Abstract
Introduction
HE4 protein (human epididymis protein-4), which is the fourth subfraction of human epithelial protein, is a glycoprotein widely used as a tumor marker in ovarian cancer. If was first discovered in the epididymal epithelium and recognized as a protease inhibitor contributing to sperm maturation. The plasma HE4 concentration may also be increased in gynecological pathologies other than ovarian cancer.
Material and methods
The study was conducted between 2016 and 2017 among patients hospitalized in the Academic Department of Gynaecology. A total of 191 women were examined. Depending on the type of pathology which was the reason for hospitalization, 4 groups of patients were identified in the study. The first of these included 30 patients with ovarian cancer, the second 33 patients with benign ovarian lesion, the third 50 patients with endometrial cancer, and the fourth 28 patients with leiomyomas.
Results
The highest concentration of HE4 protein was found in women with ovarian cancer, and it was statistically significantly higher compared to all other groups. Lower HE4 protein concentration than in women with ovarian cancer was reported in women with endometrial cancer, but it was statistically significantly higher compared to patients with uterine fibroids.
Conclusions
This marker may have significant clinical value in the differentiation of benign ovarian pathology from ovarian cancer. The study confirms the validity of using HE4 results in the assessment of potential malignancy of ovarian and endometrial lesions.
Keywords: HE4 protein, ovarian cancer, endometrial cancer, uterine fibroids
Introduction
HE4 protein (human epididymis protein-4), which is the fourth subfraction of human epithelial protein, is a glycoprotein widely used as a tumor marker in ovarian cancer. It is coded by the WFDC2 gene in the 20th chromosome. It was first discovered in the epididymal epithelium and recognized as a protease inhibitor contributing to sperm maturation. WFDC2 gene expression was confirmed in the epithelia of the female reproductive organ, breast, epididymis, vas deferens, respiratory epithelium, distal renal tubules, intestine and salivary glands [1, 2]. HE4 protein is currently used successfully as a molecular marker of ovarian cancer. A study published in 2008 by Moore et al. [3] demonstrated that it had the highest sensitivity (72.9%) and specificity (95%) from among all tested proteins as markers of ovarian cancer. Higher sensitivity was demonstrated only by correlating HE4 with the CA125 marker [4]. Currently, due to favorable results, determination of serum HE4 and CA125 protein levels is the basis of the ROMA algorithm (Risk of Ovarian Malignancy Algorithm) used in pre-operative diagnostics, which qualifies patients for low or high risk of ovarian tumor malignancy [5].
The plasma HE4 concentration may also be increased in gynecological pathologies other than ovarian cancer. Endometrial cancer is the most common cancer of the female reproductive organs in highly developed countries. Such a high incidence of this type of cancer is attributed, inter alia, to the growing percentage of obese women in society [6]. HE4 protein may prove potentially useful in prognosis as well as in assessing the degree of differentiation and clinical progression, and thus in the selection of appropriate surgical treatment of this cancer [7, 8]. There are no conclusive data on the usefulness of assays of this protein in benign reproductive organ neoplasms, such as uterine fibroids or benign ovarian lesions.
Aim of the study
The aim of the study was to evaluate the concentration of HE4 protein in women with uterine fibroids, ovarian benign pathology, and endometrial cancer, and perform comparative analysis of the values obtained in patients with ovarian cancer and a control group which consisted of women without gynecological diseases. An additional aim was to analyze the impact of surgery on the results of laboratory tests for HE4 protein concentration.
Material and methods
The study was conducted between 2016 and 2017 among patients hospitalized at the Department of Gynaecology of an academic hospital. A total of 191 women were examined. Depending on the type of pathology which was the reason for hospitalization, 4 groups of patients were identified in the study. The first of these included 30 patients with ovarian cancer, the second 33 patients with benign ovarian lesion, the third 50 patients with endometrial cancer, and the fourth 28 patients with uterine fibroids. All mentioned patients were treated surgically (all had laparotomy performed). The control group consisted of healthy women without gynecological diseases (n = 50). The approval of the Bioethical Commission was obtained for the tests. All patients were fully informed about the purpose of the study and agreed in writing to participate.
HE4 protein levels were determined by the ELISA method using the Human HE4 in PicoKine ELISA Kit. Patients in all groups were analyzed for the results of routine laboratory tests, which in women who underwent surgery were performed twice (before and after the operation) and once in the control group. Statistical analysis of obtained results of HE4 concentrations and laboratory results was carried out.
Statistica 13.1 was used for statistical analysis (Dell Inc. 2016, Dell Statistica, data analysis software system, version 13. software.dell.com).
The results for variables on the nominal scale are presented by means of frequency tables. In the case of quantitative variables, basic measures of descriptive statistics were calculated: arithmetic mean, median, quartiles and standard deviation. The consistency of the distribution of variables with the normal distribution was studied using the Shapiro-Wilk test. Since the distributions, including the key HE4 parameter, differed significantly from the normal distribution, nonparametric methods were used to verify the hypotheses. With inter-group comparisons the Mann-Whitney U test was used (in the case of two-sample comparison, the ANOVA Kruskal-Wallis rank test was used together with post hoc Dunn (for more than two groups), or, in case of qualitative variables, the chi-square test. The Wilcoxon pair order test was used to compare the values of the parameters measured before and after the surgery. Correlation analysis was performed using the Spearman rank correlation coefficient.
The significance level α = 0.05 was assumed. The results were considered statistically significant when the probability (p) value was < 0.05.
Results
The highest concentration of HE4 protein was found in women with ovarian cancer and it was higher compared to all other groups (222.7171 ±281.3557 pmol/l). Lower HE4 protein concentration than in women with ovarian cancer was reported in women with benign ovarian pathology (115.7327 ±152.8528 pmol/l), but it was higher compared to patients with endometrial cancer (75.9415 ±50.2508 pmol/l). The lowest levels of HE4 protein were obtained in the group of women with uterine fibroids (40.7888 ±18.6948 pmol/l) as well as the control group (23.0660 ±5.1591 pmol/l). The values of HE4 protein concentrations in the examined women are presented in Table 1, and the list of all significant relationships between HE4 protein concentrations in each group are found in Table 2.
Table 1.
HE4 protein concentrations in the examined groups of women
| Main HE4 concentration (pmol/l) | Median | Lower quartile | Upper quartile | Standard deviation (SD) | |
|---|---|---|---|---|---|
| Ovarian cancer (n = 30) | 222.7171 | 98.4211 | 67.7595 | 254.6587 | 281.3557 |
| Benign ovarian pathology (n = 33) | 115.7327 | 72.6372 | 56.9078 | 94.4842 | 152.8528 |
| Endometrial cancer (n = 50) | 75.9415 | 55.5565 | 50.3285 | 89.2465 | 50.2508 |
| Uterine fibroids (n = 28) | 40.7888 | 36.9906 | 28.5223 | 50.4926 | 18.6948 |
| Control group (healthy) (n = 50) | 23.0660 | 22.5500 | 20.0000 | 26.9000 | 5.1591 |
Table 2.
Summary of significant relationships (p-values) between HE4 protein concentrations in patient groups
| Subsidiary: HE4 (p/mol/l) | Ovarian cancer R: 138.57 | Benign ovarian pathology R: 118.71 | Endometrial cancer R: 106.85 Uterine fibroids | R: 67.857 | Control group R: 30.520 |
|---|---|---|---|---|---|
| Ovarian cancer | > 0.05 | > 0.05 | 0.000001 | 0.000000 | |
| Benign ovarian pathology | > 0.05 | > 0.05 | 0.000639 | 0.000000 | |
| Endometrial cancer | > 0.05 | > 0.05 | 0.027233 | 0.000000 | |
| Uterine fibroids | 0.000001 | 0.000639 | 0.027233 | 0.013978 | |
| Control group | 0.000000 | 0.000000 | 0.000000 | 0.013978 |
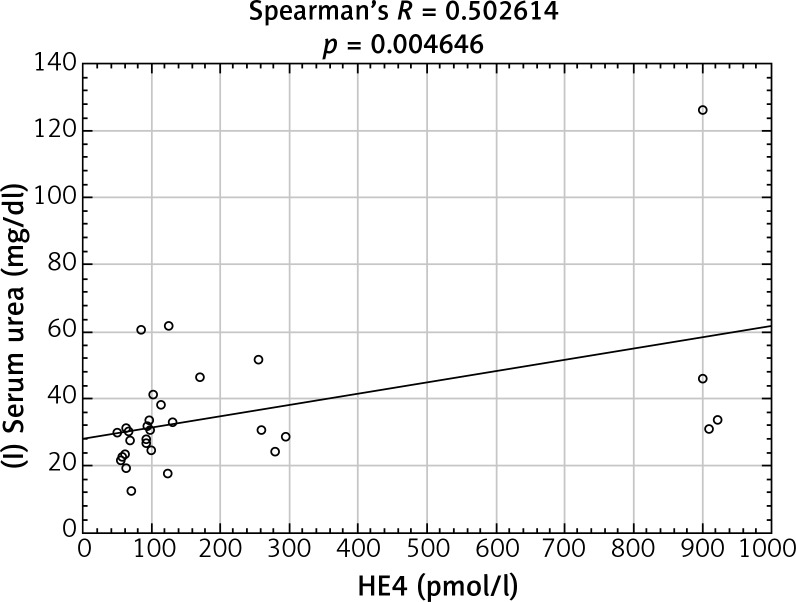
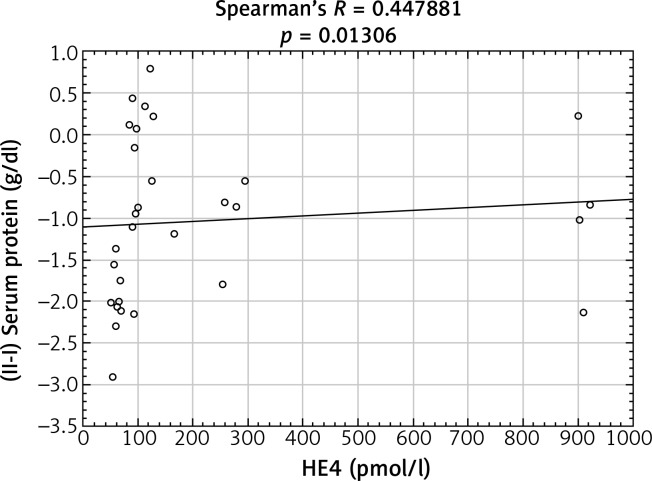
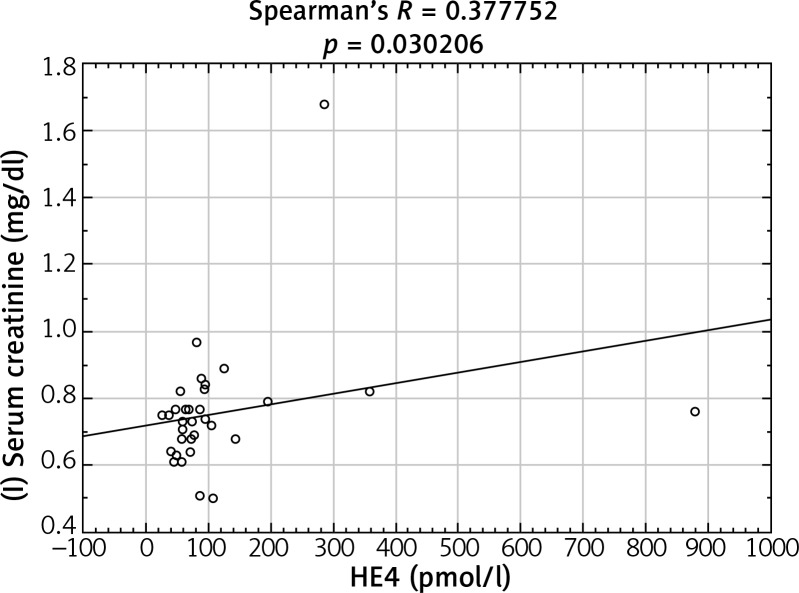
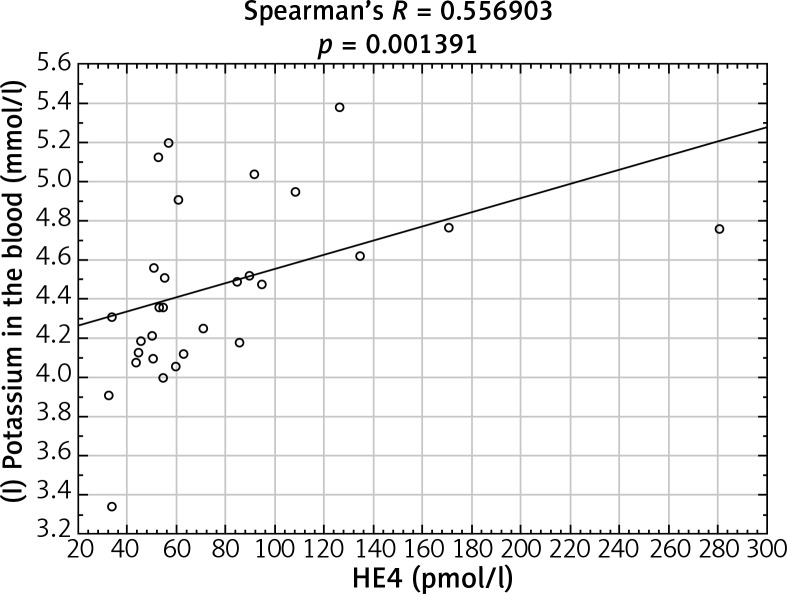
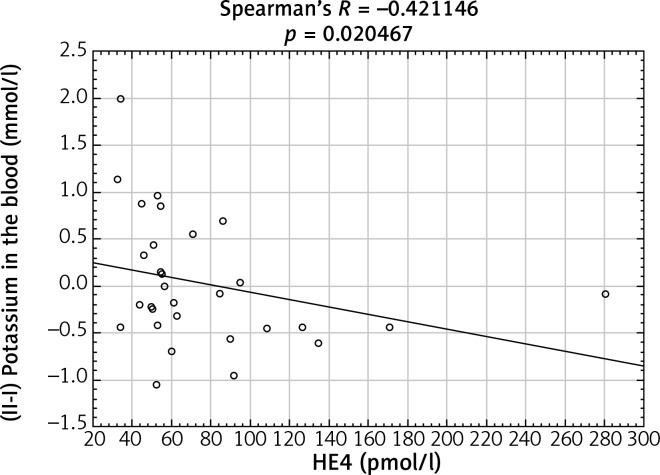
After analyzing the obtained laboratory results and performing a comparison with the concentrations of the HE4 protein in the studied women, a number of statistically significant linear correlations were obtained. In the ovarian cancer group, there were demonstrated positive linear correlations between preoperative urea and HE4 concentrations (p = 0.004646) (Fig. 1) and between the difference in protein concentration before and after surgery and HE4 (p = 0.013066) (Fig. 2). In this group of patients, negative linear correlations were found between the difference in INR after and before surgery and HE4 concentration (p = 0.006316) and between the difference in urea concentration after and before the surgery and HE4 concentration (p = 0.000958). In the group of patients with benign ovarian lesions, a positive linear relationship was found between pre-operative creatinine concentration and HE4 protein concentration (p = 0.030206) (Fig. 3). In the group of patients with endometrial carcinoma, three positive linear correlations were confirmed: between blood erythrocyte concentration before surgery and HE4 protein concentration (p = 0.043639), between potassium ion concentration prior to the operation and HE4 protein concentration (p = 0.001391) (Fig. 4), and between the INR measured after surgery and protein HE4 concentration (p = 0.022856). In the same group of patients, two negative linear correlations were found: between the difference in hematocrit after and before surgery and HE4 protein concentration (p = 0.036541), and between the difference in potassium ion concentration after and before surgery and HE4 protein concentration (p = 0.020467) (Fig. 5). In the group of women with uterine fibroids, HE4 protein concentration positively correlated with preoperative glucose concentration (p = 0.027304) and in healthy women a negative linear correlation was found between C-reactive protein concentration and HE4 protein concentration (p = 0.038470).
Fig. 1.
Positive linear correlation between preoperative urea and HE4 protein concentration in ovarian cancer group
Fig. 2.
Positive linear correlation between the difference in protein concentration before and after surgery and HE4 protein concentration in ovarian cancer group in ovarian cancer group
Fig. 3.
Positive linear correlation between pre-operative creatinine concentration and HE4 protein concentration in benign ovarian lesions group
Fig. 4.
Positive linear correlation between pre-operative potassium ions concentration and HE4 protein concentration in endometrial cancer group
Fig. 5.
Negative linear correlation between the difference in potassium ion concentration after and before surgery and HE4 protein concentration in endometrial cancer group
In addition, it was found that HE4 protein concentration in patients with uterine fibroids in whom the uterus was removed was significantly lower than in patients who had resolved to remove the uterus and only removed myomas (respectively 37.50379 ±19.62376 vs. 52.83362 ±6.95128 pmol/l, p = 0.009789). In the same group of patients whose adnexa were removed on both sides, HE4 protein concentration was significantly lower than in patients who had not had appendages removed bilaterally (respectively 32.56840 ±13.83890 vs. 47.91306 ±19.81778 pmol/l, p = 0.028718).
Discussion
The usefulness of determination of HE4 protein concentrations as a tumor marker in women with ovarian cancer has been well documented for years [5, 9, 10]. This fact is also confirmed by our research; the concentration of this protein was clearly the highest among all patients (Table 1). It seems, however, that the diagnostic potential of this glycoprotein may be much greater. In 2014, Li et al. [11] proved that the higher the HE4 level in the plasma of women with endometrial cancer, the greater their mortality, the higher the clinical stage, the lower the degree of differentiation and the worse the prognosis. The same authors also reported higher HE4 protein levels in women with atypical hypertrophy of endometrium in comparison to healthy women with normal endometrium [11]. There is a similar study concerning the clinical stage, the degree of differentiation and prognosis conducted by a Polish team, which leads to the same conclusions [12]. This may be of great clinical importance, as the initial histologic grade and the tumor size have an impact on overall survival in endometrial cancer [13]. The work of Brennan et al. [14] shows that the higher the HE4 protein concentration, the higher is the risk of cancer infiltration to the outer half of the myometrium, which substantially increases the risk of metastatic lesions in the lymph nodes. Consequently, during intra-operative examination and confirmation of a high degree of differentiation with a HE4 level below 70 pmol/l, lymphadenectomy may be aborted, as the risk of detecting tumor metastases in the lymph nodes is extremely low [14]. In addition, Abbink et al. [15] reported the usefulness of HE4 protein concentration assay in detecting distant metastatic foci in cases of endometrial cancer, when its concentration is significantly higher than during local recurrence. In this function it performs significantly better than CA125, which was found elevated only in 37% of patients, compared to 67% for HE4. Increase in HE4 heralded a recurrence at a median of 126 days before the onset of clinical symptoms. The combined efforts of Czech and Danish researchers [16] allowed for the creation of the HE4ren formula, which allows the concentration of the HE4 protein to be compared to the glomerular filtration rate. It allows comparison of HE4 marker concentration results in women with decreased glomerular filtration with results of patients with normal kidney function. This study shows the effectiveness of HE4ren in the prediction of the depth of the infiltration of the myometrium, which is of great prognostic value for patients with uterine body cancer [16]. In addition, a modern algorithm (RERT) was developed that allows highly effective determination of clinical progression according to International Federation of Gynecology and Obstetrics (FIGO), based on such data as age, BMI, fertility, menopausal status, use of oral contraception, hormone replacement therapy, hypertension and serum HE4 and CA125 concentrations. This allowed the clinical stage of the disease to be determined with sensitivity and specificity of 90% and 76%, respectively. The algorithm can be useful in determining the prognosis and selection of appropriate therapy before surgery as well as determining the necessary scope of the operation itself [17]. The cited studies prove the usefulness of the determination of HE4 protein concentrations in women with endometrial cancer, which is also confirmed by our research, but it seems that the combination of HE4 protein assays with CA125 tumor antigen is an ideal solution that can detect endometrial cancer with the greatest sensitivity and specificity, plan treatment and anticipate relapses. Dong and his colleagues are of a similar opinion [18].
The usefulness of the HE4 and CA125 protein assays in differentiating benign changes from female reproductive organ malignancy has been analyzed in many studies. According to a study by Moore et al. [19] on a group of over 1000 women with pathologies such as ovarian cancer, endometrial cancer, teratoma, myoma, inflammation or endometriosis, in the majority of benign tumors located in the reproductive organs, elevated HE4 concentrations are found in only 8% of patients, with elevated CA125 in 29%. HE4 protein levels increased mainly in ovarian cancer and endometrial cancer. Uterine fibroids, which are the most common benign tumor of this organ, were accompanied by an increase in HE4 concentration above the 95th percentile only in 5% of cases [19]. These observations confirm the results obtained in our work; HE4 protein concentrations were clearly higher in the case of malignant lesions compared to benign ones, both in the ovary and in the endometrium. This opinion is shared by researchers from the Pomeranian Medical University [20], who found that in patients with BRCA1 mutation, the concentration of HE4 marker increases in ovarian cancer and endometrial cancer, and does not grow in non-malignant changes in the female reproductive organs such as uterine fibroids, inflammation, endometriosis or benign ovarian tumors. High expression of HE4 protein in ovarian and endometrial cancer patients with higher clinical stage and lower degree of cell differentiation may also be related to the presence of ascites. It is a condition which occurs in the majority of patients with advanced ovarian and endometrial cancer. It can be a factor inducing epigenetic cell changes leading to increase in malignancy, which has been proved in vitro in HEK 293 cells [21]. Among those patients who did not have an active cancer process, HE4 levels were low, such as in the healthy population, but any (even slight) increase in the level of this protein was indicative of an active proliferative process within the ovary, fallopian tube or endometrium [20]. Another study that confirms that HE4 works well for differentiating pelvic tumors from benign to malignant is the work of Goff et al. [22].
In recent years, attempts have been made to correlate laboratory biochemical parameters with the prediction of ovarian cancer progression and predict the presence of cancer cells in the lymph nodes [23]. In addition, it is important to take into account some comorbidities that may occur in patients with ovarian or endometrial cancer as factors that can change the prognostic value of HE4 [23-26]. In our study we found a number of significant linear correlations between HE4 protein concentrations in particular groups of patients and some laboratory tests performed both before and after surgery. The most interesting appear to be correlations between concentrations of potassium ions, total protein, and INR levels and concentrations of the protein HE4 in some groups of patients (Figs. 2, 4 and 5). This is confirmed by the fact that the interpretation of the HE4 protein itself may be subject to a certain margin of error, and thus we should combine HE4 with other tumor markers in clinical practice. Positive linear correlations between HE4 protein concentration and renal function assessment parameters (urea, creatinine) may be related to the increase in HE4 concentration described by Yuan and Li [24] in women with renal dysfunction. In our studies, we observed this correlation in both women with ovarian cancer and with benign ovarian changes (Figs. 1 and 3). In women with advanced cancer, undoubtedly, a disorder of kidney function may occur, which may explain the occurrence of this relationship. In patients with benign ovarian changes, it seems that this relationship may be caused by the pressure of the lesion, often large in size, on the ureters, which makes urinary outflow from the kidney more difficult. In spite of our expanded research on these phenomena [27], there is still no reference to our results in the literature.
To sum up, we can conclude that HE4 protein concentration is increased in various tumors. Currently, the highest clinical importance of this marker is attributed to the differential diagnosis of pelvic lesions. It helps in distinguishing between benign and malignant lesions, and together with CA125, it may facilitate the diagnosis of recurrence or progression of ovarian cancer and endometrial cancer. The importance of this protein has been described in the planning of the scope of surgery in case of endometrial cancer, in determining the clinical stage of this cancer and prognosis. It is also of great importance in detecting local recurrences and distant metastases of endometrial cancer.
Conclusions
Protein concentrations of HE4 have different values in the reviewed gynecological pathologies. This marker may have significant clinical value in the differentiation of benign ovarian pathology from ovarian cancer – the results obtained by us show that the average concentrations of HE4 in a group with ovarian cancer and endometrial cancer are higher than in the group of patients with benign change of the ovary or myoma. The study confirms the validity of using HE4 results in the assessment of potential malignancy of ovarian and endometrial changes. On the other hand, we must realize the clinical imperfection of HE4 protein as a tumor marker, because an increase or a decrease can be influenced by laboratory parameters of patients and comorbidities.
Disclosure
The authors report no conflict of interest.
References
- 1.Nowak M, Janas Ł, Stachowiak G, et al. Current clinical application of serum biomarkers to detect ovarian cancer. Prz Menopauzalny. 2015;14:254–259. doi: 10.5114/pm.2015.55887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galgano MT, Hampton GM, Frierson HF. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006;19:847–853. doi: 10.1038/modpathol.3800612. [DOI] [PubMed] [Google Scholar]
- 3.Moore RG, Brown AK, Miller MC, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Hellström I, Raycraft J, Hayden-Ledbetter M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–3700. [PubMed] [Google Scholar]
- 5.Wang J, Gao J, Yao H, et al. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: a meta-analysis. Tumour Biol. 2014;35:6127–6138. doi: 10.1007/s13277-014-1811-6. [DOI] [PubMed] [Google Scholar]
- 6.Nevadunsky NS, Van Arsdale A, Strickler HD, et al. Obesity and age at diagnosis of endometrial cancer. Obstet Gynecol. 2014;124:300–306. doi: 10.1097/AOG.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 7.Capriglione S, Plotti F, Miranda A, et al. Utility of tumor marker HE4 as prognostic factor in endometrial cancer: a single-center controlled study. Tumour Biol. 2015;36:4151–4156. doi: 10.1007/s13277-015-3049-3. [DOI] [PubMed] [Google Scholar]
- 8.Angioli R, Miranda A, Aloisi A, et al. A critical review on HE4 performance in endometrial cancer: Where are we now? Tumour Biol. 2014;35:881–887. doi: 10.1007/s13277-013-1190-4. [DOI] [PubMed] [Google Scholar]
- 9.Granato T, Porpora MG, Longo F, et al. HE4 in the differential diagnosis of ovarian masses. Clin Chim Acta. 2015;446:147–155. doi: 10.1016/j.cca.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Zhuang H, Wang H, et al. Overexpression of HE4 (human epididymis protein 4) enhances proliferation, invasion and metastasis of ovarian cancer. Oncotarget. 2015;7:729–744. doi: 10.18632/oncotarget.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Gao Y, Tan M, et al. Expression of HE4 in endometrial cancer and its clinical significance. Biomed Res Int. 2015;2015:437–468. doi: 10.1155/2015/437468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdalla N, Piorkowski R, Slomka A, Kania M. Analysis of serum level of HE4 and CA125 considering selected risk factors among patients with endometrioid endometrial cancer. Contemp Oncol. 2016;20:463–467. doi: 10.5114/wo.2016.65606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekii S, Murakami N, Kato T, et al. Outcomes of salvage high-dose-rate brachytherapy with or without external beam radiotherapy for isolated vaginal recurrence of endometrial cancer. J Contemp Brachyther. 2017;9:209–215. doi: 10.5114/jcb.2017.67755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan DJ, Hackethal A, Metcalf AM, et al. Serum HE4 as a prognostic marker in endometrial cancer – a population based study. Gynecol Oncol. 2014;132:159–165. doi: 10.1016/j.ygyno.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Abbink K, Zusterzeel PL, Geurts-Moespot AJ, et al. HE4 is superior to CA125 in the detection of recurrent disease in high-risk endometrial cancer patients. Tumour Biol. 2018;40:101042831875710. doi: 10.1177/1010428318757103. [DOI] [PubMed] [Google Scholar]
- 16.Chovanec J, Selingerova I, Greplova K, et al. Adjustment of serum HE4 to reduced glomerular filtration and its use in biomarker-based prediction of deep myometrial invasion in endometrial cancer. Oncotarget. 2017;8:108213–108222. doi: 10.18632/oncotarget.22599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vezzoli M, Ravaggi A, Zanotti L, et al. RERT: a novel regression tree approach to predict extrauterine disease in endometrial carcinoma patients. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-11104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong C, Liu P, Li C. Value of HE4 combined with cancer antigen 125 in the diagnosis of endometrial cancer. Pak J Med Sci. 2017;33:1013–1017. doi: 10.12669/pjms.334.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore RG, Miller MC, Steinhoff MM, et al. Serum HE4 levels are less frequently elevated than CA125 in women with benign gynecologic disorders. Am J Obstet Gynecol. 2012;206:1–18. doi: 10.1016/j.ajog.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chudecka-Głaz A, Cymbaluk-Płoska A, Strojna A, Menkiszak J. HE4 serum levels in patients with BRCA1 gene mutation undergoing prophylactic surgery as well as in other benign and malignant gynecological diseases. Dis Markers. 2017;2017:9792756. doi: 10.1155/2017/9792756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabłowska-Gadomska I, Ducher M, Orzechowska M, et al. Peritoneal fluid stimulates neoplastic transformation of normal HEK 293 cells by high expression of pluripotent genes. Pol J Pathol. 2018;69:399–310. doi: 10.5114/pjp.2018.79550. [DOI] [PubMed] [Google Scholar]
- 22.Goff BA, Agnew K, Neradilek MB, et al. Combining a symptom index, CA125 and HE4 (triple screen) to detect ovarian cancer in women with a pelvic mass. Gynecol Oncol. 2017;147:291–295. doi: 10.1016/j.ygyno.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Xiang J, Zhou L, Li X, et al. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol. 2017;10:33–39. doi: 10.1016/j.tranon.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan T, Li Y. Human epididymis protein 4 as a potential biomarker of chronic kidney disease in female patients with normal ovarian function. Lab Med. 2017;48:238–243. doi: 10.1093/labmed/lmx036. [DOI] [PubMed] [Google Scholar]
- 25.Piek A, Meijers WC, Schroten NF, et al. HE4 serum levels are associated with heart failure severity in patients with chronic heart failure. J Card Fail. 2017;23:12–19. doi: 10.1016/j.cardfail.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Nagy B, Jr, Nagy B, Fila L, et al. Human epididymis protein 4: a novel serum inflammatory biomarker in cystic fibrosis. Chest. 2016;150:661–672. doi: 10.1016/j.chest.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Brenk A, Bodzek P, Janosz I, Olejek A. Stężenie białka HE4 u kobiet z rakiem endometrium. Gynekol Położn – Med Project. 2017;3:43–48. [Google Scholar]