Abstract
Mutations in NHE6 (also termed SLC9A6) cause the X-linked neurological disorder Christianson syndrome (CS) in males. The purpose of this study was to examine the phenotypic spectrum of female carriers of NHE6 mutations. Twenty female carriers from 9 pedigrees were enrolled, ranging from approximately age 2 to 65. A subset of female carriers was assessed using standardized neuropsychological measures. Also, the association of NHE6 expression with markers of brain age was evaluated using 740 participants in the Religious Orders Study (ROS) and Rush Memory and Aging Project (MAP). A majority, but not all, female carriers demonstrated a deficit in at least one neurocognitive domain (85%). A recognizable neuropsychological profile emerged, revealing impairments in visuospatial function, attention, and executive function. Common neuropsychiatric diagnoses included: intellectual disability/developmental delay (20%), learning difficulties (31%), speech/language delays (30%), and attention-deficit/hyperactivity disorder (20%). Notable neurological diagnoses in aging CS female carriers include corticobasal degeneration and atypical parkinsonism. In postmortem brains from the ROS/MAP dataset of normal and pathological aging, decreased NHE6 expression was correlated with greater tau deposition. Our study provides an examination of the phenotypic range in female carriers of NHE6 mutations. The findings indicate that NHE6-related disease in females represents a new neurogenetic condition.
Keywords: Na+/H+ exchanger 6 (NHE6), X-chromosome inactivation (XCI), Christianson syndrome, Female carriers
Introduction
Christianson syndrome (CS) is a monogenic, X-linked neurological disorder caused by mutations in the gene encoding endosomal Na+/H+ exchanger 6 (NHE6) (also known as SLC9A6) [1]. NHE proteins are generally 12-transmembrane-domain proteins that exchange hydrogen ions for cations (e.g., Na+ or K+ ions). Affected CS males usually harbor loss-of-function mutations and present with intellectual disability (ID)/developmental delay (DD), epilepsy, postnatal microcephaly, and ataxia [1, 2, 3, 4]. Notable neurological symptoms reported in a subset of CS patients include cerebellar/brainstem atrophy and eye movement problems (closest to Duane syndrome Type I, i.e. an inward gaze deviation), as well as regressions in adaptive and/or motor functioning [2, 3, 5]. Female carriers in these CS pedigrees exhibit heterogeneous phenotypes, ranging from unaffected to presenting with mild-moderate ID/DD, neuropsychiatric disorders including attentional difficulties, mild ataxia, and/or microcephaly [1, 2, 3, 4, 5, 6, 7, 8]. Recurrent developmental features of female carriers include learning problems, difficulty in school, speech disorder, and/or behavioral problems [2, 3, 4, 7]. However, the full phenotypic spectrum in females with NHE6 mutations, particularly across the lifespan, is currently unknown.
Progressive symptoms, some potentially reflecting neurodegeneration, have been identified in CS males. For example, symptoms that may develop and/or worsen with age include cerebellar/brainstem atrophy, motor decline/ataxia, cognitive/behavioral regressions, and low weight/height [2, 3, 9]. Postmortem examination of 2 CS males in their 40s and 50s found widespread neuronal and glial loss, as well as accumulation of tau-positive tangle-like inclusions [9]. Furthermore, neurodegenerative features have been reported in an Nhe6-null mouse model such as loss of Purkinje cells in the cerebellum, loss of tissues and synapses, and a strong microglial response [10].
The wide phenotypic spectrum reported in girls and women who are carriers of NHE6 mutations is consistent with other X-linked conditions wherein the tissues of female carriers are likely mosaic for gene expression (with a variable proportion of cells expressing from the mutant gene locus) based on random X-chromosome inactivation. Indeed, female Nhe6-heterozygous mice exhibit similar, yet less severe, neurological and motor phenotypes as Nhe6-null male mice [11]. As part of an international CS registry, we enrolled the largest cohort of female carriers (20 participants) across multiple CS pedigrees (n = 9). We conducted standardized assessments to ascertain the range and severity of neurological and adaptive symptoms in female carriers. To investigate the cognitive profile of female carriers, neuropsychological testing was conducted on 13 female carriers from 7 pedigrees. Given progressive features in CS male probands and Nhe6-null mice, neurological histories and brain imaging from CS female carriers at age 50 years or older were assessed. Finally, the relationship between NHE6 expression and neurodegenerative characteristics was examined using brain transcriptome data from two large normal aging and dementia cohorts. The brain transcriptome analysis included brain samples from 740 participants enrolled in the Religious Orders Study (ROS) [12] and the Rush Memory and Aging Project (MAP) [13].
Materials and Methods
Participants
Institutional Review Boards at Brown University and Lifespan Healthcare approved this research. All participants provided written informed consent. Female carriers were identified as part of an international CS registry [2]. Clinical information was collected by direct interviews with participants and from available medical records. DNA was isolated from either blood or saliva from all female carriers. Heterozygosity for an NHE6 mutation known to cause CS in a related male was confirmed in participating females by sequencing all coding exons (1–16) and exon/intron junctions, as previously described [2]. Some pedigree features have been changed to assure anonymity.
Neuropsychological Assessments
Neuropsychological function was assessed in a subset of available participants. Neurocognition in adults (i.e., ≥18 years old) was assessed by the following measures: Wechsler Abbreviated Scales of Intelligence-Second Edition (WASI-II), Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Trail Making Test A&B (TMT A&B), Controlled Oral Word Association Test (COWAT), and Mini-Mental State Exam (MMSE). Neurocognition in children (i.e., < 18 years old) was measured using the following assessments: A Developmental Neuropsychological Assessment-Second Edition (NEPSY-II), Wechsler Preschool Primary Scales of Intelligence-Fourth Edition (WPPSI-IV), WASI-II, Children's Memory Scales (CMS), TMT A&B, Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV), Rey Complex Figure Test (RCFT), Beery-Buktenica Developmental Test of Visual-Motor Integration (Beery), and COWAT. Domain composite scores were calculated to assess IQ (RBANS), language (RBANS, WPPSI-IV), visuospatial function (RBANS, NEPSY-II, RCFT, Beery), attention (RBANS, WPPSI-IV, WISC-IV), memory (RBANS, NEPSY-II, CMS), and executive function (TMT A&B, COWAT, NEPSY-II).
Brain Transcriptome Data
NHE6 expression in brain was analyzed from two aging and dementia cohorts – ROS [12] and MAP [13]. Postmortem brain specimens were collected from 740 participants and analyzed by researchers affiliated with these studies (N.P., T.L.Y.-P., P.L.D.J.). Data collection and methods for this analysis have been reported elsewhere [14] and are briefly summarized. RNA-Seq expression data were obtained from gray matter of the dorsolateral prefrontal cortex. RNA extraction was performed with Qiagen's miRNAeasy mini kit and RNase-free DNase. An RNA-Seq library was curated via a strand-specific dUTP method [15] with poly-A selection [16]. Finally, sequencing was performed on an Illumina HiSeq platform using 50 million paired-end reads at 101 bp.
Alzheimer's disease (AD)-related neuropathological findings such as neurofibrillary tangles, diffuse plaques, and neuritic plaques have been detailed elsewhere [14]. Immunohistochemical techniques employed the Avidin-Biotin Complex staining method, using alkaline phosphatase as the color developer. For Aβ and tau accumulation, 20-μm tissue sections from 8 brain regions were analyzed: entorhinal cortex, CA1/subiculum of the hippocampus, superior frontal cortex, mid-prefrontal cortex, inferior temporal cortex, angular gyrus, anterior cingulate cortex, and calcarine cortex. Images for neocortical (90 images) and non-neocortical (20 images) of Aβ-stained sections were quantified using an automated analysis. Aβ mean percentage was quantified as the percentage of area with amyloid and calculated both within and across each brain region. Tau abundance was quantified as the density of tangles per mm2 and averaged both within and across each brain region.
NHE6 Sequencing in Females with Idiopathic Parkinson-Related Syndromes
Female samples from the National Institute of Neurological Disorders and Stroke neurodegeneration biorepository were sequenced for NHE6 variants. A total of 156 samples were sequenced from females diagnosed with either progressive supranuclear palsy (PSP) (n = 150) or corticobasal degeneration (CBD) (n = 6) (online suppl. Table 1; see www.karger.com/doi/10.1159/000496341 for all online suppl. material). All exons and 15–20 bp into exon/intron junctions (i.e., to accurately identify splice variants) were sequenced in the NHE6 gene. PCR was performed for NHE6 target enrichment using the Fluidigm platform. GC-rich exon 1, however, was Sanger sequenced using the following primers: 5′-TTGGTTACACTGAGCCGATG-3′ (forward) and 5′-AAGCGAAAAAGGTGTGGAGA-3′ (reverse). Samples were sequenced using the MiSeq platform (Illumina). Raw read sequences were analyzed and clipped using Trimmomatic version 0.32 [17]. Reads were not included in variant analysis if they met any of the following criteria: possible adapter sequences, low quality bases of single reads (Phred score < 20), reads with low average quality threshold (window size 4 bp, Phred score < 20), reads clipped at bases with a Phred Score < 3, and clipped reads shorter than a threshold length of 80 bp. Reads were mapped using BWA-MEM version 0.7.10 (http://bio-bwa.sourceforge.net). Filtered read pairs were mapped to their amplicon sequences, not the entire human genome, to prevent mislabeling pseudogenes and additional loci with similar sequences. Variant analysis was conducted by VarScan version 2.3.6 [18]. Reads with ≥1 Phred Quality Score were analyzed. Single nucleotide polymorphisms and insertions or deletions (indels) were annotated by snpEff version 4.0 [19]. The pathogenicity of missense variants was assessed in silico using PolyPhen-2 [20]. All potentially deleterious variants identified via MiSeq were confirmed by Sanger sequencing.
Statistical Analysis
Pearson correlation analyses were conducted to examine the association between age (i.e., pediatric or adult) and neurocognition. A linear regression analysis was performed on the brain transcriptome data to determine the association between NHE6 expression in brain and multiple AD-related pathology outcomes. Aβ load and tau accumulation were added into the model as continuous measures. Covariates included age, gender, and study (i.e., ROS or MAP). To correct for multiple testing, the Benjamini-Hochberg procedure was used. Statistical analyses were performed using SAS version 9.3.
Results
Female Carriers Identified from CS Cohort
A total of 20 female carriers of NHE6 mutations were enrolled across 9 pedigrees. All mutations were predicted to be loss-of-function and were identified in pedigrees wherein there was at least one hemizygous male with the same mutation who was affected with CS. Mean age of female carriers was 30.3 ± 17.6 years, with an age range spanning from approximately 2 years to 65 years. Five non-carrier females across four CS pedigrees were also enrolled to serve as a familial control. The mean age for non-carrier females was 29.7 ± 21.1, with an age range spanning from approximately age 10 years to 75 years. When possible, neuropsychological testing was performed on a subset of female carriers and non-carriers in CS pedigrees. A total of 13 carriers and 3 non-carriers from 7 CS pedigrees were assessed. Mean carrier age of those with direct neuropsychological assessment was 30.1 ± 15.9 years, with a range of approximately 3 years to 55 years. Mean non-carrier age of those assessed was 21.7 ± 18.5 years, with a range of approximately age 10 years to 45 years.
Neuropsychological Performance in Female Carriers
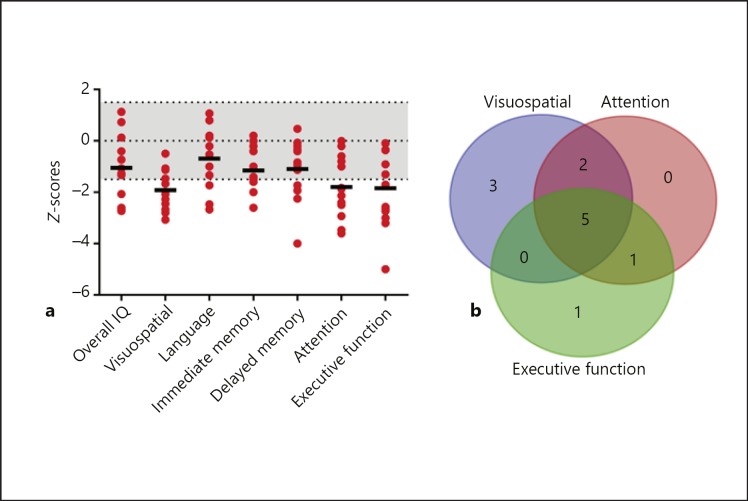
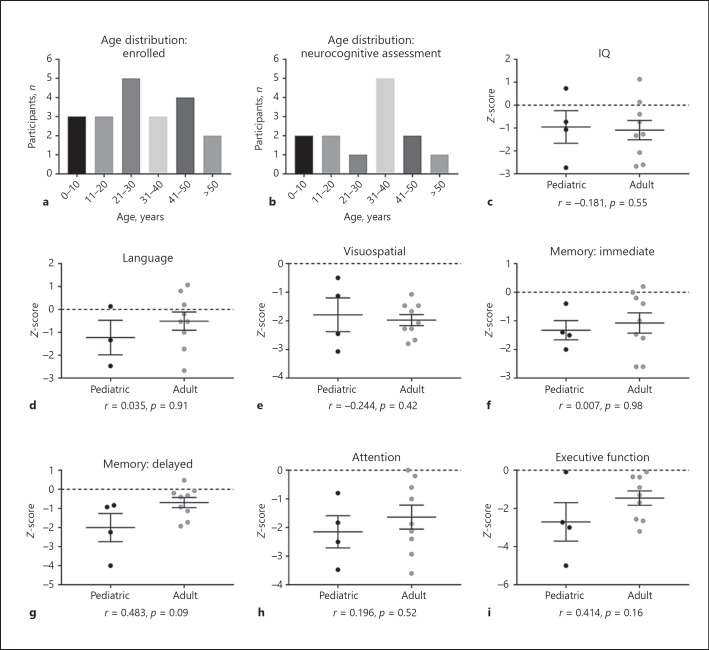
Most female carriers (85%) demonstrated deficits in at least one neurocognitive domain (Fig. 1a; Table 1). Deficits were defined as standardized, age-normed z-scores <–1.5. A majority of female carriers demonstrated deficits in two or more domains (69%). Notable impaired cognitive domains included: visuospatial function (62%), attention (62%), and executive function (54%). Almost all female carriers exhibited a deficit in at least one of these three domains (92%, 12 out of 13), while a large proportion of individuals had co-occurring deficits in all three domains (39%, 5 out of 13) (Fig. 1b). Deficits in language function were less common (25%). Approximately one-third of female carriers tested had an overall full-scale IQ < 70 (31%, 4 out of 13). We further tested if an effect of age could be discerned in this cross-sectional dataset. A distribution of participants' ages at time of enrollment and testing is shown (Fig. 2a, b). We compared neurocognitive functioning in pediatric (< 18 years old) and adult (≥18 years old) female carriers. No significant age-related correlations in performance across any domain were found (Fig. 2). Neurocognitive data were also collected on 3 non-carrier female family members, all of whom performed generally without impairment. Given the small sample size of non-carriers, it is currently difficult to establish comparison of carriers to non-carriers so as to assess deficit relative to familial expectation. Therefore, comparisons to standardized controls are presented (i.e., z-scores).
Fig. 1.
Neurocognitive profile of female carriers of NHE6 mutations from Christianson syndrome (CS) pedigrees. a Neuropsychological assessments were performed on 13 female carriers from 7 CS pedigrees. Mean female carrier age was 30.1 ± 15.9 years, with a range of approximately 3 years to 55 years. Domains with mean z-scores less than −1.5 in female carriers were visuospatial function, attention, and executive function. Individual z-scores are represented by red dots and mean z-scores per domain are denoted by a black bar. The z-score range from 1.5 to −1.5 is shaded in light gray to represent a non-clinical range. b Distribution of visuospatial, attention, and executive function deficits across individual female carriers (n = 12 participants with impairments in at least one of these domains). Impairment spanning all domains was the most common.
Table 1.
Neuropsychological profile of female carriers of NHE6 mutations
| Domain | Female carrier |
Female non-carrier |
Female carrier |
||||
|---|---|---|---|---|---|---|---|
| n | mean (SD) | % impaired | n | mean (SD) | domains impaired, n (z <−1.5) | % | |
| IQ | 13 | −1.05 (1.3) | 31 | 3 | 0.00 (0.94) | 0 | 15 |
| Language | 12 | −0.69 (1.2) | 25 | 2 | −0.24 (0.33) | 1 + | 85 |
| Visuospatial | 13 | −1.92 (o.76) | 62 | 2 | −1.14 (1.8) | 2+ | 69 |
| Immediate memory | 13 | −1.15 (o.94) | 31 | 2 | 0.07 (1.2) | 3+ | 39 |
| Delayed memory | 13 | −1.10 (1.2) | 31 | 2 | −0.67 (1.2) | 4+ | 39 |
| Attention | 13 | −1.79 (1.2) | 62 | 2 | −1.44 (3.2) | 5+ | 31 |
| Executive function | 13 | −1.84 (1.5) | 54 | 3 | −0.96 (1.6) | 6+ | 23 |
| 7+ | 8 | ||||||
Fig. 2.
Neurocognitive profile of pediatric (i.e., < 18 years old) and adult (i.e., ≥18 years old) female carriers of NHE6 mutations. a Age distribution of all enrolled female carriers (i.e., age at enrollment). b Age distribution of female carriers with neurocognitive assessments (i.e., age at testing). c–i Comparison of pediatric versus adult female carriers across neurocognitive domains. Pearson correlation analyses were conducted to examine the association between age (i.e., pediatric or adult) and neurocognition. There were no statistically significant age effects found in any domain. Ages of pediatric female carriers (n = 4) ranged from approximately 4–17 years (average age 10.3 ± 4.6 years); ages of adult female carriers (n = 9) ranged from approximately 25–55 years (average age 38.9 ± 9.3 years). All neurocognitive domains were assessed in the 4 pediatric participants, except for language (n = 3). Neurocognitive data are reported as mean ± SEM.
Neuropsychiatric and Age-Related Symptoms in Female Carriers
Notable neurological and neuropsychiatric features were also present in female NHE6 mutation carriers (Table 2). One-fifth of female carriers (20%) were diagnosed with ID/DD, which occurred in almost half of the pedigrees in this female carrier cohort (44%). Of those female carriers not diagnosed with ID/DD, 31% were noted to have learning difficulties. Additional features present in more than one female carrier included: speech/language delays (30%), attention-deficit/hyperactivity disorder (20%), eye problems such as esotropia (akin to Duane syndrome Type I), amblyopia, saccadic smooth pursuit, and myopia (20%), ataxia (10%), autism spectrum disorder (ASD, 10%), hypotonia (10%), and neuropathy (10%). One carrier was diagnosed with schizoaffective disorder and presented with masked facies. It is possible that her masked facies was caused by antipsychotic medication. Other features noted in a single female carrier included seizure disorder and microcephaly.
Table 2.
Neurological and neuropsychiatric features of female carriers of NHE6 mutations
| Diagnosis | Carriers, n | Carriers, % (n = 20) | Pedigrees, n | Pedigrees, % (n = 9) |
|---|---|---|---|---|
| Intellectual disability (ID)/developmental delay (DD) | 4 | 20 | 4 | 44 |
| Learning difficulties (if no ID/DD diagnosis) | 5 | 31a | 2 | 22 |
| Speech/language delay | 6 | 30 | 5 | 56 |
| Attention-deficit/hyperactivity disorder | 4 | 20 | 4 | 44 |
| Schizoaffective disorder | 2 | 10 | 1 | 11 |
| Autism spectrum disorder | 2 | 10 | 2 | 22 |
| Seizure disorder | 1 | 5 | 1 | 11 |
| Microcephaly | 1 | 5 | 1 | 11 |
| Ataxia | 2 | 10 | 2 | 22 |
| Hypotonia | 2 | 10 | 2 | 22 |
| Neuropathy | 2 | 10 | 2 | 22 |
| Eye problems (e.g., ophthalmoplegia) | 4 | 20 | 4 | 44 |
Percentage calculated in 16 participants (i.e., does not include carriers diagnosed with ID/DD).
Two confirmed female carriers who were enrolled, both over age 50, had histories consistent with a neurodegenerative disease, specifically atypical parkinsonism and CBD. Across the full CS cohort of families, including > 30 families, we discerned 2 additional females (likely carriers by pedigree but not confirmed) who, strictly by history, were given diagnoses of CBD or multiple systems atrophy (MSA) after age 50. Neither of these patients was available to be enrolled.
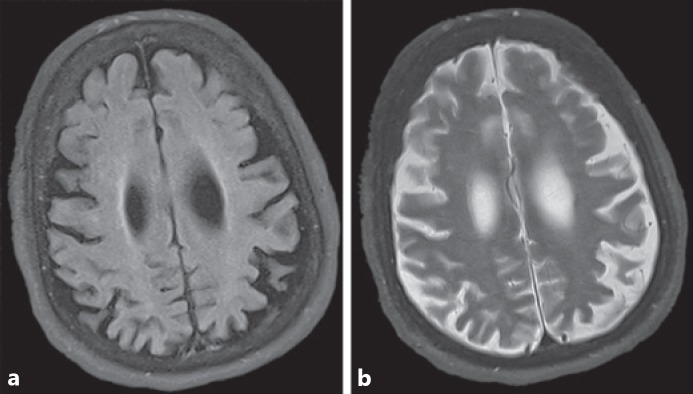
The enrolled and confirmed female carrier diagnosed with CBD was evaluated at approximately age 65. Symptom onset occurred 1–2 years prior with multiple forward falling episodes and gait disturbance. Mental status exam was notable for a rapid cognitive decline, with profound loss of activities of daily living, meaningful language, and communication skills. Dysautonomic symptoms reported included constipation and temperature dysregulation. Neurological exam was notable for masked facies. She had no upward gaze, limited downward gaze, and intact horizontal eye movements. Motor exam showed bradykinesia with abnormal tone demonstrating both cogwheeling and rigidity on her right side. She had a resting tremor at 3–6 Hz that was greater in the right hand, without a postural or intention tremor. Occasional myoclonic jerks were noted. Testing of her reflexes demonstrated hyperreflexia and sustained clonus in both ankles. While she is mostly non-ambulatory, her gait (with assistance) is notable for freezing, bradykinesia, and shuffling. This participant had been diagnosed previously with PSP due to eye movement abnormalities; however, subsequent, and in part due to results from MRI of the brain, the diagnosis was changed to CBD. The MRI scan at age 65 showed asymmetric cortical atrophy (Fig. 3). Specifically, the left frontal and parietal cortical areas were severely atrophic compared to the right hemisphere. Additional notable findings included generalized parenchymal volume loss and increased ventricular size, which was likely due to central volume loss.
Fig. 3.
a, b MRI scans of a female heterozygous for NHE6 mutation at approximately age 65 years show frontoparietal atrophy; the individual was diagnosed with corticobasal degeneration (CBD). Axial FLAIR (a) and T2-weighted (b) images show asymmetrical atrophy of the left frontal and parietal lobes.
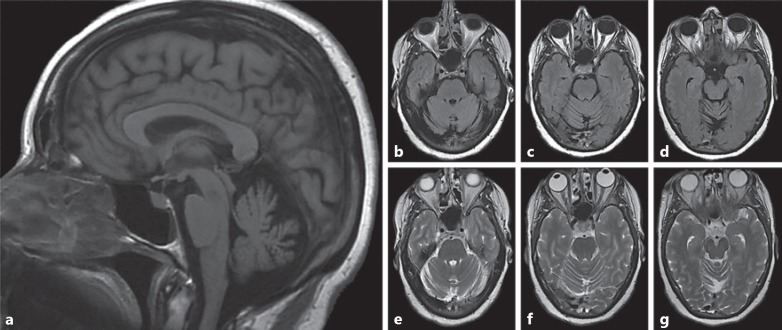
We examined a second confirmed female mutation carrier who was diagnosed with parkinsonism at approximately age 55. Symptom onset began in her early 50s, with tremors developing in her right hand and upper limbs that have worsened over time. A brief cognitive screen (i.e., MMSE) indicated intact functioning (score = 27). Examination of cranial nerves demonstrated subtle left eye hypophoria and mild reduction in convergence, as well as the presence of hypomimia and a reduced blink frequency. Motor exam was significant for bradykinesia and abnormalities in tone, including both cogwheeling and gegenhalten. Her reflexes were remarkable for clonus in the right ankle. Her gait was notable for shuffling with reduced arm swing, and she has difficulty turning. This participant had an MRI scan in her early 50s, which indicated generalized brain atrophy and notable cerebellar atrophy (Fig. 4). Cerebellar atrophy was present in both the vermis and hemispheres, and was particularly severe in the vermis. This finding is similar to cerebellar findings in CS male probands [2].
Fig. 4.
a–g MRI scans of a female heterozygous for NHE6 mutation at approximately age 51 show cerebellar atrophy; the individual was diagnosed with atypical parkinsonism. a The sagittal T1-weighted image demonstrates cerebellar atrophy. Axial FLAIR (b–d) and T2-weighted (e–g) images through the cerebellar hemispheres further highlight diffuse cerebellar volume atrophy.
Genomic and Transcriptomic Links between NHE6 and Neurodegeneration
In order to assess a potential role for NHE6 in neurodegeneration, NHE6 expression in postmortem brain was analyzed from two aging and dementia cohorts – ROS [12] and MAP [13]. Demographic and neuropathological information on the 740 participants has been previously reported [14]. To summarize, the mean age at death was 88.0 ± 6.7 years, 471 (63.6%) were female, and 447 (60.4%) met diagnostic criteria for AD. Median Aβ load was 2.2%, while the median tau tangle density was 3.7/mm2.
Brain transcriptome analysis examined correlations between aging features and NHE6 expression (Table 3). Decreased NHE6 expression was significantly associated with greater tau tangle density using a strict transcriptome-wide correction (adjusted p = 0.05). Without adjusting for multiple corrections, decreased NHE6 expression was further associated with greater Aβ load (uncorrected p = 0.04) and decreased cognitive functioning (uncorrected p = 0.03). These results did not, however, withstand correction. NHE6 expression was not correlated with neurofibrillary tangles, neuropathological severity, or AD severity. We further investigated correlations using the three main NHE6 isoforms: SLC9A6-001 (Transcript ID: ENST00000370698), SLC9A6-002 (Transcript ID: ENST00000370695), and SLC9A6-003 (Transcript ID: ENST00000370701). Notably, increased tau deposition was associated with the SLC9A6-003 isoform (adjusted p = 0.03). Finally, we sequenced NHE6 in 156 females diagnosed with Parkinson-related syndromes (e.g., 150 PSP cases and 6 CBD cases) to examine whether NHE6 mutations may be enriched in this cohort (online suppl. Table 1). No deleterious mutations (e.g., non-sense, missense, indel) were identified in this analysis.
Table 3.
Correlation between NHE6 expression in brain and Alzheimer's disease variables in aging/dementia cohorts
| NHE6 variant | Aβ load |
Tau tangle density |
||||||
|---|---|---|---|---|---|---|---|---|
| intercept | beta | unadj. p value | adj. p value | intercept | beta | unadj. p value | adj. p value | |
| NHE6 (ENSG00000198689.5) | −0.152 | −0.018 | 0.038 | 0.204 | −0.696 | −0.028 | 0.008 | 0.051 |
| NHE6: isoform 1 (ENST00000370698.3) | −0.424 | −0.016 | 0.208 | 0.664 | −1.631 | 0.032 | 0.035 | 0.213 |
| NHE6: isoform 2 (ENST00000370695.4) | −0.586 | 0.004 | 0.833 | 0.961 | −1.244 | −0.037 | 0.074 | 0.332 |
| NHE6: isoform 3 (ENST00000370701.l) | −0.482 | −0.007 | 0.259 | 0.706 | −1.028 | −0.024 | 0.002 | 0.031 |
| Neurofibrillary tangles |
Alzheimer's disease pathology |
|||||||
|---|---|---|---|---|---|---|---|---|
| intercept | beta | unadj. p value | adj. p value | intercept | beta | unadj. p value | adj. p value | |
| NHE6 (ENSG00000198689.5) | −0.485 | −0.005 | 0.155 | 0.496 | −6.189 | −0.034 | 0.053 | 0.303 |
| NHE6: isoform 1 (ENST00000370698.3) | −0.625 | 0.004 | 0.408 | 0.828 | −6.886 | −0.005 | 0.843 | 0.995 |
| NHE6: isoform 2 (ENST00000370695.4) | −0.575 | −0.005 | 0.391 | 0.820 | −6.884 | −0.020 | 0.566 | 0.923 |
| NHE6: isoform 3 (ENST00000370701.1) | −0.554 | −0.003 | 0.215 | 0.710 | −6.671 | −0.024 | 0.052 | 0.510 |
| Cognitive decline |
Neuropathological severity |
|||||||
|---|---|---|---|---|---|---|---|---|
| intercept | beta | unadj. p value | adj. p value | intercept | beta | unadj. p value | adj. p value | |
| NHE6 (ENSG00000198689.5) | −0.205 | 0.002 | 0.029 | 0.150 | −0.100 | −0.008 | 0.055 | 0.245 |
| NHE6: isoform 1 (ENST00000370698.3) | −0.143 | −0.002 | 0.177 | 0.616 | −0.317 | 0.003 | 0.614 | 0.872 |
| NHE6: isoform 2 (ENST00000370695.4) | −0.168 | 0.004 | 0.040 | 0.320 | −0.279 | −0.003 | 0.687 | 0.902 |
| NHE6: isoform 3 (ENST00000370701.1) | −0.175 | 0.001 | 0.058 | 0.385 | −0.218 | −0.006 | 0.062 | 0.408 |
Discussion/Conclusion
This study outlines novel brain-related phenotypes associated with NHE6 mutations in female carriers from CS pedigrees. Mutations in this gene cause CS in males, an X-linked disorder characterized by ID/DD, epilepsy, postnatal microcephaly, and ataxia with progressive cerebellar atrophy [1]. The current study of 20 female carriers across 9 pedigrees examines the phenotypic range in females with NHE6 mutations with regard to (1) cognitive functioning, (2) neuropsychiatric/medical conditions, and (3) age-associated features. We further examined how alterations in NHE6 brain expression correlate with neurodegenerative variables using large transcriptome datasets derived from normal and pathological aging brains at postmortem. Taken together, our results support novel neuropsychiatric phenotypes related to NHE6 mutations with a spectrum of severity in heterozygous females from CS pedigrees, including potentially age-related pathology.
Although some female carriers in CS pedigrees have previously been reported with ID/DD, speech and language problems, truncal ataxia, hyperkinesis, and psychiatric illness [1, 2, 3, 4, 7], we employed standardized neuropsychological measures across multiple CS pedigrees with inherited transmission to rigorously quantify performance across cognitive domains. Our findings suggest that female carriers of NHE6 mutations display a recognizable neurocognitive phenotype. Overall, the majority of assessed female carriers exhibited deficits in at least one cognitive domain (85%), and one-third (31%) had IQ scores < 70. Furthermore, most female carriers of NHE6 mutations demonstrated a recognizable neuropsychological profile, with specific deficits in visuospatial function (62%), attention (62%), and executive function (54%), regardless of IQ. The most common pattern across individual female carriers was impairment in all three domains (39%). Interestingly, visuospatial memory deficits have also been reported in female Nhe6-heterozygous mice using a hippocampus-dependent object placement test [11]. Therefore, visuospatial abilities may represent a core domain affected by NHE6 dysfunction across species. Additionally, we determined whether differences existed in pediatric versus adult female participants, with results indicating no differences in the current dataset. This may be due in part to the cross-sectional design or, alternatively, to the small sample size, or a combination of both. Future studies in larger cohorts of patients that are prospectively recruited and followed longitudinally will be needed to assess these questions with greater statistical power. It is also important to note that while a pattern of deficits emerged in female carriers compared to population norms, some qualitative similarities were observed in neurocognition between female carriers and non-carriers (Table 1). Unfortunately, the small sample size of female non-carriers from enrolled CS pedigrees prevents appropriate statistical analysis of these differences. A critical goal for future CS research should be a close examination of neurocognitive patterns in female carriers with reference to familial expectations by comparison to related female non-carriers in the same pedigree.
Visuospatial and executive function deficits have been found in individuals with other X-linked disorders such as Fragile X [21] and Turner [22] syndromes, as well as in female carriers of Fragile X mutations [23, 24]. Such deficits may be linked to the frontoparietal white and gray matter abnormalities [22, 25], consistent with the known localization of visuospatial and executive functions to the parietal and frontal regions, respectively. Alternatively, as the lateral and posterior cerebellar regions are involved in visuospatial and executive functions [26] and given the cerebellar atrophy observed in some CS probands [2], cerebellar involvement could be the underlying mechanism of impaired visuospatial and executive functioning in female carriers. Furthermore, visuospatial and executive dysfunction with associated frontoparietal abnormalities is also known to occur in relevant neurodegenerative conditions such as Fragile X-associated tremor/ataxia syndrome [23, 24, 27] and CBD [28, 29]. Similar to other X-linked disorders, visuospatial and executive dysfunction may represent a neurocognitive phenotype in the female carriers of NHE6 mutations.
While neurodegenerative features have been reported in CS males and an NHE6 mouse model, the extent to which female carriers may be at risk to develop these symptoms remains an ongoing question that will require larger, longitudinal studies before any conclusive statements can be made. Although we and others [7, 30] have noted cases where aging female carriers in CS pedigrees have been diagnosed with neurodegenerative diseases, it is premature, given the small sample size, to discern whether NHE6 mutations in female carriers represent a bona fide risk or to estimate what the level of risk might be. The current study directly examined 2 female carriers who were > 50 years old and were diagnosed with Parkinson-related disorders (e.g., atypical parkinsonism and CBD). Two additional aging females, considered to be likely carriers, were reported by their families to have been diagnosed with neurodegenerative diseases (e.g., CBD and MSA). Again, longitudinal studies consisting of many CS female carriers across multiple pedigrees are necessary to understand whether this cohort is at risk for age-related features or if the reported cases merely reflect false-positives that arise from small sample sizes. We also sought to investigate correlations of NHE6 expression with age-related symptoms. Notably, our analysis of two aging/dementia brain transcriptome datasets (n = 740) provides a different perspective to examine how NHE6 expression is associated with neurodegenerative features in a broader population. This analysis revealed lower NHE6 expression was significantly correlated with increased tau deposition, but not Aβ load, after use of a conservative genome-wide correction. Thus, NHE6 dysfunction may be associated with neurodegenerative symptoms, particularly in tau-related disorders. Note, transcriptional isoform 3 of NHE6 was largely responsible for this finding. Compared to the other NHE6 isoforms, isoform 3 is lacking the first 52 amino acids of NHE6; the protein region absent in isoform 3 includes the N-terminus and part of the first transmembrane domain. It is currently unknown why this isoform is associated with increased tau deposition to a greater extent than the other two isoforms. The clinical diagnoses in females ascertained to date (e.g., CBD, PSP, MSA, and atypical parkinsonism) are often associated with tau deposition. This is particularly true for glial tau inclusions, which, notably, was among the findings in one of the few CS postmortem studies, as was the absence of greater amyloid plaque burden [9]. Given the endosomal function of NHE6, it is plausible to hypothesize that processing and/or clearance of amyloid precursor protein may be altered, which has been suggested by recent published experiments [31, 32]. However, our current study of the large ROS/MAP dataset does not appear to support this. One additional finding that seems plausible is a risk of cerebellar atrophy in female NHE6 mutation carriers. This finding, as shown in Figure 4, appears to reflect a pattern that is a milder form of cerebellar atrophy, as compared to CS males. This result is also highly consistent with preclinical studies in mouse wherein Sikora et al. [11] identified Purkinje cell loss in female heterozygous mice. However, at this juncture, these interpretations regarding progressive disease in female carriers remain speculative. Also, we did not find evidence of deleterious NHE6 mutations in the DNA collection of females with idiopathic PSP, indicating that these mutations are unlikely to be a common unrecognized cause.
In summary, with regard to neurodegenerative disease, at this time we urge caution in drawing conclusions. However, we believe that our data call attention to this understudied female-specific phenotype, which warrants rigorous follow-up in larger, well-powered studies. NHE6 dysfunction may be associated with age-related features in females, possibly related to parkinsonism or tau-related syndromes. These results support the notion that endosomal proteins are involved in the pathogenesis of both neurodevelopmental and neurodegenerative diseases, and that NHE6-related human disorders may be mixed developmental and degenerative diseases. Nonetheless, follow-up studies in larger, prospectively recruited cohorts, as well as in animal models, are warranted to further define this understudied female-specific condition.
Statement of Ethics
Subjects (or their parents or guardians) included in the study for which results are reported herein gave their written informed consent. The study protocol was approved by the IRBs housed within the Brown University Office of Research Integrity and Lifespan Healthcare Research Protection Office.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
Research reported in this publication was supported in part by the following: National Institutes of Health/National Institute of Mental Health (R01MH102418 and R01MH105442) and the Hassenfeld Child Health Innovation Institute at Brown University (E.M.M.); and National Institutes of Health/National Institute of Neurological Disorders and Stroke (F31NS093880) (M.F.P.). M.F.P. was previously funded through an advanced predoctoral training grant (T32NS0624443, Lipscombe and Moore). E.M.M. has also received generous support from the Christianson Syndrome Association (CSA). The funding bodies did not play any direct role in the design of the study, in the collection, analysis, and interpretation of data, or in the writing of the manuscript.
Author Contributions
M.F.P., B.C.K., and E.M.M. designed the study, analyzed data, performed participant assessments, and wrote the manuscript. N.P., P.L.D.J., and T.L.Y.-P. contributed to the study design and analysis of the transcriptome experiment. M.S. studied DNA samples. B.A.J. performed participant assessments. J.M.R. reviewed patient neuroimaging data. J.S.L. oversaw patient neurological exams and reports. All authors have approved the manuscript.
Supplementary Material
Supplementary data
Acknowledgement
The authors would like to thank the families for their participation in the current study. E.M.M. had full access to all the data in the study. This study used samples from the National Institute of Neurological Disorders and Stroke Human Genetics Resource Center DNA and Cell Line Repository (https://catalog.coriell.org/1/ninds), as well as clinical data. Sample numbers corresponding to the samples used are listed in online supplementary Table 1.
References
- 1.Gilfillan GD, Selmer KK, Roxrud I, Smith R, Kyllerman M, Eiklid K, et al. SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angelman syndrome. Am J Hum Genet. 2008 Apr;82((4)):1003–10. doi: 10.1016/j.ajhg.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pescosolido MF, Stein DM, Schmidt M, El Achkar CM, Sabbagh M, Rogg JM, et al. Genetic and phenotypic diversity of NHE6 mutations in Christianson syndrome. Ann Neurol. 2014 Oct;76((4)):581–93. doi: 10.1002/ana.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christianson AL, Stevenson RE, van der Meyden CH, Pelser J, Theron FW, van Rensburg PL, et al. X linked severe mental retardation, craniofacial dysmorphology, epilepsy, ophthalmoplegia, and cerebellar atrophy in a large South African kindred is localised to Xq24-q27. J Med Genet. 1999 Oct;36((10)):759–66. doi: 10.1136/jmg.36.10.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroer RJ, Holden KR, Tarpey PS, Matheus MG, Griesemer DA, Friez MJ, et al. Natural history of Christianson syndrome. Am J Med Genet A. 2010 Nov;152A((11)):2775–83. doi: 10.1002/ajmg.a.33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mignot C, Héron D, Bursztyn J, Momtchilova M, Mayer M, Whalen S, et al. Novel mutation in SLC9A6 gene in a patient with Christianson syndrome and retinitis pigmentosum. Brain Dev. 2013 Feb;35((2)):172–6. doi: 10.1016/j.braindev.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi Y, Hosoki K, Matsushita M, Funatsuka M, Saito K, Kanazawa H, et al. A loss-of-function mutation in the SLC9A6 gene causes X-linked mental retardation resembling Angelman syndrome. Am J Med Genet B Neuropsychiatr Genet. 2011 Dec;156B((7)):799–807. doi: 10.1002/ajmg.b.31221. [DOI] [PubMed] [Google Scholar]
- 7.Sinajon P, Verbaan D, So J. The expanding phenotypic spectrum of female SLC9A6 mutation carriers: a case series and review of the literature. Hum Genet. 2016 Aug;135((8)):841–50. doi: 10.1007/s00439-016-1675-5. [DOI] [PubMed] [Google Scholar]
- 8.Fichou Y, Bahi-Buisson N, Nectoux J, Chelly J, Héron D, Cuisset L, et al. Mutation in the SLC9A6 gene is not a frequent cause of sporadic Angelman-like syndrome. Eur J Hum Genet. 2009 Nov;17((11)):1378–80. doi: 10.1038/ejhg.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garbern JY, Neumann M, Trojanowski JQ, Lee VM, Feldman G, Norris JW, et al. A mutation affecting the sodium/proton exchanger, SLC9A6, causes mental retardation with tau deposition. Brain. 2010 May;133((Pt 5)):1391–402. doi: 10.1093/brain/awq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M, Ouyang Q, Gong J, Pescosolido MF, Pruett BS, Mishra S, Schmidt M, Jones RN, Gamsiz Uzun ED, Lizarraga SB, Morrow EM. Mixed neurodevelopmental and neurodegenerative pathology in Nhe6-null mouse model of Christianson syndrome. eNeuro. 2017 Nov-Dec;4((6)) doi: 10.1523/ENEURO.0388-17.2017. ENEURO.0388-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikora J, Leddy J, Gulinello M, Walkley SU. X-linked Christianson syndrome: heterozygous female Slc9a6 knockout mice develop mosaic neuropathological changes and related behavioral abnormalities. Dis Model Mech. 2016 Jan;9((1)):13–23. doi: 10.1242/dmm.022780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012 Jul;9((6)):628–45. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012 Jul;9((6)):646–63. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu L, Chibnik LB, Srivastava GP, Pochet N, Yang J, Xu J, et al. Association of Brain DNA methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 with pathological diagnosis of Alzheimer disease. JAMA Neurol. 2015 Jan;72((1)):15–24. doi: 10.1001/jamaneurol.2014.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin JZ, Yassour M, Adiconis X, Nusbaum C, Thompson DA, Friedman N, et al. Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nat Methods. 2010 Sep;7((9)):709–15. doi: 10.1038/nmeth.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adiconis X, Borges-Rivera D, Satija R, DeLuca DS, Busby MA, Berlin AM, et al. Comparative analysis of RNA sequencing methods for degraded or low-input samples. Nat Methods. 2013 Jul;10((7)):623–9. doi: 10.1038/nmeth.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014 Aug;30((15)):2114–20. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012 Mar;22((3)):568–76. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cingolani P, Platts A, Wang L, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012 Apr-Jun;6((2)):80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010 Apr;7((4)):248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooper SR, Hatton D, Sideris J, Sullivan K, Hammer J, Schaaf J, et al. Executive functions in young males with fragile X syndrome in comparison to mental age-matched controls: baseline findings from a longitudinal study. Neuropsychology. 2008 Jan;22((1)):36–47. doi: 10.1037/0894-4105.22.1.36. [DOI] [PubMed] [Google Scholar]
- 22.Hong DS, Reiss AL. Cognition and behavior in Turner syndrome: a brief review. Pediatr Endocrinol Rev. 2012 May;9(Suppl 2):710–2. [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JC, Simon C, Niu YQ, Bogost M, Schneider A, Tassone F, et al. Phenotypes of hypofrontality in older female fragile X premutation carriers. Ann Neurol. 2013 Aug;74((2)):275–83. doi: 10.1002/ana.23933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraan CM, Hocking DR, Bradshaw JL, Fielding J, Cohen J, Georgiou-Karistianis N, et al. Neurobehavioural evidence for the involvement of the FMR1 gene in female carriers of fragile X syndrome. Neurosci Biobehav Rev. 2013 Mar;37((3)):522–47. doi: 10.1016/j.neubiorev.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Villalon-Reina J, Jahanshad N, Beaton E, Toga AW, Thompson PM, Simon TJ. White matter microstructural abnormalities in girls with chromosome 22q11.2 deletion syndrome, Fragile X or Turner syndrome as evidenced by diffusion tensor imaging. Neuroimage. 2013 Nov;81:441–54. doi: 10.1016/j.neuroimage.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Halloran CJ, Kinsella GJ, Storey E. The cerebellum and neuropsychological functioning: a critical review. J Clin Exp Neuropsychol. 2012;34((1)):35–56. doi: 10.1080/13803395.2011.614599. [DOI] [PubMed] [Google Scholar]
- 27.Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008 Jan;22((1)):48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 28.Graham NL, Bak TH, Hodges JR. Corticobasal degeneration as a cognitive disorder. Mov Disord. 2003 Nov;18((11)):1224–32. doi: 10.1002/mds.10536. [DOI] [PubMed] [Google Scholar]
- 29.Murray R, Neumann M, Forman MS, Farmer J, Massimo L, Rice A, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007 Apr;68((16)):1274–83. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- 30.Riess A, Rossier E, Krüger R, Dufke A, Beck-Woedl S, Horber V, et al. Novel SLC9A6 mutations in two families with Christianson syndrome. Clin Genet. 2013 Jun;83((6)):596–7. doi: 10.1111/j.1399-0004.2012.01948.x. [DOI] [PubMed] [Google Scholar]
- 31.Prasad H, Rao R. The Na+/H+ exchanger NHE6 modulates endosomal pH to control processing of amyloid precursor protein in a cell culture model of Alzheimer disease. J Biol Chem. 2015 Feb;290((9)):5311–27. doi: 10.1074/jbc.M114.602219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasad H, Rao R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc Natl Acad Sci USA. 2018 Jul;115((28)):E6640–9. doi: 10.1073/pnas.1801612115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data