Abstract
Long QT syndrome (LQTS) is a potentially severe arrhythmogenic disorder, associated with a prolonged QT interval and sudden death, caused by mutations in key genes regulating cardiac electrophysiology. Current strategies to study LQTS in vitro include heterologous systems or animal models. Despite their value, the overwhelming power of genetic tools has exposed the many limitations of these technologies. In 2010, human-induced pluripotent stem cells (hiPSCs) revolutionised the field and allowed scientists to study in vitro some of the disease traits of LQTS on hiPSC-derived cardiomyocytes (hiPSC-CMs) from LQTS patients. In this concise review we present how the hiPSC technology has been used to model three main forms of LQTS and the severe form of LQTS associated with mutations in calmodulin. We also introduce some of the most recent challenges that must be tackled in the upcoming years to successfully shift hiPSC-CMs from powerful in vitro disease modelling tools into assets to improve risk stratification and clinical decision-making.
Keywords: Long QT syndrome, cardiac arrhythmias, stem cells, human-induced pluripotent stem cells, cardiomyocytes, precision medicine, life-threatening arrhythmias, sudden cardiac death
Long QT Syndrome
Long QT syndrome (LQTS) is a potentially severe arrhythmogenic disorder, affecting more than one in 2,000 people worldwide.[1] It is characterised by a marked prolongation of the QT interval on the electrocardiogram and major cardiac events, such as syncope, cardiac arrest or sudden death, especially under conditions of physical or emotional stress.[2,3] The current diagnostic criteria and options for effective management have been recently reviewed.[4] As the first manifestation of the disease is frequently a lethal arrhythmic event, early diagnosis and therapy are extremely important and recent advances in genetics offer new opportunities.[3] Besides its central role for the discovery of the molecular basis of LQTS and for the identification of the first gene-specific therapy, genetics became pivotal for the identification of the disease-causing genes and mutations in routine diagnostic practice and for gene-specific management.[3–5] However, the low and variable penetrance exhibited by LQTS and the many confounding factors represent challenges to diagnosis and obstacles for the implementation of successful proactive treatments.[6] In this context, multiple groups including ours have started to investigate and identify factors – either genetic, pharmacological or environmental – able to modify the clinical course of the disease by acting as modifiers of gene/protein function, expression or regulation.[7] People with identical pathogenic mutations, even members of the same family, may display different degrees of clinical severity, symptoms or even be silent carriers of the mutation, which complicates the possibility of having unambiguous predictions of disease phenotypes simply based on the presence of disease-causing mutations in LQTS genes or when someone has a family history of the disease.[7–12]
Diagnosis of LQTS may also be hindered by the presence of uncharacterised rare variants; given that the pace by which modern genetic tools can extract huge amounts of information is unprecedented, the field is progressively accumulating a growing number of rare genetic variants associated with LQTS subpopulations; these, initially defined variants of unknown/uncertain significance (VUS), are variants short-listed in the genetic analysis but for which evidence of being benign or pathogenic does not allow to draw univocal conclusions.[13,14]
The complexity behind some of the puzzling LQTS clinical manifestations has still to be fully unveiled and this leaves many patients, who might need clinical attention during the course of their lives, undiagnosed and unprotected. Therapies should be personalised for these subjects, but current tools and knowledge do not allow sufficient predictivity or mutation-specific remedies. To study such a high complexity, the need for valid and reliable experimental models intensifies; traditional models as heterologous systems are incapable of completely recapitulating genetic and phenotypic features, while animal models, particularly in rodents, do have electrophysiological characteristics that are often species-specific and very different from humans.[15] This hampers the possibility of promptly translating results obtained in vitro directly to the clinics, with significant delays occurring between the discovery of promising treatments and their clinical application. For all these reasons, new experimental models, more predictive of human physiology and less limited by costs and ethics, are required and cardiomyocytes (CMs) derived from induced pluripotent stem cells (iPSC-CMs) have emerged in the past few years as a leading platform for these studies.
Almost 10 years after the first LQTS model with human iPSC-CMs (hiPSC-CMs) was published, we will briefly discuss the state of the art, some of the obstacles that need to be solved and the fascinating transition of hiPSC-CMs as precision medicine tools that are used to preliminarily investigate experimental therapies and guide pharmacological treatments.[16] We will focus on the main forms of LQTS.
Human-induced Pluripotent Stem Cells
In 2006, Shinya Yamanaka and Kazutoshi Takahashi discovered that somatic cells could be reprogrammed to a pluripotent state by transfecting four factors – OCT4, SOX2, KLF4, cMYC – subsequently called Yamanaka factors.[17,18] These reprogrammed cells, called iPSCs, can be derived from virtually anyone and represent an unprecedented source of human-based material that can be differentiated into multiple cell types through tailored differentiation protocols, including cardiomyogenic differentiation.[19] Originally, iPSCs were derived from skin fibroblasts, which requires a biopsy, often difficult to propose for young children or to vulnerable subjects often already daunted by life-threatening diseases. Thus, new methods have been developed to obtain somatic cells from more accessible and less invasive tissues samples such as blood or urine.[20,21]
In addition to the obvious electrophysiological and molecular advantages of being of human origin, these cells share genotypes with their donors; this feature makes iPSCs candidate experimental models to study diseases of genetic origin, with LQTS and cardiac channelopathies being among the first to be reproduced with this technology. In 2010, the pioneering work of Alessandra Moretti et al., demonstrated that hiPSC-CMs could become powerful tools to study the functional effects of ion channel mutations identified in LQTS patients.[16] However, as the molecular and functional characteristics of hiPSC-CMs are still far from those of adult CMs, multiple protocols were invented to promote their maturation.[22] These strategies include the use of prolonged cultures, chemically-defined media, the generation of tridimensional structures tailored for electrical stimulation protocols or co-cultures of CMs with other relevant cell types.[23–31] Overall, these experimental models are progressively improving and the techniques to study the phenotype of hiPSC-CMs have advanced in parallel.
Methods for the study of Long QT Syndrome with Human-induced Pluripotent Stem Cells
The in vitro investigation of LQTS has the primary endpoint to ascertain whether and how the CMs' electrophysiology has been modified as a consequence of LQTS-causing gene mutations. The patch clamp technique is the reference platform of choice to precisely quantify the phenotype of hiPSC-CMs. Although limited by its low throughput and disruptiveness, patch clamp allows quantitative recordings of action potentials (AP) and ion currents; furthermore, the combination of real-time computational ion current simulations with in vitro experiments has merged in the dynamic clamp technique.[32] Although not particularly new at the time hiPSC-CMs were first derived, it was embraced to compensate the intrinsically reduced inward rectifier potassium current (IK1) magnitude, typical of isolated hiPSC-CMs,[33–35] with an artificial IK1 injected in real-time. This technique transforms the action potential (AP) of hiPSC-CMs into one with more mature features as greater upstroke velocity and the presence of the AP notch, allowing voltage-gated ion channels to properly work as they were in more physiological conditions; this proved particularly relevant to capture the electrophysiological alterations caused by LQTS pathogenic mutations.[33,34]
However, since the heart is a functional syncytium, measurements of the downstream effects of LQTS mutations have greater translational power when performed on multicellular networks. At sufficient density, hiPSC-CMs do form functional beating mono- or multilayered sheets, which can be scrutinised with the multielectrode array (MEA) technology. Originally developed to study neuronal circuits, MEAs have gained interest in recent years with the development of stem cell-based cardiac disease models.[36] Furthermore, the MEA technology has also been defined as one of the platforms of choice in the context of the US Food and Drug Administration's Comprehensive in vitro Proarrhythmia Assay (CiPA), a task group which aims to identify the most suitable platform and protocols for drug screening and safety pharmacology.[37]
Other technologies with low invasiveness have been implemented as chemical or genetically-encoded voltage or calcium indicators, optogenetic actuators or imaging algorithms to investigate specific parameters of the diseased phenotype as a contraction and they all became widely used to obtain functional information with medium-high throughput.[38–44]
Long QT Syndrome Subtypes Studied with Human-induced Pluripotent Stem Cell Cardiomyocytes
As expected by the high prevalence in patients, the first three forms of LQTS are also those primarily modelled in vitro.[1] However, other LQTS types have been identified and successfully reproduced with hiPSC-CMs (Figure 1); we will focus on these three main types and on the extremely severe forms related to mutations on the genes encoding for calmodulin (CaM). Common in vitro phenotypes across all LQTS subtypes include prolonged AP durations (APDs), measured with patch clamp or voltage-sensitive indicators, prolonged field potential (FP) durations (FPD) measured with MEAs or prolonged Ca2+ and contraction transients (Figure 2). Some of the cell lines also experienced arrhythmogenic events as delayed after depolarizations (DADs) and early after depolarizations (EADs) in baseline conditions or after a pharmacological challenge.[36]
Figure 1: Number of Human-induced Pluripotent Stem Cell Lines with Long QT Syndrome Mutations Published So Far in the Literature.
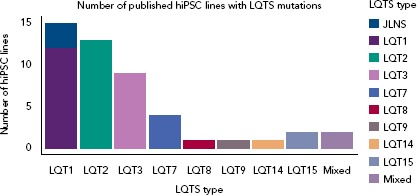
Mixed refers to hiPSC lines generated from patients with a mixed clinical phenotype that includes LQTS. hiPSC = Human-induced Pluripotent Stem Cell; LQTS = Long QT syndrome.
Figure 2: Long QT Syndrome Generates Prolongation of QT Interval at the ECG, Action Potential Duration Measured with Patch Clamp and Field Potential Duration Recorded with Multielectrode Array.
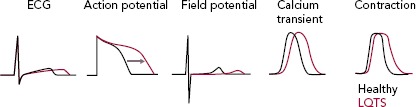
LQTS = Long QT syndrome.
Long QT Syndrome Type 1 and Jervell and Lange-Nielsen Syndrome
LQTS type 1 (LQT1) is the most prevalent form of congenital LQTS, accounting for approximately 40–55% of all cases and its heterozygous form, called Romano-Ward syndrome, was the first LQTS type to be recapitulated with hiPSC-CMs.[45–48] The severe homozygous form, when associated with deafness, is defined as Jervell and Lange-Nielsen syndrome (JLNS).[49,50] People with LQT1 and JLNS are affected by mutations in KCNQ1 that induce loss of functions in Kv7.1, an important potassium channel responsible for the slow delayed rectifier potassium current (IKs). Although IKs is not straightforward to record in hiPSC-CMs due to their limited cell capacitance, which reduces the total current density, and to a late IKs onset during CM development,[51] partially mitigated by certain maturation strategies,[25] in vitro models of LQT1/JLNS were able to recapitulate the pathological phenotype and exhibited marked reductions in IKs magnitude when compared to hiPSC-CMs from healthy donors or to isogenic controls[52–54]; detailed investigation of IKs with patch clamp also revealed the possibility for hiPSC-CMs to perfectly discriminate between homozygosity and heterozygosity.[51–55] The similarities between hiPSC-CMs and clinical disease features extend also to triggers of arrhythmias; as previously described in humans, an increased activation of the sympathetic cascade is a clear arrhythmogenic trigger for LQT1/JLNS.[56] When this is reproduced in vitro by stimulating hiPSC-CMs with beta-adrenergic agonists, their effect on L-type Ca2+ current outweighs the repolarisation capacity of the limited IKs magnitude available in LQT1/JLNS cells, leading to APD prolongation, abnormal Ca2+ transients and arrhythmogenic events (Figure 3).
Figure 3: Effect of Beta-adrenergic Stimulation on Cardiomyocytes From Long QT Syndrome Type 1/Jervell and Lange-Nielsen Syndrome Patients.
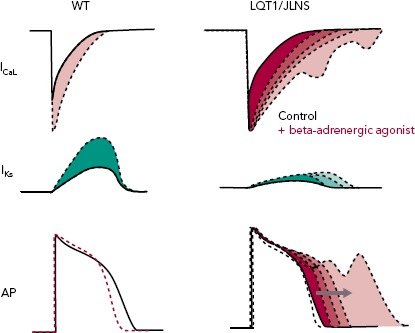
Beta-adrenergic stimulation increases both the L-type Ca2+ current (ICaL) and the slow delayed rectifier potassium current (IKs). In cardiomyocytes from healthy people, the increase in IKs is sufficient to counterbalance that of ICaL, leading to APD shortening in humans. In the presence of loss-of-function mutations in KCNQ1, as in LQT1/JLNS patients, the IKs is not sufficient to counterbalance the ICaL enhancement, leading to APD prolongation and arrhythmias. AP = action potential; LQT1 = Long QT syndrome type 1; JLNS = Jervell and Lange-Nielsen Syndrome Patients; WT = wild-type (healthy control).
Long QT Syndrome Type 2
LQTS type 2 (LQT2) is the second most common form of LQTS, accounting for 30–45% of cases.[45] It is characterised by mutations in the KCNH2 gene, which encodes for the human ether a-go-go-related gene (hERG, Kv11.1), a potassium channel responsible for the rapid delayed rectifier potassium current (IKr), pivotal for the cardiac repolarisation phase.[46] LQT2 mutations may induce loss of function in IKr through four main mechanisms.[57] The in vitro phenotype in hiPSC-CMs is generally clear, with marked reductions in IKr coupled to consistent APD prolongation.[58–68] Clinical LQT2 triggers of arrhythmias include sudden auditory stimuli or loud noises and while these cannot be properly mimicked in vitro, alternatives as a stimulation with positive inotropes, alone or in combination with IKs blockers, are a widely used strategy to exacerbate arrhythmias in these cells.[56,69]
Since the majority of LQT2 mutations affect IKr through defective protein trafficking with hERG channels incapable of maturing and reaching the CMs' membrane, researchers have adopted several strategies to restore proper hERG expression or function.[70] Some rescued the LQT2 phenotype with compounds able to increase the conductance of wild type hERGs, other with proteasome inhibitors[59,61,67] or, more recently, through a drug repurposing strategy which promoted the maturation of mutant hERGs in vitro.[58,62] This drug repurposing strategy was very rapidly translated to the two LQT2 patients whose iPSC-CMs were previously tested in vitro and proved that, besides the expected discrepancies between the clinical and in vitro conditions, the data obtained in patient-specific iPSC-CMs do offer a certain degree of predictivity in the clinical situation.[71]
Long QT Syndrome Type 3
LQTS type 3 (LQT3) is the third most common form of LQTS, accounting for 5–13% of all congenital LQTS cases.[45,46] It is the consequence of gain-of-function mutations in the SCN5A gene, leading to an increase in the cardiac sodium current (INa); different biophysical changes induced by LQT3 mutations may increase either the peak INa or also affect its late component (INa,L), the latter involved in electrophysiological abnormalities in multiple tissues, such as pancreatic beta-cells, cardiomyocytes or neurons.[72,73] Despite INa overall current magnitude is lower in hiPSC-CMs compared with adult CMs and published hiPSC-CM models of LQT3 did correctly recapitulate increases in INa and other features of LQTS as EADs or prolonged calcium transients; APD was prolonged in the majority of models even though some authors described larger than ideal inter-line and inter-cell phenotypic variability.[66,74–77] Furthermore, hiPSC-CMs with LQT3 mutations reproduced the expected effect of pharmacological therapies based on sodium channel blockers, which were able to rescue the prolonged APD and FPD and to suppress arrhythmogenic events in a dose-dependent way.[74,76,78] Of note, some authors were also able to model through hiPSC-CMs some cardiac disorders with overlapping phenotypes of LQT3 and Brugada syndrome.[75]
Long QT Syndrome Type 14 and 15
LQTS types 14 (LQT14), 15 (LQT15), and the upcoming LQTS type 16 (LQT16), which is not yet officially approved, are characterised by mutations in one of the three genes (CALM1–3) encoding for CaM.[79] Discovered by our group as associated with severe forms of LQTS and later investigated with hiPSC-CMs, these mutations impair the activity of CaM by either reducing its Ca2+-binding affinity or by altering its capacity to interact with downstream targets.[33,80–82] Since CaM is a ubiquitous protein, present in CMs in limited pools, the consequences are dramatic, with very young people experiencing severe arrhythmias since birth and for whom current therapies are insufficient or inappropriate.[83–85] These severe phenotypic characteristics have been perfectly captured by the hiPSC technology, with hiPSC-CMs from patients with CALM mutations exhibiting pathologically slowed ICaL Ca2+-dependent inactivation and massively prolonged APDs. A phenotypic rescue has been attempted in hiPSC-CMs through novel pharmacological or biotechnological strategies, with promising results that have already obtained preliminary translation to patients.[33,81,82,86]
Latest Challenges
The aforementioned disease models represent a good approximation of the clinical condition and have pushed scientists to explore potential applications for hiPSC-CMs. A few of the most recent challenges of the cardiac hiPSC field will be discussed below.
Data Variability and Gene Editing
The correlation between in vitro and in vivo data is still either uncharted territory or has resulted in contradictory findings in the literature. Although evidence has demonstrated that a fair correlation between clinical and in vitro recordings exists (i.e. LQTS cell lines generally do have prolonged APD/FPD in vitro), we still do not know precisely its extent and whether and how different reprogramming or maturation strategies could modify the magnitude and the time course of the in vitro equivalents of clinically relevant parameters as the QT interval or the RR interval. In the years since the application of hiPSC technology to disease modelling, it has become clear that cell lines from different donors have innate characteristics that are independent of the specific pathogenic mutation, thus they are likely to be associated with donor-specific characteristics.[87,88]
Electrophysiological properties as APD, which length should directly correlate with the presence of LQTS mutations, seem cell-line specific or differentiation-method specific rather than disease-specific and the same applies to FPD-RR relationships measured with MEAs; this can be attributed to potential confounding factors as manual pipetting, cell passage number and donor's gender or ethnicity, suggesting the need of great care when data have to be interpreted on a translational perspective.[62,88,89] In this vein, one of the most important additions in the LQTS field has been the concept of isogenic lines[61]; gene editing techniques, either based on zinc finger nucleases (ZFN), transcription activator-like effector nuclease (TALEN) or clustered regularly Interspaced short palindromic repeats (CRISPR)/Cas9.[90–93] This technology can introduce pathogenic mutations, homozygous or heterozygous, into hiPSC lines from healthy donors, or correct them without altering the cell's genetic background. This has proved to be important for introducing more robust standards to evaluate the functional consequences of point mutations and for precision medicine approaches.[12,55,61,62,82,94,95]
Precision Medicine
Genetics, hiPSCs and cellular electrophysiology are progressively providing better chances of tailoring therapies to the specific mutation of the patient, and it is clear that the partnership between clinical cardiologists and basic scientists can be very fruitful[96]. One of the biggest advantages of hiPSC-CMs is that they share their genetic background with patients. This link can be harnessed to correlate clinically relevant information as genetic data to in vitro physiological and pharmacological responses. Patients with similar characteristics can be clustered according to their phenotype or genetic data to obtain homogenous cohorts of people who share similar characteristics. HiPSC-CMs are generated from these subjects and subsequently screened with molecular and functional assays that are able to generate data of translational relevance. Gene editing tools are used here to validate the results in the presence of identical genetic backgrounds.[95] This strategy may have important consequences for risk stratification and therapeutic responses, as patients with certain VUSs would ideally be given different drug dosages based on the preliminary results of the in vitro screenings in hiPSC-CMs.
In the context of precision medicine, the identification of novel clinical applications of drugs that have already received approval from regulatory agencies (drug repurposing) has increased. When combined with hiPSCs, it allows marketed drugs that are used to treat other disorders to be tested in vitro with a remarkable throughput on differentiated cells of human origin. In this view, our group has identified that lumacaftor (LUM), a compound currently prescribed for the treatment of specific mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, associated with defects trafficking in CFTR channels, could also rescue the disease phenotype in hiPSC-CMs derived from a specific class of people with LQT2 affected by hERG trafficking problems.[58] Since LUM is already marketed for the treatment of cystic fibrosis in combination with ivacaftor and its safety profile is known, we were sufficiently confident to start a clinical trial.[71] The mechanisms by which LUM may rescue hERG trafficking defects are still unknown and these results will require extensive confirmation from clinical data and from other hiPSC lines from more LQT2 donors to verify whether these effects could be cell-line- or mutation-specific or whether their mechanism could be exploited for broader applications.
Conclusion
hiPSC technology has brought significant contributions to the way LQTS and cardiac arrhythmias are modelled at preclinical level[97]; however, more efforts are required to offer an exhaustive comprehension of the translational relevance of hiPSC findings. In our view, shared and open protocols, tighter standards from researchers, journals, funding agencies as well as the implementation of large publicly available informatic tools and datasets are the sole way to revolutionise how the iPSC technology interacts with the clinics and shift hiPSC-CMs from useful in vitro preclinical tools to something able to produce relevant information for prompt clinical decision-making. It is also clear that genetics, combined with the most recent advances in hiPSC-CMs technology, bioinformatics and artificial intelligence, will unveil future possibilities and targets for the diagnosis, risk stratification and treatment of LQTS.
Clinical Perspective
LQTS is a potentially lethal arrhythmogenic disorder that affects more than 1 in every 2,000 children. Our knowledge of genetics in LQTS has progressively increased over the years, and we reached a mismatch between the amount of genetic information available and the functional data that allow us to interpret this genetic information.
The emerging role of variants of unknown/uncertain significance (VUS) require in vitro experimental models capable of effectively integrating the genetic component and to extract predictive information relevant for clinical use.
Human induced pluripotent stem cell-derived cardiomyocytes from LQTS patients, as they share the patients' genotype, have proven to be a useful platform for in vitro investigation of the effect of pathogenic mutations of LQTS and VUS on the cardiac phenotype, and may represent the optimal tool, in combination with gene editing strategies, for large-scale investigation of the functional consequences of genetic variants.
Large precision medicine approaches could combine the benefits of large-scale genetic studies with reliable functional readouts operating at medium to high throughput.
Some of the current limitations of the hiPSC technology can be addressed only with combined and bidirectional efforts between clinicians and scientists.
Acknowledgments
Maria-Christina Kotta for discussion and suggestions on the genetic aspects of LQTS. This work was supported by a Marie Skłodowska-Curie Individual Fellowship (H2020-MSCA-IF-2017 No. 795209) to Luca Sala, and by the Leducq Foundation for Cardiovascular Research grant 18CVD05 “Towards Precision Medicine with Human iPSCs for Cardiac Channelopathies”. Pinuccia De Tomasi for expert editorial support.
References
- 1.Schwartz PJ, Stramba-Badiale M, Crotti L et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–7. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. 1975;89:378–90. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- 3.Crotti L, Kotta MC. The role of genetics in primary ventricular fibrillation, inherited channelopathies and cardiomyopathies. Int J Cardiol. 2017;237:45–8. doi: 10.1016/j.ijcard.2017.03.119. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Ackerman MJ. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013;34:3109–16. doi: 10.1093/eurheartj/eht089. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz PJ, Priori SG, Locati EH et al. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–6. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 6.Priori SG, Napolitano C, Schwartz PJ. Low penetrance in the long-QT syndrome: clinical impact. Circulation. 1999;99:529–33. doi: 10.1161/01.CIR.99.4.529. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Crotti L, George AL. Modifier genes for sudden cardiac death. Eur Heart J. 2018;39:3925–31. doi: 10.1093/eurheartj/ehy502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brink PA, Crotti L, Corfield V et al. Phenotypic variability and unusual clinical severity of congenital long-QT syndrome in a founder population. Circulation. 2005;112:2602–10. doi: 10.1161/CIRCULATIONAHA.105.572453. [DOI] [PubMed] [Google Scholar]
- 9.Crotti L, Monti MC, Insolia R et al. NOS1AP is a genetic modifier of the long-QT syndrome. Circulation. 2009;120:1657–63. doi: 10.1161/CIRCULATIONAHA.109.879643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duchatelet S, Crotti L, Peat RA et al. Identification of a KCNQ1 polymorphism acting as a protective modifier against arrhythmic risk in long-QT syndrome. Circ Cardiovasc Genet. 2013;6:354–61. doi: 10.1161/CIRCGENETICS.113.000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Villiers CP, van der Merwe L, Crotti L et al. AKAP9 is a genetic modifier of congenital long-QT syndrome type 1. Circ Cardiovasc Genet. 2014;7:599–606. doi: 10.1161/CIRCGENETICS.113.000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai S, Wan X, Ramirez-Navarro A et al. Physiological genomics identifies genetic modifiers of long QT syndrome type 2 severity. J Clin Invest. 2018;128:1043–56. doi: 10.1172/JCI94996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards S, Aziz N, Bale S et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–23. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giudicessi JR, Ackerman MJ. Genotype- and phenotype-guided management of congenital long QT syndrome. Curr Probl Cardiol. 2013;38:417–55. doi: 10.1016/j.cpcardiol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sala L, Hegyi B, Bartolucci C et al. Action potential contour contributes to species differences in repolarization response to beta-adrenergic stimulation. Europace. 2017;67:615. doi: 10.1093/europace/eux236. [DOI] [PubMed] [Google Scholar]
- 16.Moretti A, Bellin M, Welling A et al. Patient-specific induced pluripotent stem-cell models for Long-QT Syndrome. N Engl J Med. 2010;363:1397–409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Tanabe K, Ohnuki M et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Denning C, Borgdorff V, Crutchley J et al. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim Biophys Acta. 2016;1863:1728–48. doi: 10.1016/j.bbamcr.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loh Y-H, Agarwal S, Park I-H et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–9. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jouni M, Si-Tayeb K, Es-Salah-Lamoureux Z et al. Toward personalized medicine: using cardiomyocytes differentiated from urine-derived pluripotent stem cells to recapitulate electrophysiological characteristics of type 2 Long QT Syndrome. J Am Heart Assoc. 2015;4:e002159. doi: 10.1161/JAHA.115.002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verkerk AO, Veerman CC, Zegers JG et al. Patch-clamp recording from human induced pluripotent stem cell-derived cardiomyocytes: Improving action potential characteristics through dynamic clamp. Int J Mol Sci. 2017;18:pii–E1873. doi: 10.3390/ijms18091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundy SD, Zhu W-Z, Regnier M et al. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burridge PW, Matsa E, Shukla P et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–60. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro MC, Tertoolen LG, Guadix JA et al. Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro Correlation between contraction force and electrophysiology. Biomaterials. 2015;51:138–50. doi: 10.1016/j.biomaterials.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 26.van den Berg CW, Elliott DA, Braam SR et al. Differentiation of human pluripotent stem cells to cardiomyocytes under defined conditions. Methods Mol Biol. 2016;1353:163–80. doi: 10.1007/7651_2014_178. [DOI] [PubMed] [Google Scholar]
- 27.Giacomelli E, Bellin M, Sala L et al. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development. 2017;144:1008–17. doi: 10.1242/dev.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannhardt I, Breckwoldt K, Letuffe-Brenière D et al. Human engineered heart tissue: analysis of contractile force. Stem Cell Reports. 2016;7:29–42. doi: 10.1016/j.stemcr.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nunes SS, Miklas JW, Liu J et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10:781–7. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronaldson-Bouchard K, Ma SP, Yeager K et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–43. doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kane C, Terracciano CM. Human cardiac fibroblasts engage the sarcoplasmic reticulum in induced pluripotent stem cell-derived cardiomyocyte excitation contraction coupling. J Am Coll Cardiol. 2018;72:1061–3. doi: 10.1016/j.jacc.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Wilders R. Dynamic clamp: a powerful tool in cardiac electrophysiology. J Physiol. 2006;576:349–59. doi: 10.1113/jphysiol.2006.115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocchetti M, Sala L, Dreizehnter L et al. Elucidating arrhythmogenic mechanisms of long-QT syndrome CALM1-F142L mutation in patient-specific induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc Res. 2017;113:531–41. doi: 10.1093/cvr/cvx006. [DOI] [PubMed] [Google Scholar]
- 34.Meijer van Putten RME, Mengarelli I, Guan K et al. Ion channelopathies in human induced pluripotent stem cell derived cardiomyocytes: a dynamic clamp study with virtual IK1. Front Physiol. 2015;6:7. doi: 10.3389/fphys.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horváth A, Lemoine MD, Löser A et al. Low resting membrane potential and low inward rectifier potassium currents are not inherent features of hiPSC-derived cardiomyocytes. Stem Cell Reports. 2018;10:822–33. doi: 10.1016/j.stemcr.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sala L, Ward-van Oostwaard D, Tertoolen LGJ Electrophysiological analysis of human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) using multi-electrode arrays (MEAs) J Vis Exp. 2017. [DOI] [PMC free article] [PubMed]
- 37.Sager PT, Gintant G, Turner JR et al. Rechanneling the cardiac proarrhythmia safety paradigm: A meeting report from the Cardiac Safety Research Consortium. Am Heart J. 2014;167:292–300. doi: 10.1016/j.ahj.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Shaheen N, Shiti A, Huber I et al. Human induced pluripotent stem cell-derived cardiac cell sheets expressing genetically encoded voltage indicator for pharmacological and arrhythmia studies. Stem Cell Reports. 2018;10:1879–94. doi: 10.1016/j.stemcr.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shinnawi R, Huber I, Maizels L et al. Monitoring Human-induced pluripotent stem cell-derived cardiomyocytes with genetically encoded calcium and voltage fluorescent reporters. Stem Cell Reports. 2015;5:582–96. doi: 10.1016/j.stemcr.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dempsey GT, Chaudhary KW, Atwater N et al. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J Pharmacol Toxicol Methods. 2016;81:240–50. doi: 10.1016/j.vascn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Kijlstra JD, Hu D, Mittal N et al. Integrated analysis of contractile kinetics, force generation, and electrical activity in single human stem cell-derived cardiomyocytes. Stem Cell Reports. 2015;5:1226–38. doi: 10.1016/j.stemcr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribeiro AJS, Schwab O, Mandegar MA et al. Multi-imaging method to assay the contractile mechanical output of micropatterned human iPSC-derived cardiac myocytes. Circ Res. 2017;120:1572–83. doi: 10.1161/CIRCRESAHA.116.310363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sala L, van Meer BJ, Tertoolen LGJ et al. MUSCLE MOTION: A versatile open software tool to quantify cardiomyocyte and cardiac muscle contraction in vitro and in vivo. Circ Res. 2018;122:e5–16. doi: 10.1161/CIRCRESAHA.117.312067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Meer BJ, Sala L, Tertoolen LGJ et al. Quantification of muscle contraction in vitro and in vivo using MUSCLEMOTION software: From stem cell-derived cardiomyocytes to zebrafish and human hearts. Curr Protoc Hum Genet. 2018;99:e67. doi: 10.1002/cphg.67. [DOI] [PubMed] [Google Scholar]
- 45.Kapplinger JD, Tester DJ, Salisbury BA et al. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 2009;6:1297–303. doi: 10.1016/j.hrthm.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz PJ, Crotti L, Insolia R. Long-QT syndrome: from genetics to management. Circ Arrhythmia Electrophysiol. 2012;5:868–77. doi: 10.1161/CIRCEP.111.962019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romano C, Gemme G, Pongiglione R. Rare cardiac arrhythmias of the pediatric age. Minerva Pediatr. 1963;15:1155–64. [PubMed] [Google Scholar]
- 48.Ward OC. A new familial cardiac syndrome in children. J Ir Med Assoc. 1964;54:103–6. [PubMed] [Google Scholar]
- 49.Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J. 1957;54:59–68. doi: 10.1016/0002-8703(57)90079-0. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz PJ, Spazzolini C, Crotti L et al. The Jervell and Lange-Nielsen Syndrome. Circulation. 2006;113:783–90. doi: 10.1161/CIRCULATIONAHA.105.592899. [DOI] [PubMed] [Google Scholar]
- 51.Obreztchikova MN, Sosunov EA, Plotnikov A et al. Developmental changes in IKr and IKs contribute to age-related expression of dofetilide effects on repolarization and proarrhythmia. Cardiovasc Res. 2003;59:339–50. doi: 10.1016/S0008-6363(03)00360-2. [DOI] [PubMed] [Google Scholar]
- 52.Kasai-Brunswick THH, Silva Dos Santos D, Ferreira RPP et al. Generation of patient-specific induced pluripotent stem cell lines from one patient with Jervell and Lange-Nielsen syndrome, one with type 1 long QT syndrome and two healthy relatives. Stem Cell Res. 2018;31:174–80. doi: 10.1016/j.scr.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 53.Mura M, Ginevrino M, Zappatore R et al. Generation of the human induced pluripotent stem cell (hiPSC) line PSMi003-A from a patient affected by an autosomal recessive form of Long QT Syndrome type 1. Stem Cell Res. 2018;29:170–3. doi: 10.1016/j.scr.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Wuriyanghai Y, Makiyama T, Sasaki K et al. Complex aberrant splicing in the induced pluripotent stem cell-derived cardiomyocytes from a patient with long QT syndrome carrying KCNQ1-A344Aspl mutation. Heart Rhythm. 2018;15:1566–74. doi: 10.1016/j.hrthm.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, D'Aniello C, Verkerk AO et al. Recessive cardiac phenotypes in induced pluripotent stem cell models of Jervell and Lange-Nielsen syndrome: disease mechanisms and pharmacological rescue. Proc Natl Acad Sci USA. 2014;111:E5383–92. doi: 10.1073/pnas.1419553111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz PJ, Priori SG, Spazzolini C et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.CIR.103.1.89. [DOI] [PubMed] [Google Scholar]
- 57.Delisle BP, Anson BD, Rajamani S et al. Biology of cardiac arrhythmias: ion channel protein trafficking. Circ Res. 2004;94:1418–28. doi: 10.1161/01.RES.0000128561.28701.ea. [DOI] [PubMed] [Google Scholar]
- 58.Mehta A, Ramachandra CJA, Singh P et al. Identification of a targeted and testable antiarrhythmic therapy for long-QT syndrome type 2 using a patient-specific cellular model. Eur Heart J. 2018;39:1446–55. doi: 10.1093/eurheartj/ehx394. [DOI] [PubMed] [Google Scholar]
- 59.Mehta A, Sequiera GL, Ramachandra CJA et al. Re-trafficking of hERG reverses long QT syndrome 2 phenotype in human iPS-derived cardiomyocytes. Cardiovasc Res. 2014;102:497–506. doi: 10.1093/cvr/cvu060. [DOI] [PubMed] [Google Scholar]
- 60.Matsa E, Dixon JE, Medway C et al. Allele-specific RNA interference rescues the long-QT syndrome phenotype in human-induced pluripotency stem cell cardiomyocytes. Eur Heart J. 2014;35:1078–87. doi: 10.1093/eurheartj/eht067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bellin M, Casini S, Davis RP et al. Isogenic human pluripotent stem cell pairs reveal the role of a KCNH2 mutation in long-QT syndrome. EMBO J. 2013;32:3161–75. doi: 10.1038/emboj.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sala L, Yu Z, Ward-van Oostwaard D et al. A new hERG allosteric modulator rescues genetic and drug-induced long-QT syndrome phenotypes in cardiomyocytes from isogenic pairs of patient induced pluripotent stem cells. EMBO Mol Med. 2016;8:1065–81. doi: 10.15252/emmm.201606260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terrenoire C, Wang K, Tung KWC et al. Induced pluripotent stem cells used to reveal drug actions in a long QT syndrome family with complex genetics. J Gen Physiol. 2013;141:61–72. doi: 10.1085/jgp.201210899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lahti AL, Kujala VJ, Chapman H et al. Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis Model Mech. 2012;5:220–30. doi: 10.1242/dmm.008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Itzhaki I, Maizels L, Huber I et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–9. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 66.Spencer CI, Baba S, Nakamura K et al. Calcium transients closely reflect prolonged action potentials in iPSC models of inherited cardiac arrhythmia. Stem Cell Reports. 2014;3:269–81. doi: 10.1016/j.stemcr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mura M, Mehta A, Ramachandra CJ et al. The KCNH2 -IVS9-28A/G mutation causes aberrant isoform expression and hERG trafficking defect in cardiomyocytes derived from patients affected by Long QT Syndrome type 2. Int J Cardiol. 2017;240:367–71. doi: 10.1016/j.ijcard.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 68.Fatima A, Ivanyuk D, Herms S et al. Generation of human induced pluripotent stem cell line from a patient with a long QT syndrome type 2. Stem Cell Res. 2016;16:304–7. doi: 10.1016/j.scr.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 69.Matsa E, Rajamohan D, Dick E et al. Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. Eur Heart J. 2011;32:952–62. doi: 10.1093/eurheartj/ehr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson CL, Delisle BP, Anson BD et al. Most LQT2 Mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–73. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz PJ, Gnecchi M, Dagradi F From patient-specific induced pluripotent stem cells to clinical translation in Long QT Syndrome type 2. Eur Heart J. 2019. [DOI] [PubMed]
- 72.Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac ‘late sodium current’. Pharmacol Ther. 2008;119:326–39. doi: 10.1016/j.pharmthera.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Rizzetto R, Rocchetti M, Sala L et al. Late sodium current (INaL) in pancreatic β-cells. Pflügers Arch – Eur J Physiol. 2015;467:1757–68. doi: 10.1007/s00424-014-1613-0. [DOI] [PubMed] [Google Scholar]
- 74.Ma D, Wei H, Zhao Y et al. Modeling type 3 long QT syndrome with cardiomyocytes derived from patient-specific induced pluripotent stem cells. Int J Cardiol. 2013;168:5277–86. doi: 10.1016/j.ijcard.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 75.Okata S, Yuasa S, Suzuki T et al. Embryonic type Na+ channel β-subunit, SCN3B masks the disease phenotype of Brugada syndrome. Sci Rep. 2016;6:34198. doi: 10.1038/srep34198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malan D, Zhang M, Stallmeyer B et al. Human iPS cell model of type 3 long QT syndrome recapitulates drug-based phenotype correction. Basic Res Cardiol. 2016;111:11–4. doi: 10.1007/s00395-016-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fatima A, Kaifeng S, Dittmann S et al. The disease-specific phenotype in cardiomyocytes derived from induced pluripotent stem cells of two long QT syndrome type 3 patients. PLoS One. 2013;8:e83005. doi: 10.1371/journal.pone.0083005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Portero V, Casini S, Hoekstra M et al. Anti-arrhythmic potential of the late sodium current inhibitor GS-458967 in murine Scn5a-1798insD+/- and human SCN5A-1795insD+/- iPSC-derived cardiomyocytes. Cardiovasc Res. 2017;113:829–38. doi: 10.1093/cvr/cvx077. [DOI] [PubMed] [Google Scholar]
- 79.Badone B, Ronchi C, Kotta M-C et al. Calmodulinopathy: functional effects of CALM mutations and their relationship with clinical phenotypes. Front Cardiovasc Med. 2018;5:176. doi: 10.3389/fcvm.2018.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crotti L, Johnson CN, Graf E et al. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 2013;127:1009–17. doi: 10.1161/CIRCULATIONAHA.112.001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamamoto Y, Makiyama T, Harita T et al. Allele-specific ablation rescues electrophysiological abnormalities in a human iPS cell model of long-QT syndrome with a CALM2 mutation. Hum Mol Genet. 2017;26:1670–7. doi: 10.1093/hmg/ddx073. [DOI] [PubMed] [Google Scholar]
- 82.Limpitikul WB, Dick IE, Tester DJ et al. A precision medicine approach to the rescue of function on malignant calmodulinopathic Long-QT Syndrome. Circ Res. 2017;120:39–48. doi: 10.1161/CIRCRESAHA.116.309283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu X, Bers DM. Free and bound intracellular calmodulin measurements in cardiac myocytes. Cell Calcium. 2007;41:353–64. doi: 10.1016/j.ceca.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maier LS, Ziolo MT, Bossuyt J et al. Dynamic changes in free Ca-calmodulin levels in adult cardiac myocytes. J Mol Cell Cardiol. 2006;41:451–8. doi: 10.1016/j.yjmcc.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 85.Crotti L, Spazzolini C, Tester DJ Calmodulin mutations and life-threatening cardiac arrhythmias: Insights from the International Calmodulinopathy Registry. Eur Heart J. (In press) [DOI] [PMC free article] [PubMed]
- 86.Webster G, Schoppen ZJ, George AL. Treatment of calmodulinopathy with verapamil. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-220568. pii: bcr-2017-220568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang P, Sallam K, Wu H et al. Patient-specific and genome-edited induced pluripotent stem cell-derived cardiomyocytes elucidate single-cell phenotype of Brugada syndrome. J Am Coll Cardiol. 2016;68:2086–96. doi: 10.1016/j.jacc.2016.07.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kilpinen H, Goncalves A, Leha A et al. Common genetic variation drives molecular heterogeneity in human iPSCs. Nature. 2017;546:370–5. doi: 10.1038/nature22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sala L, Bellin M, Mummery CL. Integrating cardiomyocytes from human pluripotent stem cells in safety pharmacology: Has the time come? Br J Pharmacol. 2017;174:3749–65. doi: 10.1111/bph.13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Urnov FD, Rebar EJ, Holmes MC et al. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–46. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 91.Mussolino C, Morbitzer R, Lütge F et al. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 2011;39:9283–93. doi: 10.1093/nar/gkr597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–78. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freedman LP, Gibson MC, Ethier SP et al. Reproducibility: changing the policies and culture of cell line authentication. Nat Methods. 2015;12:493–7. doi: 10.1038/nmeth.3403. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, Liang P, Lan F et al. Genome editing of isogenic human induced pluripotent stem cells recapitulates long QT phenotype for drug testing. J Am Coll Cardiol. 2014;64:451–9. doi: 10.1016/j.jacc.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma N, Zhang JZ, Itzhaki I et al. Determining the pathogenicity of a genomic variant of uncertain significance using CRISPR/Cas9 and human-induced pluripotent stem cells. Circulation. 2018;138:2666–81. doi: 10.1161/CIRCULATIONAHA.117.032273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schwartz PJ, Sala L. Precision vs traditional medicine. Clinical questions trigger progress in basic science: a favor not always returned. Circ Res. 2019;124(459) doi: 10.1161/CIRCRESAHA.119.314629. [DOI] [PubMed] [Google Scholar]
- 97.Gnecchi M, Stefanello M, Mura M. Induced pluripotent stem cell technology: Toward the future of cardiac arrhythmias. Int J Cardiol. 2017;237:49–52. doi: 10.1016/j.ijcard.2017.03.085. [DOI] [PubMed] [Google Scholar]


