Abstract
Ventricular arrhythmias are challenging to manage in athletes with concern for an elevated risk of sudden cardiac death (SCD) during sports competition. Monomorphic ventricular arrhythmias (MMVA), while often benign in athletes with a structurally normal heart, are also associated with a unique subset of idiopathic and malignant substrates that must be clearly defined. A comprehensive evaluation for structural and/or electrical heart disease is required in order to exclude cardiac conditions that increase risk of SCD with exercise, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. Unique issues for physicians who manage this population include navigating athletes through the decision of whether they can safely continue their chosen sport. In the absence of structural heart disease, therapies such as radiofrequency catheter ablation are very effective for certain arrhythmias and may allow for return to competitive sports participation. In this comprehensive review, we summarise the recommendations for evaluating and managing athletes with MMVA.
Keywords: Ablation, arrhythmogenic right ventricular cardiomyopathy, athlete, hypertrophic cardiomyopathy, premature ventricular contraction, sports cardiology, sudden cardiac death, ventricular tachycardia
Monomorphic ventricular arrhythmias (MMVA) are not uncommon in athletes,[1,2] yet their presence appropriately raises concern among practitioners for possible increased risk of sudden cardiac death (SCD) during sports activity and competition. While all MMVA detected in athletes warrant further evaluation,[1] a majority of MMVA in this population are likely to be benign. In some instances of so called ‘idiopathic’ premature ventricular contractions (PVCs)/ventricular tachycardia (VT), the arrhythmia may be unrelated to athletic status or may be a benign manifestation of the physiological changes that may occur in the ‘athlete's heart’.[2] However, this is a matter of debate,[3] and the presence of MMVA may also be a manifestation of structural pathology, such as hypertrophic cardiomyopathy (HCM), myocarditis, and arrhythmogenic right ventricular cardiomyopathy (ARVC), or electrical abnormalities such as long QT syndrome or Brugada syndrome, which are associated with increased risks of SCD with exercise.
The focus of this review is to discuss the management of athletes found to have MMVA, including diagnostic workup, advanced treatment options and guidance recommendations for this unique population.
Epidemiology
MMVA can range from PVCs to non-sustained VT (NSVT) and sustained VT. MMVA can be seen in trained athletes on 12-lead ECG or ambulatory ECG monitoring and in most cases there are no cardiovascular abnormalities detected on further workup.[4] The overall prevalence of MMVA does not appear to be higher in the athlete population. A recent study found that the prevalence of MMVA in a group of young athletes (16–35 years) in Italy (10%) was similar to that of sedentary controls (11%).[5] Similar findings were observed in an older population of athletes (>30 years), although the overall prevalence of MMVA was higher in the older population overall (26% in athletes and 23% in sedentary controls).[3] Interestingly, in both studies, the prevalence of MMVA was not associated with the amount and duration of exercise. While there are some data suggesting that MMVA are often either abolished or substantially reduced in frequency with detraining,[6] the similar prevalence between athletes and non-athletes suggests that in most patients there is no cause-effect relationship between athlete status and arrhythmia in persons without known structural heart disease.
PVCs are not uncommon in athletes, yet whether there is a higher prevalence of PVCs specifically in athletes compared with the general population is unclear. One of the early studies comparing PVC frequency between endurance athletes and healthy sedentary people found a higher prevalence of ventricular ectopy in the athlete group, which included both any ectopy (70% versus 55%) and complex ectopy (25% versus 5%).[7] However, subsequent studies have not seen a significant difference in athletes.[3,5,8] Younger athletes (mean age 21 years) were found to be more likely to have isolated, rare ectopy (<10 PVCs/24 hours) compared with their sedentary counterparts (49% versus 28%),[5] but this difference was not seen in a study of middle-aged athletes and sedentary controls (53% versus 50%).[3]
Interestingly, while idiopathic PVCs are often thought to be sympathetically mediated, there appears to be variability in the circadian distribution of PVC occurrence among patients. In a recent study of 101 consecutive unselected patients with frequent monomorphic PVCs referred for radiofrequency catheter ablation (RFA), 50.5% were found to have fast heart rate-dependent PVCs (more PVCs occurred at higher heart rates), whereas 9.9% had slow heart rate-dependent PVCs (more PVCs at lower heart rates).[9] No correlation between heart rate and PVC frequency was seen in the rest of the patients (39.6%). However, the circadian variability of PVCs in athletes has not been specifically studied and further data is needed to determine how different sub-types of PVCs/VT should be managed in athletes.
Evaluation
The initial diagnosis of MMVA in athletes may present in a variety of ways, including incidental detection on screening ECGs or after workup performed for symptoms such as palpitations. While most of these arrhythmias are likely to be benign, it is crucial to identify features that may be suggestive of underlying structural heart disease, warranting not only further workup but also potentially restriction from sports activity or competition. Beyond the routine history and physical examination that should be performed in all patients presenting with MMVA, important features to assess in athletes include arrhythmia frequency, response of the arrhythmia to exercise and electrocardiographic patterns (Figure 1).
Figure 1: Flow Diagram of Proposed Evaluation for Athletes with Monomorphic Ventricular Arrhythmias.
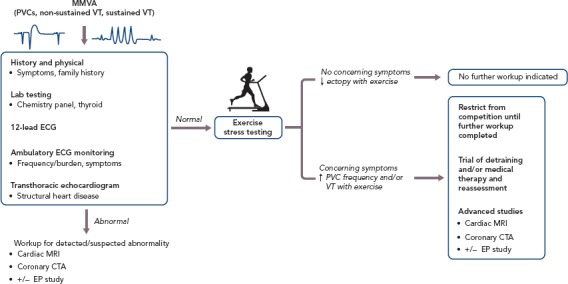
General recommendations for initial evaluation of an athlete who presents with MMVA, with further management depending on the results of exercise stress testing. CTA = computed tomographic angiography; EP = electrophysiology; MMVA = monomorphic ventricular arrhythmias; PVC = premature ventricular contractions; VT = ventricular tachycardia.
Clinical Assessment
While some athletes who are referred for evaluation of MMVA may be asymptomatic with an incidental finding of irregular heartbeat,[3,4,8] it is imperative to perform a detailed history to identify any concerning symptoms that the athlete may not otherwise mention unprompted. Specific symptoms to inquire about include palpitations, light-headedness, dizziness/presyncope, syncopal episodes, typical and atypical chest pain, dyspnoea that is sudden in onset or out of proportion to the degree of exercise, sudden fatigue, nausea, abdominal pain, or decreased exercise performance. Furthermore, family history should be queried for sudden death, syncope, or known cardiac disease, and basic laboratory tests – including a chemistry panel and thyroid function tests – should be performed to assess for possible contributions from electrolyte or endocrine abnormalities.
In addition to the physical exam, non-invasive studies that should be performed in athletes with MMVA are a 12-lead ECG, ambulatory ECG monitor (i.e. 24-hour Holter or 2–4 week event monitor), exercise stress testing and a transthoracic echocardiogram.[10] On the 12-lead ECG, the most recent version of the international recommendations on ECG interpretation in athletes should be used to identify abnormal findings,[11] which may be suggestive of pathologies (e.g. HCM and ARVC) that can predispose an athlete to MMVA.[12] Prior ECGs should be reviewed when available, and it may also be useful to obtain serial ECGs. It is also important to differentiate normal variants associated with different racial groups, such as early repolarisation seen in athletes of Afro-Caribbean descent where the ECG commonly shows elevated ST segments with upward concavity followed by negative T wave in V2-V4.[13] The exercise stress testing protocol should be based on maximal effort, not a target heart rate, and attempts should be made to replicate the level and form of exercise achieved in the athlete's sport.[1] The findings on these initial studies may prompt further evaluation, which may include but are not limited to coronary computed tomographic angiography and cardiac MRI.
Arrhythmia Frequency/Burden
The frequency/burden of PVCs/VT can be quantified by ambulatory ECG monitoring including 24–48 hour Holter monitoring or 14–30-day event recorders. The type of monitor chosen should be determined by the frequency with which the athlete experiences symptoms; if asymptomatic, a 24–48-hour Holter monitor is a reasonable first option. In a study of 355 athletes with ventricular arrhythmias detected on a 24-hour Holter monitor obtained because of palpitations and/or ≥3 PVCs on a resting ECG, 30% of athletes who had ≥2000 PVCs in the 24-hour period were found to have cardiac abnormalities on further workup; of those athletes with abnormalities, ARVC (10%) and mitral valve prolapse (9%) were most common. Conversely, cardiac abnormalities were seen in only 3% and 0% of athletes with ≥100–2000 PVCs or <100 PVCs, respectively.[4] Thus, a higher frequency of PVCs (>2000 in a 24-hour period) should raise suspicion for structural heart disease and warrants further evaluation. Furthermore, the presence of multiple (≥2) PVCs on a resting 12-lead ECG should prompt further evaluation, with at least an echocardiogram, an ambulatory ECG monitor and exercise stress testing.[11]
Response of Arrhythmia to Exercise
The response of PVCs to exercise can offer insight into the likelihood of underlying cardiac disease.[14] Monomorphic PVCs that decrease or disappear with exercise are generally considered to be benign.[1] Conversely, a recent study found that athletes who had increased ventricular arrhythmia (VA) with exercise were more likely to have pathological myocardial substrate on cardiac MRI than those who had decreased VA with exercise.[15] In older athletes, the primary concern of increasing ectopy with exercise is an ischaemic aetiology. In a recent meta-analysis of ten studies evaluating exercise-related PVCs during clinical stress testing, exercise-related PVCs correlated with an increased risk of adverse cardiac events, even in asymptomatic patients without overt cardiovascular disease.[16] Interestingly, the sensitivity analyses found that only PVCs seen during the recovery phase of stress testing were associated with adverse outcomes. Notably, these studies did not focus on athletes.
Electrocardiographic Patterns in Risk Stratification
Attempts to localise the origin of the PVC/VT may be helpful for risk stratification. PVCs originating from the right ventricular outflow tract (RVOT), suggested by a left bundle branch block (LBBB) pattern, inferior axis and precordial transition at or after V3 are likely to be benign (although this pattern can sometimes be seen in ARVC). The precordial transition is the V-lead in which the QRS changes from a predominantly negative S wave deflection to a positive R wave pattern. PVCs of left ventricular outflow tract (LVOT) origin (i.e. LBBB pattern and inferior axis with early precordial transition) and fascicular origin (i.e. RBBB pattern and superior axis for posterior fascicular sites) are less common, but also generally considered to be benign.[17] On the other hand, morphological features that raise concern for pathology include any pattern not typical of a classic idiopathic site of origin, markedly widened QRS intervals (>140 ms) and/or a pleomorphic pattern of PVCs, as these suggest an atypical site of origin or multiple sites of origin. Additionally, the PVC coupling interval can be assessed, and a short coupling interval may raise concern for an increased risk of an R-on-T phenomenon and polymorphic ventricular arrhythmias. When assessing exercise-induced ventricular arrhythmias, Cipriani and colleagues demonstrated that repolarisation abnormalities at baseline, complex VA (couplets, triplets, NSVT) on 24-hour ambulatory ECG monitoring, and repetitive exercise-induced VA with RBBB or polymorphic morphology have been associated with higher risk of structural heart disease on cardiac MRI.[15] Slower (<150 BPM), monomorphic patterns are more likely to be benign compared with faster and polymorphic patterns,[1] but there are exceptions. For instance, benign idiopathic outflow tract VT can often be very fast (Figure 1), while pathological scar-mediated VT in ARVC may have a slower rate (Figure 3).
Figure 3: Monomorphic Ventricular Arrhythmias in the Setting of Arrhythmogenic Right Ventricular Cardiomyopathy.
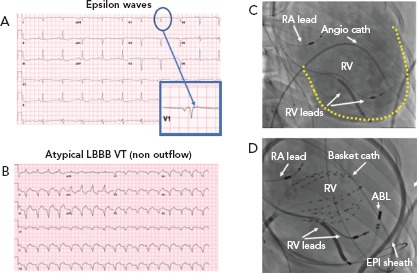
A: Electrocardiographic findings of epsilon waves visualised in lead V1 when the athlete is in normal sinus rhythm; B: Atypical LBBB VT originating from the apical aspect of the right ventricular free wall; C: RAO angiographic view at the time of EP study/RFA demonstrating markedly enlarged RV on angiography (yellow outline); D: Catheter positions during mapping and RFA of VT shown in panel B. RFA was undertaken using a combined endocardial and epicardial approach. ABL = ablation catheter; ANGIO = angiographic; ARVC = arrhythmogenic right ventricular cardiomyopathy; cath = catheter; EPI = epicardial; LBBB = left bundle branch block; MMVA = monomorphic ventricular arrhythmias; RA = right atrium; RV = right ventricle; VT = ventricular tachycardia.
Figure 2: Idiopathic (Outflow) Ventricular Tachycardia.
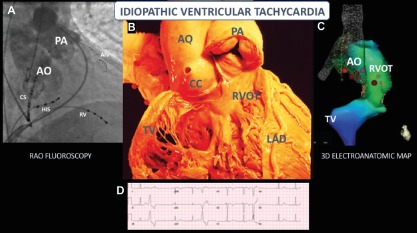
A: RAO fluoroscopic view of outflow tracts after simultaneous pulmonary artery and aortic root angiography; B: A similar anatomic view with the RV free wall removed; C: A similar view after completion of a 3D electro-anatomic map (Biosense Webster) prior to RFA; D: 12-lead ECG of a PVC with a left bundle, inferior axis morphology, with precordial transition at lead V3, consistent with outflow origin. AIV = anterior interventricular vein; AO = aorta; CC = coronary cusp; CS = coronary sinus; HIS, = bundle of HIS; LAD = left anterior descending artery; PA = pulmonary artery; RAO = right anterior oblique; RV = right ventricle; RVOT = right ventricular outflow tract; TV = tricuspid valve. Image B is reproduced with permission from the UCLA Cardiac Arrhythmia Center, Wallace MacAlpine Collection.
Differential Diagnosis
Age has a strong influence on the differential diagnosis: older athletes (>35 years) are more likely to have atherosclerotic coronary artery disease (CAD) and ischaemic heart disease as an aetiology, whereas younger athletes (<35 years) are more likely to have congenital abnormalities, such as anomalous origin of a coronary artery, familial cardiomyopathy or a genetic predisposition to arrhythmia.[14]
Structural Heart Disease
Familial cardiomyopathies include HCM, ARVC, and left ventricular non-compaction cardiomyopathy. These conditions need to be considered in all athletes with VA, but particularly in younger athletes given that HCM and ARVC may manifest as VA at an early age. In a seminal study from 1996 by Maron et al. on SCD in young athletes, HCM was found to be the most common structural cardiovascular cause of death, followed by anomalous origin of the coronary arteries.[18] A more recent 10-year study from Australia similarly found that a familial cardiomyopathy (HCM or ARVC) contributed to approximately 33% of sport-related SCD in children,[19] and an analysis of a UK registry found that myocardial disease (including left ventricular hypertrophy and fibrosis, HCM and ARVC) accounted for 40% of SCD in athletes.[20] Interestingly, HCM was found to be a more common cause of SCD in male athletes, while anomalous origin of the coronary arteries was the more common cause in female athletes.[21] However, the SCD reported in studies of patients with HCM is often related to polymorphic VT/VF, although they can also present with MMVA.
In older athletes with MMVA, atherosclerotic CAD should be considered, as it is the most common aetiology of sport-related SCD in this group, presumably due to ischaemic scar-related VA.[19] Other structural pathologies that can lead to MMVA in athletes of any age include arrhythmogenic inflammatory cardiomyopathy (including sarcoidosis and myocarditis)[22,23] and valvular abnormalities (e.g. mitral valve prolapse).[24,25]
Electrical Heart Disease
While structural heart disease needs to be evaluated in athletes with MMVA, the most common finding in athletes who experienced SCD is a structurally normal heart,[26] and the most common cause of SCD in children and adolescent athletes is sudden arrhythmic death syndrome (SADS).[20] Presumably, SADS is because of undiagnosed inherited arrhythmia disorders, such as ion channelopathies.[27] These channelopathies include long QT syndrome, Brugada syndrome and catecholaminergic polymorphic ventricular tachycardia, but more commonly manifest as polymorphic VA and are thus outside of the scope of this review on MMVA.
Management
The management of any athlete with known or possible cardiovascular disease, especially MMVA, can be particularly challenging because of the large role that physical activity and competitive sports plays in the athlete's personal and/or professional life. Management decisions therefore require a shared-decision making approach that emphasises the need to prioritise the athlete's safety, including providing a temporary or indefinite recommendation to refrain from strenuous exercise or competition. However, it should be noted that certain sporting organisations may require medical ‘clearance’ for competitive participation and the athlete may have less autonomy in these situations.
Restriction from Competitive Sports
Before an athlete with known or suspected MMVA can be cleared to return to competitive sport participation, a cardiac evaluation – comprising at least of an exercise stress test targeting maximal performance, echocardiogram and ambulatory ECG monitoring – is recommended.[1,10] Based on the American Heart Association (AHA) and American College of Cardiology (ACC) recommendations, absent of any structural or electrical heart disease or high-risk ECG features, athletes who demonstrate no greater than PVC couplets during maximal exercise testing, ideally recapitulating their sport of choice, can participate in all competitive sports.[1] The European Society of Cardiology (ESC) recommendations also include stipulations that there be no family history of SCD, no increased PVC frequency during exercise, and overall PVC burden <2000 per 24 hour for clearance to participate in all sports.[28] If exercise testing results in increased VA frequency, repetitive forms of PVCs, NSVT or sustained VT, then the athlete should be given the recommendation to refrain from participation in competitive sports until further evaluation and/or treatment are performed.[1,28]
Athletes found to have structural heart disease or evidence of myocarditis should be advised to restrict themselves to playing low intensity (Class IA) sports, such as bowling and golf (Figure 4). In particular, while expert opinion suggests that those with myocarditis may potentially be able to return to higher-intensity sports after evaluation for complete resolution of their condition,[1,28] athletes with a diagnosis of ARVC should be counselled extensively on the importance of refraining from strenuous exercise indefinitely, as exercise in these patients is associated with a high risk of a lethal VT.[29–31] High-intensity exercise, independent of exercise duration, increases risk in patients with ARVC,[32] and exercise restriction has indeed been found to reduce VT frequency.[33] Athletes without overt ARVC, but who have a desmosomal mutation associated with ARVC, are at increased risk of developing ARVC, heart failure, and/or lethal VT with endurance and/or frequent exercise.[34] These athletes should also be counselled to refrain from endurance exercise and high-intensity sports (above Class IA), but can likely still safely adhere to the AHA's minimal exercise recommendations (450 to 750 metabolic equivalent-minutes weekly).[35]
Figure 4: Classification of Sports by Static and Dynamic Components.
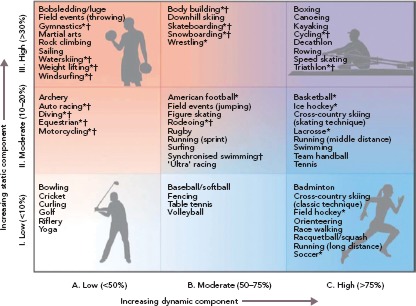
General guidelines classifying sports based on the peak static and dynamic components typically reached during competition (although higher values can be achieved). *Sport presents danger of bodily collision. †Sport carries increased risk if syncope occurs.
Source: Levine et al., 2015.[59] Reproduced with permission of Levine et al. and the Journal of the American College of Cardiology.
Interestingly, it has been reported that high-intensity endurance exercise itself may result in ARVC-like pathology, including an increased risk of VT, without any detected genetic mutation (‘gene-elusive’).[36–38] In addition, a recent study found a distinct pattern of isolated subepicardial RVOT scar associated with MMVA in high-level endurance athletes that is amenable to RFA.[39] However, little is known about the potential need for post-ablation exercise limitations in this patient population. Further, a small study of seven asymptomatic athletes with extensive subepicardial late gadolinium enhancement on cardiac MRI found that six of the seven developed symptomatic VT or progressive LV dysfunction.[40] The aetiology of the subepicardial scar was unknown in these athletes, and this study supports comprehensive evaluation and close follow-up in those athletes found to have myocardial fibrosis on cardiac MRI.
Prescribed Deconditioning
In athletes without structural or electrical heart disease, prescribed periods of deconditioning (ranging from 12–24 weeks) have been shown to reduce the frequency of MMVA.[6] The beneficial effects of periods of deconditioning on frequency reduction appear to persist after a resumption of intensive training, when compared with the MMVA frequency prior to deconditioning.[41] However, in practice, athletes are often reluctant to participate in deconditioning periods. Reassuringly, one study assessed 120 athletes with no obvious structural heart disease found to have a modest burden of PVCs (>100/24 hours) on routine pre-participation screening.[42] Continued sports activity in these athletes did not result in any deaths or development of overt heart disease, and overall modest PVC burden actually decreased over the median follow-up period of 84 months. Thus, as long as there is no evidence of overt structural or electrical heart disease, deconditioning is an optional recommendation for many athletes with MMVA.
Medical Management
In symptomatic athletes, medical therapy can be considered to reduce symptoms and MMVA frequency. For athletes with symptomatic PVCs or a high PVC burden, beta-blocker therapy can be effective at reducing PVC frequency, and the ESC recommends beta-blockers as first-line medical therapy for the treatment of MMVA.[43] Additionally, beta-blockers can be considered for athletes with NSVT induced by exercise, and documentation of resolution of NSVT with exercise on beta-blocker therapy is required before an athlete can be cleared to return to competitive sport participation.[1] However, it should be noted that beta-blockers may have limited utility/tolerance in athletes given the high baseline parasympathetic tone (and associated resting bradycardia) seen in elite athletes, as well as the possible adverse effects these medications may have on athletic performance. Further, some sporting organisations actually prohibit the use of beta-blockers in athletes during competition, and physicians should review and remind their athlete patients to review the list of prohibited classes of medications prior to prescribing any new medication. Of note, there is no evidence at this time to support the use of antiarrhythmic medications to suppress benign PVCs in athletes.[14] For patients with PVC-induced cardiomyopathy, however, there are recent data to suggest the safety and efficacy of class IC antiarrhythmic medications, such as flecainide in an unselected patient population.[44]
For athletes with idiopathic MMVT, medical therapy can be considered if RFA is not possible or not desired,[45,46] and the location of VT origin can be influential in guiding therapy. For instance, fascicular VT has been found to be responsive to the non-dihidropyridine calcium channel blocker verapamil, as well as beta-blockers. For outflow tract VT, class IC and class III antiarrhythmic medications are more effective than beta-blockers or calcium channel blockers, and can be considered as long as there is no evidence of structural heart disease.[46] However, the side effect profiles of antiarrhythmic medications need to be strongly considered prior to initiation in any athlete and beta-blockers are usually the first choice given the safety profile.
Radiofrequency Catheter Ablation
Many athletes, particularly younger ones, are reluctant to take medications because of concerns about their side effects and their possible impact on athletic performance. Fortunately, RFA of MMVT – previously limited to experienced academic centres – is now being increasingly performed internationally as more cardiac electrophysiologists gain advanced training in the procedure.[47] For athletes with symptomatic PVCs, RF ablation of PVCs may be considered if the PVCs are refractory to medications or if the athlete is unable or unwilling to take medications.[1] In contrast to medications, RFA can be curative of MMVAs. In fact, for PVCs with an RVOT origin, RFA was found to be more effective than antiarrhythmic drug therapy in preventing recurrence,[48] and a multi-centre study found RFA to have an 84% acute success rate with a low complication rate (2.4% major complications and 2.8% minor complications).[49] Complications were most commonly related to vascular access, but rare complications including cardiac tamponade and atrioventricular block can occur. Notably no procedure-related stroke or deaths were seen in the 1,185 patients included in the study.[49]
Furthermore, given the association of PVC frequency with an increased risk of heart failure (PVC-induced cardiomyopathy),[50] it is reasonable to consider RFA in athletes with a high PVC burden and/or a reduction in systolic function due to PVCs.[43] RFA of PVCs originating from the LVOT, aortic cusp, or epicardial origin should only be undertaken at experienced centres. For athletes with idiopathic, monomorphic sustained VT, RFA is also a reasonable therapeutic option.[1]
In patients with MMVT associated with structural heart disease, such as ARVC and HCM, RFA can be considered, particularly if they are highly symptomatic or are experiencing frequent ICD therapies. A recent retrospective study of VT ablation in patients with non-ischaemic cardiomyopathy found that the outcomes of VT ablation differed based on the underlying etiology.[51] Patients with ARVC had the highest VT-free survival 1 year after RFA (82%), while patients with HCM had a relatively higher rate of VT recurrence after ablation. In another recent study focused on patients with ARVC VT, RF ablation was associated with similar outcomes compared to drug therapy, although combined epicardial-endocardial RF ablation resulted in lower VT recurrence compared with an endocardial alone approach.[52] Similarly, in a small, highly selected group of patients with MMVT associated with HCM, combined epicardial-endocardial RFA was effective at preventing recurrence of ICD shocks in 78% of patients at a median follow-up of 37 months.[53] Thus, RFA can be an effective option for select patients with ARVC and HCM. However, given the presence of structural heart disease, RFA therapy does not allow for return to athletic competition, even if successful, as the procedure may eliminate the clinical arrhythmia but does not resolve the underlying pathology and future development of new VT circuits is possible.
Device Implantation
The decision to implant an ICD in athletes is a particularly challenging one, as it may impact their ability to continue competition in their chosen sports.[54] Additionally, potential complications of ICDs include inappropriate shocks, device-related infections and lead failure. In particular, with transvenous ICD systems, repetitive arm motion during athletic activity may result in lead dislodgement with resulting sensing failure or in lead fracture and inappropriate shocks.[55] The recently available subcutaneous ICD may have a lower risk of lead malfunction.[56] The recommendations for ICD implantation in athletes follow the same guideline recommendations for primary and secondary prevention in the general population. The AHA/ACC guidelines state that athletes who have survived a cardiac arrest due to MMVA should have an ICD placed.[1] Additionally, athletes who have had documented symptomatic rapid monomorphic VT from a non-reversible cause should also have an ICD placed if not idiopathic in origin.[1] In a multinational registry of 440 athletes with implanted transvenous ICDs and median follow-up of 44 months, the proportion receiving appropriate shocks during competition/practice was 11%; ARVC was the only clinical factor associated with receiving a shock. The estimated lead survival free of malfunction was 95% at 5 years and 85% at 10 years; there were no generator malfunctions.[57] Notably, the AHA/ACC guidelines reiterate that the desire of an athlete to continue participation in athletic competition does not qualify as an indication for ICD implantation, and it is inappropriate to implant an ICD solely to allow for return to athletic competition.[1]
Return to Competitive Sports and Physical Activity
After comprehensive evaluation of an athlete with MMVA, the recommendations on continued participation in competitive sports hinges on the presence of structural and/or electrical heart disease as well as response to therapy. In athletes without structural heart disease who have single PVCs and no greater than ventricular couplets during maximal exercise testing, participation can be allowed in all competitive sports without further evaluation.[1] If PVCs either increase in frequency or cause symptoms during exercise (such as light-headedness, excessive fatigue, or excessive dyspnoea), the athlete should be restricted to sports below the exertional level at which these occurred until further evaluation (e.g. echocardiography, cardiac MRI, electrophysiological testing) has been performed and structural heart disease ruled out and treatment initiated. If the athlete has been started on drug therapy such as beta-blockers, return to participation should only occur if there is documentation on either exercise or electrophysiological testing that the MMVA no longer occurs under the physiological conditions in which it previously occurred prior to medical therapy. In athletes who have had successful RFA of their MMVA, return to full competitive activities can be allowed if there is no evidence of spontaneous or induced VA at least 3 months after the date of their procedure in the setting of a structurally normal heart.[1]
Athletes with structural heart disease with documented NSVT should be restricted to low-intensity class IA sports (Figure 4), even if they have undergone successful RFA of the MMVA.[1] Notably, the data supporting this recommendation are limited (Class IC recommendation from the AHA/ACC). As mentioned above, continued high-intensity physical activity can promote progression of conditions such as ARVC, and athletes with these diagnoses should be counselled extensively on these risks. However, in athletes whose MMVA are a result of a transient inflammatory process such as myocarditis, re-evaluation for return to competition is recommended after there is clinical and laboratory evidence of resolution of the inflammation; if there is reasonable evidence to suggest that the pathology has fully resolved, the athlete may return to competition a minimum of 3 months after clinical resolution.[1] However, in inflammatory conditions such as cardiac sarcoidosis, there can be recurrence of the inflammatory process after dormant periods and complete resolution may not occur.
For athletes who have had an ICD, participation in class IA sports is permitted if there is no evidence of MMVA requiring device therapy for at least 3 months. Participation in higher-intensity sports may be considered after extensive discussion with the athlete on the risks involved, including ICD pocket-site injury in impact sports.[1] These decisions will be diagnosis-dependent, as athletes with diagnoses that are known to be negatively impacted by exercise, such as ARVC, should be advised against participation in higher-intensity sports. The ICD Sports Safety Registry of athletes with ICDs implanted who continue to participate in competitive sports, including high-risk sports, has helped to inform sports cardiologists and patients on the outcomes of these athletes.[57,58] In longer-term follow-up of the 440 participants in the registry, while there were appropriate and inappropriate ICD shocks in some of the athletes, there were no failures to terminate arrhythmias and no physical injuries due to the ICD. Freedom from lead malfunction (definite or possible) was 85% at 10 years.[57]
Conclusion
Athletes with MMVA warrant comprehensive evaluation by a sports cardiology program, with experience and expertise as needed in paediatric cardiology, adult congenital cardiology, advanced cardiac imaging, interventional cardiology, electrophysiology and advanced exercise testing. While most of these MMVA will be of a benign nature, it is crucial to evaluate for the presence of structural and/or electrical heart disease, as continued competitive sport participation may expose the athlete to an increased risk of SCD in conditions such as ARVC and HCM. In the absence of structural heart disease, the improved outcomes of therapeutic procedures such as RFA and improved understanding of the management of MMVA in athletes have allowed many athletes to be guided safely back towards competition with the guidance of a sports cardiologist. In all cases, shared decision making should be central to formulating the treatment plan for each athlete.
Clinical Perspective
MMVA, including premature ventricular complexes as well as non-sustained and sustained VT, are not uncommon in athletes and always raise concern about whether an athlete can continue to participate in competitive sports.
Initial assessment includes a comprehensive history and physical examination and review of all available ECGs.
Identifying high-risk features, particularly response to maximal exercise testing and presence of structural heart disease, is critical to determine safety of continued competitive sport participation.
Echocardiography and frequently cardiac MRI are required to evaluate for structural heart disease, as certain conditions (i.e. ARVC, HCM) pose an elevated risk of sudden cardiac death during competition.
Treatment options include observation and monitoring, exercise restriction, medical therapy, and interventions such as RFA and ICD implantation.
References
- 1.Zipes DP, Link MS, Ackerman MJ et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 9: Arrhythmias and conduction defects: A scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2412–23. doi: 10.1016/j.jacc.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114:1633–44. doi: 10.1161/CIRCULATIONAHA.106.613562. [DOI] [PubMed] [Google Scholar]
- 3.Zorzi A, Mastella G, Cipriani A et al. Burden of ventricular arrhythmias at 12-lead 24-hour ambulatory ECG monitoring in middle-aged endurance athletes versus sedentary controls. Eur J Prev Cardiol. 2018;25:2003–11. doi: 10.1177/2047487318797396. [DOI] [PubMed] [Google Scholar]
- 4.Biffi A, Pelliccia A, Verdile L et al. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2002;40:446–52. doi: 10.1016/S0735-1097(02)01977-0. [DOI] [PubMed] [Google Scholar]
- 5.Zorzi A, De Lazzari M, Mastella G et al. Ventricular arrhythmias in young competitive athletes: Prevalence, determinants, and underlying substrate. J Am Heart Assoc. 2018;7:e009171. doi: 10.1161/JAHA.118.009171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biffi A, Maron BJ, Verdile L et al. Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2004;44:1053–58. doi: 10.1016/j.jacc.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 7.Palatini P, Maraglino G, Sperti G et al. Prevalence and possible mechanisms of ventricular arrhythmias in athletes. Am Heart J. 1985;110:560–7. doi: 10.1016/0002-8703(85)90075-4. [DOI] [PubMed] [Google Scholar]
- 8.Bjørnstad H, Storstein L, Meen HD, Hals O. Ambulatory electrocardiographic findings in top athletes, athletic students and control subjects. Cardiology. 1994;84:42–50. doi: 10.1159/000176327. [DOI] [PubMed] [Google Scholar]
- 9.Hamon D, Abehsira G, Gu K et al. Circadian variability patterns predict and guide premature ventricular contraction ablation procedural inducibility and outcomes. Heart Rhythm. 2018;15:99–106. doi: 10.1016/j.hrthm.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Steriotis AK, Nava A, Rigato I et al. Noninvasive cardiac screening in young athletes with ventricular arrhythmias. Am J Cardiol. 2013;111:557–62. doi: 10.1016/j.amjcard.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S, Drezner JA, Baggish A et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. 2018;39:1466–80. doi: 10.1093/eurheartj/ehw631. [DOI] [PubMed] [Google Scholar]
- 12.Katritsis GD, Katritsis DG. The Electrocardiogram in athletes revisited. Arrhythm Electrophysiol Rev. 2013;2:99–104. doi: 10.15420/aer.2013.2.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corrado D, Biffi A, Basso C et al. 12-lead ECG in the athlete: physiological versus pathological abnormalities. Br J Sports Med. 2009;43:669–76. doi: 10.1136/bjsm.2008.054759. [DOI] [PubMed] [Google Scholar]
- 14.Singh TK, Baggish AL. Premature ventricular beats in the athlete: management considerations. Expert Rev Cardiovasc Ther. 2018;16:277–86. doi: 10.1080/14779072.2018.1443395. [DOI] [PubMed] [Google Scholar]
- 15.Cipriani A, Zorzi A, Sarto P et al. Predictive value of exercise testing in athletes with ventricular ectopy evaluated by cardiac magnetic resonance. Heart Rhythm. 2019;16:239–48. doi: 10.1016/j.hrthm.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Lee V, Perera D, Lambiase P. Prognostic significance of exercise-induced premature ventricular complexes: a systematic review and meta-analysis of observational studies. Heart Asia. 2017;9:14–24. doi: 10.1136/heartasia-2016-010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verdile L, Maron BJ, Pelliccia A et al. Clinical significance of exercise-induced ventricular tachyarrhythmias in trained athletes without cardiovascular abnormalities. Heart Rhythm. 2015;12:78–85. doi: 10.1016/j.hrthm.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Maron BJ, Shirani J, Poliac LC et al. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. doi: 10.1001/jama.1996.03540030033028. [DOI] [PubMed] [Google Scholar]
- 19.Dennis M, Elder A, Semsarian C et al. A 10-year review of sudden death during sporting activities. Heart Rhythm. 2018;15:1477–83. doi: 10.1016/j.hrthm.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Finocchiaro G, Papadakis M, Robertus J-L et al. Etiology of Sudden Death in Sports: Insights From a United Kingdom Regional Registry. J Am Coll Cardiol. 2016;67:2108–15. doi: 10.1016/j.jacc.2016.02.062. [DOI] [PubMed] [Google Scholar]
- 21.Maron BJ, Haas TS, Ahluwalia A et al. Demographics and epidemiology of sudden deaths in young competitive athletes: From the United States National Registry. Am J Med. 2016;129:1170–7. doi: 10.1016/j.amjmed.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Bauer BS, Li A, Bradfield JS. Arrhythmogenic inflammatory cardiomyopathy: A review. Arrhythm Electrophysiol Rev. 2018;7:181–6. doi: 10.15420/aer.2018.26.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tung R, Bauer B, Schelbert H et al. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: The potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015;12:2488–98. doi: 10.1016/j.hrthm.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonow RO, Nishimura RA, Thompson PD, Udelson JE. American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology, Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and American College of Cardiology. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 5: Valvular heart disease: A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e292–7. doi: 10.1161/CIR.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 25.Nalliah CJ, Mahajan R, Elliott AD et al. Mitral valve prolapse and sudden cardiac death: a systematic review and meta-analysis. Heart. 2019;105:144–51. doi: 10.1136/heartjnl-2017-312932. [DOI] [PubMed] [Google Scholar]
- 26.Harmon KG, Drezner JA, Maleszewski JJ et al. Pathogeneses of sudden cardiac death in national collegiate athletic association athletes. Circ Arrhythm Electrophysiol. 2014;7:198–204. doi: 10.1161/CIRCEP.113.001376. [DOI] [PubMed] [Google Scholar]
- 27.Raju H, Behr ER. Unexplained sudden death, focussing on genetics and family phenotyping. Curr Opin Cardiol. 2013;28:19–25. doi: 10.1097/HCO.0b013e32835b0a9e. [DOI] [PubMed] [Google Scholar]
- 28.Heidbüchel H, Corrado D, Biffi A et al. Study Group on Sports Cardiology of the European Association for Cardiovascular Prevention and Rehabilitation. Recommendations for participation in leisure-time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions. Part II: ventricular arrhythmias, channelopathies and implantable defibrillators. Eur J Cardiovasc Prev Rehabil. 2006;13:676–86. doi: 10.1097/01.hjr.0000239465.26132.29. [DOI] [PubMed] [Google Scholar]
- 29.Corrado D, Basso C, Judge DP. Arrhythmogenic Cardiomyopathy. Circ Res. 2017;121:784–802. doi: 10.1161/CIRCRESAHA.117.309345. [DOI] [PubMed] [Google Scholar]
- 30.Guasch E, Mont L. Diagnosis pathophysiology, and management of exercise-induced arrhythmias. Nat Rev Cardiol. 2017;14:88–101. doi: 10.1038/nrcardio.2016.173. [DOI] [PubMed] [Google Scholar]
- 31.Mazzanti A, Ng K, Faragli A et al. Arrhythmogenic right ventricular cardiomyopathy: Clinical course and predictors of arrhythmic risk. J Am Coll Cardiol. 2016;68:2540–50. doi: 10.1016/j.jacc.2016.09.951. [DOI] [PubMed] [Google Scholar]
- 32.Lie ØH, Dejgaard LA, Saberniak J et al. Harmful effects of exercise intensity and exercise duration in patients with arrhythmogenic cardiomyopathy. JACC Clin Electrophysiol. 2018;4:744–53. doi: 10.1016/j.jacep.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Orgeron G, Tichnell C et al. Impact of exercise restriction on arrhythmic risk among patients with arrhythmogenic right ventricular cardiomyopathy. J Am Heart Assoc. 2018;7:e008843. doi: 10.1161/JAHA.118.008843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James CA, Bhonsale A, Tichnell C et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–7. doi: 10.1016/j.jacc.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawant AC, Te Riele AS, Tichnell C et al. Safety of American Heart Association-recommended minimum exercise for desmosomal mutation carriers. Heart Rhythm. 2016;13:199–207. doi: 10.1016/j.hrthm.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 36.La Gerche A, Claessen G, Dymarkowski S et al. Exercise-induced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur Heart J. 2015;36:1998–2010. doi: 10.1093/eurheartj/ehv202. [DOI] [PubMed] [Google Scholar]
- 37.Sawant AC, Bhonsale A, te Riele AS et al. Exercise has a disproportionate role in the pathogenesis of arrhythmogenic right ventricular dysplasia/cardiomyopathy in patients without desmosomal mutations. J Am Heart Assoc. 2014;3:e001471. doi: 10.1161/JAHA.114.001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heidbuchel H, Prior DL, La Gerche A. Ventricular arrhythmias associated with long-term endurance sports: what is the evidence? Br J Sports Med. 2012;46 Suppl 1:i44–50. doi: 10.1136/bjsports-2012-091162. [DOI] [PubMed] [Google Scholar]
- 39.Venlet J, Piers SR, Jongbloed JD et al. Isolated subepicardial right ventricular outflow tract scar in athletes with ventricular tachycardia. J Am Coll Cardiol. 2017;69:497–507. doi: 10.1016/j.jacc.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 40.Schnell F, Claessen G, La Gerche A et al. Subepicardial delayed gadolinium enhancement in asymptomatic athletes: let sleeping dogs lie? Br J Sports Med. 2016;50:111–7. doi: 10.1136/bjsports-2014-094546. [DOI] [PubMed] [Google Scholar]
- 41.Biffi A, Maron BJ, Culasso F et al. Patterns of ventricular tachyarrhythmias associated with training, deconditioning and retraining in elite athletes without cardiovascular abnormalities. Am J Cardiol. 2011;107:697–703. doi: 10.1016/j.amjcard.2010.10.049. [DOI] [PubMed] [Google Scholar]
- 42.Delise P, Sitta N, Lanari E et al. Long-term effect of continuing sports activity in competitive athletes with frequent ventricular premature complexes and apparently normal heart. Am J Cardiol. 2013;112:1396–402. doi: 10.1016/j.amjcard.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 43.Priori SG, Blomström-Lundqvist C, Mazzanti A et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 44.Hyman MC, Mustin D, Supple G et al. Class IC antiarrhythmic drugs for suspected premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 2018;15:159–63. doi: 10.1016/j.hrthm.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 45.Lai E, Chung EH. Management of arrhythmias in athletes: Atrial fibrillation, premature ventricular contractions, and ventricular tachycardia. Curr Treat Options Cardiovasc Med. 2017;19:86. doi: 10.1007/s11936-017-0583-x. [DOI] [PubMed] [Google Scholar]
- 46.Saeid AK, Klein GJ, Leong-Sit P. Sustained ventricular tachycardia in apparently normal hearts: Medical therapy should be the first step in management. Card Electrophysiol Clin. 2016;8:631–9. doi: 10.1016/j.ccep.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Bradfield JS, Shivkumar K. Anatomy for ventricular tachycardia ablation in structural heart disease. Card Electrophysiol Clin. 2017;9:11–24. doi: 10.1016/j.ccep.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Ling Z, Liu Z, Su L et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: prospective randomized study. Circ Arrhythm Electrophysiol. 2014;7:237–43. doi: 10.1161/CIRCEP.113.000805. [DOI] [PubMed] [Google Scholar]
- 49.Latchamsetty R, Yokokawa M, Morady F et al. Multicenter Outcomes for Catheter Ablation of Idiopathic Premature Ventricular Complexes. JACC Clin Electrophysiol. 2015;1:116–23. doi: 10.1016/j.jacep.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Dukes JW, Dewland TA, Vittinghoff E et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol. 2015;66:101–9. doi: 10.1016/j.jacc.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaseghi M, Hu TY, Tung R et al. Vet Outcomes of catheter ablation of ventricular tachycardia based on etiology in nonischemic heart disease: An international ventricular tachycardia ablation center collaborative study. JACC Clin Electrophysiol. 2018;4:1141–50. doi: 10.1016/j.jacep.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahida S, Venlet J, Saguner AM et al. Ablation compared with drug therapy for recurrent ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy: Results from a multicenter study. Heart Rhythm. 2019;16:536–43. doi: 10.1016/j.hrthm.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Dukkipati SR, d'Avila A, Soejima K et al. Long-term outcomes of combined epicardial and endocardial ablation of monomorphic ventricular tachycardia related to hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:185–94. doi: 10.1161/CIRCEP.110.957290. [DOI] [PubMed] [Google Scholar]
- 54.Heidbuchel H, Carré F. Exercise and competitive sports in patients with an implantable cardioverter-defibrillator. Eur Heart J. 2014;35:3097–102. doi: 10.1093/eurheartj/ehu130. [DOI] [PubMed] [Google Scholar]
- 55.Lampert R, Cannom D, Olshansky B. Safety of sports participation in patients with implantable cardioverter defibrillators: a survey of heart rhythm society members. J Cardiovasc Electrophysiol. 2006;17:11–15. doi: 10.1111/j.1540-8167.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 56.Bardy GH, Smith WM, Hood MA et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36–44. doi: 10.1056/NEJMoa0909545. [DOI] [PubMed] [Google Scholar]
- 57.Lampert R, Olshansky B, Heidbuchel H et al. Safety of Sports for Athletes With Implantable Cardioverter-Defibrillators: Long-term results of a prospective multinational registry. Circulation. 2017;135:2310–2. doi: 10.1161/CIRCULATIONAHA.117.027828. [DOI] [PubMed] [Google Scholar]
- 58.Lampert R, Olshansky B, Heidbuchel H et al. Safety of sports for athletes with implantable cardioverter-defibrillators: results of a prospective, multinational registry. Circulation. 2013;127:2021–30. doi: 10.1161/CIRCULATIONAHA.112.000447. [DOI] [PubMed] [Google Scholar]
- 59.Levine BD, Baggish AL, Kovacs RJ et al. American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology, Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and American College of Cardiology. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 1: Classification of Sports: Dynamic, static, and impact: A scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e262–6. doi: 10.1161/CIR.0000000000000237. [DOI] [PubMed] [Google Scholar]


