.g., vascular dementia, Lewy body dementia) without quantitative analysis.
Therefore, the aim of this study was to investigate whether the CHM is more effective than the conventional drug therapies to improving cognitive and ADL deterioration for adults with AD.
2. Methods
2.1. Search strategy
Five electronic databases (Medline, Embase, Cinahl, PsycArticles, and CNKI) were searched for potential publications from their inception to January 2019. We used two groups of search terms to retrieve potentially relevant articles: (1) “Chinese herbal medicine” OR “Chinese herb” OR “traditional Chinese medicine” OR “Chinese medicine”; (2) “Alzheimer's disease” OR “Alzheimer*” OR “Senile Dementia”. In addition, reference lists of initially retrieved documents were also screened to identify potential publications that were related to our interesting topic. If so, manual search was performed.
For example, the search strategy was conducted in Medline database:
#1: Chinese herbal medicine
#2: Chinese herb
#3: traditional Chinese medicine
#4: Chinese medicine
#5: #1 OR #2 OR #3 OR #4
#6: Alzheimer's disease
#7: Alzheimer*
#8: #6 OR #7
#9: #5 AND #8
2.2. Inclusion and exclusion criteria
The inclusion criteria were like following.
2.2.1. Participants
Participants diagnosed with AD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) III, III-R or IV were taken into consideration, regardless of gender, age, duration of the disease. We excluded individuals diagnosed with other types of dementia (e.g., vascular dementia, Lewy body disease), or mild cognitive impairment.
2.2.2. Interventions
CHM was defined as the prescription consisting of multiple herbs or patent herbal products. We excluded the combination of CHM with injections or acupuncture.
2.2.3. Control
We included conventional drug therapies (including donepezil, memantine, and galantamine), placebo or no intervention as control interventions.
2.2.4. Outcomes
The outcome measurements were Mini-Mental State Examination (MMSE) and ADL.
We excluded conference proceeding, case-study, cross-sectional studies, controlled study with no randomization, or review.
2.3. Data extraction
The key information of all eligible studies were independently extracted by two reviewers; author and year of publication, characteristics of subjects, sample size, gender and age, study design, intervention protocol, outcome measurements, and statistical data (mean and standard deviation and the number of participants of each group) needed for computing effect size.
2.4. Risk of bias assessment
The risk of bias of all randomized controlled trials were evaluated according to the Cochrane risk of bias tool.22 This scale involves 7-domain assessment: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
2.5. Statistical analysis
This meta-analysis was performed using the Comprehensive Meta-Analysis Software. As the cognition and ADL were measured by different scales across the eligible studies, we used the standardized mean difference (SMD) with random-effects model with 95% confidence interval (CI). Three levels of effect size (small [0.2–0.49], medium [0.5–0.79], and large [0.8 or more]) were taken as the corresponding magnitudes of intervention effect. The I2 statistic was employed to assess heterogeneity, and it was classified as 25% (low heterogeneity), 50% (moderate heterogeneity), and 75% (high heterogeneity).23 The Egger's regression test was used to examine the publication bias. In addition, subgroup meta-analysis was performed based on the type of CHM interventions.
3. Results
3.1. Search results
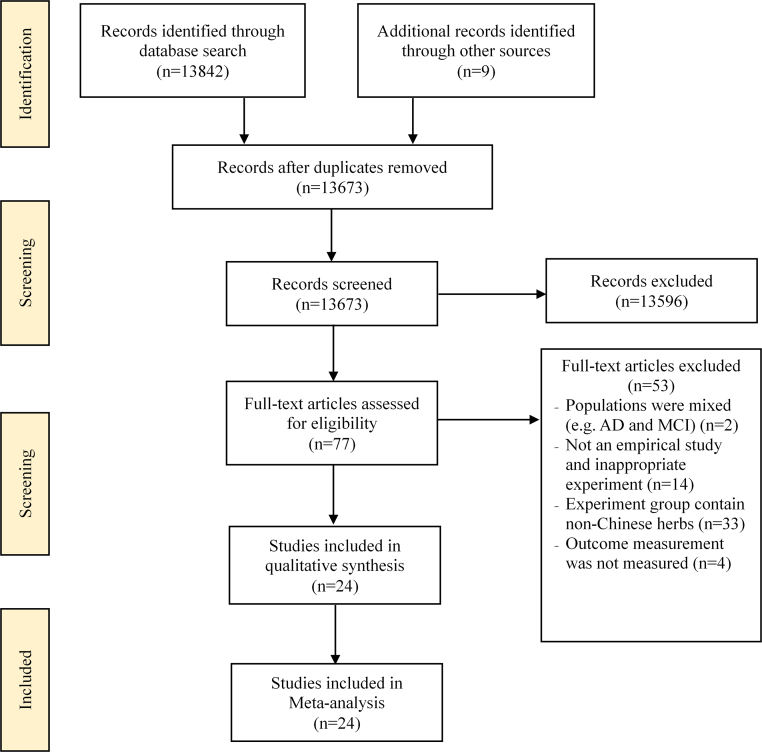
The search and selection process of this review was described based on the PRISMA guideline in Fig. 1.24 A total of 13,851 records were identified at the initial stage. After removing duplicates (n = 178), the titles and abstracts (n = 13,673) of remaining articles were screened by two independent reviewers to further determine the eligibility of potential articles; this led to elimination of 13,596 articles. Seventy-seven full-text articles were further assessed and 53 articles were excluded. Finally, 24 RCT articles regarding to the effects of CHM on cognitive function and ADL were included in this review.14, 15, 16, 17, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44
Fig. 1.
Flow diagram of the literature search process based on the PRISMA guideline. PRISMA, preferred reporting items for systematic reviews and meta-analyses; AD, Alzheimer's disease; MCI, mild cognitive impairment.
3.2. Study characteristics
Details of the included studies are shown in Table 1, Table 2. The studies were published from 2001 to 2018. In total, 1711 participants (mean age from 60 to 84 years) with mild to severe degree of AD were included in this review along with sample size across studies ranging from 20 to 186.
Table 1.
Summary of Randomized Controlled Trials of Chinese Herbal Medicine Studies
| First authors year, reference | Severity of AD Age (years) Total sample size (M/F) |
Diagnostic criteria | Duration | Intervention |
Outcomes | ||
|---|---|---|---|---|---|---|---|
| CHM (Formula)/Dosage | Control | Results | |||||
| Zhang (2018)14 | Mild to mod AD 72.9 E;72.9C 88(53/35) |
DSM-IV and image test | 2 months | Qingxin Yizhi decoction (1 dose/2 times/d) | Donepezil (mg/time, 2 times/d) | 1) MMSE | 1) E > C, p < 0.05 |
| Liu (2013)15 | Mild to mod AD 74.0E; 75.0C 60(37/23) |
DSM-IV | 12 weeks | Bushenhuatanyizhi instant granules (6 g/time, 2 times/d) | Piracetam (0.8 g/3 times/d) | 1) MMSE 2) ADL |
1) E = C, NS 2) E = C, NS |
| Wang (2015)16 | n.r. 73.2E; 74.4C 66(30/36) |
DSM-IV | 6 months | Congrong Yizhi decoction (1.2 g/time, 3 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE 2) ADL |
1) E > C, p < 0.05 2) E > C, p < 0.05 |
| Yang (2013)17 | Mild to mod AD 84.2E; 82.7C 60 (45/15) |
DSM-IV | 12 weeks | Yizhi Jiannao Granule (5.5 g/time, 2 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE 2) ADL |
1) E = C, NS 2) E = C, NS |
| Ding (2009)25 | n.r. 73.5 (NR in details) 56(33/23) |
DSM-IV | 3 months | Shenghuang Yizhi decoction (16 g/time, 2 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE | 1) E = C, NS |
| Li (2002)26 | Mild to severe AD 66.0E; 65.0C 32(NR) |
DSM-IV | 8 weeks | Danggui, shaoyaosan (1 dose/2 times/d) | Nimodipine (20–40 mg/3 times/d) | 1) MMSE 2) ADL |
1) E = C, NS 2) E < C, p < 0.05 |
| Jia (2018)27 | Mild to mod AD 59.7E; 60.1C 93(42/51) |
Imaging test | 3 months | Rehmanniae Decoction (1 dose/2 times/d) | Donepezil (1st month: 5 mg/1 time/d; 2nd–3rd month: 10 mg/1 time/d) | 1) MMSE | 1) E > C, p < 0.05 |
| Jin (2017)28 | Mild to mod AD 76.5E; 75.6C 120(41/79) |
DSM-IV | 6 months | Tongqiaohuoxuetang (100 mL/time, 2 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE 2) ADL |
1) E > C, p < 0.05 2) E < C, p < 0.05 |
| Wang (2013)29 | n.r. 66.3E; 68.7C 40 (21/19) |
NINCDS-ADRDA | 24 weeks | Jiaweizuoguiwan (100 mL/time, 3 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE | 1) E > C, p < 0.05 |
| Fu (2012)30 | n.r. 70.5E; 71.1C 30 (14/16) |
DSM-IV | 24 weeks | Yishen Huazhuo granules (1 sachet/1 time/d) | Donepezil (5 mg/1 time/d) | 1) MMSE 2) ADL |
1) E = C, NS 2) E = C, NS |
| Zhou (2001)31 | n.r. 75.3E; 73.4C 68 (29/39) |
DSM-IV | 12 weeks | Bushen Fang (10 mL/time, 2 times/d) | Donepezil (5 mg/1 time/d) | 1) ADL | 1) E = C, NS |
| Zhu (2010)32 | Mild to mod AD 60–80 40 (NR) |
DSM-IV | 8 weeks | Yizhi Jiannao granules (5.5 g/time, 3 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE | 1) E < C, p < 0.05 |
| He (2013)33 | Mild to severe AD 66.5E; 67.1C 70 (35/35) |
DSM-IV | 8 weeks | Buyanghuanwutang (200 mL/time, 2 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE 2) ADL |
1) E > C, p < 0.05 2) E > C, p < 0.05 |
| Zhou (2007)34 | n.r. 76.1E; 74.8C 44 (22/22) |
NINCDS-ADRDA | 6 months | Reinhartdt and sea cucumber capsule (0.9 g/time, 3 times/d) | Donepezil (5–10 mg/1 time/d) | 1) MMSE 2) ADL |
1) E < C, p < 0.05 2) E < C, p < 0.05 |
| Gao (2004)35 | n.r. 78.2E; 78.1C 62 (55/7) |
NINCDS-ADRDA | 12 weeks | Reinhartdt and sea cucumber capsule (0.9 g/time, 3 times/d) | Sanlexi (0.2 g/time, 3 times/d) | 1) MMSE 2) ADL |
1) E > C, p < 0.05 2) E > C, p < 0.05 |
| Chang (2013)36 | Mild to mod AD 78.9E; 77.2C 73 (54/19) |
NINCDS-ADRDA | 12 weeks | Refined Xingnao powder (100 mL/time, 2 times/d) | Donepezil (10 mg/1 time/d) | 1) MMSE 2) ADL |
1) E = C, NS 2) E = C, NS |
| Li (2010)37 | Mild to mod AD 67.6E; 67.1C 120(58/62) |
DSM-IV | 12 weeks | Naolingtang (1 dose/2 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE | 1) E = C, NS |
| Lin (2002)38 | Mild to mod AD 76.4E; 72.6C 40 (9/21) |
NINCDS-ADRDA |
12 weeks | Tiaoxinfang (10 mL/time, 2 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE 2) ADL |
1) E = C, NS 2) E = C, NS |
| Wang (2002)39 | Mild to severe AD 76.9E; 73.4C 68(30/38) |
DSM-IV |
12 weeks | Tiaoxinfang (10 mL/time, 2 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE 2) ADL |
1) E = C, NS 2) E = C, NS |
| Chen (2013)40 | n.r. 67.4E; 68.1C 80 (56/24) |
DSM-IV and imaging test | 8 weeks | Wenpitongluokaiqiaotang (1 dose/2 times/d) | Donepezil (1st month: 5 mg/1 time/d; 2nd month: 10 mg/1 time/d) | 1) MMSE | 1) E > C, p < 0.05 |
| Liu (2010)41 | n.r. 61.5E; 62.3C 20 (8/12) |
DSM-IV | 12 weeks | Yizhijiannao granules (5.5g/time, 3 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE | 1) E > C, p < 0.05 |
| Zhang (2009)42 | AD 66.8E; 67.6C 73 (41/32O |
DSM-IV | 3 months | Yizhitang (150 mL/time, 2 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE 2) ADL |
1) E > C, p < 0.05 2) E > C, p < 0.05 |
| Peng (2009)43 | AD 67.2E; 67.5C 56(34/22) |
DSM-IV | 12weeks | Yizhijiannao granules (5.5 g/time, 3 times/d) | Donepezil (5 mg/1 time/d) | 1) MMSE 2) ADL |
1) E = C, NS 2) E = C, NS |
| Yan (2007)44 | AD 65.0E; 66.0C 186(131/55) |
NINCDS-ADRDA | 24 weeks | Zhijingkoufuye (20 mL/time, 3 times/d) | Donepezil (1st month: 5 mg/1 time/d; 2nd–6 month 2–6: 10 mg/1 time/d) |
1) MMSE 2) ADL |
1) E = C, NS 2) E = C, NS |
ADAS-Cog: Alzheimer's Disease Assessment Scale Cognitive; ADL: activity of daily living; C: control group; d: day; E: experiment group; MMSE: Mini-Mental State Examination; F: female; M: male. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition; NINCDS-ADRDA: Neurobiological and Communication Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; NR: not reported; NS: not significant.
Table 2.
The Ingredients of Chinese Herbal Medicines Used in the Included Studies
| First author Year, references | Name of Chinese herbal medicines | Ingredients |
|---|---|---|
| Zhang (2018)14 | Qingxin Yizhi decoction | Astragali radix 15 g, Fried jujube kernel 20 g, Lumbricus 15 g, Fructus cannabis 15 g, Red peony 12 g, Bitter cardamon 12 g, Angelica 10 g, Peach kernel 10 g, Cassia twig 10 g, Prepared liquorice root 10 g |
| Liu (2013)15 | Bushenhuatanyizhi instant granules | Radix polygoni multiflori, Rhizoma panacis japonici, Rhizoma acori tatarinowii, Caulis bambusae in taeniam, Rhizoma pinelliae, Poria cocos, Radix Palygalae |
| Wang (2015)16 | Congrong Yizhi decoction | Radix polygoni multiflori, Lumbricus, Lotus leaf, Cistanche, Rhaponticum) |
| Yang (2013)17 | Yizhi Jiannao Granule | Epimedium, Cynomorium, Radix dipsaci, Acanthopanax, Platycladi seed, Leech, Radix notoginseng |
| Ding (2009)25 | Shenghuang Yizhi decoction | Stiff silkworm, Cicada, Zedoary, Rhubarb, Phellodendron, Coptis, Gardenia |
| Li (2002)26 | Danggui shaoyaosan | Chinese angelica 6 g, Ligusticum wallichii 6 g, Herbaceous peony 9 g, Atractylodes 9 g, Poria cocos 9 g, Rhizoma alismatis 10 g |
| Jia (2018)27 | Rehmanniae decoction | Dendrobium 12 g, Polygala tenuifolia 12 g, Cinnamon 10 g, Processed radix aconiti lateralis 10 g, Cornus officinalis 30 g, Rhizoma acori tatarinowii 15 g, Radix ophiopogonis 15 g, Poria cocos 15 g, Schisandra chinensis 15 g, Cistanche 15 g, Atractylodes 15 g, Radix codonopsis 15 g |
| Jin (2017)28 | Tongqiaohuoxuetang | Radix paeoniae rubra 15 g, Ligusticum wallichii 15 g, Peach kernel 15 g, Carthamus tinctorious 15 g, Acorus gramineus 12 g, Radix curcumae 12 g, Bile arisaema 12 g, Caulis bambusae in Taeniis 12 g |
| Wang (2013)29 | Jiaweizuoguiwan | Salvia miltiorrhiza 20 g, Ligusticum wallichii 20 g, Chinese yam 15 g, Radix rehmanniae preparata 10 g, Deerhorn glue 10 g, Medlar 15 g, Radix achyranthis bidentatae 15 g, Semen cuscutae 10 g, Fructus corni 10 g |
| Fu (2012)30 | Yishen Huazhuo granules | Epimedium 9 g, Fructus psoraleae 10 g, Radix polygoni multiflori 10 g, Glossy privet fruit 9 g, Astragali radix 10 g, Ligusticum wallichii 6 g, Acorus gramineus 6 g |
| Zhou (2001)31 | Bushen Fang | Prepared rehmannia root, Ophiopogon, Fructus corni, Fructus psoraleae |
| Zhu (2010)32 | Yizhi Jiannao granules | Epimedium, Radix polygoni multiflori, Dipsacus, Cynomorium songaricum, Acanthopanax senticosus, Semen platycladi, Leech, Turmeric, Panax notoginseng |
| He (2013)33 | Buyanghuanwutang | Astragali radix 30–120 g, Chinese angelica 10 g, Lumbricus 12 g, Ligusticum wallichii 10 g, Peach kernel 10 g, Carthamus tinctorious 6 g, Red paeony root 10 g |
| Zhou (2007)34 | Reinhartdt and sea cucumber capsule | Sea snake, Trepang, Polygala amflra, Acorus gramineus |
| Gao (2004)35 | Reinhartdt and sea cucumber capsule | Sea snake, Trepang, Polygala amflra, Acorus gramineus |
| Chang (2013)36 | Refined Xingnao powder | Epimedium, Radix polygoni multiflori, Astragali radix, Cassia twig, Ligusticum wallichii |
| Li (2010)37 | Naolingtang | Radix polygoni multiflori 20 g, Epimedium 10 g, White ginseng 15 g, Rhodiola rosea 15 g, Eucommia ulmoides 10 g, Fructus psoraleae 5 g, Acorus gramineus 10 g, Fructus Cnidii 10 g |
| Lin (2002)38 | Tiaoxinfang | Polygala tenuifolia, Codonopsis pilosula, Cassia twig, Poria cocos, Crude drug 4.67 g/mL |
| Wang (2002)39 | Tiaoxinfang | Polygala tenuifolia, Codonopsis pilosula, Cassia twig, Poria cocos, Crude drug 4.67 g/mL |
| Chen (2013)40 | Wenpitongluokaiqiaotang | Astragali radix 30 g, Radix polygoni multiflori 10 g, Acorus gramineus 10 g, Bitter cardamon 10 g, Gynostemma pentaphylla 10 g, Prepared rehmannia root 20 g, Panax notoginseng 10 g |
| Liu (2010)41 | Yizhijiannao granules | Epimedium, Dipsacus, Cynomorium songaricum, Acanthopanax senticosus, Semen platycladi, Leech, Panax notoginseng, Crude drug 5.5 g |
| Zhang (2009)42 | Yizhitang | Epimedium 30 g, Acorus gramineus 10 g, Ligusticum wallichii 15 g, Salvia miltiorrhiza 30 g, Bitter cardamon 15 g, Radix paeoniae rubra 10 g, bile arisaema 10 g, Rheum officinale 3 g, Fructus aurantii 15 g, Liquorice 10 g, Panax notoginseng 6 g |
| Peng (2009)43 | Yizhijiannao granules | Epimedium, Dipsacus, Cynomorium songaricum, Acanthopanax senticosus, Semen platycladi, Leech, Panax notoginseng, Crude drug 5.5 g |
| Yan (2007)44 | Zhijingkoufuye | Polygonatum 15 g, Ganoderma 15 g, Semen juglandis 15 g, Pine nut kernel 15 g, Prepared rehmannia root 12 g, Radix ophiopogonis 12 g, Schisandrin 12 g, Angelica sinensis 12 g, Radix curcumae 12 g, Salvia miltiorrhiza 30 g |
The type of CHM treatment varied greatly across the included studies and its intervention duration lasted 2- to 6-month. In the control group where participants were treated with either Donepezil or Piracetam on cognitive function14, 15, 16, 17, 25, 26, 27, 28, 29, 30, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 and ADL15, 16, 17, 25, 27, 29, 30, 31, 32, 33, 34, 37, 38, 39, 40, 41 were the main outcome measures across the studies included.
3.3. Risk of bias assessment
Details of the quality assessment for each study are presented in Table 3. In term of random sequence generation, the random number approach was presented in five studies,26, 28, 31, 35 and 18 studies did not report clearly the random allocation strategies.14, 15, 16, 17, 26, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 41, 44 Two studies were high risk of bias due to lack of the random sequence generation.27, 33 In aspect of allocation concealment, one study reported the allocation concealment,28 and 18 studies did not state the details of allocation concealment.14, 15, 16, 17, 26, 27, 28, 29, 30, 32, 33, 34, 37, 38, 39, 40, 41, 44 The methods of blinding of participants and personnel were only reported in one study16 the rest of studies were not described. For blinding of outcome assessment, 23 studies did not provide the detailed information,14, 15, 17, 25, 27, 28, 29, 30, 31, 32, 33, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 and only one study were low risk of bias.16 The incomplete outcome data was described in all studies. Finally, for the aspect of selective reporting and other bias, none studies reported the clear risk of bias.
Table 3.
Methodological Quality of the Included Studies
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Zhang (2018)14 | ? | ? | − | ? | + | ? | ? |
| Liu (2013)15 | ? | ? | − | ? | + | ? | ? |
| Wang (2015)16 | ? | ? | + | + | + | ? | ? |
| Yang (2013)17 | ? | ? | − | ? | + | ? | ? |
| Ding (2009)25 | + | + | − | ? | + | ? | ? |
| Li (2002)26 | ? | ? | − | ? | + | ? | ? |
| Jia (2018)27 | + | ? | − | ? | + | ? | ? |
| Jin (2017)28 | ? | ? | − | ? | + | ? | ? |
| Wang (2013)29 | ? | ? | − | ? | + | ? | ? |
| Fu (2012)30 | ? | ? | − | ? | + | ? | ? |
| Zhou (2001)31 | ? | − | − | ? | + | ? | ? |
| Zhu (2010)32 | ? | ? | − | ? | + | ? | ? |
| He (2013)33 | + | ? | − | ? | + | ? | ? |
| Zhou (2007)34 | ? | ? | − | ? | + | ? | ? |
| Gao (2004)35 | ? | − | − | ? | + | ? | ? |
| Chang (2013)36 | ? | − | − | ? | + | ? | ? |
| Li (2010)37 | ? | ? | − | ? | + | ? | ? |
| Lin (2002)38 | ? | ? | − | ? | + | ? | ? |
| Wang (2002)39 | ? | ? | − | ? | + | ? | ? |
| Chen (2013)40 | + | ? | − | ? | + | ? | ? |
| Liu (2010)41 | ? | ? | − | ? | + | ? | ? |
| Zhang (2009)42 | − | − | − | ? | + | ? | ? |
| Peng (2009)43 | − | − | − | ? | + | ? | ? |
| Yan (2007)44 | ? | ? | − | ? | + | ? | ? |
Note:“+” low risk of bias; “?” unclear risk of bias; “−” high risk of bias.
3.4. Synthesized results
3.4.1. Cognitive function
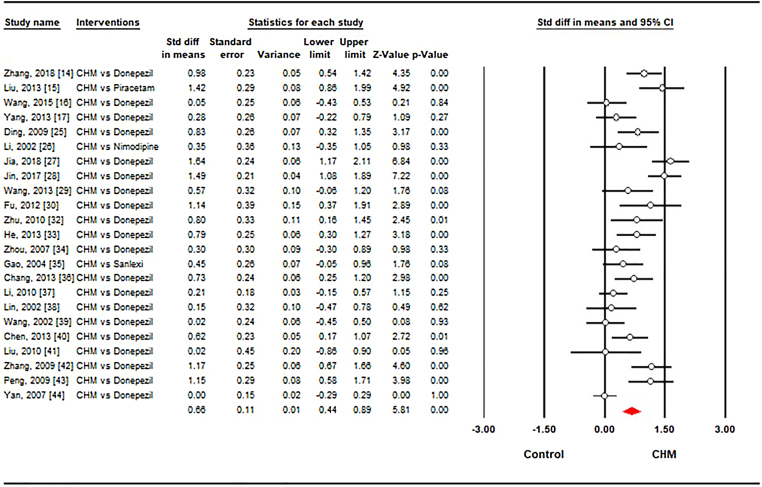
A total of 23 studies 14, 25, 26, 27, 28, 29, 30, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 were to investigate the effect CHM versus conventional drug in term of cognitive function, measured by MMSE. The pooled results from 23 studies indicated that CHM had significant effect on cognition (SMD = 0.66, 95% CI [0.44, 0.89]) with high heterogeneity (I2 = 77.9%, p < 0.001) in Fig. 2. Of which there were 20 trials 14, 25, 27, 28, 29, 30, 32, 33, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44 that assessed the effect of CHM on the cognition compared with Donepezil (SMD = 0.65, 95% CI [0.41, 0.89], I2 = 79.1%, p < 0.001). One study evaluated the effect of CHM on the cognition compared with Nimodipine and Sanlexi respectively but there were no statistically significant differences between CHM and Nimodipine and Sanlexi.
Fig. 2.
Forest plot showing the effect sizes of Chinese herbal medicine on cognitive function compared to conventional drugs. CHM: Chinese herbal medicine. The white circles represent the point of standardized mean difference. Lines represent 95% confidence intervals. The diamond shows the summary statistic. And p-value <0.05 represents that the Chinese medicine is significantly more effective than control group (conventional drugs) in treating cognition.
3.4.2. ADL
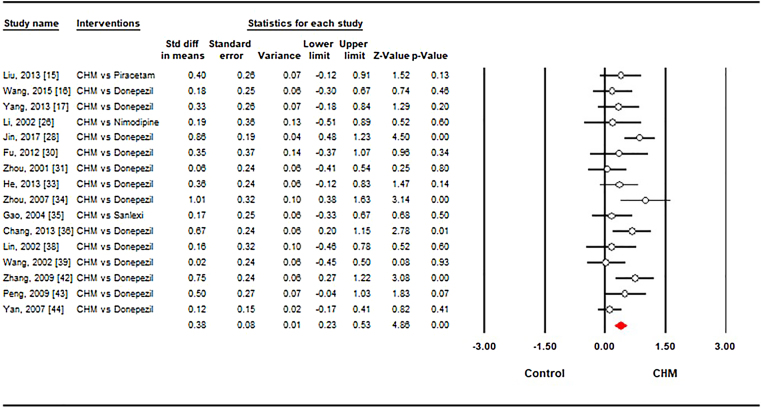
A total of 16 studies15, 16, 17, 25, 27, 29, 30, 31, 32, 33, 34, 37, 38, 39, 40, 41 compared the CHM with conventional drug therapies in this meta-analysis. The aggregated results showed improvement in ADL in favor of CHM (SMD = 0.38, 95% CI [0.25, 0.49], I2 = 35.3%, p < 0.001) in Fig. 3. Of which the pooled result from 13 trials indicated that the cognitive improvement was in favor of the CHM compared with the Donepezil (SMD = 0.40, 95% CI [0.22, 0.59], I2 = 46%, p < 0.001). CHM did not have statistically significant differences when compared with Nimodipine (SMD = 0.19, 95% CI [−0.51, 0.89], I2 = 0%, p = 0.60), Piracetam (SMD = 0.40, 95% CI [−0.12, 0.91], I2 = 0%, p = 0.13) and Sanlexi (SMD = 0.17, 95% CI [−0.33, 0.67], I2 = 0%, p = 0.50).
Fig. 3.
Forest plot showing the effect sizes of Chinese herbal medicine on activity of daily living compared to conventional drugs. CHM: Chinese herbal medicine. The white circles represent the point of standardized mean difference. Lines represent 95% confidence intervals. The diamond shows the summary statistic. And p-value <0.05 represent that the Chinese medicine is significantly more effective than control group (conventional drugs) in treating activity of daily living.
3.5. Publication bias
The result of Egger's test represented no statistically significant publication bias (p = 0.31). In terms of ADL performance, the result of Egger's test revealed no statistically significant publication bias (p = 0.64).
4. Discussion
We conducted a systematic review and meta-analysis to evaluate the efficacy of CHM on cognition and ADL in patients with AD. Our findings indicated that the CHM may enhance the cognitive function and ADL compared with conventional drug therapy, at least as effective as other drugs. However, overall risk of bias prevents the firm conclusion. The results need to be interpreted with caution, especially for practitioners who recommend prescriptions to patients.
Pooled analysis indicated that after taking CHM, AD patients can improve their abilities in terms of cognition and activity with significant effect. Although several studies failed to show the superior effects of CHM on cognitive function and ADL compared with conventional drugs, these results may be interpreted as the equivalent effects of CHM with conventional drug therapies.15, 17, 20, 36, 46 In the Chinese medical theories, the multi-herbs of CHM may have synergetic effects on to regulate the Amyloid beta and calcium disorder, as accumulating Amyloid beta and calcium disorder are associated with the physiology pathology of AD.47, 48, 49 For example, CHM (i.e., Yizhi formula) can reduce the Aβ aggregation and calcium ion activity, and further improve the memory abilities in Aβ-induced mice.50, 51 Not only CHM regulated immune-neuroendocrine functions and hemorrheology, but also altered cognitive dysfunction.52 Taken together, the CHMs seem to have great benefits to the relief of patients with AD though changing or regulating signaling pathways (e.g., calcium, enzyme), neuroregeneration, and even reducing Aβ concentration.53, 54
We would like to acknowledge several limitations in this current review study. First, although the present systematic review and meta-analysis was not registered in PROSPERO, we tried to avoid the bias in the post hoc analysis. Second, there are several intrinsic caveats in the primary studies and they may affect the quality of this study. Third, original authors did use proper sample size calculation, and this may underestimate the efficacy of intervention. Fourth, we did not perform meta-regression to find the cause of heterogeneities and this may exaggerate the effects of the treatment. Finally the prescription and doses of CHMs were vary according to the studies and these may occur the heterogeneity of meta-analysis.
In conclusion, the results of this systematic review suggest that CHM treatment may have potential effects on improving cognitive function and ADL for individuals with AD. However, the evidence is limited because of high risk of bias of included studies. Further rigorous studies are needed to confirm the efficacy.
Funding
This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
Yanjie Zhang and Wook Song designed the whole study; Kyoungmin Noh and Yanjie Zhang completed the literature search and data collection; Yanjie Zhang and Kyoungmin Noh conducted the meta-analysis; all authors contributed to the interpretation of data and revision.
Conflict of interest
The authors declare no conflict of interest.
Data availability
All extracted data was provided as supplement Table 1.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.imr.2019.04.006.
Supplementary
The following are the supplementary data to this article:
References
- 1.Alzheimer's Association 2018 Alzheimer's disease facts and figures. Alzheimer's Dement. 2018;14:367–429. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Ahn N., Kim K. Effects of an elastic band resistance exercise program on lower extremity muscle strength and gait ability in patients with Alzheimer's disease. J Phys Ther Sci. 2015;27:1953–1955. doi: 10.1589/jpts.27.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noetzli M., Eap C.B. Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of alzheimer's disease. Clin Pharmacokinet. 2013;52:225–241. doi: 10.1007/s40262-013-0038-9. [DOI] [PubMed] [Google Scholar]
- 4.Prince M., Comas-Herrera A., Knapp M., Guerchet M., Karagiannidou M. World Alzheimer Report 2016 improving healthcare for people living with dementia. Coverage, Quality and costs now and in the future. Alzheimer's Dis Int. 2016:1–140. [Google Scholar]
- 5.Number of dementia patients to surpass 2.7 million by 2050. BOOKIT. http://somslife.tistory.com/15?category=637581. Accessed July 20, 2018.
- 6.Knight R., Khondoker M., Magill N., Stewart R., Landau S. A systematic review and meta-analysis of the effectiveness of acetylcholinesterase inhibitors and memantine in treating the cognitive symptoms of dementia. Dement Geriatr Cogn Disord. 2018;45:131–151. doi: 10.1159/000486546. [DOI] [PubMed] [Google Scholar]
- 7.Ströhle A., Schmidt D.K., Schultz F., Fricke N., Staden T., Hellweg R. Drug and exercise treatment of alzheimer disease and mild cognitive impairment: a systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am J Geriatr Psychiatry. 2015;23:1234–1249. doi: 10.1016/j.jagp.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Raina P., Santaguida P., Ismaila A., Patterson C., Cowan D., Levine M. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 9.Landi F., Russo A., Liperoti R., Cesari M., Barillaro C., Pahor M. Anticholinergic drugs and physical function among frail elderly population. Clin Pharmacol Ther. 2007;81:235–241. doi: 10.1038/sj.clpt.6100035. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J., Peng W., Xu M., Li W., Liu Z. The effectiveness and safety of acupuncture for patients with alzheimer disease. Medicine (Baltimore) 2015;94:e933. doi: 10.1097/MD.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X. China Academy Press; Beijing: 2011. Shen Nong Ben Cao Jing; pp. 60–80. [Google Scholar]
- 12.Bi M., Tong S., Zhang Z., Ma Q., Zhang S., Luo Z. Changes in cerebral glucose metabolism in patients with mild-to-moderate Alzheimer's disease: a pilot study with the Chinese herbal medicine fuzhisan. Neurosci Lett. 2011;501:35–40. doi: 10.1016/j.neulet.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Lin C., Zhang L., Cui Y., Gu Y., Guo J. Cognitive improvement during treatment for mild Alzheimer's disease with a Chinese herbal formula: a randomized controlled trial. PLoS One. 2015;10:e0130353. doi: 10.1371/journal.pone.0130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L., Peng J. Qingxin Yizhi decoction on improving the quality of life, cognitive level and the biochemical indexes of SOD and MDA in AD patient. Shanxi J Tradit Chinese Med. 2018;39:593–595. [Google Scholar]
- 15.Liu P., Kong M., Liu S., Chen G., Wang P. Effect of reinforcing kidney-essence, removing phlegm, and promoting mental therapy on treating Alzheimer's disease. J Tradit Chinese Med. 2013;33:449–454. doi: 10.1016/s0254-6272(13)60147-8. [DOI] [PubMed] [Google Scholar]
- 16.Wang S. Clinical effect of Congrong Yizhi decoction on people with Alzheimer's Disease. Zhejiang J Chinese Med. 2015;50:386–387. [Google Scholar]
- 17.Yang P., Dong K. Clinical Effects of Yizhi Jiannao granule on Alzheimer’ Disease. J New Chinese Med. 2013;45:56–58. [Google Scholar]
- 18.May B.H., Lit M., Xue C.C.L., Yang A.W., Zhang A.L., Owens M.D. Herbal medicine for dementia: a systematic review. Phytother Res. 2009;23:447–459. doi: 10.1002/ptr.2656. [DOI] [PubMed] [Google Scholar]
- 19.Fu L.-M., Li J.-T. A systematic review of single chinese herbs for Alzheimer's disease treatment. Evid Based Complement Alternat Med 2011. 2011:640284. doi: 10.1093/ecam/nep136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos-Neto L.L., de Vilhena Toledo M.A., Medeiros-Souza P., de Souza G.A. The use of herbal medicine in Alzheimer's disease—a systematic review. Evidence-Based Complement Altern Med. 2006;3:441–445. doi: 10.1093/ecam/nel071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyde A.J., May B.H., Dong L., Feng M., Liu S., Guo X. Herbal medicine for management of the behavioural and psychological symptoms of dementia (BPSD): a systematic review and meta-analysis. J Psychopharmacol. 2017;31:169–183. doi: 10.1177/0269881116675515. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P.T. The Cochrane Collaboration; 2011. Cochrane Handbook for systematic reviews of interventions version; pp. 2–3. [Google Scholar]
- 23.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding X., Zang Q., Sun Q., Zhang J. Clinical research of Shenghuangyizhi granule on Alerzheimer's disease. Chinese J Gerontol. 2009;29:2023–2024. 25. [Google Scholar]
- 26.Li Z., Mu Y., Ouyang Q. Clinic control research of Alzheimer's disease by the combination of acupuncture and Danggui Shaoyao San (DGSYS) of TCM. Chinese J Clin Rehabil. 2002;6:2848–2849. [Google Scholar]
- 27.Jia X., Luo H., Xu W., Miao Y. Observation on efficacy of rehmanniae decoction in modified treatment of Alzheimer. Eval Anal drug-use Hosp China. 2018;18:499–501. [Google Scholar]
- 28.Jin X., Duan Y., Zhang D., Liu Z., Mei Q., Huang H. Observation on the effect of tongqiaohuoxue decoction on Alzheimer's disease. Chinese J Integr Med Cardio-/Cerebrovascular Dis. 2017;15:15–17. [Google Scholar]
- 29.Wang E. Clinical observation on 20 cases of Alzheimer's disease treated by Jiaweizuoguiwan. Jiangsu Tradit Chinese Med. 2013;45:38–39. [Google Scholar]
- 30.Fu K., Lin C., Zhang Y., Guo J., Wang X., Cui Y. Clinical study on treatment of 15 cases of mild Alzheimer's disease with Yishenhuazhuofang. Jiangsu Tradit Chinese Med. 2012;44:28–29. [Google Scholar]
- 31.Zhou R., Lin S., Wang J., Yu Z. Clinical study on life ability improved by “Kidney-Tonifying Formula” in Alzheimer's disease. Mod Rehabil. 2001;5:35–38. [Google Scholar]
- 32.Zhu H., Dong K., Wu Y., Zhang T., Li R., Hu S. Effect of Bushenhuoxue method on cognitive function improvement of Alzheimer's disease patients. Chinese J Gerontol. 2010;30:1493–1495. [Google Scholar]
- 33.He H., Zhou R. Revised Buyang Huanwu decoction treat senile dementia (SD) 35 cases. Zhejiang Chinese Med Univ. 2013;37:723–724. [Google Scholar]
- 34.Zhou Z., Liang L., Yan Y. Clinical study of reinhartdt and sea cucumber capsule combined with donepezil in treating Alzheimer's disease. Chinese J Integr Tradit West Med. 2007;21:110–113. [PubMed] [Google Scholar]
- 35.Gao P., Wen S., Qin S., Cai X. A clinical study of the efficacy and safety of Fufanghaishe capsule in the treatment of Alzheimer disease. Chinese J Clin Healthc. 2004;7:401–402. [Google Scholar]
- 36.Chang F., Yuan Y., Sun Y., Lai J., Song X., Lin Y. Clinical research on refined Xingnao power in treatment of senile dementia. Chinese Arch Tradit Chinese Med. 2013;30:1253–1255. [Google Scholar]
- 37.Li X., He M. Effect of Naoling decoction on MMSE and serum pro-inflammatory cytokines in patients with Alzheimer's disease. Pract Prev Med. 2010;17:959–961. [Google Scholar]
- 38.Lin S., Zhou R., Wang J., Yu Z. Comparative study of “heart-regulating formula” and “kidney-tonifying formula” in treating the cognition and daily life ability of Alzheimer's disease. Chinese J Gerontol. 2002;22:434–436. [Google Scholar]
- 39.Wang J. A clinical study on the treatment of Alzheimer's disease with Diaoxinfang. J Beijing Univ Tradit Chinese Med. 2002;25:51–53. [Google Scholar]
- 40.Chen W., Jiang L., Liu T., Gu L., Wu P., Wang J. Clinical observation on 40 cases of senile dementia with phlegm resistant obstruction syndrome treated by Wenpitongluokaiqiao decoction. J Tradit Chinese Med. 2013;54:1759–1761. [Google Scholar]
- 41.Liu P., Dong K. Effect of Yizhijiannao granule on expression of IL-1β and TNF-α in peripheral serum of patients with Alzheimer's disease. Hunan J Tradit Chinese Med. 2010;26:1–3. [Google Scholar]
- 42.Zhang F. Treatment of 38 cases of Alzheimer's disease with Yizhi decoction. J Chinese Med. 2009;24:45–46. [Google Scholar]
- 43.Peng X.W., Dong K.L. Clinical observation on acupuncture combined with Yizhi Jiannao granules for treatment of Alzheimer's disease. Chinese Acupunct Moxibust. 2009;29:269–271. [PubMed] [Google Scholar]
- 44.Yan S., Yan S., Qi D., Niu Y., Wang M. Clinical observation on 93 cases of Alzheimer's disease treated by Zhijing oral liquid. Guangxi Tradit Chinese Med. 2007;30:30–32. [Google Scholar]
- 45.May B.H., Lu C., Lu Y., Zhang A.L., Xue C.C.L. Chinese herbs for memory disorders: a review and systematic analysis of classical herbal literature. JAMS J Acupunct Meridian Stud. 2013;6:2–11. doi: 10.1016/j.jams.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 46.May B.H., Feng M., Hyde A.J., Hügel H., Chang S., Dong L. Comparisons between traditional medicines and pharmacotherapies for Alzheimer disease: a systematic review and meta-analysis of cognitive outcomes. Int J Geriatr Psychiatry. 2018;33:449–458. doi: 10.1002/gps.4830. [DOI] [PubMed] [Google Scholar]
- 47.Yang H., Wen S.R., Zhang G.W., Wang T.G., Hu F.X., Li X.L. Effects of Chinese herbal medicine Fuzhisan on autologous neural stem cells in the brain of SAMP-8 mice. Exp Gerontol. 2011;46:628–636. doi: 10.1016/j.exger.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Fu X., Wang Q., Wang Z., Kuang H., Jiang P. Danggui-Shaoyao-San: new hope for Alzheimer's disease. Aging Dis. 2016;7:502–513. doi: 10.14336/AD.2015.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Z., Xiao Z., Zhu D., Yan Y., Yu B., Wang Q. Aqueous extracts of FBD, a Chinese herb formula composed of Poria cocos, Atractylodes macrocephala, and Angelica sinensis reverse scopolamine induced memory deficit in ICR mice. Pharm Biol. 2009;47:396–401. [Google Scholar]
- 50.Cai H., Luo Y., Yan X., Ding P., Huang Y., Fang S. The mechanisms of bushen-yizhi formula as a therapeutic agent against Alzheimer's disease. Sci Rep. 2018;8:3104. doi: 10.1038/s41598-018-21468-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woods N.K., Padmanabhan J. vol. 740. Springer; Dordrecht: 2012. Neuronal calcium signaling and Alzheimer's disease; pp. 1193–1217. (Advances in experimental medicine and biology). [DOI] [PubMed] [Google Scholar]
- 52.Kou J., Zhu D., Yan Y. Neuroprotective effects of the aqueous extract of the Chinese medicine Danggui-Shaoyao-san on aged mice. J Ethnopharmacol. 2005;97:313–318. doi: 10.1016/j.jep.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Sreenivasmurthy S., Liu J.-Y., Song J.-X., Yang C.-B., Malampati S., Wang Z.-Y. Neurogenic traditional Chinese medicine as a promising strategy for the treatment of Alzheimer's disease. Int J Mol Sci. 2017;18:272. doi: 10.3390/ijms18020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z.-Y., Liu J.-G., Li H., Yang H.-M. Pharmacological effects of active components of chinese herbal medicine in the treatment of Alzheimer's disease: a review. Am J Chin Med. 2016;44:1525–1541. doi: 10.1142/S0192415X16500853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All extracted data was provided as supplement Table 1.