Abstract
Diffuse large B cell lymphoma (DLBCL) is classified as an aggressive lymphoma due to its poor prognosis regardless of the treatment. Almost all cases of DLBCL are treated using rituximab-combination chemotherapy, but spontaneous regression without any therapeutic modalities may rarely occur. A 35-year-old man complained of abdominal pain and discomfort. Positron emission tomography-computed tomography (PET-CT) demonstrated abnormal accumulation of fluorodeoxyglucose in the thickened wall of the small intestine and multiple lymphadenopathy. Laparoscopic lymph node biopsy was performed, and the diagnosis of DLBCL was made based on the biopsy findings. Soon after the laparoscopic biopsy, the patient felt free from any symptoms. Approximately three months later, no abnormal accumulation of fluorodeoxyglucose in the entire body was found on PET-CT. He has remained in complete metabolic remission for over three years according to PET-CT. We discuss the mechanism of this rare phenomenon.
Keywords: diffuse large B-cell lymphoma, spontaneous regression, CD8, PD-1
INTRODUCTION
Diffuse large B cell lymphoma (DLBCL) is an aggressive lymphoproliferative disorder commonly involving the lymph nodes. Extranodal DLBCL, that is, DLBCL found outside of lymph nodes, occasionally localizes in the small intestine. The accepted standard treatment is rituximab-combination chemotherapy, but the prognosis is poor due to the high relapse rate of the disease.1 Spontaneous regression (SR) has been defined as the complete disappearance of malignant tumors without any treatment modalities. SR occasionally occurs for all cancer types, including malignant lymphoma.2 Although SR commonly occurs for low-grade lymphomas, SR rarely occurs for aggressive lymphomas such as DLBCL.3 We report a case of SR of DLBCL approximately three months after laparoscopic lymph node biopsy. Our patient did not receive any medications or radiotherapy. Although the detailed mechanism of SR remains unknown, we assumed that the marked infiltration of CD8-positive T cells and programmed cell death-1-positive T cells around lymphoma cells played a role in the SR in our patient.
CASE PRESENTATION
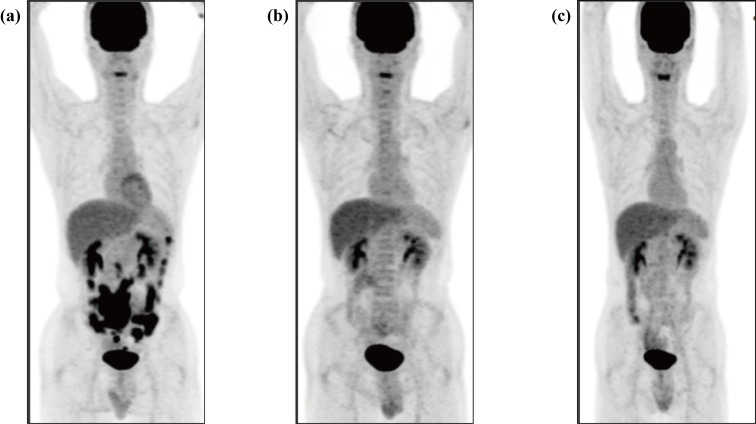
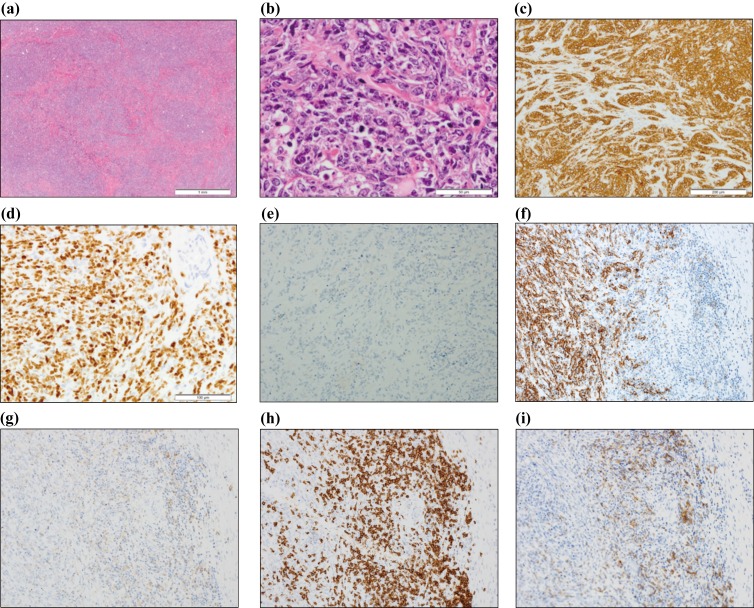
A 35-year-old man was admitted to our hospital for evaluation of an intra-abdominal mass. He had no remarkable medical history. He developed abdominal pain and visited a local clinic in May 2014. Abdominal ultrasonography revealed a large mass on the right side of the umbilical region. He was admitted to our hospital several days later. At that time, he did not have any abdominal pain, but he noted discomfort around the upper area of the umbilical region. Physical examinations revealed a palpable soft mass on the right side of the umbilical region, which was consistent with the ultrasonography findings. Laboratory examinations found no abnormalities, including in the levels of C-reactive protein, lactate dehydrogenase, and soluble interleukin-2 receptor. He was also negative for the human immunodeficiency virus (HIV). Contrast-enhanced computed tomography (CECT) demonstrated thickening of the entire circumference of the wall of the small intestine on the right side of the umbilical region and multiple soft tissue density nodules in the mesentery. On positron emission tomography-computed tomography (PET-CT), abnormal accumulation of fluorodeoxyglucose was localized in the small intestine and multiple nodules (Fig. 1a). However, no abnormal accumulation of fluorodeoxyglucose was observed outside of the abdominal region. Total double-balloon endoscopy revealed multiple areas with small raised reddish mucosa in the lumen of the entire circumference of the small intestine. To examine these abnormalities, laparoscopic lymph node biopsy was performed one month later. Multiple lymph nodules were found in the mesentery, and a tumor was also found in the small intestine. One lymph node in the mesentery was removed. On histopathological examination, diffuse proliferation of large-sized abnormal cells with irregular nuclei and scant cytoplasm in the biopsied lymph node were observed (Fig. 2a, b). Immunohistochemical analysis revealed that these abnormal cells were positive for CD19, CD20 (Fig. 2c, f), and Bcl-6, and negative for CD10, Bcl-2, Myc (below 5%), and MUM-1, consistent with the germinal center (GC) origin according to Hans’ criteria.4 The Epstein-Barr virus encoded small RNA (EBER) in situ hybridization was negative. The Ki-67 index was very high (Fig. 2d). According to immunohistochemical analysis, abnormal cells did not express programmed cell death ligand 1 (PD-L1) (Fig. 2e). Proliferation of many collagen fibers suggested interstitial fibrosis, and apoptotic abnormal cells were also found. Both infiltrating CD4- and CD8-positive T cells were found in the background of a lymph node, with CD8-positive T cells being predominant (Fig. 2g, h). According to immunohistochemical analysis, the CD4- and CD8-positive T cells surrounding the abnormal cells expressed programmed cell death-1 (PD-1) (Fig. 2i). Flow cytometric analysis demonstrated that the abnormal cells were positive only for CD19 and CD20, consistent with the immunohistochemical analysis. JH rearrangement examined by Southern blotting exhibited a monoclonal band, suggesting clonal proliferation. These findings led to the diagnosis of DLBCL, not otherwise specified according to the World Health Organization (WHO) 2017 classification.1 Chromosomal analysis using G banding was unsuccessful. Bone marrow examination revealed no abnormal cells. Thus, he was determined to be at clinical stage IIA. The international prognostic index (IPI) was classified as low risk. After the laparoscopic biopsy, the patient felt that his abdominal discomfort gradually improved, and approximately one month later, he felt free from any symptoms. Of note, there was no abnormal accumulation of fluorodeoxyglucose on PET-CT three months later (Fig. 1b). He did not take any medications, such as anti-cancer agents or prednisolone, nor did he receive radiotherapy. We considered that his DLBCL spontaneously regressed after the laparoscopic biopsy. At the time of writing this report, the patient was free from any symptoms. DLBCL has not recurred for over three years and no abnormal accumulation of fluorodeoxyglucose has been observed by PET-CT (Fig. 1c).
Fig 1.
Temporal changes on PET-CT images
(a) Abnormal accumulation of fluorodeoxyglucose in the thickened walls of the small intestine (SUVmax 12.33) and in multiple lymphadenopathy (SUVmax 7.53) before the laparoscopic biopsy. (b) No abnormal accumulation of fluorodeoxyglucose in the entire body three months later. (c) No abnormal accumulation of fluorodeoxyglucose in the entire body, demonstrating that he remained in complete metabolic remission three years later.
Fig 2.
Histopathological findings.
(a) Diffuse proliferation of abnormal cells was observed in the biopsied lymph node (Hematoxylin-Eosin staining, X2). (b) Large abnormal cells with irregular nuclei, clear nucleoli, and scant cytoplasm were found. Many apoptotic cells and fibrosis were also observed (Hematoxylin-Eosin staining, X40). (c) Immunohistochemical analysis revealed that the abnormal cells were positive for CD20 (X20). (d) The Ki-67 index was above 90% (X20). (e) Abnormal cells were negative for PD-L1 (X200). (f) Abnormal cells were positive for CD20 (X200). (g) CD4-positive cells were found around the tumor (X200). (h) Many CD8-positive cells were found around the tumor (X200). (i) Both CD4- and CD8-positive cells around the tumor were positive for PD-1 (X200).
DISCUSSION
Here, we report a case of SR of DLBCL in the small intestine with multiple lymphadenopathy. SR has been defined as the complete disappearance of malignant tumors without any treatment modalities. SR may occur for any cancer type, including malignant lymphoma.2 The average duration of SR has been reported to be thirteen months.5 For aggressive lymphomas, such as DLBCL, SR may rarely occur, although SR has been commonly reported for low-grade lymphomas such as follicular lymphoma and mucosal-associated lymphoid tissue lymphoma.3,6 SR has been observed mostly for extranodal lymphoma in regions, such as the breast7-9 and skin,10 but rarely for nodal lymphoma.3 However, to the best of our knowledge, SR of DLBCL involving the small intestine has not been previously reported. SR of primary gastrointestinal lymphoma excluding the small intestine without any treatment modalities is markedly rare, and to the best of our knowledge, only one case of SR of primary gastric diffuse large lymphoma was reported in 1989. Shigematsu et al.11 reported the case of a 73-year-old man diagnosed with primary gastric diffuse large cell lymphoma. Gastroscopy revealed an isolated protuberant tumor on the posterior wall of the antrum, and biopsy confirmed the diagnosis of malignant lymphoma. Sixty days later, gastroscopy demonstrated the disappearance of the tumor. Shigematsu et al. did not mention the mechanism of SR. In our patient, both thickening of the wall of the small intestine and multiple lymphadenopathy disappeared without any treatment, although in previous case reports, one lesion in, for example, the lymph node,3 breast,7-9 and prostate,12 disappeared spontaneously. Our patient exhibited the rare SR of an aggressive lymphoma involving both extranodal and nodal lesions.
The mechanism of SR remains unknown, but immunomodulation has been proposed. Other iatrogenic immunodeficiency-associated lymphoproliferative disorders,13 such as post-transplant and methotrexate-related lymphoproliferative disorders, may regress after the cessation of immunosuppressant agents. Our patient did not take any medications, including immunosuppressants, and his lymphoma cells were negative for EBER, as determined by in situ hybridization. Thus, immune system suppression may not be related to the SR in our patient. Recently, immuno-editing has been considered to be synonymous with immunomodulation, and several immune escape mechanisms have been proposed.14 One such mechanism is the expression of inhibitory signal molecules, such as CD80, PD-L1, and indoleamine 2,3-dioxygenase (IDO), on tumor cells. Another is the induction of regulatory T (Treg) cells and myeloid-derived suppressor cells (MDSCs) around tumor cells. We did not examine the expression of CD80 or IDO, or Treg cells or MDSCs because of the lack of monoclonal antibodies. Thus, we focused on the PD-L1/PD-1 axis because the expression of PD-L1 on tumor cells is thought to be one of the most powerful and frequent immune escape mechanisms. PD-L1 expression is stimulated by exogenous inflammatory cytokines, endogenous AKT/mTOR or JAK/STAT3 signaling, or by structural variations in the 3’-untranslated region of the PD-L1 gene.15 However, we did not examine the expression of these molecules or structural variations.
When tumor cells express PD-L1 on their surface, PD-1/CD8-double-positive T lymphocytes do not attack them through the inhibitory signals of the PD-L1/PD-1 axis. Therefore, apoptosis of tumor cells does not occur. In contrast, when tumor cells do not express PD-L1, PD-1/CD8-double-positive T lymphocytes attack them and induce their apoptosis. This is one of the mechanisms of immune escape in cancer. In our patient, many tumor-infiltrating CD8-positive T cells were found in the biopsied lymph node. Immunohistochemical analysis revealed that the lymphoma cells did not express PD-L1, whereas the surrounding lymphocytes, including CD4- and CD8-positive T cells, expressed PD-1.16 Moreover, apoptotic cells were found in the biopsied lymph node. Thus, PD-1-expressing lymphocytes were thought to have attacked the lymphoma cells, which did not express PD-L1, and apoptosis of lymphoma cells may have been induced through the PD-L1/PD-1 axis. We consider this to have been the mechanism of SR. Similar findings have been described in other reports. Iihara et al.7 and Oya et al.8 reported the marked infiltration of CD8-positive T cells into the residual breast tissue in the SR of primary breast lymphoma. Takahashi et al.17 reported that in the case of SR of intravascular large B cell lymphoma, apoptosis of lymphoma cells was observed in biopsied samples. The predictive factors for the SR of lymphoma have not been clarified; however, many tumor-infiltrating CD8-positive T cells expressing PD-1, lymphoma cells that do not express PD-L1, and apoptosis of lymphoma cells may be the predictive factors for SR. This is the first report of a case in which the PD-L1/PD-1 axis was possibly involved in the SR of aggressive lymphoma.
There are several reports on the positivity rate of PD-L1 in malignant lymphoma, especially DLBCL. Kiyasu et al.16 reported that the prevalence rate of PD-L1-positive DLBCL was 10.5%, as determined using 30% or more as the threshold for PD-L1 positivity. Furthermore, using the same threshold as Kiyasu, Xing et al.18 reported that 16% of 86 patients were considered positive for PD-L1. These authors reported that PD-L1 positivity was significantly associated with the non-GC origin of DLBCL and poor prognosis. In our patient, DLBCL was classified to be of GC origin in accordance with Hans’ criteria and spontaneously regressed, indicating a good prognosis. These findings were consistent with previous reports. Casey et al.19 found that MYC binds directly to the promoters of PD-L1, and regulates the expression of PD-L1 on the tumor cell surface. In our patient, his lymphoma cells did not express MYC or PD-L1 based on immunohistochemical analysis. We considered that the PD-L1 negativity in the lymphoma cells to be due to the lack of myc expression. Thus, myc positivity may be a surrogate marker of PD-L1 expression in DLBCL. Further study of many cases may clarify this point.
In summary, we report the rare phenomenon of SR of DLBCL. Our patient has remained in complete metabolic remission, as demonstrated by PET-CT, over three years without any therapeutic modalities. The PD-L1/PD-1 axis and marked amount of tumor-infiltrating CD8-positive T cells may have played a role in the SR in our patient.
ACKNOWLEDGMENTS
We verbally received informed consent from the patient for the publication of this case report and accompanying images.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflicts of interest.
REFERENCES
- 1.Gascoyne RD, Campo E, Jaffe ES, et al. Diffuse large B-cell lymphoma, NOS. In: Swerdlow SH, Campo E, Harris NL, et al. (eds): WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. revised 4th ed, Lyon, IARC. 2017; pp. 291-297. [Google Scholar]
- 2.Ricci SB, Cerchiari U. Spontaneous regression of malignant tumors: importance of the immune system and other factors (Review) [Review]. Oncol Lett. 2010; 1: 941-945. 10.3892/ol.2010.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe R, Ogawa K, Maruyama Y, Nakamura N, Abe M. Spontaneous regression of diffuse large B-cell lymphoma harbouring Epstein-Barr virus: a case report and review of the literature. J Clin Exp Hematop. 2007; 47: 23-26. 10.3960/jslrt.47.23 [DOI] [PubMed] [Google Scholar]
- 4.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103: 275-282. 10.1182/blood-2003-05-1545 [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Bhargava P, Zhuang H, et al. Spontaneous regression of follicular, mantle cell, and diffuse large B-cell non-Hodgkin’s lymphomas detected by FDG-PET imaging. Clin Nucl Med. 2004; 29: 685-688. 10.1097/00003072-200411000-00002 [DOI] [PubMed] [Google Scholar]
- 6.Fukushima K, Hirosako S, Tenjin Y, et al. Pulmonary mucosa-associated lymphoid tissue lymphoma with spontaneous regression after computed tomography-guided needle biopsy: a case report and summary of 8 reported cases. Intern Med. 2016; 55: 3655-3660. 10.2169/internalmedicine.55.6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iihara K, Yamaguchi K, Nishimura Y, et al. Spontaneous regression of malignant lymphoma of the breast. Pathol Int. 2004; 54: 537-542. 10.1111/j.1440-1827.2004.01652.x [DOI] [PubMed] [Google Scholar]
- 8.Oya M, Hirahashi M, Ochi M, et al. Spontaneous regression of primary breast lymphoma. Pathol Int. 2009; 59: 664-669. 10.1111/j.1440-1827.2009.02424.x [DOI] [PubMed] [Google Scholar]
- 9.Iwatani T, Kawabata H, Miura D, Ota Y, Ohashi K. Complete spontaneous regression of primary diffuse large B-cell lymphoma of the breast. J Clin Oncol. 2011; 29: e113-e115. 10.1200/JCO.2010.31.2801 [DOI] [PubMed] [Google Scholar]
- 10.Graham PM, Richardson AS, Schapiro BL, Saunders MD, Stewart DM. Spontaneous regression of primary cutaneous diffuse large B-cell lymphoma, leg type with significant T-cell immune response. JAAD Case Rep. 2018; 4: 305-309. 10.1016/j.jdcr.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigematsu A, Iida M, Lien GS, et al. Spontaneous regression of primary malignant lymphoma of the stomach in two nontreated Japanese. J Clin Gastroenterol. 1989; 11: 511-517. 10.1097/00004836-198910000-00006 [DOI] [PubMed] [Google Scholar]
- 12.Monzen Y, Nakahara M, Nishisaka T. Spontaneous regression of primary malignant lymphoma of the prostate. Case Rep Urol. 2013; 2013: 1-3. 10.1155/2013/363072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaulard P, Swerdlow SH, Harris NK, Sundstrӧm C, Jaffe ES. Other iatrogenic immunodeficiency-associated lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al. (eds): WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. revised 4th ed, Lyon, IARC. 2017; pp. 462-464. [Google Scholar]
- 14.Okazaki T, Okazaki IM. [Regulation of autoimmunity and tumor immunity by immunoinhibitory co-receptor, PD-1]. Seikagaku. 2015; 87: 693-704 [Article in Japanese]. [PubMed] [Google Scholar]
- 15.Pizzi M, Boi M, Bertoni F, Inghirami G. Emerging therapies provide new opportunities to reshape the multifaceted interactions between the immune system and lymphoma cells. Leukemia. 2016; 30: 1805-1815. 10.1038/leu.2016.161 [DOI] [PubMed] [Google Scholar]
- 16.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015; 126: 2193-2201. 10.1182/blood-2015-02-629600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi T, Ikejiri F, Takami S, et al. Spontaneous regression of intravascular large B-cell lymphoma and apoptosis of lymphoma cells: A case report. J Clin Exp Hematop. 2015; 55: 151-156. 10.3960/jslrt.55.151 [DOI] [PubMed] [Google Scholar]
- 18.Xing W, Dresser K, Zhang R, et al. PD-L1 expression in EBV-negative diffuse large B-cell lymphoma: clinicopathologic features and prognostic implications. Oncotarget. 2016; 7: 59976-59986. 10.18632/oncotarget.11045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casey SC, Tong L, Li Y, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016; 352: 227-231. 10.1126/science.aac9935 [DOI] [PMC free article] [PubMed] [Google Scholar]